Abstract
Highly functionalized, high value added bioactive molecules are generally obtained by synthetic procedures that are highly selective, economical, with high atom economy, and environmentally friendly. Following these guidelines, the use of recoverable solid catalysts, nonpolluting substrates, or toxic organic solvent contributes greatly to these demands. In the last three decades, heteropolyacids (HPAs) and its derivatives have received great attention as recyclable solid catalysts, due to their strong Brönsted acidity, excellent oxidizing capacity under mild conditions, and various reuse cycles without appreciable loss of their catalytic activity. However, new activation methods should be investigated to improve the sustainability of a process using HPAs. In this review, we report the latest advances associated with the synthesis of potentially bioactive molecules using more energy efficient alternatives such as microwaves, ultrasound, mechanochemistry, and photochemistry to minimize the energy consumption associated with organic synthesis. The transformations studied include construction reaction, heterocycle synthesis, selective oxidation, and biomass recovery.
1 Introduction
In the 1990’s, a new discipline in chemistry, called green chemistry, emerged, focusing all its efforts on solving the problems associated with pollution. The evolution of this new discipline focused on presenting a change in the strategies for solving environmental problems, associated with the regulatory and mandatory reduction in industrial emissions at the “end of the pipe,” toward the prevention of pollution through the design of production technologies themselves [1]. The 12 principles of green chemistry proposed by Anastas and Warner established the ways to minimize the environmental impacts of chemical production, and also indicate research priorities for the development of green chemistry technologies [2].
Since its inception, green chemistry has grown notably aided by different areas of research. Among them, heterogeneous catalyst-assisted organic synthesis has been one of the fastest growing sciences. Although, some catalytic technologies are almost 100 years old [1], they have been strengthened in the last decade since the principles of green chemistry promote their development in replacement of stoichiometric or catalytic processes. These catalysts, such as NaOH, H2SO4, HCl, HF, AlCl3, BF3, and ZnCl2, pose handling, containment, disposal, and regeneration risks due to their corrosive and toxic nature [3]. Heterogeneous catalysis using solid catalysts has great advantages that include the easy separation of the reaction medium, the possibility of reuse and, in general, it tends to be more selective, reducing the formation of secondary products [4]. A particular class of catalysts are heteropolyoxometalates, particularly heteropolyacids (HPAs) due to the broad range of reactions in which they can be employed.
Heteropolyoxometalates are a very large group of compounds associated with units of transition metal-oxygen octahedral anions, which can join to form innumerable structures. Among all the structures discovered, the most investigated and used HPAs correspond to those of the Keggin-type structure. Their most common structure is represented by the formula α[XM12O40] n−, where X is Si4+ or P5+, and M is Mo6+ or W6+. However, other representative HPAs include Wells–Dawson and Preyssler structure [5].
Another relevant aspect in the optimization of chemical processes in order to make them more sustainable is related to energy consumption. Energy consumption represents a major global challenge to achieve a sustainable planet. For this reason, developing alternative sources of safer, efficient, cleaner, renewable, and controllable energy is a goal of green chemistry, and the utilization of safe and efficient energy is being explored vigorously [6,7].
Taking into account the principles of sustainable chemistry applied to the synthesis of products, notable efforts are being made aimed at the use of heterogeneous catalytic processes with the minimization of energy consumption during transformations through alternative techniques to conventional heating such as microwaves, ultrasounds, photochemistry, electrochemistry, and mechanochemistry, among others [6,7,8].
On the other hand, in synthetic organic chemistry at the laboratory level or on an industrial scale, transformations are normally carried out using catalysts under homogeneous reaction conditions with conventional heating. Thus, these obsolete procedures can be redesigned through the use of catalysts that operate in heterogeneous conditions and also optimize the process through the use of alternative energy techniques to assist in these transformations [9]. Especially in the synthesis of heterocycles, about 90% of the new drugs contain this type of ring in their structures, which increases the interest in redesigning the classic syntheses of this type of compound using cleaner technologies and better yields, as well as obtaining new skeletons, and studying their activities [10]. The literature describes several review articles about the use of HPAs as catalysts in organic chemistry [5,10,11,12,13,14,15]. In addition to the transformations associated with the synthesis of heterocycles, this article also refers to other transformations typical of organic chemistry that include construction reactions, oxidation, and biomass recovery.
In this review, we report the advances associated with different organic transformations, through green procedures, developing more energy-efficient alternatives such as microwaves, ultrasound, mechanochemistry, and photochemistry, plus the use of different materials based on HPAs, updated with the contributions made in the area for the period 2000–2020.
2 Microwave synthesis
The synthetic processes of organic chemistry are perfected every day through new techniques that promote substantial improvements in the yields and selectivity of these transformations. In particular, organic synthesis assisted by the use of a microwave source has revolutionized organic synthesis. Many molecules can be prepared in substantially shorter reaction times compared to classical thermal heating procedures [16,17]. This notably promoted the discovery of new drugs and products of technological interest.
The use of microwave irradiation in organic transformation includes several advantages, collaborating with the development of cleaner synthetic processes, e.g., high heating efficiency, uniform heating throughout the material, increased process speed, low operating cost, final product purity, reduction in unwanted secondary reaction, and no ambient heat loss, among others [16,17].
The importance of this technique as an aid to green chemistry is reflected in the number of publications in the last decade that can be addressed through numerous review articles [18,19,20,21,22,23,24,25,26,27,28]. The different contributions in the synthesis of organic compounds using HPA catalysts and microwaves as an energy source are indicated below.
Simple transformations such as esterification, ammoxidation, and acetylation can be easily performed using distinct HPAs assisted with microwave irradiation. For example, Heravi group reported a friendly procedure for the catalytic salicylic acid esterification using aliphatic and benzylic alcohols with a Preyssler HPA supported on nanosilica H14[NaP5W30O110]–SiO2, under microwave irradiation (Scheme 1). This methodology allows obtaining high reaction yields (14 examples, 66–98%) under solvent-free conditions, and the catalyst was reused for several times without appreciable loss in its activity [29].
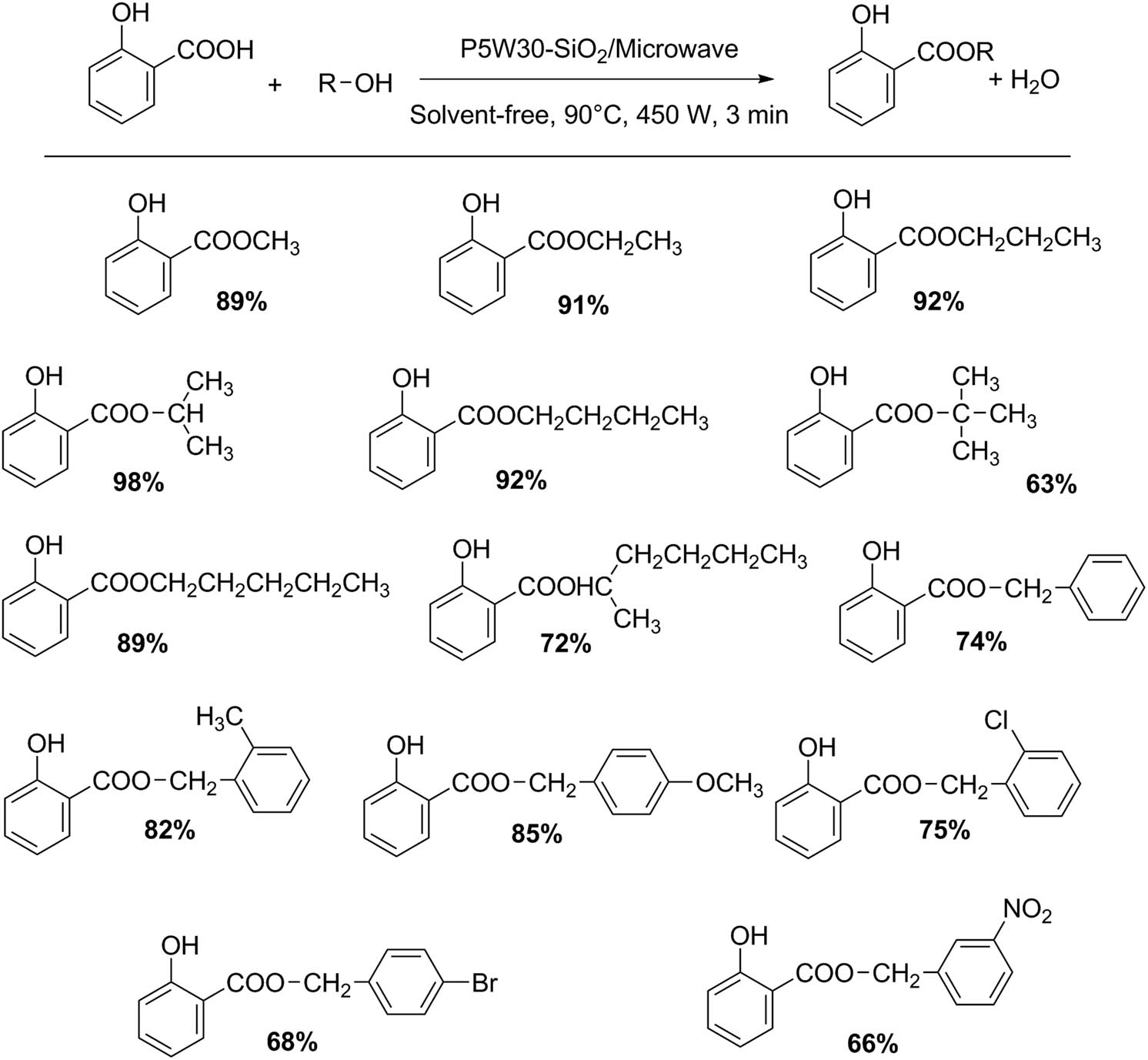
Esterification of salicylic acid with alcohols.
The esterification of acetic acid and fatty acids also has been studied using Keggin HPAs. The influence of several reaction conditions was monitored for the reaction between 1-butanol and acetic acid (Scheme 2). Five alcohols were acetylated with very good yields in shorter reaction times (2–12 min) with a selectivity greater than 97%. To analyze the catalyst recycling and stability, at the reaction end, the catalyst was filtered, washed with dichloromethane, dried at room temperature, and finally activated at 130°C for 3 h. The recovered catalyst retained catalytic activity after several cycles [30,31].
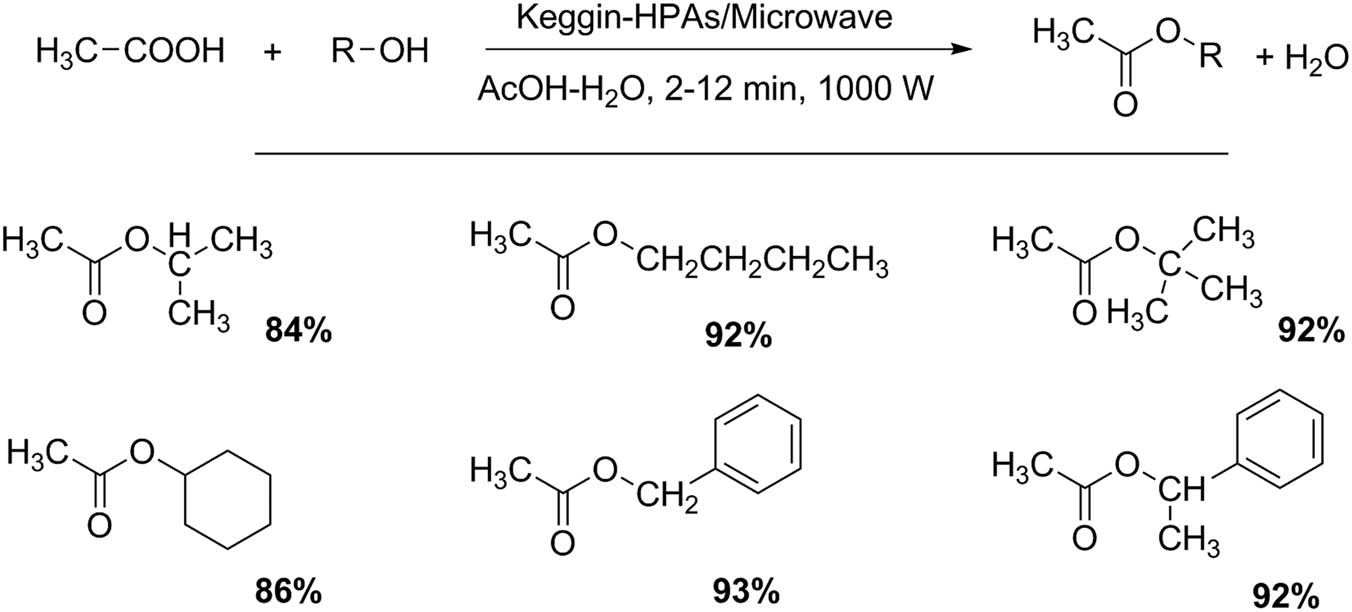
Esterification of acetic acid with alcohols.
Duan et al. reported the preparation and characterization of a new type of HPA ionic liquid [(CH3)3NCH2CH2OH]H2PW12O40 (phosphotungstic acid [PW12]-IL) using choline chloride and H3PW12O40 (PW12) as precursors. Excellent conversions (97%) for esterification of palmitic acid with methanol were obtained under microwave-accelerated conditions (65°C, 50 min) (Scheme 3) [32]. Besides, this catalyst can be easily removed by lowering the reaction temperature without appreciable activity loss.

Palmitic acid esterification with methanol.
A catalyst with Preyssler structure, H14[NaP5W30O110], promotes the ammoxidation of aldehydes for the synthesis of Z-isomer oximes under microwave irradiation (Scheme 4). The simple methodology involves the addition of a catalytic amount of P5W30 to a mixture of hydroxylamine hydrochloride (3 mmol) and aldehyde (2 mmol), in solvent-free conditions. The reaction mixture was irradiated for 2 min with microwaves. Six examples of Z-oximes were prepared with a variable yield (21–71%) [33].
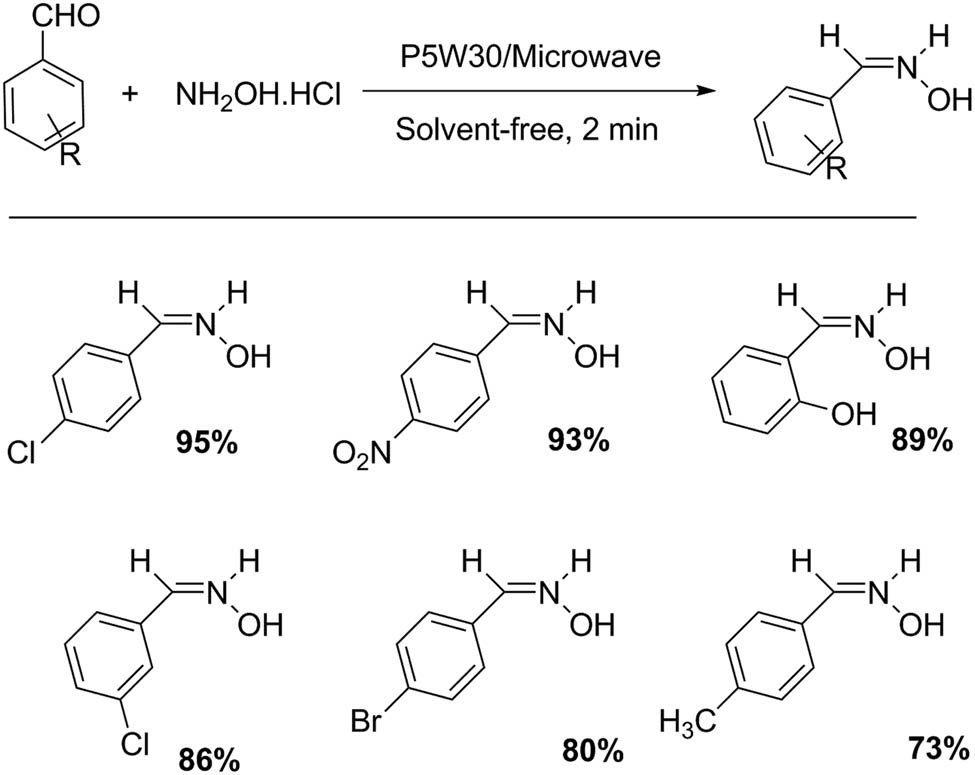
Ammoxidation of aromatic aldehydes.
Another interesting example under solvent-free conditions, microwave irradiation, and different Keggin-type HPAs is shown in Scheme 5. Ighilahriz-Boubchir et al. [34] demonstrated that an equimolar mixture of 2-phenyl-3,1-(4H)-benzoxazin-4-one (10 mmol) and amines (10 mmol) a one HPA (1.2 mol%) such as H3PW12O40·13H2O, H4SiW12O40·13H2O, H4SiMo12O40·13H2O, and H3PMo12O40·13H2O can give higher yields to 2-benzoylamino-N-phenylbenzamide derivatives (66–92%) with short reaction times (in some cases, 3 min).
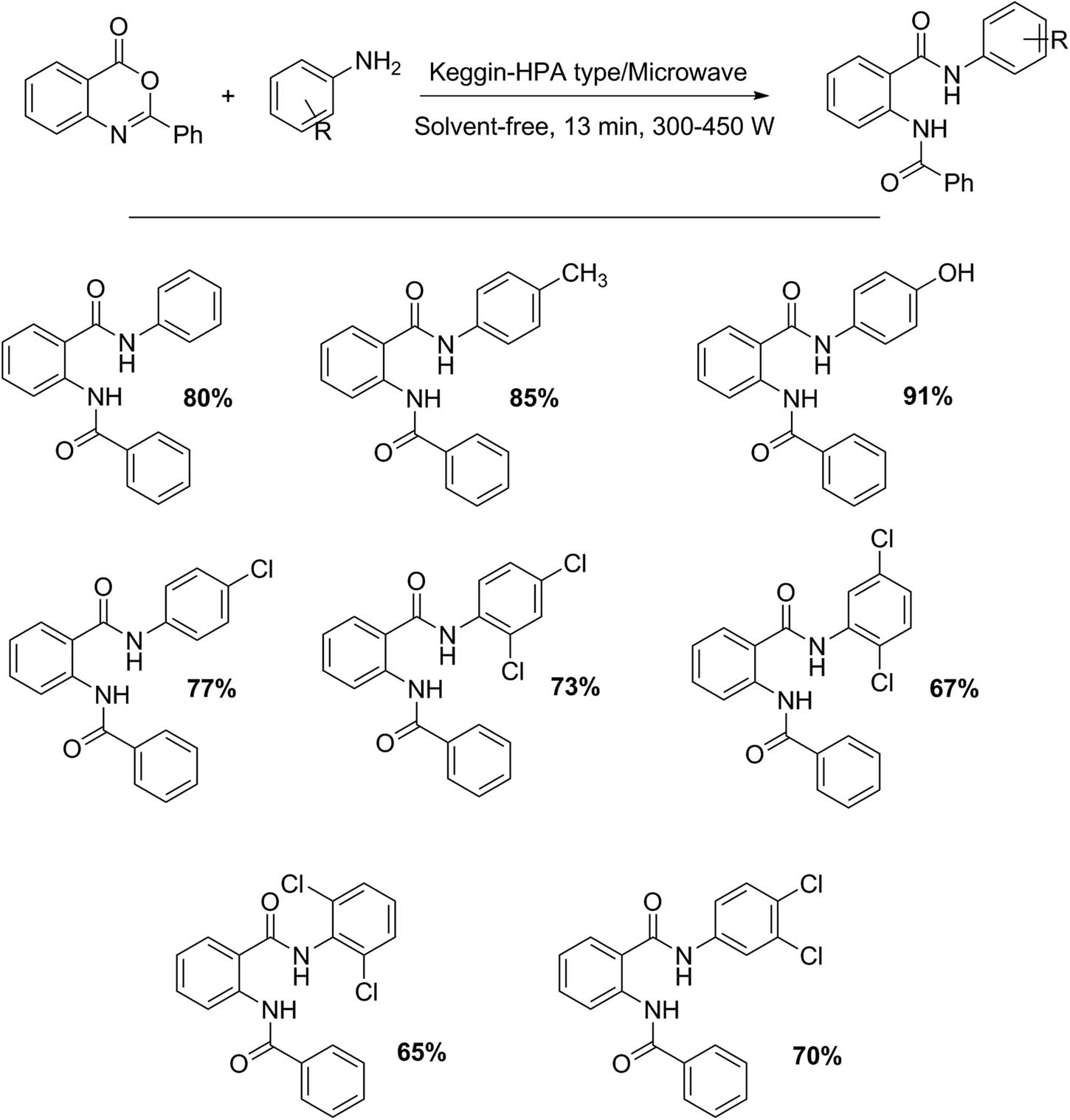
2 Benzoylamino-N-phenyl-benzamide derivative synthesis.
On the other hand, the functionalization of HPAs on mesoporous materials is an attractive strategy to increase proton availability, maintaining the high yields, short reaction time, easy processing, and reusability of the catalysts. Thus, PW12 catalysts supported on amino-functionalized Zr and La-incorporated SBA-15 mesoporous materials (PW12/H N-ZLS) have resulted in efficient heterogeneous catalysts for acetylation of anisole with acetic anhydride under microwave irradiation (Scheme 6). The catalytic tests were performed employing the following amount of reagents: anisole (10 mmol), acetic anhydride (20 mmol), dichloromethane (10 mL), and PW12/H2N-ZLS, or PW12/ZLS (0.6 g). The reaction mixture was irradiated with microware (450 W, 8 min). The 40%PW12/H2N-ZLS-N was the most selective catalyst, showing 84% conversion of anisole and p-methoxyacetophenone selectivity of about 99%. Meanwhile, when using 30% PW12/H2N-ZLS-N as catalyst, the conversion was 88% (10 min) and the selectivity was 96% [35]. Similarly, Ping and Yuchun presented a procedure for p-methoxyacetophenone using PW12 acid catalyst [36].
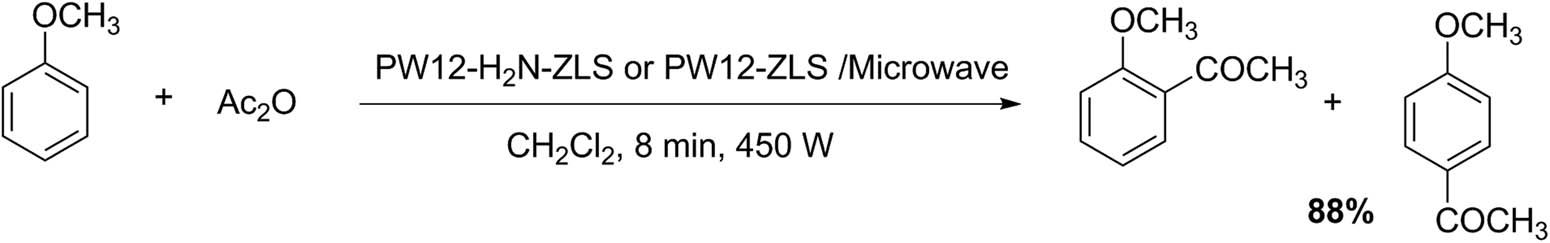
Anisole acetylation.
Although the transformations described so far represent single bond formation reactions, there is also an abundance of literature about heterocycle synthesis.
3,4-Dihydropyrimidine-2(1H)-ones (a particular class of dihydropyrimidines) are interesting compounds that play an important role in therapeutic and bioorganic chemistry. Some relevant activities include antibacterial and antihypertensive effects, and they also act as calcium channel blockers, α-1a-antagonists, and neuropeptide Y antagonist [37,38]. The Biginelli reaction is the conventional method for preparing these compounds. This multicomponent reaction needs acidic catalysts and involves the condensation of β-dicarbonyl compounds, an aldehyde, and urea or thiourea [37,38].
Several variants of Biginelli reaction have been studied during the last two decades. Spiro-fused heterocycles can be obtained from the reaction of cyclic β-diamides with β-ketoesters, Meldrum’s acid, or barbituric acid derivatives with one equivalent of urea and two equivalents of aldehydes, giving the corresponding compounds in the presence of H6GeW10V2O40·22H2O (GeW10V2) as catalyst, under solvent-free conditions at 80°C (900 W, 7 min) (Scheme 7). Twelve compounds were reported with yields between 66% and 87%, free of secondary products; besides, the recovered catalyst retained its catalytic activity after 4 cycles [39].
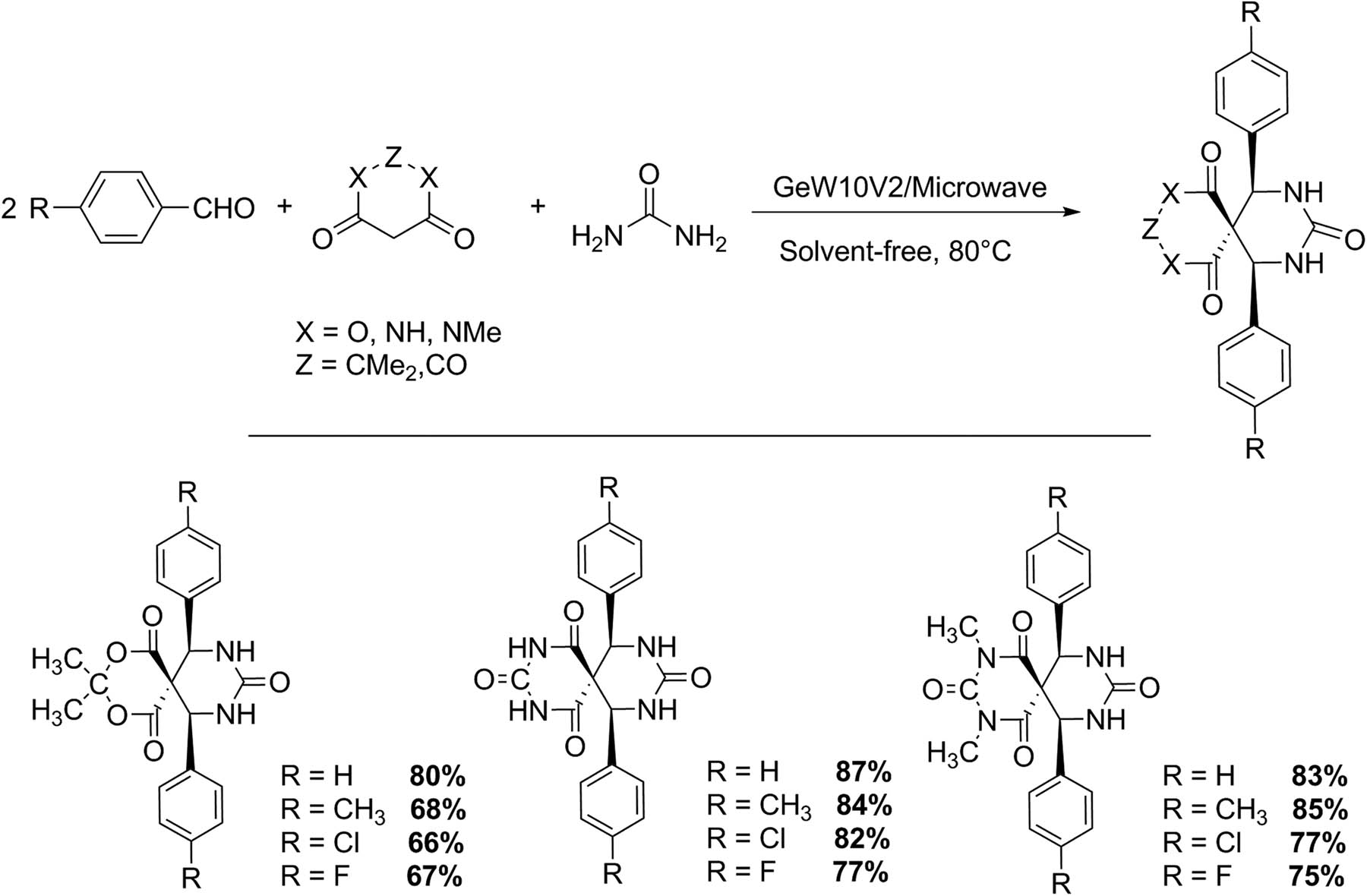
Synthesis of spiro-fused Biginelli derivatives.
Dihydropyrimidinone derivatives from biomass platform molecules have been studied by our research group. A three-component domino reaction through a combination of furfurals, β-ketoesters, and urea/thiourea using Preyssler HPA H14NaP5W29MoO110 (P5W29Mo) encapsulated in a silica framework as the catalyst can provide moderate yields using microwave irradiation under solvent-free conditions [40]. Eight dihydropyrimidinones were obtained with yields between 45% and 68%. A representative experiment involves a mixture of methyl acetoacetate (1 mmol), 2-furaldehyde (1 mmol), urea (1 mmol), and (P5W29Mo-SiO2) (100 mg, 1 mmol%). The mixture was strongly mixed and heated on microwave at 120°C for 15 min (Scheme 8).
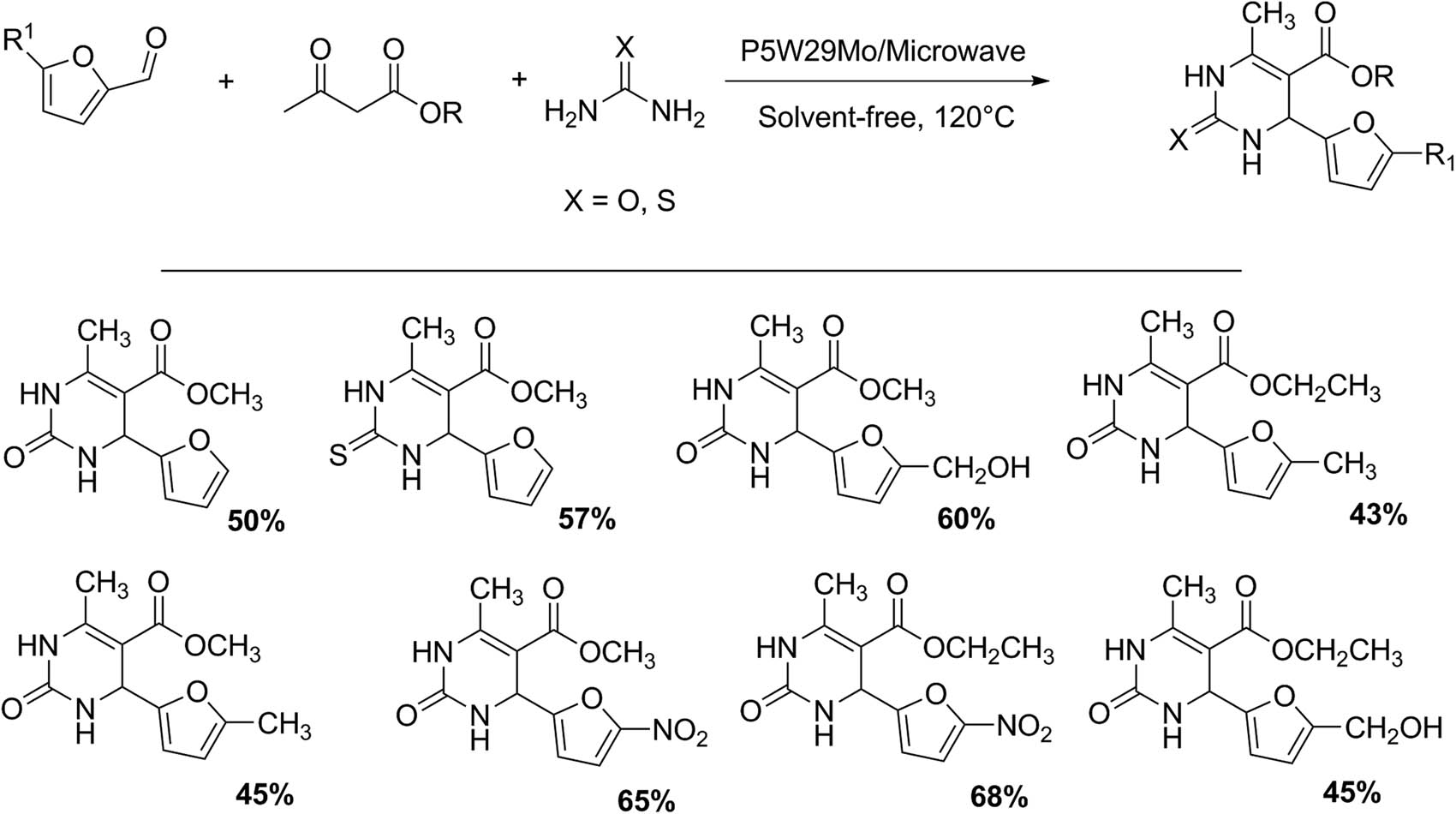
Synthesis of dihydropyrimidinones.
The synthesis of 3,4-dihydropyrimidin-2(1H)-one/thione analogs of curcumin can be performed by a one-pot multicomponent cyclocondensation using curcumin, substituted aromatic aldehydes, and urea/thiourea in ethanol catalyzed by commercial Keggin-type HPA H3PMo12O40 (PMo12) under microwave irradiation (Scheme 9). In a conventional test, a mixture of curcumin (2 mmol), substituted aldehydes (2 mmol), and urea/thiourea (3 mmol) dissolved in ethanol (2 mL) and PMo12 (5 mmol%) was irradiated under microwaves for 150–210 s. After completion of the reaction, the solvent was removed, and the mixture was poured into crushed ice. The precipitate was filtered, washed with hot water, dried, and crystallized with diethyl ether. Fourteen examples of 3,4-dihydropyrimidin-2(1H)-one/thione analogs of curcumin were synthesized with excellent yield (93–98%), using a simple, efficient, and improved Biginelli protocol [41]. These compounds were evaluated for their antioxidant and antibacterial activity, which was excellent and moderate, respectively.
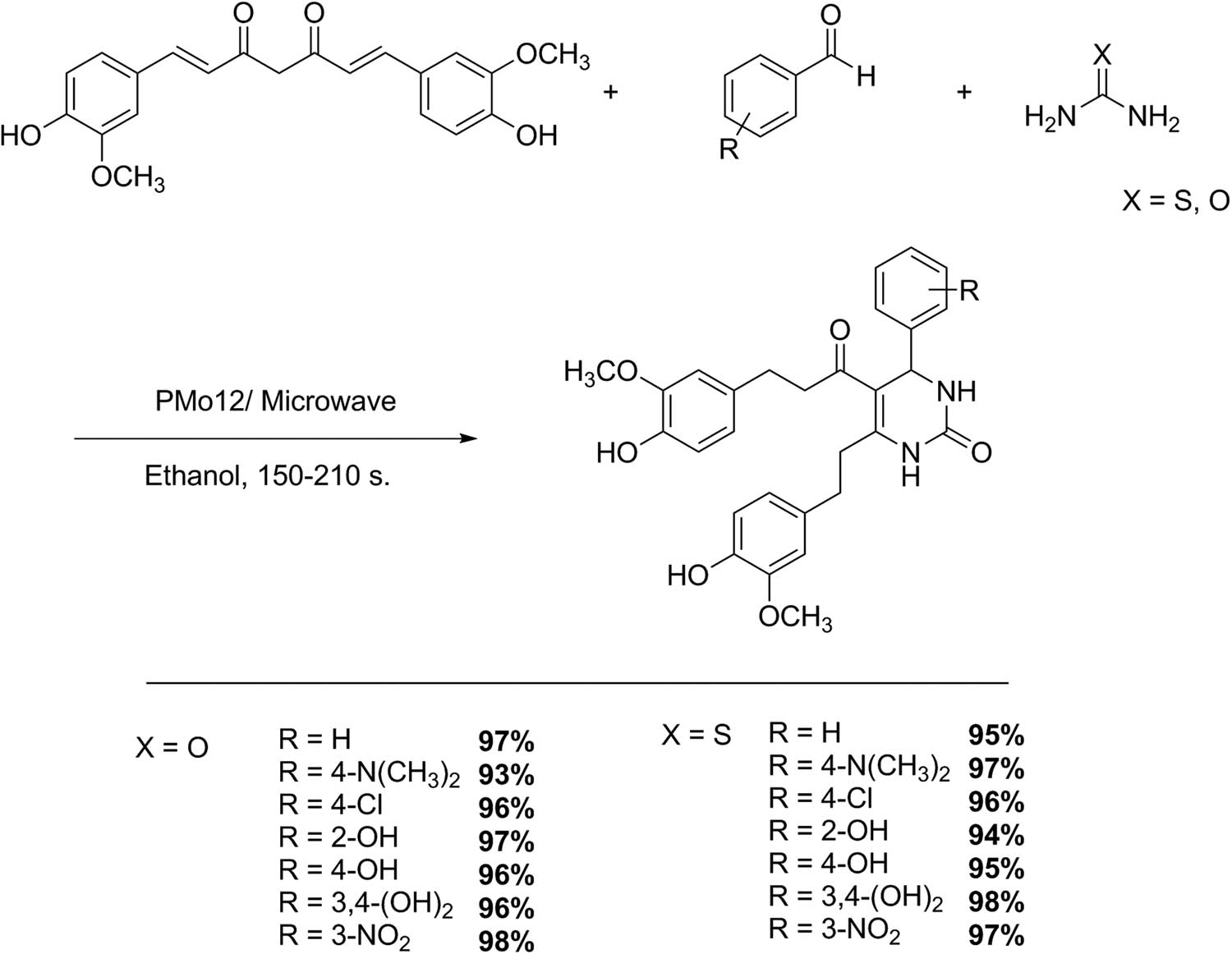
Synthesis of 3,4-dihydropyrimidinones/thiones of curcumin.
The Biginelli reaction can be slightly modified to obtain pyrazolopyranopyrimidines. This approach has been studied by Heravi group obtaining higher yields (>90%), in times between 35 and 50 min, at 100°C with a tungstophosphoric acid supported on amine-functionalized halloysite nano clay (PW12-HAL-CLAY) (Scheme 10). The procedure only involves the aqueous mixture of barbituric acid (1 mmol), hydrazine hydrate (1.1 mmol), ethyl acetoacetate (1 mmol), and aldehydes (1 mmol) in the presence of a catalytic amount of catalyst (0.03 g). The heterogeneous catalyst was reused for three cycles without significant loss of activity [42].
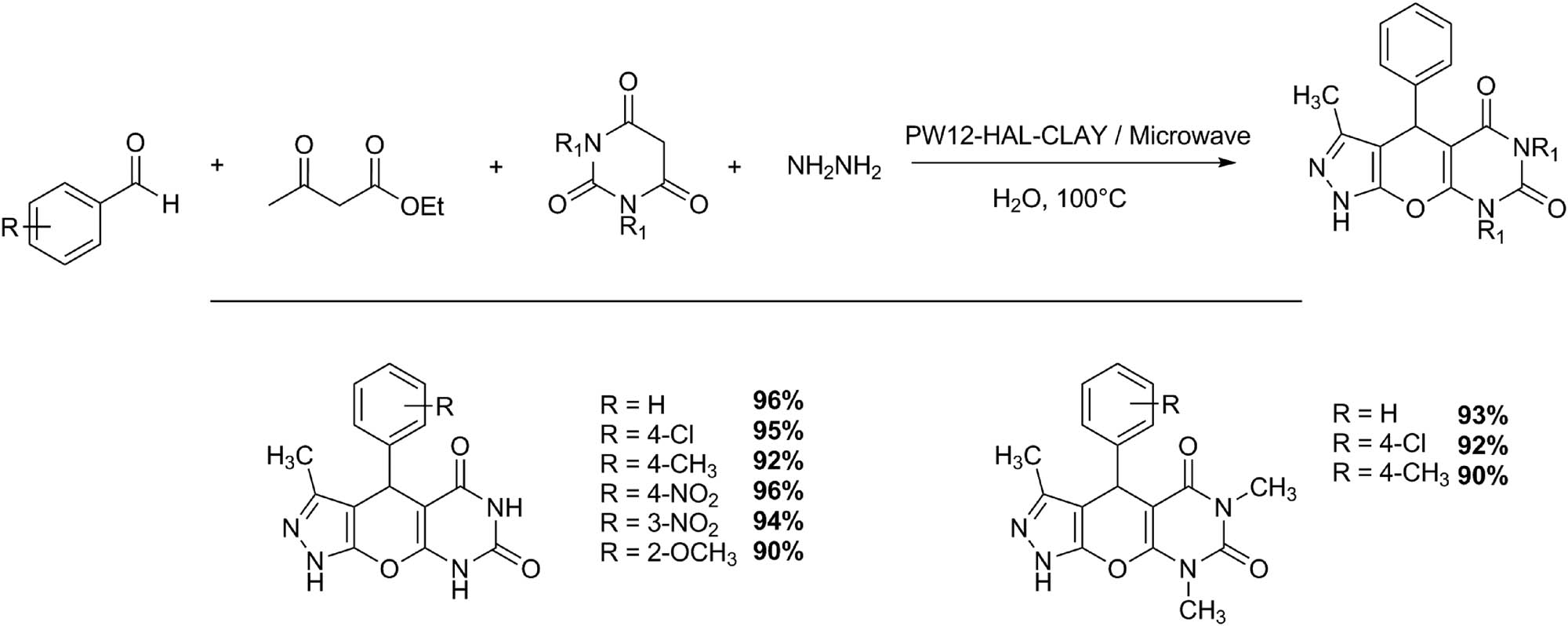
Synthesis of pyrazolopyranopyrimidines.
This same research group reported the reaction of pyrazoles and formamide under microwave irradiation using various solid acid catalysts such as HY-zeolite, silica-supported sulfuric acid, and silica-supported HPAs as catalyst for the synthesis of 4-amino-pyrazolo[3,4-d] pyrimidine derivatives. H3PW12O40 (PW12) showed the best catalytic activity in terms of yields and reaction times (Scheme 11). A typical procedure involves an appropriate amount of pyrazole (1 mmol) mixed with excess amounts of formamide (2 mmol) and a catalytic amount of PW12. The reaction mixture was placed in a microwave oven for the indicated time (8–12 min). Upon reaction completion, the catalyst was filtered and reused, and the diethyl ether extract was concentrated. The products were purified by liquid chromatography, and recrystallization of ethanol. Five examples of 4-amino-pyrazolo[3,4-d]pyrimidines were obtained with very good yields, between 71% and 88% [43]. Some advantages of this methodology are: reaction performed in solvent-free conditions, short reaction time using microwave irradiation, and use of inexpensive and recyclable catalyst.
![Scheme 11
Synthesis of 4-amino-pyrazolo[3,4-d] pyrimidines.](/document/doi/10.1515/gps-2022-0068/asset/graphic/j_gps-2022-0068_fig_011.jpg)
Synthesis of 4-amino-pyrazolo[3,4-d] pyrimidines.
The synthesis of quinoline derivatives by a one-pot reaction of anilines with crotonaldehyde or methyl vinyl ketone using phosphotungstic acid, under microwave irradiation conditions, have also been studied with some detail (Scheme 12) [44,45]. Several quinoline-core compounds have pharmaceutical activities such as antitumor, antimalarial, analgesic, antihypertensive, anti-inflammatory, antibacterial, antiasthmatic, and antiplatelet agents, among others. In addition, quinoline derivatives are a valuable substructure used in a variety of compounds with electronic and photonic functions, polymer chemistry, and agrochemical applications, due to their notable mechanical properties [44,45]. The synthesis using a Keggin HPA (PW12) requires aniline (2 mmol) previously adsorbed on silica gel (0.4 g) and mixed with catalyst (0.2 g). Then crotonaldehyde (3 mmol) is added and mixed again. The mixture is irradiated with microwaves at a power of 80% at a pulse rate of 45 s for 10 min. When the process has finished, the product is extracted with ethyl acetate and purified by liquid chromatography. Under this methodology, 19 quinoline derivatives produced by the Doebner–Miller reaction using PW12 as catalyst and under microwave irradiation conditions of 300 W for 10–15 min can be obtained with very good yields (79–94%) [46].
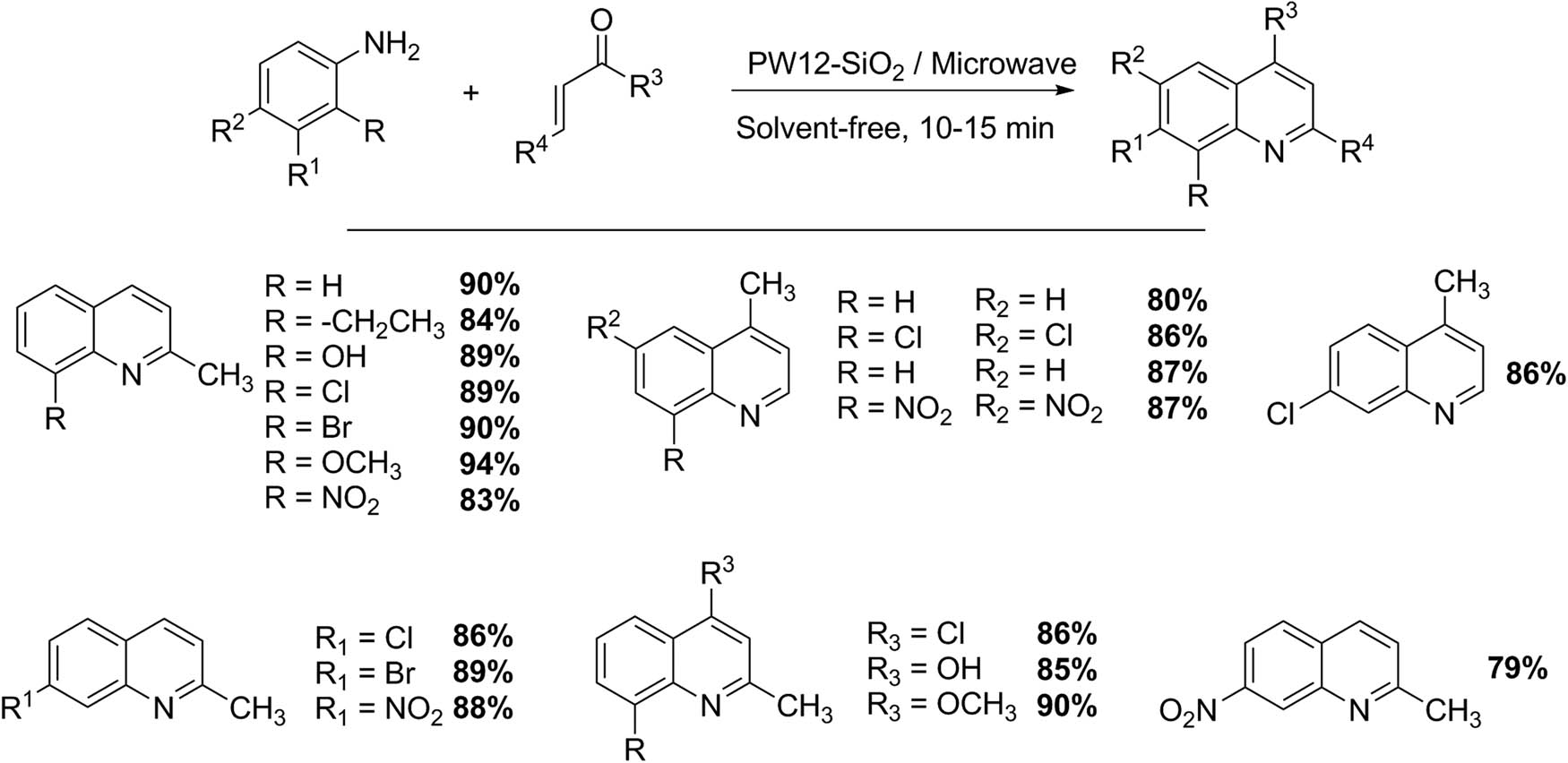
Synthesis of quinoline derivatives.
The quinazolinone system is a particular class of quinoxaline derivatives that possess a carbonyl group. This substructure and its derivatives are present in a large family of compounds with important biological activities. They have valuable pharmacological and therapeutic properties such as anticonvulsant, anticancer, anti-inflammatory, antihypertensive, antimalarial, antibacterial, antifungal, and antiviral activities [47,48]. The synthesis of quinazolin-4(3H)-ones in the presence of HPAs (1.2 mol%) under microwave irradiation was carried out from condensation of anthranilic acid (10 mmol), amine (10 mmol), and formic acid or orthoesters (14 mmol) using 2-ethoxyethanol (5 mL) as reaction solvent (Scheme 13). The power of microwave irradiation was initially set to 300 W for 3 min, and was increased to 450 W for 10 min. Similarly, the authors performed a suitable method in solvent-free conditions. Initially, several Keggin HPAs were tested (H3PW12O40·13H2O, H4SiW12O40·13H2O, H4SiMo12O40·13H2O, or H3PMo12O40·13H2O) as catalysts. However, the use of PW12 acid coupled with microwave irradiation showed better yields in solvent-free conditions in 13 min approximately. Ten compounds were obtained with yields of the order of 80% in most of the cases [48].
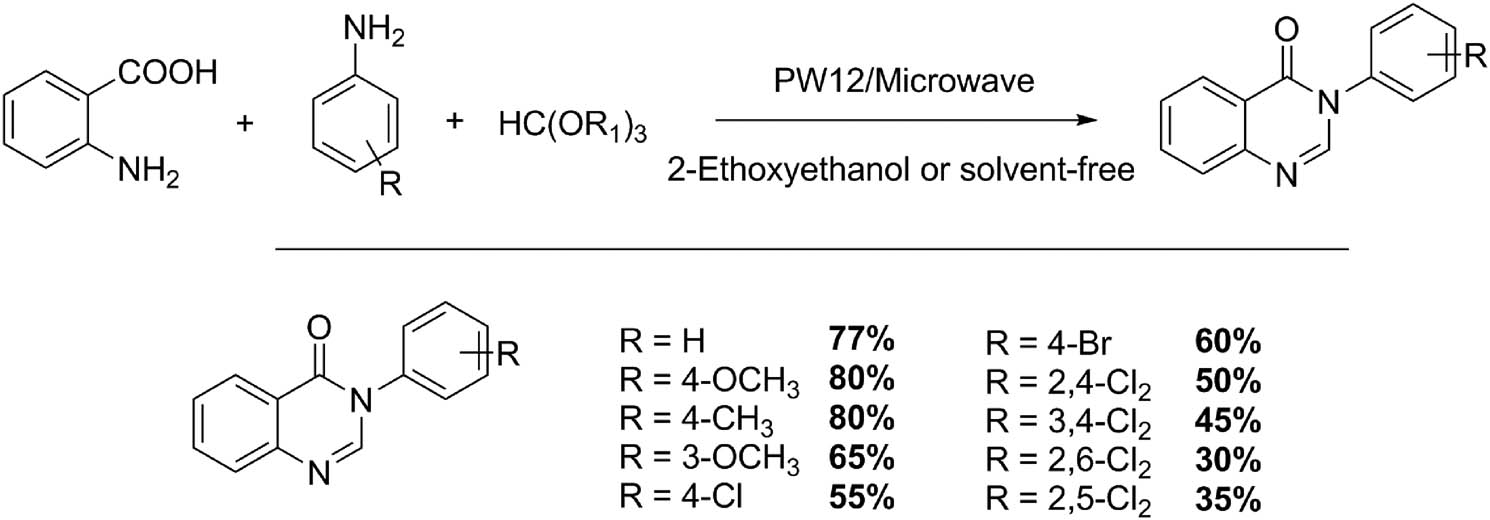
Synthesis of 4(3H)-quinazolinones.
The imidazole substructure has received much attention because of its anti-inflammatory, antimicrobial, antiallergenic, anticonvulsant, analgesic, antithrombotic, hypnotic, and proton pump inhibitor activities. Imidazole derivatives have also been reported in compounds that are used for electronics, fungicides, photography, and as fire retardants [49,50,51,52,53]. Das et al. [53] established the use of defective Keggin-type HPA PAlMo11O40H6 (PAlMo11) as catalyst for efficient one-pot four-component synthesis of 1,2,4,5-tetrasubstituted imidazoles assisted by microwave (Scheme 14). Particularly, a mixture of 1,2-diketone (1 mmol), primary amines (1 mmol), aldehydes (1 mmol), ammonium acetate (or urea) (1.5 mmol), and PAlMo11 (5 mol%) was irradiated with microwave (560 W) for 10 min. The crude products were extracted with CH2Cl2 and then purified by recrystallization from ethanol. Nineteen tetrasubstituted imidazole derivatives were obtained with excellent yields (87–94%). The procedure represents an environmentally friendly alternative: a multicomponent process with high atom economy, reaction in solvent-free conditions, reusable catalyst, absence of secondary products, product of high purity obtained by simple filtration, and an extremely short reaction time (10 min) by using microwave irradiation.
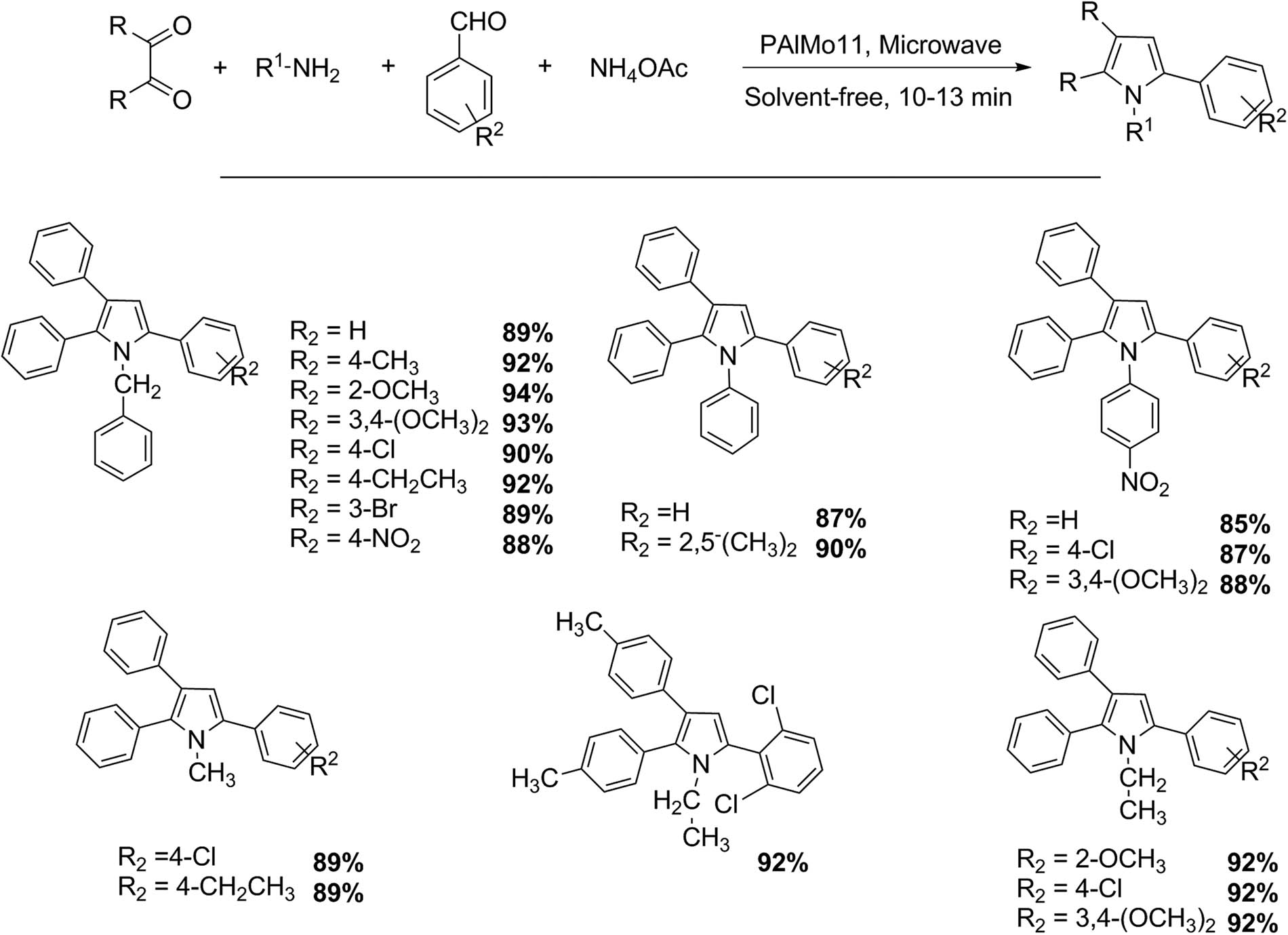
Tetrasubstituted imidazole synthesis.
A similar procedure to that developed previously using p-phenylenediamine allows obtaining tetrasubstituted imidazoles with luminescent properties. In this procedure, a solvent-free microwave-assisted four-component synthesis of 1,2,4,5-tetrasubstituted imidazoles containing a 4-aminophenyl substituent was tested by condensation of p-phenylenediamine (1 mmol), aryl diketone (1 mmol), benzaldehyde derivatives (2 mmol), and ammonium acetate (8 mmol) in the presence of PW12 supported on silica (0.7 mmol%). When the microwave-assisted reaction ended, the residue was cooled to room temperature and purified by liquid chromatography and recrystallized in ethanol to give the pure product. Twelve 1,2,4,5-imidazoles with a 4-amino-phenyl substituent (auxochrome in 1 position) were successfully synthesized (57–74%) using PW12 and silica gel as solid support. The authors highlighted the possibility of using these new types of compounds with luminescence properties as potential applications in metal ion detection, biological, pharmaceutical, and material fields [54].
Keri et al. [55] reported an interesting work about an efficient method for 2-arylbenzimidazole preparation from o-phenylenediamines and phenoxyacetic acids under microwave conditions using a HPA with Wells–Dawson structure such as H6P2W18O62·24H2O (P2W18) as catalyst (Scheme 15). The reaction was carried out with o-phenylenediamine (1.0 mmol), different phenoxyacetic acids (1.0 mmol), and a catalytic amount of P2W18 HPA (1 mol%) in 2 mL of toluene. The reaction mixture was irradiated in a microwave oven for an appropriate time (2.5–4.5 min), and then the catalyst was recovered quantitatively after washing with toluene. The reaction mixture was purified by liquid chromatography. Thirty examples were obtained with excellent yields (82–95%) practically free of secondary products.
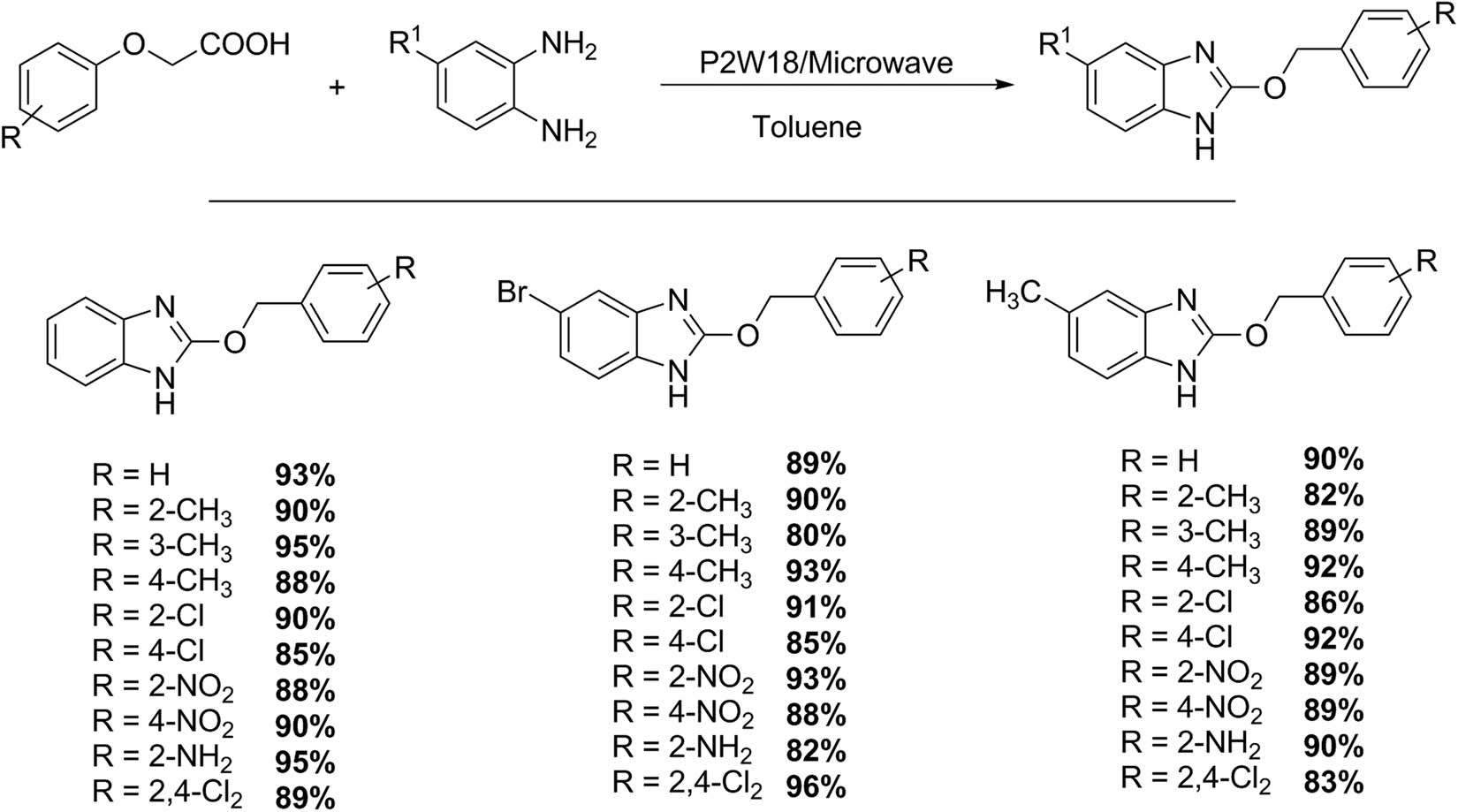
Synthesis of benzimidazoles.
Coumarins are other highly relevant heterocyclic compounds due to their diversity of applications at industrial level, especially in the pharmaceutical industry. Compounds possessing the coumarin subunit in their structure have been reported to exhibit different biological activities such as inhibitors of platelet aggregation, compounds with antibacterial, antiparasitic, antifungal, and anticancer activities, and selective enzyme inhibitors of HIV-1 protease, among others [56,57]. They are also widely used in the preparation of fragrances, flavors, fluorescent dyes and sensors, food additives, and as starting compounds for the synthesis of other bioactive compounds such as chromones, furocoumarins, and others. The structural modification of the coumarin nucleus leads to changes in the application of the molecule, since this gives it the ability to target various action points, which leads to variations in the normal action of the organism to be treated, and the modification of the molecule increases its cytotoxicity and selectivity.
Previous work by our research group allowed obtaining, in a simple way and in friendly environmental conditions, simple coumarins using a domestic microwave oven using silica-supported Wells–Dawson acid (P2W18-SiO2). Solvent-free reactions were performed using phenols (1 mmol) and β-ketoesters (1 mmol) or phenylpropiolic acid (1 mmol) by the Pechmann reaction (Scheme 16a and b). The crude products were extracted with hot toluene (3 mL × 2 mL), and recrystallized from hot ethanol. Nine compounds were obtained with yields between 54% and 99% (10–25 min) [58].
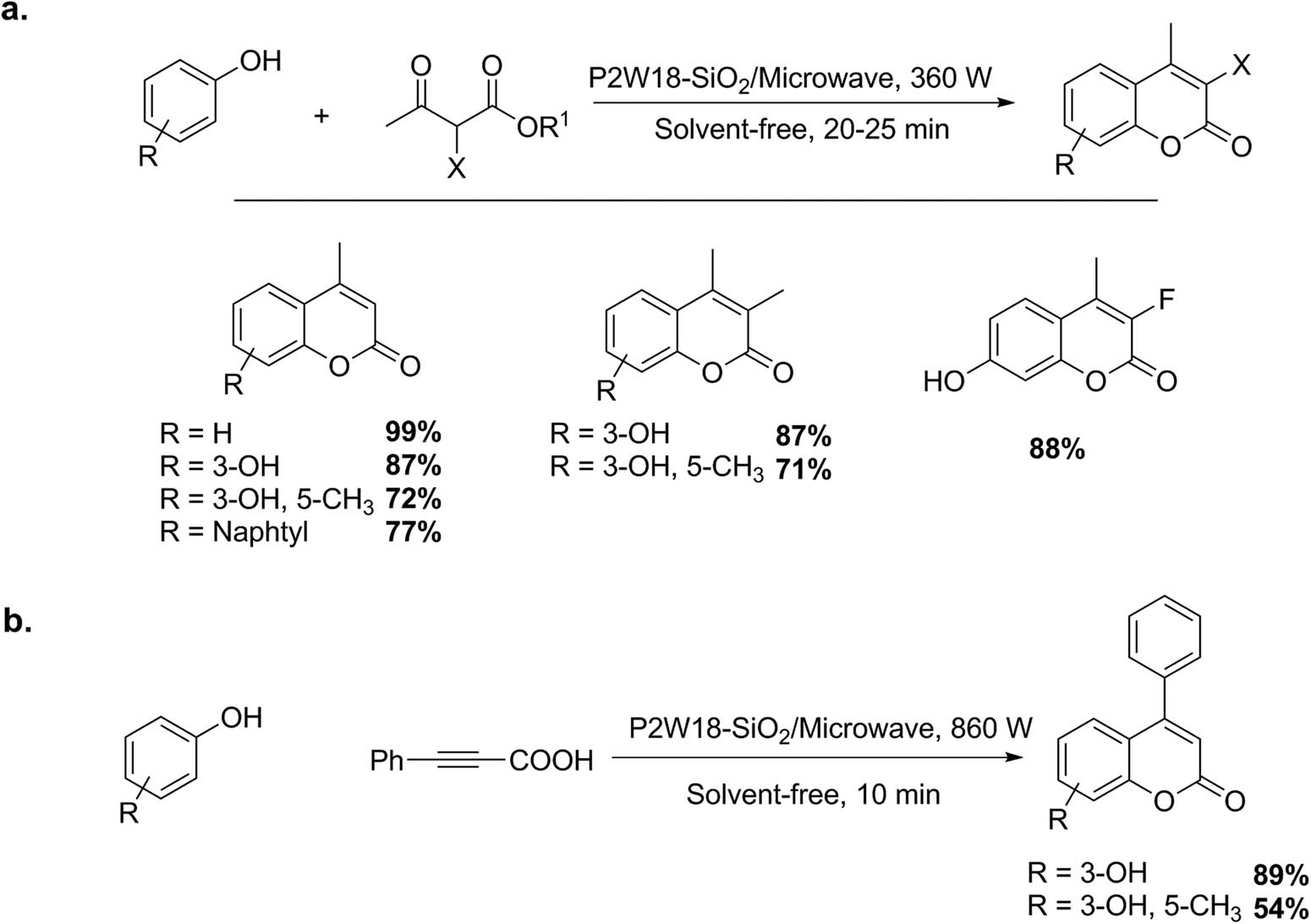
(a and b) Synthesis of simple coumarins.
A recent work of Bennini-Amroun et al. [59] reported a similar procedure for 4-methylcoumarin synthesis using Keggin-type polyoxometalates (POMs) and microwave irradiation. This study was completed with theoretical calculations and the analysis of a probable reaction mechanism. The authors found the best reaction condition using a Keggin-type HPA, H4SiMo12O40, and SiMo12, and obtained 7-hydroxy-4-methylcoumarin with a yield of 97% from and ethyl acetoacetate. Nine 4-methylcoumarin derivatives were obtained with variable yields (25–90%) when the reaction mixture was irradiated between 25% and 90%. Similarly, Cáceres group reported the synthesis of coumarins from phenols and ethylacetoacetate using H3PMo12O40 (PMo12) and H3PW12O40 (PW12) supported on silica. 3,5-Dimethoxyphenol and ethyl acetoacetate in solvent-free conditions were used as test reaction. Comparing the classic thermal procedure, the use of microwave considerably reduced the reaction (60 vs 4 min) with a high yield (95%) [60].
The preparation of 3,4-dihydrocoumarins from α,β-unsaturated carboxylic acids and phenols can be conducted using P2W18-SiO2 as catalyst and solvent-free conditions with microwave irradiation (Scheme 16b). The experiments performed under conventional heating gave null or poor results. Only good yields of the products were obtained using microwave heating in short times (5–15 min) and a power level of 200–960 W depending on the substrate. Five examples of dihydrocoumarins were obtained with a yield of 40–82% [58].
Our research group has also studied the synthesis of dihydrofurocoumarins through the strategy of cycloaddition of allyloxycoumarins under sustainable conditions: absence of solvents, application of microwave irradiation, and insoluble HPAs with Preyssler structure H14(NaP5MoW29O110)] (P5MoW29). Scheme 17 shows a representative example of their preparation [61].
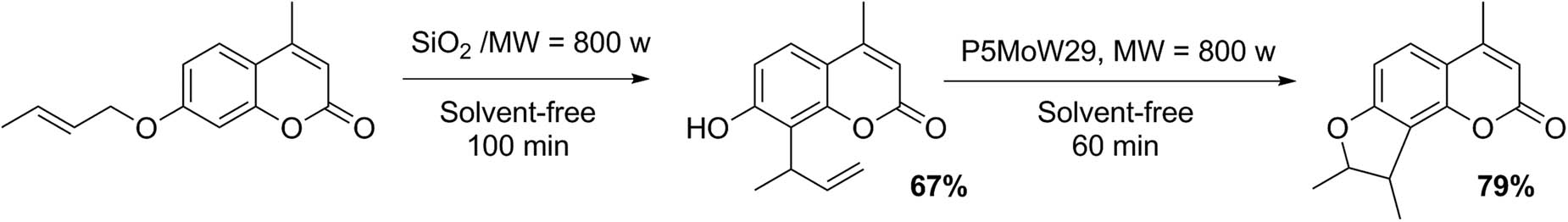
Synthesis of dihydrofurocoumarins.
The synthesis of potentially active organic compounds is actually based on the combination of diverse substructures that enhance the biological activity of already known substances [62]. We have already described the different bioactivities of coumarins; for their part, benzothiazole compounds have received considerable attention in organic synthesis and medicinal chemistry. These substructures were found in various natural marine and terrestrial compounds that are widely used in a great number of applications as antioxidants, plant growth promoters, enzyme inhibitors, imaging reagents, and fluorescence materials. These substances have varied pharmacological activities including anticancer, antiviral, antifungal, antibacterial, antituberculosis, antidiabetic, anthelmintic, anti-inflammatory, antiparkinsonism effects, and so on. Similarly, coumarin derivatives have several pharmacological activities such as antibacterial, antifungal, antiviral, inhibition of HIV-1 protease, anti-inflammatory, neuroprotective, antidiabetic, and others. Their relevance is also clear in the food or pest industry where their fungicide and antioxidant activities are investigated [63,64].
Shafiee and collaborators presented an elegant synthesis of 3-benzothiazolo coumarin and 3-benzothiazolino coumarin compounds, using o-aminothiophenol with 3-acetyl and 3-cyanochromen-2-one catalyzed by Keggin HPAs with very good to excellent isolated yields (62–97%) under microwave irradiation (Schemes 18 and 19). The 3-benzothiazolino coumarins were synthesized from 3-acetylcoumarins 1 (1 mmol) and 2-aminothiophenols 2 (1.2 mmol) in 3 mL of AcOH as solvent and HPA catalyst (1–5 mmol%). The reaction was irradiated with microwaves at 300 W for 7 min. Similarly, the reaction could be performed in absolute ethanol (3 mL). In these cases, the mixture was irradiated for 10 min (5 pulses of 2 min). The compounds were purified by liquid chromatography, and the catalyst was filtered and reused without loss of the catalytic activity. Six examples of 3-benzothiazolino coumarins were presented (73–95%) [64].
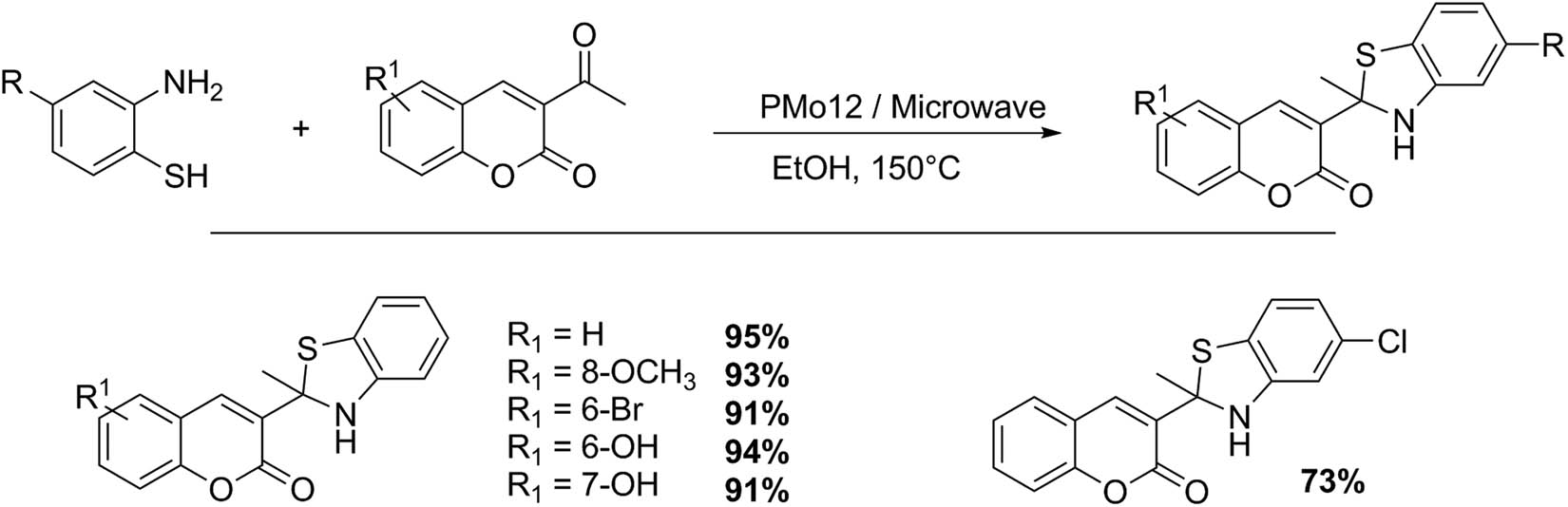
3-Benzothiazolino coumarin synthesis.
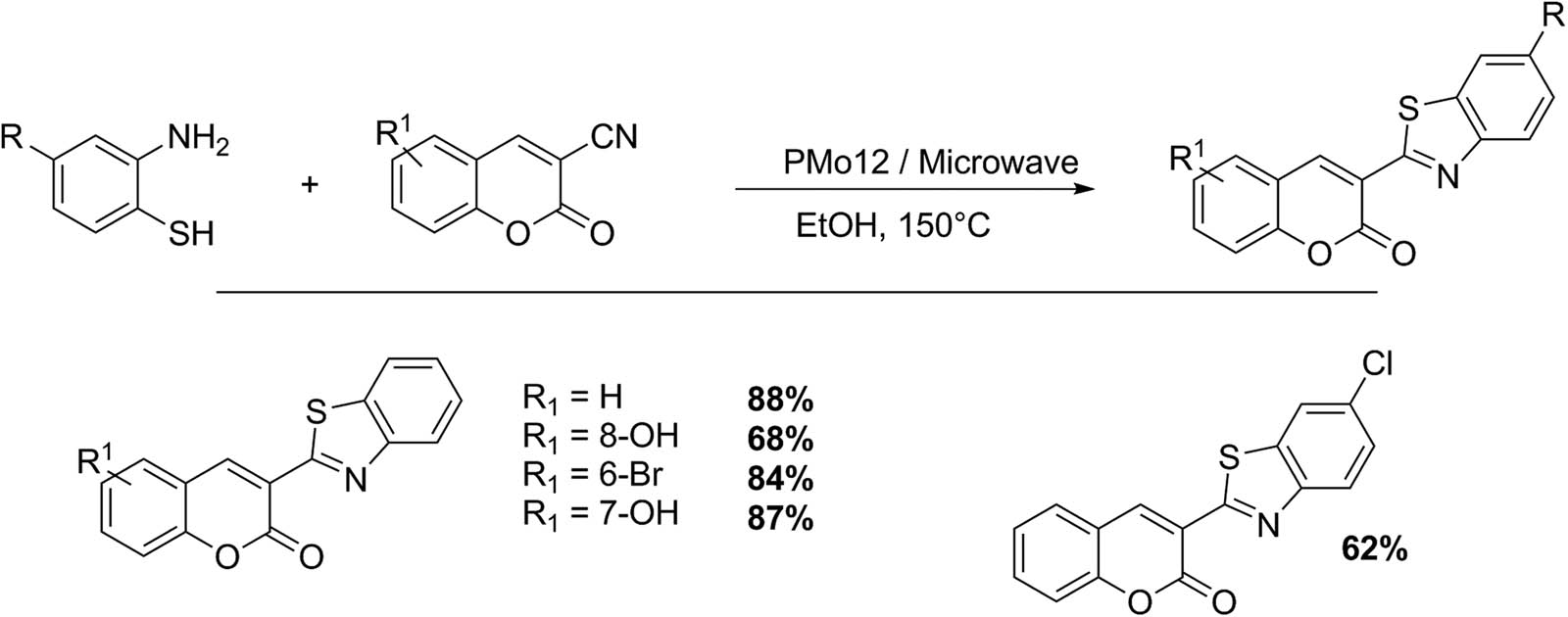
3-Benzothiazolo coumarin synthesis.
Working under similar reaction conditions and substituting 3-acetyl coumarins for 3-cyano coumarins, the same authors obtained five examples of 3-thiazolo coumarins with very good yields (66–88%) (Scheme 19) [64].
Motamedi group reported a multicomponent procedure that involved an aldehyde, a 1,3-dicarbonyl compound, and an amine as nitrogen source at short reaction time, solvent-free conditions, and free of secondary products [65]. In this reaction, chromeno[4,3-b]quinoline derivatives were obtained with operational simplicity and very good performances (Scheme 20). In a representative experiment, 4-aminocoumarin1 (1 mmol), 1,3-cyclohexadione 3 (1 mmol), aldehyde 4 (1 mmol), and HPA (PW12) (10 mmol%) under solvent-free conditions were maintained for 5 min in a microwave oven at 100°C. The product was extracted with acetone and purified by liquid chromatography. Ten quinolone derivatives were obtained with yields between 80% and 95% [65].
![Scheme 20
Synthesis of chromeno[4,3-b]quinolones.](/document/doi/10.1515/gps-2022-0068/asset/graphic/j_gps-2022-0068_fig_020.jpg)
Synthesis of chromeno[4,3-b]quinolones.
The preparation of xanthene derivatives is important in synthetic organic chemistry due to the great range of bioactivity properties of these compounds, such as bactericide, antiviral, and anti-inflammatory. They have been employed for photodynamic therapy and as fluorescent material for visualization of biomolecules [66]. Their preparation via microwave can be performed using encapsulated molybdophosphoric acid in dealuminated zeolites-Y (PMo12-DAZY) [67] (Scheme 21). The synthesis requires mixing aldehyde (1 mmol), 2-naphthol (2 mmol), and acid catalyst (0.0125 mmol), irradiating at 800 W for 2–3.5 min. The scope of this methodology allowed obtaining 13 compounds with yields between 75% and 95%.
![Scheme 21
Synthesis of 14-substituted-14H-dibenzo[a,j] xanthenes.](/document/doi/10.1515/gps-2022-0068/asset/graphic/j_gps-2022-0068_fig_021.jpg)
Synthesis of 14-substituted-14H-dibenzo[a,j] xanthenes.
Chromones constitute a group of compounds of the flavonoid family. Their biological and pharmacological properties have been widely documented. Flavones have a repelling property against some phytophagous insects and a Coptotermes sp. subterranean termite [58,68]. In this sense, our research group carried out a green and solvent-free synthesis of substituted flavones and 2-arylchromones via the cyclization of 1-(2-hydroxyphenyl)-3-aryl-1,3-propanediones using a Wells–Dawson HPA (P2W18-SiO2) as catalyst (Scheme 22). When 1-(2-hydroxyphenyl)-3-aryl-1,3-diketone (0.5 mmol) and P2W18-SiO2 catalyst (1% mmol) are submitted to microwave irradiation at 840 W for 5–8 min, higher yields can be reached. In fact, using this methodology 14 examples of chromones and flavones were prepared with excellent yields (82–96%) free of secondary products [58].
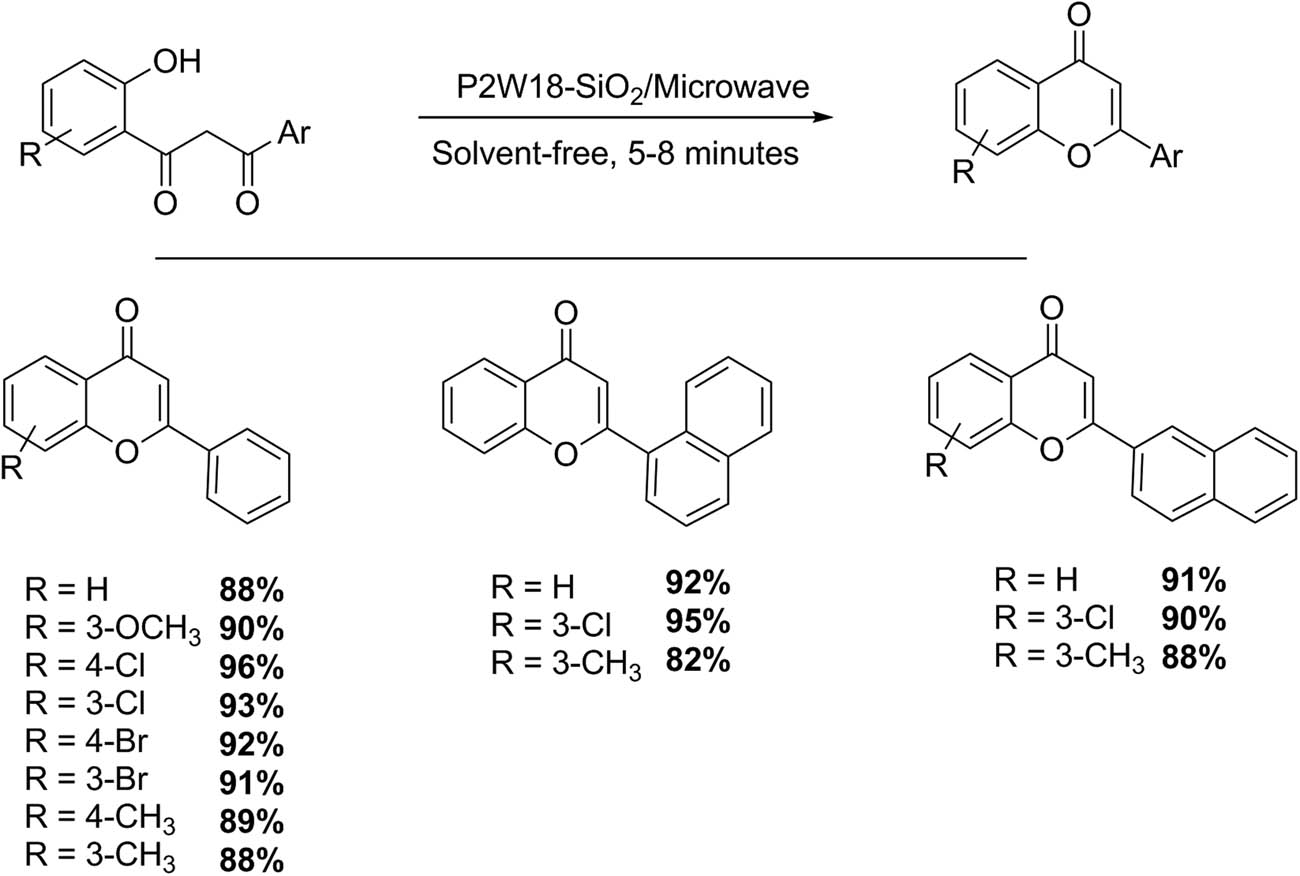
Synthesis of chromones and flavones.
During the last decade, the chemistry of calixarenes has aroused increasing interest. Resorcinarene derivatives have been used as stationary phase in achiral capillary gas chromatography (GC) and self-assembled on gold surfaces for nanoscale advances. However, the principal applications of these compounds are in the catalysis area where p-sulfonic acid calix[n]arenes are used as organocatalysts in allylic alkylation, Mannich-type reactions, Biginelli reaction, and in esterification procedures [69].
Calix [4] resorcinarenes by cyclocondensation reaction of resorcinol and aldehydes catalyzed in the presence of PW12 can be carried out via microwave-assisted synthesis [4] (Scheme 23). In a typical procedure, a solution of resorcinol (10 mmol) and aldehydes (10 mmol) was added to a solution of PW12 (1 mmol%) in 2-ethoxyethanol (2 mL). This mixture was irradiated by microwaves for 5 min at a fixed power of 300 W. Excellent isolated yields (up to 88%) were attained without relevant secondary product formation. The pure product was obtained by cooling the reaction mixture. The formed solid was filtered and identified by NMR. Nine products were obtained (88–91% yields) [70].
![Scheme 23
Synthesis of calix (4] resorcinarenes.](/document/doi/10.1515/gps-2022-0068/asset/graphic/j_gps-2022-0068_fig_023.jpg)
Synthesis of calix (4] resorcinarenes.
On the other hand, POMs can in general act as reversible multielectron oxidant catalysts. In the last decade, procedures involving catalysis and clean oxidants such as hydrogen peroxide, organic peroxides, and molecular oxygen have been developed [71,72,73,74,75,76,77]. These are useful systems to oxidize organic substrates and present high efficiency due to their low cost, safety in storage and operation, and mild reaction conditions. Our research group has demonstrated the versatility of these materials in the selective oxidation of organic substrates including alcohols, phenols, amines, and sulfides, among others [78,79,80,81,82,83,84,85]. In this review, we also present the selective oxidation of organic substrates using HPAs as well as microwave irradiation to reduce reaction times.
Chatel et al. [86] reported the oxidation of five- to eight-membered cyclanols to the corresponding cyclic ketone, with aqueous hydrogen peroxide in the presence of tungstic acid (H2WO4) as a catalyst and an ammonium-based ionic liquid under organic solvent-free conditions (Scheme 24). In a typical procedure for cyclohexanol oxidation, a mixture of this substrate (10 mmol), tungstic acid (2.4 mol%), Aliquat 336 (6.8 mol%), and 30% hydrogen peroxide (20 mmol) was used. The mixture was stirred under microwave irradiation at 90°C for 2.5 min (P = 80 W). The use of Aliquat 336 associated with a nonconventional method such as microwave led to excellent yields, e.g., cyclohexanone oxidation: 94% [83].
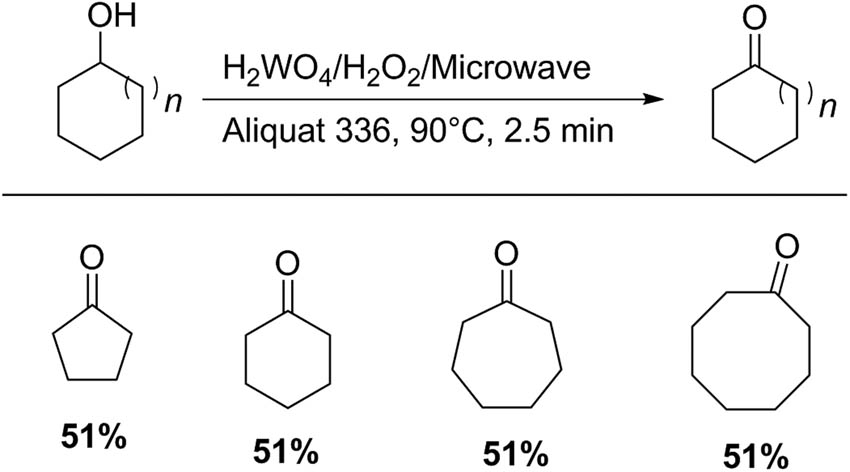
Oxidation of cycloalkanols to cycloalkanones.
Liu and Hou [87] reported the direct benzene oxidation to phenol over a Keggin HPA containing vanadium in the primary structure and pyridine in the secondary structure (PMoVPy), using microwave irradiation. They found that the optimal conditions were: 1.0 mL of benzene, 0.15 g catalyst, 1.8 mL 30% aqueous solution of H2O2, 15 mL of acetonitrile, 70°C reaction temperature, and 20 min reaction time (Scheme 25). The use of microwave energy and a modified Keggin HPA gives a phenol yield of 24.7% (conventional heating gives only 7.8% of phenol yield) [84].
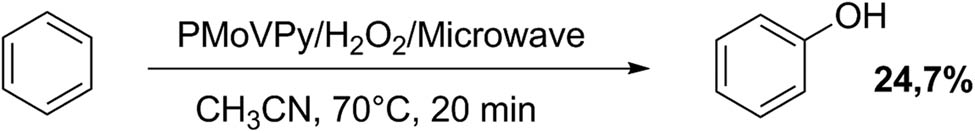
Direct benzene oxidation to phenol.
Bamoharram et al. [88] reported the selective oxidation of aldehydes, particularly benzaldehydes to carboxylic acid derivatives, using tetrabutylammoniumhexatungstate (VI), [(n-C4H9)4N]2[W6O19] (W6O19A2) (Scheme 26). For comparative purposes the reactions were alternatively tested using conventional heating and microwave radiation/irradiation. In a representative test, benzaldehyde (1 mmol), catalyst (0.03 g), and hydrogen peroxide (30 mmol) were mixed thoroughly in a small beaker. The reaction mixture was placed in a microwave oven and irradiated for 4–5 min at 350 W. The reaction product was recovered by adding aqueous HCl and filtered. Seven examples of aldehydes were tested, and the carboxylic acids were obtained (45–70%) in a very short time (4–5 min vs 6–8 h, in conventional heating). The reaction yield under microwave irradiation was: 4-nitrobenzaldehyde > 4-chlorobenzaldehyde > 4-bromobenzaldehyde > 3-nitrobenzaldehyde > 3-chlorobenzaldehyde > benzaldehyde > 4-methyl benzaldehyde, indicating that substrates with strong electron-attracting groups lead to better reaction yields.

Aldehyde oxidation to carboxylic acids.
The same group described a suitable procedure for aldehyde oxidation, but in this case the study was carried out using a Preyssler catalyst and H2O2 as oxidizing agent, under microwave irradiation or by conventional heating (70°C) [89]. The catalyst was used in bulk and supported on silica [89,90]. In a classic experiment, benzaldehyde (1 mmol), supported catalyst (0.04 g), and hydrogen peroxide (2 mL) were mixed in a small beaker, and the reaction mixture was irradiated for 1–2 min at 1,100 W. Very good yields of the corresponding carboxylic acids were obtained for 5 aldehydes (70–100%) in less than 2 min, 8 h being the necessary time to reach the maximum yields by means of conventional heating at 70°C. Only traces of the product were obtained by carrying out the reaction using aldehydes that contain −OH groups in their structure [89]. Similarly, these same authors reported the use of nano-SiO2-supported Preyssler (P5W30-SiO2), obtained by sol–gel method (Scheme 27). Benzyl alcohol (1 mmol), P5W30-SiO2 (0.03 g), and hydrogen peroxide (6 mL) were irradiated for 3 min. After cooling to 20°C, the mixture was filtered and analyzed by GC. Five examples of benzaldehydes were obtained with a yield between 15% and 70% depending on the substituent, in a short time of 3 min compared to the classical heating method (6–10 h) [90].

Benzyl alcohol oxidation to benzaldehydes.
Adipic acid is a relevant precursor in nylon manufacture. Idrissou et al. [91] studied cyclohexanone oxidation using a PMo12 heteropolyacid as catalyst in the presence of H2O2 (Scheme 28). Both procedures, conventional (90°C) and microwave irradiation (100 W) were checked, and several parameters that include solvent, temperature, hydrogen peroxide concentration, and cyclohexanone/catalyst were tested. For both the procedures, the best adipic acid yields (26–28%) were obtained in solvent-free conditions, with H2O2 (30%) as oxidant. Secondary products such as glutaric and succinic acids were also obtained. Microwave technology saves time (30 min vs 20 h) and energy when compared to the usual heating method.
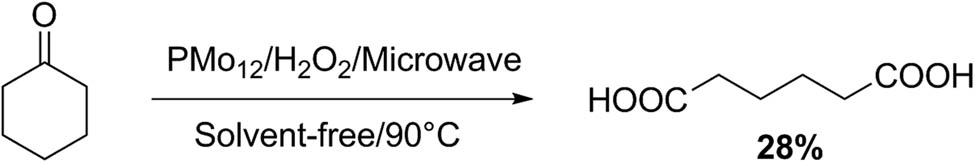
Cyclohexane oxidation to adipic acid.
Adipic acid can be obtained from cyclohexanone in moderate yields under microwave irradiation using ammonium phosphomolybdate with Wells–Dawson structure as catalyst and hydrogen peroxide. The results show that under the optimized conditions, i.e., temperature of 100°C, microwave power of 400 W, irradiation time of 3.5 h, the yield of pure adipic acid was about 73% using a catalytic amount (1%) of catalyst. The reaction yield after five reuses of the catalyst exceeded 43% [92].
Quasim et al. [93] reported the preparation and characterization of peroxo-cerium-containing POMs via the synthesis of 9-peroxo-6-cerium(iv)-containing 30-tungsto-3-germanate, [Ce iv 6(O2)9(GeW10O37)3]24 − (GeW10Ce). This recyclable homogeneous catalyst was employed successfully in the selective microwave-activated sulfoxidation of the model substrate methionine to sulfoxide (in the absence of hydrogen peroxide), and to sulfone in the presence of hydrogen peroxide/catalyst (Scheme 29). The oxidation of dl-methionine (S) to the corresponding sulfoxide was performed by dissolving the substrate (0.067 mmol) in 2.5 mL of water, then the catalyst (0.009 mmol) was added, and the solution was heated by microwave irradiation (power of 30 W) at 50°C for 50 min. Moreover, the oxidation of dl-methionine to the corresponding sulfone was performed by dissolving the substrate S (0.087 mmol), catalyst (0.0084 mmol), and 0.05 mL of 30% H2O2 (0.75 mmol) in 2.5 mL of water. The reaction mixture was heated by microwave irradiation at 50°C (power of 30 W) for 35 min. The yields of two procedures were practically quantitative [93].
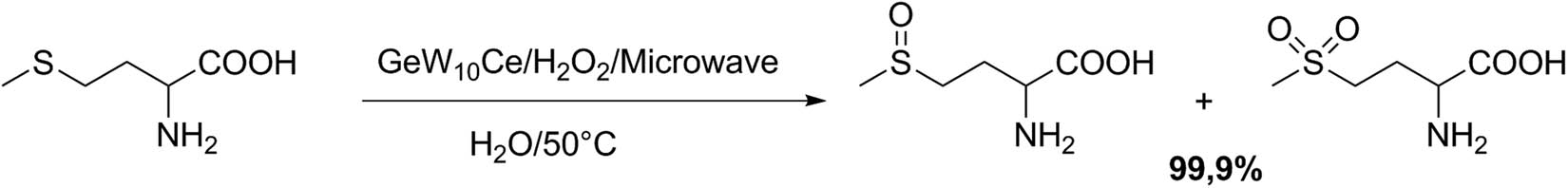
Selective oxidation of methionine.
Bonchio et al. [94,95] studied the organic–inorganic hybrid complexes based on lacunary polyoxotungstates and organosilanes or phosphonic reagents in the selective oxidation of several organic substrates that include alkenes, sulfides, sulfoxides, alcohols, and ketones, using hydrogen peroxide as suitable oxidant. In a representative test for sulfide oxidation, POM (0.8 µmol), substrate (0.25 mmol), 35% H2O2 (0.1 mmol), and CH3CN (0.6 mL) were irradiated with microwave (120°C, 240 W, 25 min). Yields up to 99%, with high selectivity, were obtained in 25–50 min depending both on the POM structure and on the organic moiety, under microwave irradiation [94]. The same group reported the use of transition metal substituted POMs with redox active center (Ru, Fe, and Mn). The microwave irradiation induces an efficient and selective oxidation of DMSO and cyclohexanone oxidation, using different oxidants that include NaIO, KHSO4, and PyClNO, in oxygen atmosphere [95,96]. The inclusion of this type of POM complex salt in bicontinuous microemulsion allowed obtaining materials with self-cleaning properties which enabled to displace fouling, thanks to the oxygenic activity of chemically anchored POM [97]. The surfactants may be used to anchor nanoparticles on the surface of the polymeric membrane surface through noncovalent interactions [98].
Another topic of current relevance is biomass valorization. The preparation and characterization of novel catalytic materials with relevant activity and stability for biomass depolymerization and valorization in chemical products have been a hot topic in chemistry for the last years. Solid catalysts are often the most viable alternative to liquid mineral acid catalysts for waste generation reduction and a very simple catalyst separation. Numerous works have highlighted the use of HPAs and derivatives in biomass valorization. However, there are few works that refer to the use of alternative heat sources such as microwaves [12,99,100,101,102].
One of the most important biomass transformations using microwave irradiation and HPAs as catalysts is the cascade conversion of cellulose to levulinic acid (LA) or alkyl levulinates. In this regard, Zhang et al. [103] reported a series of Lewis metal-substituent phosphotungstic acids PW12L (where, L = Cu, Sn, Cr, Zn, Fe, oxygen is 39) and PW11L (L = Ti, Zr, oxygen is 40) in LA production. PW12Ti was the most active catalyst with the highest cellulose conversion, giving the highest glucose yield in water and the highest LA yield in a mixture of methyl isobutyl ketone (MIBK) and water. The reaction was tested in the XH-800C computer microwave apparatus. In a test experiment, the reaction mixture contained 0.1 g cellulose, 5 mL of MIBK, 0.5 mL of H2O, and 0.08 mmol catalyst. Heterogeneous HOCH2CH2N(CH3)3H4PW11TiO40 (ChH4PWTi) had been synthesized using choline chloride as organic precursor to achieve higher yields of LA due to its temperature-responsive property, and amphiphilic assembly of the nanoreactor to concentrate substrates, stabilization of LA by its environment surroundings. The conversion and yield of LA using ChH4PWTi as catalyst were 93.7% and 70.9%, respectively, using microwave irradiation (100°C, 180 min) [103].
The same group reported a synthesis of a triple-functional POM Cs [HGeNbO] (CsGeNb) and its evaluation for its catalytic activity in the synthesis of methyl levulinate (ML). High conversion of cellulose (86.0%) and a higher yield of ML (55.4%) were obtained under the optimized reaction conditions: 0.1 g cellulose, 0.04 mmol catalyst, and 6 mL of methanol at 165°C for 3 h [104].
Analogously, this group reported the use of a series of Lewis acid metals monosubstituted phosphotungstic acids PW12L and PW11L in the direct production of ML from cellulosic biomass. PW11Ti was found to be highly efficient for the generation of ML from mono- or polysaccharides, reaching 62.6% ML yield directly from cellulose (microwave irradiation, 2 h) [105].
Finally, Quereshi et al. reported the use of Keggin silicotungstic acid (SiW12) catalyst over zirconia support in LA production. The catalyst evaluation tests were performed using LA and anhydrous ethanol as substrates, under microwave irradiation. Under optimum operating conditions, more than 90% LA conversion with 100% of ethyl levulinate selectivity was obtained at 110°C temperature, 30 min, in the presence of 100 mg of ESZN-4 in a solution containing LA and ethanol in a 1:43 ratio [106].
3 Sonochemistry
Sonochemistry is a broad area of research in chemistry, characterized by the study of chemical transformations associated with the use of ultrasound irradiation (20 kHz to 10 MHz). The chemical effects of ultrasound do not originate from a direct coupling of the acoustic field to the molecular species. Instead, they come from nonlinear acoustic phenomena, primarily acoustic cavitation. The acoustic cavitation generates bubbles in liquids, and the violent bubble collapse during cavitation causes extreme local temperatures (up to ∼5,000°C), heating/cooling rates (up to ∼10 billion°C·s−1), and pressures (up to ∼1,000 atm), giving rise to many chemical (sonochemical) reactions [107,108,109].
During the last three decades, substantial academic and industrial efforts have been made to protect the environment by redesigning polluting processes and reducing waste generation. Sonochemistry has gained considerable importance in a lot of research areas including nanomaterial synthesis, spent nuclear fuel, biodiesel production, and food chemistry. Due to these versatile properties, ultrasound is a powerful tool to carry out different chemical organic reactions, for example, bioactive heterocycles in high yield and selectivity, shorter reaction times, and easier work-up conditions compared to the conventional procedure. The literature reports several review articles associated with the use of microwaves in organic synthesis and more recently, environmentally friendly conditions as well [6,110,111,112,113,114,115,116,117,118].
In addition, the important thing about this technology is that it not only allows assisting in chemical transformations, but also in the synthesis of materials that can be used as catalysts in the respective transformations, contributing to the reduction in energy consumption in both stages of the process [119,120,121].
The different contributions in the synthesis of organic compounds using HPA catalysts, and the use of ultrasound as an energy source are indicated below.
Several simple transformations have been studied using HPAs as catalysts and ultrasound as promoter, for example, Ai et al. [122] obtained acylals from different aldehydes and acetic anhydride in the presence of Keggin-type stannum(iv) phosphomolybdate at 20°C under ultrasound irradiation (Scheme 30). In an experiment test, a mixture of aldehyde (30 mmol), Ac2O (60 mmol), and 0.50 g SnPMo12 was irradiated with ultrasound. The products were monitored by GC, and purified by recrystallization. Twelve acylals were obtained with excellent yields (75–98%, 0.2–2.5 h). In the same conditions, aldehyde containing hydroxyl groups were triacetylated [122].
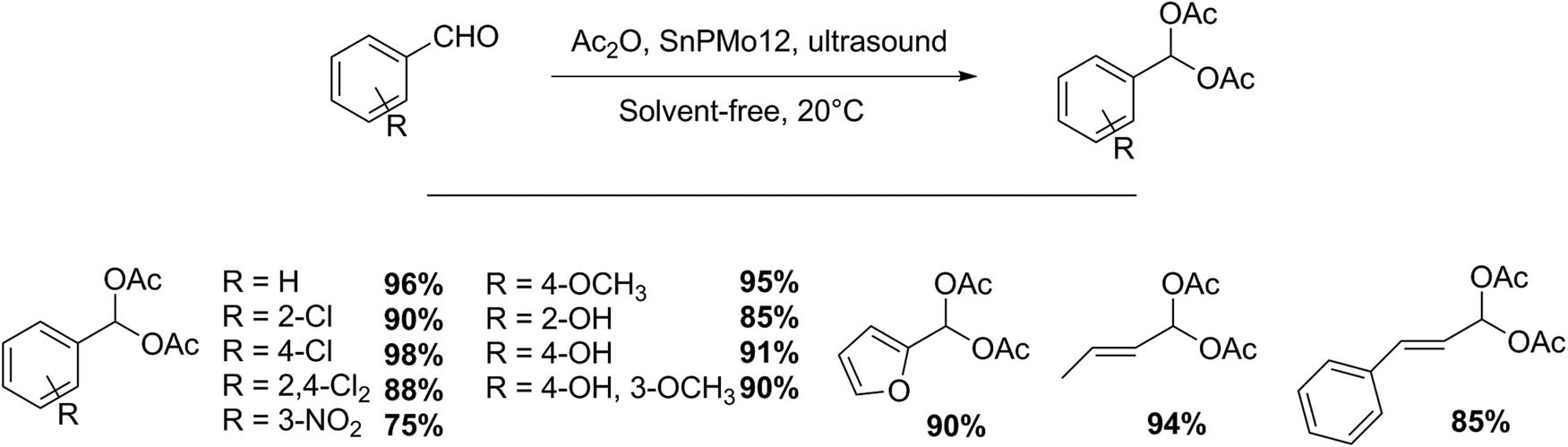
Acylal synthesis.
Chen et al. [123] reported a kinetic study about the preparation of benzyl acetate from benzyl alcohol and glacial acetic acid using SiW12 supported on silica (Scheme 31). Under optimized conditions, the esterification yield was 95.3%. The optimized conditions were 10 kHz ultrasonic frequency and 1.0 W·cm−2 ultrasonic intensity; reaction temperature, 110°C; amount of catalyst used, 1.5 g; molar ratio of benzyl alcohol to glacial acetic acid, 1.5; reaction time, 75 min.

Esterification reaction for benzyl acetate synthesis.
Hamidian et al. [124] reported a synthesis procedure of hydroxytriarylmethane derived from the reaction between 2-sulfobenzoic anhydride and phenols with Preyssler HPAs under ultrasonic irradiation (Scheme 32). The general procedure involves a mixture of 2-sulfobenzoic anhydride (20 mmol), phenol derivatives (60 mmol), and a catalytic amount of P5W30 (0.03 mmol) in acetonitrile (10 mL). The reaction mixture was irradiated under ultrasound at 55–65°C. When the reaction ended (TLC), the catalyst was filtered, and the isolated product was purified and characterized. Six products were obtained with yields between 54% and 72% (reaction time: 60–150 min). The P5W30 catalyst was recovered and reused without appreciable loss of the catalytic activity [124].
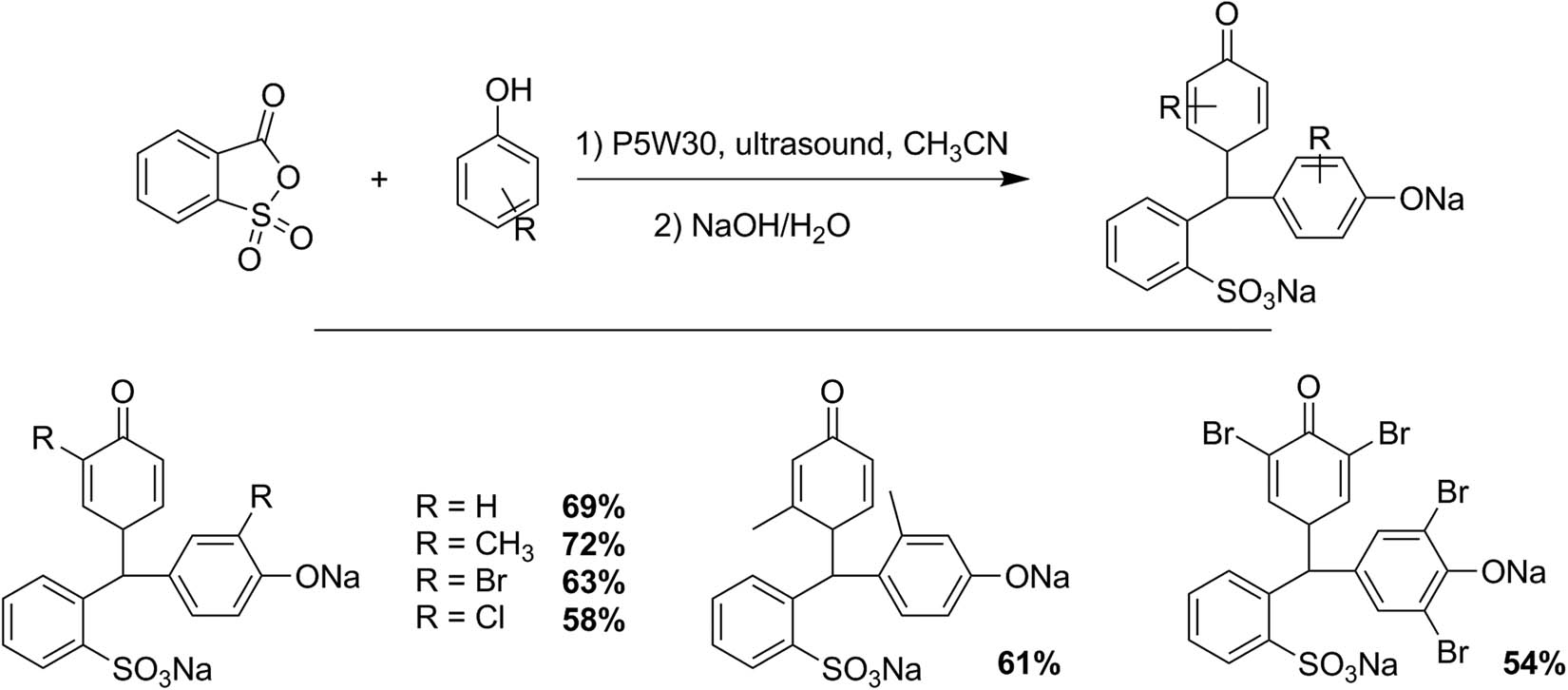
Hydroxytriarylmethane synthesis.
Undoubtedly, the great contribution of ultrasound coupled with HPAs is given in the synthesis of potentially active heterocycles. Different heterocycle structures have been prepared through sustainable processes such as multicomponent reactions with high atom economy. Some of the synthesized heterocycles include: pyridines, pyrimidines, thiazoles, indoles, quinoxalines, and pyrans.
Compounds containing pyridine substructure have great importance in organic synthesis due to their medicinal and biological activities, for example, neuroprotective, anti-inflammatory, antitubercular, hepatoprotective, cardiovascular, antidiabetic, and antianginal activities, among others [5,125]. Particularly, 2-amino-3-cyanopyridines possess different bioactivities, such as antibacterial, antimicrobial, antiviral, antifungal, and antihypertensive, among others [126,127].
These compounds were obtained via one-pot, four-component condensation reaction (Scheme 33) using hybrid catalysts composed of vanadium Keggin HPA anchored on mesoporous materials such as SBA-15 under ultrasound green approach [126]. The HPA (H5PW10V2O40) was immobilized on SBA-15@ADMPT, where AMDMT (4,6-bis(3,5-dimethyl-1H-pyrazol-1-yl)-1,3,5-triazine) acts as anchoring agent. The test reaction was studied with malononitrile (1 mmol), ketone (1 mmol), and ammonium acetate or amine (1.5 mmol) in 5 mL ethanol solution containing the aromatic aldehyde 1 (1 mmol) with SBA-15-ADMPT-PW10V2. The reaction mixture was irradiated with ultrasound with a power of 50 W (3–5 min). The product was recrystallized from aqueous ethanol, and the catalyst was recycled, dried at room temperature, and activated at 80°C for 2 h. The catalyst reuse gave similar results in comparison to the fresh one. By using this methodology, 15 examples (77–95%) were reported, which include several operating advantages compared to other previous methods, for example, very short reaction time (3–5 min), mild reaction conditions, and simple catalyst recycling [126].
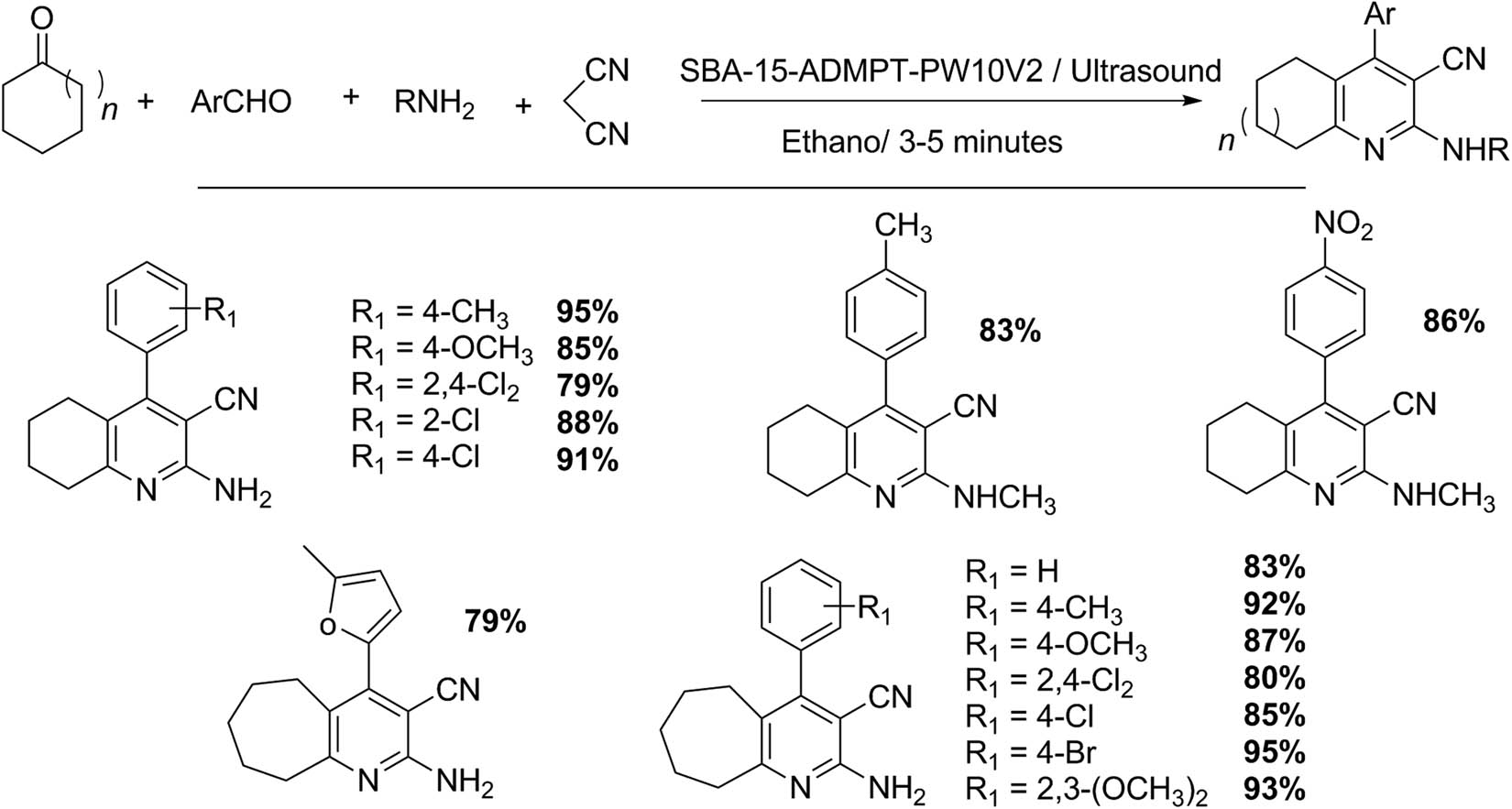
2-Amino-3-cyanopyridine synthesis.
The synthesis of 3,4-dihydropyrimidin 2(1H)-ones through Biginelli reaction between aldehydes, urea, and ethyl acetoacetate (Scheme 34) was also performed using a kegging HPA H4PMo11VO40 (PMo11V) that was prepared and characterized appropriately and assisted with ultrasonic irradiation. The mixture of ethyl acetoacetate (1 mmol), aromatic aldehyde (1 mmol), and urea (1.5 mmol) in the presence of PMo11V (0.1 g), and ethanol, as reaction solvent, was irradiated with ultrasonic waves for 20–42 min at 20°C. The catalyst was recovered from the reaction mixture by filtration and dried (4 h at 120°C) in an oven prior to its reuse. The conversion decreased from 90% to 81% between the first and third catalytic cycles. The product was recovered from the reaction mixture by adding water and filtering the resulting solid. Then, it was recrystallized from ethanol to give the pure product. Eleven 3,4-dihydropyrimidin 2(1H)-ones were obtained with very good yields (88–96%) and excellent selectivity [128].
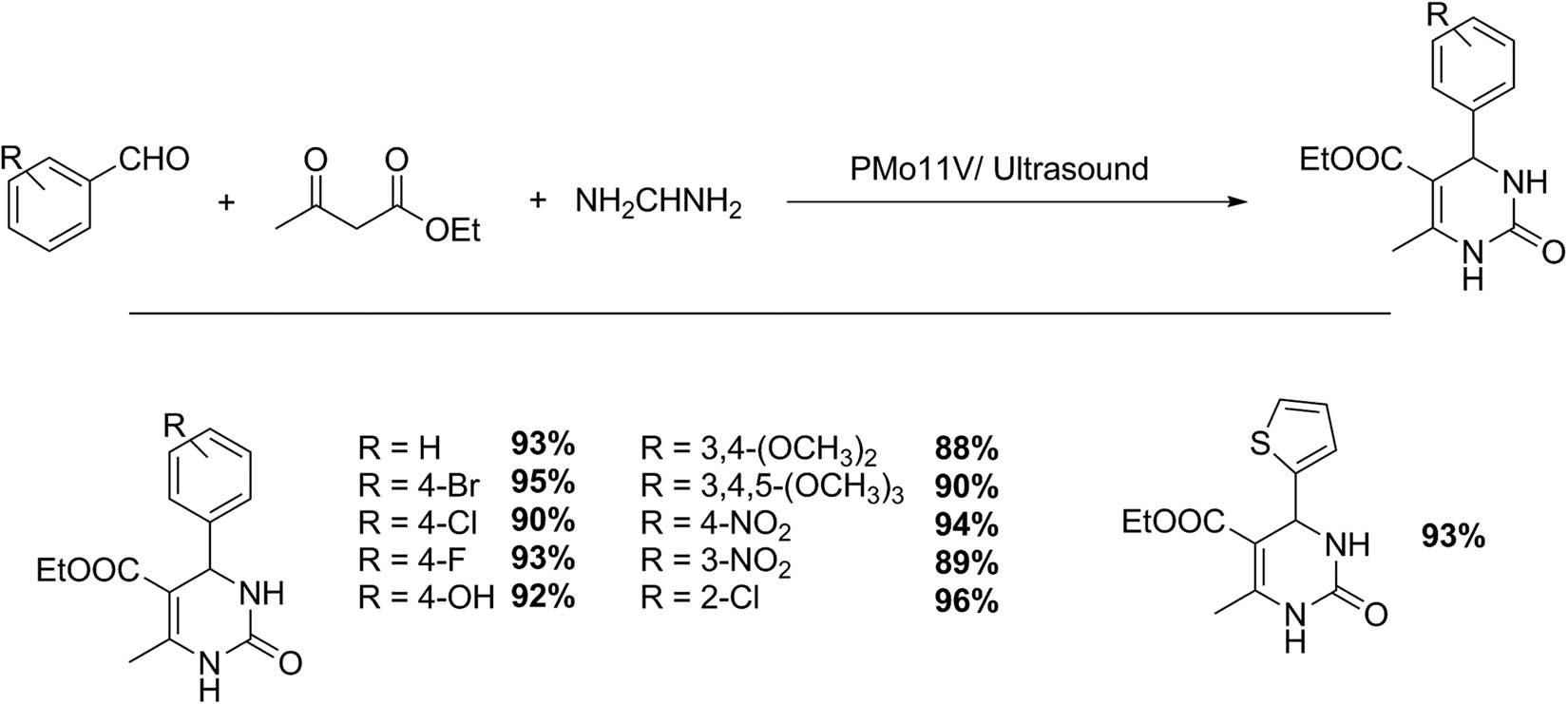
3,4-Dihydropyrimidin 2(1H)-ones.
Heravi et al. [42] reported the synthesis and characterization of a Keggin HPA PW12 supported on amine-functionalized halloysite nano clay (PW12–H2N–HAL–CLAY) as a suitable catalyst for the pyrazolopyranopyrimidine synthesis through a domino multicomponent reaction. The four-component procedure involved the reaction between an aldehyde, hydrazine hydrate, ethylacetoacetate, and barbituric acid (Scheme 35). In a classic test for pyrazolopyranopyrimidine synthesis, a solution of hydrazine hydrate (1.1 mmol) was added to ethyl acetoacetate (1 mmol). Then, barbituric acid (1 mmol), the aldehyde (1 mmol), and the PW12–H2N–HAL–CLAY catalyst were added, and the mixture was refluxed in water (similarly, for comparative purposes, the reaction was tested using ultrasound and microwave irradiation). The pyrazole derivative was purified by recrystallization from ethanol. The catalyst was easily recycled, and no changes in the catalytic activity were detected [42].
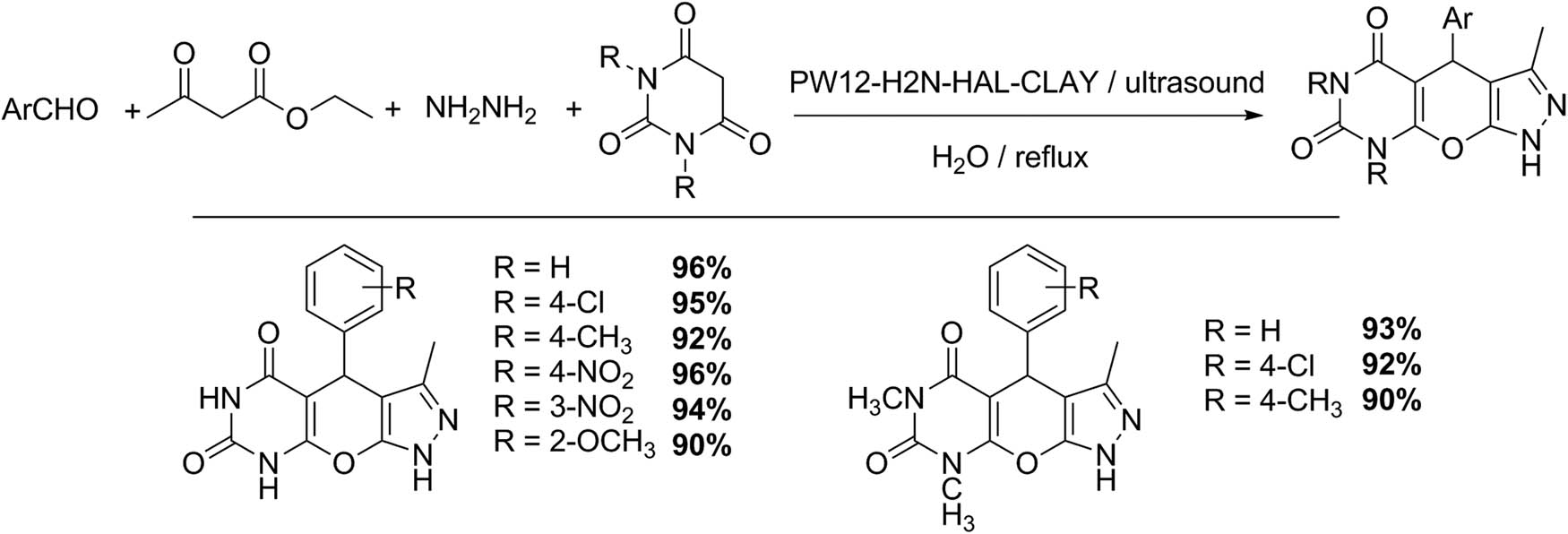
Pyrazolopyranopyrimidine synthesis.
Nine examples of pyrazolopyranopyrimidines were obtained with excellent yields (90–96%), and a reaction time between 30 and 50 min.
Similarly, the same group studied the synthesis of two families of benzopyranopyrimidines with a hybrid catalyst based on the incorporation of Keggin HPA into creatin-functionalized halloysite clay (PW12-CRE-CLAY). Ultrasonic irradiation and the use of water as a solvent were two main aspects to consider this process more environmentally friendly (Schemes 36 and 37). The first series of benzopyranopyrimidines was synthetized by the following protocol: a mixture of a corresponding aldehyde (1 mmol), urea or thiourea (1 mmol), 4-hydroxycoumarin (1 mmol), and a catalytic amount of Keggin-based materials, in water (10 mL), was irradiated with ultrasonic irradiation at 20°C (4–22 min). Ethanol was used to obtain the recrystallized product, showing seven examples with yields of 80–95% [129].
Benzopyranopyrimidine synthesis.
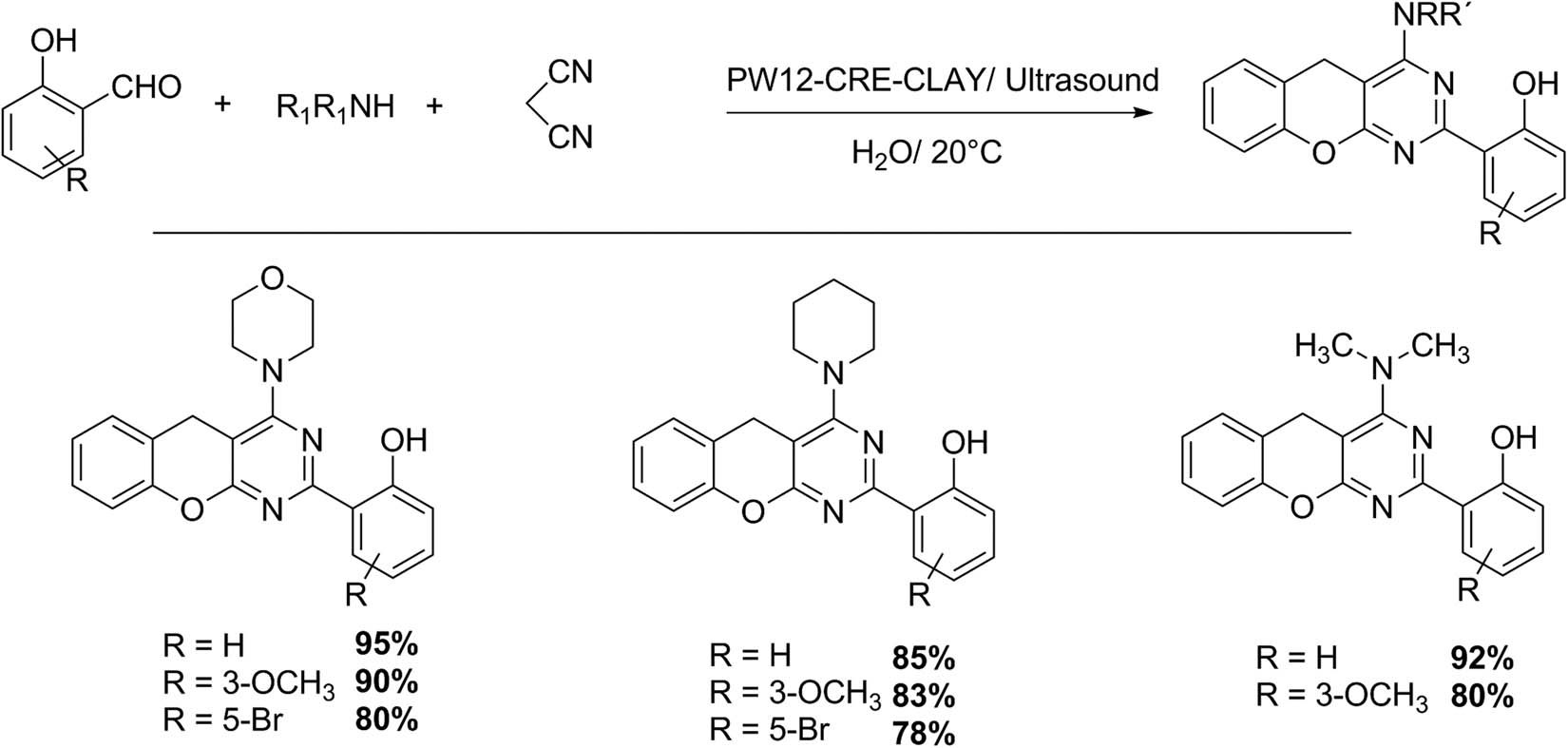
Benzopyranopyrimidine synthesis (family two).
The second series of benzopyranopyrimidines was synthesized using malononitrile (1 mmol), 2-hydroxybenzaldehydes (2 mmol), and amines (1 mmol) in the presence of PW12-CRE-CLAY. Eight examples illustrate the scope of this procedure, obtaining yields of 78–95% [129]. Both protocols represent a clean alternative for benzopyranopyrimidine synthesis in terms of short reaction time, high yields, environmentally friendly solvent, and reusable catalyst [129].
It is well known that the indole skeleton is present in natural products of the alkaloid family. Indoles and their related molecules have a wide range of pharmacological properties, for example, antibacterial, antihypertensive, antitumor, and anti-Alzheimer’s, among others. Recently, bis-indoles (molecules that contain two interconnected indole substructure residues), presenting novel and very relevant properties, such as powerful anticancer agents, have been found. They are also intermediate products in research and medicinal industry, and important building blocks in pharmaceutical ingredients [130,131].
Rahimi et al. [131] reported the reaction of indole with electron-deficient alkenes (Scheme 38). This protocol allows the bis(indole) compound synthesis using water as solvent and ultrasound irradiation. This methodology is assisted by 12-tungstophosphoric (PW12). A mixture of indole (1 mmol), alkene (0.5 mmol), and PW12 (3 mmol) was irradiated (70 W) at 90°C to obtain a solid product after purification by liquid chromatography. Sixteen examples were obtained with excellent yields (77–93%) in 10–20 min. The methodology reported has several advantages such as short reaction time, operational simplicity, environmentally friendly solvent and catalyst, and high selectivity and product yields.
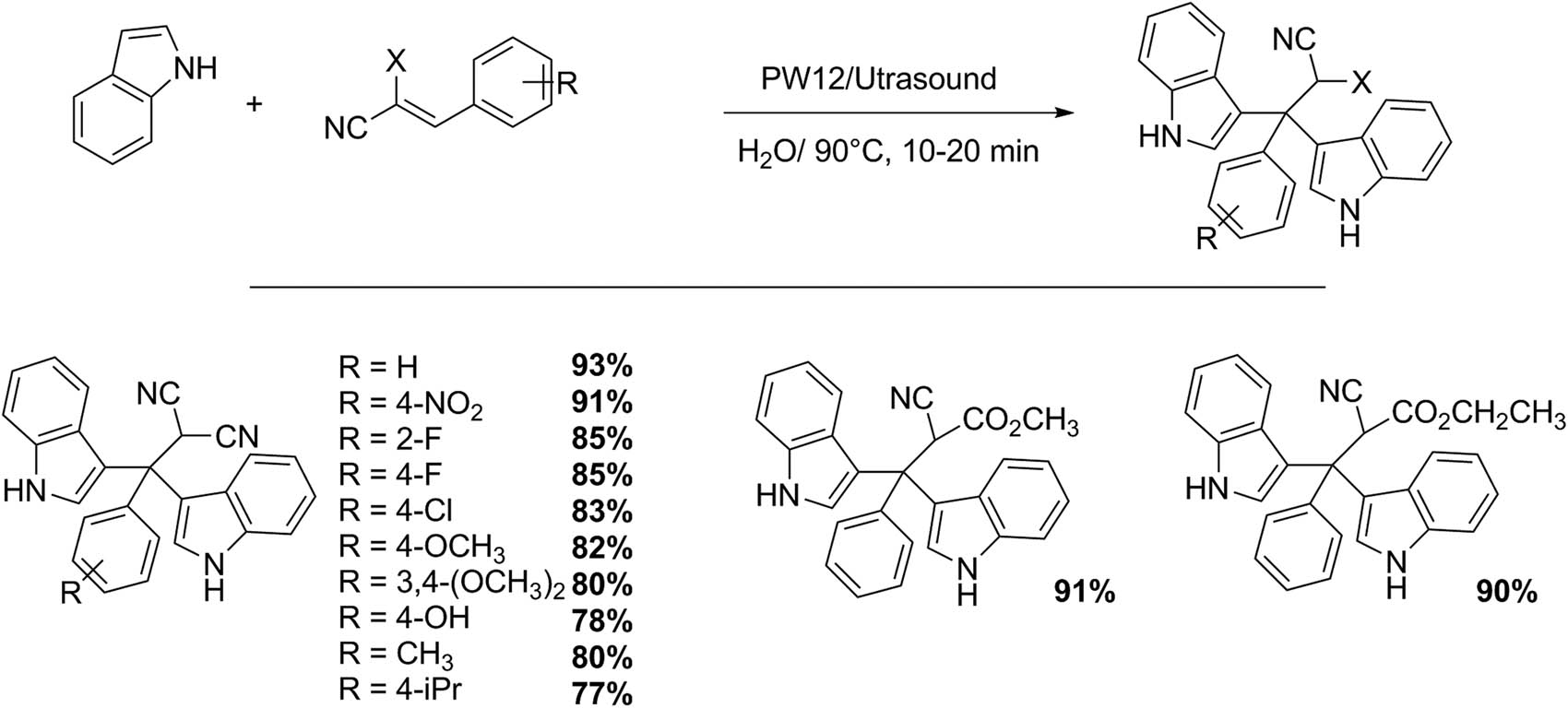
Bis-indole synthesis.
Spirooxindoles can be obtained by multicomponent preparation with isatin (1 mmol), malononitrile (1 mmol), and cyclohexane-1,3-dione (1 mmol) in the presence of a Keggin HPA supported on silica (PW12-SiO2, 8 mmol%) as catalyst, and ethanol as solvent (5 mL) (Scheme 39). The reaction mixture was irradiated by ultrasound. At the end of reaction, the catalyst was separated by filtration, and the residue was recrystallized from a mixture of ethanol/dioxane. Seven spirooxindoles were prepared in a time (6–10 min) with good yields (75–86%). The catalyst can be reused without loss of catalytic activity [132].
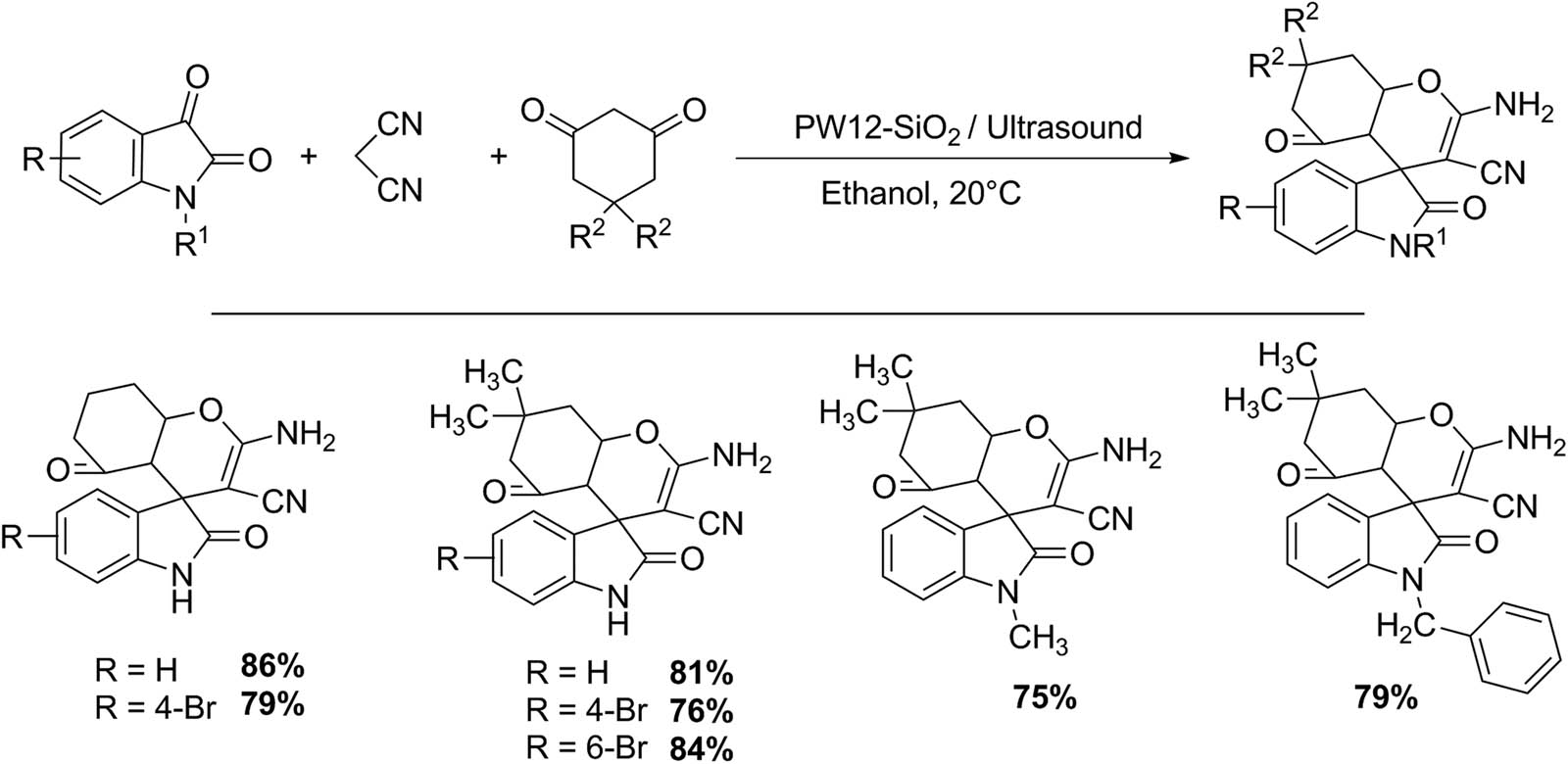
Spirooxindole synthesis.
The molecules with benzothiazole core play a vital role in pharmacology, and a great number of compounds present this moiety, including fungicides, antibiotics, anti-inflammatory, antiviral, and anticarcinogenic drugs [133,134].
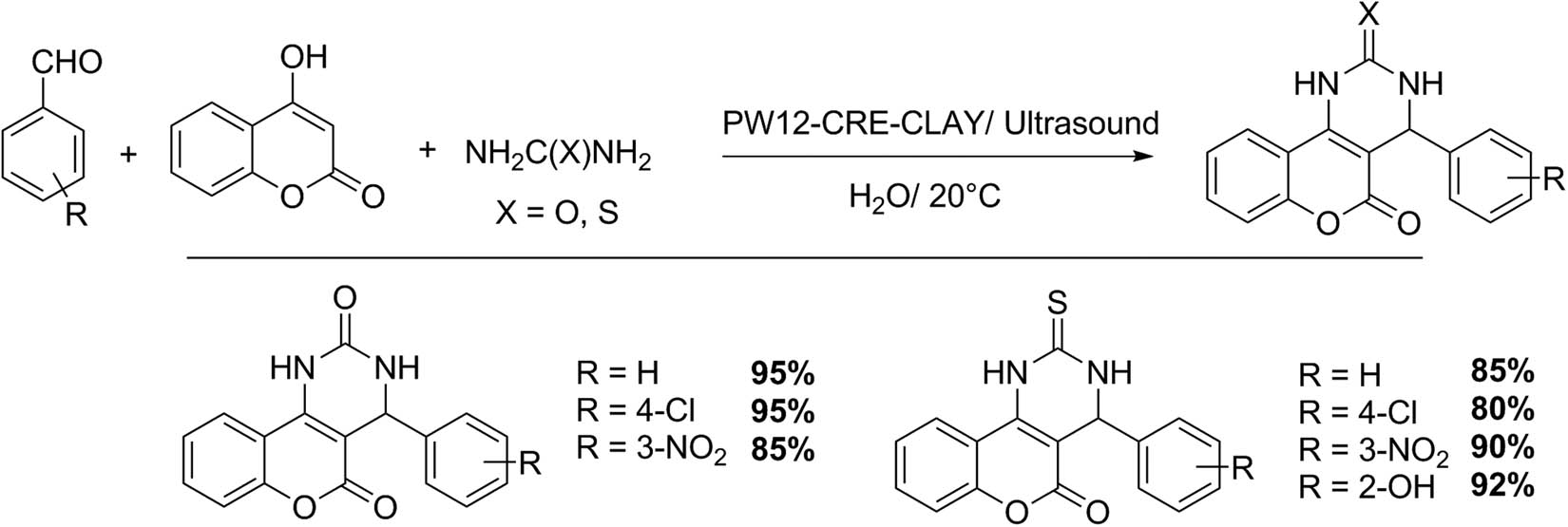
Javanshir et al. [133], using 2-aminobenzothiazole, different aldehydes, 2-naphthol and Wells–Dawson HPA (P2W18) as catalyst at 45°C under ultrasound irradiation, were able to obtain 2-aminobenzothiazolomethylnaphthols (Scheme 40). In a representative experiment, the equimolar mixture of the reagents, in the presence of a 3 mmol% of P2W18 in water (3 mL) as reaction solvent, was sonicated at 45°C for 80–120 min. The reaction mixture was washed with water, and the crude was crystallized from a mixture of water/acetone (Scheme 40). The procedure is very simple and provides very good yields of benzothiazole derivatives (15 examples, 62–92%).
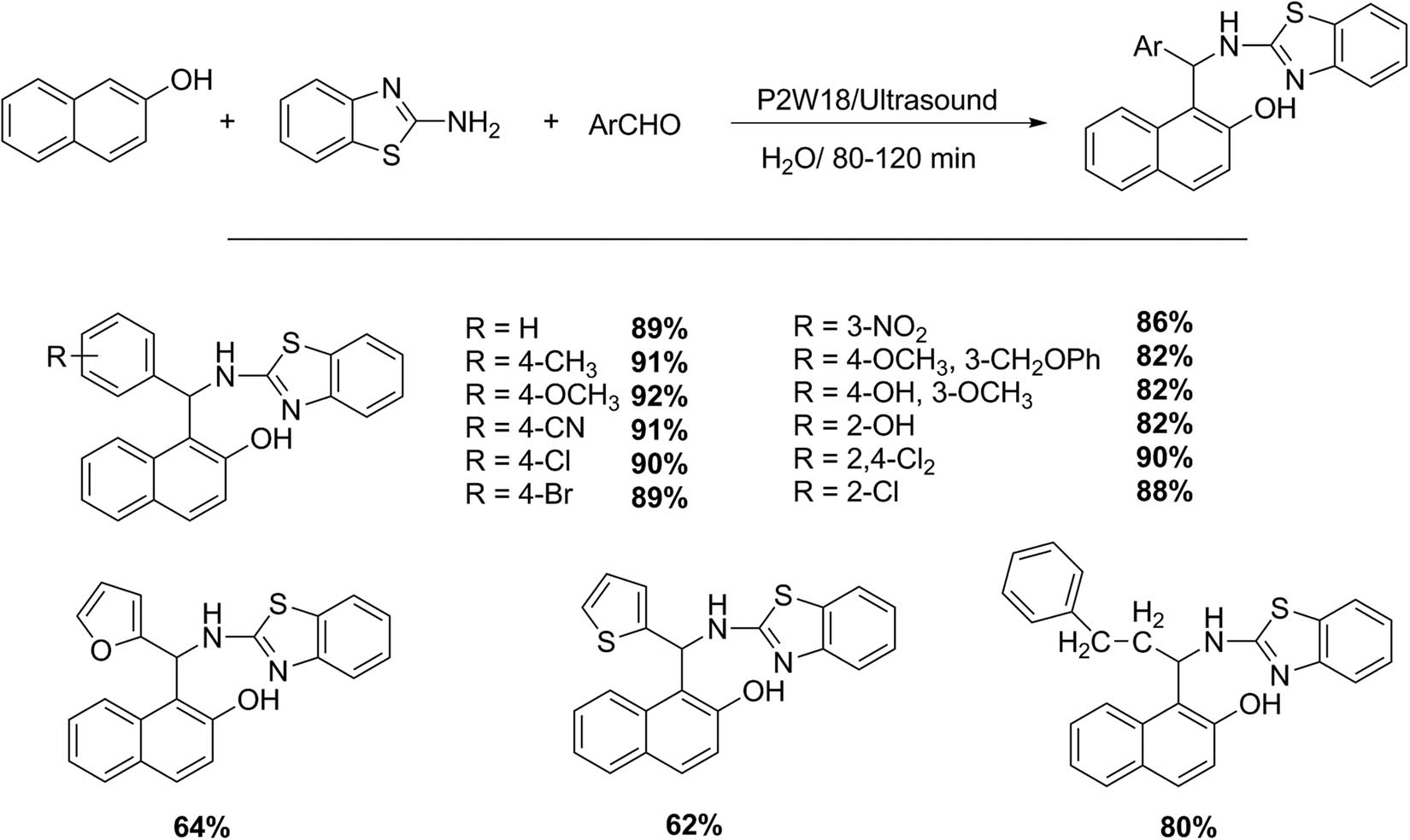
2-Aminobenzothiazolomethylnaphthols.
Bougheloum et al. [135] reported a very interesting and efficient work on the synthesis of benzothiazole derivatives containing sulfonamide or cyclic imide moieties (Scheme 41). The reaction was assisted by a Wells–Dawson catalyst (cesium salt of Wells–Dawson HPA, Cs5HP2W18O62 or P2W18Cs5), and ultrasound irradiation in water as reaction solvent. In a representative test, sulfonamide (1 mmol) was added to a mixture of cyclic anhydride (2 mmol) and P2W18Cs5 (10 mmol%), and the mixture was irradiated by ultrasound (35 kHz at 25°C). Then, 2-aminobenzothiazole derivatives were added (additional irradiation, 10 min). At the reaction end, the catalyst was recovered by filtration, and the product was purified by liquid chromatography and recrystallization. Through this protocol, twenty two derivatives of benzothiazoles were prepared, many of which are not reported in the literature (48–92%) [135]. The procedure represents a clean, useful and efficient alternative: one-pot reaction, under ultrasound, and water as reaction solvent.

Benzothiazole synthesis.
Quinazolinones can be obtained from the reaction between 2-amino-benzamide and acyl chlorides in the presence of silica-supported Preyssler catalyst (Scheme 42). In a test reaction, a mixture of 2-amino-benzamide (10 mmol), acyl chlorides (10 mmol), and silica-supported Preyssler catalyst (3 mmol%), in acetonitrile (10 mL), was irradiated by ultrasound at 20°C (7–10 min). The catalyst was filtered off and reused three times with low loss of catalytic activity. Five examples of quinazolinones were obtained with excellent yields (88–95%), without secondary product formation in a short reaction time. The performance of the Preyssler catalyst included in silica (P5W30-SiO2) was significantly higher than that obtained with the bulk Preyssler HPA (88–95% vs 81–91%) [136].
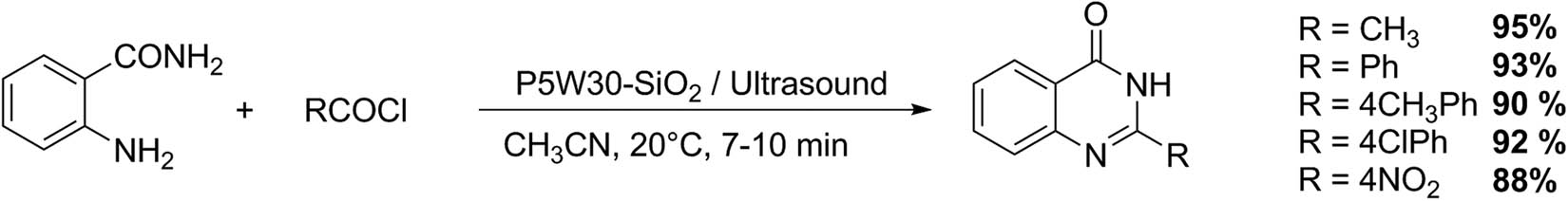
Quinazolinone synthesis.
The pyran heterocycle represents a very important type of 6-membered heterocycles containing oxygen and are an outstanding scaffold for preparing a wide range of heterocycles with biochemical and technological applications. In particular, 4-H-chromenes and pyrazoles are promising medicament precursors [5].
Azarifar group reported the preparation of a new nanotitania-supported Preyssler-type HPA and presented its application in efficient procedures for the synthesis of 4-H-chromene and pyrazole derivatives (Schemes 43 and 44). The reaction is performed using ultrasound, giving quantitative yields of both products. In a representative test, a mixture of malononitrile (1 mmol), dimedone (1 mmol) or 3-methyl-1-phenylpyrazolin-5-one, and an aldehyde (1 mmol), in absolute ethanol (5 mL), was stirred in the presence of catalyst (P5W30-TiO2, 0.0056 mmol%). The reaction mixture was sonicated at 40°C (15–40 min for 4-H-chromenes, and 10–30 min for pyrazole derivatives). After reaction completion the products were purified by ethanol crystallization, and the catalyst was recovered and reused for three consecutive batches without loss of catalytic activity.
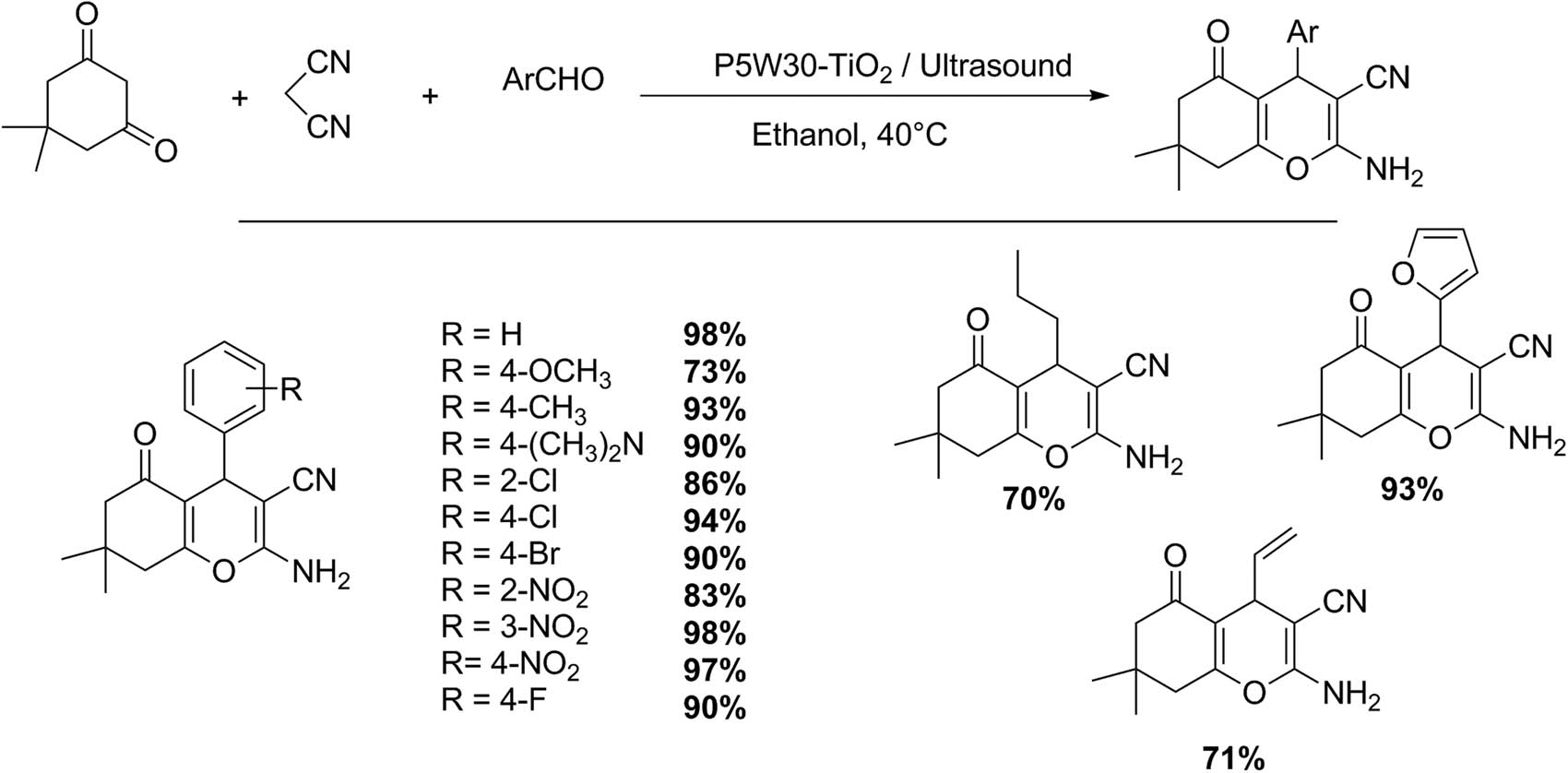
4-H-Chromene synthesis.
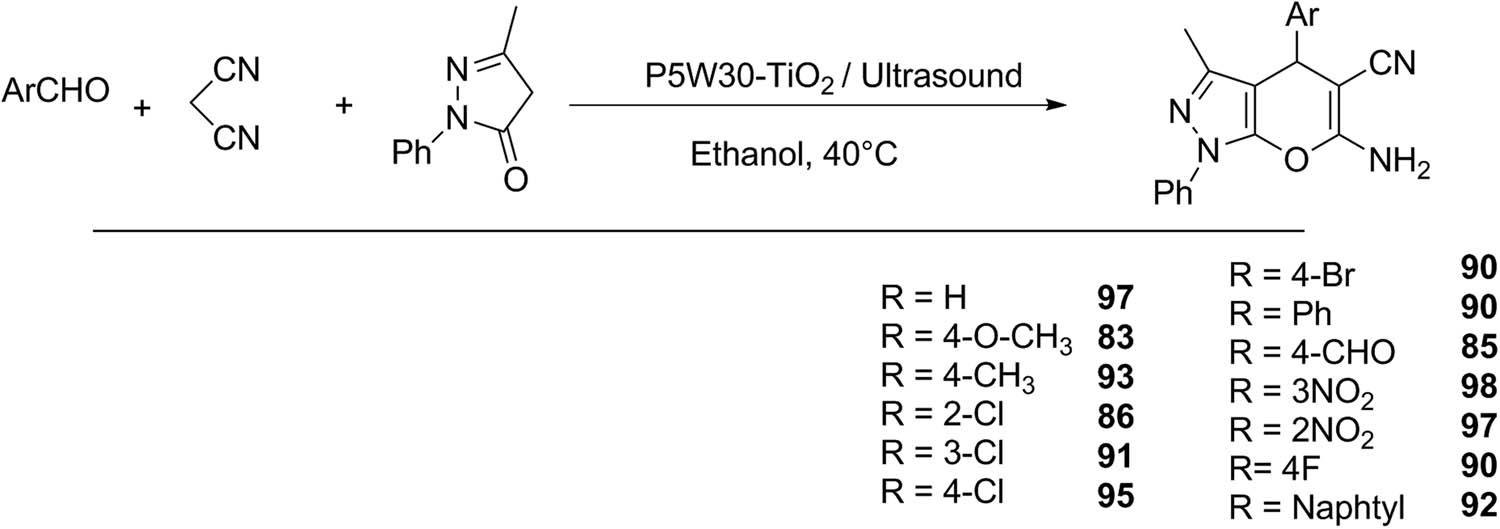
2,3-Dihydropyranopyrazole synthesis.
In this methodology ultrasound accelerated the multicomponent synthesis of tetrahydrobenzo[b]pyrans (15 examples, 70–98%), and 1,4-dihydropyrano[2,3-c]pyrazole derivatives (13 examples, 83–97%), in the presence of nanotitania-supported Preyssler-type HPA as heterogeneous catalyst [137].
Maleki et al. [138] studied the one-pot synthesis of different functionalized pyran derivatives with NiFe2O4–SiO2–P5W30 in ethanol, at 20°C, under ultrasonic irradiation. The HPA P5W30 (H14[NaP5W30O110]) was immobilized on a nanoparticle core shell with magnetic properties. The authors carried out different types of transformations by alternately changing one or two substrates. In this way, they obtained four families of highly functionalized pyrans.
Initially, the authors optimized the procedure for the synthesis of tetrahydrobenzo[b]pyrans and 2-amino-3-cyano-4H-pyrans, and later used the optimal reaction conditions for the synthesis of various family compounds (Scheme 45). In a representative experiment, ethanol (5 mL) was added to a mixture of aldehyde (1 mmol), malononitrile (1.2 mmol), dimedone (1 mmol), and the catalyst (0.02 g), and the mixture was irradiated under sonication (ultrasonic power 250 W, irradiation frequency 40 kHz), at 20°C. The magnetic catalyst was separated by an external magnetic field. The solution was poured into crushed ice, and the formed product was separated by filtration, and recrystallization from ethanol to give the pure products. Fifteen tetrahydrobenzo[b]pyrans (5–10 min, 80–94%) and six examples (15–35 min, 83–92%) were obtained with very good yield free of secondary products [138].
![Scheme 45
Tetrahydrobenzo[b]pyran and 2-amino-3-cyano-4H-pyran synthesis.](/document/doi/10.1515/gps-2022-0068/asset/graphic/j_gps-2022-0068_fig_045.jpg)
Tetrahydrobenzo[b]pyran and 2-amino-3-cyano-4H-pyran synthesis.
Similarly, through a three-component process that in this particular case involves the reaction between an aldehyde, malononitrile, and 3-methyl-1-phenyl-2-pyrazoline-5-one, six examples of pyrano[2,3-c]pyrazoles were obtained with excellent yields (89–94%, ultrasonic irradiation, 5 min) (Scheme 46) [138].
![Scheme 46
Ultrasound assisted one pot three component synthesis of pyrano[2,3-c]pyrazoles.](/document/doi/10.1515/gps-2022-0068/asset/graphic/j_gps-2022-0068_fig_046.jpg)
Ultrasound assisted one pot three component synthesis of pyrano[2,3-c]pyrazoles.
Finally, the authors explored a one-pot tetracomponent procedure replacing 3-methyl-1-phenyl-2-pyrazoline-5-one with hydrazine hydrate and ethyl acetoacetate in similar reaction conditions. A series of 7 pyrano[2,3-c]pyrazoles was obtained with yields between 90% and 94% (ultrasonic irradiation, 10 min) (Scheme 47) [138].
![Scheme 47
Ultrasound assisted one pot tetra-component synthesis of pyrano[2,3-c]pyrazoles.](/document/doi/10.1515/gps-2022-0068/asset/graphic/j_gps-2022-0068_fig_047.jpg)
Ultrasound assisted one pot tetra-component synthesis of pyrano[2,3-c]pyrazoles.
Tetrahydrobenzo-[b]-pyrans using ultrasonic irradiations and HPA deposited on a magnetic support have been reported by Mozafari and Heidarizadeh (Scheme 48) [139]. The catalyst was prepared using silica-coated manganese ferrite nanoparticles modified previously with diamine groups to obtain a core shell structure labelled MnFe2O4–SiO2@NHPhNH2. The phosphotungstic acid (PW12) was anchored on this support. The reaction is very simple and only requires the mixture of the aromatic aldehyde (1 mmol), malononitrile (1.2 mmol), dimedone (1 mmol), and catalyst (0.04 g). The reaction mixture was sonicated at 80°C in water as reaction solvent. The catalyst was magnetically separated, and the product was extracted with ethyl acetate, and recrystallized from ethanol. Nine tetrahydrobenzo-[b]-pyrans were obtained with excellent yields and a short time (87–94%, 8–12 min) [139]. This same catalyst was used to obtain indazolo[2,1-b]phthalazine-triones. Phthalazine and its derivatives exhibit some bioactivity such as anticancer, antifungal, anticonvulsant, and anti-inflammatory, and are also used in hypertensive therapy [139].
![Scheme 48
Tetrahydrobenzo-[b]-pyrans.](/document/doi/10.1515/gps-2022-0068/asset/graphic/j_gps-2022-0068_fig_048.jpg)
Tetrahydrobenzo-[b]-pyrans.
Then, using the same catalyst, another multicomponent procedure was evaluated. In this case, 2H-indazolo[2,1-b]phthalazine-triones were obtained using easily available starting materials (dimedone, phthalhydrazide, and an aromatic aldehyde) (Scheme 49). In a representative test a mixture of aldehyde (1 mmol), dimedone (1 mmol), phthalhydrazide (1 mmol), and the catalyst (0.03 g) was sonicated at 80°C in the presence of H2O. When the reaction was complete, the catalyst was separated magnetically, the product was extracted with ethyl acetate, and recrystallized. Nine examples of 2H-indazolo[2,1-b]phthalazine-triones were obtained with very good yields (86–95%, 6–12 min) [139].
![Scheme 49
2H-indazolo[2,1-b]phthalazine-triones.](/document/doi/10.1515/gps-2022-0068/asset/graphic/j_gps-2022-0068_fig_049.jpg)
2H-indazolo[2,1-b]phthalazine-triones.
Through this procedure, the synthesis was done under environmentally friendly conditions that include the use of a recoverable magnetic catalyst, ultrasound irradiation as an energy source, and the use of water as a reaction solvent. Furthermore, both procedures were carried out through a one-pot-multicomponent process with high atom economy.
The use of ultrasound irradiation is not limited to organic synthesis; on the contrary, it is a very useful technique in different types of processes that include the manufacture of materials, the degradation of contaminants, assistance in emulsification and extraction processes, and sustainable organic synthesis processes [121,140–143]. The bibliography presents some reviews about synthesis processes related to the oxidation of organic substrates [118,144–146].
The literature reports some work-related selective oxidation processes in organic synthesis assisted by the use of ultrasound and materials based on HPAs as catalysts. Among them, it is important to highlight the oxidation of alkenes, alcohols, and sulfides, which is of special interest in oxidative desulfurization (ODS) processes of fossil fuels. The approach consists in the deposition of a Keggin-type heteropolyanion compound (PMo11V) on clay supports such as montmorillonite (MONT). Salavati et al. [147] reported that this type of solid (PMo11V–MONT) resulted in efficient and selective catalysts for oxidation of alkenes under ultrasonic irradiation conditions (Scheme 50). The reactions were conducted with 4.76 mmol% of PMo11V-MONT, 0.8 mmol of alkene, 5 mL of acetonitrile, and 1 mL of H2O2. The degree of advancement of the reaction and the selectivity toward the formation of the epoxide were determined by GC analysis. When the reaction was complete, the catalyst was filtered, and the product was purified by liquid chromatography. For cyclooctene oxidation the results showed a conversion of 58% with an epoxide selectivity of 65%. Similarly, the cyclohexene oxidation showed 58% epoxide selectivity with a conversion of 72%. In this case, cyclohexene-1-one and cyclohexene-1-ol were detected as secondary products [147].
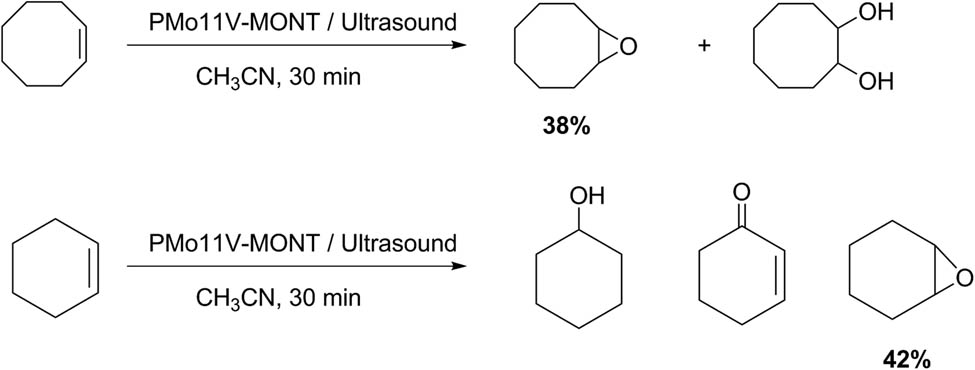
Selective alkene oxidation.
By supporting heteropolyanion nanoparticles on materials such as MMT, a recoverable and reusable catalyst was obtained for the selective oxidation of alkenes with hydrogen peroxide. Ultrasonic irradiation notably increased the conversions and reduced the reaction times (for example, from 10 h to 30 min). Seven examples with structural variety were tested with good conversion (28–92%) and selectivity (30–96%).
The same authors prepared new materials composed of vanadium-containing polyphosphomolybdate immobilized on multiwall carbon nanotubes (PMo11V-CNTs). Then, the obtained composites were characterized and were found to be an efficient catalyst for selective alkene oxidation under ultrasonic irradiation conditions. In a typical experiment, for example, hydrogen peroxide (1 mL, 30%) was added to styrene (0.8 mmol) and the supported catalysts (2.86 mmol% of PMo11V) in acetonitrile (5 mL), and the mixture was exposed to ultrasound irradiation (frequency, 24 kHz, and output power, 0–400 W). The reaction was monitored by GC. The products were purified by liquid chromatography and identified by 1H and 13C-NMR. For the reaction of styrene oxidation, a yield of epoxide of 43% was detected in a reaction time of 40 min (conversion of 71% and selectivity to epoxide of 60%). The optimal reaction conditions were extended to other substrates of different structural nature, obtaining yields to epoxides between 39% and 84% (Scheme 51) [148].
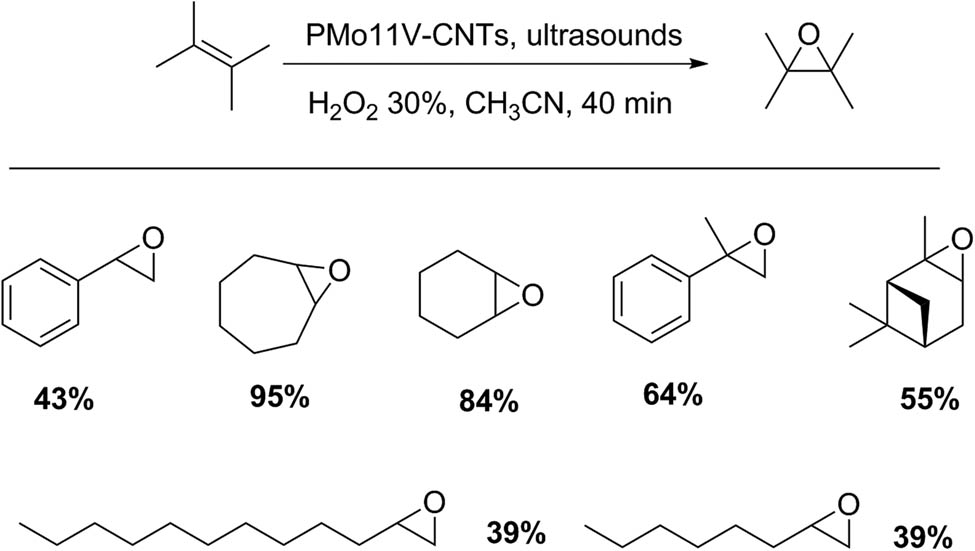
Epoxidation of olefins.
Lin group reported a suitable procedure for adipic acid preparation using a system formed by aqueous hydrogen peroxide and SiW12 under ultrasonication (Scheme 52). In a representative experiment, a round flask was charged with cyclohexene (2 mmol), SiW12 (0.04 mmol), and 30% H2O2 (10 mmol). The reaction flask was irradiated in a cleaning bath for 4 h. The reaction products were isolated by filtration of the reaction mixture, with a yield of 92% [149].
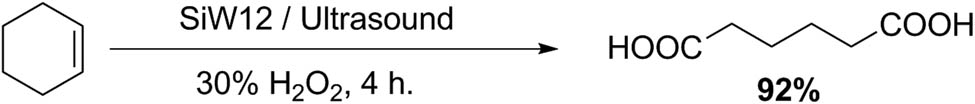
Adipic acid synthesis.
Azarifar et al. reported the use of a Preyssler-type HPA supported on TiO2 nanoparticles as catalyst in the selective oxidation of primary aromatic amines to azoxy derivatives using trans-3,5-dihydroperoxy-3,5-dimethyl-1,2-dioxolane as oxidant (Scheme 53). The transformation proceeded in green conditions assisted by ultrasound irradiation to afford the product with high selectivity. The general procedure consisted in the dissolution of an aromatic amine (1 mmol) and P5W30 nano-TiO2 catalyst (25 mg) in absolute ethanol (5 mL), sonication at 20°C for 5 min, and then trans-3,5-dihydroperoxy-3,5-dimethyl-1,2-dioxolane (2 mmol) was added to the mixture in one portion. After completion (TLC), the catalyst was separated by filtration, and the products were purified by liquid chromatography and recrystallization. Twelve examples were obtained with excellent yields and selectivity (reaction yields between 81% and 98%, reaction time 5–20 min) [150].
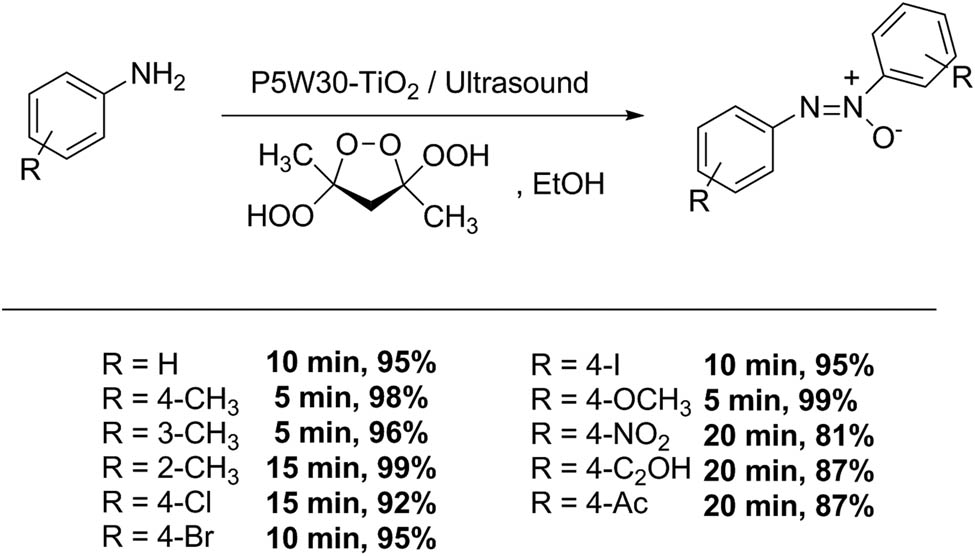
Aromatic amine oxidation.
Rezvani et al. [151] described the synthesis and characterization of nanocomposite mixed-addenda vanadium substituted POM-TiO2 and its use as suitable catalyst for a rapid and efficient synthesis of symmetric disulfides for thiol oxidation under ultrasound irradiation, and hydrogen peroxide as green oxidant (Scheme 54). In a representative test of a mixture of thiol (0.5 mmol), and PMo11V–TiO2 (16 mg) in EtOH (8 mL), 2 mL of 30% hydrogen peroxide was added. The reaction was monitored by TLC, and the reaction product was purified by liquid chromatography. Nine examples were obtained (78–97%, 10–40 min) [151].
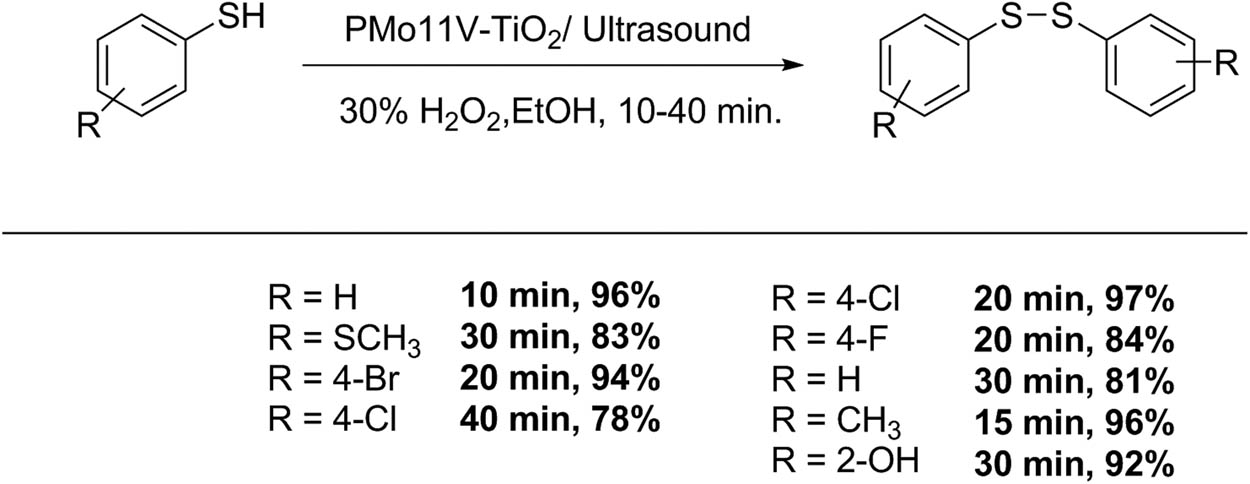
Disulfide synthesis.
The selective oxidation of compounds containing sulfur in their structure is undoubtedly the most important oxidative process, especially the selective oxidation of sulfides to sulfoxides and sulfones. This is substantially associated with the need to eliminate sulfur-containing compounds that are present in fossil fuels. The elimination of refractory compounds, such as derivatives of thiophene or dibenzothiophenes, present in fuels, is necessary, due to the requirements imposed by current governments related to caring for the environment. Although there are numerous processes that help to comply with these regulations, for example, selective adsorption, extractive distillation, hydrodesulfurization, biodesulfurization, and ODS, among others, in this review we will focus on those associated with ODS, in which HPAs and ultrasounds are used to assist in the process. Most of the proposed works promote the synthesis and characterization of new materials based on HPAs, with the aim of improving the process (Scheme 55).
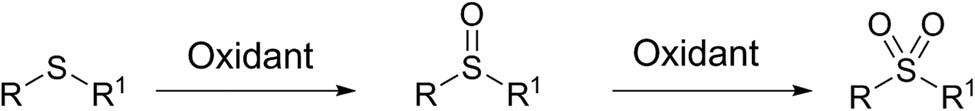
Oxidation of sulfides to sulfoxides and sulfones.
Specifically, we will focus on highlighting the contributions associated with the oxidation of sulfides assisted with HPAs and ultrasounds [152,153]. Afzalinia et al. [154] reported the ultrasound-assisted oxidative desulfurization (UAOD) of liquid fuels performed with a novel material based on a Keggin-type phosphotungstic acid (PW12) encapsulated into an amino-functionalized MOF (TMU-17-NH2). The experiment tests were performed in a biphasic system, for example, acetonitrile and model fuel, using BT, DBT, or 4,6-DMDBT as substrates. For example, 5 mg of PW12-TMU-17-NH2 was placed in the vessel, then a mixture of acetonitrile (5 mL) and model fuel (5 mL, 2.5 mg of DBT) and finally, H2O2 30% (1:1 oxidant/S-containing compound molar ratio) were added. The mixture was irradiated for 5 min at room temperature, and the reaction progress was evaluated by GC analysis. The best result indicated that the conversions of DBT to DBTO2 achieved 98% after 15 min. The catalyst retained its activity even after six cycles.
Liu et al. [155] reported the application of PW12 supported on activated carbon (AC) in the oxidation-desulfurization of fuel oil assisted by ultrasound. DBT was 100% converted using a volume ratio of H2O2:model oil of 1:10 catalyzed by 1.25% of PW12-AC-10 after ultrasound irradiation for 9 min at 70°C. The reaction catalyzed by supported HPW avoided the use of a phase transfer agent, and the materials were easily separated and reused [155].
Similarly, Gildo et al. [156] presented the use of AC-supported phosphotungstic acid as oxidation synthesis in the selective oxidation of sulfide to sulfoxides and sulfones. In an experiment test, the fuel was prepared using dibenzothiophene, benzothiophene, and thiophene. About 2,800 ppm of sulfur with a fixed composition of DBT, BT, and T was prepared by dissolving certain amounts of these compounds in toluene, then the catalyst and hydrogen peroxide were added, and the reaction was irradiated with ultrasound. The optimal conditions were found to be 25.52 wt% H2O2, 983.9 mg catalyst, 9.52 mL of aqueous phase per 10 mL of organic phase, and 76.6 min of ultrasonication producing 94.7% conversion. Also, the authors reported the use of kerosene and, in this case, the sulfur content was reduced from 1,370 to 14 ppm S, equivalent to 99.0% desulfurization [156].
Wang et al. [157] performed a systematic ozonation (assisted by ultrasound) procedure for desulfurization of diesel oil with some metal salts of Keggin-type HPAs, H3PW12O40. Fe0.8H0.6PW12O40 exhibited the best catalytic activity, and the difficulty of removing sulfur compounds depended on the electron density of sulfur atoms. Sulfur compounds with high electron density were more easily removed. The catalyst could be used four times without appreciable loss of catalytic activity [157].
Yu et al. [158] reported the use of ultrasound in the oxidative desulfurization of diesel fuel in a mixture formed by commercial aqueous hydrogen peroxide and methanol. When the system containing 2 mmol% PW12 was irradiated with 400 W ultrasound for 10 min, the sulfur content dropped from 211 to 19 ppm. The protocol is very simple, rapid, and inexpensive.
Zhao and Wang [159] reported a procedure for ultrasound-assisted catalytic ozonation combined with an extraction process for the removal of dibenzothiophene using acetonitrile as extracting agent. Keggin-type heteropolycompounds, including H3PW12O40, H5BW12O40, Cs2.5H0.5PW12O40, and Ce0.8H0.6PW12O40, were used. The Ce0.8H0.6PW12O40 catalyst exhibited the best catalytic activity (93.2% in 60 min). The catalyst was also recoverable, reusable, and showed activity similar to the fresh material. The result indicates that the assisted ultrasonic-catalytic ozonation process combined with extraction is promising for deep desulfurization of diesel oil [159].
The transformations associated with the valorization of biomass are also important, including processing of biomass and the valorization of its building blocks. The literature reports several examples of the use of catalysis with HPAs assisting in esterification procedures for fuel production. However, there are practically no reports on the use of HPAs-ultrasound for the valorization of building blocks present in biomass [160,161].
Lee group performed numerous studies on the transesterification of crude Jatropha oil to fatty acid methyl esters in an ultrasound-assisted process, in the presence of different HPA-based catalysts [162]. The first studies involve the fatty acid methyl ester synthesis from crude Jatropha oil using an ultrasound-assisted process with different gamma alumina (Al) supported tungstophosphoric acid (PW12-γAl2O3) catalysts. The highest reaction yield of 84% was achieved under the optimum conditions: ultrasonic amplitude of about 60%, a molar ratio of 19:1, and a reaction temperature of 65°C, for 50 min.
The mathematical representation of fatty acid methyl ester (FAME) yield was successfully generated and statistically validated [163]. In this case, the crude Jatropha oil transformation was performed in the presence of tungstophosphoric acid supported on AC (PW12-AC) using ultrasound-assisted process. The catalyst with 20% TPA loading achieved the best methyl ester yield (87.3%, 40 min). The catalysts showed 1% w/w water tolerance, and their reusability and leaching were analyzed on PW12(20)-AC, revealing that the reaction was mainly heterogeneous in nature with an appreciable contribution of homogeneous reaction [164].
Another similar study about transesterification of crude Jatropha oil in the presence of cesium-doped HPA catalyst and assisted by ultrasonic irradiation was conducted by these authors. Different Cs HPA catalysts with different levels of cesium exchange were synthesized, characterized, and tested in order to identify the most relevant catalyst. The most active catalyst (Cs1.5H1.5PW12O40) showed the highest FAME yield of 90.5% (34 min). The reaction was principally of heterogeneous nature, and the yield was constant after three successive reaction cycles [165].
Dadhania et al. [166] presented the synthesis of a magnetically separable heteropolyanion based ionic liquid (MNP@HPAIL, Figure 1), and its catalytic activity was evaluated for biodiesel production through the esterification of long-chain fatty acid under ultrasonic irradiation [166].
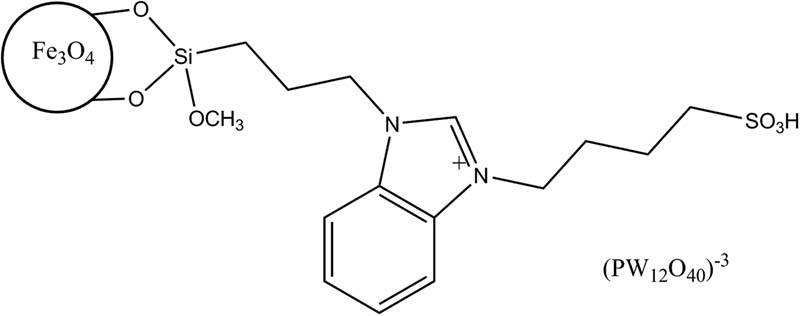
Magnetically separable heteropolyanion-based ionic liquid (MNP@HPAIL).
The MNP@HPAIL catalyst showed the maximum conversion in the presence of 150 mg catalyst, an acid-alcohol ratio of 1:10 at 70°C and 60 min. The new material was used in 6 consecutive reaction cycles with the same biodiesel yield.
Another aspect of great interest in the recovery of biomass is the depolymerization of products derived from lignin to monomers of the phenolic type. Du et al. [167] reported the effect of a catalyst (MCM-41 supported phosphotungstic acid) on the yields and characteristics of various depolymerization products of organosolv lignin, using ultrasound irradiation. In general, ultrasound irradiation played important roles in promoting lignin depolymerization and reducing char yield. The best reaction conditions were: temperature, 310°C; reaction time, 6 h; solvent, isopropanol; ultrasound frequency, 30%, and catalyst, 50%. In these conditions, conversions of 95.52% and 98.54% were obtained using liquid fuel and lignin, respectively. The catalyst could be reused five times without loss of catalytic activity.
4 Mechanochemistry
Solvent-free conditions under favorable environmental conditions are the main characteristic of mechanochemical methods [168,169,170,171]. Several organic reactions using solid acid catalysts have been studied in solvent-free mechanochemical processes that reduce contamination and costs, simplify treatment steps, and eliminate wasteful and excessive heating [172,173].
The mechanochemical process depends on the mill type, ball material (quantity and size), scale of the synthesis, frequency, reaction time, and energy dissipation. In this sense, the activation energy necessary to initiate a chemical reaction is directly related to the amount of energy in the system, and could be provided by grinding, shearing, or extrusion. Of these three energy input ways, grinding is the most useful technique that describes the mechanical action by hard surfaces and could be employed through a manual or ball-milling method. Several reactions can be promoted by mechanosynthesis, including those mediated or catalyzed by metals, condensation reactions, nucleophilic addition, cascade reactions, oxidations, halogenation, and cycloaddition reaction, among others [174–177].
Urethanes or carbamates are compounds of great interest in the field of agrochemicals, especially as pesticides, fungicides, and herbicides. Likewise, they are used as synthesis intermediates in the pharmaceutical industry and as precursors for the synthesis of polyurethanes and peptides in the polymer industry. In general, their preparation involves highly polluting processes and the use of highly toxic reagents [178].
Gharib et al. [179] reported a very simple procedure for the synthesis of carbamates using Preyssler HPA, in the absence of solvent and at room temperature, using grinding technology. The methodology provides an alternative suitable for carbamate synthesis of alcohols and phenol [179]. The procedure used for primary carbamates involves the reaction of either alcohol or phenol with sodium isocyanate in the presence of P5W30 (Scheme 56). The activation reaction is provided by the friction of molecules in solid phase (in solvent-free conditions). Seventeen primary carbamates were obtained with very good yields (58–98%).
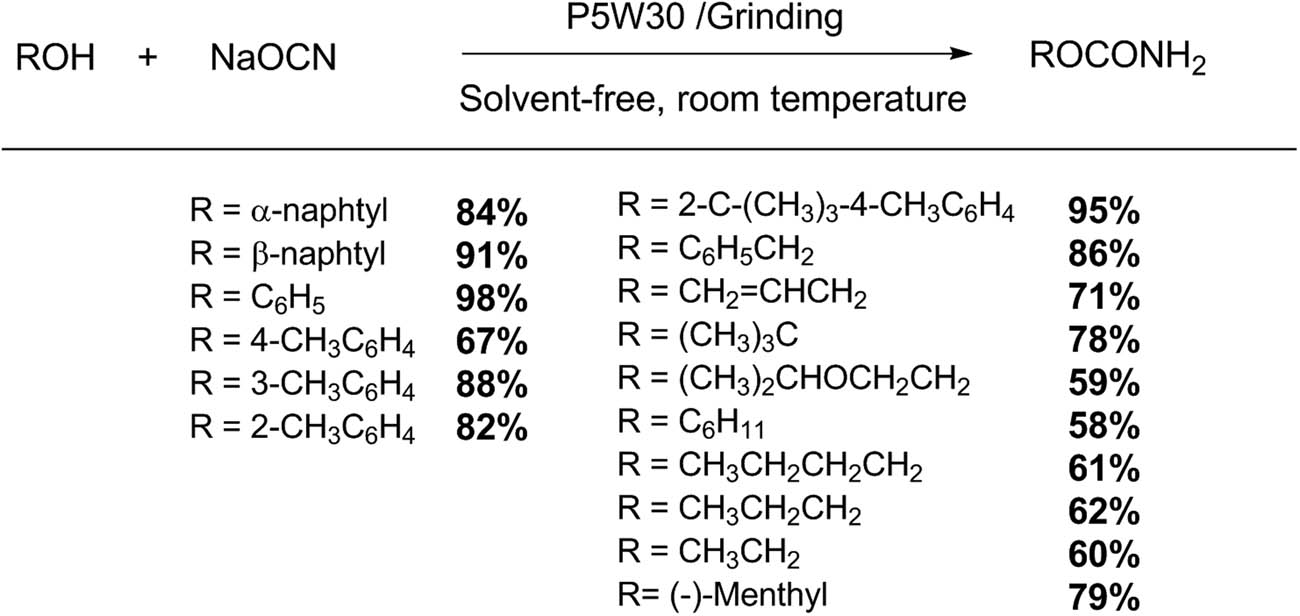
Primary carbamate synthesis.
The N-formylation of aromatic amines in solvent-free and grinding conditions has been studied with heteropolyanion-based sulfated ionic liquid (HIL-[Ch-OSO3H]3W12PO40). This solid was prepared by pairing phosphotungstic acid anion (W12PO40 3−) with sulfate functionalized cholinium cation [N,N,N-trimethyl-2-(sulfooxy)ethanaminium]. The test reaction was studied using a 1 mmol aniline, 3 mmol formic acid (98%), and 20 mg catalyst, which were thoroughly mixed in a mortar and then crushed at 27°C, within 5–10 min (Scheme 57). The catalyst is insoluble in ethanol, allowing its recovery during six reaction cycles without loss of activity. Various examples of N-formylamines were obtained with excellent yields (87–99%, 5–15 min) [180].
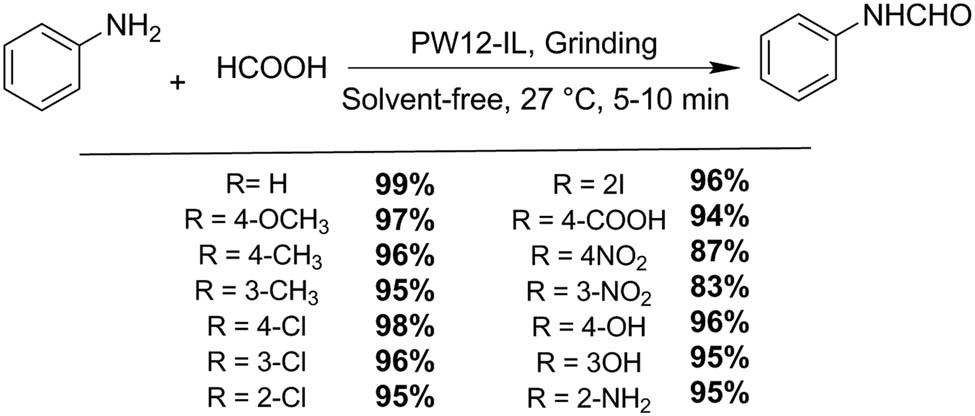
N-formylation of amines.
Additionally, the paper reported the N-formylation of heterocyclic secondary amines, for example, imidazole in similar conditions (Scheme 58); five examples were reported (82–88%, 45–85 min) [180].
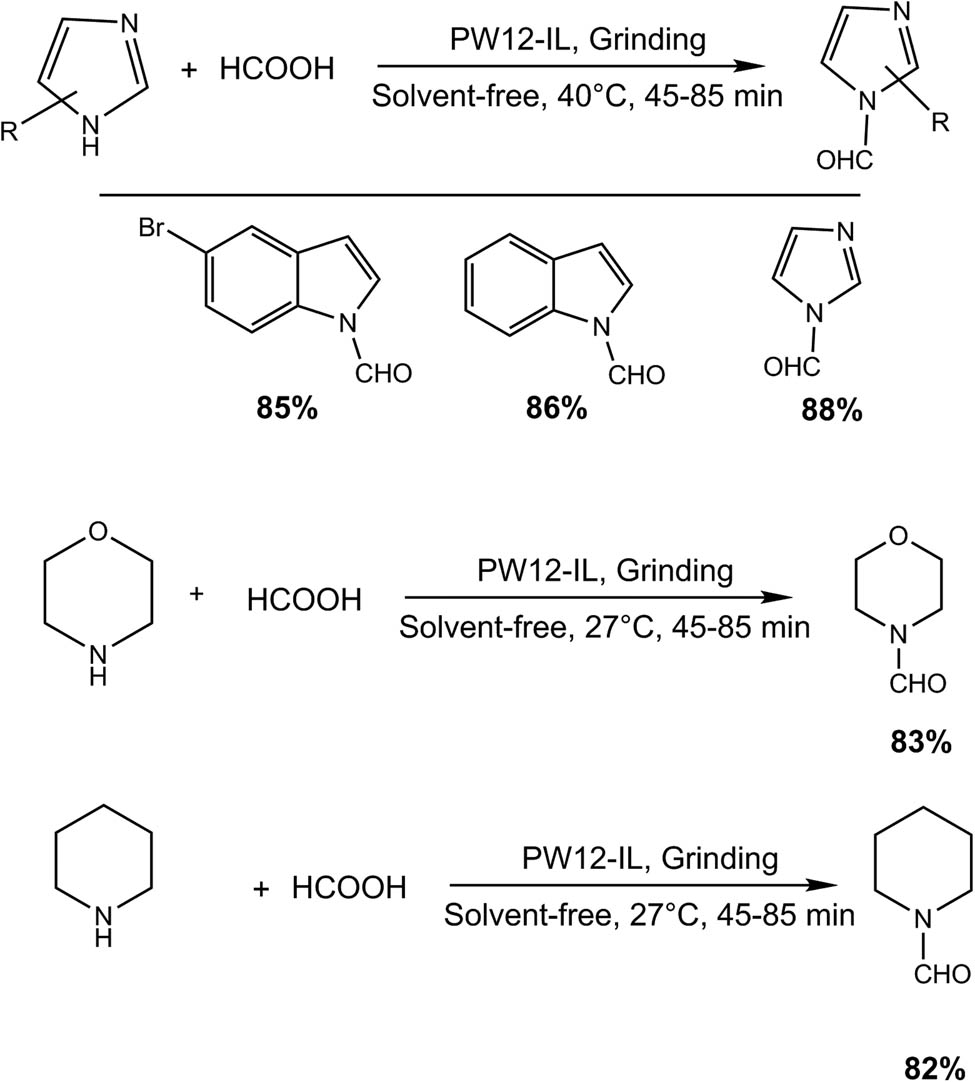
N-formylation of heterocyclic secondary amines.
Li and Sun group reported a very fast procedure for diindolylmethane synthesis via pseudo-multicomponent condensation of indole and aromatic aldehydes catalyzed by SiW12 at room temperature within 1–5 min by grinding. The general procedure involves mixing aldehydes (0.5 mmol), indole (1 mmol), and SiW12 (1 mmol%) in a mortar, and then grinding at 20°C for less than 5 min. The product was purified by liquid chromatography. Eleven examples were obtained with very good yields using aromatic aldehydes as substrates (68–98%) (Scheme 59). However, the reaction of indole and ketones or aliphatic aldehydes gave lower yields (16–49%) [181].
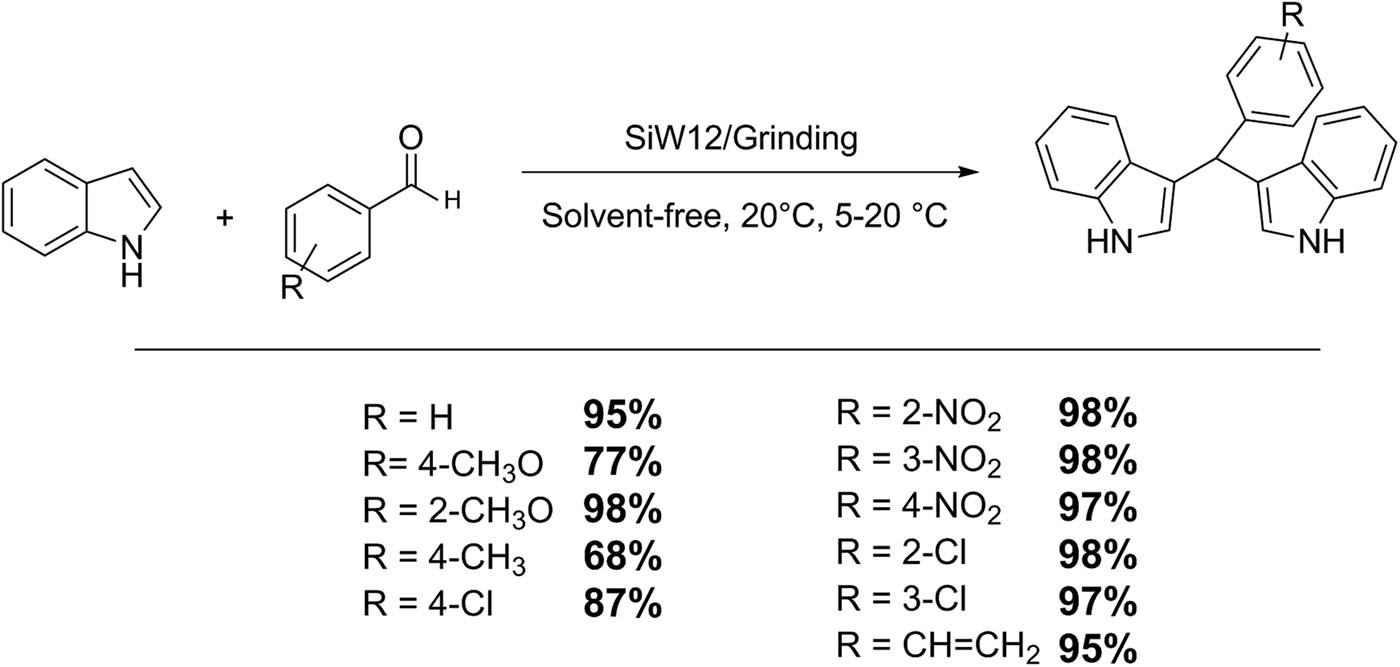
Diindolylmethane synthesis.
Patil et al. [182] reported a suitable procedure for benzoxazole derivative synthesis through a grinding approach. The authors used PW12 supported on silica as catalyst in the synthesis of 2-arylbenzoxazole from 2-aminophenols with different aromatic aldehydes using grinding, at room temperature. Excellent yields were obtained (85–92%), using both aromatic aldehydes that have electron-donating or electron-withdrawing groups (Scheme 60). The protocol has several advantages that include: simple work-up, short reaction times, mild reaction conditions, high yields of products, and the non-toxicity of the catalyst.
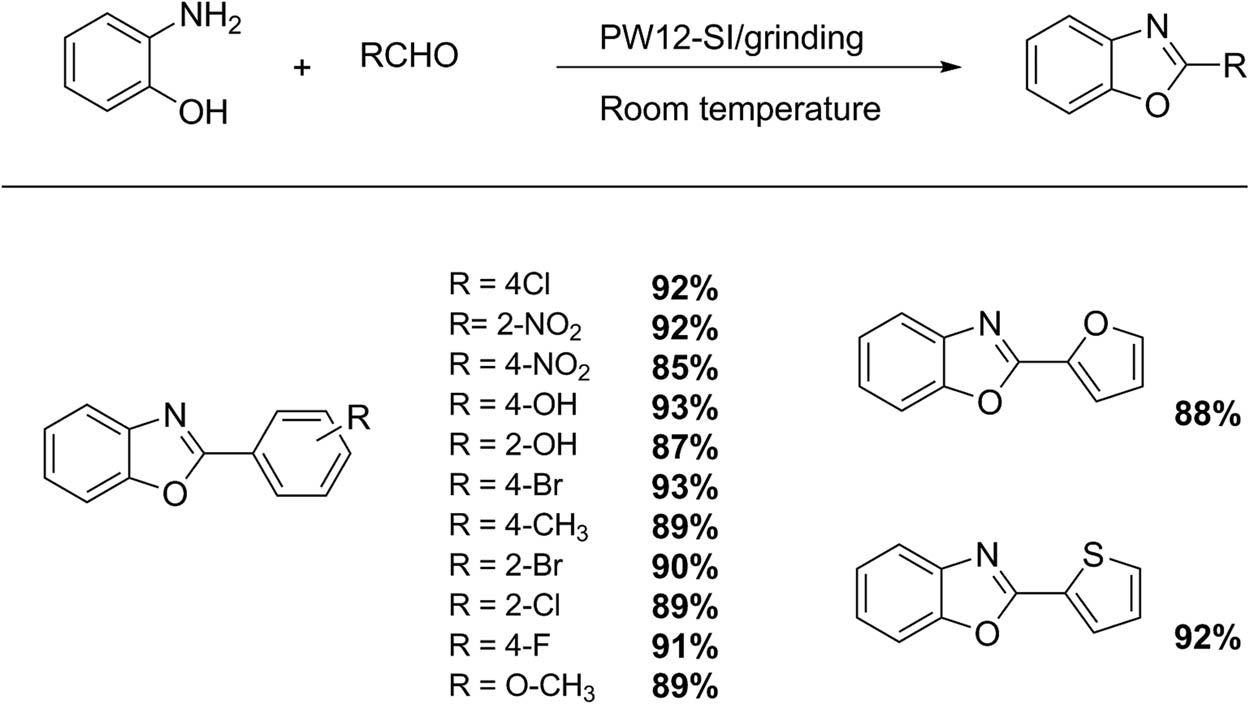
2-Arylbenzoxazole synthesis.
Kouznetsov group reported a suitable method for the synthesis of cis-4-amido-N-yl-2-methyl-tetrahydroquinolines in solvent-free, phosphomolybdic acid-catalyzed and mechanochemical conditions. The authors employed two different alternative mechanochemical methodologies: high-speed vibratory ball mill and high-speed mixer ball mill, obtaining slightly better results using the former technique. In a representative experiment, aniline (1 mmol), N-vinyl amide (2.1 mmol), and PMo12 (10 mmol%) were placed in an open-air agate dish, and one ball (d = 51.6 mm, 186.25 g) was put over the mixture, the reaction was launched to the maximum mill frequency (80 Hz), until the reaction was complete (TLC). The products were purified by liquid chromatography [174]. Nine examples of 2-Me-1,2,3,4-tetrahydroquinolines were obtained in diastereoselective manner by means of a Diels–Alder type reaction with very good yields (56–76%) (Scheme 61).
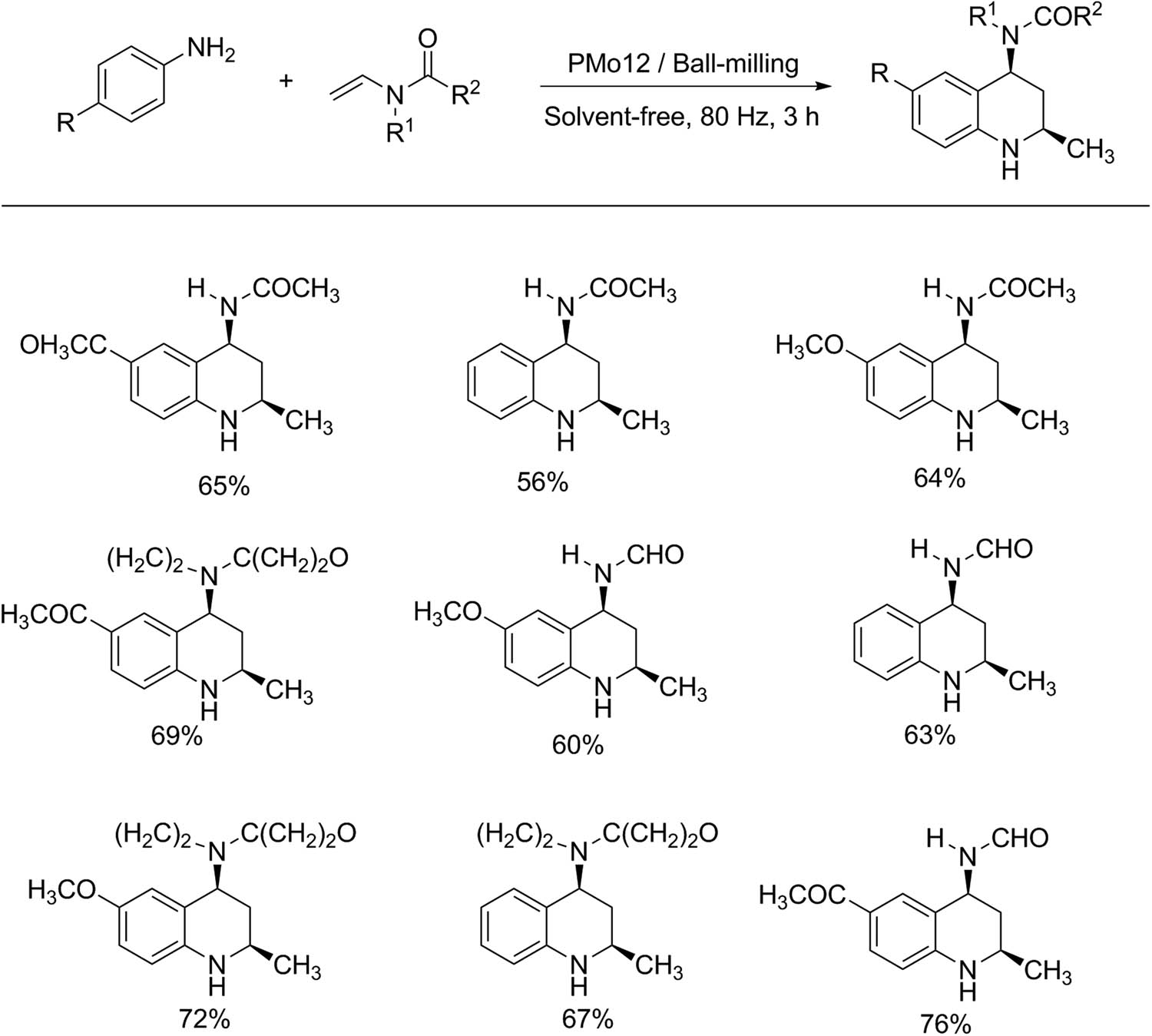
Cis-4-amido-N-yl-2-methyl-tetrahydroquinoline synthesis.
A mechanochemical methodology, solvent-free conditions, and the use of solid catalysts are desirable actions to perform the reaction in suitable conditions. The valorization of biomass by depolymerization processes has had a tremendous development in recent years. In general, different procedures have been tested in order to achieve optimal results. Numerous recent works have dealt with the degradation of biopolymers in order to obtain useful building blocks in bioindustries [183–188]. In particular, the use of HPAs as catalytic materials associated with mechanochemical processes to improve biomass depolymerization processes has already begun to be studied [102,188].
Guerrero Ruiz group reported a combined method for cellulose conversion into renewable biorefinery feedstock using a heterogeneous bifunctional catalyst based on HPAs and Ru nanoparticles supported on carbon for the acid hydrolysis and hydrogenation/hydrogenolysis. The protocol involves one-pot tandem conversion of cellulose, mainly propylene glycol and ethylene glycol. It is also important to note that the cellulose pretreatment is necessary, since during the ball-milling pretreatment the crystallinity and particle size of cellulose are reduced, which results in a much higher conversion of cellulose [102].
Xiao et al. [188] reported an efficient integrated protocol for sacharization of cellulose and hemicellulose from lignocellulosic biomass. In general, the transformation of raw carbohydrate–lignin matrix is very low due to the existence of crystalline zones and inhibition of lignin. To solve this problem, the authors proposed the development of an integrated process that includes the synergistic effect of HPAs as catalysts, ball milling, and enzymatic hydrolysis. A previous ball milling process provides a pretreatment that reduces the crystallinity and particle size of bamboo cellulose (crystallinity index for cellulose, 71.3–9.5%, and particles 283–29 µm in D50 size). Then, they studied the combined hydrolysis procedure, phosphotungstic acid PW12 hydrolysis at 150°C for 2.0 h and subsequently, enzymatic hydrolysis at 50°C for 7.0 h being the optimal conditions. The results indicate that BSS milled without PW12 produces 763.6 mg·g−1 of monomeric sugars, employing 0.7 g PW12/g substrate, while BSS milled with 2.5% PW12 can achieve 741.6 mg·g−1 of monomeric sugars but only employing 0.1 g PW12/g substrate in the reaction. The no-milling process produces 763.6 mg·g−1 of monomeric sugars using a substrate/HPA ratio of 0.7. However, the amount of HPAs necessary to produce a similar amount of monomeric sugars, but using the sample with the grinding pretreatment, is substantially reduced (0.1 g·g−1) [188].
5 Photocatalysis
The use of light as a source of activation in chemical transformations has gained tremendous importance in the last decade. The design of new photoactive materials has undoubtedly been the determining contribution in this discipline. It is certainly an area of strong growth due to the advent of new, high-efficiency, low-cost light sources with defined wavelengths such as LEDs and diodes [18,189].
The combined use of light and catalytic materials based on HPAs has developed intensively in the past decade. The main applications in this regard are aimed at obtaining robust, recoverable, and reusable materials in environmental remediation areas. Thus, there are numerous works on the use of TiO2 doped with HPAs for the remediation of water, the degradation of pesticides, and the elimination of industrial products, mainly drugs [190–194].
Different reactions have been studied through the use of photoactive materials in organic synthesis, for example, transformation of functional groups, redox processes, reactions of construction of carbon–carbon and carbon–heteroatom bonds, degradations, and more recently, driven by green chemistry, multicomponent processes of high atom economy, and reactions in the absence of solvent or with environmentally friendly solvents [195–198].
However, the literature describes very few examples of the use of both ultraviolet and visible light in organic synthesis using HPAs as catalysts. Most of them are simple transformations, fundamentally oxidation procedures. The literature reported some redox processes, especially of selective photo-oxidation of alcohols, assisted by materials based on HPAs; however, the central objectives pointed to the mechanistic study of the reaction and not for organic synthesis purposes. For example, Nomiya et al. [199] reported a mechanism study about the photocatalytic oxidation of isopropyl alcohol to acetone using tetrabutylammonium decatungstate (an isopolyoxometalate).
More recently, Wu et al. [200] reported an environmentally friendly process for obtaining benzaldehyde. The authors prepared a photocatalyst (UCNS-PW12), based on a tungstophosphoric Keggin HPA (PW12) incorporated on graphitic nitride (g-C3N4) nanosheets. This catalyst showed higher selectivity (99% at 2 h) with moderate conversion (58%) in the photo-oxidation reaction of benzyl alcohol in water at 20°C.
García-López et al. [201] reported the preparation of several materials based on Wells–Dawson (PW12) and Keggin (P2W18) HPAs, supported on boron nitride (BN) or carbon nitride (C3N4). The new catalysts were well characterized, and their photocatalytic activity (acid and redox) was studied particularly in 2-propanol dehydration. The activity of these supported HPAs increased when the reaction was carried in the presence of light, due to a considerable decrease in the activation energy in the photo-assisted process. The materials PW12/BN and P2W18/BN showed the highest turnover frequency (TOF), at the highest temperature (120°C, and LED radiation at 365 nm). The stabilization of the W octahedral coordination (XAS data) allowed the highest activity in the case of PW12/BN.
The same author reported the preparation and characterization of Keggin HPA (PW12) supported on SiO2, TiO2, and N-doped TiO2 in the hydration of propene to 2-propanol assisted by UV irradiation. Similarly, the catalytic activity was higher when the reaction was assisted with light, mainly due to the ability of photoexcited PW to trap electrons when it is included in the support, in agreement with EPR spectroscopy results [202]. In this same sense, this author reported propene hydration to 2-propanol and glycerol dehydration to acrolein, using Keggin HPAs supported on TiO2 Evonik P25 under UV irradiation. The material showed excellent catalytic activity due to the strong acidity and the ability to form oxidative sites [203].
Carboxylic acids can be obtained by photo-oxidation of aromatic aldehydes using molecular oxygen or aqueous hydrogen peroxide, in the presence of Preyssler HPA P5W30 [204]. In the present study it was found that it was very active when hydrogen peroxide and UV light were used. In a test reaction, 4 mL of acetonitrile and 6 mL of water, substrate (2 mmol), and P5W30 (0.5 mmol%) were added, and the mixture was stirred for 30 min. Then, the reaction was irradiated with UV light, and oxygen gas (99.99%) was introduced to the reaction mixture. The reaction product (carboxylic acid) was recovered by filtration, and purified by recrystallization. Five examples of the respective carboxylic acid were obtained with variable yields (28–97%, 6 h), using the bulk catalyst (Scheme 62). Benzaldehydes substituted with –OH group gave only traces of reaction product. Also, the use of the catalyst supported on silica led to lower reaction yields (19–54%, respectively), attributable to an acid–base interaction between the catalyst and the support. Finally, the photocatalytic oxidation of p-chlorobenzaldehyde was investigated in the presence of aqueous H2O2 in similar conditions over 6 h. In these conditions, the reaction yield was 42% [204].
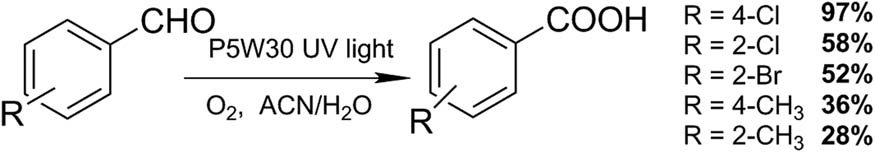
Benzaldehyde oxidation to benzoic acids.
The same author reported a similar study about the selective benzyl alcohol oxidation to benzaldehyde using Preyssler acid in bulk form, H14[NaP5W30O110], and silica-supported with different loadings, under O2 and H2O2 (Scheme 63). High selectivity and moderate yields were obtained, the bulk catalyst being more active than the one supported on silica (80% vs 52%, 6 h). In a typical experiment, to 2 mL of acetonitrile and 3 mL water, substrate (2 mmol) and H14[NaP5W30O110] (0.5 mmol%) were added, and the mixture was stirred for 30 min. Then, the mixture was irradiated for 6 h in the presence of oxygen gas (6 h). Similarly, aqueous hydrogen peroxide was used as clean oxidant, although the yields were lower with H2O2 than O2 (80% vs 35%). The results show that this photocatalytic system (HPAs/oxygen) is an effective system for oxidation of other benzyl alcohols (five examples, 50–80%) [205].
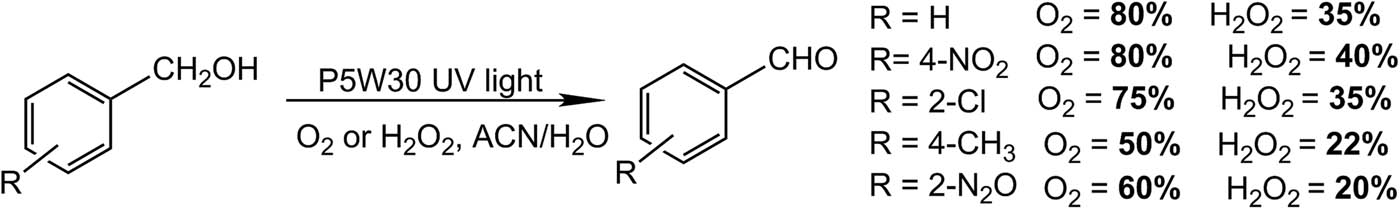
Benzyl alcohol oxidation to benzaldehydes.
6 Conclusion
In this review, we have explored the different advances associated with green chemistry and related to the use of alternative sources of energy to carry out different chemical transformations, using catalytic materials based on HPAs and related compounds.
Each one of the contributions was covered in different sections considering the different alternative sources of energy such as microwaves, ultrasound, photochemistry, and mechanochemistry. Some specific sections include a description of the combined use of these techniques (technological tandem) since it substantially improves the efficiency of certain processes.
Different chemical transformations were explored, including organic synthesis reactions (especially heterocycle synthesis), redox oxidation processes, and biomass valorization. In particular, HPA-assisted degradation of chemical compounds was not considered in this review.
From the analysis of the different contributions in this subject, it is concluded that there is abundant bibliography associated with the use of catalytic materials based on HPAs for the synthesis of heterocycles using microwaves and ultrasound. However, the advances associated with the use of photocatalysis and mechanochemistry are much smaller, especially related to the synthesis of heterocycles in the case of photocatalysis, and the valorization of biomass by mechanochemical methods that include ball milling and grinding.
It concludes by establishing the need to multiply the use of these technologies using environmentally friendly reaction media and designing new materials based on HPAs that are robust and can be easily reused in this type of transformation.
Acknowledgment
We thank Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Universidad Nacional de La Plata (UNLP) for financial support.
-
Funding information: This research work was supported by CONICET (PIP 0084), Agencia Nacional de Promoción Científica y Técnica ANPCyT (0157), UNLP, and Comisión de Investigaciones Científicas Provincia de Buenos Aires CICPBA.
-
Author contributions: Diego M. Ruiz: writing – original draft and formal analysis; Gustavo A. Pasquale: bibliographic search, writing – original draft, and formal analysis; José J. Martínez: writing – review and editing; Gustavo P. Romanelli: conceptualization, writing – review and editing, supervision, and project administration. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: Authors state no conflict of interest.
References
[1] Sheldon RA, Arends IWCE, Hanefeld U. Green chemistry and catalysis. Weinheim, Germany: Wiley; 2007. ISBN 9783527611003. 10.1002/9783527611003.Suche in Google Scholar
[2] Anastas P, Warner J. Green chemistry: theory and practice. New York, NY, USA: Oxford University Press; 1998. ISBN 9780198502340. 10.1021/op000054t.Suche in Google Scholar
[3] Kokel A, Schäfer C. Application of green chemistry in homogeneous catalysis. In: Török B, Dransfield T, editors. Green chemistry–an inclusive approach. Boston, MA, USA: Elsevier; 2018. p. 375–414. ISBN 9780128095492. 10.1039/C7GC01393K.Suche in Google Scholar
[4] Pálinkó I. Heterogeneous catalysis: a fundamental pillar of sustainable synthesis. In: Török B, Dransfield T, editors. Green chemistry–an inclusive approach. Boston, MA, USA: Elsevier; 2018. p. 415–47. ISBN 9780128095492.10.1016/B978-0-12-809270-5.00017-0Suche in Google Scholar
[5] Escobar AM, Blustein G, Luque R, Romanelli GP. Recent applications of heteropolyacids and related compounds in heterocycle synthesis. Contributions between 2010 and 2020. Catalysts. 2021;11:291. 10.3390/catal11020291.Suche in Google Scholar
[6] Mandal B. Alternate energy sources for sustainable organic synthesis. Chem Sel. 2019;4:8301–10. 10.1002/slct.201901653.Suche in Google Scholar
[7] Stefanidis G, Stankiewicz A. Alternative energy sources for green chemistry royal society of chemistry. Cambridge, UK: RSC Publishing; 2016. p. 1–33. ISBN 9781782623632. 10.1039/9781782623632.Suche in Google Scholar
[8] Veitia MSI, Ferroud C. New activation methods used in green chemistry for the synthesis of high added value molecules. Int J Energy Env Eng. 2015;6:37–46. 10.1007/s40095-014-0148-7.Suche in Google Scholar
[9] Freitas EF, Souza RY, Passos STA, Dias JA, Dias SCL, Neto BAD. Tuning the Biginelli reaction mechanism by the ionic liquid effect: the combined role of supported heteropolyacid derivatives and acidic strength. RSC Adv. 2019;9:27125–35. 10.1039/C9RA03336J.Suche in Google Scholar PubMed PubMed Central
[10] Balci M. Recent advances in the synthesis of fused heterocycles with new skeletons via alkyne cyclization. Tetrahedron Lett. 2020;61(24):151994. 10.1016/j.tetlet.2020.151994.Suche in Google Scholar
[11] Romanelli G, Autino J. Recent applications of heteropolyacids and related compounds in heterocycles synthesis. Mini Rev Org Chem. 2009;6(4):359–66. 10.2174/157019309789371578.Suche in Google Scholar
[12] Palermo V, Sathicq A, Romanelli G. Suitable transformation of compounds present in biomass using heteropolycompounds as catalysts. Curr Opin Green Sust Chem. 2020;25:100362. 10.1016/j.cogsc.2020.100362.Suche in Google Scholar
[13] Hu YH, Shi JH, Wang GL, Lin BP, Jiang F. Application of heteropolyacids or their salt catalysts in organic synthesis. Xiandai Huagong/Modern Chem Ind. 2004;24:35–9.Suche in Google Scholar
[14] Heravi MM, Sadjadi S. Recent developments in use of heteropolyacids, their salts and polyoxometalates in organic synthesis. JICS. 2009;6(3):1–54. 10.1007/BF03246501.Suche in Google Scholar
[15] Gharib A. Application of heteropolyacids and nano-catalyst in heterocycles synthesis. Org Med Chem Int J. 2016;1(1):29–35. 10.19080/OMCIJ.2016.01.555554.Suche in Google Scholar
[16] Ravichandran S, Karthikeyan E. Microwave synthesis – a potential tool for green chemistry. Int J Chem Tech Res. 2011;3(1):466–70.Suche in Google Scholar
[17] Vavsari VF, Balalaie S. Recent advances in green synthesis of chromones. Chem Heterocycl Comp. 2020;56:404–7. 10.1007/s10593-020-02675-8.Suche in Google Scholar
[18] Avila-Ortiz CG, Juaristi E. Novel methodologies for chemical activation in organic synthesis under solvent-free reaction conditions. Molecules. 2020;25:3579. 10.3390/molecules25163579.Suche in Google Scholar PubMed PubMed Central
[19] Lambat TL, Chopra PKPG, Mahmood SH. Microwave: a green contrivance for the synthesis of N-heterocyclic compounds. Curr Org Chem. 2020;24:2527–54. 10.2174/1385272824999200622114919.Suche in Google Scholar
[20] Priyanka M, Garg SL. Benefits of microwave-assisted organic synthesis over conventional methods in synthetic chemistry. Res J Chem Env. 2019;23(4):103–8.Suche in Google Scholar
[21] Banik BK. Microwave-induced organic reactions toward biologically active molecules. Curr Med Chem. 2019;26(24):4492–4. 10.2174/092986732624190927114808.Suche in Google Scholar PubMed
[22] Mahato A, Sahoo BM, Banik BK. Microwave-assisted synthesis: paradigm of green chemistry. J Indian Chem Soc. 2018;95:1–13.Suche in Google Scholar
[23] Kumari K, Vishvakarma VK, Singh P, Patel R, Chandra R. Microwave: an important and efficient tool for the synthesis of biological potent organic compounds. Curr Med Chem. 2017;24(41):4579–95. 10.2174/0929867324666170529100929.Suche in Google Scholar PubMed
[24] Kokel A, Schäfer C, Török B. Application of microwave-assisted heterogeneous catalysis in sustainable synthesis design. Green Chem. 2017;19:3729–51. 10.1039/C7GC01393K.Suche in Google Scholar
[25] Gupta M, Paul S, Gupta R. General characteristics and applications of microwaves in organic synthesis. Acta Chim Slovenica. 2009;56:749–64. 10.1002/CHIN.201020219.Suche in Google Scholar
[26] Strauss CR, Varma RS. Microwaves in green and sustainable chemistry. Top Curr Chem. 2006;266:199–231. 10.1007/128_060.Suche in Google Scholar
[27] El Ashry ESH, Kassem AA. Account of microwave irradiation for accelerating organic reactions. Arkivoc. 2006;9:1–16. 10.3998/ark.5550190.0007.901.Suche in Google Scholar
[28] Roberts BA, Strauss CR. Toward rapid, “green,” predictable microwave-assisted synthesis. Acc Chem Res. 2005;38:653–61. 10.1021/ar040278m.Suche in Google Scholar
[29] Bamoharram FF, Heravi MM, Ebrahimi J, Ahmadpour A, Zebarjad M. Catalytic performance of nano-SiO2-supported Preyssler heteropolyacid in esterification of salicylic acid with aliphatic and benzylic alcohols. Chin J Catal. 2011;32:782–8. 10.1016/S1872-2067(10)60219-7.Suche in Google Scholar
[30] Zonoz FM, Tayebee R. Synthesis of some alkyl acetates from alcohols catalyzed by H5PW10V2O40 and H5PMo10V2O40 under microwave irradiation (Part 2). J Korean Chem Soc. 2011;55:541–5. 10.5012/jkcs.2011.55.3.541.Suche in Google Scholar
[31] Rezaei-Seresht E, Zonoz FM, Estiri M, Tayebee R. Microwave-assisted solvent-free acetylation of some alcohols catalyzed by Keggin type heteropoly acids. Ind Eng Chem Res. 2011;50:1837–46. 10.1021/ie101641t.Suche in Google Scholar
[32] Duan X, Sun G, Sun Z, Li J, Wang S, Wang X, et al. A heteropolyacid-based ionic liquid as a thermoregulated and environmentally friendly catalyst in esterification reaction under microwave assistance. Catal Commun. 2013;42:125–8. 10.1016/j.catcom.2013.08.014.Suche in Google Scholar
[33] Bamoharram FF, Heravi MM, Roshani M, Jalal S, Rashki N. Catalytic performance of preyssler anion in selective oximation of aromatic aldehydes. Asian J Chem. 2010;22:4421–5.Suche in Google Scholar
[34] Ighilahriz-Boubchir K, Boutemeur-Kheddis B, Rabia C, Makhloufi-Chebli M, Hamdi M, Silva AMS. Recyclable Keggin heteropolyacids as an environmentally benign catalyst for the synthesis of new 2-benzoylamino-n-phenyl-benzamide derivatives under microwave irradiations at solvent-free conditions and the evaluation of biological activity. Molecules. 2018;23:8. 10.3390/molecules23010008.Suche in Google Scholar PubMed PubMed Central
[35] Liu J, Liu Y, Yang W, Guo H, Fang F, Tang Z. Immobilization of phosphortungstic acid on amino-functionalized bimetallic Zr-La-SBA-15 and its highly catalytic performance for acetylation. J Mol Catal A Chem. 2014;393:1–7. 10.1016/j.molcata.2014.04.011.Suche in Google Scholar
[36] Ping C, Yuchun Z. Synthesis of p-Methoxyacetophenone with phosphotungstic acid catalyst. Petrochem Technol. 2007;36(9):919–23.Suche in Google Scholar
[37] Amini MM, Shaabani A, Bazgir A. Tungstophosphoric acid (H3PW12O40): an efficient and eco-friendly catalyst for the one-pot synthesis of dihydropyrimidin-2(1H)-ones. Catal Commun. 2006;7:843–7. 10.1016/j.catcom.2006.02.027.Suche in Google Scholar
[38] Kappe CO. Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog. Acc Chem Res. 2000;33(12):879–88. 10.1021/ar000048h.Suche in Google Scholar PubMed
[39] Jetti SR, Verma D, Jain S. Microwave-assisted synthesis of spirofused heterocycles using decatungstodivanadogermanic heteropoly acid as a novel and reusable heterogeneous catalyst under solvent-free conditions. J Catal. 2013;2:2013–8. 10.1155/2013/392162.Suche in Google Scholar
[40] Portilla-Zuñiga OM, Sathicq ÁG, Martínez JJ, Fernandes SA, Rezende TR, Romanelli GP. Synthesis of Biginelli adducts using a Preyssler heteropolyacid in silica matrix from biomass building block. Sustain Chem Pharm. 2018;10:50–5. 10.1016/j.scp.2018.09.002.Suche in Google Scholar
[41] Khaldi-Khellafi N, Makhloufi-Chebli M, Oukacha-Hikem D, Bouaziz ST, Lamara KO, Idir T, et al. Green synthesis, antioxidant and antibacterial activities of 4-aryl-3, 4-dihydropyrimidinones/thiones derivatives of curcumin. Theoretical calculations and mechanism study. J Mol Struct. 2019;1181:261–9. 10.1016/j.molstruc.2018.12.104.Suche in Google Scholar
[42] Sadjadi S, Heravi MM, Daraie M. Heteropolyacid supported on amine-functionalized halloysite nano clay as an efficient catalyst for the synthesis of pyrazolopyranopyrimidines via four-component domino reaction. Res Chem Intermed. 2017;43(4):2201–14. 10.1007/s11164-016-2756-8.Suche in Google Scholar
[43] Heravi MM, Rajabzadeh G, Bamoharram FF, Seifi N. An eco-friendly catalytic route for synthesis of 4-amino-pyrazolo [3,4-d] pyrimidine derivatives by Keggin heteropolyacids under classical heating and microwave irradiation. J Mol Catal A: Chem. 2006;256(1–2):238–41. 10.1016/j.molcata.2006.04.016.Suche in Google Scholar
[44] Bennardi DO, Blanco MN, Pizzio LR, Autino JC, Romanelli GP. An efficient and green catalytic method for Friedländer quinoline synthesis using tungstophosphoric acid included in a polymeric matrix. Curr Catal. 2015;4(1):65–72. 10.2174/2211544704666150424234036.Suche in Google Scholar
[45] Chaudhuri MK, Hussain S. An efficient synthesis of quinolines under solvent-free conditions. J Chem Sci. 2006;118(2):199–202. 10.1007/BF02708474.Suche in Google Scholar
[46] Sivaprasad G, Rajesh R, Perumal PT. Synthesis of quinaldines and lepidines by a Doebner–Miller reaction under thermal and microwave irradiation conditions using phosphotungstic acid. Tetrahedron Lett. 2006;47(11):1783–5. 10.1016/j.tetlet.2006.01.034.Suche in Google Scholar
[47] Sharif M. Quinazolin-4(3H)-ones: a tangible synthesis protocol via an oxidative olefin bond cleavage using metal-catalyst free conditions. Appl Sci. 2020;10(8):2815. 10.3390/app10082815.Suche in Google Scholar
[48] Ighilahriz K, Boutemeur B, Chami F, Rabia C, Hamdi M, Hamdi SMA. Microwave-assisted and heteropolyacids-catalysed cyclocondensation reaction for the synthesis of 4(3H)-quinazolinones. Molecules. 2008;13(4):779–89. 10.3390/molecules13040779.Suche in Google Scholar PubMed PubMed Central
[49] Javid A, Heravi MM, Bamoharram FF, Nikpour M. One-pot synthesis of tetrasubstituted imidazoles catalyzed by preyssler-type heteropoly acid. E-J Chem. 2011;8:547–52. 10.1155/2011/980546.Suche in Google Scholar
[50] Naureen S, Ijaz F, Munawar MA, Asif N, Chaudhry F, Ashraf M, et al. Synthesis of tetrasubstituted imidazoles containing indole and their antiurease and antioxidant activities. J Chil Chem Soc. 2017;62:3583–7. 10.4067/s0717-97072017000303583.Suche in Google Scholar
[51] Tzani MA, Gabriel C, Lykakis IN. Selective synthesis of benzimidazoles from o-phenylenediamine and aldehydes promoted by supported gold nanoparticles. Nanomaterials. 2020;10(12):2405. 10.3390/nano10122405.Suche in Google Scholar PubMed PubMed Central
[52] Chakrabarty M, Mukherji A, Mukherjee R, Arima S, Harigaya YA. Keggin heteropoly acid as an efficient catalyst for an expeditious, one-pot synthesis of 1-methyl-2-(hetero)arylbenzimidazoles. Tetrahedron Lett. 2007;48:5239–42. 10.1016/j.tetlet.2007.05.144.Suche in Google Scholar
[53] Das PJ, Das J, Ghosh M, Sultana S. Solvent free one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles catalyzed by secondary amine based ionic liquid and defective keggin heteropoly acid. Green Sustain Chem. 2013;3:6–13. 10.4236/gsc.2013.34A002.Suche in Google Scholar
[54] Yan L, Chen Y, Sun X, You M, Chen X, Gu Q, et al. Microwave-assisted solvent-free catalyzed synthesis and luminescence properties of 1,2,4,5-tetrasubstituted imidazoles bearing a 4-aminophenyl substituent. Chem Pap. 2017;71:627–37. 10.1007/s11696-016-0051-1.Suche in Google Scholar
[55] Keri RS, Hosamani KM, Seetharama RHR, Shingalapur RV. Wells–Dawson heteropolyacid: an efficient recyclable catalyst for the synthesis of benzimidazoles under microwave condition. Catal Lett. 2009;131(3):552–9. 10.1007/s10562-009-9966-2.Suche in Google Scholar
[56] Annunziata F, Pinna C, Dallavalle S, Tamborini L, Pinto A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int J Mol Sci. 2020;21(13):4618. 10.3390/ijms21134618.Suche in Google Scholar PubMed PubMed Central
[57] Romanelli GP, Bennardi D, Ruiz DM, Baronetti G, Thomas HJ, Autino JC. A solvent-free synthesis of coumarins using a Wells–Dawson heteropolyacid as catalyst. Tetrahedron Lett. 2004;45(48):8935–9. 10.1016/j.tetlet.2004.09.183.Suche in Google Scholar
[58] Bennardi DO, Ruiz DM, Romanelli GP, Baronetti GT, Thomas HJ, Autino JC. Efficient microwave solvent-free synthesis of flavones, chromones, coumarins and dihydrocoumarins. Lett Org Chem. 2008;5(8):607–15. 10.2174/157017808786857570.Suche in Google Scholar
[59] Bennini-Amroun L, Mazari-Hachi T, Bouaziz-Terrachet S, Makhloufi-Chebli M, Rabia C. Keggin-type polyoxometalates as efficient catalysts for the synthesis of 4-methylcoumarins in solvent-free conditions, under conventional heating and microwave irradiations: Theoretical calculations and mechanism studies. Chem Data Coll. 2020;28:100436. 10.1016/j.cdc.2020.100436.Suche in Google Scholar
[60] Torviso R, Mansilla D, Belizán A, Alesso E, Moltrasio G, Vázquez P, et al. Catalytic activity of Keggin heteropolycompounds in the Pechmann reaction. Appl Catal A: Gen. 2008;339(1):53–60. 10.1016/j.apcata.2008.01.020.Suche in Google Scholar
[61] Ruiz DM, Autino JC, Romanelli GP. A solvent-free method for synthesis of dihydroangelicins using microwaves. Curr Green Chem. 2017;3(3):242–7. 10.2174/2213346104666170224101037.Suche in Google Scholar
[62] Atanasov AG, Zotchev SB, Dirsch VM, International Natural Product Sciences Taskforce, Supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20(3):200–16. 10.1038/s41573-020-00114-z.Suche in Google Scholar PubMed PubMed Central
[63] Gao X, Liu J, Zuo X, Feng X, Gao Y. Recent advances in synthesis of benzothiazole compounds related to green chemistry. Molecules. 2020;25(7):1675. 10.3390/molecules25071675.Suche in Google Scholar PubMed PubMed Central
[64] Khoobi M, Ramazani A, Foroumadi AR, Hamadi H, Hojjati Z, Shafiee A. Efficient microwave-assisted synthesis of 3-benzothiazolo and 3-benzothiazolino coumarin derivatives catalyzed by heteropoly acids. JICS. 2011;8(4):1036–42. 10.1007/BF03246560.Suche in Google Scholar
[65] Motamedi R, Sobhani S, Barani F. H3PW12O40 as an efficient catalyst for one-pot-tricomponent synthesis of chromeno[4,3-b] quinolones under microwave irradiation. Q J Iran Chem Commun. 2017;5(3):237–363.Suche in Google Scholar
[66] Mohammadi Z, Badiei AR, Azizi M. The one-pot synthesis of 14-aryl-14H-dibenzo[a,j]xanthene derivatives using sulfonic acid functionalized silica (SiO2-Pr-SO3H) under solvent free conditions. Sci Iran. 2011;18(3):453–7. 10.1016/j.scient.2011.05.008.Suche in Google Scholar
[67] Moghadam M, Tangestaninejad S, Mirkhani V, Mohammadpoor-Baltork I, Moosavifar M. Host (nanocavity of dealuminated zeolite-Y)-guest (12-molybdophosphoric acid) nanocomposite material: An efficient and reusable catalyst for synthesis of 14-substituted-14-H-dibenzo[a,j] xanthenes under thermal and microwave irradiation conditions. C R Chim. 2011;14(5):489–95. 10.1016/j.crci.2010.10.006.Suche in Google Scholar
[68] Bennardi DO, Romanelli GP, Sathicq AG, Autino JC, Baronetti GT, Thomas HJ. Wells–Dawson heteropolyacid as reusable catalyst for sustainable synthesis of flavones. Appl Catal A Gen. 2011;404(1–2):68–73. 10.1016/j.apcata.2011.07.011.Suche in Google Scholar
[69] Fernandes SA, Natalino R, Rodrigues Gazolla PA, da Silva MJ, Jham JN. p-Sulfonic acid calix[n]arenes as homogeneous and recyclable organocatalysts for esterification reactions. Tetrahedron Lett. 2012;53:1630–3. 10.1016/j.tetlet.2012.01.078.Suche in Google Scholar
[70] Hedidi M, Hamdi SM, Mazari T, Boutemeur B, Rabia C, Chemat F, et al. Microwave-assisted synthesis of calix[4]resorcinarenes. Tetrahedron. 2006;62(24):5652–5. 10.1016/j.tet.2006.03.095.Suche in Google Scholar
[71] Lancaster M. Green Chemistry: An Introductory Text. Royal Society of Chemistry. Cambridge, UK: RSC Publishing; 2020. ISBN 9781839162947.10.1039/9781839168888Suche in Google Scholar
[72] Marcì G, García-López EI, Palmisano L. Heteropolyacid-based materials as heterogeneous photocatalysts. Eur J Inorg Chem. 2014;1:21–35. 10.1002/ejic.201300883.Suche in Google Scholar
[73] Liu S, Shao S, Qin H, Guo Y. Application of Peroxo-heteropolyacids (salts) in catalytic organic reaction. Chem Bull/Huaxue Tongbao. 2012;75:239–44.Suche in Google Scholar
[74] Misono M. Recent topics in heterogeneous catalysis of heteropolyacids. Kor J Chem Eng. 1997;14(11):427–31. 10.1007/BF02706587.Suche in Google Scholar
[75] Jansen RJJ, van Veldhuizen HM, Schwegler MA, van Bekkum H. Recent (1987‐1993) developments in heteropolyacid catalysts in acid catalyzed reactions and oxidation catalysis. Recl Trav Chim Pays‐Bas. 1994;113:115–35. 10.1002/recl.19941130302.Suche in Google Scholar
[76] Yu X, De Waele V, Löfberg A, Ordomsky V, Khodakov AY. Selective photocatalytic conversion of methane into carbon monoxide over zinc-heteropolyacid-titania nanocomposites. Nat Commun. 2019;10:700. 10.1038/s41467-019-08525-2.Suche in Google Scholar PubMed PubMed Central
[77] Chafran LS, Paiva MF, Freitas JOC, Sales MJA, Dias SCL, Dias JA. Preparation of PLA blends by polycondensation of D,L-lactic acid using supported 12-tungstophosphoric acid as a heterogeneous catalyst. Heliyon. 2019;5(5):e01810. 10.1016/j.heliyon.2019.e01810.Suche in Google Scholar PubMed PubMed Central
[78] Villabrille P, Romanelli G, Vázquez P, Cáceres C. Vanadium-substituted Keggin heteropolycompounds as catalysts for ecofriendly liquid phase oxidation of 2,6-dimethylphenol to 2,6-dimethyl-1,4-benzoquinone. Appl Catal A Gen. 2004;270(1–2):101–11. 10.1016/j.apcata.2004.04.028.Suche in Google Scholar
[79] Romanelli GP, Vazquez PG, Tundo P. New heteropolyacids as catalysts for the selective oxidation of sulfides to sulfoxides with hydrogen peroxide. Synlett. 2005;2005(1):75–8. 10.1055/s-2004-837195.Suche in Google Scholar
[80] Sathicq A, Romanelli G, Palermo V, Vázquez P, Thomas H. Heterocyclic amine salts of Keggin heteropolyacids used as catalyst for the selective oxidation of sulfides to sulfoxides. Tetrahedron Lett. 2008;49(9):1441–4. 10.1016/j.tetlet.2008.01.009.Suche in Google Scholar
[81] Egusquiza G, Romanelli G, Cabello C, Botto C, Thomas H. Arene and Phenol oxidation with hydrogen peroxide using “sandwich” type substituted polyoxometalates as catalysts. Catal Commun. 2008;9(1):45–50. 10.1016/j.catcom.2007.04.026.Suche in Google Scholar
[82] Villabrille P, Romanelli G, Vázquez P, Cáceres C. Supported heteropolycompounds as ecofriendly catalysts for 2,6-dimethylphenol oxidation to 2,6-dimethyl-1,4-benzoquinone. Appl Catal A Gen. 2008;334(1–2):374–88. 10.1016/j.apcata.2007.10.025.Suche in Google Scholar
[83] Tundo P, Romanelli G, Vázquez P, Loris P, Aricó F. Multiphase oxidation of aniline to nitrosobenzene with hydrogen peroxide catalyzed by heteropolyacids. Synlett. 2008;2008:667–70. 10.1055/s-2008-1072502.Suche in Google Scholar
[84] Palacio M, Villabrille P, Romanelli G, Vázquez P, Cáceres C. Ecofriendly liquid phase oxidation with hydrogen peroxide of 2,6-dimethylphenol to 2,6-dimethyl-1,4-benzoquinone catalyzed by TiO2-CeO2 mixed xerogels. Appl Catal A Gen. 2009;359(1–2):62–8. 10.1016/j.apcata.2009.02.032.Suche in Google Scholar
[85] Palermo V, Vázquez P, Romanelli G. Simple and friendly sulfones synthesis using aqueous hydrogen peroxide with reusable Keggin molibdenun heteropolyacid immobilized on aminopropyl-functionalized silica. Phosphorus Sulfur Silicon Relat Elem. 2009;184(12):3258–68. 10.1080/10426500903299885.Suche in Google Scholar
[86] Chatel G, Monnier C, Kardos N, Voiron C, Andrioletti B, Draye M. Green, selective and swift oxidation of cyclic alcohols to corresponding ketones. Appl Catal A Gen. 2014;478:157–64. 10.1016/j.apcata.2014.03.033.Suche in Google Scholar
[87] Liu T, Hou JH. Direct hydroxylation of benzene to phenol over pyridine-modified vanadium-substituted heteropoly acid under microwave condition. Asian J Chem. 2014;26:2683–85. 10.14233/ajchem.2014.15799.Suche in Google Scholar
[88] Bamoharram FF, Heravi MM, Omidinia R, Tavakoli-Hoseini N. Tetrabutylammoniumhexatungstate (VI), [(n-C4H9)4N]2[W6O19]: As a green and recylable isopolyanion catalyst in oxidation of benzaldehydes. Synth React Inorg Metal-Org Nano-Metal Chem. 2013;43(2):125–30. 10.1080/15533174.2012.680122.Suche in Google Scholar
[89] Bamoharram FF, Alizadeh MH, Razavi H, Moghayadi M. Novel oxidation of aromatic aldehydes catalyzed by Preyssler’s anion, [NaP5W30O110]14−. J Braz Chem Soc. 2006;17(3):505–09. 10.1590/S0103-50532006000300011.Suche in Google Scholar
[90] Bamoharram FF, Heravi MM, Teymouri H, Zebarjad M, Ahmadpour A. Preyssler heteropolyacid supported on nano-SiO2: A green and reusable catalyst in selective oxidation of benzyl alcohols to benzaldehydes. Synth React Inorg Metal-Org Nano-Metal Chem. 2011;41:1221–8. 10.1080/15533174.2011.591873.Suche in Google Scholar
[91] Idrissou Y, Mouanni S, Amitouche D, Mazari T, Marchal-Roch C, Rabia C. Cyclohexanone oxidation over H3PMo12O40 heteropolyacid via two activation modes microwave irradiation and conventional method. Bull Chem React Eng Catal. 2019;14(2):427–35. 10.9767/bcrec.14.2.3054.427-435.Suche in Google Scholar
[92] Cao X, Ren J, Xu C, Xie B, Sun D. Clean oxidation of cyclohexanone to adipic acid catalyzed by ammonium phosphomolybdate with Dawson structure under microwave irradiation. Adv Mater Res. 2012;550–553:170–4. 10.4028/www.scientific.net/AMR.550-553.170.Suche in Google Scholar
[93] Qasim HM, Ayass WW, Donfack P, Mougharbel AS, Bhattacharya S, Nisar T, et al. Peroxo-Cerium(IV)-containing polyoxometalates: [CeIV6(O2)9(GeW10O37)3]24−, a recyclable homogeneous oxidation catalyst. Inorg Chem. 2019;58(17):11300–7. 10.1021/acs.inorgchem.9b01164.Suche in Google Scholar PubMed
[94] Carraro M, Sandei L, Sartorel A, Scorrano G, Bonchio M. Hybrid polyoxotungstates as second-generation POM-based catalysts for microwave-assisted H2O2 activation. Org Lett. 2006;8(17):3671–4. 10.1021/ol061197o.Suche in Google Scholar PubMed
[95] Bonchio M, Carraro M, Scorrano G, Kortz U. Microwave-assisted fast cyclohexane oxygenation catalyzed by iron-substituted polyoxotungstates. Adv Synth Catal. 2005;347(15):1909–12. 10.1002/adsc.200505111.Suche in Google Scholar
[96] Bonchio M, Carraro M, Sartorel A, Scorrano G, Kortz U. Bio-inspired oxidations with polyoxometalate catalysts. J Mol Catal A Chem. 2006;251(1–2):93–9. 10.1016/j.molcata.2006.02.034.Suche in Google Scholar
[97] Galiano F, Mancuso R, Carraro M, Bundschuh J, Hoinkis J, Bonchio M, et al. A polyoxometalate-based self-cleaning smart material with oxygenic activity for water remediation with membrane technology. Appl Mater Today. 2021;21:101002. 10.1016/j.apmt.2021.101002.Suche in Google Scholar
[98] De Luca G, Bisignano F, Figoli A, Galiano F, Furia E, Mancuso R, et al. Bromide ion exchange with a Keggin polyoxometalate on functionalized polymeric membranes: a theoretical and experimental study. J Phys Chem B. 2014;18:2396–404. 10.1021/jp411401v.Suche in Google Scholar PubMed
[99] Rodríguez-Padrón D, Balu AM, Romero AA, Luque R. Chapter two – biomass valorization: catalytic approaches using benign-by-design nanomaterials. Adv Inorg Chem. 2021;77:27–58. 10.1016/bs.adioch.2020.12.003.Suche in Google Scholar
[100] Luque R. Benign-by-design catalysts and processes for biomass conversion. Curr Opin Green Sustain Chem. 2016;2:6–9. 10.1016/j.cogsc.2016.09.004.Suche in Google Scholar
[101] Zhang Y, Zhao M, Wang H, Hu H, Liu R, Huang Z, et al. Damaged starch derived carbon foam-supported heteropolyacid for catalytic conversion of cellulose: improved catalytic performance and efficient reusability. Bioresour Technol. 2019;288:121532. 10.1016/j.biortech.2019.121532.Suche in Google Scholar PubMed
[102] Almohalla M, Rodríguez-Ramos I, Ribeiro LS, Órfão JJM, Pereira MFR, Guerrero-Ruiz A. Cooperative action of heteropolyacids and carbon supported Ru catalysts for the conversion of cellulose. Catal Today. 2018;301:65–71. 10.1016/j.cattod.2017.05.023.Suche in Google Scholar
[103] Zhang X, Zhang X, Sun N, Wang S, Wang X, Jiang Z. High production of levulinic acid from cellulosic feedstocks being catalyzed by temperature-responsive transition metal substituted heteropolyacids. Renew Energy. 2019;141:802–13. 10.1016/j.renene.2019.04.058.Suche in Google Scholar
[104] Zhang X, Zhang H, Li Y, Bawa M, Wang S, Wang X, et al. First triple-functional polyoxometalate Cs10.6[H2.4GeNb13O41] for highly selective production of methyl levulinate directly from cellulose. Cellulose. 2018;25:6405–19. 10.1007/s10570-018-2037-3.Suche in Google Scholar
[105] Zhang X, Li Y, Xue L, Wang S, Wang X, Jiang Z. Catalyzing cascade production of methyl levulinate from polysaccharides using heteropolyacids HnPW11Mo39 with Brønsted/Lewis acidic sites. ACS Sustain Chem Eng. 2018;681(1):165–76. 10.1021/acssuschemeng.7b02042.Suche in Google Scholar
[106] Quereshi S, Ahmad E, Pant KK, Dutta S. Synthesis and characterization of zirconia supported silicotungstic acid for ethyl levulinate production. Ind Eng Chem Res. 2019;58(35):16045–54. 10.1021/acs.iecr.9b01659.Suche in Google Scholar
[107] Gedanken A. Ultrasonic processing to produce nanoparticles. In: Buschow KHJ, [and others], Encyclopedia of materials: science and technology. 2nd edn. Amsterdam, New York: Elsevier; 2001. p. 9450–6. ISBN: 9780080431529.10.1016/B0-08-043152-6/01708-3Suche in Google Scholar
[108] Gong C, Hart DP. Ultrasound induced cavitation and sonochemical yields. J Acoust Soc Am. 1998;104:1–16.10.1121/1.423851Suche in Google Scholar
[109] Gawande MB, Bonifácio VDB, Luque R, Branco PS, Varma RS. Solvent-free and catalysts-free chemistry: a benign pathway to sustainability. Chem Sus Chem. 2014;7(1):24–44. 10.1002/cssc.201300485.Suche in Google Scholar PubMed
[110] Draye M, Chatel G, Duwald R. Ultrasound for drug synthesis: a green approach. Pharmaceuticals. 2020;13:23. 10.3390/ph13020023.Suche in Google Scholar PubMed PubMed Central
[111] Sharma A, Wakode S, Sharma S, Fayaz F, Pottoo FH. Methods and strategies used in green chemistry: a review. Curr Org Chem. 2020;24(22):2555–65. 10.2174/1385272824999200802025233.Suche in Google Scholar
[112] Sathishkumar P, Mangalaraja RV, Anandan S. Review on the recent improvements in sonochemical and combined sonochemical oxidation processes–a powerful tool for destruction of environmental contaminants. Renew Sustain Energ Rev. 2016;55:426–54. 10.1016/j.rser.2015.10.139.Suche in Google Scholar
[113] Kaur N. Ultrasound-assisted green synthesis of five-membered O- and S-heterocycles. Synth Commun. 2018;48(14):1715–38. 10.1080/00397911.2018.146067.Suche in Google Scholar
[114] Lupacchini M, Mascitti A, Giachi G, Tonucci L, d'Alessandro N, Martinez J, et al. Sonochemistry in non-conventional, green solvents or solvent-free reactions. Tetrahedron. 2017;73(6):609–53. 10.1016/j.tet.2016.12.014.Suche in Google Scholar
[115] Banerjee B. Recent developments on ultrasound-assisted organic synthesis in aqueous medium. J Serb Chem Soc. 2017;82:755–90. 10.2298/JSC170217057B.Suche in Google Scholar
[116] Draye M, Kardos N. Advances in green organic sonochemistry. In: Colmenares J, Chatel G, editors. Sonochemistry. Topics in current chemistry collections. Cham: Springer; 2017. 29–57. 10.1007/978-3-319-54271-3_2.Suche in Google Scholar
[117] Chatel G, Macfarlane DR. Ionic liquids and ultrasound in combination: Synergies and challenges. Chem Soc Rev. 2014;43:8132–49. 10.1039/C4CS00193A.Suche in Google Scholar PubMed
[118] Puri S, Kaur B, Parmar A, Kumar H. Applications of ultrasound in organic synthesis – a green approach. Curr Org Chem. 2013;17(16):1790–828. 10.2174/13852728113179990018.Suche in Google Scholar
[119] Kharissova OV, Kharisov BI, Oliva González CM, Peña Méndez Y, López I. Greener synthesis of chemical compounds and materials. R Soc Open Sci. 2019;6(11):191378. 10.1098/rsos.191378.Suche in Google Scholar PubMed PubMed Central
[120] Colmenares JC, Kuna E, Lisowski P. Synthesis of photoactive materials by sonication: application in photocatalysis and solar cells. In: Colmenares J, Chatel G, editors. Sonochemistry. Topics in current chemistry collections. Vol. 374, Cham: Springer; 2016. p. 59. https://doi.org/10.1007/978-3-319-54271-3_4. 10.1007%2Fs41061-016-0062-y.Suche in Google Scholar
[121] Li Z, Zhuang T, Dong J, Wang L, Xia J, Wang H, et al. Sonochemical fabrication of inorganic nanoparticles for applications in catalysis. Ultrason Sonochem. 2021;71:105384. 10.1016/j.ultsonch.2020.105384.Suche in Google Scholar PubMed PubMed Central
[122] Ai HM, Liu Q. Ultrasound-assisted synthesis of acylals catalyzed by stannum (IV) phosphomolybdate under solvent-free condition. J Chem Soc Pak. 2012;34:299–301.Suche in Google Scholar
[123] Chen X, Wang J, Han Y, Lu XP, Han PF. Ultrasound assisted heteropoly acid catalyst SiW12/SiO2 for synthesis of benzyl acetate. Asian J Chem. 2013;25(2):623–7. 10.14233/ajchem.2013.11621.Suche in Google Scholar
[124] Hamidian H. Rapid and efficient synthesis of hydroxytriarylmethanes under ultrasonic irradiation using keggin heteropolyacids and preyssler catalysts in green conditions. Org Chem Int. 2013;2013:502343–5. 10.1155/2013/502343.Suche in Google Scholar
[125] Patil P, Sethy SP, Sameena T, Shailaja K. Pyridine and its biological activity: a review. Asian J Res Chem. 2013;6(10):888–99. 10.5958/0974-4150.Suche in Google Scholar
[126] Dastjerdi NM, Ghanbari M. Ultrasound-promoted green approach for the synthesis of multisubstituted pyridines using stable and reusable SBA-15@ADMPT/H5PW10V2O40 nanocatalyst at room temperature. Green Chem Lett Rev. 2020;13(3):192–205. 10.1080/17518253.2020.1797183.Suche in Google Scholar
[127] Khalifeh R, Ghamari MA. Multicomponent synthesis of 2-amino-3-cyanopyridine derivatives catalyzed by heterogeneous and recyclable copper nanoparticles on charcoal. J Braz Chem Soc. 2016;27(4):759–68. 10.5935/0103-5053.20150327.Suche in Google Scholar
[128] Chavan LD, Deshmukh SN, Shankarwar SG. A simple and green protocol for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones using 11-Molybdo-1-vanado phosphoric acid as a catalyst under ultrasound irradiation. Orbital: Electron J Chem. 2019;11(5):314–20. 10.17807/orbital.v11i5.1423.Suche in Google Scholar
[129] Sadjadi S, Heravi MM, Malmir M. Heteropolyacid@creatin-halloysite clay: an environmentally friendly, reusable and heterogeneous catalyst for the synthesis of benzopyranopyrimidines. Res Chem Intermed. 2017;43:6701–17. 10.1007/s11164-017-3016-2.Suche in Google Scholar
[130] Song B, Qu X, Zhang L, Han K, Wu D, Xiang C, et al. Synthesis and antitumor properties of bis-indole derivatives. J Chem Pharm Res. 2014;6(10):239–43.Suche in Google Scholar
[131] Rahimi S, Amrollahi MA, Kheilkordi Z. An efficient ultrasound-promoted method for the synthesis of bis(indole) derivatives. C R Chimie. 2015;18(5):558–63. 10.1016/j.crci.2014.10.005.Suche in Google Scholar
[132] Samadizadeh M. H3PW12O40: an efficient catalyst for the synthesis of spirooxindoles under ultrasound irradiation. Asian J Chem. 2013;25(12):6619–21. 10.14233/ajchem.2013.14390.Suche in Google Scholar
[133] Javanshir S, Ohanian A, Heravi MM, Naimi-Jamal MR, Bamoharram FF. Ultrasound-promoted, rapid, green, one-pot synthesis of 2′-aminobenzothiazolomethylnaphthols via a multi-component reaction, catalyzed by heteropolyacid in aqueous media. J Saudi Chem Soc. 2014;18(5):502–6. 10.1016/j.jscs.2011.10.013.Suche in Google Scholar
[134] Srivastava SD, Sen JP. Synthesis and biological evaluation of 2-aminobenzothiazole derivatives. Indian J Chem. 2008;47B:1583–6.10.1002/chin.200906147Suche in Google Scholar
[135] Bougheloum C, Alioua S, Belghiche R, Benali N, Messalhi A. An efficient green synthesis of new benzothiazoles containing sulfonamide or cyclic imide moieties. J Heterocycl Chem. 2020;57:120–31. 10.1002/jhet.3753.Suche in Google Scholar
[136] Heravi MM, Sadjadi S, Sadjadi S, Oskooie HA, Bamoharram FF. Rapid and efficient synthesis of 4(3H)-quinazolinones under ultrasonic irradiation using silica-supported Preyssler nano particles. Ultrason Sonochem. 2009;16(6):708–10. 10.1016/j.ultsonch.2009.02.010.Suche in Google Scholar PubMed
[137] Azarifar D, Khatami SM, Nejat-Yami R. Nano-titania-supported Preyssler-type heteropolyacid: an efficient and reusable catalyst in ultrasound-promoted synthesis of 4H-chromenes and 4H-pyrano [2, 3-c] pyrazoles. J Chem Sci. 2014;126(1):95–101.10.1007/s12039-013-0548-xSuche in Google Scholar
[138] Maleki B, Baghayeri M, Jannat Abadi SA, Tayebee R, Khojastehnezhad A. Ultrasound promoted facile one pot synthesis of highly substituted pyran derivatives catalyzed by silica-coated magnetic NiFe2O4 nanoparticles-supported H14[NaP5W30O110] under mild conditions. RSC Adv. 2016;6(99):96644–61. 10.1039/C6RA20895A.Suche in Google Scholar
[139] Mozafari R, Heidarizadeh F. Phosphotungstic acid supported on SiO2@NHPhNH2 functionalized nanoparticles of MnFe2O4 as a recyclable catalyst for the preparation of tetrahydrobenzo[b]pyran and indazolo [2,1-b] phthalazine-triones. Polyhedron. 2019;162:263–76. 10.1016/j.poly.2019.01.065.Suche in Google Scholar
[140] Bradley M, Ashokkumar M, Grieser F. Sonochemical production of fluorescent and phosphorescent latex particles. J Am Chem Soc. 2003;125(2):525–9. 10.1021/ja0268581.Suche in Google Scholar PubMed
[141] Rahman MM, Lamsal BP. Ultrasound-assisted extraction and modification of plant-based proteins: Impact on physicochemical, functional, and nutritional properties. Compr Rev Food Sci Food Saf. 2021;20:1457–80. 10.1111/1541-4337.12709.Suche in Google Scholar PubMed
[142] Lu X, Qiu W, Peng J, Xu H, Wang D, Cao Y, et al. Review on additives-assisted ultrasound for organic pollutants. Degrad J Hazard Mater. 2021;403:123915. 10.1016/j.jhazmat.2020.123915.Suche in Google Scholar PubMed
[143] Fu X, Belwal T, Cravotto G, Luo Z. Sono-physical and sono-chemical effects of ultrasound: Primary applications in extraction and freezing operations and influence on food components. Ultrason Sonochem. 2020;60:104726. 10.1016/j.ultsonch.2019.104726.Suche in Google Scholar PubMed
[144] Cravotto G, Borretto E, Oliverio M, Procopio A, Penoni A. Organic reactions in water or biphasic aqueous systems under sonochemical conditions. A review on catalytic effects. Catal Commun. 2015;63:2–9. 10.1016/j.catcom.2014.12.014.Suche in Google Scholar
[145] Anandan S, Ponnusamy KV, Ashokkumar M. A review on hybrid techniques for the degradation of organic pollutants in aqueous environment. Ultrason Sonochem. 2020;67:105130. 10.1016/j.ultsonch.2020.105130.Suche in Google Scholar PubMed
[146] Martín-Aranda RM, Calvino-Casilda V. Last decade of research in sonochemistry for green organic synthesis. Recent Pat Chem Eng. 2010;3(2):82–98. 10.2174/2211334711003020082.Suche in Google Scholar
[147] Salavati H, Tangestaninejad S, Moghadam M, Mirkhani V, Mohammadpoor-Baltork I. Sonocatalytic oxidation of olefins catalyzed by heteropolyanion montmorillonite nanocomposite. Ultrason Sonochem. 2010;17(1):145–52. 10.1016/j.ultsonch.2009.05.009.Suche in Google Scholar PubMed
[148] Salavati H, Tangestaninejad S, Moghadam M, Mirkhani V, Mohammadpoor-Baltork I. Sonocatalytic epoxidation of alkenes by vanadium-containing polyphosphomolybdate immobilized on multi-wall carbon nanotubes. Ultrason Sonochem. 2010;17(2):453–545. 10.1016/j.ultsonch.2009.09.011.Suche in Google Scholar PubMed
[149] Lin Z-P, Wan L. Synthesis of adipic acid oxidized by H2O2-silicotungstic acid under ultrasonication. Asian J Chem. 2013;25:6008–10. 10.14233/ajchem.2013.14230.Suche in Google Scholar
[150] Azarifar D, Khatami S-M, Najminejad Z. Ultrasound-accelerated selective oxidation of primary aromatic amines to azoxy derivatives with trans-3,5-dihydroperoxy-3,5-dimethyl-1,2-dioxolane catalyzed by Preyssler acid-mediated nano-TiO2. J Iran Chem Soc. 2014;11:587–92. 10.1007/s13738-013-0328-z.Suche in Google Scholar
[151] Rezvani MA, Asli MAN, Abdollahi L, Oveisi M. Nano composite mixed-addenda vanadium substituted polyoxometalate-TiO2 as a green, reusable and efficient catalyst for rapid and efficient synthesis of symmetric disulfides under ultrasound irradiation. Chem Solid Mat. 2014;2(1):41–51.Suche in Google Scholar
[152] Hossain MN, Park HC, Choi HS. Comprehensive review on catalytic oxidative desulfurization of liquid fuel oil. Catalysts. 2019;9:229. 10.3390/catal9030229.Suche in Google Scholar
[153] Wu Z, Ondruschka B. Ultrasound-assisted oxidative desulfurization of liquid fuels and its industrial application. Ultrason Sonochem. 2010;17(6):1027–32. 10.1016/j.ultsonch.2009.11.005.Suche in Google Scholar PubMed
[154] Afzalinia A, Mirzaie A, Nikseresht A, Musabeygi T. Ultrasound-assisted oxidative desulfurization process of liquid fuel by phosphotungstic acid encapsulated in a interpenetrating amine-functionalized Zn(II)-based MOF as catalyst. Ultrason Sonochem. 2017;34:713–20. 10.1016/j.ultsonch.2016.07.006.Suche in Google Scholar PubMed
[155] Liu L, Zhang Y, Tan W. Ultrasound-assisted oxidation of dibenzothiophene with phosphotungstic acid supported on activated carbon. Ultrason Sonochem. 2014;21(3):970–4. 10.1016/j.ultsonch.2013.10.028.Suche in Google Scholar PubMed
[156] Gildo PJ, Dugos N, Roces S, Wan MW. Optimized ultrasound-assisted oxidative desulfurization process of simulated fuels over activated carbon-supported phosphotungstic acid. MATEC Web Conf. 2018;156:03045. 10.1051/matecconf/201815603045.Suche in Google Scholar
[157] Wang R, Zhao Y, Kozhevnikov IV, Zhao J. An ultrasound enhanced catalytic ozonation process for the ultra-deep desulfurization of diesel oil. N J Chem. 2020;44:15467–74. 10.1039/D0NJ03368E.Suche in Google Scholar
[158] Yu GX, Zhong Q, Jin M, Lu P. Ultrasound-assisted oxidative desulfurization of diesel Fuel in H2O2/heteropoly acid/system. Adv Mater Res. 2014;1033–1034:85–9. 10.4028/www.scientific.net/AMR.1033-1034.85.Suche in Google Scholar
[159] Zhao Y, Wang R. Deep desulfurization of diesel oil by ultrasound-assisted catalytic ozonation combined with extraction process. Pet Coal. 2013;55:62–7.Suche in Google Scholar
[160] Thangaraj B, Solomon PR, Muniyandi B, Ranganathan S, Lin L. Catalysis in biodiesel production-a review. Clean Energ. 2019;3(1):2–23. 10.1093/ce/zky020.Suche in Google Scholar
[161] Luo J, Fang Z, Smith Jr , RL. Ultrasound-enhanced conversion of biomass to biofuels. Prog Energ Combust Sci. 2014;41:56–93. 10.1016/j.pecs.2013.11.001.Suche in Google Scholar
[162] Badday AS, Abdullah AZ, Lee KT. Artificial neural network approach for modeling of ultrasound-assisted transesterification process of crude Jatropha oil catalyzed by heteropolyacid based catalyst. Chem Eng Process. 2014;75:31–7. 10.1016/j.cep.2013.10.008.Suche in Google Scholar
[163] Badday AS, Abdullah AZ, Lee KT. Ultrasound-assisted transesterification of crude Jatropha oil using alumina-supported heteropolyacid catalyst. Appl Energy. 2013;105:380–8. 10.1016/j.apenergy.2013.01.028.Suche in Google Scholar
[164] Badday AS, Abdullah AZ, Lee KT. Transesterification of crude Jatropha oil by activated carbon-supported heteropolyacid catalyst in an ultrasound-assisted reactor system. Renew Energy. 2014;62:10–7. 10.1016/j.renene.2013.06.037.Suche in Google Scholar
[165] Badday AS, Abdullah AZ, Lee KT. Ultrasound-assisted transesterification of crude Jatropha oil using cesium doped heteropolyacid catalyst: Interactions between process variables. Energy. 2013;60:283–91. 10.1016/j.energy.2013.08.002.Suche in Google Scholar
[166] Dadhania H, Raval D, Dadhania A. Magnetically separable heteropolyanion based ionic liquid as a heterogeneous catalyst for ultrasound mediated biodiesel production through esterification of fatty acids. Fuel. 2021;296:120673. 10.1016/j.fuel.2021.120673.Suche in Google Scholar
[167] Du B, Chen C, Sun Y, Yang M, Yu M, Liu B, et al. Efficient and controllable ultrasound-assisted depolymerization of organosolv lignin catalyzed to liquid fuels by MCM-41 supported phosphotungstic acid. RSC Adv. 2020;10:31479. 10.1039/D0RA05069E.Suche in Google Scholar
[168] Jia X-C, Li J, Ding Y, Zhang B, Wang N, Wang Y-H. A simple and green protocol for 2H-indazolo[2,1-b]phthalazine-triones using grinding method. J Chem. 2013;2013:634510–5. 10.1155/2013/634510.Suche in Google Scholar
[169] Kaupp G. Organic solid-state reactions with 100% yield. In: Toda F, editor. Organic solid state reactions. Topics in current chemistry. Vol. 254, Berlin, Heidelberg: Springer; 2005. 95–183. 10.1007/b100997.Suche in Google Scholar
[170] Rodríguez B, Bruckmann A, Rantanen T, Bolm C. Solvent-free carbon-carbon bond formations in ball mills. Adv Synth Catal. 2007;349(14–15):2213–33. 10.1002/adsc.200700252.Suche in Google Scholar
[171] Bruckmann A, Krebs A, Bolm C. Organocatalytic reactions: effects of ball milling, microwave and ultrasound irradiation. Green Chem. 2008;10(11):1131–41. 10.1039/B812536H.Suche in Google Scholar
[172] Borah R, Dutta P, Sarma P. Investigation of efficient synthesis of 1,8-dioxo-octahydroxanthene derivatives under solvent-free grinding method. Curr Chem Lett. 2013;2(4):159–66. 10.5267/j.ccl.2013.08.003.Suche in Google Scholar
[173] Bose AK, Pednekar S, Ganguly SN, Chakraborty G, Manhas MS. A simplified green chemistry approach to the Biginelli reaction using “Grindstone Chemistry.” Tetrahedron Lett. 2004;45(45):8351–3. 10.1016/j.tetlet.2004.09.064.Suche in Google Scholar
[174] Kouznetsov VV, Merchán-Arenas DR, Martínez-Bonilla CA, Macías MA, Roussel P, Gauthier GH. Grinding and Milling: two efficient methodologies in the solvent-free phosphomolybdic acid-catalyzed and mechanochemical synthesis of cis-4-Amido-N-yl-2-methyl-tetrahydroquinolines. J Braz Chem Soc. 2016;27(12):2246–55. 10.5935/0103-5053.20160117.Suche in Google Scholar
[175] Wang G-W. Mechanochemical organic synthesis. Chem Soc Rev. 2013;42:7668–700. 10.1039/C3CS35526H.Suche in Google Scholar
[176] Hsu C, Lai C, Chiu S. Using diels–alder reactions to synthesize [2] rotaxanes under solvent-free conditions. Tetrahedron. 2009;65(14):2824–9. 10.1016/j.tet.2008.12.082.Suche in Google Scholar
[177] McKissic KS, Caruso JT, Blair RG, Mack J. Comparison of shaking versus baking: further understanding the energetics of a mechanochemical reaction. Green Chem. 2014;16:1628–32. 10.1039/C3GC41496E.Suche in Google Scholar
[178] Martin LL, Davis L, Klein JT, Nemoto P, Olsen GE, Bores GM, et al. Synthesis and preliminary structure-activity relationships of 1-[(3-fluoro-4-pyridinyl)amino]-3-methyl-1H-indol-5-yl methyl carbamate (P10358), a novel acetylcholinesterase inhibitor. Bioorg Med Chem Lett. 1997;7(2):157–62. 10.1016/S0960-894X(96)00592-6.Suche in Google Scholar
[179] Gharib A, Jahangir M, Roshani M. Efficient catalytic synthesis of primary carbamates using Preyssler heteropolyacids catalyst, H14[NaP5W30O110] under solvent-free and green conditions. 13rd International Conference on Synthesis Organic Chemistry; 2009. p. a00410.3390/ecsoc-13-00164Suche in Google Scholar
[180] Satasia SP, Kalaria PN, Raval DK. Heteropolyanion-based sulfated ionic liquid catalyzed formamides synthesis by grindstone chemistry. J Mol Catal A: Chem. 2014;391:41–7. 10.1016/j.molcata.2014.04.005.Suche in Google Scholar
[181] Li J-T, Sun S-F. Synthesis of diindolylmethanes (DIMs) catalyzed by silicotungstic acid by grinding method. E-J Chem. 2010;7:986980–926. 10.1155/2010/986980.Suche in Google Scholar
[182] Patil MR, Yelamaggad A, Keri RS. A mild, efficient and reusable solid phosphotungstic acid catalyst mediated synthesis of benzoxazole derivatives: a grinding approach. Lett Org Chem. 2016;13(7):474–81. 10.2174/2212717803666160728170600.Suche in Google Scholar
[183] Wang J, Minami E, Asmadi M, Kawamoto H. Thermal degradation of hemicellulose and cellulose in ball-milled cedar and beech. J Wood Sci. 2021;67:32. 10.1186/s10086-021-01962-y.Suche in Google Scholar
[184] Liu X, Yan P, Xu Z, Zhang ZC. The effect of mix-milling with P2O5 on cellulose physicochemical properties responsible for increased glucose yield. Carbohydr Polym. 2021;258:117652. 10.1016/j.carbpol.2021.117652.Suche in Google Scholar PubMed
[185] Wu Y, Ge S, Xia C, Mei C, Kim KH, Cai L, et al. Application of intermittent ball milling to enzymatic hydrolysis for efficient conversion of lignocellulosic biomass into glucose. Renew Sustain Energy Rev. 2020;136:110442. 10.1016/j.rser.2020.110442.Suche in Google Scholar
[186] Asada C, Sasaki Y, Nakamura Y. Production of eco-refinery pulp from moso bamboo using steam treatment followed by milling treatment. Waste Biomass Valoriz. 2020;11:6139–46. 10.1007/s12649-019-00847-y.Suche in Google Scholar
[187] Ribeiro LS, Órfão JJM, Pereira MFR. An overview of the hydrolytic hydrogenation of lignocellulosic biomass using carbon-supported metal catalysts. Mater Today Sustain. 2021;11-12:100058. 10.1016/j.mtsust.2020.100058.Suche in Google Scholar
[188] Xiao Z, Yang Q, Wu X, He Y. Ball milling promotes saccharification of agricultural biomass by heteropolyacid and enzyme: unlock the lignin cage for sugars recovery. Biomass Conv Bioref. 2020. 10.1007/s13399-020-00950-4.Suche in Google Scholar
[189] Levine LH, Richards JT, Coutts JL, Soler R, Maxik F, Wheeler RM. Feasibility of ultraviolet-light-emitting diodes as an alternative light source for photocatalysis. J Air Waste Manag Assoc. 2011;61(9):932–40. 10.1080/10473289.2011.596746.Suche in Google Scholar PubMed
[190] Nikoonahad A, Djahed B, Norzaee S, Eslami H, Derakhshan Z, Miri M, et al. An overview report on the application of heteropoly acids on supporting materials in the photocatalytic degradation of organic pollutants from aqueous solutions. Peer J. 2018;6:e5501. 10.7717/peerj.5501.Suche in Google Scholar PubMed PubMed Central
[191] García-López EI, Marcì G, Palmisano L. Heteropolyacid-based heterogeneous photocatalysts for environmental application. In: Colmenares J, Xu YJ, editors. Heterogeneous photocatalysis. Green chemistry and sustainable technology. Berlin, Heidelberg: Springer; 2015. p. 63–107. 10.1007/978-3-662-48719-8_3.Suche in Google Scholar
[192] Taghavi M, Ehrampoush MH, Ghaneian MT, Tabatabaee M, Fakhri Y. Application of a Keggin-type heteropoly acid on supporting nanoparticles in photocatalytic degradation of organic pollutants in aqueous solutions. J Clean Prod. 2018;197:1447–53. 10.1016/j.jclepro.2018.06.280.Suche in Google Scholar
[193] Rezaei Ghalebi H, Aber S, Karimi A. Keggin type of cesium phosphomolybdate synthesized via solid-state reaction as an efficient catalyst for the photodegradation of a dye pollutant in aqueous phase. J Mol Catal A: Chem. 2016;415:96–103. 10.1016/j.molcata.2016.01.031.Suche in Google Scholar
[194] Marcì G, García-Lõpez EI, Palmisano L. Heteropolyacid-based materials as heterogeneous photocatalysts. Eur J Inorg Chem. 2014;2014:21–35. 10.1002/ejic.201300883.Suche in Google Scholar
[195] Michelin C, Hoffmann N. Photocatalysis applied to organic synthesis – a green chemistry approach . Curr Opin Green Sustain Chem. 2018;10:40–5. 10.1016/j.cogsc.2018.02.009.Suche in Google Scholar
[196] Reischauer S, Pieber B. Emerging concepts in photocatalytic organic synthesis. iScience. 2021;24:102209. 10.1016/j.isci.2021.102209.Suche in Google Scholar
[197] Liu M, Peng T, Li H, Zhao L, Sang Y, Feng Q, et al. Photoresponsive nanostructure assisted green synthesis of organics and polymers. Appl Catal B: Env. 2019;249:172–210. 10.1016/j.apcatb.2019.02.071.Suche in Google Scholar
[198] Radhika NP, Selvin R, Kakkar R, Umar A. Recent advances in nano-photocatalysts for organic synthesis. Arab J Chem. 2019;12:4550–78. 10.1016/j.arabjc.2016.07.007.Suche in Google Scholar
[199] Nomiya K, Sugie Y, Miyazaki T, Miwa M. Catalysis by heteropolyacids-ix. Photocatalytic oxidation of isopropyl alcohol to acetone under oxygen using tetrabutylammonium decatungstate. Polyhedron. 1986;5(7):1267–71. 10.1016/S0277-5387(00)83470-1.Suche in Google Scholar
[200] Wu L, An S, Song Y-F. Immobilized graphitic carbon nitride: highly efficient photo oxidation of benzyl alcohol in the aqueous phase. Eng. 2021;7(1):94–102. 10.1016/j.eng.2020.07.025.Suche in Google Scholar
[201] García-López EI, Pomilla FR, Krivtsov I, Serrano A, Liotta LF, Villar-Rodil S, et al. Heteropolyacids supported on boron nitride and carbon nitride for catalytic and catalytic photo-assisted alcohol dehydration. Catal Today. 2021;380:209–22. 10.1016/j.cattod.2021.01.015.Suche in Google Scholar
[202] García‐López EI, Marcì G, Pomilla FR, Liotta LF, Megna B, Paganini MC, et al. Improved (Photo)catalytic propene hydration in a gas/solid system by using heteropolyacid/oxide composites: electron paramagnetic resonance, acidity, and role of water. Eur J Inorg Chem. 2017;13:1900–7. 10.1002/ejic.201601396.Suche in Google Scholar
[203] Marcì G, García-López E, Vaiano V, Sarno G, Sannino D, Palmisano L. Keggin heteropolyacids supported on TiO2 used in gas-solid (photo)catalytic propene hydration and in liquid-solid photocatalytic glycerol dehydration. Catal Today. 2017;281:60–70. 10.1016/j.cattod.2016.04.037.Suche in Google Scholar
[204] Bamoharram FF, Heravi MM, Mehdizadeh S. Preyssler anion as a green and eco-friendly catalyst for photocatalytic oxidation of aromatic aldehydes. Synth React Inorg Metal-Org Nano-Metal Chem. 2009;39(10):746–50. 10.1080/15533170903433287.Suche in Google Scholar
[205] Bamoharram FF, Heravi MM, Heravi HM, Dehghan M. Photocatalytic oxidation of benzyl alcohols in the presence of H14[NaP5W30O110] as a green and reusable catalyst. Synth React Inorg Metal-Org Nano-Metal Chem. 2009;39(7):394–9. 10.1080/15533170903129745.Suche in Google Scholar
© 2022 Diego M. Ruiz et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Artikel in diesem Heft
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

