Abstract
The nano VO2 powders were prepared by hydrothermal synthesis. The effects of Gd and Nd element doping on the structure and phase transition temperature of VO2 were studied. The X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Scanning electron microscopy (SEM), and Transmission electron microscopy (TEM) results showed that Gd element and Nd element will affect the structure of VO2. Gd3+ and Nd3+ can occupy partial position of V4+ lattice and form solid solution, increasing the lattice parameters of VO2. Both the doped and un-doped VO2 powders exhibit a monoclinic structure at room temperature. Due to the lattice deformation caused by Gd or Nd doping, the aggregation of particles is prevented, and the grain is refined obviously. Differential scanning calorimetry curves showed that both Gd doping and Nd doping can reduce the phase transition temperature of VO2(M). When the Gd doping concentration is 6 at%, the phase transition temperature can be reduced from 71.7°C to 60.3°C, and the infrared transmittance before and after the phase transition also changes significantly, reaching more than 40%. Nd doping is similar, and the phase transition temperature decreased to 55.6°C with the addition of 9 at% Nd.
1 Introduction
To the best of our knowledge, there are about 20 kinds of vanadium oxides, each of which has different crystal structures and different properties [1,2]. According to incomplete statistics, at least eight kinds of vanadium oxides can achieve a mutual semiconductor-to-metal transition (SMT) in a temperature range from –147°C to 257°C. As representative vanadium oxide, the phase transition temperature of VO2 is about 68°C. It is close to room temperature, which is a first-order phase transition [3,4].
VO2 behaves as a semiconductor below the phase transition temperature, and as a metal above the phase transition temperature. Mutations with 4 or 5 orders of magnitude in the resistance (rate) can be obtained, accompanied by a sudden change in magnetic susceptibility, optical refractive index, transmittance, and reflectance during the SMT. Especially in the infrared and near-infrared bands, the optical transmittance changes most obviously [5,6,7]. These changes are because when the temperature is lower than the phase transition temperature, VO2 structure transformed from tetragonal rutile structure to monoclinic rutile structure, in which the electron position of dⅡ orbital and the π* orbital is changed. It will result in a change in the electron motion from a continuous state to a discontinuous state [8,9]. The thermotropic phase-transition characteristics of VO2 enable it to have high application value in the field of energy-saving windows [10,11]. But the phase transition temperature, ∼68°C, is still relatively high. To meet the requirements of the practical applications, a variety of approaches have been taken to decrease the phase transition temperature of VO2 [12], such as element doping [13], electric fields [14], surface and interface engineering [15], preparation of VO2 nanocomposites [16,17,18], and structural modification [19,20]. Among these strategies, element doping can lower SMT effectively. A series of studies on element doping have been carried out, mainly including: W [21,22,23,24,25], Mg [26], Mo [27,28], F [29,30], Zr [31], Sb [32], Cr [33], Nb [34], Al [35], etc.
It is well known that rare earth (RE)-doping has been used as an effective approach for modulating the microstructures and photoelectric performance of optoelectronic materials and photocatalytic materials. It was reported that Eu-doping decreases the SMT temperature of monoclinic VO2 polycrystalline thin films from 68°C to 47.5°C due to substitution of trivalent Eu3+ ions in VO2 lattice [36]. Similar decreasing phenomena in SMT temperature of VO2 polycrystalline thin films with Y3+-doping are also observed [37].
The microstructure change caused by doping can obviously adjust the physical properties of vanadium oxide, while the symmetric local structure can help to reduce the thermal barrier of SMT, thus reducing the SMT temperature [38]. Considering the high symmetry of cubic Gd2O3 and Nd2O3 lattice [39], it can be inferred that Gd3+ or Nd3+ may be a promising rare earth dopant. Based on this motivation, we explored the influence of Gd3+ doping and Nd3+ doping on the structure and SMT properties of VO2 powders.
2 Experimental
10 mmol oxalic acid (C2H2O4·2H2O, analytically pure) and 5 mmol vanadium pentoxide (V2O5, analytical reagent) powders were mixed with 80 mL of deionized water. The mixture was continuously stirred at 50°C until the suspension liquid turned into dark blue. Then 10 mL of urea precipitant (H2NCONH2, analytically pure, 0.15 mol‧L−1) and a certain proportion of Gd(NO3)3 (analytically pure) or Nd(NO3)3 (analytically pure) were added to the dark blue suspension liquid. After sufficient stirring, the mixed suspension was transferred to a closed polytetrafluoroethylene reactor with heat preservation at 190°C for 60 h. After the completion of the reaction, the product was filtered and washed to obtain VO2(B) precursor. The precursor VO2(B) was placed in a tube furnace with heat treatment at 600°C for 5 h under nitrogen atmosphere and then cooled down to ambient temperature in the furnace. The product was dispersed with ultrasonic treatment to obtain nano VO2(M) powder. The effect of Gd doping and Nd doping on the microstructure and phase transition temperature of VO2 powder was investigated. Gd atomic ratio of 2%, 4%, and 6% and Nd/V atomic ratio of 3%, 6%, and 9% were designed to conduct the comparative study, respectively.
X-ray diffractometer (D8 ADVANCE A25, Cu Kα as the source of radiation, output power was 3 kW) was employed to analyze the phase composition. The chemical valences of elements in the powders were detected by X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi). Scanning electron microscope (SEM, JSM-6390A) was applied to observe the microstructure. The TEM and HRTEM images of the powders were obtained by using high-resolution transmission electron microscopy (HRTEM, JEM–2100F STEM/EDS). Laser particle size analyzer (Mastersizer 2000) was used to test the particle size distribution of the nano powder particles. Synchronous thermal analyzer (STA449F3) and Fourier transform infrared spectrometer (Thermo Fisher Scientific IS5) was used to analyze the phase transition characteristics of the nano VO2(M) powders.
3 Results and discussion
3.1 Effect of Gd/Nd doping on the phase and structure of VO2
Gd/Nd-doped VO2(M) powders were prepared with Gd(NO3)3 or Nd(NO3)3 as dopant. X-ray diffraction (XRD) results of different samples are shown in Figure 1. All the Gd-doped and Nd-doped samples exhibited obvious monoclinic VO2 structure, which indicates that moderate Gd doping or Nd doping does not significantly change the crystal structure of VO2(M). After Gd3+ or Nd3+ is incorporated, it can form a substitutional solid solution with VO2.
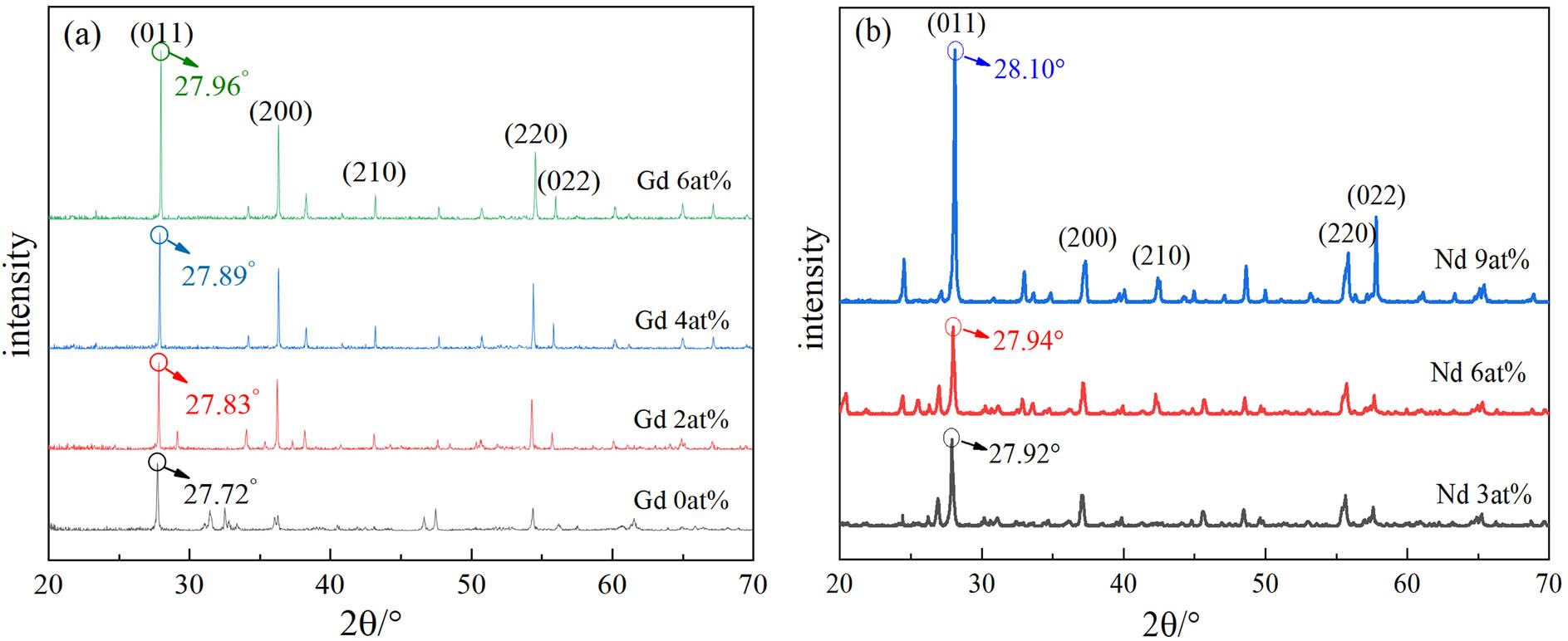
XRD analysis of VO2(M) powders doped with different dopants: (a) Gd3+ and (b) Nd3 +.
However, it can also be seen from Figure 1 that Gd/Nd doping affects the microstructure of VO2. For un-doped samples, the angular position of the strongest Bragg peak, the (011)-peak, is located at 27.72°. After doping 2, 4, and 6 at% Gd in the powder samples, the angular position is shifted to 27.83°, 27.89°, and 27.96°, respectively. These small shifts to higher diffraction angles indicate the decrease in the crystal lattice spacing according to Bragg’s equation:
where d – spacing, λ – wavelength of X-ray, and θ – diffraction angle. With the increase in Nd doping amount, the diffraction angle also changed slightly. After doping 3, 6, and 9 at% Nd in the powder samples, the angular position is shifted to 27.92°, 27.94°, and 28.10°, respectively. The main reason is that the ion radii of Gd3+ and Nd3+ are larger than V4+. The main reason is that the ion radii of Gd3+ and Nd3+ are larger than V4+. After replacing V4+ with Gd3+ or Nd3+, the lattice spacing of (011) plane is compressed, resulting in the diffraction peak deviation to a higher angle.
In order to verify whether the Gd/Nd atoms existed in the M-phase VO2 lattice, XPS characterizations were performed on the 4 at% Gd-doped and 6 at% Nd-doped powder samples, and the results are shown in Figures 2 and 3. Figure 2a shows four elements including C, V, Gd, and O in 4 at% Gd-doped powder sample, indicating that the obtained powder product is Gd-doped VO2. A peak appears in the O1s region with the major peak at 530.17 eV, which is assigned to O2– ions in the V–O bonding. The V2p3/2 peak splits into two peaks at 517.37 eV [V(V)] and 516.07 eV [V(IV)], as shown in Figure 2b. The peak of V5+ was stronger than that of V4+, which was mainly due to the exposure of powder materials in the air, leading to the oxidation of some particle surfaces, while XPS detected the main surface composition of the particle materials.

XPS spectra for the 4 at% Gd-doped VO2(M) powder samples: (a) survey spectrum, (b) core-level spectrum for V 2p, and (c) core-level spectrum for Gd 4d.

XPS spectra for the 6 at% Nd-doped VO2(M) powder samples: (a) survey spectrum, (b) core-level spectrum for V 2p, and (c) core-level spectrum for Nd 3d.
Two peaks of Gd 4d are shown in Figure 2c at positions of 141.17 and 142.07 eV, respectively, which is corresponding to Gd 4d, and it indicates that the valence state of Gd3+ exists in the Gd-doped VO2 powder.
The O and V peaks of Nd-doped sample were similar to those in Figure 2. In Figure 3c, there are two obvious peaks, which are binding energy 983.77 and 1,003.0 eV, respectively, corresponding to Nd 3d. It indicates that the valence state of Nd3+ exists in the Nd-doped VO2 powder.
Figure 4 is a topographical view of nano VO2 powders with different dopants. The influence of heat treatment on the micro-structure morphology was researched. It can be seen from Figure 4a that the sample without heat treatment exhibits a typical VO2(B) nanoneedle morphology. After heat treatment at 600°C for 5 h, the particle size of the sample increased significantly, showing a micron-scale rod shape, as shown in Figure 4b. This is mainly because the V element diffusion among the grains promotes the interparticle fusion and grain boundary migration during the high-temperature heat treatment process, thereby the particle size is increased. It can also be seen from Figure 4b–e that Gd doping can play a certain role in refining grains. After 4 and 6 at% Gd doping, the original shapes of VO2 are changed into the spheroidal shape, and the particle sizes are reduced to about 200 nm. The same phenomenon was observed for 6 at% Nd-doped sample, which can be seen from Figure 4f. This is probably due to the more lattice deformation of recrystallization caused by Gd/Nd doping during the heat treatment process. It will provide more chances to generate new nucleation, thus creating appropriate conditions for grain refinement. In addition, Gd–O and Nd–O bonding strength are higher than that of V–O bond, the diffusion of V atom is inhibited, thus the grain growth is prevented to a certain extent.

SEM images of nano VO2(M) powders doped with different dopants: (a) 0 at% without heat treatment, (b) 0 at%, heated at 600°C for 5 h, (c) 2 at% Gd, (d) 4 at% Gd, (e) 6 at% Gd, and (f) 6 at% Nd.
To further understand the element distribution of the powder samples, elemental mapping analyses were performed, as shown in Figures 5 and 6. It is clearly seen that V, Gd, and O elements are distributed uniformly in the sample, which suggested that the Gd element is well dispersed into VO2 lattice. The Nd element is also mostly dispersed in the sample, but some segregation for Nd can be observed obviously. The reason for the uneven compositions can be derived from the above given analysis. This indicates that although Nd can also be doped into VO2 lattice, the doping uniformity is not as good as that of Gd.

Elements distribution on the Gd-doped VO2 powder: (a) SEM and (b–d) distribution of V, Gd, and O.

Elements distribution on the Nd-doped VO2 powder: (a) SEM and (b–d) distribution of V, Nd, and O.
Particle size distribution diagrams of VO2 powder samples doped with different dopants are shown in Figure 7. It can be seen that the particle size of each sample is substantially normally distributed. The average particle size of the un-doped samples was 898.6 nm. After adding 2, 4, and 6 at% Gd doping, the average particle size of VO2 powder samples dropped to 733.8, 332.4, and 167.3 nm, respectively. These particle size distribution data are similar to the SEM results of Figure 4. After adding 3, 6, and 9 at% Nd doping, the average particle size of VO2 powder samples dropped to 656.3, 296.2, and 263.8 nm, respectively. The results of the particle size analysis show that Gd or Nd doping has an obvious effect of grain refinement. Among all the doping level, the effect of grain refinement at 6 at% Gd doping is more obvious. Smaller average particle size and narrower distribution range can be obtained, which indicate that the particle size is relatively uniform.

Particle size distribution of nano Gd/Nd-doped VO2 powders: (a) Gd and (b) Nd.
In order to further analyze the lattice structure of the nano VO2 powder samples, the TEM test was performed on the sample with 6 at% Gd doping, and the results are shown in Figure 8. It can be seen from Figure 8a that the shape of the particles is mainly spherical-like particles, the particle size is substantially less than 300 nm, and the particle size distribution range is wide, which is consistent with the observation by SEM. In Figure 8b, the clear lattice planes can be seen, indicating a good crystallinity of the obtained nano VO2 powder. The interplanar distance of (011) plane is 0.321 nm, which is in accordance with the HRTEM image of VO2(M). Energy spectrum analysis was carried out for different regions in Figure 8a, and the results were shown in Figure 8c. Besides the copper elements in the micro-grid of copper mesh, the main elements in different regions were only Gd, O, and V, indicating that the nano VO2 powder prepared by this method had little impurity content.

(a) TEM, (b) HRTEM images, and (c) EDS for the 6 at% Gd-doped VO2 powders.
3.2 Effect of Gd/Nd doping on phase transformation of VO2
Differential scanning calorimetry (DSC) was applied to investigate the Gd/Nd concentrations on the phase transition temperature of VO2(M) powder samples, and the results are shown in Figure 9.

DSC curves of VO2(M) powders doped with different dopants: (a) Gd and (b) Nd.
It can be seen from Figure 9 that obvious endothermic peaks correspond to the phase transitions for all the tested powder samples during environmental heating process. The phase-transition temperature decreases with increase in the Gd doping amount. When adding 2 at% Gd, the phase-transition of VO2(M) reduced from 71.7°C to 65.9°C. Further increasing Gd concentration up to 6 at%, the phase-transition temperature dropped down to 60.3°C. Nd doping also plays a role in reducing the phase-transition temperature of VO2(M). When adding 6 and 9 at% Nd, the phase-transition of VO2(M) reduced to 58.8°C and 55.6°C, respectively. Analysis of its reasons, mainly because the Gd or Nd doping changes the energy level structure of VO2. The introduction of impurity elements and the difference in radius of Gd/Nd and V ions destroy the stable phase structure combining V4+–V4+. With increase in the Gd3+ or Nd3+ amount, the lattice deformation is more serious, which will result in reducing the phase-transition temperature of nano VO2 powder.
In order to investigate the change in the transmission properties of Gd/Nd-doped VO2 before and after the phase transition, infrared transmittance tests were performed on 6% Gd-doped VO2 (T c1 = 60.3°C) and 6% Nd-doped VO2 (T c2 = 58.8°C). The test temperatures were 25°C and 65°C, separately. The obtained results are shown in Figure 10.

Infrared transmittance of VO2 powders: (a) with 6 at% Gd doping and (b) with 6 at% Nd doping.
It can be clearly seen in Figure 10a that the infrared absorption spectra of 6 at% Gd-doped VO2 samples at 25°C and 65°C is quite different. 25°C is lower than T c1, and its infrared transmittance is higher, with the highest infrared transmittance reaching about 60%. 65°C is higher than T c1, the infrared transmittance is basically maintained within 10%. Under the two conditions, the change in infrared transmittance ΔT is more than 50%. This indicates that under the condition of 65°C, VO2 underwent a phase transition and was transformed into a metallic phase, which had a strong reflection on infrared light and reduced the transmittance. At 65°C, the 6 at% Nd-doped sample also underwent metallic phase transformation, and the infrared transmittance is generally less than 30%. Compared with the condition of 25°C, the infrared transmittance is reduced by about 30%.
4 Conclusion
For VO2(M) powders doped with Gd(NO3)3 and Nd(NO3)3, Gd3+ and Nd3+ will occupy some certain positions in V4+ lattice, forming substitutional solid solution and increasing the lattice parameters of VO2.
The lattice deformation of VO2 caused by Gd or Nd doping will prevent the particles from accumulating, thus refining the grains.
Due to the difference in ion radius, Gd or Nd doping will destroy the stable phase structure of V4+–V4+, thus reducing the phase transition temperature.
With increase in the doping amount, the phase transition temperatures of VO2(M) powders decrease gradually. With the introduction of 6 at% Gd or 9 at% Nd into VO2(M) powders, the phase transition temperature can be reduced from 71.7°C to 60.3°C and 55.6°C, respectively.
-
Funding information: This work is supported by the Natural Science Foundation of Shaanxi Province (No. 2019JQ–761 and No. 2020JQ–681), the Scientific and Technological Innovation Team Project of Shaanxi Innovation Capability Support Plan (2022TD–30), the Foundation of Shaanxi Educational Committee (no. 20JK0702), and the Regional Innovation Capability Guidance Plan of Shaanxi Province (No. 2022QFY10–05 and No. 2022QFY10–03).
-
Author contributions: Bin Wang and Dandan Zhao: writing – original draft, writing – review and editing, conception, and design of the study; Jinjing Du, Linbo Li, and Jun Zhu: writing – original draft, methodology, analysis, and interpretation of data; Chao Wang: writing – original draft and methodology.
-
Conflict of interest: Authors state no conflict of interest.
References
[1] Guinneton F, Sauques L, Valmalette JC, Cros F, Gavarri JR. Role of surface defects and microstructure in infrared optical properties of thermochromic VO2 materials. J Phys Chem Solids. 2005;66:63–73. 10.1016/j.jpcs.2004.08.032.Search in Google Scholar
[2] Park IS, Choi SY, Ha JS, Khim J. Synthesis and characterization of visible-light absorbing ordered mesoporous titanosilicate incorporated with vanadium oxide. Chem Phys Lett. 2007;444(1–3):161–6. 10.1016/j.cplett.2007.07.007.Search in Google Scholar
[3] Zerov VY, Kulikov YV, Malyarov VG, Khrebtov IA, Shaganov II, Shadrin EB. Vanadium oxide films with improved characteristics for IR microbolometric matrices. Tech Phys Lett. 2001;27(5):378–80. 10.1134/1.1376757.Search in Google Scholar
[4] Goodenough GB. The two components of the crystallographic transition in VO2. J Solid State Chem. 1971;3(4):490–500. 10.1016/0022-4596(71)90091-0.Search in Google Scholar
[5] Wang SF, Liu MS, Kong LB, Long Y, Jiang XC, Yu AB. Recent progress in VO2 smart coatings: strategies to improve the thermochromic properties. Prog Mater Sci. 2016;81:1–54. 10.1016/j.pmatsci.2016.03.001.Search in Google Scholar
[6] Gao Y, Luo H, Zhang Z, Kang L, Chen Z, Du J, et al. Nanoceramic VO2 thermochromic smart glass: a review on progress in solution processing. Nano Energy. 2012;1:221–46. 10.1016/j.nanoen.2011.12.002.Search in Google Scholar
[7] Yuce H, Alaboz H, Demirhan Y, Ozdemir M, Ozyuzer L, Aygun G. Investigation of electron beam lithography effects on metal-insulator transition behavior of vanadium dioxide. Phys Scr. 2017;92(11):114007. 10.1088/1402-4896/aa90a3.Search in Google Scholar
[8] Park JH, Coy JM, Kasirga TS, Huang C, Fei Z, Hunter S, et al. Measurement of a solid-state triple point at the metal-insulator transition in VO2. Nature. 2013;500(7463):431–4. 10.1038/nature12425.Search in Google Scholar PubMed
[9] Budai JD, Hong J, Manley ME, Specht ED, Li CW, Tischler JZ, et al. Metallization of vanadium dioxide driven by large phonon entropy. Nature. 2014;515(7528):535–9. 10.1038/nature13865.Search in Google Scholar PubMed
[10] Li M, Magdassi S, Gao YF, Long L. Hydrothermal synthesis of VO2 polymorphs: advantages, challenges and prospects for the application of energy efficient smart windows. Small. 2017;13(36):1701147. 10.1002/smll.201701147.Search in Google Scholar PubMed
[11] Granqvist CG, Niklasso GA, Buratti C. Thermochromic oxide-based thin films and nanoparticle composites for energy-efficient glazings. Buildings. 2016;7:3. 10.3390/buildings7010003.Search in Google Scholar
[12] Cui YY, Ke YJ, Liu C, Chen Z, Wang N, Zhang LM, et al. Thermochromic VO2 for energy-efficient smart windows. Joule. 2018;2:1–40. 10.1016/j.joule. 2018.06.018.Search in Google Scholar
[13] Wu C, Feng F, Feng J, Dai J, Peng L, Zhao J, et al. Hydrogen-incorporation stabilization of metallic VO2(R) phase to room temperature, displaying promising low-temperature thermoelectric effect. J Am Chem Soc. 2011;133(35):13798–801. 10.1021/ja203186f.Search in Google Scholar PubMed
[14] Jeong J, Aetukuri N, Graf T, Schladt TD, Samant MG, Parkin SSP. Suppression of metal-insulator transition in VO2 by electric field-induced oxygen vacancy formation. Science. 2013;339(6126):1402–5. 10.1126/science.1230512.Search in Google Scholar PubMed
[15] Xu G, Huang CM, Tazawa M, Jin P, Chen DC, Miao L. Electron injection assisted phase transition in a nano-Au-VO2 junction. Appl Phys Lett. 2008;93:061911. 10.1063/1.2972106.Search in Google Scholar
[16] Zhu JT, Huang AB, Ma HB, Chen YX, Zhang SP, Ji SD, et al. Hybrid film of VO2 nanoparticles and nickel(II)-based ligand exchange thermochromic system: excellent optical performance with a temperature responsive colour change. N J Chem. 2017;41(2):830–5. 10.1039/C6NJ03369E.Search in Google Scholar
[17] Zhu J, Zhou Y, Wang B, Zheng J, Ji S, Yao H, et al. Vanadium dioxide nanoparticle-based thermochromic smart coating: high luminous transmittance, excellent solar regulation efficiency, and near room temperature phase transition. ACS Appl Mater Inter. 2015;7(50):27796–803. 10.1021/acsami.5b09011.Search in Google Scholar PubMed
[18] Liu C, Cao X, Kamyshny A, Law JY, Magdassi S, Long Y. VO2/Si-Al gel nanocomposite thermochromic smart foils: largely enhanced luminous transmittance and solar modulation. J Colloid Interf Sci. 2014;427:49–53. 10.1016/j.jcis.2013.11.028.Search in Google Scholar PubMed
[19] Wu S, Tian S, Liu B, Tao H, Zhao X, Palgrave RG, et al. Facile synthesis of mesoporous VO2 nanocrystals by a cotton-template method and their enhanced thermochromic properties. Sol Energy Mat Sol C. 2018;176:427–34. 10.1016/j.solmat.2017.11.001.Search in Google Scholar
[20] Wang M, Xue YQ, Cui ZX, Zhang R. Size-dependent crystal transition thermodynamics of nano-VO2 (M). J Phys Chem C. 2018;122(15):8621–7. 10.1021/acs.jpcc.8b01183.Search in Google Scholar
[21] Li B, Tian SQ, Tao HZ, Zhao XJ. Tungsten doped M-phase VO2 mesoporous nanocrystals with enhanced comprehensive thermochromic properties for smart windows. Ceram Int. 2019;45(4):4342–50. 10.1016/j.ceramint.2018.11.109.Search in Google Scholar
[22] Zomaya D, Xu WZ, Grohe B, Mittler S, Charpentier PA. W-doped VO2/PVP coatings with enhanced thermochromic performance. Sol Energy Mat Sol C. 2019;200:109900. 10.1016/j.solmat.2019.04.022.Search in Google Scholar
[23] Cao CX, Gao YF, Luo HJ. Pure single-crystal rutile vanadium dioxide powders: synthesis, mechanism and phase-transformation property. J Phys Chem C. 2011;48:18810–4. 10.1021/jp8073688.Search in Google Scholar
[24] Li J, Liu CY, Mao LJ. The character of W-doped one-dimensional VO2(M). J Solid State Chem. 2009;182:2835–9. 10.1016/j.jssc.2009.07.031.Search in Google Scholar
[25] Liang S, Shi Q, Zhu H, Peng B, Huang W. One-step hydrothermal synthesis of W doped VO2(M) nanorods with a tunable phase-transition temperature for infrared smart windows. ACS Omega. 2016;1:1139–48. 10.1021/acsomega.6b00221.Search in Google Scholar PubMed PubMed Central
[26] Mlyuka NR, Niklasson GA, Granqvist CG. Mg doping of thermochromic VO2 films enhances the optical transmittance and decreases the metal-insulator transition temperature. Appl Phys Lett. 2009;95(17):171909. 10.1063/1.3229949.Search in Google Scholar
[27] Zhang YF, Zhang JC, Zhang XZ, Huang C, Zhong YL, Deng Y. The additives W, Mo, Sn and Fe for promoting the formation of VO2(M) and its optical switching properties. Mater Lett. 2013;92:61–4. 10.1016/j.matlet.2012.10.054.Search in Google Scholar
[28] Mai LQ, Chen W, Xu Q, Peng GF, Zhu QY. Mo doped vanadium oxide nanotubes: microstructure and electrochemistry. Chem Phys Lett. 2003;382(3–4):307–12. 10.1016/j.cplett.2003.10.067.Search in Google Scholar
[29] Zhang Y, Zhang J, Zhang X, Deng Y, Zhong Y, Huang C, et al. Influence of different additives on the synthesis of VO2 polymorphs. Ceram Int. 2013;39(7):8363–76. 10.1016/j.ceramint.2013.04.016.Search in Google Scholar
[30] Dai L, Chen S, Liu J, Gao Y, Zhou J, Chen Z, et al. F-doped VO2 nanoparticles for thermochromic energy-saving foils with modified color and enhanced solar-heat shielding ability. Phys Chem Chem Phys. 2013;15(28):11723–9. 10.1039/c3cp51359a.Search in Google Scholar PubMed
[31] Shen N, Chen S, Chen Z, Liu XL, Cao CX, Dong BR, et al. The synthesis and performance of Zr-doped and W-Zr-codoped VO2 nanoparticles and derived flexible foils. J Mater Chem A. 2014;2(36):15087–93. 10.1039/c4ta02880e.Search in Google Scholar
[32] Gao Y, Cao C, Dai L, Luo H, Kanehira M, Ding Y, et al. Phase and shape controlled VO2 nano-structures by antimony doping. Energy Env Sci. 2012;5(9):8708–13. 10.1039/c2ee22290f.Search in Google Scholar
[33] Ji C, Wu Z, Wu X, Wang J, Liu X, Gou J, et al. Terahertz transmittance and metal-insulator phase transition properties of M2 phase VO2 films induced by Cr doping. Appl Surf Sci. 2018;455:622–8. 10.1016/j.apsusc.2018.05.085.Search in Google Scholar
[34] Wang ZL, Zhang R, Chen XH, Fu QS, Li CL, Yuan SL, et al. Nb doping effect in VO2 studied by investigations of magnetic behavior. Ceram Int. 2018;44(7):8623–7. 10.1016/j.ceramint.2018.02.079.Search in Google Scholar
[35] Wan JY, Ren QH, Wu NN, Gao YF. Density functional theory study of M-doped (M = B, C, N, Mg, Al) VO2 nanoparticles for thermochromic energy-saving foils. J Alloy Compd. 2016;662:621–7. 10.1016/j.jallcom.2015.12.100.Search in Google Scholar
[36] Cao X, Wang N, Madassi S, Mandler D, Long Y. Europium doped vanadium dioxide material: reduced phase transition temperature, enhanced luminous transmittance and solar modulation. Sci Adv Mater. 2014;6(3):558–61. 10.1166/sam.2014.1777.Search in Google Scholar
[37] Gu D, Sun ZH, Zhou X, Guo R, Wang T, Jiang YD. Effect of yttrium-doping on the microstructures and semiconductor-metal phase transition characteristics of polycrystalline VO2 thin films. Appl Surf Sci. 2015;359:819–25. 10.1016/j.apsusc.2015.10.179.Search in Google Scholar
[38] Gu DE, Zhou X, Sun ZH, Jiang YD. Influence of Gadolinium-doping on the microstructures and phase transition characteristics of VO2 thin films. J Alloy Compd. 2017;705:64–9. 10.1016/j.jallcom.2017.02.138.Search in Google Scholar
[39] Mejai N, Debelle A, Thomé L, Sattonnay G, Gosset D, Boulle A, et al. Depth-dependent phase change in Gd2O3 epitaxial layers under ion irradiation. Appl Phys Lett. 2015;107(13):131903. 10.1063/1.4932089.Search in Google Scholar
© 2022 Bin Wang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

