Abstract
In this paper, the Cu-0.52Ag-0.22Cr alloy was prepared by hot horizontal continuous casting. The effects of aging process on micro-hardness, electrical conductivity, and nanoscale precipitates of Cu-0.52Ag-0.22Cr alloy were studied. The electrical conductivity and micro-hardness increase significantly in the early aging time. With the extension of aging time, the electrical conductivity is basically unchanged and remains at a high level. While, the micro-hardness increases slowly, the change trend is different at 623 K, 723 K, and 773 K. The optimisation of process parameters occurs in 723 K for 2 h. At this time, the electric conductivity is 95.8% IACS and the hardness is 104.1 HV0.1. The XRD result shows that the Ag and Cr are precipitated in elemental form copper matrix. Further TEM shows that, Cr exists at the sub-boundary in the form of larger nanoscale precipitates (100-200 nm). While a large number of Ag nanoscale precipitates (8-10 nm) is dispersed on the copper matrix. The synergistic effect of Ag and Cr nanoscale precipitates significantly improved the properties of the alloy.
1 Introduction
Multi-component Cu-based alloys have been explored as important functional materials for industrial application owing to their high strength, controllable micro-hardness and superior electrical conductivity. Sometimes they can also be used as bacteriostatic biomaterials [1, 2, 3, 4, 5, 6, 7, 8]. Microal-loying is usually used by material researchers to maintain the alloy’s high electric conductivity and mechanical properties [9, 10]. The high strength of alloys is due to nanoscale precipitates in the copper matrix during the aging process. The high electrical conductivity results from the low solid solubility of alloying elements [11, 12, 13, 14, 15, 16, 17, 18, 25]. Krishna et al. [13] found that the presence of nanoscale precipitates in the soft matrix significantly improves the strength by resistance the motion of dislocations in the aging process of Cu-3Ag-Zr alloy. The size of the Ag and Zr nanoscale precipitates was measured to be 7 nm and 80 nm, respectively. By analyzing the results, they found that the major contribution to strength was form coherency and dislocation strengthening of nanoscale precipitates. Nanoscale precipitates were found through the research Precipitation behavior and properties of aged Cu-0.23Be-0.84Co alloy by Zhou et al. [14]. A coherent nanometer precipitation results with the best strengthening effect. Peng et al. [15] pointed out that the morphology and phase relationship of nanoscale precipitates change with the aging process, having a great influence on the properties of the alloy. The above results indicate that the properties of the alloy depend on the morphology, distribution of the precipitated phase, and the relationship of phase relations. Moreover, both Ag and Cr are nanoscale precipitates.
Cu-Ag-Cr alloy as an extremely important Cu-based alloy with obvious aging strengthening effect and good comprehensive mechanical properties [19, 20, 21, 22, 23, 24, 25, 26, 27, 28], is known as a “pearl” because of its widely application in electrified railway contact lines, high fidelity video, and audio lines. When Ag and Cr are used as nanoscale precipitates of copper alloy, what is the effect on the properties of the alloy. Jia et al. [29] prepared Cu-0.1Ag-0.3Cr alloy by using vacuum induction furnace, and studied the relationship between heat treatment process and performances. The performance of the obtained alloy was the best after aging for 4 h at 723 K, with the electrical conductivity of 82% and the micro-hardness of 144HV. At this point, the elemental Cr is present in the form of nanoscale sediments. The size of nanoscale precipitates is 6-7 nm, and the dislocation cutting mechanism has a great influence on the micro-hardness and strength of the alloy. Therefore, the electrical conductivity increased significantly due to the fine and well dispersed nanoscale precipitates. These nanoscale precipitates are caused by the decomposition of supersaturated solid solution during aging. Liu et al. [30]. prepared Cu-Ag-Cr alloys by vacuum melting furnace. Through deformation heat treatment (the shape variable was 40%), the electrical conductivity was close to 90% IACS after aging for 4 h at 753 K. It is found that Cr nanoscale precipitates exist in the form of single substance after TTRT and have a certain phase relationship. But it is considered that trace Ag exists in the matrix in the form of solid soluble copper,which has not been explained and proved. Chen et al. [31] prepared Cu-Ag-Cr (Ce) alloy by means of medium frequency induction furnace and studied the microstructure of Cu-Cr-Ag alloy under different treatment conditions. The addition of Ce element makes the electric conductivity of the alloy reach 93±0.1% IACS after 4 h of insulation at 823 K. Alloy grain refinement’s is not an important factor affecting the properties of the alloy. Xu et al. [32] adopted medium frequency induction furnace. They found that the micro-hardness and electrical conductivity of the alloy increased with the increase of Ag content under different heat treatments, and the electrical conductivity reached to 83.2% IACS. With the addition of Ag, Cu-Cr alloys exhibit obvious solution strengthening and significantly depressed chain precipitation of Cr nanoscale precipitates. It was explained as the relationship between the amount of Ag and the improvement of mechanical properties. The above study presents a little about Ag and focuses mainly on the impact of Cr on the properties of the alloy. It has good mechanical properties, but it presents high loses of electrical conductivity. Therefore, in order to obtain high strength and high electric conductivity alloy, it is more important to control the nanoscale precipitates of Ag and Cr and to play a synergistic role.
Therefore, in this work, a hot horizontal continuous casting was used to produce the new Cu-Ag-Cr alloy. Then, the effect of aging treatment on properties and nanoscale precipitates of Cu-Ag-Cr alloy was studied by optical, SEM, XRD, TEM, and property testing. Simultaneously, the optimum aging process parameters were determined within the scope of the experiment.
2 Experimental procedure
First a small piece of high purity Cu was melted with Ag and Cr, respectively at 1473-1573 K in a 7 kg vacuum inductive furnace to obtain Cu-3.5Ag and Cu-1.0Cr intermediate alloys. Then, the intermediate alloys were added to the graphite crucible of hot horizontal continuous casting equipment. The Cu-0.52Ag-0.22Cr alloy bar (Φ = 16 mm) could be obtained. The continuous casting rate was 20 mm/min and the temperature of the graphite crucible was maintained at 1423-1473 K.
The solid solution and aging process for the alloy was carried out in heat treatment furnace. Briefly, After the solid solution process operated under 1223 K for 1 h, the as-prepared alloy was aging at 673 K, 723 K, and 773 K for 0.5 h, 2 h, 4 h, and 8 h, respectively. The dimension of the specimen was Φ = 16 mm × (15-20) mm. The micro-hardness was measured on HVS-1000 digital micro-hardness tester with a load of 100 g and a loading time of 10 s. Each measurement was repeated for 6 times and the g error is less than 10%. The electric conductivity of the sample was measured using a Sigma 2008B1 digital eddy current metal conductometer. Each sample was tested for 5 times and the measurement error was less than 0.02%IACS. X-ray diffraction (XRD) was used to analyze the phase compositions of alloys obtained under different conditions. Morphologies of the alloys and the distribution of the precipitates in the copper matrix were observed by scanning electron microscopy (SEM) and the JEM2000 high resolution transmission electron microscopy (HRTEM).
3 Results
3.1 Properties of the alloy
Figure 1 shows the change of micro-hardness of the Cu-0.52Ag-0.22Cr alloy prepared at different aging temperatures for various times between 0 h and 8 h. As shown in Figure 1, the micro-hardness of the alloy obtained after solid solution treatment is only 40.3 HV0.1, which greatly enhanced after the aging process. As seen, after aging at 673 K, 723 K, and 773 K for 30 min, the micro-hardness of the alloys significantly increased to 73.7 HV0.1, 101.1 HV.01, and 88.6 HV0.1, respectively, which was 1.8, 2.5, 2.2 times that of alloy obtained after solid solution treatment.
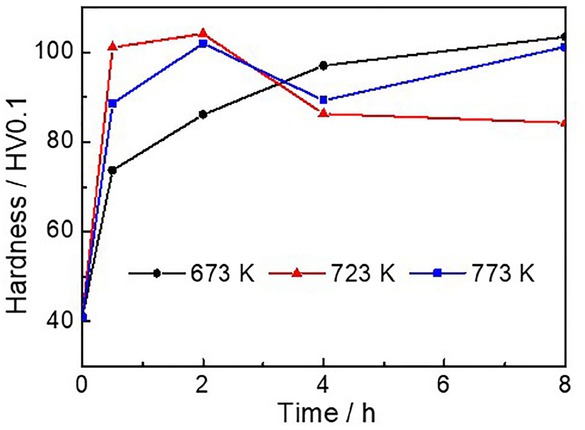
Comparisons of the micro-hardness of the Cu-0.52Ag-0.22Cr alloy after aging at different temperature for different time
Subsequently, micro-hardness continuously increased when the aging time prolonged to 2 h. But the change trend was different under different temperature, when the aging time extended to 8 h. Under relatively low temperature (673 K), one can observe the sustained growth of the micro-hardness to 103.5 HV0.1 with the prolonged aging time to 8 h. When the aging temperature was 723 K and 773 K, the highest micro-hardness was 104.1 HV0.1 and 102.0HV0.1, both of which were obtained when the aging time was 2 h.
Figure 2 shows the electrical conductivity of the alloys obtained after diverse aging temperatures for different times. Within a short period of 30 min, there was an initial burst increase for the electrical conductivity of the alloys, with electric conductivity of the 93.3, 93.9 and 94.4% IACS after 30 min when the aging temperature was 673 K, 723 K, and 773 K, respectively. Compared to the initial electric conductivity of 51% IACS, the electric conductivity increased 82.9%, 84.1%, and 85.1%. Then the electrical conductivity was almost unchanged when the aging time extended to 2, 4, 6 and 8 h, respectively. So, the effect of temperature on electric conductivity is not very obvious. The maximum difference of electric conductivity reached 1.9% IACS (96.5-94.6% IACS).
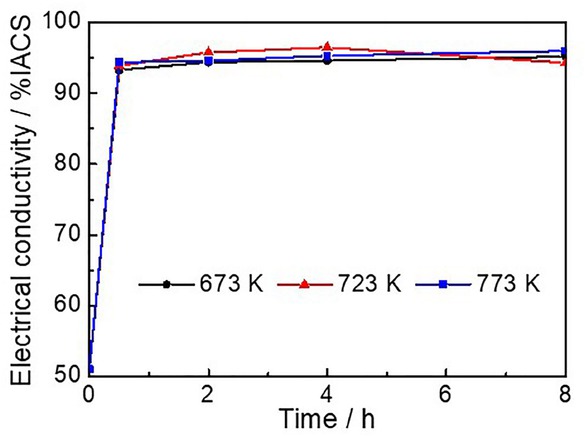
Variation of electrical conductivity with the aging time
In practical applications, the alloys should be equipped with both excellent hardness and electrical conductivity at the same time. So we introduce a new formula (R/Rmax)×(E/Emax), where R is the electric conductivity, E is the micro-hardness, and max is the maximum value. The obtained result was presented in Figure 3. When the aging process was performed under 723 K for 2 h, the maximum value is obtained. At this moment, the alloy has the best comprehensive properties (electrical conductivity and micro-hardness), with the electric conductivity of 95.8% IACS and the micro-hardness of 104.1 HV0.1.
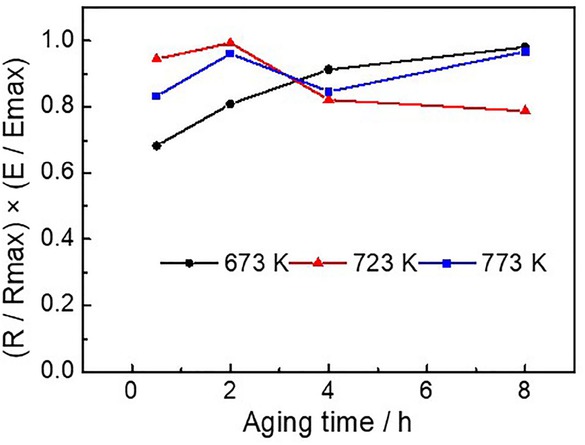
Standardized curves of micro-hardness and conductivity under different aging condition
3.2 Microstructure and nanoscale precipitates of alloy
The properties of alloys are closely related to microstructure. Thus, the evolution of macroscopic phase was evaluated using XRD. Based on the XRD spectra in Figure 4, it is clear that the alloy with face-centered cubic crystal structure could be obtained after solid solution treatment at 1223 K for 1 h, which was confirmed by the characteristic peaks of FCC-(110). As the sample was heated at 723 K for 0.5 h, the FCC-(110) peak dramatically weakened. The XRD pattern was composed of the characteristic peaks of Cu (200), Cr (211) and Ag (103), indicating that Ag and Cr precipitated from the copper matrix and formed a new single phase. When the aging time continued, prolonged to 8 h, the crystal phase of the alloys was almost unchanged, which was in agreement with the results presented in Figures 1 and 2. Under the aging temperature of 723 K, the microstructure changed with the aging time and the results are shown in Figure 5. The microstructures of the alloys are all isometric crystals, and the grain surface gradually changed rough with the extension of aging time. Meanwhile, the grain grows with aging time. In order to further explore the occurrence of this situation, TEM was used to observe the nanoscale precipitates.
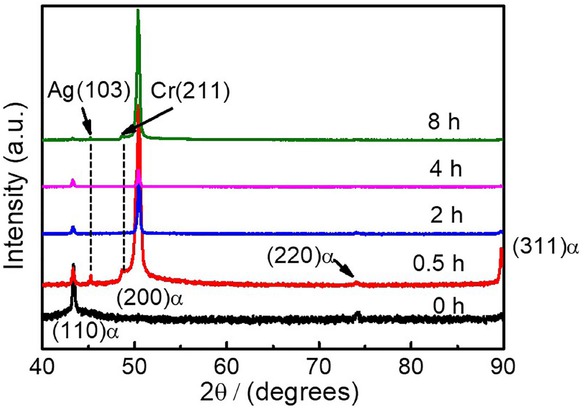
XRD pattern of the alloy at 723 K changes with time
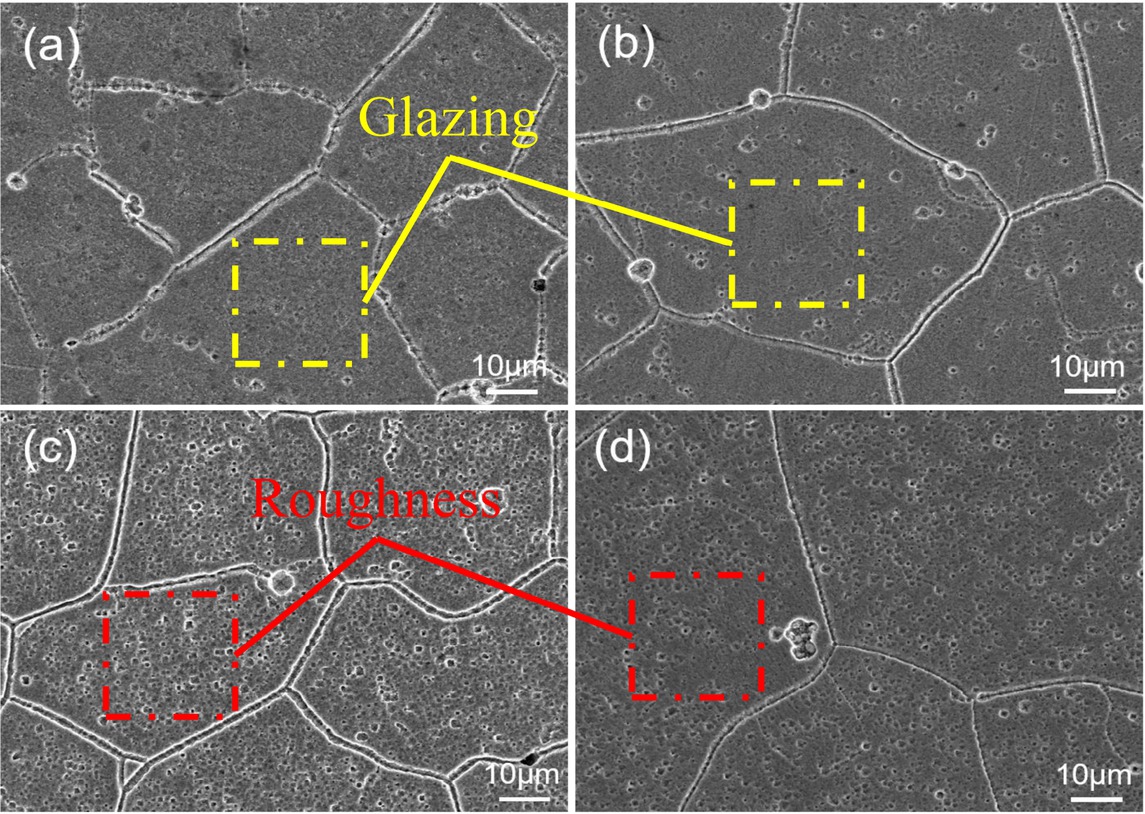
SEM images of the alloys obtained after aging at 723 K for different times: (a) 0.5 h; (b) 2 h; (c) 4 h; (d) 8 h
Figure 6a shows a typical transmission electron microscopy (TEM) image of the alloy obtained after solid solution. As seen, there are only dislocations in the TEM image and no precipitated phase can be observed. Only a set of diffraction patterns of the copper matrix can be seen from the Figure 6b.

The TEM image for Cu-0.52 Ag-0.22 Cr alloy after solid solution: (a) bright field image; (b) SADE pattern
Figure 7 shows the TEM images of alloy after aging at 723 K for 0.5 h. A small number of elliptic particles with a size of about 100 nm can be seen from Figure 7 and most of them distributed on the sub-grain boundary. The EDX spectra in Figure 7b further confirmed that the main component of the particles was Cr. In addition, a large number of nanoscale precipitates with the size of 4-5 nm can be observed from the bright field image in Figure 7c. The lattice parameter of the copper matrix was 0.1729, and the parameter of the precipitation phase is 0.1802 (Figure 7d). From the corresponding FFT diagram obtained by Fourier transform it can be found that the diffraction is relatively complex and the nanoscale precipitates and matrix are coherent. The phase relationship between the precipitated phase and the copper matrix can be expressed as (110)α//(110)γ, [001] α//[001]𝛾 (α : copper matrix; γ : Ag). Relatively to the solid solution state, a large number of dispersive Ag particles and Cr particles with large size were precipitated after 0.5 h of aging (Figure 7c and Figure 7a).
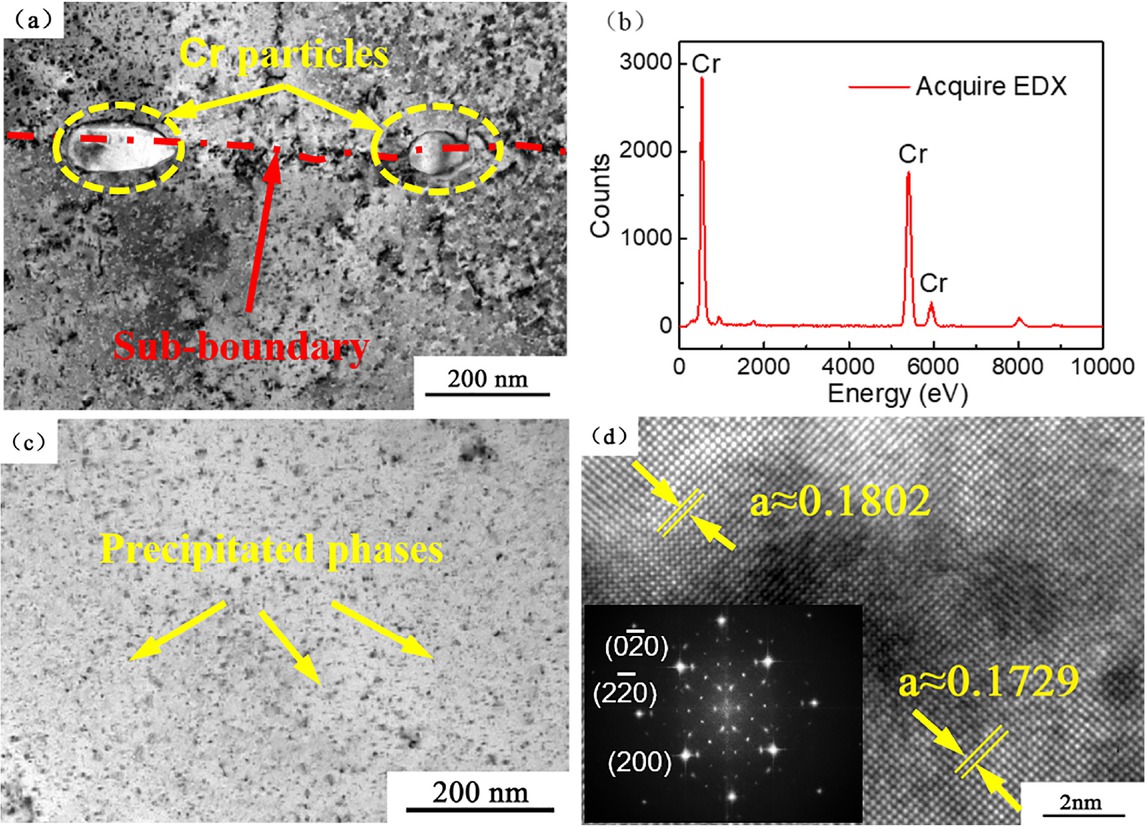
TEM images of the alloy at 723 K×0.5 h (a and c) bright field image; (b) energy spectrum; (d) HRTEM
The microstructure of product obtained after aging at 723 K for 2 h was shown in Figure 8a. The dimension of the nanoscale precipitates grew up to 8-10 nm. The structure of the nanoscale precipitates did not change. It can be seen from Figure 8b that the matrix parameters of copper matrix decreased and the matrix parameters of nanoscale precipitates increased. It revealed the growth of the precipitated phase and continuous precipitation. Through the calibration of the SADE and the calculation of the mismatch, the coherent relationship between the nanoscale precipitates and the matrix was lost. The phase relationship can be expressed as (110)α//(110)γ, [001] α//[111]γ.
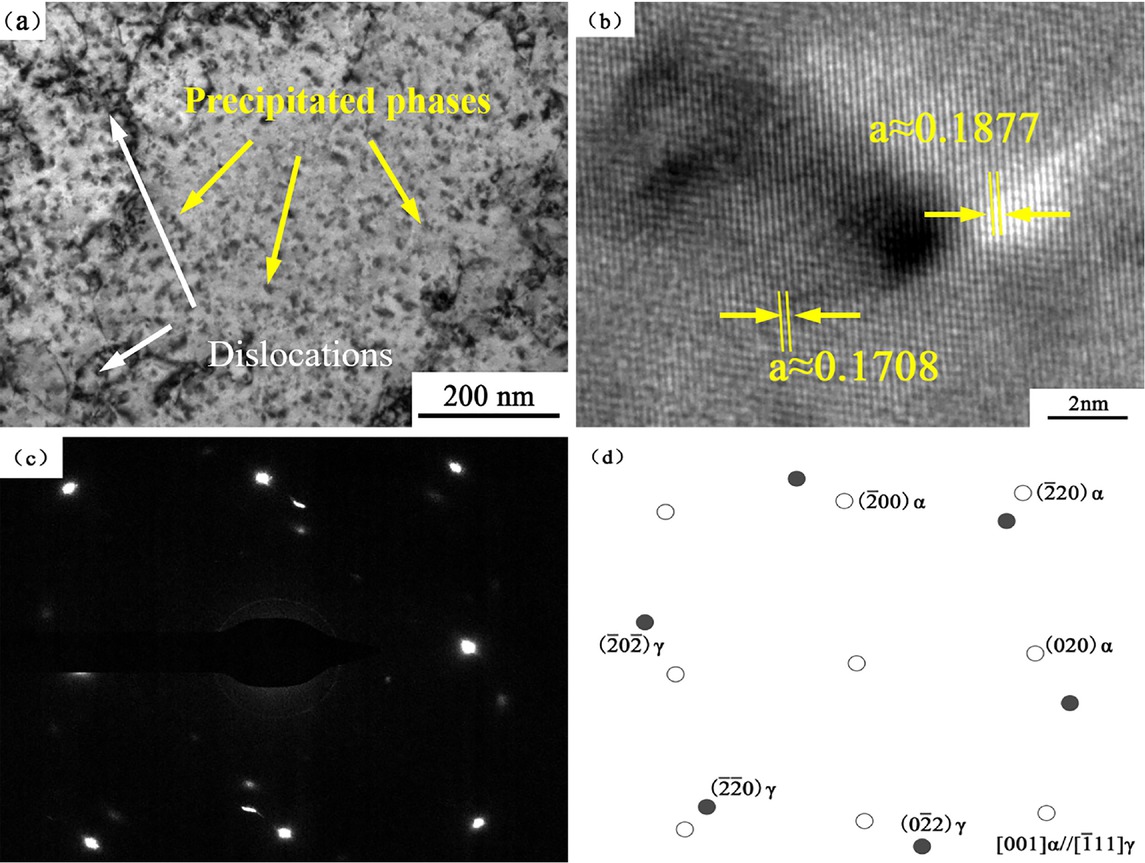
TEM of the alloy at 723 K×2 h: (a) bright field image; (b) HRTEM; (c) SADE pattern of the precipitate; (d) index of SADE pattern
4 Discussion
4.1 Effect of aging on properties of alloy
The alloy kept steady supersaturated solid solution stage after solid solution treatment. Thus, the precipitation capacity of the second phase is large, and the precipitation rate is fast in the in the early stage of aging [18, 23], which leads to quick increase for the hardness of the alloys in the initial 30 min. With the extension of aging time (30 min to 2 h), the saturation gradually decreased, resulting the slow precipitation rate and the growth of precipitation phase [18]. This can affect the micro-hardness of the alloy, so the micro-hardness increases slowly. When the temperature is 673 K, the diffusion rate for all elements in the alloy and the precipitation of the second phase is slow, causing unobvious strengthening effect [19]. When the temperature is 773 K, the saturation of the alloy will be destroyed, and the precipitation power of the second phase will be insufficient. Moreover, the precipitated second phase will not stop growing. Thus, it can destroy the coherence phenomenon of the matrix, and the micro-hardness is inevitably declined when the aging time pro-longed from 2 to 8 h [20, 31].
The scattering effect of solid solution elements on electrons is the main reason that influences the electric conductivity of the alloys. The higher content of alloy elements in the matrix, the stronger the scattering effecton electrons, and the electric lower the conductivity of the alloy. Therefore, the electric conductivity and micro-hardness basically follow the same law. With the extension of time, the solid solution elements continuously precipitate and the matrix is closer to the copper matrix. The electric conductivity maintains at a high level. In the early stage of aging (before 0.5 h), it has a higher saturation. As the result, the higher the temperature (less than 773 K), the higher the electric conductivity is. In the later stage of aging, when the temperature is lower than 723 K, the diffusion ability of solid solution elements decreases and the precipitation is slower [32, 35]. When the temperature is higher than 723 K, the saturation is low and the precipitation is slow. When the temperature is 723 K, the precipitation is normal. The precipitation phase grows slowly with the extension of time, which will have a certain impact on the electric conductivity [35].
4.2 Effect of nanoscale precipitates on properties
After high-temperature solid solution treatment, it can be seen from both Figure 4 and Figure 6 that Ag and Cr are fully integrated into the copper matrix. Thus, the electric conductivity and microhardness of the alloy are relatively low. There are no nanoscale precipitates. After aging process, Cr accumulates in the sub-grain boundary in large nanoscale precipitates. It proves that the addition of Ag element can change the chain precipitation of Cr element [33]. It has a strong blocking effect on dislocation, as shown in Figure 8. The fine and dispersed Ag nanoscale precipitates gradually coarsened with the aging, and the phase connection with the matrix also changed. It can block the dislocation motion. The increase of electric conductivity is caused by the precipitation of Ag and Cr nanoscale precipitates from the copper matrix. The characteristic parameters of nanoscale precipitates have limited influence on the electric conductivity. Nevertheless, nanoscale precipitates have a significant effect on microhardness.
As it is well known, the strengthening mechanism of hardness is the nail-rolling effect on dislocation [34]. According to the calculated results of the mismatch degree, it can be concluded that the nanoscale precipitates and matrix are semi-coherent. At this point, through the study of the second phase strengthening mechanism (9.9%), the Orowan strengthening mechanism can be adopted. The strengthening due to the Orowan model is described by the Orowan–Ashby equation [34]:
Where the τ0A is the shear stress of the Orowan–Ashby mechanism, r is the average radius of the Ag particle, r0 is the dislocation center size, α is the hard particle spacing. In over-aging alloys, the Ag particle size will increase. While τ0A will reduce. Therefore, alloys will have lower micro-hardness. When dislocation cuts through the nanoscale precipitates [34]:
The relationship between α and the radius and volume fraction of the nanoscale precipitates can be calculated using the following equation [34]:
Putting the relevant parameters into formula. (G = 44.1MPa; b = 0.2556nm; v = 0.35; θ = 0.5; ∈ = 0.015; r0 = 2b) Since the electric conductivity remains high level, we assume that Ag precipitates entirely from the copper matrix. According to alloy composition, the volume fraction of the precipitated phase was calculated to be 0.044%. So, one can get the following formula:
Working out as 4<r<5. Therefore, when the aging time is prolonged, the nanoscale precipitates increases and the hardness decreases.
5 Conclusion
In the early stage of aging, the electric conductivity and micro-hardness increased significantly. With the extension of aging time, the electric conductivity remains at a high level and basically unchanged. While the micro-hardness increases slowly, the change trend is different at variuos temperatures. Therefore the micro-hardness is more sensitive to temperature than electric conductivity.
The optimum aging parameters are obtained within the experimental range. The best alloy can be obtained after aging at 723 K for 2 h, with the micro-hardness of 104.1HV0.1 and electric conductivity of 95.8% IACS.
The XRD result shows that the value changed greatly after aging. Only the diffraction front of copper matrix can be observed after solid solution. The peak of Cu (200), Ag (103) and Cr (211) are beginning to appear after aging. This means that Ag and Cr are precipitated from copper matrix.
Nanometer precipitates have a significant effect on the improvement of alloy properties. In the aging process, Cr exists at the sub-boundary in the form of larger nanoscale precipitates (100-200 nm). At the same time, a large number of Ag particles dispersed on copper matrix. The size of Ag nanoscale precipitates is 8-10 nm. The nanoscale precipitates are semi-congruent with the matrix. The phase relationship between the precipitated phase and the copper matrix can be expressed as (110)α//(110)γ, [001] α//[111]γ. The synergistic effect of Ag and Cr nanoscale precipitates greatly improved the properties of the alloy.
Acknowledgement
The present work is financially supported by the National key research and development program (2016YFB0301400), Henan innovation leading project (191110210400), Key scientific research projects of institutions of higher learning in Henan province (19A430012), and Innovation fund for outstanding talents of Henan province (182101510003).
References
[1] Horie H., Copper-titanium alloy for electronic component, U.S. Patent 10, 100, 387, 2018-10-16.Suche in Google Scholar
[2] Zhang X.H., Zhang Y., Tian B.H., Jia Y.L., Liu Y., Song K.X., Volinsky A.A., Xue H.H., Cr effects on the electrical contact properties of the Al2O3-Cu/15W composites, Nanotechnol. Rev., 2019, 8(1), 128-135.10.1515/ntrev-2019-0012Suche in Google Scholar
[3] Yin Z.M., Zhang S.L., Hotspots and Developing Tendency on High-strength and High-conductivity Copper Alloys, Min. Metall. Eng., 2002, 2.Suche in Google Scholar
[4] Rai M., Ingle A.P., Pandit R., Pandit R., Shende S., Gupta I., Biswas J.K., Silva S.S., Copper and copper nanoparticles: role in management of insect-pests and pathogenic microbes, Nanotechnol. Rev., 2018, 7(4), 303-315.10.1515/ntrev-2018-0031Suche in Google Scholar
[5] Okafuji Y., Copper-cobalt-silicon alloy for electrode material, U.S. Patent 10, 056, 166, 2018-8-21.Suche in Google Scholar
[6] Scott A., Vadalasetty K.P., Chwalibog A., Sawosz E., Copper nanoparticles as an alternative feed additive in poultry diet: a review, Nanotechnol. Rev., 2018, 7(1), 69-93.10.1515/ntrev-2017-0159Suche in Google Scholar
[7] Liu Y., Liu P., LiW., Tian B.H., Aging behavior and electrical sliding wear properties of Cu-Cr-Zr-Ce alloy,Trlbol. Int., 2005, 25(3), 265-269.Suche in Google Scholar
[8] Ban I., Stergar J., Maver U., NiCu magnetic nanoparticles: review of synthesis methods, surface functionalization approaches, and biomedical applications, Nanotechnol. Rev., 2018, 7(2), 187-207.10.1515/ntrev-2017-0193Suche in Google Scholar
[9] Bracey C.L., Ellis P.R., Hutchings G.J., ChemInform Abstract: Application of Copper—Gold Alloys in Catalysis: Current Status and Future Perspectives, Chem. Soc. Rev., 2009, 38(8), 2231-2243.10.1002/chin.200947257Suche in Google Scholar
[10] Zuo X., Qu L., Zhao C., An B., Wang E., Niu R., Xin Y., Lu J., Han K., Nucleation and growth of γ-Fe precipitate in Cu-2% Fe alloy aged under high magnetic field, J. All. Compd., 2016, 662, 355-360.10.1016/j.jallcom.2015.12.046Suche in Google Scholar
[11] Prokoshkina D., Esin V.A., Divinski S.V., Experimental evidence for anomalous grain boundary diffusion of Fe in Cu and Cu-Fe alloys, Acta Mater., 2017, 133, 240-246.10.1016/j.actamat.2017.05.024Suche in Google Scholar
[12] Guo F.,Wang C., Cheng D., Current Status and Development Trend of Rolling Technology of Copper Alloy Plate and Strip, Hot Work. Tec., 2016, 19, 10-13.Suche in Google Scholar
[13] Krishna S.C., Abhay K., Properties and Strengthening Mechanisms in Cold-Rolled and Aged Cu-3Ag-0.5Zr Alloy, Metall. Mi-crost. An., 2014, 3(4), 323-327.10.1007/s13632-014-0147-3Suche in Google Scholar
[14] Zhou Y.J., Song K.X., Xing J.D., Zhang Y.M., Precipitation behavior and properties of aged Cu-0.23 Be-0.84 Co alloy, J. All. Compd., 2016, 658, 920-930.10.1016/j.jallcom.2015.10.290Suche in Google Scholar
[15] Mishnev R., Shakhova I., Belyakov A., Kaibyshev R., Deformation microstructures, strengthening mechanisms, and electrical conductivity in a Cu–Cr–Zr alloy, Mat. Sci. Eng. A - Struct., 2015, 629, 29-40.10.1016/j.msea.2015.01.065Suche in Google Scholar
[16] Jia S.G., Liu P., Tian B.H., Zheng M.S., Zhou G.S., Lou H.F., Strengthening mechanism in high-strength and high-conductivity Cu-0.1Ag-0.11Cr alloy, China J. Nonferrous, 2004, 14(7), 1144-1148.Suche in Google Scholar
[17] Li R.G., Kang H.J., Chen Z.G., Fan G.H., Zou C.L., Wang W., Zhang S.J., Lu Y.P., Jie J.C., Cao Z.Q., Li T.J., A promising structure for fabricating high strength and high electrical conductivity copper alloys, Sci. Rep., 2016, 6, 20799.10.1038/srep20799Suche in Google Scholar PubMed PubMed Central
[18] Li H.W., Dai J.Y., Strengthen mechanism and ration analysis of Cu-Ag-Cr alloy, China J. Rare Metals, 2010, 34(6), 828-832.Suche in Google Scholar
[19] Wang M.H., Huang L., Chen M.L., Wang Y.L., Processing map and hot working mechanisms of Cu-Ag alloy in hot compression process, J. Cent. South Univ., 2015, 22(3), 821-828.10.1007/s11771-015-2588-5Suche in Google Scholar
[20] Gubicza J., Sitarama R.K., Subramanya S.V., Alexander K., Zoltan H., Martin P., The Effect of Thermomechanical Treatment on the Microstructure and the Mechanical Behavior of a Supersaturated Cu-Ag Alloy, Mater. Sci. Forum, 2015, 812, 6.10.4028/www.scientific.net/MSF.812.53Suche in Google Scholar
[21] Du D., Fautrelle Y., Dong A., Shu D., Zhu G., Sun B., Effect of Ag Content on the Microstructure and Crystallization of Coupled Eutectic Growth in Directionally Solidified Al-Cu-Ag Alloys, Metall. Mater. Trans. A, 2018, 49(10), 4735-4747.10.1007/s11661-018-4799-5Suche in Google Scholar
[22] Wang S., Zhang Y., Yao D., Micro-structure and Properties of Cu– 0.3 wt% Ag Alloy Ultra-Fine Wires, TMS 2019 148th Annual Meeting and Exhibition Supplemental Proc. Springer, Cham, 2019, 629-635.10.1007/978-3-030-05861-6_60Suche in Google Scholar
[23] Kim S.W., Wang Y., Jung T.K., Jin C.Y., Choung J., Lee J.W., Pore Characteristics of Lotus-Type Porous Cu–Fe and Cu–Cr Alloys Fabricated by Unidirectional Solidification, J. Nanosci. Nanotechnol., 2018, 18(3), 2262-2265.10.1166/jnn.2018.14984Suche in Google Scholar PubMed
[24] Tian W., Bi L., Ma F., Du J., Effect of Zr on as-cast microstructure and properties of Cu-Cr alloy, Vacuum, 2018, 149, 238-247.10.1016/j.vacuum.2017.12.011Suche in Google Scholar
[25] Feng X., Xie H., Li Z., Mi X., Huang G., Peng L., et al, Comparison of Ag and Zr with same atomic ratio in Cu-Cr alloy, IOP Conf. Series: Mat. Sci. Eng. IOP Pub., 2018, 397(1), 012053.10.1088/1757-899X/397/1/012053Suche in Google Scholar
[26] Su J.H., Dong Q.M., Liu P., LI H.J., Kang B.X., Aging hardening and recrystallization of the deformed Cu-0.3Cr-0.15Zr-0.05Mg alloy, Heat Treat. Met. C, 2004, 29(2).Suche in Google Scholar
[27] Gubicza J., Hegedűs Z., Lábár J.L., Kauffmann A., Freudenberger J., Subramanya S.V., Solute redistribution during annealing of a cold rolled Cu–Ag alloy, J. All. Compd., 2015, 623, 96-103.10.1016/j.jallcom.2014.10.093Suche in Google Scholar
[28] Li W.B., Pan Q.L., Xiao Y.P., He T.B., Liu X.Y., Effect of aging processes on microstructure and properties of Al-Zn-Cu-Mg-Sc-Zr alloy, T. Mater. Heat Treat., 2011, 32(8), 47-53.Suche in Google Scholar
[29] Jia S.G., Zheng M.S., Liu P., Ren F.Z., Tian B.H., Zhou G.S., Lou H.F., Aging properties studies in a Cu-Ag-Cr Alloy, Mat. Sci. Eng. A - Struct., 2006, 419(1-2), 8-11.10.1016/j.msea.2005.09.118Suche in Google Scholar
[30] Liu J.B., Zhang L., Meng L., Effects of rare-earth additions on the microstructure and strength of Cu-Ag composites, Mat. Sci. Eng. A - Struct., 2008, 498(1-2), 392-396.10.1016/j.msea.2008.08.014Suche in Google Scholar
[31] Chen H.M., Yuan D.W., Wu S.J., Wang H., Xie W.B., Yang B., Relationship between Microstructure and Properties of Cu-Cr-Ag-(Ce) Alloy Using Microscopic Investigation, Scanning, 2017, 2017, 1-8.10.1155/2017/4646581Suche in Google Scholar
[32] Xu S., Fu H., Wang Y., Xie J.X., Effect of Ag addition on the microstructure and mechanical properties of Cu-Cr alloy, Mat. Sci. Eng. A – Struct., 2018, 726, 508-214.10.1016/j.msea.2018.04.077Suche in Google Scholar
[33] Liu Y., Li Z., Jiang Y.X., Zhang Y., Zhou Z.Z., Lei Q., The microstructure evolution and properties of a Cu-Cr-Ag alloy during thermal-mechanical treatment, Mater. Res., 2017, 32, 1324-1332.10.1557/jmr.2017.17Suche in Google Scholar
[34] Correia J.B., Davies H.A., Sellars C.M., Strengthening in rapidly solidified age hardened Cu-Cr and Cu-Cr-Zr alloys, J. Acta Mater., 1997, 45(1), 177-190.10.1016/S1359-6454(96)00142-5Suche in Google Scholar
[35] Bao G., Xu Y., Huang L., Lu X., Zhang L., Fang Y., Strengthening Effect of Ag Precipitates in Cu-Ag Alloys: A Quantitative Approach, Mater. Res. Lett., 2016, 4(1), 37-42.10.1080/21663831.2015.1091795Suche in Google Scholar
© 2020 L. Kong et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Generalized locally-exact homogenization theory for evaluation of electric conductivity and resistance of multiphase materials
- Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide
- Characterization of mechanical properties of epoxy/nanohybrid composites by nanoindentation
- Graphene and CNT impact on heat transfer response of nanocomposite cylinders
- A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites
- Ultrasmall Fe3O4 nanoparticles induce S-phase arrest and inhibit cancer cells proliferation
- Effect of aging on properties and nanoscale precipitates of Cu-Ag-Cr alloy
- Effect of nano-strengthening on the properties and microstructure of recycled concrete
- Stabilizing effect of methylcellulose on the dispersion of multi-walled carbon nanotubes in cementitious composites
- Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites
- Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation
- Preparation and properties of 3D interconnected CNTs/Cu composites
- On factors affecting surface free energy of carbon black for reinforcing rubber
- Nano-silica modified phenolic resin film: manufacturing and properties
- Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete
- Halloysite nanotubes in polymer science: purification, characterization, modification and applications
- Cellulose hydrogel skeleton by extrusion 3D printing of solution
- Crack closure and flexural tensile capacity with SMA fibers randomly embedded on tensile side of mortar beams
- An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites
- Utilization of red mud for producing a high strength binder by composition optimization and nano strengthening
- One-pot synthesis of nano titanium dioxide in supercritical water
- Printability of photo-sensitive nanocomposites using two-photon polymerization
- In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries
- Effect of nano and micro conductive materials on conductive properties of carbon fiber reinforced concrete
- Tribological performance of nano-diamond composites-dispersed lubricants on commercial cylinder liner mating with CrN piston ring
- Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance
- Genetic mechanisms of deep-water massive sandstones in continental lake basins and their significance in micro–nano reservoir storage systems: A case study of the Yanchang formation in the Ordos Basin
- Effects of nanoparticles on engineering performance of cementitious composites reinforced with PVA fibers
- Band gap manipulation of viscoelastic functionally graded phononic crystal
- Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution
- Manipulating conductive network formation via 3D T-ZnO: A facile approach for a CNT-reinforced nanocomposite
- Microstructure and mechanical properties of WC–Ni multiphase ceramic materials with NiCl2·6H2O as a binder
- Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite
- Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function
- Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing
- Corrosion behaviour of multilayer CrN coatings deposited by hybrid HIPIMS after oxidation treatment
- Surface hydrophobicity and oleophilicity of hierarchical metal structures fabricated using ink-based selective laser melting of micro/nanoparticles
- Research on bond–slip performance between pultruded glass fiber-reinforced polymer tube and nano-CaCO3 concrete
- Antibacterial polymer nanofiber-coated and high elastin protein-expressing BMSCs incorporated polypropylene mesh for accelerating healing of female pelvic floor dysfunction
- Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films
- A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination
- Arrangement structure of carbon nanofiber with excellent spectral radiation characteristics
- Effect of different particle sizes of nano-SiO2 on the properties and microstructure of cement paste
- Superior Fe x N electrocatalyst derived from 1,1′-diacetylferrocene for oxygen reduction reaction in alkaline and acidic media
- Facile growth of aluminum oxide thin film by chemical liquid deposition and its application in devices
- Liquid crystallinity and thermal properties of polyhedral oligomeric silsesquioxane/side-chain azobenzene hybrid copolymer
- Laboratory experiment on the nano-TiO2 photocatalytic degradation effect of road surface oil pollution
- Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage
- Conversion of sub-µm calcium carbonate (calcite) particles to hollow hydroxyapatite agglomerates in K2HPO4 solutions
- Exact solutions of bending deflection for single-walled BNNTs based on the classical Euler–Bernoulli beam theory
- Effects of substrate properties and sputtering methods on self-formation of Ag particles on the Ag–Mo(Zr) alloy films
- Enhancing carbonation and chloride resistance of autoclaved concrete by incorporating nano-CaCO3
- Effect of SiO2 aerogels loading on photocatalytic degradation of nitrobenzene using composites with tetrapod-like ZnO
- Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles
- Hydration activity, crystal structural, and electronic properties studies of Ba-doped dicalcium silicate
- Microstructure and mechanical properties of brazing joint of silver-based composite filler metal
- Polymer nanocomposite sunlight spectrum down-converters made by open-air PLD
- Cryogenic milling and formation of nanostructured machined surface of AISI 4340
- Braided composite stent for peripheral vascular applications
- Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre–reinforced starch biopolymer composites
- Study on influencing factors of photocatalytic performance of CdS/TiO2 nanocomposite concrete
- Improving flexural and dielectric properties of carbon fiber epoxy composite laminates reinforced with carbon nanotubes interlayer using electrospray deposition
- Scalable fabrication of carbon materials based silicon rubber for highly stretchable e-textile sensor
- Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery
- Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries
- Synthesis of functionalized carbon nanotubes for fluorescent biosensors
- Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete
- Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage
- Microstructural evolution and properties of Cu–20 wt% Ag alloy wire by multi-pass continuous drawing
- Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene
- Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications
- Preparation and piezoresistivity of carbon nanotube-coated sand reinforced cement mortar
- Vibrational analysis of an irregular single-walled carbon nanotube incorporating initial stress effects
- Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials
- Single pulse laser removal of indium tin oxide film on glass and polyethylene terephthalate by nanosecond and femtosecond laser
- Mechanical reinforcement with enhanced electrical and heat conduction of epoxy resin by polyaniline and graphene nanoplatelets
- High-efficiency method for recycling lithium from spent LiFePO4 cathode
- Degradable tough chitosan dressing for skin wound recovery
- Static and dynamic analyses of auxetic hybrid FRC/CNTRC laminated plates
- Review articles
- Carbon nanomaterials enhanced cement-based composites: advances and challenges
- Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide
- Review on modeling and application of chemical mechanical polishing
- Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites
- Advances in modelling and analysis of nano structures: a review
- Mechanical properties of nanomaterials: A review
- New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics
- A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials
- Recent development and applications of nanomaterials for cancer immunotherapy
- Advances in biomaterials for adipose tissue reconstruction in plastic surgery
- Advances of graphene- and graphene oxide-modified cementitious materials
- Theories for triboelectric nanogenerators: A comprehensive review
- Nanotechnology of diamondoids for the fabrication of nanostructured systems
- Material advancement in technological development for the 5G wireless communications
- Nanoengineering in biomedicine: Current development and future perspectives
- Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview
- Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review
- Carbon nanotube–reinforced polymer composite for electromagnetic interference application: A review
- Functionalized layered double hydroxide applied to heavy metal ions absorption: A review
- A new classification method of nanotechnology for design integration in biomaterials
- Finite element analysis of natural fibers composites: A review
- Phase change materials for building construction: An overview of nano-/micro-encapsulation
- Recent advance in surface modification for regulating cell adhesion and behaviors
- Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions
- Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review
- Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications
- A review of passive methods in microchannel heat sink application through advanced geometric structure and nanofluids: Current advancements and challenges
- Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices
- Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging
- Synthesis of graphene: Potential carbon precursors and approaches
- A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE)
- Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water
- Analysis of functionally graded carbon nanotube-reinforced composite structures: A review
- Application of nanomaterials in ultra-high performance concrete: A review
- Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer
- Advanced nickel nanoparticles technology: From synthesis to applications
- Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable?
- Progress in construction of bio-inspired physico-antimicrobial surfaces
- From materials to devices using fused deposition modeling: A state-of-art review
- A review for modified Li composite anode: Principle, preparation and challenge
- Naturally or artificially constructed nanocellulose architectures for epoxy composites: A review
Artikel in diesem Heft
- Research Articles
- Generalized locally-exact homogenization theory for evaluation of electric conductivity and resistance of multiphase materials
- Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide
- Characterization of mechanical properties of epoxy/nanohybrid composites by nanoindentation
- Graphene and CNT impact on heat transfer response of nanocomposite cylinders
- A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites
- Ultrasmall Fe3O4 nanoparticles induce S-phase arrest and inhibit cancer cells proliferation
- Effect of aging on properties and nanoscale precipitates of Cu-Ag-Cr alloy
- Effect of nano-strengthening on the properties and microstructure of recycled concrete
- Stabilizing effect of methylcellulose on the dispersion of multi-walled carbon nanotubes in cementitious composites
- Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites
- Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation
- Preparation and properties of 3D interconnected CNTs/Cu composites
- On factors affecting surface free energy of carbon black for reinforcing rubber
- Nano-silica modified phenolic resin film: manufacturing and properties
- Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete
- Halloysite nanotubes in polymer science: purification, characterization, modification and applications
- Cellulose hydrogel skeleton by extrusion 3D printing of solution
- Crack closure and flexural tensile capacity with SMA fibers randomly embedded on tensile side of mortar beams
- An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites
- Utilization of red mud for producing a high strength binder by composition optimization and nano strengthening
- One-pot synthesis of nano titanium dioxide in supercritical water
- Printability of photo-sensitive nanocomposites using two-photon polymerization
- In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries
- Effect of nano and micro conductive materials on conductive properties of carbon fiber reinforced concrete
- Tribological performance of nano-diamond composites-dispersed lubricants on commercial cylinder liner mating with CrN piston ring
- Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance
- Genetic mechanisms of deep-water massive sandstones in continental lake basins and their significance in micro–nano reservoir storage systems: A case study of the Yanchang formation in the Ordos Basin
- Effects of nanoparticles on engineering performance of cementitious composites reinforced with PVA fibers
- Band gap manipulation of viscoelastic functionally graded phononic crystal
- Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution
- Manipulating conductive network formation via 3D T-ZnO: A facile approach for a CNT-reinforced nanocomposite
- Microstructure and mechanical properties of WC–Ni multiphase ceramic materials with NiCl2·6H2O as a binder
- Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite
- Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function
- Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing
- Corrosion behaviour of multilayer CrN coatings deposited by hybrid HIPIMS after oxidation treatment
- Surface hydrophobicity and oleophilicity of hierarchical metal structures fabricated using ink-based selective laser melting of micro/nanoparticles
- Research on bond–slip performance between pultruded glass fiber-reinforced polymer tube and nano-CaCO3 concrete
- Antibacterial polymer nanofiber-coated and high elastin protein-expressing BMSCs incorporated polypropylene mesh for accelerating healing of female pelvic floor dysfunction
- Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films
- A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination
- Arrangement structure of carbon nanofiber with excellent spectral radiation characteristics
- Effect of different particle sizes of nano-SiO2 on the properties and microstructure of cement paste
- Superior Fe x N electrocatalyst derived from 1,1′-diacetylferrocene for oxygen reduction reaction in alkaline and acidic media
- Facile growth of aluminum oxide thin film by chemical liquid deposition and its application in devices
- Liquid crystallinity and thermal properties of polyhedral oligomeric silsesquioxane/side-chain azobenzene hybrid copolymer
- Laboratory experiment on the nano-TiO2 photocatalytic degradation effect of road surface oil pollution
- Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage
- Conversion of sub-µm calcium carbonate (calcite) particles to hollow hydroxyapatite agglomerates in K2HPO4 solutions
- Exact solutions of bending deflection for single-walled BNNTs based on the classical Euler–Bernoulli beam theory
- Effects of substrate properties and sputtering methods on self-formation of Ag particles on the Ag–Mo(Zr) alloy films
- Enhancing carbonation and chloride resistance of autoclaved concrete by incorporating nano-CaCO3
- Effect of SiO2 aerogels loading on photocatalytic degradation of nitrobenzene using composites with tetrapod-like ZnO
- Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles
- Hydration activity, crystal structural, and electronic properties studies of Ba-doped dicalcium silicate
- Microstructure and mechanical properties of brazing joint of silver-based composite filler metal
- Polymer nanocomposite sunlight spectrum down-converters made by open-air PLD
- Cryogenic milling and formation of nanostructured machined surface of AISI 4340
- Braided composite stent for peripheral vascular applications
- Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre–reinforced starch biopolymer composites
- Study on influencing factors of photocatalytic performance of CdS/TiO2 nanocomposite concrete
- Improving flexural and dielectric properties of carbon fiber epoxy composite laminates reinforced with carbon nanotubes interlayer using electrospray deposition
- Scalable fabrication of carbon materials based silicon rubber for highly stretchable e-textile sensor
- Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery
- Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries
- Synthesis of functionalized carbon nanotubes for fluorescent biosensors
- Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete
- Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage
- Microstructural evolution and properties of Cu–20 wt% Ag alloy wire by multi-pass continuous drawing
- Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene
- Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications
- Preparation and piezoresistivity of carbon nanotube-coated sand reinforced cement mortar
- Vibrational analysis of an irregular single-walled carbon nanotube incorporating initial stress effects
- Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials
- Single pulse laser removal of indium tin oxide film on glass and polyethylene terephthalate by nanosecond and femtosecond laser
- Mechanical reinforcement with enhanced electrical and heat conduction of epoxy resin by polyaniline and graphene nanoplatelets
- High-efficiency method for recycling lithium from spent LiFePO4 cathode
- Degradable tough chitosan dressing for skin wound recovery
- Static and dynamic analyses of auxetic hybrid FRC/CNTRC laminated plates
- Review articles
- Carbon nanomaterials enhanced cement-based composites: advances and challenges
- Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide
- Review on modeling and application of chemical mechanical polishing
- Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites
- Advances in modelling and analysis of nano structures: a review
- Mechanical properties of nanomaterials: A review
- New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics
- A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials
- Recent development and applications of nanomaterials for cancer immunotherapy
- Advances in biomaterials for adipose tissue reconstruction in plastic surgery
- Advances of graphene- and graphene oxide-modified cementitious materials
- Theories for triboelectric nanogenerators: A comprehensive review
- Nanotechnology of diamondoids for the fabrication of nanostructured systems
- Material advancement in technological development for the 5G wireless communications
- Nanoengineering in biomedicine: Current development and future perspectives
- Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview
- Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review
- Carbon nanotube–reinforced polymer composite for electromagnetic interference application: A review
- Functionalized layered double hydroxide applied to heavy metal ions absorption: A review
- A new classification method of nanotechnology for design integration in biomaterials
- Finite element analysis of natural fibers composites: A review
- Phase change materials for building construction: An overview of nano-/micro-encapsulation
- Recent advance in surface modification for regulating cell adhesion and behaviors
- Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions
- Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review
- Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications
- A review of passive methods in microchannel heat sink application through advanced geometric structure and nanofluids: Current advancements and challenges
- Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices
- Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging
- Synthesis of graphene: Potential carbon precursors and approaches
- A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE)
- Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water
- Analysis of functionally graded carbon nanotube-reinforced composite structures: A review
- Application of nanomaterials in ultra-high performance concrete: A review
- Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer
- Advanced nickel nanoparticles technology: From synthesis to applications
- Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable?
- Progress in construction of bio-inspired physico-antimicrobial surfaces
- From materials to devices using fused deposition modeling: A state-of-art review
- A review for modified Li composite anode: Principle, preparation and challenge
- Naturally or artificially constructed nanocellulose architectures for epoxy composites: A review

