Abstract
High belite cement has a wide application potential due to its low energy consumption, low CO2 emission, and excellent durability performance. Due to the low hydration rate and strength development at an early age, the activation of beta-dicalcium silicate (β-C2S) crystallographic structure is essential to improve the early strength of high belite cement. In this study, the β-C2S phase is activated by dissolving Ba2+ ions into the crystal lattice to improve the hydration rate. Unlike the traditional analysis methods of thermodynamics and dynamics theory, the first principle and density functional theory were applied to study the effect of Ba2+ ions on the activation of β-C2S, especially on the crystallographic structure, lattice parameters, and electronic structure change. The crystallographic structure of β-C2S can be activated by doping Ba atom and the crystal formation energy increases and the bandgap between VBM and CBM become narrow in the activated β-C2S crystallographic structure. Comparing the Ca2+ substitution in [CaO6] or [CaO8], the lattice deformation and hydraulic reactivity is more significant in Ba2-C2S and Ba22-C2S. The first principle and density functional theory explains the change of the electronic structure of the activated crystallographic structure and provides a theoretical basis for the purposeful design of material structures.
Graphical abstract
Highlights
The electron structure variation is related to the variation of charge exchange and atomic hybridization orbital in Ba-doped β-C2S.
The lattice deformation and hydraulic reactivity are more significant in Ba-doped β-C2S with Ba → Ca/[CaO8].
The crystal formation energy increases and the band gap between VBM and CBM become narrow in Ba-doped β-C2S.
1 Introduction
The traditional cement manufacture has been lasting for less than 200 years. Due to the consumption of high-grade limestones, the high-grade limestone resource in our country is not enough for the next 40 years [1,2]. Therefore, low-grade ore should be used in cement manufacture in point of view of sustainable development. High belite cement has a wide application potential due to its low energy consumption, low CO2 emission, and excellent durability performance [3,4,5,6,7,8]. Compared with Ordinary Portland cement, high belite cement consists of more than 40% dicalcium silicate (C2S) [9,10]. Due to its low CaO consumption, the cement calcining process becomes more energy-saving and a large sum of low-grade ore can be recycled [11,12,13].
β-C2S belongs to an island structure, due to the lack of coplanar structure in the [CaO x ]2x−2 polyhedron, the length of the Ca2+ ion migration path is quite long, thus inhibits the hydraulic reaction of β-C2S during the hydration process [14,15]. Due to the low hydration rate and strength development in an early age, the application of high-belite cement is limited. The activation of the C2S crystallographic structure is essential to improve the early strength of high belite cement. By calcining at high temperature, dopant ions are incorporated in the crystal lattice of β-C2S in the form of the lattice vacancy or site substitution [16,17]. With the reduction of the crystal symmetry and the formation of crystal lattice distortion, the microstress in the lattice increases and the lattice activation of β-C2S is achieved. Activators such as As2O5, V2O5, Cr2O3, MgO, BaO, CrO, P2O5, R2O, etc. are commonly used in cement industry production [18,19]. At the same time, the addition of activators can effectively reduce the formation energy in chemical reaction thermodynamics [3].
Many researches have studied the activation mechanism of doped ions on the crystal structure transformation of β-C2S [16,20,21,22,23]. Cuesta et al. [24] studied the activation mechanism of doped B3+ ions on belite cement and found that Ca2+ ions and Si4+ ions in the [SiO4] tetrahedron have been replaced, which contributes to the hydraulic reaction during cement hydration. Wang et al. [25] studied the activation effect of BaSO4 on β-C2S in belite cement clinker during calcination, and the results showed that BaSO4 could dissolve into the crystal structure of β-C2S and improve the early compressive strength.
The first principle and density functional theory can explain the change of the electronic structure of the activated crystal structure and also provide a theoretical basis for the purposeful design of the material structure [26,27,28]. Based on the traditional thermodynamics and kinetics theory, first-principles quantum mechanics calculations have been applied to study the relationship between electronic structure and reaction activity of C2S polymorphs [29,30]. In this study, the β-C2S phase is activated by dissolving ions into the crystal lattice to improve the hydration rate. The first principle and density functional theory were applied to study the effect of different ions on the activation of C2S, especially on the crystallographic structure and lattice parameters and electronic structure change.
2 Materials and methods
2.1 Synthesis of Ba-doped C2S
Analytical reagents and deionized water are applied during the solution preparation. β-C2S is synthesized according to ref. [16]. Analytical SiO2 and CaO with a stoichiometric proportion of Ca:Si = 2:1, 4% BaSO4 with total mass were mixed and ground uniformly by a small ball mill for 20 min until the sieve residue is less than 5%. After mixing anhydrous ethanol, the samples were made into a pill with a diameter of 15 mm and dried in a vacuum drying oven at 105℃. The samples were calcined in the furnace with the calcination temperature 1250°C, the heating rate 10°C/min, and heat preservation for 3 h. The samples were rapid cooling in the case of crystal transformation. The clinker was crushed and ground into a fine powder, and the chemical composition of β-C2S was analyzed by X-ray fluorescence spectrometry (ThermoFisher, ESCALAB 250Xi).
2.2 Characterization method
The structural variation of β-C2S doping Ba2+ ions was further analyzed by Nuclear Magnetic Resonance (29Si MAS-NMR) and Fourier transform infrared (FT-IR) spectroscopy. 29Si MAS-NMR was measured by a Bruker Avance II 400 MHz spectrometer with a field strength of 9.4 T, operating at 99.2 MHz. The chemical shifts were referenced to tetramethylsilane (TMS). FT-IR patterns were obtained by a Fourier transform infrared spectrometer (Nicolet is5003) with a wave length range from 250 to 4,000 cm−1.
2.3 Molecular dynamics simulation
In this paper, the first principle calculation is based on the density functional theory (DFT) and the plane-wave pseudopotential method (PWP) [31], and the CASTEP module of molecular structure and mechanics simulation software Materials Studio@ (Accelrys 6.2) is employed to calculate the electronic structure and cohesive energy β-C2S with/without doping Ba ions.
The state of the electronic structure is described by density functional and the generalized gradient approximation (GGA) [32]. The exchange–correlation functionals were calculated using Generalized Gradient Approximation (GGA) with the Perdew–Burke–Ernzerhof functional (PBE functional) [33]. The Brillouin zone was sampled with 4 × 4 × 4 k-points in the primitive cell. The values of kinetic energy cutoff E c and the k-points number are increased until the calculated energy converges within the required tolerance, where E c determines the number of plane waves and k points does the sampling of the irreducible wedge of the Brillouin zone [34]. The cutoff energy of plane-wave (PW) was 380 eV, the energy tolerance was 5 × 10−7 eV/atom, the force tolerance was 0.03 eV/Å, the stress tolerance was 0.05 GPa, and the displacement tolerance was 0.001 Å.
3 Results and analysis
3.1 Activation of Ba-doped β-C2S
The chemical composition of the calcined Ba-doped C2S is shown in Table 1, which shows that 2.55% Ba2+ is stabilized in the system. The NMR spectrum can reflect the atomic coordination and adjacent atomic effect. The crystallographic structure variation of C2S reported can be described by the shift of 29Si NMR spectrum [35,36]. Figure 1 shows 29Si spectrum peak shifts and the appearance of the secondary peak right beside the main peak in the NMR spectrum. It infers to the atom subordination change and octahedral transformation in the Ba-doped C2S.
Chemical composition of Ba-doped C2S (wt%)
| Al2O3 | CaO | SiO2 | Fe2O3 | K2O | MgO | P2O5 | SO3 | BaO | LOI |
|---|---|---|---|---|---|---|---|---|---|
| 1.89 | 62.12 | 30.06 | 1.12 | 0.23 | 0.67 | 0.21 | 0.45 | 2.55 | 0.7 |

29Si NMR patterns of β-C2S phases.
FI-IR analysis was further carried out to verify the lattice variation in Ba-doped C2S. FT-IR patterns of hydrated β-C2S and Ba-doped β-C2S at different curing age are shown in Figure 2. New peaks at 900 and 845 cm−1 in Ba-doped β-C2S are ascribed to the asymmetric stretching vibration of the [SiO4] tetrahedron, which confirmed that Ba2+ enters the crystal lattice after calcination and is consisted with the observation in NMR analysis [37]. The [SiO4] tetrahedral stretching vibration at 3,440, 1,420, and 1,020 cm−1 can be attributed to the formation of C–S–H gel [38]. The appearance of a stronger absorption at 1420 cm−1 is attributed to the faster β-C2S hydration rate after the Ba doping. It can be concluded that Ba2+ doping can significantly promote the β-C2S hydration at an early age.

FT-IR patterns of hydrated β-C2S (left) and Ba-doped β-C2S (right) at different curing age.
3.2 Crystal structure and lattice parameters
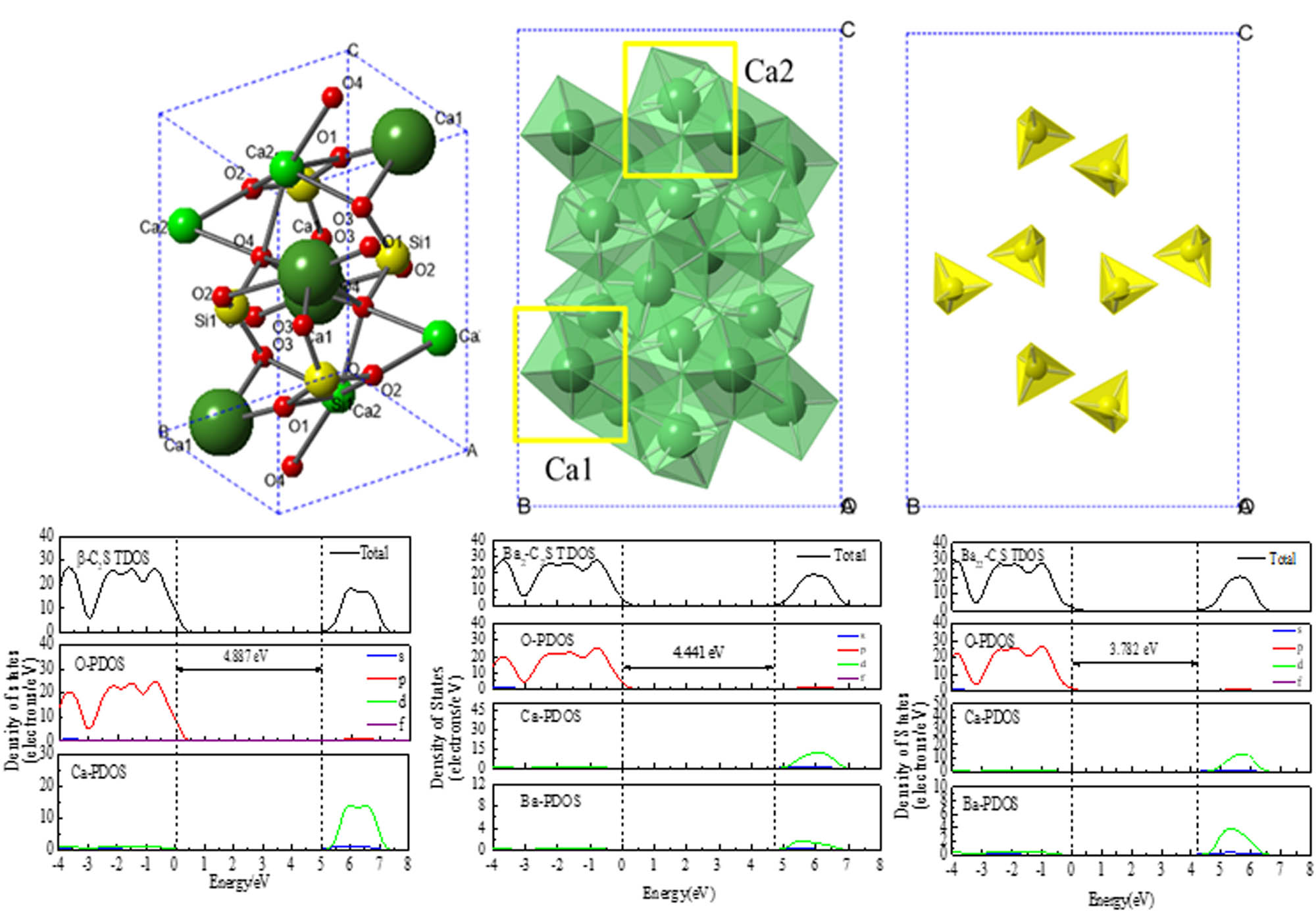
The lattice parameters of β-C2S are a = 5.502 Å, b = 6.745 Å, c = 9.297 Å, α = γ = 90.00°, and β = 94.59°, which belongs to the monoclinic system with the P21/n 1 space group [39]. There are 8Ca atoms, 4Si atoms, and 16O atoms in a single Ca2SiO4 lattice. According to the lattice model present in Figure 3 by Crystal Maker@ software, Ca atoms can combine with O atom to form [CaO6] octahedron and [CaO8] hexahedron. With Ba substituted Ca in β-C2S lattice, a new solid/solution Ca1−x Ba x SiO4 (x corresponds to the weight percentage) is formed [15]. Two solid/solution ratios of doped Ba are investigated, Ca1.969Ba0.031SiO4 with single Ba substitution and Ca1.938Ba0.062SiO4 with double Ba substitution [40]. Therefore, Ba1(2)-C2S and Ba11(12,22)-C2S in Table 2 represent two substitution sites of Ca atoms, where Ba1 and Ba2 represent Ca atom in the [CaO6] octahedron and the [CaO8] hexahedron, respectively. According to the lattice parameters listed in Table 1, the calculated parameters (a = 5.57 Å, b = 6.81 Å, c = 9.37 Å, α = γ = 90.00°, β = 94.66°) are basically the same as the theoretical parameters. The error is less than 2%, which confirms the accuracy of the model [41].
![Figure 3
Crystalline structure of β-C2S (Red balls represent O atoms; green balls represent Ca atoms; yellow balls represent Si atoms.) Ca1 and Ca2 atoms in β-C2S can combine with O atom to form [CaO6] octahedron and [CaO8] hexahedron. Si atom in β-C2S can combine with O atom to form [SiO4] tetrahedron.](/document/doi/10.1515/ntrev-2020-0082/asset/graphic/j_ntrev-2020-0082_fig_003.jpg)
Crystalline structure of β-C2S (Red balls represent O atoms; green balls represent Ca atoms; yellow balls represent Si atoms.) Ca1 and Ca2 atoms in β-C2S can combine with O atom to form [CaO6] octahedron and [CaO8] hexahedron. Si atom in β-C2S can combine with O atom to form [SiO4] tetrahedron.
Lattice parameters of β-C2S and Ba-doped C2S space group P21/n 1
| a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | Formation energy (eV) | |
|---|---|---|---|---|---|---|---|
| C2Stheo [39] | 5.50 | 6.75 | 9.30 | 90 | 94.59 | 90 | −15480.52 |
| C2Scal | 5.57 | 6.81 | 9.37 | 90 | 94.66 | 90 | −15481.62 |
| Ba1-C2S | 5.63 | 6.99 | 9.39 | 90.91 | 90.93 | 89.93 | −15177.56 |
| Ba2-C2S | 5.60 | 6.92 | 9.63 | 90.88 | 93.38 | 89.55 | −15177.47 |
| Ba11-C2S | 5.77 | 7.09 | 9.42 | 91.05 | 89.22 | 89.99 | −14873.37 |
| Ba22-C2S | 5.67 | 7.06 | 9.81 | 91.36 | 91.42 | 89.08 | −14873.21 |
| Ba12-C2S | 5.59 | 7.10 | 9.71 | 91.47 | 92.17 | 89.66 | −14873.29 |
When compared with general β-C2S, the addition of Ba leads to the lattice deformation and the reduction of formation energy in Ba-doped C2S. The spacing of each crystal plane is increased, and the inner holes are enlarged accordingly. Higher degrees of Ba2+ substitution leads to higher variants of crystalline structure. Comparing the Ca2+ substitution in [CaO6] or [CaO8], the lattice deformation and hydraulic reactivity are more significant in Ba2-C2S and Ba22-C2S.
The low hydraulic reactivity of β-C2S is ascribed to two aspects, one is the H2O molecules cannot enter the cavity in the β-C2S island structure, the other is that Ca attached to the tetrahedron [SiO4] is not easily dissolved out [28,42]. Pritts et al. [43] have found that Ba, Fe, Al, and Pb doped in β-C2S clinkers can reduce crystalline symmetry and increase the hydraulic reactivity [40,41,42,43,44]. Due to the limitations of microscopic characterization methods, the correlation between the mechanism of hydration kinetics and the crystallographic structure of Ba-doped C2S remains to be further studied; therefore, the electronic structure Ba-doped C2S is further studied in the following.
3.3 Electronic structure of Ba-doped C2S
The total and partial density of states (TDOS and PDOS) for β-C2S, Ba1(2)-C2S and Ba11(12,22)-C2S calculated are shown in Figure 4. The main contribution to valence band in β-C2S arises from the O-2p located from −4 to 0 eV below the Fermi energy, the main contribution to the conduction band in β-C2S arises from the Ca-3d located from 5 to 7 eV above the Fermi energy. The band gap between VBM and CBM is 4.887 eV. In the case of Ba1(2)-C2S, the original bond state in the crystal structure has changed with Ba-adopted. Partial contribution to the conduction band comes from the Ba-3d located from 5 to 7 eV. The band gaps in the Ba1-C2S and Ba2-C2S are 4.762 and 4.441 eV correspondingly. In the case of Ba11(12,22)-C2S, the band gaps between VBM and CBM for each crystal structure are 4.31, 3.78, and 4.41 eV respectively, which indicates that the band gap decreases with the increment of the solid/solution ratios of doped Ba in the system.

The total and partial density of states (TDOS and PDOS) of β-C2S and Ba-doped C2S. Dashed lines represent Fermi energy.
According to the coordination theory of crystal chemistry, the cations are filled in the [CaO6] octahedron when r +/ r − = 0.414–0.732; the cations are filled in the [CaO8] hexahedron when r +/ r − = 0.732–1.0. Due to r Ca 2+/ r O 2− = 0.75 and r Ba 2+/ r O 2− = 1.35/1.32 ≈ 1.0, β-C2S can incorporate Ba2+ by substitution of Ca2+ in the [CaO8] hexahedron instead of [CaO6] octahedron, with the solid solution of general formula Ca2−x Ba x SiO4 [37,45]. The formation of coplanar structures of [Ca(Ba)O x ]2x−2 polyhedra leads to the structures of the coplanar polyhedron change from spiral chains to three-dimensional network structures [46]. It is suggested that the hydraulic activity of Ba-doped β-C2S is greatly improved with the formation of Ba–O–Si chains instead of Ca–O–Si chains. When β-C2S contact with water, the Ca2+/[CaO x ]2x−2 polyhedra is dissolved, where OH− and Si-OH are formed accordingly [40]. Due to the internal forces in the [CaO x ]2x−2 are unbalanced, a component force points to the location with Ca2+ extracted, which accelerates the continuous dissolution of Ca2+ ions. In conclusion, the electron structure variation is related to the variation of charge exchange and atomic hybridization orbital in Ba-doped β-C2S with different doping positions.
4 Conclusions
The following conclusions can be drawn based on the laboratory investigations:
Doping Ba2+ in β-C2S can significantly promote the early hydration. According to the electronic structure and hydraulic reactivity results, the electron structure variation is related to the variation of charge exchange and atomic hybridization orbital in Ba-doped β-C2S with different doping positions.
The hydration kinetics and electronic structure of β-C2S can be established systematically and intuitively by the first principle calculation. Comparing the Ca2+ substitution in [CaO6] or [CaO8], the lattice deformation and hydraulic reactivity is more significant in Ba2-C2S and Ba22-C2S.
The crystallographic structure of β-C2S can be activated by doping Ba atom and the crystal formation energy increases and the band gap between VBM and CBM become narrow in the activated β-C2S crystallographic structure.
Acknowledgments
The authors would like to appreciate the financial sponsored by Shanghai Sailing Program No. 20YF1431800 and National Natural Science Foundation of China (No.51872064).
-
Author contributions: Investigation, writing – original draft preparation, visualization, software: L. C.; Investigation, formal analysis: A. Z., L. C., D. Z.; Writing – review & editing: S. L., Z. Q.; Supervision: Z. W.
-
Conflict of interest: The authors declare no conflict of interest regarding the publication of this paper.
References
[1] Benhelal E, Zahedi G, Shamsaei E, Bahadori A. Global strategies and potentials to curb CO2 emissions in cement industry. J Clean Prod. 2013;51:142–61.10.1016/j.jclepro.2012.10.049Search in Google Scholar
[2] Skalamprinos S, Jen G, Galan I, Whittaker M, Elhoweris A, Glasser F. The synthesis and hydration of ternesite, Ca5(SiO4)2SO4. Cement Concrete Res. 2018;113:27–40.10.1016/j.cemconres.2018.06.012Search in Google Scholar
[3] Stanek T, Sulovsky P. Active low-energy belite cement. Cement. Concrete. Res. 2015;68:203–10.Search in Google Scholar
[4] Huang Y, Qian J, Kang X, Yu J, Fan Y, Dang Y, et al. Belite-calcium sulfoaluminate cement prepared with phosphogypsum: Influence of P2O5 and F on the clinker formation and cement performances. Constr Build Mater. 2019;203:432–42.10.1016/j.conbuildmat.2019.01.112Search in Google Scholar
[5] Liu Y, Jia M, Song C, Lu S, Wang H, Zhang G, et al. Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide. Nanotechnol Rev. 2020;9:1.10.1515/ntrev-2020-0002Search in Google Scholar
[6] Da Costa EB, Rodríguez ED, Bernal SA, Provis JL, Gobbo LA, Kirchheim AP. Production and hydration of calcium sulfoaluminate-belite cements derived from aluminium anodising sludge. Constr Build Mater. 2016;122:373–83.10.1016/j.conbuildmat.2016.06.022Search in Google Scholar
[7] Meng T, Zhang J, Wei D, Shen J. Effect of nano-strengthening on the properties and microstructure of recycled concrete. Nanotechnol Rev. 2020;9:1.10.1515/ntrev-2020-0008Search in Google Scholar
[8] Zhuang C, Chen Y. The effect of nano-SiO2 on concrete properties: A review. Nanotechnol Rev. 2019;8(1):562–72.10.1515/ntrev-2019-0050Search in Google Scholar
[9] Rungchet A, Chindaprasirt P, Wansom S, Pimraksa K. Hydrothermal synthesis of calcium sulfoaluminate-belite cement from industrial waste materials. J Clean Prod. 2016;115:273–83.10.1016/j.jclepro.2015.12.068Search in Google Scholar
[10] Chi L, Lu S, Yao Y. Damping additives used in cement-matrix composites: A review. Compos Part B. 2019;164(1):26–36.10.1016/j.compositesb.2018.11.057Search in Google Scholar
[11] Piekkari K, Ohenoja K, Isteri V, Tanskanen P, Illikainen M. Immobilization of heavy metals, selenate, and sulfate from a hazardous industrial side stream by using calcium sulfoaluminate-belite cement. J Clean Prod. 2020;258:120560.10.1016/j.jclepro.2020.120560Search in Google Scholar
[12] Zheng Y, Li Q, Liu Y. Mine tailing as alternative to clay for producing belite cement clinker. Adv Mater Res. 2013;2704–13.10.4028/www.scientific.net/AMR.726-731.2704Search in Google Scholar
[13] Ávalos-Rendón TL, Chelala EAP, Mendoza Escobedo CJ, Figueroa IA, Lara VH, Palacios-Romero LM. Synthesis of belite cements at low temperature from silica fume and natural commercial zeolite. Mater Sci Eng. 2018;229:79–85.10.1016/j.mseb.2017.12.020Search in Google Scholar
[14] Chi L, Wang Z, Lu S, Zhao DZ, Yao Y. Development of mathematical models for predicting the compressive strength and hydration process using the EIS impedance of cementitious materials. Constr Build Mater. 2019;208:652–68.10.1016/j.conbuildmat.2019.03.056Search in Google Scholar
[15] Qi C, Spagnoli D, Fourie A. DFT-D study of single water adsorption on low-index surfaces of calcium silicate phases in cement. Appl Surf Sci. 2020;518:146255.10.1016/j.apsusc.2020.146255Search in Google Scholar
[16] Thomas JJ, Ghazizadeh S, Masoero E. Kinetic mechanisms and activation energies for hydration of standard and highly reactive forms of beta-dicalcium silicate (C2S). Cement Concrete Res. 2017;100:322–28.10.1016/j.cemconres.2017.06.001Search in Google Scholar
[17] Martínez IM, Meseguer-Olmo L, Bernabeu-Esclapez A, Velásquez PA, De Aza PN. In vitro behavior of α-tricalcium phosphate doped with dicalcium silicate in the system Ca2SiO4–Ca3(PO4)2. Mater Charact. 2012;63:47–55.10.1016/j.matchar.2011.10.013Search in Google Scholar
[18] Pritts IM, Daugherty KE. The effect of stabilizing agents on the hydration rate of β-C2S. Cement Concrete Res. 1976;6(6):783–95.10.1016/0008-8846(76)90008-9Search in Google Scholar
[19] Chen W, Lv G, Hu W, Li D, Chen S, Dai Z. Synthesis and applications of graphene quantum dots: A review. Nanotechnol Rev. 2018;7(2):157–85.10.1515/ntrev-2017-0199Search in Google Scholar
[20] Popescu CD, Muntean M, Sharp JH. Industrial trial production of low energy belite cement. Cement Concrete Comp. 2003;25(7):689–93.10.1016/S0958-9465(02)00097-5Search in Google Scholar
[21] Maiti SC, Ghoroi C. Influence of catalytic nano-additive for stabilization of beta-dicalcium silicate and its hydration rate with different electrolytes. Cement Concrete Res. 2017;98:111–21.10.1016/j.cemconres.2017.04.008Search in Google Scholar
[22] Manohar CS, Kumar BS, Sadhu SPP, Srimadh SK, Varma KBR. Novel Lead-free biocompatible piezoelectric Hydroxyapatite (HA)-BCZT (Ba0.85Ca0.15Zr0.1Ti0.9O3) nanocrystal composites for bone regeneration. Nanotechnol Rev. 2019;8(1):61–78.10.1515/ntrev-2019-0006Search in Google Scholar
[23] Li H, Zhang X, Liu Q, Liu Y, Liu H, Wang X, et al. First-principles calculations of mechanical and thermodynamic properties of tungsten-based alloy. Nanotechnol Rev. 2019;8(1):258–65.10.1515/ntrev-2019-0024Search in Google Scholar
[24] Cuesta A, Losilla ER, Aranda MAG, Sanz J, De la Torre AG. Reactive belite stabilization mechanisms by boron-bearing dopants. Cement Concrete Res. 2012;42(4):598–06.10.1016/j.cemconres.2012.01.006Search in Google Scholar
[25] Wang Z, Ma X, Niu J. Solutionizing of Additional Ions in Belite-rich Clinkers and the Properties of the Resulting Cement. J Wuhan Univ Technol. 2009;24(5):834–37.10.1007/s11595-009-5834-6Search in Google Scholar
[26] Pala MG, Esseni D. Full-band quantum simulation of electron devices with the pseudopotential method: Theory, implementation, and applications. Phys Rev B. 2018;97:12531012.10.1103/PhysRevB.97.125310Search in Google Scholar
[27] Sellier JM, Sviercoski RF, Dimov I. On the Wigner Monte Carlo method coupled to pseudopotential models. J Comput Appl Math. 2016;293:217–22.10.1016/j.cam.2015.01.033Search in Google Scholar
[28] Wang Q, Li F, Shen X, Shi W, Li X, Guo Y, et al. Relation between reactivity and electronic structure for α′L-, β- and γ-dicalcium silicate: A first-principles study. Cement Concrete Res. 2014;57:28–32.10.1016/j.cemconres.2013.12.004Search in Google Scholar
[29] Durgun E, Manzano H, Pellenq RJM, Grossman JC. Understanding and Controlling the Reactivity of the Calcium Silicate phases from First Principles. Chem Mater. 2012;24(7):1262–67.10.1021/cm203127mSearch in Google Scholar
[30] Wei L, Cui S, Guo H, Zhang L. The effect of alkali metal over Mn/TiO2 for low-temperature SCR of NO with NH3 through DRIFT and DFT. Comp Mater. 2018;144:216–22.10.1016/j.commatsci.2017.12.013Search in Google Scholar
[31] Kuellmer K, Kraemer A, Reith D, Joppich W, Foysi H. Numerical optimisation of the pseudopotential-based lattice Boltzmann method. J Compu Sci. 2016;17:384–93.10.1016/j.jocs.2016.04.005Search in Google Scholar
[32] Dharmawardhana CC, Misra A, Ching W. Theoretical investigation of C-(A)-S-H(I) cement hydrates. Constr Build Mater. 2018;184:536–48.10.1016/j.conbuildmat.2018.07.004Search in Google Scholar
[33] Li H, Zhang X, Liu Q, Liu Y, Liu H, Wang X, et al. First-principles calculations of mechanical and thermodynamic properties of tungsten-based alloy. Nanotechnol Rev. 2019;8:258–65.10.1515/ntrev-2019-0024Search in Google Scholar
[34] Shein IR, Ivanovskii AL. Electronic properties of the novel 18-K superconducting Y2C3 as compared with 4-K YC2 from first principles calculations. Solid State Commun. 2004;131(3):223–27.10.1016/j.ssc.2004.04.048Search in Google Scholar
[35] Schneider J, Cincotto MA, Panepucci H. Si-29 and Al-27 high-resolution NMR characterization of calcium silicate hydrate phases in activated blast-furnace slag pastes. Cement Concrete Res. 2001;31(7):993–01.10.1016/S0008-8846(01)00530-0Search in Google Scholar
[36] Pardal X, Brunet F, Charpentier T, Pochard I, Nonat A. Al-27 and Si-29 Solid-State NMR Characterization of Calcium-Aluminosilicate-Hydrate. Inorg Chem. 2012;51(3):1827–36.10.1021/ic202124xSearch in Google Scholar PubMed
[37] Guan W, Zhao X. Fluoride recovery using porous calcium silicate hydrates via spontaneous Ca2 + and OH− release. Sep Purif Technol. 2016;165:71–7.10.1016/j.seppur.2016.03.050Search in Google Scholar
[38] Bernard E, Dauzères A, Lothenbach B. Magnesium and calcium silicate hydrates, Part II: Mg-exchange at the interface “low-pH” cement and magnesium environment studied in a C-S-H and M-S-H model system. Appl Geochem. 2018;89:210–18.10.1016/j.apgeochem.2017.12.006Search in Google Scholar
[39] Taylor HFW. Cement chemistry. 2nd ed. London: Thomas Telford Publishing; 1990.Search in Google Scholar
[40] Bulina NV, Chaikina MV, Prosanov IY, Dudina DV. Strontium and silicate co-substituted hydroxyapatite: Mechanochemical synthesis and structural characterization. Mater Sci Eng B. 2020;262:114719.10.1016/j.mseb.2020.114719Search in Google Scholar
[41] Wang F, Zhang Y, Jiang J, Yin B, Li Z. Effect of temperature on the capillary transport of sodium sulfate solution in calcium silicate hydrate nanopore: A molecular dynamics study. Constr Build Mater. 2020;231:117111.10.1016/j.conbuildmat.2019.117111Search in Google Scholar
[42] Azeem M, Azhar Saleem M. Role of electrostatic potential energy in carbon nanotube augmented cement paste matrix. Constr Build Mater. 2020;239:117875.10.1016/j.conbuildmat.2019.117875Search in Google Scholar
[43] Pritts IM, Daugherty KE. The effect of stabilizing agents on the hydration rate of β-C2S. Cement Concrete Res. 1976;6(6):783–95.10.1016/0008-8846(76)90008-9Search in Google Scholar
[44] Zhu G, Li H, Li S, Hou X, Wang X. Crystallization of calcium silicate at elevated temperatures in highly alkaline system of Na2O-CaO-SiO2-H2O. Chinese J Chem Eng. 2017;25(10):1539–44.10.1016/j.cjche.2017.02.012Search in Google Scholar
[45] Tavakoli D, Tarighat A. Molecular dynamics study on the mechanical properties of Portland cement clinker phases. Comp Mater Sci. 2016;119:65–73.10.1016/j.commatsci.2016.03.043Search in Google Scholar
[46] Zhang X, Zhang Yi, Tian B, Jia Y, Liu Y, Song K, et al. Cr effects on the electrical contact properties of the Al2O3-Cu/15W composites. Nanotechnol Rev. 2019;8(1):128–35.10.1515/ntrev-2019-0012Search in Google Scholar
© 2020 Lin Chi et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Generalized locally-exact homogenization theory for evaluation of electric conductivity and resistance of multiphase materials
- Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide
- Characterization of mechanical properties of epoxy/nanohybrid composites by nanoindentation
- Graphene and CNT impact on heat transfer response of nanocomposite cylinders
- A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites
- Ultrasmall Fe3O4 nanoparticles induce S-phase arrest and inhibit cancer cells proliferation
- Effect of aging on properties and nanoscale precipitates of Cu-Ag-Cr alloy
- Effect of nano-strengthening on the properties and microstructure of recycled concrete
- Stabilizing effect of methylcellulose on the dispersion of multi-walled carbon nanotubes in cementitious composites
- Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites
- Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation
- Preparation and properties of 3D interconnected CNTs/Cu composites
- On factors affecting surface free energy of carbon black for reinforcing rubber
- Nano-silica modified phenolic resin film: manufacturing and properties
- Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete
- Halloysite nanotubes in polymer science: purification, characterization, modification and applications
- Cellulose hydrogel skeleton by extrusion 3D printing of solution
- Crack closure and flexural tensile capacity with SMA fibers randomly embedded on tensile side of mortar beams
- An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites
- Utilization of red mud for producing a high strength binder by composition optimization and nano strengthening
- One-pot synthesis of nano titanium dioxide in supercritical water
- Printability of photo-sensitive nanocomposites using two-photon polymerization
- In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries
- Effect of nano and micro conductive materials on conductive properties of carbon fiber reinforced concrete
- Tribological performance of nano-diamond composites-dispersed lubricants on commercial cylinder liner mating with CrN piston ring
- Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance
- Genetic mechanisms of deep-water massive sandstones in continental lake basins and their significance in micro–nano reservoir storage systems: A case study of the Yanchang formation in the Ordos Basin
- Effects of nanoparticles on engineering performance of cementitious composites reinforced with PVA fibers
- Band gap manipulation of viscoelastic functionally graded phononic crystal
- Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution
- Manipulating conductive network formation via 3D T-ZnO: A facile approach for a CNT-reinforced nanocomposite
- Microstructure and mechanical properties of WC–Ni multiphase ceramic materials with NiCl2·6H2O as a binder
- Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite
- Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function
- Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing
- Corrosion behaviour of multilayer CrN coatings deposited by hybrid HIPIMS after oxidation treatment
- Surface hydrophobicity and oleophilicity of hierarchical metal structures fabricated using ink-based selective laser melting of micro/nanoparticles
- Research on bond–slip performance between pultruded glass fiber-reinforced polymer tube and nano-CaCO3 concrete
- Antibacterial polymer nanofiber-coated and high elastin protein-expressing BMSCs incorporated polypropylene mesh for accelerating healing of female pelvic floor dysfunction
- Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films
- A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination
- Arrangement structure of carbon nanofiber with excellent spectral radiation characteristics
- Effect of different particle sizes of nano-SiO2 on the properties and microstructure of cement paste
- Superior Fe x N electrocatalyst derived from 1,1′-diacetylferrocene for oxygen reduction reaction in alkaline and acidic media
- Facile growth of aluminum oxide thin film by chemical liquid deposition and its application in devices
- Liquid crystallinity and thermal properties of polyhedral oligomeric silsesquioxane/side-chain azobenzene hybrid copolymer
- Laboratory experiment on the nano-TiO2 photocatalytic degradation effect of road surface oil pollution
- Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage
- Conversion of sub-µm calcium carbonate (calcite) particles to hollow hydroxyapatite agglomerates in K2HPO4 solutions
- Exact solutions of bending deflection for single-walled BNNTs based on the classical Euler–Bernoulli beam theory
- Effects of substrate properties and sputtering methods on self-formation of Ag particles on the Ag–Mo(Zr) alloy films
- Enhancing carbonation and chloride resistance of autoclaved concrete by incorporating nano-CaCO3
- Effect of SiO2 aerogels loading on photocatalytic degradation of nitrobenzene using composites with tetrapod-like ZnO
- Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles
- Hydration activity, crystal structural, and electronic properties studies of Ba-doped dicalcium silicate
- Microstructure and mechanical properties of brazing joint of silver-based composite filler metal
- Polymer nanocomposite sunlight spectrum down-converters made by open-air PLD
- Cryogenic milling and formation of nanostructured machined surface of AISI 4340
- Braided composite stent for peripheral vascular applications
- Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre–reinforced starch biopolymer composites
- Study on influencing factors of photocatalytic performance of CdS/TiO2 nanocomposite concrete
- Improving flexural and dielectric properties of carbon fiber epoxy composite laminates reinforced with carbon nanotubes interlayer using electrospray deposition
- Scalable fabrication of carbon materials based silicon rubber for highly stretchable e-textile sensor
- Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery
- Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries
- Synthesis of functionalized carbon nanotubes for fluorescent biosensors
- Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete
- Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage
- Microstructural evolution and properties of Cu–20 wt% Ag alloy wire by multi-pass continuous drawing
- Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene
- Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications
- Preparation and piezoresistivity of carbon nanotube-coated sand reinforced cement mortar
- Vibrational analysis of an irregular single-walled carbon nanotube incorporating initial stress effects
- Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials
- Single pulse laser removal of indium tin oxide film on glass and polyethylene terephthalate by nanosecond and femtosecond laser
- Mechanical reinforcement with enhanced electrical and heat conduction of epoxy resin by polyaniline and graphene nanoplatelets
- High-efficiency method for recycling lithium from spent LiFePO4 cathode
- Degradable tough chitosan dressing for skin wound recovery
- Static and dynamic analyses of auxetic hybrid FRC/CNTRC laminated plates
- Review articles
- Carbon nanomaterials enhanced cement-based composites: advances and challenges
- Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide
- Review on modeling and application of chemical mechanical polishing
- Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites
- Advances in modelling and analysis of nano structures: a review
- Mechanical properties of nanomaterials: A review
- New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics
- A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials
- Recent development and applications of nanomaterials for cancer immunotherapy
- Advances in biomaterials for adipose tissue reconstruction in plastic surgery
- Advances of graphene- and graphene oxide-modified cementitious materials
- Theories for triboelectric nanogenerators: A comprehensive review
- Nanotechnology of diamondoids for the fabrication of nanostructured systems
- Material advancement in technological development for the 5G wireless communications
- Nanoengineering in biomedicine: Current development and future perspectives
- Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview
- Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review
- Carbon nanotube–reinforced polymer composite for electromagnetic interference application: A review
- Functionalized layered double hydroxide applied to heavy metal ions absorption: A review
- A new classification method of nanotechnology for design integration in biomaterials
- Finite element analysis of natural fibers composites: A review
- Phase change materials for building construction: An overview of nano-/micro-encapsulation
- Recent advance in surface modification for regulating cell adhesion and behaviors
- Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions
- Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review
- Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications
- A review of passive methods in microchannel heat sink application through advanced geometric structure and nanofluids: Current advancements and challenges
- Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices
- Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging
- Synthesis of graphene: Potential carbon precursors and approaches
- A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE)
- Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water
- Analysis of functionally graded carbon nanotube-reinforced composite structures: A review
- Application of nanomaterials in ultra-high performance concrete: A review
- Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer
- Advanced nickel nanoparticles technology: From synthesis to applications
- Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable?
- Progress in construction of bio-inspired physico-antimicrobial surfaces
- From materials to devices using fused deposition modeling: A state-of-art review
- A review for modified Li composite anode: Principle, preparation and challenge
- Naturally or artificially constructed nanocellulose architectures for epoxy composites: A review
Articles in the same Issue
- Research Articles
- Generalized locally-exact homogenization theory for evaluation of electric conductivity and resistance of multiphase materials
- Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide
- Characterization of mechanical properties of epoxy/nanohybrid composites by nanoindentation
- Graphene and CNT impact on heat transfer response of nanocomposite cylinders
- A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites
- Ultrasmall Fe3O4 nanoparticles induce S-phase arrest and inhibit cancer cells proliferation
- Effect of aging on properties and nanoscale precipitates of Cu-Ag-Cr alloy
- Effect of nano-strengthening on the properties and microstructure of recycled concrete
- Stabilizing effect of methylcellulose on the dispersion of multi-walled carbon nanotubes in cementitious composites
- Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites
- Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation
- Preparation and properties of 3D interconnected CNTs/Cu composites
- On factors affecting surface free energy of carbon black for reinforcing rubber
- Nano-silica modified phenolic resin film: manufacturing and properties
- Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete
- Halloysite nanotubes in polymer science: purification, characterization, modification and applications
- Cellulose hydrogel skeleton by extrusion 3D printing of solution
- Crack closure and flexural tensile capacity with SMA fibers randomly embedded on tensile side of mortar beams
- An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites
- Utilization of red mud for producing a high strength binder by composition optimization and nano strengthening
- One-pot synthesis of nano titanium dioxide in supercritical water
- Printability of photo-sensitive nanocomposites using two-photon polymerization
- In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries
- Effect of nano and micro conductive materials on conductive properties of carbon fiber reinforced concrete
- Tribological performance of nano-diamond composites-dispersed lubricants on commercial cylinder liner mating with CrN piston ring
- Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance
- Genetic mechanisms of deep-water massive sandstones in continental lake basins and their significance in micro–nano reservoir storage systems: A case study of the Yanchang formation in the Ordos Basin
- Effects of nanoparticles on engineering performance of cementitious composites reinforced with PVA fibers
- Band gap manipulation of viscoelastic functionally graded phononic crystal
- Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution
- Manipulating conductive network formation via 3D T-ZnO: A facile approach for a CNT-reinforced nanocomposite
- Microstructure and mechanical properties of WC–Ni multiphase ceramic materials with NiCl2·6H2O as a binder
- Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite
- Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function
- Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing
- Corrosion behaviour of multilayer CrN coatings deposited by hybrid HIPIMS after oxidation treatment
- Surface hydrophobicity and oleophilicity of hierarchical metal structures fabricated using ink-based selective laser melting of micro/nanoparticles
- Research on bond–slip performance between pultruded glass fiber-reinforced polymer tube and nano-CaCO3 concrete
- Antibacterial polymer nanofiber-coated and high elastin protein-expressing BMSCs incorporated polypropylene mesh for accelerating healing of female pelvic floor dysfunction
- Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films
- A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination
- Arrangement structure of carbon nanofiber with excellent spectral radiation characteristics
- Effect of different particle sizes of nano-SiO2 on the properties and microstructure of cement paste
- Superior Fe x N electrocatalyst derived from 1,1′-diacetylferrocene for oxygen reduction reaction in alkaline and acidic media
- Facile growth of aluminum oxide thin film by chemical liquid deposition and its application in devices
- Liquid crystallinity and thermal properties of polyhedral oligomeric silsesquioxane/side-chain azobenzene hybrid copolymer
- Laboratory experiment on the nano-TiO2 photocatalytic degradation effect of road surface oil pollution
- Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage
- Conversion of sub-µm calcium carbonate (calcite) particles to hollow hydroxyapatite agglomerates in K2HPO4 solutions
- Exact solutions of bending deflection for single-walled BNNTs based on the classical Euler–Bernoulli beam theory
- Effects of substrate properties and sputtering methods on self-formation of Ag particles on the Ag–Mo(Zr) alloy films
- Enhancing carbonation and chloride resistance of autoclaved concrete by incorporating nano-CaCO3
- Effect of SiO2 aerogels loading on photocatalytic degradation of nitrobenzene using composites with tetrapod-like ZnO
- Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles
- Hydration activity, crystal structural, and electronic properties studies of Ba-doped dicalcium silicate
- Microstructure and mechanical properties of brazing joint of silver-based composite filler metal
- Polymer nanocomposite sunlight spectrum down-converters made by open-air PLD
- Cryogenic milling and formation of nanostructured machined surface of AISI 4340
- Braided composite stent for peripheral vascular applications
- Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre–reinforced starch biopolymer composites
- Study on influencing factors of photocatalytic performance of CdS/TiO2 nanocomposite concrete
- Improving flexural and dielectric properties of carbon fiber epoxy composite laminates reinforced with carbon nanotubes interlayer using electrospray deposition
- Scalable fabrication of carbon materials based silicon rubber for highly stretchable e-textile sensor
- Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery
- Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries
- Synthesis of functionalized carbon nanotubes for fluorescent biosensors
- Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete
- Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage
- Microstructural evolution and properties of Cu–20 wt% Ag alloy wire by multi-pass continuous drawing
- Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene
- Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications
- Preparation and piezoresistivity of carbon nanotube-coated sand reinforced cement mortar
- Vibrational analysis of an irregular single-walled carbon nanotube incorporating initial stress effects
- Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials
- Single pulse laser removal of indium tin oxide film on glass and polyethylene terephthalate by nanosecond and femtosecond laser
- Mechanical reinforcement with enhanced electrical and heat conduction of epoxy resin by polyaniline and graphene nanoplatelets
- High-efficiency method for recycling lithium from spent LiFePO4 cathode
- Degradable tough chitosan dressing for skin wound recovery
- Static and dynamic analyses of auxetic hybrid FRC/CNTRC laminated plates
- Review articles
- Carbon nanomaterials enhanced cement-based composites: advances and challenges
- Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide
- Review on modeling and application of chemical mechanical polishing
- Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites
- Advances in modelling and analysis of nano structures: a review
- Mechanical properties of nanomaterials: A review
- New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics
- A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials
- Recent development and applications of nanomaterials for cancer immunotherapy
- Advances in biomaterials for adipose tissue reconstruction in plastic surgery
- Advances of graphene- and graphene oxide-modified cementitious materials
- Theories for triboelectric nanogenerators: A comprehensive review
- Nanotechnology of diamondoids for the fabrication of nanostructured systems
- Material advancement in technological development for the 5G wireless communications
- Nanoengineering in biomedicine: Current development and future perspectives
- Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview
- Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review
- Carbon nanotube–reinforced polymer composite for electromagnetic interference application: A review
- Functionalized layered double hydroxide applied to heavy metal ions absorption: A review
- A new classification method of nanotechnology for design integration in biomaterials
- Finite element analysis of natural fibers composites: A review
- Phase change materials for building construction: An overview of nano-/micro-encapsulation
- Recent advance in surface modification for regulating cell adhesion and behaviors
- Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions
- Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review
- Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications
- A review of passive methods in microchannel heat sink application through advanced geometric structure and nanofluids: Current advancements and challenges
- Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices
- Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging
- Synthesis of graphene: Potential carbon precursors and approaches
- A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE)
- Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water
- Analysis of functionally graded carbon nanotube-reinforced composite structures: A review
- Application of nanomaterials in ultra-high performance concrete: A review
- Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer
- Advanced nickel nanoparticles technology: From synthesis to applications
- Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable?
- Progress in construction of bio-inspired physico-antimicrobial surfaces
- From materials to devices using fused deposition modeling: A state-of-art review
- A review for modified Li composite anode: Principle, preparation and challenge
- Naturally or artificially constructed nanocellulose architectures for epoxy composites: A review

