Abstract
Thermal plasma is a promising technology widely used in materials processing and waste treatment due to its unique properties including high temperature, high energy density, high chemical activity, and high quench rate. Air-plasma is preferentially used because air is of low price as plasma gas. The content of NO x in off-gas from air-plasma was determined using a gas analyzer, and a treatment unit was designed for the green air-plasma process. Results show that the concentration of NO x in off-gas from air-plasma was 2,489 and 9,112 ppm when the plasma input power was 50 and 150 kW, respectively. O2 in the off-gas would act as an oxidant to promote NO x absorption; thus, alkali absorption method was directly used for the treatment of the present off-gas from air-plasma. The absorption efficiency could be increased to 62.2% when additional O2 was provided into the off-gas to change its O2 content from 20% to 50%. The absorption rate was estimated based on the experimental data and a multistage absorption unit design, which could be reduced below 100 ppm and meet the emission standard. This article presents the feasibility of thermal plasma off-gas purification, so as to truly realize the green plasma process.
1 Introduction
Plasma is usually considered the fourth state of matter that is different from solid, liquid, and gaseous substances. It is a quasi-neutral gas composed of electrons, ions, neutrals in the ground state, excited species, and photons, in which the total number of positive charges is equal to that of negative charges [1,2]. Plasma can be mainly divided into high-temperature plasma and low-temperature plasma according to the ionization degree and temperature [3,4]. High-temperature plasma exhibits similar temperatures for electrons and ions and a very high ionization degree (≈1), while low-temperature plasma exhibits higher electron temperature T e and lower ion temperature T i, in this case, a partially ionized medium [5]. Low-temperature plasma can be further classified as thermal and non-thermal plasma, where plasma with a comparable temperature of heavy ions to that of electrons is known as thermal plasma [6].
Thermal plasma is usually generated by way of making electric current pass-through gases. Many routes, such as direct current (DC), alternating current (AC), radio frequency (RF) and microwaves, have been employed to generate thermal plasma [7]. The plasma torch can be classified according to the generating source, calling DC plasma, AC plasma, and RF plasma. The DC arc plasma is usually used in two modes as non-transferred arc and transferred arc [8]. Thermal plasma has distinct properties compared to ordinary chemical reaction media, including high temperature, high energy density, and high chemical activity, which makes plasma a promising technology widely used in material processing and waste treatment, usually known as “plasma enhancement” [2,5].
Plasma processing of materials can be classified into four categories [9,10]. (i) Plasma spheroidization: powders with irregular shape fed into plasma flame were melted in the high-temperature region to form spherical liquid drops and then fast quenched (with sufficient water-cooling or gas-cooling out of the plasma flame) to form spherical powders under sharp quenching rate [11,12,13]. (ii) Thermal plasma evaporation and condensation: powders with large sizes were fed into plasma flame and vaporized, and ultra-fine powders were produced after fast quenching by physical vapor deposition [14,15,16]. (iii) Plasma-enhanced chemical vapor deposition: reaction precursors are fed in the form of gases of vapors, and plasma is introduced into the reaction zone to activate the precursors [17,18,19,20,21]. (iv) Plasma treatment of solid phases: target material is prepared by simply exposing the solid precursor to plasma through plasma–solid interaction [22,23].
Thermal plasma is also successfully applied to treat a variety of wastes. On the one hand, plasma can thermally decompose hazardous organic compounds into simpler fuel gas which in turn was employed for diverse applications [24]. On the other hand, plasma also helps melt solid inorganic components or, with the addition of glass former, vitrify waste to form a stable glassy slag product in which hazardous substances are trapped within the glass network. The inert glassy slag which is obtained as a by-product in the plasma-assisted waste treatment can be upgraded to value-added products such as glass ceramics, road filler material, and building construction material [25,26]. Different waste types such as municipal wastes (paper, biomass, plastic, cloth, etc.) and hazardous wastes (from industrial, agriculture, and hospitals) were processed via thermal plasma technology [27,28,29,30,31].
In a project of thermal plasma processing, one of the main parameters which need to take into account is the system pressure. In principle, atmospheric-pressure plasma devices can provide a crucial advantage over low-pressure plasma because they eliminate complications introduced by the need for vacuum. Therefore, atmospheric pressure plasma has been mostly employed for material processing and waste treatment. Air is the most common gas used, but several inert gases (N2, He, Ar, etc.) have also been widely used as plasma gas [32,33,34]. When air is used, it must be subjected to a large differential in electrical potential in order for air to conduct electricity. Otherwise, Ar is usually used at the initial stage of the arc ignition process and switched to air after the plasma operates stably.
Another problem with the use of air as a thermal plasma gas is that N2 and O2 would react with each other to form NO x in the unordinary chemical reaction media provided by thermal plasma. When a plasma wind tunnel was used for ablation of ultrahigh temperature ceramic materials, there existed NO x in the exhaust gas which could be preliminarily judged through the color and smell.
This article will discuss the exhaust gas treatment based on the data accumulation. The content of NO x in off-gas from air-plasma was determined using a gas analyzer, removal methods and their important parameters were examined according to the characteristics of plasma off-gas, and a treatment unit was designed so as to truly realize the green plasma process.
2 Experimental
Different thermal plasma devices including DC plasma, RF plasma, and three-phase AC plasma were designed and used in our laboratory. The DC plasma and three-phase AC plasma are usually applied for waste treatment, and the RF plasma is commonly used for functional powder material synthesis. DC plasma is also used to test the ablation behavior of ultra-high-temperature materials. When the air was used as plasma gas during these processes, the off-gas was a light brown pungent gas. We supposed that something had to be conducted for the plasma technique both in experimental conduction and in future application.
Figure 1 illustrates the off-gas treatment unit connected with the thermal plasma reactor. The green lines exhibit the gas flow path, while the blue lines are the circulating absorption liquid pipeline. Air is supplied by the air compressor as the plasma gas, and the off-gas is exhausted by an air pump and sent into the off-gas treatment unit. The gas to be treated enters from the bottom of the absorption tower and runs upward, while the absorption liquid enters from the top of the absorption tower and runs downward driven by a circulating pump. In order to enhance their contact and absorption efficiency, we set up sieve plates and porous suspended ball fillers in the absorption tower. The gas before and after absorption was tested using a gas analyzer. The off-gas after absorption was exhausted to the atmosphere or introduced into a secondary stage absorption.

Illustration of off-gas treatment unit connected with thermal plasma reactor.
Figure 2 shows the pictures of off-gas treatment unit connected with RF (Figure 2a) and DC (Figure 2b) plasma reactor, respectively. Plasma is usually ignited using Ar as a working gas and switched to air afterward. The off-gas is pumped out of the plasma reactor so that the system pressure maintains at negative pressure, which is conducive to the stable operation of thermal plasma for a long time. In the present work, the plasma equipment runs without any materials treated, so that all NO x comes from the plasma itself. The off-gas was tested online using a flue gas analyzer (Testo-350, Testoterm).

Picture of off-gas treatment unit connected with (a) RF and (b) DC plasma reactor.
3 Results and discussion
3.1 Formation of NO x in air-plasma
NO x includes N2O, NO, NO2, N2O3, N2O4, and N2O5, in which NO and NO2 are often referred to as air pollutants. The formation of NO x can be ruled by the following reactions between N2 and O2:
Whether a chemical reaction occurs spontaneously is usually judged by the change of the Gibbs free energy ΔG. Thermodynamic calculation has been made based on the data given in the literature [35]. Figure 3 shows ΔG of the above reactions as a function of temperature.

ΔG of the above reactions as a function of temperature.
ΔG value for reaction in Eq. 1 decreases as the temperature increases and becomes lower than zero at the temperature around 7,300°C, indicating that reaction in Eq. 1 could take place spontaneously when the temperature is above 7,300°C. ΔG value for other reactions increases as the temperature increases and keeps positive. Thermodynamic calculation shows that the earlier reactions to form NO x cannot occur under conventional conditions.
NO x in nature mainly originates from lightning, which is a form of plasma. Plasma can provide highly chemically active species, promoting reactions that cannot occur under normal conditions. That is the reason why NO x can be detected in the off-gas of thermal plasma.
3.2 Detection of NO x from air-plasma
Detection of NO x was first conducted using the 50 kW air-plasma. Detailed plasma parameters are listed in Table 1.
Detailed parameters for 50 kW air-plasma
| Parameters | Values |
|---|---|
| Rated power | 50 kW |
| Operating voltage | 297 V |
| Operating current | 165 A |
| Total airflow | 16 m3·h−1 |
Figure 4 shows the NO x concentration in the off-gas from the 50 kW air-plasma. The concentration increased and reached a stable value 12 s after the beginning of the test. The time lag is because one needs to pump the off-gas into the analyzer for analysis.
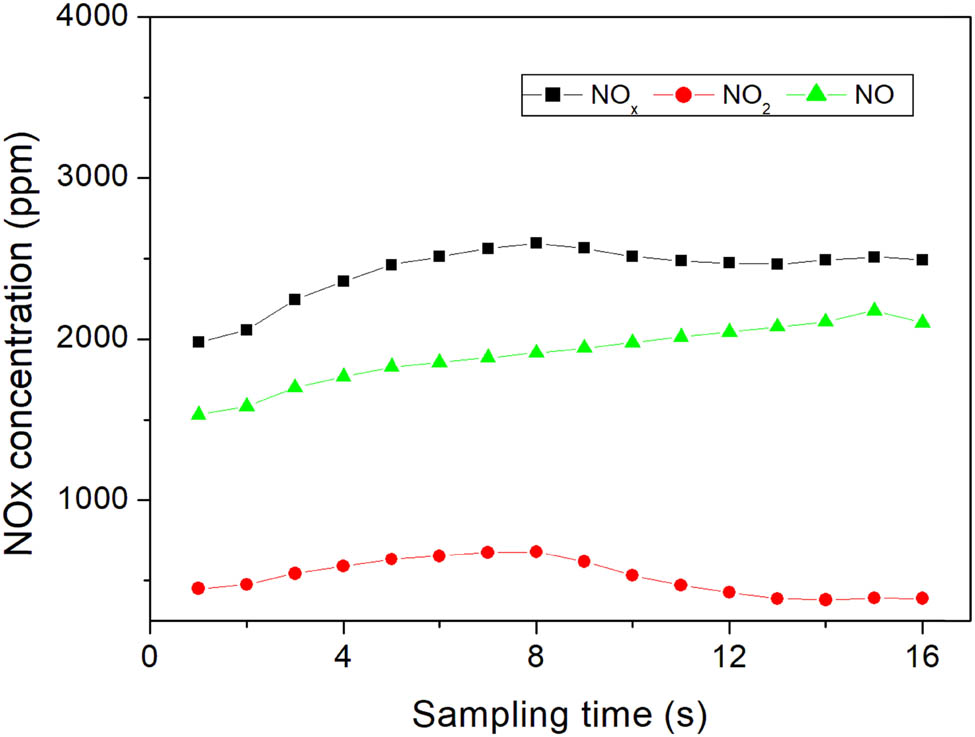
NO x concentration in the off-gas from air-plasma at different sampling times.
The mean concentration was calculated based on the last four data as shown in Table 2. The mean concentration of NO and NO2 was 2,101 and 388 ppm, respectively, and the total NO x content is 2,489 ppm. The ratio of NO/NO2 after stabilization is about 5.4.
NO x concentration and the calculated mean value
| Serial number | NO (ppm) | NO2 (ppm) | NO x (ppm) |
|---|---|---|---|
| 01 | 2,077 | 389 | 2,466 |
| 02 | 2,107 | 381 | 2,488 |
| 03 | 2,117 | 392 | 2,509 |
| 04 | 2,104 | 389 | 2,493 |
| Mean value | 2,101 | 388 | 2,489 |
| Standard deviation | 219.2 | 16.7 | 236.5 |
In order to examine the influence of the plasma power on the NO x concentration in off-gas, a 150 kW air-plasma was applied. Detailed plasma parameters are listed in Table 3.
Detailed parameters for 150 kW air-plasma
| Parameters | Values |
|---|---|
| Rated power | 150 kW |
| Operating voltage | 410 V |
| Operating current | 348 A |
| Total airflow | 50 m3·h−1 |
Figure 5 shows the NO x concentration in the off-gas from the 150 kW air-plasma. The air-plasma with the input power of 150 kW was equipped with a plasma wind tunnel, and the detection point was 30 m away from the plasma flame. There was less than 3 m between the plasma flame and detection point when the laboratory had 50 kW plasma. The long distance provided more time for the conversion from NO to NO2 in the air.
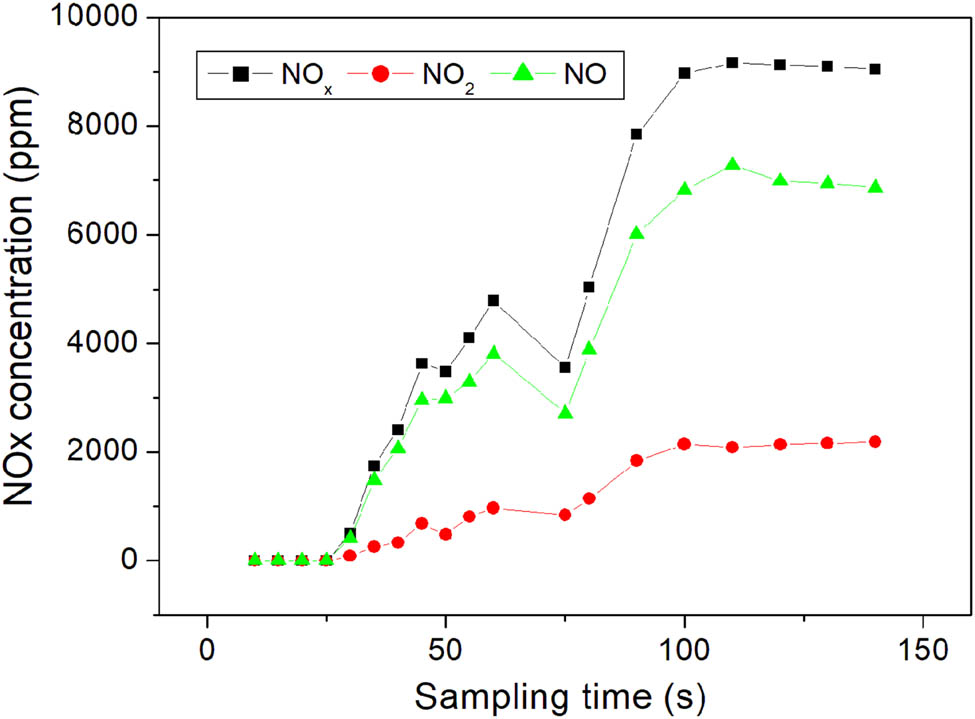
NO x concentration in the off-gas from air-plasma at different sampling times.
Table 4 shows the NO x concentration in the off-gas from the air-plasma with the input power of 150 kW. This test was started from the moment when the plasma ignited. It can be seen that the concentration of NO and NO2 raised from zero and reached a stable value of 100 s after the beginning of the test.
NO x concentration in the off-gas from air-plasma with the input power of 150 kW
| Serial number | Time (s) | NO (ppm) | NO2 (ppm) | NO x (ppm) |
|---|---|---|---|---|
| 01 | 10 | 0.0 | 0.0 | 0.0 |
| 02 | 15 | 0.0 | 0.0 | 0.0 |
| 03 | 20 | 0.0 | 0.2 | 0.2 |
| 04 | 25 | 0.0 | 0.0 | 0.0 |
| 05 | 30 | 414.0 | 86.2 | 500.2 |
| 06 | 35 | 1,484.0 | 257.6 | 1,741.6 |
| 07 | 40 | 2,068.0 | 336.1 | 2,404.1 |
| 08 | 45 | 2,952.0 | 680.0 | 3,632.0 |
| 09 | 50 | 2,995.0 | 482.3 | 3,477.3 |
| 10 | 55 | 3,289.0 | 812.1 | 4,101.1 |
| 11 | 60 | 3,819.0 | 971.6 | 4,790.6 |
| 12 | 75 | 2,711.0 | 844.6 | 3,555.6 |
| 13 | 80 | 3,890.0 | 1,142.1 | 5,032.1 |
| 14 | 90 | 6,008.0 | 1,837.5 | 7,845.5 |
| 15 | 100 | 6,821.0 | 2,145.8 | 8,966.8 |
| 16 | 110 | 7,084.0 | 2,083.6 | 9,167.6 |
| 17 | 120 | 6,987.0 | 2,139.3 | 9,126.3 |
| 18 | 130 | 6,938.0 | 2,164.1 | 9,102.1 |
| 19 | 140 | 6,866.0 | 2,186.1 | 9,052.1 |
The mean concentration was also calculated based on the last four data provided in Table 4. The mean concentration of NO and NO2 was 6,968 and 2,143 ppm, respectively, and the total NO x concentration was 9,112 ppm. The ratio of NO/NO2 after stabilization was about 3.25. The decrease in NO/NO2 ratio from 5.4 to 3.25 was related to the detection position.
3.3 Removal of NO x from air-plasma
3.3.1 Selection of adsorption method
NO x removal technology mainly includes selective catalytic reduction, selective non-catalytic reduction, solid adsorption method, liquid absorption method, and microbiological method [36]. Among them, the liquid absorption method uses simple equipment to obtain high removal efficiency and large adsorption capacity. According to the type of absorbent and purification principle, the liquid absorption method can be divided into water absorption method, acid absorption method, alkali absorption method, oxidation absorption method, and absorption reduction method [37,38].
At the beginning of this work, the water absorption method was first selected in consideration that water is cheap and pollution-free. Otherwise, it could avoid the corrosive effect of acid and alkali solutions on the equipment. A water circulating pump was used to extract the plasma tail gas and maintain the negative pressure state of the system. The water in the circulating pump also acted as the absorption liquid for NO x removal. It turned out that the absorption effect was not obvious, because the laboratory was still filled with a pungent smell after water absorption. We terminated the experiment immediately and used alkali absorption method instead.
The alkali solution absorption method uses the alkali solution (NaOH, KOH, or Na2CO3) to react with NO x and generate nitrate and nitrite, so as to remove NO x . However, the solubility of NO in water and solution is very low, so the alkali solution absorption method is generally only applicable to off-gas with high NO2 content. The off-gas from thermal air-plasma is typically of high NO content as the testing results in Section 3.2, which does not seem apparently to be suitable for alkali absorption treatment.
Oxidation absorption is a method that oxidizes NO to NO2 with an oxidant, in order to promote NO x absorption in an alkali solution. Oxidants mainly include gas-phase oxidants such as O2, O3, Cl2, and ClO2, and liquid-phase oxidants such as KMnO4, NaClO2, NaClO, H2O2, HNO3, and Na2CrO4. The reactions can be formulated as follows when O2 is used as oxidant and NaOH is used in absorption solution:
It is gratifying that the off-gas from thermal air-plasma is still mainly composed of O2 and N2. In order to determine the O2 and N2 content, the off-gas was collected from the plasma reactor using gas bags and characterized by gas chromatography. Table 5 shows the ratio of O2/N2 in pure air and the off-gas from air-plasma with the input power of 150 kW. The values are 0.276 and 0.253, respectively. The atomic ratio of O/N in NO x (whether 1/1 in NO or 2/1 in NO2) is greater than that in the air (0.276). The reaction between O2 and N2 to form NO x would consume more O2 than N2 and result in a decrease of O2 and an increase of N2 content. That is the reason why the off-gas exhibited a lower O2 content and higher N2 content than air.
Ratio of O2/N2 in pure air to the off-gas
| Sample | O2 (vol%) | N2 (vol%) | Ratio of O2/N2 |
|---|---|---|---|
| Pure air | 21.6 | 78.4 | 0.276 |
| Off-gas | 20.2 | 79.8 | 0.253 |
Now that there is O2 in the off-gas that can act as an oxidant to promote NO absorption, the alkali absorption method can be directly used for treatment of the present off-gas from air-plasma. In consideration that the alkali solution would corrode the water circulating pump, a spray tower (shown in Figure 1) was built.
3.3.2 Effect of gas flow
The concentration of NaOH solution as absorption liquid is 16 wt%. The flow of the liquid pump for NaOH solution circulation was 30 L·min−1. The torch power was kept constant, and the off-gas was pumped and introduced into the absorption tower through a branch connected to the exhaust pipeline. The gas flow was regulated by a rotameter from 2.5 to 20.0 m3·h−1. Figure 6 shows the effect of gas flow on the absorption efficiency.

Effect of gas flow on the absorption efficiency.
The absorptivity of NO x increased obviously from 6.5% to 61.2% when the gas flow decreased from 20 to 2.5 m3·h−1. The absorptivity of NO also increased from 5.3% to 52.5%. However, the concentration of NO2 increased instead of decreased and showed an opposite changing trend. The absorptivity of NO2 was −4.7% with the gas flow of 20 m3·h−1 and decreased to −16.0% with the gas flow of 2.5 m3·h−1.
Both the decrease of NO and increase of NO2 during the absorption process were related to the conversion rate of NO to NO2. When the gas flow decreased from 20 to 2.5 m3·h−1, the residence time was prolonged by eight times, which provided more sufficient time for the conversion from NO to NO2. In order to give a clear explanation, we suppose that the conversion from NO to NO2 is divided into two stages, before and after absorption. The residence time was prolonged for both stages. At the first stage before absorption, a longer residence time was attributed to the higher conversion from NO to NO2, resulting in an absorption efficiency, which is the reason why the absorptivity of NO increased obviously with the gas flow decrease. At the second stage after absorption, a longer residence time was still attributed to the higher conversion from NO to NO2, resulting in an increase in NO2 concentration.
3.3.3 Effect of O2 content
It is demonstrated from the above results that O2 in the off-gas must have played an important role so that alkali absorption method can be directly used for the treatment of the off-gas from air-plasma with high NO content. Inspired by this O2-enhanced absorption process, we provided additional O2 into the off-gas to increase its O2 content. Figure 7 shows the absorption efficiency with and without additional O2. The O2 flow was 1.5 m3·h−1 when the off-gas was 2.5 m3·h−1. The concentration of O2 was increased from about 20% to about 50%. The absorptivity of NO increased slightly from 61.2% to 69.7%, while the absorptivity of NO2 increased obviously from −16% to 16%. The obvious increase in NO2 absorptivity cannot be explained clearly through the change of residence time. It indicated that O2 might have played a synergistic absorption role except that it can convert NO to NO2 as an oxidant during different stages. The total NO x absorptivity increased from 52.5% to 62.2%.
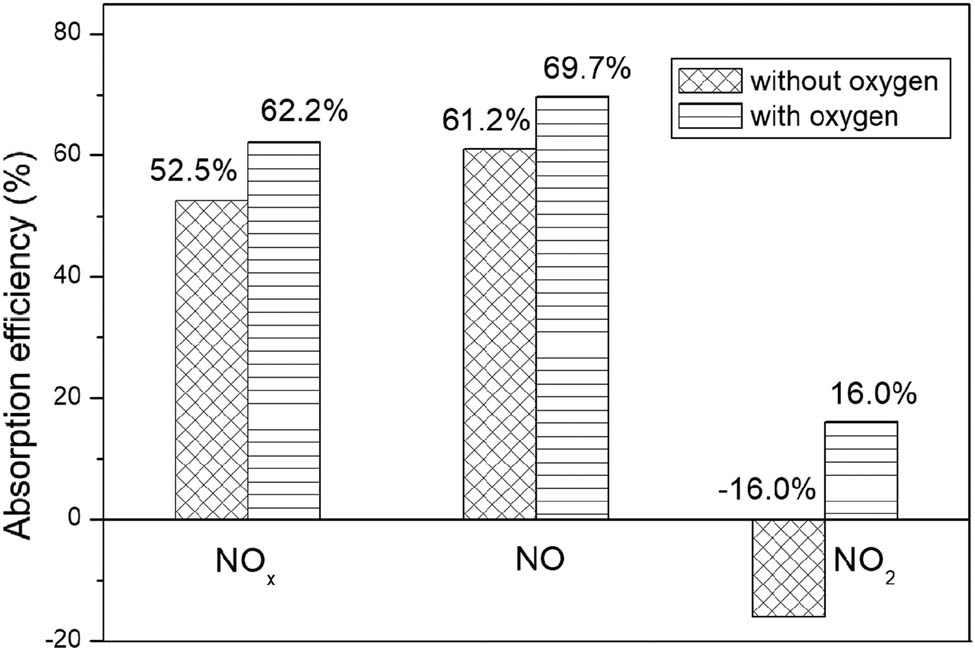
The absorption efficiency with and without additional O2.
3.3.4 Design of multistage unit
Taking the gas flow and O2 content effects into account, the content of NO and NO2 was reduced to a fairly low level at 436 and 197.2 ppm, respectively. The total content of NO x was 633.2 ppm, which has not reached the emission standard of 200 ppm. Multistage absorption is a commonly used strategy in industrial production to increase absorption efficiency.
The number of cycles of absorption can be estimated from the absorption rate. In the above absorption experiment, the initial concentration of NO x was 1,674.9 ppm, and the concentration after one cycle absorption was 633.2 ppm. The absorption rate was 62.2%. The NO x concentration was detected at different positions as shown in Figure 8: the inlet of the first absorption tower, the outlet of the first absorption tower, the outlet of the second absorption tower, and the outlet of the third absorption tower. The NO x concentration and the absorption rate after different cycles of absorption were estimated, and the results are shown in Table 6.
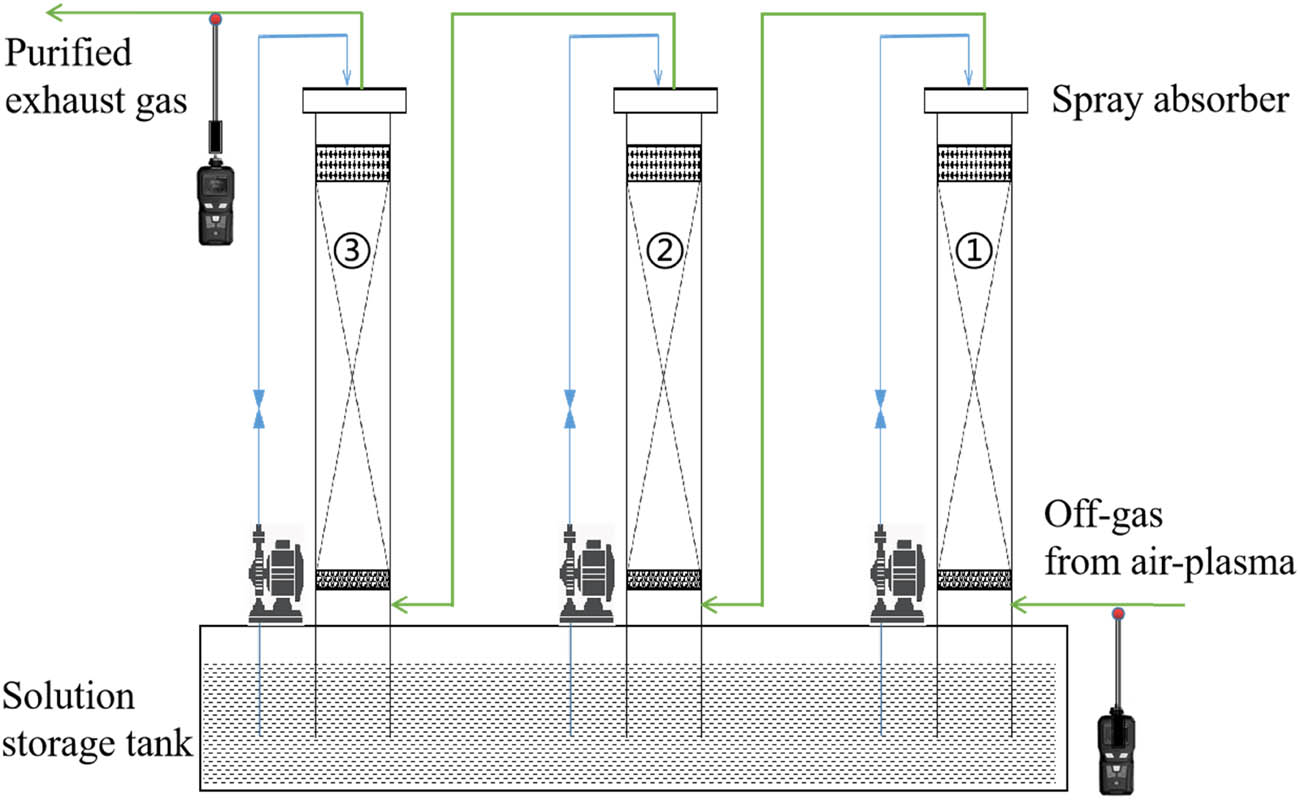
Illustration of a multistage off-gas treatment unit.
Estimated concentration of NO x after different cycles of absorption
| Cycle number | Cumulative absorption rate (%) | NO x concentration (ppm) |
|---|---|---|
| 0 | 0 | 1,674.9 |
| 1 | 62.2 | 633.2 |
| 2 | 85.7 | 239.2 |
| 3 | 94.6 | 90.5 |
It can be estimated that NO x concentration after a three-cycle absorption can be reduced below 100 ppm which can meet the emission standard. Based on the assessment, we have designed a multistage unit for the treatment of off-gas from air plasma, as shown in Figure 8. This article intends to present the feasibility of thermal plasma off-gas purification. Further testing and application according to detailed plasma running parameters are still in progress.
4 Conclusions
The concentration of NO x in off-gas from air-plasma was determined by a gas analyzer, removal methods were selected according to the characteristics of plasma off-gas, different influencing parameters were examined, and a treatment unit was designed for the green air-plasma process. Several conclusions can be drawn from the results and discussion:
N2 and O2 would react and form NO x in the unordinary chemical reaction media provided by thermal plasma, and the concentration of NO x in off-gas from air-plasma increased with the increase in plasma power.
The content of O2 in the off-gas was close to that in air, which would act as an oxidant to promote NO x absorption; thus, alkali absorption method can be directly used for the treatment of the present off-gas from air-plasma.
The absorption efficiency was 52.5% when the gas flow was 2.5 m3·h−1, and it could be further increased to 62.2% when additional O2 was provided into the off-gas to increase its O2 content from 20% to 50%.
It was estimated that the NO x concentration can be reduced below 100 ppm when a multistage absorption unit was designed, which can meet the emission standard and present a feasible technical scheme for the green plasma process.
-
Funding information: This research was sponsored by the National Natural Science Foundation of China (No. 11875284), the China Central Guidance on Local Science Technology Development Fund of Henan Province (No. Z20221343028), Program for Science & Technology Innovation Talents in Universities of Henan Province (No. 21HASTIT020), and Henan Science and Technology Development Program (No. 222102230036).
-
Author contributions: Liuyang Bai: conceptualization, methodology, investigation, writing – original draft preparation, writing – review and editing, project administration, funding acquisition; Yuge Ouyang: methodology, writing – original draft preparation, writing – review and editing; Hongbing Wang: investigation, visualization; Min Wang: investigation, visualization; Fangli Yuan: conceptualization, supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Boulos MI, Fauchais P, Pfender E. Thermal plasmas: Fundamentals and applications. New York: Plenum Press; 1994.10.1007/978-1-4899-1337-1Search in Google Scholar
[2] Sikarwar VS, Hrabovský M, Van OG, Pohořelý M, Jeremiáš M. Progress in waste utilization via thermal plasma. Prog Energy Combust Sci. 2020;81:100873. 10.1016/j.pecs.2020.100873.Search in Google Scholar
[3] Prado ES, Miranda FS, de Araujo LG, Petraconi G, Baldan MR. Thermal Plasma Technology for radioactive waste treatment: A Review. J Radioanal Nucl Chem. 2020;325(2):331–42. 10.1007/s10967-020-07269-4.Search in Google Scholar
[4] Schumacher U. Basics of plasma physics. In: Dinklage A, Klinger T, Marx G, Schweikhard L, (eds). Plasma Physics. Lecture Notes in Physics. Berlin, Heidelberg: Springer; 2005. 10.1007/11360360_1.Search in Google Scholar
[5] Petitpas G, Rollier J, Darmon A. A comparative study of non-thermal plasma assisted Reforming Technologies. Int J Hydrog Energ. 2007;32(14):2848–67. 10.1016/j.ijhydene.2007.03.026.Search in Google Scholar
[6] Huang H, Tang L. Treatment of organic waste using thermal plasma pyrolysis technology. Energy Convers Manag. 2007;48:1331–7. 10.1016/j.enconman.2006.08.013.Search in Google Scholar
[7] Chen FF. Introduction to plasma physics. New York: Springer Science & Business Media; 1974.10.1007/978-1-4757-0459-4_1Search in Google Scholar
[8] Zhukov MF, Zasypkin IM, Timoshevskii AN. Thermal plasma torches: design, characteristics, application. Cambridge: Cambridge International Science Publishing; 2007.Search in Google Scholar
[9] Yuan FL, Jin HC, Hou GL. Progress on preparation of special powders using HF thermal plasma (in Chinese). Chin J Process Eng. 2018;18(6):1139–45. 10.12034/j.issn.1009-606X.218240.Search in Google Scholar
[10] Zheng J, Yang R, Xie L, Qu J, Liu Y, Li X. Plasma-assisted approaches in inorganic nanostructure fabrication. Adv Mater. 2010;22(13):1451–73. 10.1002/adma.200903147.Search in Google Scholar PubMed
[11] He J, Bai L, Jin H, Yuan F. Optimization of tungsten particles spheroidization with different size in thermal plasma reactor based on numerical simulation. Powder Technol. 2016;302:288–97. 10.1016/j.powtec.2016.08.067.Search in Google Scholar
[12] Zhu H, Li X, Chen Q. Three-dimensional simulation and experimental investigation on spheroidization of stainless steel powders using radio frequency thermal plasma. J Mater Eng Perform. 2022;31:6606–16. 10.1007/s11665-022-06714-7.Search in Google Scholar
[13] Hu P, Yan S, Yuan F, Bai L, Li J, Chen Y. Effect of plasma spheroidization process on the microstructure and crystallographic phases of silica, alumina and nickel particles. Plasma Sci Technol. 2007;9(5):611–5. 10.1088/1009-0630/9/5/20.Search in Google Scholar
[14] He J, Bai L, Jin H, Jia Z, Hou G, Yuan F. Simulation and experimental observation of silicon particles’ vaporization in RF thermal plasma reactor for preparing si nano-powder. Powder Technol. 2017;313:27–35. 10.1016/j.powtec.2017.02.062.Search in Google Scholar
[15] Bai L, He J, Ouyang Y, Liu W, Liu H, Yao H, et al. Modeling and selection of RF thermal plasma hot-wall torch for large-scale production of nanopowders. Materials. 2019;12(13):2141. 10.3390/ma12132141.Search in Google Scholar PubMed PubMed Central
[16] Hou G, Cheng B, Ding F, Yao M, Cao Y, Hu P, et al. Well dispersed silicon nanospheres synthesized by RF thermal plasma treatment and their high thermal conductivity and dielectric constant in polymer nanocomposites. RSC Adv. 2015;5(13):9432–40. 10.1039/c4ra14212h.Search in Google Scholar
[17] Bai L, Yuan F, Fang Z, Wang Q, Ouyang Y, Jin H, et al. RF thermal plasma synthesis of ultrafine ZrB2-ZrC composite powders. Nanomaterials. 2020;10(12):2497. 10.3390/nano10122497.Search in Google Scholar PubMed PubMed Central
[18] Bai L, Fan J, Hu P, Yuan F, Li J, Tang Q. RF plasma synthesis of nickel nanopowders via hydrogen reduction of nickel hydroxide/carbonate. J Alloy Compd. 2009;481(1–2):563–7. 10.1016/j.jallcom.2009.03.054.Search in Google Scholar
[19] Zhang H, Yao M, Bai L, Xiang W, Jin H, Li J, et al. Synthesis of uniform octahedral tungsten trioxide by RF induction thermal plasma and its application in gas sensing. CrystEngComm. 2013;15(7):1432. 10.1039/c2ce26514a.Search in Google Scholar
[20] Zhang X, Wang Y, Min BI, Kumai E, Tanaka M, Watanabe T. A controllable and byproduct-free synthesis method of carbon-coated silicon nanoparticles by induction thermal plasma for Lithium Ion Battery. Adv Powder Technol. 2021;32(8):2828–38. 10.1016/j.apt.2021.06.003.Search in Google Scholar
[21] Yang Z, Du Y, Yang Y, Jin H, Shi H, Bai L, et al. Large-scale production of highly stable silicon monoxide nanowires by radio-frequency thermal plasma as anodes for high-performance Li-Ion Batteries. J Power Sources. 2021;497:229906. 10.1016/j.jpowsour.2021.229906.Search in Google Scholar
[22] Li X, Xin W, Zheng X, Ren Z, Sun D, Lu W. Microstructural characterization and formation mechanism of nitrided layers on aluminum substrates by thermal plasma nitriding. Metals. 2019;9(5):523. 10.3390/met9050523.Search in Google Scholar
[23] Rajendran I, Shreeram B. Corrosion, adhesion and erosion study of MZ and ML system using thermal plasma. Int J Heavy Veh Syst. 2018;25(3/4):406. 10.1504/ijhvs.2018.10016136.Search in Google Scholar
[24] Moustakas K, Fatta D, Malamis S, Haralambous K, Loizidou M. Demonstration plasma gasification/vitrification system for effective hazardous waste treatment. J Hazard Mater. 2005;123(1–3):120–6. 10.1016/j.jhazmat.2005.03.038.Search in Google Scholar PubMed
[25] Cheng TW, Tu CC, Ko MS, Ueng TH. Production of glass–ceramics from incinerator ash using lab-scale and pilot-scale thermal plasma systems. Ceram Int. 2011;37(7):2437–44. 10.1016/j.ceramint.2011.05.088.Search in Google Scholar
[26] Károly Z, Mohai I, Tóth M, Wéber F, Szépvölgyi J. Production of glass–ceramics from fly ash using Arc Plasma. J Eur Ceram Soc. 2007;27(2–3):1721–5. 10.1016/j.jeurceramsoc.2006.05.015.Search in Google Scholar
[27] Morrin S, Lettieri P, Chapman C, Mazzei L. Two stage fluid bed-plasma gasification process for solid waste valorisation: technical review and preliminary thermodynamic modelling of sulphur emissions. Waste Manag. 2012;32:676–84. 10.1016/j.wasman.2011.08.020.Search in Google Scholar PubMed
[28] Fabry F, Rehmet C, Rohani V, Fulcheri L. Waste gasification by thermal plasma: a review. Waste Biomass Valor. 2013;4(421):439. 10.1007/s12649-013-9201-7.Search in Google Scholar
[29] Sanlisoy A, Carpinlioglu MO. A review on plasma gasifcation for solid waste disposal. Int J Hydrog Energy. 2017;42:1361–5. 10.1016/j.ijhydene.2016.06.008.Search in Google Scholar
[30] Changming D, Chao S, Gong X. Plasma methods for metals recovery from metal-containing waste. Waste Manag. 2018;77:373–87. 10.1016/j.wasman.2018.04.026.Search in Google Scholar PubMed
[31] Bai L, Sun W, Yang Z, Ouyang Y, Wang M, Yuan F. Laboratory research on design of three-phase AC arc plasma pyrolysis device for recycling of waste printed circuit boards. Processes. 2022;10(5):1031. 10.3390/pr10051031.Search in Google Scholar
[32] Abdulkarim BI, Hassan MAA. Thermal plasma treatment of wastes: a review. Aust J Basic Appl Sci. 2015;9(31):322–33.Search in Google Scholar
[33] Hrabovský M. Generation of thermal plasmas in liquid-stabilized and hybrid DC-arc torches. Pure Appl Chem. 2002;74(3):429–33. 10.1351/pac200274030429.Search in Google Scholar
[34] Watanabe T, Shimbara S. Halogenated hydrocarbon decomposition by steam thermal plasmas. High Temp Mater Process. 2003;7(4):455–74. 10.1615/hightempmatproc.v7.i4.30.Search in Google Scholar
[35] Knacke O, Kubaschewski O, Hesselmann K. Thermochemical properties of inorganic substances. Berlin/NewYork; Verlag Stahleisen, Dsseldorf: Springer-Verlag; 1991.Search in Google Scholar
[36] Wang D, Li S, Yang S, Ji L. Current status and prospects of the application of industrial denitration technology. Sci Technol Chem Indus. 2020;28(2):77–82.Search in Google Scholar
[37] Si M, Shen B, Adwek G, Xiong L, Liu L, Yuan P, et al. Review on the NO removal from flue gas by oxidation methods. J Env Sci. 2021;101:49–71. 10.1016/j.jes.2020.08.004.Search in Google Scholar PubMed
© 2022 Liuyang Bai et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

