Abstract
This work aimed to study the preparation of cerium oxide (CeO2) used as the catalyst for biodiesel production from palm oil. The precipitation method was used in the catalyst synthesis. The effects of oxalic concentrations and stirring rates in the precipitation process were investigated. Oxalic acid was added into cerium (Ce) in ethylenediaminetetraacetic acid solution to form Ce oxalate before the Ce oxalate was calcined to obtain CeO2. The results showed that oxalic concentrations and stirring rates slightly affect the morphology of CeO2. However, these parameters considerably affect the amount of basic sites of CeO2. The basicity of CeO2 plays the main role in catalyzing the transesterification reaction for biodiesel production. When CeO2 was used as the catalyst in biodiesel production from palm oil under operating conditions using a 5% catalyst, methanol-to-oil molar ratio of 30:1, reaction temperature of 150°C, 13.8 bars, and 3-h reaction time, CeO2 obtained from 3% oxalic concentration and 400 rpm stirring rates in the precipitation process provided the highest %FAME in the range of 93.9–94.2% since it had higher basicity. In addition, the decrease in surface area of CeO2 after the use was less severe than that of basicity due to catalyst deactivation.
1 Introduction
At the present, there is a potential for the problem of fossil energy shortages to occur in the future due to development in several sectors such as industries, transportation, and logistics. Furthermore, the use of fossil energy such as benzene and diesel can generate environmental problems, such as air pollution, global warming, and PM2.5 dust (particulate matter with the size of 2.5 µm or smaller) [1,2]. Therefore, it is necessary to find other energy sources which are renewable, sustainable, and environmental-friendly for environmental and energy security. There are several types of alternative energy, such as wind energy, solar energy, water energy, hydrogen energy, and biodiesel, which can be used instead of fossil energy. Biodiesel is one such alternative energy that has shown its suitability for use in agricultural countries such as Thailand. Biodiesel can be produced from various oil plants. For example, rice bran oil was proposed as a promising renewable source for biodiesel production as well as those from palm, jatropha, coconut, and soybean plants, which are abundant in nature [3,4,5,6,7,8]. However, some properties of biodiesel such as density and viscosity, which affect the fuel supply system and spray characteristics, need to be improved in order to be efficiently used in diesel engines [9]. The combustion of biodiesel does not emit sulfur dioxide, and biodiesel is also labeled as “carbon-neutral.” The net amount of CO2 emission from the combustion of biodiesel does not increase in the atmosphere since the oil plants consume CO2 for photosynthesis and are used as raw materials for biodiesel production. Thus, biodiesel is green, eco-friendly, and sustainable energy that can be practically used.
Biodiesel can be synthesized using several methods. Biodiesel production on the industrial scale mostly uses NaOH or KOH as the catalyst. However, the use of NaOH and KOH, which are homogeneous catalysts, generates large amounts of wastewater, and the separation of catalysts for reuse was not easily carried out [10]. Therefore, heterogeneous catalysts are expected to solve these drawbacks from the use of homogeneous catalysts. Several heterogeneous catalysts such as MgO, CaO, and SrO were studied in biodiesel production [11,12,13]. However, the leaching problem was found when these catalysts mentioned earlier were used [14]. The reuse of these catalysts cannot be carried out to reduce the production cost. It might affect the purification process of biodiesel from catalyst leaching. Thus, it is necessary to study other catalysts which reduced the leaching problem. Therefore, cerium oxide (CeO2) has gradually gained attention for use as the catalyst in transesterification reactions due to its good thermal stability [15]. This might reduce the leaching problem, and the catalyst can be reused several times.
CeO2 was one type of heterogeneous catalyst which has been studied. CeO2 has wide applications in various areas such as microelectronics, optoelectronics, fuel cell technologies, gas sensors, oxygen storage, ceramics, and biomedical applications [16,17]. As CeO2 in nanoparticle form provides a good thermal property, large oxygen storage, and flexible capacity in valence transformation, it can be used as an additive that is added to biodiesel. The CeO2-containing biodiesel helps diesel engines to reduce toxic emissions such as soot, smoke opacity, NO x , CO, and HC [18]. In addition, CeO2 can be used as the catalyst in several reactions such as photocatalytic reactions, oxidation reactions, and transesterification reactions [19,20,21]. For transesterification reaction in biodiesel production, CeO2 as a heterogeneous catalyst has been more studied since CeO2 showed strong durability, resulting in several reuses. For transesterification reaction in biodiesel production, CeO2 has been studied for use as the catalyst. CeO2 contained basic sites which are active sites to catalyze the transesterification reaction [10]. However, most works have studied mixed oxide between other elements and cerium (Ce) [22,23,24,25]. In this work, CeO2 in the form of oxide was used to catalyze the transesterification reaction.
Ce is an element in the lanthanide group. Ce compounds can be found in natural minerals such as alanite, bastanite, monazite, cerite, and samarskite. The main source of Ce is bastanite and monazite [26]. CeO2 synthesis can be carried out using several methods such as precipitation, hydrothermal, solvothermal, sonochemical, spray pyrolysis, microemulsification, sol–gel, plant-mediated, and fungus-mediated methods [27,28,29]. In this work, the precipitation method was used to synthesize CeO2 since this method is simple, cheap, and easy to scale up [30]. There are several Ce sources used as the precursor in the synthesis such as Ce nitrate hexahydrate, Ce carbonate, Ce hydroxide, and Ce chloride [29,30,31,32]. Here, the Ce source was obtained from the Thailand Institute of Nuclear Technology. The Ce source was in the form of Ce in ethylenediaminetetraacetic acid solution (Ce in ethylenediaminetetraacetic acid [EDTA] solution), which was obtained and then purified using the ion exchange method [33]. The Ce source in this work was originally from natural minerals such as monazite ore. After decomposition and purification of monazite ore, a large amount of Ce in EDTA solution was obtained. Therefore, this Ce source can be suitably used to prepare adequate amounts of CeO2 catalyst for large-scale production of biodiesel. In addition, the precipitation method was used for CeO2 preparation, which is simple, cheap, and easy to scale up. Therefore, the catalyst cost could be economical when CeO2 is used as the catalyst for commercial biodiesel production.
Oxalic acid was used as the precipitant. Oxalic acid is one of the chemicals which is commonly used to separate rare earth elements from solutions since it has an affinity with rare earth elements [34]. After the addition of oxalic acid into Ce in EDTA solution, Ce oxalate was formed and then calcined to obtain CeO2. In this work, the effect of oxalic concentrations and stirring rates in the precipitation which affected the properties of CeO2 used as the catalyst in transesterification reaction for biodiesel production were studied.
2 Materials and methods
2.1 Materials
Ce in ethylenediaminetetraacetic acid solution (Ce in EDTA solution) was obtained from the Thailand Institute of Nuclear Technology. Oxalic acid (C2H2O4·2H2O, 99.5%, AR/ACS) was from Loba Chemie PVT. Ltd., and methanol (CH3OH, 99.5%, AR) was from QREC company. Palm oil was purchased from a commercial company (Morakot brand, Thailand).
2.2 Catalyst preparation and its characterization
Ce in the form of dissolved Ce in EDTA (called, Ce in EDTA solution) was obtained from the purification process of mixed rare earth ores using ion exchange resin [33]. The concentration of Ce in EDTA solution was measured using ICP-OES. The main rare earth elements in the solution were praseodymium (Pr) and Ce. The concentrations of Pr and Ce in the solution were 36.49 and 524.6 mg L−1, and the pH of Ce in the EDTA solution was 8. For the precipitation process, the Ce in EDTA solution was filtered using a filter paper (No. 1, Whatman). Then, filtered Ce in EDTA of 1,400 mL was added to a 2,000 mL beaker. Next, the 3% w/v oxalic solution was dropped into the beaker at the feed rate of 20 mL·min−1 under a stirring rate of 400 rpm, using a magnetic bar. The addition of oxalic solution was stopped when the final pH of the solution was 4. After that, the precipitates were obtained and the mixture was left at room temperature (28°C) for 16 h (aging time). The precipitates were then filtered using filter paper (No. 1, Whatman) with the help of a vacuum pump. The precipitates were dried in an oven at 110°C for 12 h. CeO2 was obtained after the precipitates were calcined at 900°C for 3 h with a heat rate of 5°C·min−1. The oxalic concentrations (0.5% and 3% w/v) and the stirring rates (200, 400, 600, and 800 rpm) for the precipitation process were studied. The characterization of CeO2 was carried out, using Brunauer–Emmett–Teller (BET), temperature-programmed desorption of CO2 (CO2-TPD), X-ray diffraction (XRD), and scanning electron microscopy (SEM) measurements. The surface area of the CeO2 catalyst was determined using N2-adsorption in BET measurement (BELSORP-mini II, MicrotracBEL). The CO2-TPD (Autochem 2910, Micromeritics) was used to determine the basicity of CeO2. For the SEM measurement (Nira 3, TESCAN), CeO2 was covered with gold film before it was placed in the chamber of the instrument using an acceleration voltage at 15 kV. The crystal structure of CeO2 was measured using an XRD instrument (D8 Advance, Bruker), equipped with Cu Kα radiation at 40 kV and 40 mA. The XRD pattern was recorded using 2-theta in the range of 10–90°.
2.3 Biodiesel production
The palm oil of 113 mL and 5 g CeO2 were added in a Parr stirred batch reactor (Model 4568) with the stirring rate of 600 rpm under an N2 atmosphere at 13.8 bars. The mixture was heated to 150°C. Then, the methanol of 142.6 mL was fed into the reactor. The reaction was carried out for 3 h. After that, the mixture was separated to obtain biodiesel, using a centrifuge at 3,000 rpm for 30 min. Biodiesel was baked at 110°C for 12 h to remove the residue of methanol. The %FAME was measured using GC-FID (Clarus 600, PerkinElmer), following the EN14103-2011 standard method. The catalyst was washed two times using methanol and was then measured using BET, CO2-TPD, XRD, and SEM techniques.
3 Results and discussion
Oxalic acid is one of the chemicals which is commonly used as the precipitant to separate rare earth elements from the solution. In general, oxalic acid has an affinity with rare earth elements. The precipitation of dissolved rare earth elements using oxalic acid can be performed for all pH ranges [34]. In this work, Ce in EDTA solution was added to an oxalic solution. The white precipitates (Ce oxalate) were formed immediately after the oxalic solution was added. The precipitates were filtered and then calcined. After the calcination, Ce oxalate was transformed into CeO2, which was pale yellow. For the precipitation process, the addition of oxalic solution was stopped when the final pH of the solution was 4. At pH = 4 of the solution, CeO2 provided the larger basic sites than those of pH = 3 and 2, respectively (see Supplementary material). The basic sites of CeO2 play the main role in catalyzing the transesterification reaction for biodiesel production [35,36,37]. As a result, CeO2 obtained from pH = 4 offered more %FAME than those of pH = 3 and 2. Therefore, the final pH = 4 of the solution in the precipitation process was suitable and chosen for the precipitation in the next experiment.
Although the use of the final pH = 4 in the precipitation showed a higher %FAME than those of lower pH, a smaller amount of CeO2 was obtained. In addition, a larger amount of Ce in EDTA solution was consumed during the precipitation using the final pH = 4, in order to obtain the same amount of CeO2 from those of lower pH. It was expected that the use of higher pH than pH = 4 consumed a greater amount of Ce in EDTA solution and obtained less CeO2. Therefore, it might not be suitable to use higher pH than pH = 4 in the precipitation for use on a commercial scale. Therefore, the final pH = 4 of the solution in the precipitation was chosen to be used in the next experiment.
3.1 The effect of oxalic concentration
In this section, the concentrations of oxalic concentration used were 0.5% and 3% w/v. The XRD patterns of CeO2 synthesized using 0.5% and 3% oxalic concentrations are shown in Figure 1. The peaks were observed at 28.5°, 33.0°, 47.5°, 56.5°, 59.1°, 69.5°, 76.9°, 79.2°, and 88.7°, assigned to crystal planes of (111), (200), (220), (311), (222), (400), (331), (420), and (422), respectively [38]. Although the use of 0.5% and 3% oxalic provided different Ce to oxalic ratios, XRD patterns of CeO2 from both samples were similar. The precipitates of Ce oxalate were calcined at a high temperature of 900°C [39]. All amounts of Ce oxalate were completely transformed into CeO2. Therefore, the different XRD patterns from both samples were not clearly observed. From Figure 2a and b, SEM micrographs of CeO2 synthesized using 0.5% and 3% oxalic concentration are illustrated. The CeO2 particles were spherical and were attached via a rod. As listed in Table 1, surface area and pore size of CeO2 synthesized using 0.5% and 3% oxalic concentrations were not much different. It was reported that the solubility of Ce oxalate decreased in the presence of higher oxalic concentrations [40]. Therefore, the use of 3% oxalic concentration decreased the solubility of Ce oxalate more than that of 0.5% oxalic concentration in a shorter time, affecting the precipitate formation. It could be said that the use of 3% oxalic concentration lowers the ratio of Ce to oxalic acid more than that of 0.5% oxalic concentration. Therefore, the use of 3% oxalic concentration offered low supersaturation, and there were few nuclei in the system. The remaining ions can improve the growth of nuclei. However, the use of 0.5% oxalic concentration might lead to the agglomeration of Ceoxalate due to the higher ratio of Ce to oxalic acid. As a result, CeO2 synthesized using 0.5% oxalic concentration might have a little larger size and less surface area than that of 3% oxalic concentration. The concentration of oxalic acid had little effect on the morphology of CeO2 [41].
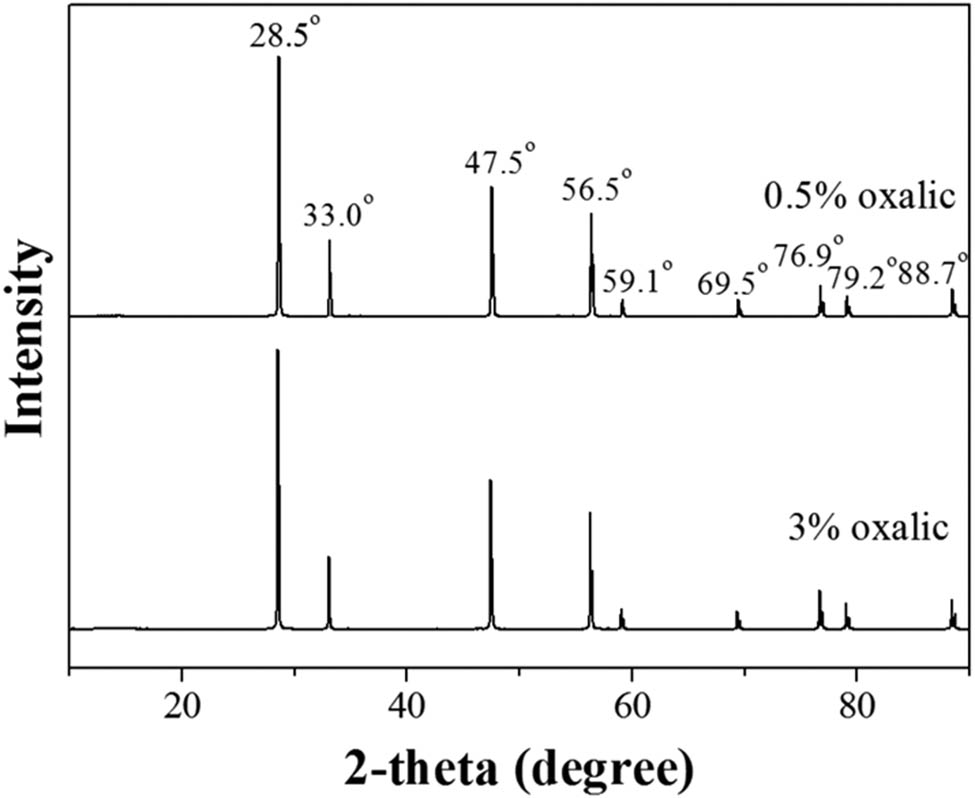
XRD patterns of CeO2 synthesized using 0.5% and 3% oxalic concentration at 400 rpm stirring rate.

SEM micrograph (26,700× magnification) of CeO2 synthesized using different oxalic concentration: (a) 0.5% oxalic and (b) 3% oxalic concentration at 400 rpm stirring rate.
Properties of CeO2 synthesized using 0.5% and 3% oxalic concentrations at 400 rpm stirring rate
| Catalysts | Surface area | Pore size | Basicity | %FAME |
| (m2·gcat −1) | (nm) | (µmol·CO2·gcat −1) | ||
| CeO2 at 0.5% oxalic concentration | 6.53 | 15.3 | 517.99 | 82.7 |
| CeO2 at 3% oxalic concentration | 6.75 | 13.12 | 613.09 | 94.2 |
However, the basicity of CeO2 synthesized using 3% oxalic concentration (613.09 µmol·CO2·gcat −1) was considerably more than that of CeO2 synthesized using 0.5% oxalic concentration (517.99 µmol·CO2·gcat −1), as shown in Table 1. The peaks of desorption of CO2 between 600°C and 800°C as shown in Figure 3 indicate that basic sites of CeO2 synthesized using 0.5% and 3% oxalic concentration provided strong basic sites [42]. As the final pH = 4 of the solution was controlled, the use of 3% oxalic concentration showed less ratio of Ce to oxalic acid than that of 0.5% oxalic concentration in a shorter time. This might affect the structure of Ce oxalate particles during the formation of precipitates, resulting in different amounts of basic sites of CeO2 as the final product [43,44]. The greater amount of basic sites of CeO2 from the use of 3% oxalic concentration in precipitation provided 94.2% FAME, which was more than that of 82.7% FAME obtained from 0.5% oxalic concentration, as shown in Table 1. Therefore, 3% oxalic concentration is the suitable concentration and so was chosen for the next experiment due to the higher basicity of CeO2. The basicity of the catalyst plays the main role in the catalysis of transesterification reaction. The high activity of catalysts in transesterification reaction was attributed to high numbers of basic sites and the basic strength [35,36,37]. Therefore, the high %FAME can be obtained using high basicity catalysts in the transesterification reaction.
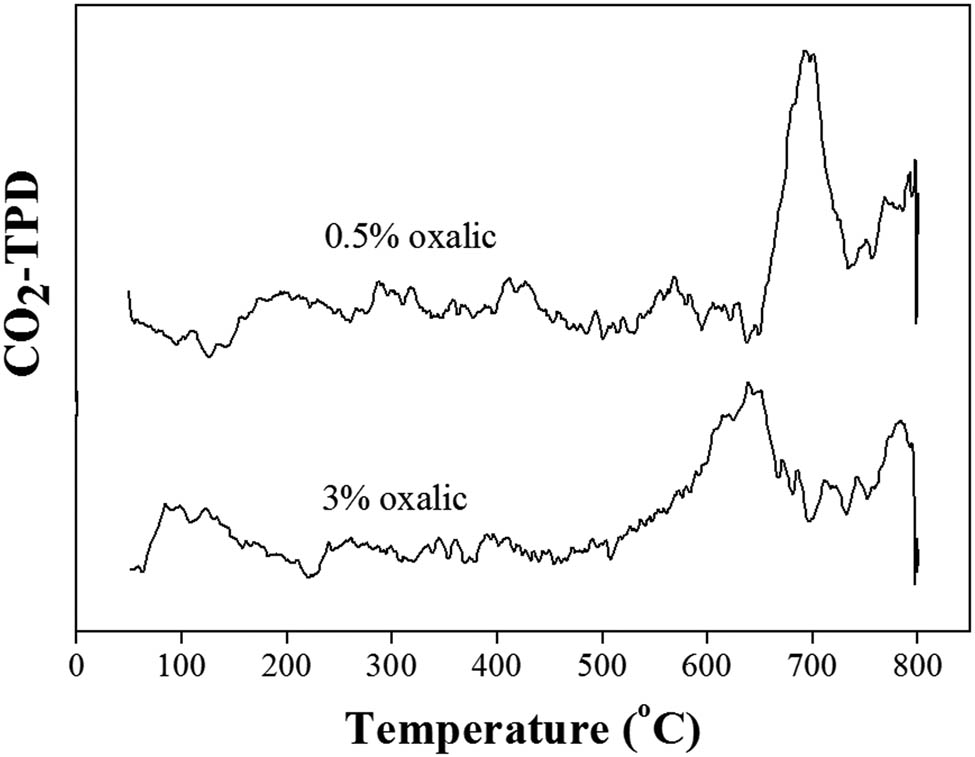
CO2 desorption of CeO2 synthesized using a 3% oxalic concentration at a 400 rpm stirring rate.
The 94.2% FAME obtained from CeO2 using 3% oxalic concentration in precipitation was slightly lower than the standard value (96.5%) [45]. In comparison with other works, mixed oxide CaO–CeO2 used as the catalyst provided %FAME in the range of 95–98% under mild operating conditions [46,47,48]. One work by Soodjit et al. [21] used CeO2 obtained from the precipitation method and using the same Ce source of this work. Their results showed that 88.92% FAME was obtained under more crucial operating conditions, compared to this work. However, the precipitation conditions were not similar to this work. This showed that the precipitation conditions should be controlled to improve the catalytic activity of CeO2 for transesterification reaction. Therefore, the other parameters in the precipitation conditions should be further investigated in order to add more potential to CeO2 for use as catalysts in large-scale biodiesel production.
3.2 The effect of stirring rates in precipitation
In this section, the effect of stirring rates was studied using 200, 400, 600, and 800 rpm in the precipitation with a 3% oxalic concentration. The XRD patterns of CeO2 from different stirring rates are shown in Figure 4 and are assigned to the crystal planes of CeO2, as mentioned above [38]. The morphology of CeO2 using different stirring rates looked similar for all samples, as shown in Figure 5. Table 2 shows the surface areas and pore sizes of CeO2 obtained using different stirring rates. The surface area slightly increased at higher stirring rates. Stirring improves mass transfer and prevents local supersaturation [39]. In addition, the use of high stirring rates can break up the particles into small particles. The surface area of CeO2 at 200 rpm was 5.11 m2·gcat −1, and the surface area of CeO2 increased to 5.54 m2·gcat −1 at 600 rpm. At higher stirring rates, the shear force could break up the particles, leading to smaller particles and higher surface area [39,49]. However, the surface area of CeO2 decreased to 5.35 m2·gcat −1 at 800 rpm, possibly because high stirring rates facilitate particle collision to form particle aggregate [50]. The sizes of CeO2 particles from all stirring rates were not much different from those observed in Figure 5. However, CeO2 particles at 800 rpm stirring rate agglomerated more tightly than those at lower stirring rates. This resulted in less surface area at the higher stirring rate of 800 rpm. The pore sizes of CeO2 decreased as stirring rates increased. As shown in Table 2, the basicity of CeO2 was the highest at the stirring rate of 400 rpm. The CO2 desorption of CeO2 at 400 rpm is illustrated in Figure 6, which shows the desorption peaks of strong basicity between 600°C and 800°C. The stirring rates might affect the morphology of CeO2 which was mentioned above, leading to the change in the basicity of CeO2. The high basicity of the catalyst provided a high activity to catalyze transesterification reaction. Therefore, CeO2 at 200, 400, and 600 rpm showed %FAME, which was similar as they provided high basicity. The highest 93.9% FAME was obtained from CeO2 at 400 rpm. As CeO2 at 800 rpm had low basicity, the lowest %FAME was offered at 83.4%, as given in Table 2.
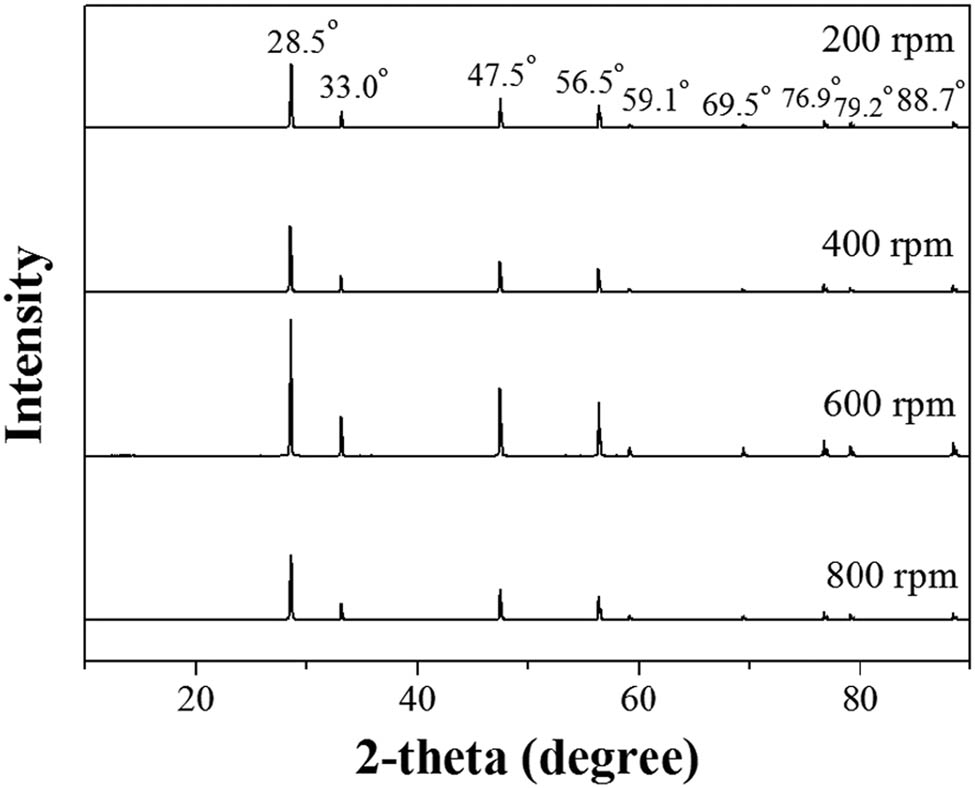
XRD patterns of CeO2 synthesized using 200, 400, 600, and 800 rpm stirring rates at 3% oxalic concentration.
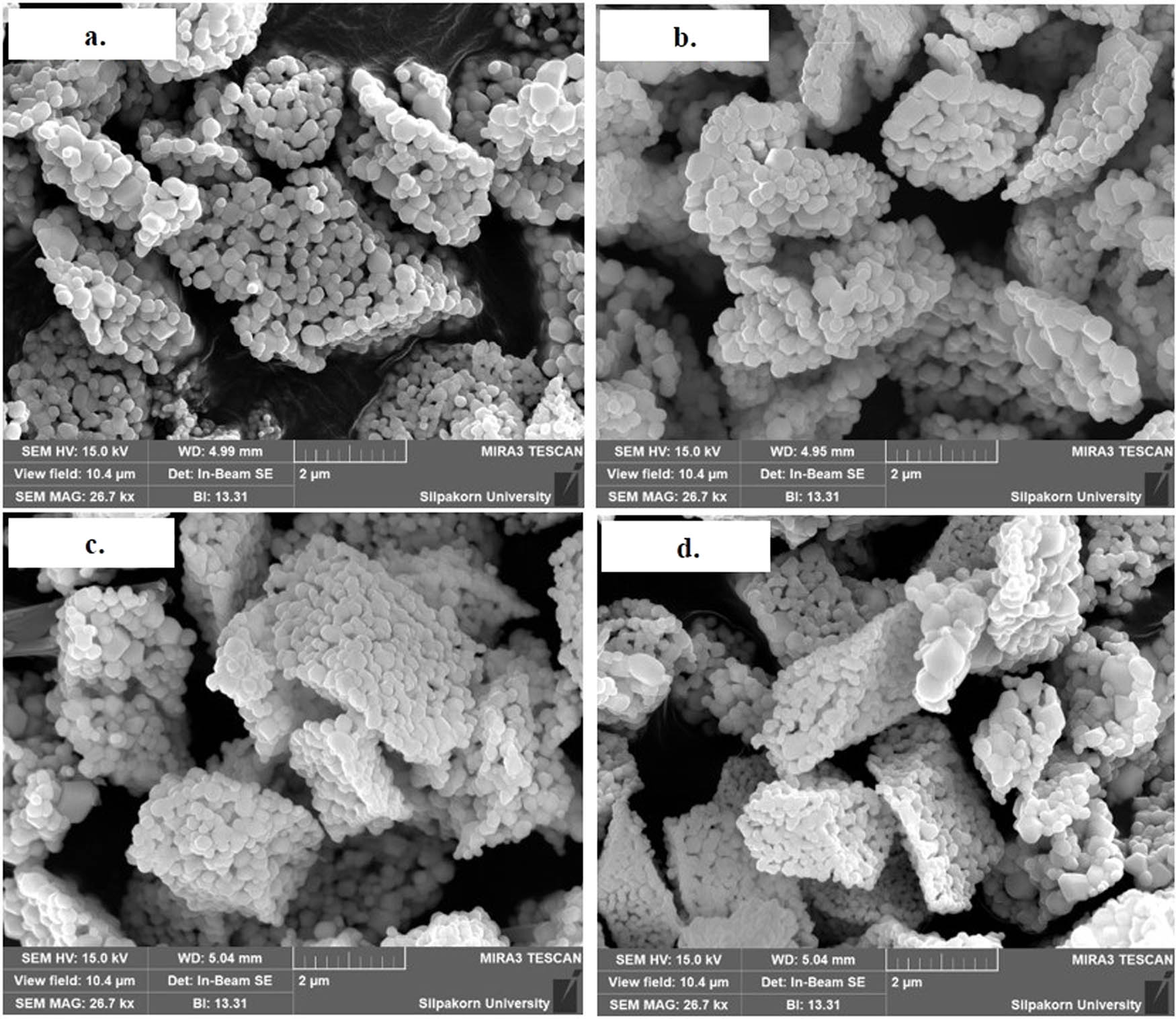
SEM micrograph (26,700× magnification) of CeO2 synthesized using different stirring rates: (a) 200 rpm, (b) 400 rpm, (c) 600 rpm, and (d) 800 rpm at 3% oxalic concentration.
Properties of CeO2 using different stirring rates at 3% oxalic concentration
| Catalysts | Surface area | Pore size | Basicity | %FAME |
|---|---|---|---|---|
| (m2·gcat −1) | (nm) | (µmol·CO2·gcat −1) | ||
| CeO2 at 200 rpm | 5.11 | 39.35 | 490.41 | 92 |
| CeO2 at 400 rpm | 5.11 | 17 | 531.1 | 93.9 |
| CeO2 at 600 rpm | 5.54 | 13.51 | 431.53 | 91.9 |
| CeO2 at 800 rpm | 5.35 | 15.25 | 288.59 | 83.4 |

CO2 desorption of CeO2 synthesized using a 400 rpm stirring rate at 3% oxalic concentration.
3.3 The change in properties of CeO2 before and after reaction
In this section, CeO2, after use as the catalyst for transesterification reaction, was measured and compared to CeO2 before the reaction. The XRD patterns and morphology of CeO2 using 3% oxalic concentration and 400 rpm stirring rate are presented in Figures 7 and 8. It was found that the XRD peaks and the morphology of CeO2 before and after the reaction looked similar. The difference was not clearly observed from XRD and SEM results. The XRD patterns and the morphology of CeO2 from other samples are presented in Supplementary information. However, the surface area and basicity of CeO2 decreased after the reaction, as shown in Table 3. The surface areas of most samples were reduced moderately. In addition, most samples considerately lost basicity after the reaction. These might be the result of the leaching of the catalyst and the effect of surface poisoning such as the adsorption of compounds on the active sites of the catalysts [12,48].

XRD patterns of CeO2 before and after the reaction using 3% oxalic concentration (at 400 rpm stirring rate) and using 400 rpm stirring rate (at 3% stirring rate).

SEM micrograph (26,700× magnification) of CeO2: (a) 3% oxalic before reaction, (b) 3% oxalic after reaction (the sample in (a) and (b) used 400 rpm stirring rate), (c) 400 rpm before reaction, and (d) 400 rpm after reaction (the samples in (c) and (d) used 3% oxalic concentration).
Comparison of surface area and basicity of CeO2 before and after transesterification reaction
| Sample | Surface area (m2·gcat −1) | %Decreased surface area | Basicity (µmol·CO2·gcat −1) | %Decreased basicity | ||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| 0.5% Oxalic (400 rpm) | 6.53 | 5.85 | −10.41 | 517.99 | 274.70 | −46.97 |
| 3% Oxalic (400 rpm) | 6.75 | 3.49 | −48.3 | 613.09 | 338.21 | −44.84 |
| 200 rpm (3% oxalic) | 5.11 | 3.91 | −23.48 | 490.41 | 310.22 | −36.74 |
| 400 rpm (3% oxalic) | 5.11 | 4.71 | −7.83 | 531.1 | 384.34 | −27.63 |
| 600 rpm (3% oxalic) | 5.54 | 4.56 | −17.68 | 431.53 | 141.8 | −67.14 |
| 800 rpm (3% oxalic) | 5.35 | 5.03 | −5.98 | 288.59 | 182.32 | −36.82 |
4 Conclusions
In conclusion, oxalic concentrations and stirring rates used in the precipitation slightly affect the morphology but considerately influence the basicity of CeO2. As the basicity of catalysts is the main factor in catalyzing transesterification reaction, the greatest amount of basic sites from CeO2 obtained using 3% oxalic concentration and 400 rpm stirring rates provided the highest %FAME in the range of 93.9–94.2%. After the reaction, the change in surface area and basic sites of CeO2 were observed. It was found that the decrease in surface area was less severe than that of the basicity of CeO2. Therefore, the precipitation conditions in CeO2 preparation are one way to improve CeO2 properties to be suitable for use as the catalyst in biodiesel production. Other parameters in the precipitation such as aging times, precipitation temperatures, and calcination temperatures, including the reuse of CeO2 and reaction kinetics, should be further studied, in order to obtain biodiesel qualities within the standard values and be able to enlarge the biodiesel production for commercial use in the future.
Acknowledgment
SP would like to thank Silpakorn University Research, Innovation and Creative Fund for financial support and Thailand Institute of Nuclear Technology for supporting Ce in EDTA solution as the Ce source. Colin Liddle was also thanked for the English correction.
-
Funding information: Silpakorn University Research, Innovation and Creative Fund.
-
Author contributions: Teerapat Hasakul: doing the experiments and writing original draft; Sunthon Piticharoenphun: writing manuscript, formal analysis, and project administration; Dussadee Rattanaphra: formal analysis, review, and measurement; Sasikarn Nuchdang: measurement; Wilasinee Kingkam: measurement.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analysed during this study are included in this published article and tis supplementary files.
References
[1] Atabani AE, Silitonga AS, Badruddin IA, Mahlia TMI, Masjuki HH, Mekhilef S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew Sust Energ Rev. 2012;16:2070–93.10.1016/j.rser.2012.01.003Search in Google Scholar
[2] Liu Y, Zhang W, Yang W, Bai Z, Zhao X. Chemical compositions of PM2.5 emitted from diesel trucks and construction equipment. Aerosol Sci Eng. 2018;2(51):51–60.10.1007/s41810-017-0020-2Search in Google Scholar
[3] Pleanjai S, Gheewala SH. Full chain energy analysis of biodiesel production from palm oil in Thailand. Appl Energy. 2009;86:S209–14.10.1016/j.apenergy.2009.05.013Search in Google Scholar
[4] Prueksakorn K, Gheewala SH. Full chain energy analysis of biodiesel from Jatropha curcas L. in Thailand. Environ Sci Technol. 2008;42:3388–93.10.1021/es7022237Search in Google Scholar PubMed
[5] Nakpong P, Wootthikanokkhan S. High free fatty acid coconut oil as a potential feedstock for biodiesel production in Thailand. Renew. Energy. 2010;35:1682.10.1016/j.renene.2009.12.004Search in Google Scholar
[6] Tippayawong N, Kongjareon E, Jompakdee W. Ethanolysis of soybean oil into biodiesel: Process optimization via central composite design. J Mech Sci Technol. 2005;19(10):1902–9.10.1007/BF02984269Search in Google Scholar
[7] Khwanpruk S, U-tapao C. Potential biodiesel production from palm oil, coconut oil and soybean oil for Thailand. MATEC Web of Conference (ICEAST). vol. 192; 2018. p. 03062.10.1051/matecconf/201819203062Search in Google Scholar
[8] Hoang AT, Tabatabaei M, Aghbashlo M, Carlucci AP, Olcer AI, Le AT, et al. Rice bran oil-based biodiesel as a promising renewable fuel alternative to petrodiesel: A review. Renew Sust Energ Rev. 2021;135:110204.10.1016/j.rser.2020.110204Search in Google Scholar
[9] Hoang AT. Prediction of the density and viscosity of biodiesel and the influence of biodiesel properties on a diesel engine fuel supply system. J Mar Eng Technol. 2021;20(5):299–311.10.1080/20464177.2018.1532734Search in Google Scholar
[10] Atadashi IM, Aroua MK, Abdul AAR, Sulaiman NMN. The effects of catalysts in biodiesel production: A review. J Ind Eng Chem. 2013;19:14–26.10.1016/j.jiec.2012.07.009Search in Google Scholar
[11] Zabeti M, Daud WMAW, Aroua MK. Activity of solid catalysts for biodiesel production: A review. Fuel Process Technol. 2009;90:770–7.10.1016/j.fuproc.2009.03.010Search in Google Scholar
[12] Boey PL, Maniam GP, Hamid SA. Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: A review. Chem Eng J. 2011;168:15–22.10.1016/j.cej.2011.01.009Search in Google Scholar
[13] Queda N, Bonzi-Coulibaly YL, Ouedraogo IWK. Deactivation processes, regeneration conditions and reusability performance of CaO or MgO based catalysts used for biodiesel production-Review. Mater Sci Appl. 2017;8:94.10.4236/msa.2017.81007Search in Google Scholar
[14] de Sousa FP, dos Reis GP, Cardoso CC, Mussel WN, Pasa VMD. Performance of CaO from different sources as a catalyst precursor in soybean oil transesterification: kinetics and leachning evaluation. J Environ Chem Eng. 2016;4(2):1970–7.10.1016/j.jece.2016.03.009Search in Google Scholar
[15] Laha SC, Ryoo R. Synthesis of thermally stable mesoporous cerium oxide with nanocrystalline frameworks using mesoporous silica templates. Chem Commun. 2003;17:2138–9.10.1039/B305524HSearch in Google Scholar
[16] Liying H, Yumin S, Lanhong J, Shikao S. Recent advances of cerium oxide nanoparticles in synthesis, luminescence and biomedical studies: a review. J Rare Earths. 2015;33(8):791–9.10.1016/S1002-0721(14)60486-5Search in Google Scholar
[17] Ramachandran M, Subadevi R, Sivakumar M. Role of pH on synthesis and characterization of cerium oxide (CeO2) nanoparticles by modified co-precipitation method. Vacuum. 2019;161:220–4.10.1016/j.vacuum.2018.12.002Search in Google Scholar
[18] Hoang AT. Combustion behavior, performance and emission characteristics of diesel engine fuelled with biodiesel containing cerium oxide nanoparticles: A review. Fuel Process Technol. 2021;218:106840.10.1016/j.fuproc.2021.106840Search in Google Scholar
[19] Chung KH, Park DC. Water photolysis reaction on cerium oxide photocatalysts. Catal Today. 1996;30:157–62.10.1016/0920-5861(96)00006-5Search in Google Scholar
[20] Ntainjua NE, Garcia T, Solsona B, Taylor SH. The influence of cerium to urea preparation ratio of nanocrystalline ceria catalysts for the total oxidation of naphthalene. Catal Today. 2008;137:373–8.10.1016/j.cattod.2007.12.140Search in Google Scholar
[21] Soodjit P, Srinohakun P, Rattanaphra D, Yoosuk B, Egashira R. Synthesis of biodiesel catalysed by rare earth solid catalyst. International Conference on Innovations in Engineering and Technology (ICIET’2013). Bangkok, Thailand; 2013 Dec 25th–26th. p. 229–32.Search in Google Scholar
[22] Kunjachan C, Kurian M. Cerium oxide-based nanostructures as efficient catalysts for transesterification of methylacetate with n-butanol. Cleaner Eng Technol. 2021;4:100232.10.1016/j.clet.2021.100232Search in Google Scholar
[23] Reyero I, Moral A, Bimbela F, Radosevic J, Sanz O, Montes M, et al. Metallic monolithic catalysts based on calcium and cerium for the production of biodiesel. Fuel. 2016;182:668–76.10.1016/j.fuel.2016.06.043Search in Google Scholar
[24] Kim M, DiMaggio C, Yan S, Salley SO, Ng KYS. The effect of support material on the transesterification activity of CaO-La2O3 and CaO-CeO2 supported catalysts. Green Chem. 2011;13:334–9.10.1039/C0GC00828ASearch in Google Scholar
[25] Russbueldt BME, Hoelderich WF. New rare earth oxide catalysts for the transesterification of triglycerides with methanol resulting in biodiesel and pure glycerol. J Catal. 2010;271:290–304.10.1016/j.jcat.2010.02.005Search in Google Scholar
[26] Kokare BN, Mandhare M, Anuse MA. Liquid-liquid extraction of cerium (IV) from salicylate media using n-n-octylaniline in xylene as an extractant. J Chil Chem Soc. 2010;55(4):431–5.10.4067/S0717-97072010000400004Search in Google Scholar
[27] Yin L, Wang Y, Pang G, Koltypin Y, Gedanken A. Sonochemical synthesis of cerium oxide nanoparticles-Effect of additives and quantum size effect. J Colloid Interface Sci. 2002;246:78–84.10.1006/jcis.2001.8047Search in Google Scholar PubMed
[28] Dhall A, Self W. Cerium oxide nanoparticles: A brief review of their synthesis methods and biomedical applications. Antioxidants. 2018;7:97.10.3390/antiox7080097Search in Google Scholar PubMed PubMed Central
[29] Nyoka M, Choonara YE, Kumar P, Kondiah PPD, Pillay V. Synthesis of cerium oxide nanoparticles using various methods: Implications for biomedical application. Nanomaterials. 2020;10:242.10.3390/nano10020242Search in Google Scholar PubMed PubMed Central
[30] Yu JB, Yang ZG, Wang ZH, Tu TS, Zhang ZQ, Li JY, et al. Synthesis of cerium oxalate hydrate by precipitation technique under external magnetic field. Rare Metals. 2017:1–8.10.1007/s12598-017-0986-6Search in Google Scholar
[31] Kovacevic M, Agarwal S, Mojet BL, Ommen JGV, Lefferts L. The effects of morphology of cerium oxide catalysts for dehydrogenation of ethylbenzene to styrene. Appl Catal A-Gen. 2015;505:354–64.10.1016/j.apcata.2015.07.025Search in Google Scholar
[32] Yamashita M, Kameyama K, Yabe S, Yoshida S, Fujishiro Y, Kawai T, et al. Synthesis and microstructure of calcia doped ceria as UV filters. J Mater Sci. 2002;37:683–7.10.1023/A:1013819310041Search in Google Scholar
[33] Rattanaphra D, Chuoykaew P. Purification process and characterization of cerium oxide from mixed rare earth. TIChE International Conference. Nakornratchasima, Thailand: 2012 Oct 25th-26th.Search in Google Scholar
[34] Han KN. Characteristics of precipitation of rare earth elements with various precipitants. Minerals. 2020;10:178.10.3390/min10020178Search in Google Scholar
[35] Umdu ES, Seker E. Transesterification of sunflower oil on single step sol-gel made Al2O3 supported CaO Catalysts: Effect of basic strength and basicity on turnover frequency. Bioresour Technol. 2012;106:178–81.10.1016/j.biortech.2011.11.135Search in Google Scholar
[36] Sun J, Yang J, Xu X. Basicity-FAME yield correlations in metal cation modified MgAl mixed oxides for biodiesel synthesis. Catal Commun. 2014;52:1–4.10.1016/j.catcom.2014.03.023Search in Google Scholar
[37] AVRK R, Dudhe P, Chelvam V. Role of oxygen defects in basicity of Se doped ZnO nanocatalyst for enhanced triglyceride transesterification in biodiesel production. Catal Commun. 2021;149:106258.10.1016/j.catcom.2020.106258Search in Google Scholar
[38] Poggio E, Jobbagy M, Moreno M, Laborde M, Marino F, Baronetti G. Influence of the calcination temperature on the structure and reducibility of nanoceria obtained from crystalline Ce(OH)CO3 precursor. Int J Hydrog Energy. 2011;36:15899–905.10.1016/j.ijhydene.2011.09.026Search in Google Scholar
[39] Zhaogang L, Mei L, Yanhong H, Mitang W, Zhenxue S. Preparation of large particle rare earth oxides by precipitation with oxalic acid. J Rare Earths. 2008;26(2):158–62.10.1016/S1002-0721(08)60057-5Search in Google Scholar
[40] Chung CY, Kim EH, Lee EH, Yoo JH. Solubility of rare earth oxalate in oxalic and nitric acid media. J Ind Eng Chem. 1998;4(4):277.Search in Google Scholar
[41] Yan M, Wei W, He S, Zuoren N. Preparation and morphology of nano-size ceria by a stripping precipitation using oxalic acid as a precipitating agent. J Rare Earths. 2012;30(12):1265–9.10.1016/S1002-0721(12)60218-XSearch in Google Scholar
[42] Ali B, Yusup S, Quitain AT, Alnarabiji MS, Kamil RNM, Kida T. Synthesis of novel graphene oxide/bentonite bi-funtional heterogeneous catalyst for one-pot esterification and transesterification reactions. Energy Convers Manag. 2018;171:1801–12.10.1016/j.enconman.2018.06.082Search in Google Scholar
[43] Hellgardt K, Chadwick D. Effect of pH of precipitation on the preparation of high surface area aluminas from nitrate solutions. Ind Eng Chem Res. 1998;37:405–11.10.1021/ie970591aSearch in Google Scholar
[44] Yan M, Wei W, Zuoren N. Influence of pH on morphology and formation mechanism of CeO2 nanocrystalline. J Rare Earths. 2007;28:53–7.10.1016/S1002-0721(07)60044-1Search in Google Scholar
[45] Tsoutsos T, Tournaki S, Gkouskos Z, Paraiba O, Giglio F, Garcia PQ, et al. Quality characteristics of biodiesel produced from used cooking oil in Southern Europe. ChemEngineering. 2019;3:19.10.3390/chemengineering3010019Search in Google Scholar
[46] Thitsartarn W, Kawi S. An active and stable CaO-CeO2 catalyst for transesterification of oil to biodiesel. Green Chem. 2011;13:3423.10.1039/c1gc15596bSearch in Google Scholar
[47] Zheng YC, Yu XH, Yang J. Synthesis of CaO-CeO2 catalysts by soft template method for biodiesel production. IOP Conf Ser: Earth Environ Sci. 2017;69:012048.10.1088/1755-1315/69/1/012048Search in Google Scholar
[48] Zhang N, Xue H, Hu R. The activity and stability of CeO2@CaO catalysts for the production of biodiesel. RSC Adv. 2018;8:32922–9.10.1039/C8RA06884DSearch in Google Scholar
[49] Mei L, Mitang W, Zhaogang L, Yanhong H, Jinxiu W. Cerium oxide with large particle size prepared by continuous precipitation. J Rare Earths. 2009;27(6):991–6.10.1016/S1002-0721(08)60376-2Search in Google Scholar
[50] Sung MH, Choi IS, Kim JS, Kim WS. Agglomeration of yttrium oxalate particles produced by reaction precipitation in semi-batch reactor. Chem Eng Sci. 2000;55:2173–84.10.1016/S0009-2509(99)00480-7Search in Google Scholar
© 2022 Teerapat Hasakul et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

