Abstract
The surface activity of carbon black (CB) is an important factor affecting the reinforcement of rubber. The quantitative determination of the surface activity (surface free energy) of CB is of great significance. A simplified formula is obtained to determine the free energy of CB surface through theoretical analysis and mathematical derivation. The surface free energy for four kinds of industrial CBs were measured by inverse gas chromatography, and the influential factors were studied. The results showed that the aging time of the chromatographic column plays an important role in accurate measurement of the surface free energy of CB, in comparison with the influences from the inlet pressure and carrier gas flow rate of the chromatographic column filled with CB. Several kinds of industrial CB were treated at high temperature, and the surface free energy of CB had a significant increase. With the increase of surface free energy, the maximum torque was decreased significantly, the elongation at break tended to increase, the heat generation of vulcanizates was increased, and the wear resistance was decreased.
1 Introduction
As an industrial raw material, carbon black (CB) is a fluffy black powder with diameter of small size. It is widely used for various applications such as reinforcement for rubber [1], anti-aging and anti-static additives for plastic [2], anode materials for lithium ion battery [3, 4, 5], support for catalyst [6], pigment for printing and dyeing industries [7], etc. Recently, some literatures have reported that the carbon fibers or graphene were used as reinforcing agents for rubber. However, due to the high cost and low reinforcing effect, they have not been widely used [8]. In fact, the CB used for reinforcing rubber occupies its 94% production in industry.
According to previous studies, the particle size, structure and surface activity of CB are the main factors that affect the reinforcement of rubber. In the chemical sense, the surface activity of CB depends on different chemical groups attached on the surface, such as carboxyl, hydroxyl, quinonyl and lactone [9]. In the physical sense, it is reflected by the surface free energy of CB, especially its non-polar parts, that is, the dispersive free energy. Due to the high surface activity and small size, CB is prone to agglomerate, making it difficult to disperse in some polymer matrices. As a polar inorganic particle, it is also difficult to disperse uniformly in some non-polar or low polar rubbers, such as styrene-butadiene and butadiene rubbers. The dispersion of CB affects the performance of its properties and limits its varieties of applications. Therefore, the surface of CB is generally modified by chemical or physical methods before being used to meet the actual needs. The major modification methods include dispersant treatment, surface oxidation, surface graft and plasma [10, 11, 12, 13, 14]. Through surface modification, the surface free energy of CB will be changed accordingly to meet the specific requirements. For example, hydrophilic groups such as carboxyl and hydroxyl are formed on the surface of CB by oxidation treatment, which enhances the dispersion of CB in aqueous solution [15]. It can reduce the intermolecular force and improve the compatibility of CB and medium by the graft modification.
Previous studies [16, 17] showed that the bound rubber has a crucial influence on the CB-reinforced rubber, while the bound rubber is affected by the surface activity of CB. The disfigurements of structure, impurity, hydrogen and chemical groups on the surface of CB are all accessible positions with high activity, and some of these positions plays an important role [18]. The reduction of highly active positions will decrease the amount of bound rubbers, leading to the decrease of stress at definite elongation, tensile strength, abrasion resistance, and increase of tan δ of gross rubber [19]. The study on the surface activity of CB is indispensable. However, there is little study on the surface free energy of CB, and most of the previous studies only focused on the investigation of structural and functional groups of CB.
At present, the main calculation model of surface free energy is the Owens-Wendt-Rabel-Kaelble (OWRK) model, which is based on Fowkes’ sum rule of solid surface free energy. The surface free energy can be divided into the dispersive and the polar components, reflecting the different molecular types of forces [20, 21, 22]. The main methods used to determine the surface free energy are inverse gas chromatography (IGC) and contact angle measurements [23, 24]. In the contact angle measurement, the dispersive and polar components of the surface free energy are calculated by measuring the contact angles of a known small molecular liquid with solid material. However, the users sometimes were perplexed by the unreproducible values under repeated measurements and the high requirement of samples preparation [25, 26].
The IGC is one of the simplest and most reliable methods to measure the surface properties of materials, and hence widely used for providing information about some surface physicochemical properties such as diffusion kinetics, surface energy heterogeneity, polar functionality and so on. This chromatographic method is different from the conventional gas chromatography. Prior to test, the sample is used as a stationary phase, and the known small molecular liquid is used as the probe molecule. The gasification probe molecules are taken into the chromatographic column by the carrier gas under certain conditions. The surface free energy of the stationary phase is studied by measuring the retention time of the probe molecules required to pass through the chromatographic column[27, 28]. Compared with the contact angle measurement, no special sample preparation and elaborated apparatus are needed, so accurate measurements are possible over a wide range of temperatures [27]. The sample could be nanoparticles, a fibrous composition, polymer and so on [29, 30].
High surface activity is the main feature of CB, different from other fillers, which has an essential effect on the interaction between filler surface and polymer matrix. Therefore, accurate determination of the surface activity (surface free energy) of CB becomes important. However, there is still limited study on the factors affecting the surface activity evaluated by inverse gas chromatography.
In this study, the effects of carrier gas flow rate, inlet pressure and aging time of the packed column on the surface activity of various CB samples are systematically studied through theoretical analysis and experimental verification. Moreover, the surface free energy of CB treated at different temperature was measured using the inverse gas chromatography. The performance of the rubber filled by the treated CB was investigated.
2 Experimental
2.1 Materials and methods
The CB samples A, B, C, D, N234, N375, N550 and N660 are all industrial CB from Suzhou Baohua Carbon Black Co., Ltd. The insoluble sulphur with the size of 200 mesh was obtained from Eastman. Styrene-butadiene rubber (SBR) was supplied by Baling Petrochemical Company. The probe molecules: n-pentane, n-hexane, n-heptane, n-octane and acetone are all chromatographical grade.
The following apparatus were used: Box type atmosphere furnace (JQF1400-40, Shanghai Jigong Electric Co., Ltd.), Gas chromatograph (GC-17A Gas Chromatograph, Shimadzu), Data acquisition (CSC-100D, Beijing Huibo Elite Technology Co., Ltd.), Twin-roll mill for rubber (XK-152.5, Guangdong Zhanjiang Experimental Machinery Factory), Internal mixer (Haake Torque Rheometer with a volume of 500 mL, Thermo Fisher Scientific Inc.), Flat vulcanizing machine (630kN, Huzhou Rubber Machinery Co., Ltd.), Pneumatic automatic slicer (GT-7016-AR, High-Speed Rail Testing Instrument Co., Ltd.), Electronic universal material testing machine (double column desktop 2365, Instron Corporation), Rubber processing analyzer (RPA2000, Alpha), Rotorless vulcanizer (MRD2000, Alpha), Mooney viscometer (MV2000, Alpha), Akron grinding machine (High-Speed Rail Testing Instrument Co., Ltd.), and Compression Heat Test Machine (GT-RH-2000, High-Speed Rail Testing Instrument Co., Ltd.).
2.2 Heat treatment of CB in vacuum
In order to avoid the oxidization of CB by air, the untreated sample N375 was placed in a tube furnace connected to a vacuum pump. The CB sample was heated in the vacuum tube at 300°C for 1 h. After that, the sample was cooled down to room temperature, subsequently taken out from the vacuum tube and kept in a vacuum dryer.
2.3 Experimental condition for inverse gas chromatography
The stainless columns with 2 mm inner size and 30 cm long were chosen for the measurement of CB surface free energy. The packed column filled with CB was installed in the gas chromatographic system. The air tightness was checked first, then the pressure stabilizer was adjusted, and a soap film flow meter was used to measure the carrier gas flow rate corresponding to different column front pressures at room temperature. After that, the gas chromatograph, data acquisition and chromatograph workstations were switched on, so the rising temperature was programmed and stabilized at 180°C. Then highly pure helium passed through the chromatographic column with the flow rate of 30 mL/min, aging for a certain period of time. Finally, a 1 μL micro-injector was used to inject various probe molecules, including n-hexane, n-heptane and n-octane.
2.4 CB surface free energy
The surface free energy of CB is divided into two parts, non-polar and polar components, and can be expressed by a formula:
2.4.1 Nonpolar and polar components of CB surface free energy
Calculation of retention volume VN [31]
where, VN is the net retention volume, D is the volume of carrier gas, j is the gas compressibility factor, tr is the retention time, tm is the dead time, pw is the equilibrium pressure, po is the pressure in the outlet of the column, Tc is the column temperature, and Tf is the flowmeter temperature.
The value of j is given by [32]:
where, pi is the pressure at the inlet of the column.
Calculation of adsorption energy –ΔG [21]
where, −ΔG is the minus value of adsorption free energy, R is the gas constant, T is the column temperature, C = 299, S is the specific surface area of the filler, and g is the mass of the filler in the column.
Calculation of
Calculation of
where,
Calculation of the polar part of adsorption free energy
Using the −ΔG of n-alkanes to plot their molecular cross-sectional areas, a linear relationship is obtained. Then, the molecular cross-sectional area of the polar probe molecule is substituted into the relational formula to obtain the theoretical non-polar adsorption free energy of the polar probe molecule. Afterwards, the total free energy of the polar molecule minus the total free energy of non-polar part, that is, the polar part of its adsorption free energy, expressed as −ΔΔG.
Calculation of polar parameter of surface free energy rsp [33]
where, rsp is the polar parameter of surface free energy, −ΔΔG is the polar part of the absorbing free energy of the polar probe molecules and ap is the area occupied by a probe molecule on the CB surface.
2.4.2 Theoretical deduction of CB surface free energy
2.4.2.1 Mathematical model
During the measurement of surface free energy of CB, each sample is tested under the respective column temperature, carrier gas flow rate, inlet pressure, etc.
First, suppose that n-pentane, n-hexane, n-heptane and n-octane are corresponding to x=5, x=6, x=7, x=8, respectively, in that, x is an independent variable which stands for different alkanes, and t stands for the adjusted retention time, that is, t = tr − tm. Because different alkane results in a particular adjusted retention time, under fixed inlet pressure of column and volume of carrier gas, the correlation between t and x can be decribed as follows,
According to formula (1), the relationship between adjusted retention volume and adjusted retention time can be expressed as follows,
According to formula (3), the relationship between Gibbs free energy and adjusted retention volume can be given:
According to the definition of
2.4.2.2 Mathematical deduction
Combination of formulas (5), (6), (7) and (8) results in:
According to formula (4), the dispersive free energy
2.5 Gross rubber preparation and characterization
The gross rubber was prepared by a two-stage process. The first stage of the mixing was performed in an Haake internal mixer at a speed of 60 r/min with the initial temperature of 90°C. The rubber, additives and filler (CB) were added, and the temperature of the mixer is controlled at about 150°C. The second stage of the mixing was performed in an open mill with twin-rollers. The above blended rubber, sulfur and accelerator were added into the open mill to obtain the gross rubber.
Vulcanization curve: The vulcanization curve of the gross rubber was obtained using a MRD2000 rotorless vulcanizer at the temperature of 150°C for 35 min.
Mooney viscosity: The test was carried out using a disc-like viscometer according to the national standard GB/T1232.1-2000which is similar to the ISO 289-1:1994.
The specimens for other measurements were vulcanized using the gross rubber on a plate vulcanizer. The plate was heated by electrothermal calefactors at a temperature of 150°C and driven by hydraulic cylinder at a pressure of 10 MPa for 90 min. The performance of the specimens was tested after 24 hours (after vulcanization).
Tensile performance: The test was carried out using a universal material testing machine with the stretching speed of 500 mm/min at room temperature according to GB/ T 528-2009 which is similar to the ISO 37: 2005.
Tear resistance: The test was carried out using the machine with a stretching speed of 500 mm/min at room temperature according to GB/ T 529-2008 which is similar to the ISO 34-1: 2004.
Hardness: The test was carried out using a hardness tester at room temperature according to GB/ T 531.1-2008 which is similar to the ISO 7619-1:2004.
Rebound elasticity: The rubber elasticity tester was used at room temperature according to GB/T 1681-2009 which is similar to the ISO 4662: 1986.
Compressed heat generation: The compression heat generator was used at (55±1)°C. The specimen was preheated for 30 min, compressed for 25 min with a frequency of 30 Hz, stroke length of 4.45 mm, pressure of 1 MPa, according to GB/T7759-1996 which is similar to the ISO 815:1991.
DIN wear: The specimen was tested using an abrasion machine with rotating rollers at room temperature according to GB/T9867-2008 which is similar to the ISO 4649: 2002.
3 Results and discussion
3.1 Effect of inlet pressure on surface free energy
Table 1 shows the dispersive free energy of samples A and B at different inlet pressures. The Figures 1 and 2 show the relationship between the adsorption energy and carbon number of probe molecules. The column temperature was 180°C, the carrier gas flow rate was 12.24 mL/min and the aging time was 12 h, while the inlet pressures of column was varied.

The effect of inlet pressure of column (Sample A)

The effect of inlet pressure of column (Sample B)
| Sample | Pi (kPa) | |
Error |
|---|---|---|---|
| A | 30 50 |
85.05 83.24 |
2.17% |
| B | 27 35 |
163.15 168.51 |
3.18% |
As shown in Table 1, the dispersive free energy
Figures 1 and 2 show that at different inlet pressures, the probe molecules have a good linear correlation between the adsorption free energy and the number of carbon atoms, and the slope
3.2 Effect of column aging time on surface free energy
Table 2 shows the dispersive free energy
| Inlet pressure (kPa) | Aging time (h) | |
Error |
|---|---|---|---|
| 30 | 12 24 |
85.05 88.96 |
4.6% |
| 50 | 12 32 |
83.24 87.65 |
5.3% |

Effect of aging time of column on adsorption energy (30 kPa)

Effect of aging time of column on adsorption energy (50kPa)
Table 2 shows that the dispersive free energy after the aging time of 24 hours and 32 hours is 4.6% and 5.3%, respectively, which is higher than the result from the aging time of 12 h. Figures 3 and 4 show that the probe molecules have a good linear correlation between the free energy of adsorption and the number of carbon atoms at different aging time. However, the slopes of different lines are different, which indicates that the aging time has an impact on the
3.3 Effect of carrier gas flow rate on dispersive free energy
By changing the carrier gas flow rate, the dispersive free energy,
| Sample | D(mL/min) | Error | |
|---|---|---|---|
| A | 6.87 12.24 |
86.82 88.96 |
2.46% |
| B | 12.26 131 |
168.51 164.78 |
2.21% |
| C | 11.16 20.22 |
104.86 106.77 |
1.82% |
| D | 12.56 21.51 |
116.45 118.17 |
1.48% |
3.4 Effect of carrier gas flow rate on surface free energy
According to the experimental results above, it can be seen that the influence of carrier gas flow rate on the free energy of a specific CB surface is below 2.5%, which is almost negligible.
However, it seems that carrier gas flow rate makes some difference according to our experience on the basis of a large number of experimental measurements in the past few years. In order to clarify this point, four types of CB with different values of surface free energy have been tested.
3.4.1 Adjusted retention time under different carrier gas flow rates
By measuring the adjusted retention time under different carrier gas flow rates, the logarithm of the adjusted retention time is plotted against the number of carbon atoms for each n-alkane probe molecule, and the results are shown in Figures 5 to 8.

Adjustment retention time of probe molecules for sample N550

Adjustment retention time of probe molecules for sample N375

Adjustment retention time of probe molecules for sample N375-300°C

Adjustment retention time of probe molecules for sample N234
It should be noted that the retention time of n-octane is much longer than other n-paraffin hydrocarbons, due to the strong interaction between n-octane and CB surface. Furthermore, the shape of n-octane peak is also irregular, especially for samples with high surface energy. Therefore, the measurement of retention time using n-octane is canceled in this test to avoid some obvious errors and unnecessary time consumption.
From Figure 5 to Figure 8, it can be seen that the logarithmic values of the adjusted retention time of each n-alkane probe molecule obey a good linear relationship with the carbon numbers at different carrier gas flow rates. In addition, for the same kind of CB samples, the straight lines at different flow rates maintain a good parallel relationship, that is, the straight line slope
3.4.2 Surface free energy at different carrier gas flow rates
According to formula (1) to formula (5), the CB surface free energy of four samples was calculated at different carrier gas flow rates, and the results are summarized in Table 4. The dispersive free energy and polar parameter of different CB samples versus the carrier gas flow are shown in Figures 9 and 10. The surface free energy is shown in Figure 11.

Dispersive free energy of different samples versus carrier gas flow rate

Polar parameter of different samples versus carrier gas flow rate
CB surface free energy measured at different carrier gas flow rates
| Dispersive free energy (mJ/m2) | Polar parameter (mJ/m2) | |||||||
|---|---|---|---|---|---|---|---|---|
| CGFR Samples | N550 | N375 | N375 −300 °C | N234 | N550 | N375 | N375 −300 °C | N234 |
| 11 | 104.1 | 160.9 | 218.7 | - | 56.6 | 57.5 | 74.2 | - |
| 15 | 98.1 | 158.7 | 225.9 | - | 54.1 | 62.9 | 75.9 | - |
| 20 | 109.8 | 160.5 | 229.3 | 238.8 | 60.9 | 62.8 | 76.1 | 72.1 |
| 26 | 107.8 | 164.0 | 230.0 | 242.0 | 59.1 | 64.1 | 76.0 | 72.9 |
| 35 | 99.4 | 167.4 | 226.3 | 238.6 | 59.7 | 63.5 | 76.6 | 72.2 |
| 41 | 103.1 | 166.9 | 235.3 | 245.1 | 59.0 | 62.1 | 78.6 | 73.7 |
| 50 | 104.4 | 174.1 | 240.8 | 238.8 | 60.8 | 62.3 | 82.0 | 72.9 |
| 60 | 109.0 | 173.8 | 229.2 | 241.0 | 61.0 | 67.6 | 79.8 | 74.3 |
| 75 | 103.0 | 173.3 | 216.6 | 238.3 | 56.9 | 68.5 | 76.9 | 73.1 |
| 86 | 107.2 | 160.8 | 212.7 | 234.6 | 61.0 | 62.4 | 77.7 | 72.8 |
| 100 | 106.4 | 160.9 | 212.3 | 239.9 | 62.5 | 64.9 | 76.5 | 74.5 |
| 113 | 107.4 | 155.4 | 211.2 | 238.7 | 60.9 | 64.2 | 77.5 | 74.2 |
| Max. Value | 109.8 | 174.1 | 240.8 | 245.1 | 62.5 | 68.5 | 82.0 | 74.5 |
| Min. Value | 98.1 | 155.4 | 211.2 | 234.6 | 54.1 | 57.5 | 74.2 | 72.1 |
| Average Value | 105.0 | 164.7 | 224.0 | 239.6 | 59.4 | 63.6 | 77.3 | 73.3 |
| RSD | 3.5% | 3.8% | 4.3% | 1.1% | 4.1% | 4.4% | 2.7% | 1.2% |
(CGFR: Carrier gas flow rate, mL/min; RSD: relative standard deviation)
It can be seen that the relative standard deviations (RSD) from Table 4 are below 5% for all CB samples. Accordingly, the change of carrier gas flow rate has no obvious effect on the surface free energy of CB. It is also indicative from Figure 9 that the trends of dispersive free energy for different CB samples are consistent at different carrier gas flow rates, demonstrating that the influence of carrier gas flow rate on the dispersive free energy of different CB samples is the same. According to Figure 10, in case of the slow carrier gas flow rate, the vibration values of N550 and N375 are large. Consequently, small carrier gas flow rate should be avoided during experimental measurements. Under the condition of higher carrier gas flow rate, the fluctuation of the polar parameters is smaller, indicating that the influence of the carrier gas flow rate on the polar parameter of CB is not significant.According to the data of N375 and N375-300°C, we know both dispersive free energy and polar parameter of N375 CB after 300°C treatment have a significant increase of about 36% compared with the results from untreated CB, which indicates that the surface free energy of CB will change after heating. However, no matter the CBs have been heated or not, the effect of carrier gas flow rate on the experimental results is not significant when the surface free energy is measured by inverse gas chromatography.
According to Figure 11, it can be seen that the trends of surface free energy are very similar for any types of CB samples, indicating that the effect of carrier gas flow rate on the surface free energy is insignificant.

Surface free energy of different samples versus carrier gas flow rate
3.5 Effect of heat treatment of CB on rubber performance
According to the comparison of CB N375 and N375-300°C, the surface free energy of CB has a significant increase after heat treatment at high temperature. The surface free energy (surface activity) is a major factor affecting the reinforcement of rubber. Therefore, it is important to further investigate the effect of heat treatment on the performance of the rubber reinforced by CB. We performed a series of experiment, the industrial CB N660 was subjected to vacuum heat treatment at different temperatures, and applied in SRB rubber. The performance of the reinforced rubber was tested.
3.5.1 Effect of heat treatment on surface free energy of CB
The dispersive free energy and polarity parameters of CB N660 increase with the increase of temperature (Table 5). The dispersive free energy increases particularly fast, which leads to the increase of CB surface free energy. It can be concluded that temperature has an important effect on the surface activity of CB. It may be due to the reduction of surface adsorbates and some highly active sites exposed after heat treatment [35].
Effect of heat treatment on surface free energy of CB
| rsp | r (mJ/m2) | ||
|---|---|---|---|
| (mJ/m2) | (mJ/m2) | ||
| N660 (untreated) | 123.14 | 63.24 | 186.38 |
| N660 (300°C) | 273.52 | 70.35 | 343.87 |
| N660 (400°C) | 323.24 | 80.52 | 403.76 |
| N660 (500°C) | 389.45 | 87.43 | 476.90 |
3.5.2 Effect of heat-treated CB on Mooney viscosity and vulcanization
It can be seen from Table 6 that for the rubber filled by CB N660 the maximum torque MH during vulcanization is gradually reduced from 20.89 to 18.54 with the increasing treatment temperature of CB, while the scorching time T10 and the positive vulcanization time T90 change little. The main effect of heat treatment on the CB is the reduction of the surface active groups on CB surface which affects the surface properties of CB. Therefore, the maximum torque MH is affected, while it has little effect on the curing time.
Effect of high temperature treatment of CB on vulcanization characteristics of gross rubber
| Minimum torque | Maximum torque | Scorching time | Positive vulcanization | |
|---|---|---|---|---|
| (ML/dN·m) | (MH/dN·m) | (T10/min) | time (T90/min) | |
| N660 (untreated) | 2.19 | 20.89 | 15.60 | 37.25 |
| N660 (300°C) | 2.28 | 20.10 | 16.71 | 39.30 |
| N660 (400°C) | 2.22 | 19.51 | 17.05 | 37.91 |
| N660 (500°C) | 2.23 | 18.54 | 15.60 | 39.11 |
It can be seen from Figure 12 that the Mooney viscosity of the rubber filled with CB N660 has little change after treatment at 300°C, 400°C and 500°C. The CB under-goes high temperature treatment, which could affect the surface activity and reduce the active groups on the surface of CB. However, heat treatment has little effect on the particle size and pore structure of CB. Therefore, the bound rubber content in the blend is not influenced significantly.

Effect of high temperature treatment on Mooney viscosity for carbon black filled SBR rubber
3.5.3 Effect of heat-treated CB on mechanical properties of vulcanizate
It can be seen from Table 7 that when the industrial CB N660 is subjected to high-temperature treatment, the tensile strength of the reinforced rubber after vulcanization changes little, the tensile stress is slightly reduced, and the tear strength, hardness and resilience energy are not changed obviously, while the elongation at break has an increasing trend. The reason is that the active groups on the surface of the particles was changed after high-temperature treatment. However, the size and structure of CB changed little. Therefore, the tensile stress of vulcanized rubber was reduced and the elongation at break was increased.
Effect of high temperature treatment of N660 CB on physical and mechanical properties of vulcanizate
| N660 (untreated) | N660 (300°C) | N660 (400°C) | N660 (500°C) | |
|---|---|---|---|---|
| Tensile strength (MPa) | 19.82 | 21.28 | 21.52 | 21.97 |
| Strength at 100% elongation (MPa) | 2.52 | 2.49 | 2.38 | 2.34 |
| Strength at 300% elongation (MPa) | 12.42 | 12.11 | 11.65 | 11.75 |
| Elongation at break (%) | 495 | 512 | 545 | 553 |
| Tear strength (MPa) | 46.7 | 50.2 | 47.8 | 48.6 |
| Hardness (Shore A) | 66 | 66 | 66 | 65 |
| Resilience (%) | 45.5 | 45.7 | 45.4 | 45.6 |
The compression heat and compression set of SBR reinforced by N660 increase with the increase of temperature (Figure 13). The reason could be that the high-temperature treatment weakened the surface activity of the CB, so that the interaction among the CB particles and the rubber polymer chains is weakened. The molecular chains and the CB particles cannot simultaneously move, resulting in hysteresis under the changing stress, which enhanced the friction between the CB and rubber. As a result, the heat generation was increased, and also the permanent deformation rate.
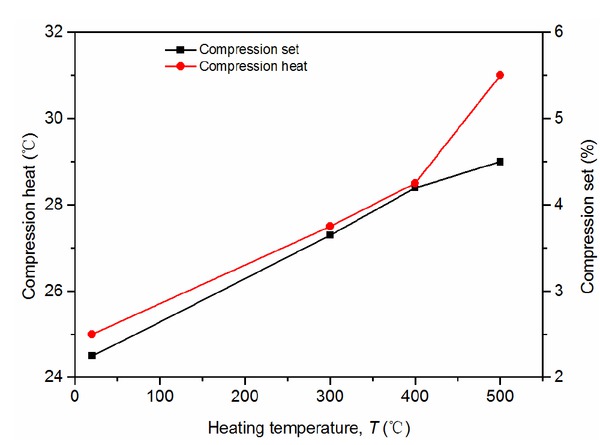
Compression heat and compression set versus temperature of vulcanized rubber filled with N660
The abradability of the vulcanized rubbers filled with the CB N660 was tested using a DIN (German Industrial Standard) and an Akron abrasion machine. The results in Figure 14 show that the abrasion value of vulcanized rubber increases with the increase of temperature, which is due to the decrease of surface activity of CB particles, leading to the decrease of interaction between CB particles and rubber molecular chains. As a result, the wear resistance of vulcanized rubber decreased.

Effect of temperature on abradability of vulcanized rubber filled with N660
4 Conclusions
The surface free energy of CB was measured by inverse gas chromatography and a formula was proposed to calculate the surface free energy through theoretical derivation. The following conclusions could be drawn.
The inlet pressure and the carrier gas flow rate of the column have little effect on the dispersive free energy, and the variation trend of dispersive free energy and polar parameters with carrier gas flow rate are basically consistent. The aging time of the chromatographic column has a certain influence on the experimental results, since the adsorbates on the CB surface will be reduced as the aging time increases.
The high-temperature treatment of industrial CB has little influence on its structure, while mainly affects the surface activity of CB. The Mooney viscosity, the scorching time and vulcanization rate of the blend are affected little by the high-temperature treatment of CB. However, the maximum torque is decreased significantly with the increase of temperature.
With the increase of heating temperature for CB, the change of tensile strength and stress at definite elongation of CB-reinforced SBR is small, the elongation at break tends to increase, the heat generation of vulcanizates is increased, while the wear resistance is decreased.
-
Data Availability: The data used to support the findings of this study are included within the article. Any more specific details in the data will be delivered by the corresponding author upon request.
-
Conflict of Interest: The authors declare no conflict of interest regarding the publication of this paper.
Acknowledgement
This research was financially supported by Minjiang Scholarship of Fujian Province (No. Min-Gaojiao[2010]-117), Central-government Guided Fund for Local Economic Development (No. 830170778), R&D Fund for Strategic Emerging Industry of Fujian Province (No. 82918001), International Cooperation Project of Fujian Science and Technology Department (No. 830170771) and Teaching and Researching Fund for Young Staff of Fujian Educational Department (No. JT180040).
References
[1] Hoshikawa Y., An B., Kashihara S., Ishii T., Ando M., Fujisawa S., Hayakawa K., Hamatani S., Yamada H., Kyotani T., Analysis of the interaction between rubber polymer and carbon black surfaces by efficient removal of physisorbed polymer from carbon-rubber composites, Carbon, 2016, 99, 148-156.10.1016/j.carbon.2015.12.003Search in Google Scholar
[2] Wang F., Hong R. Y., Feng W. G., Badami D., Zeng K., Electrical and mechanical properties of ABS/EPDM composites filled with carbon black, Mater. Lett., 2014, 125, 48-50.10.1016/j.matlet.2014.03.136Search in Google Scholar
[3] Ossai C. I., Raghavan N., Nanostructure and nanomaterial characterization, growth mechanisms, and applications, Nanotechnol. Rev., 2018, 7, 209-231.10.1515/ntrev-2017-0156Search in Google Scholar
[4] Jaffe A., Valdes A. S., Karunadasa H. I., Quinone-functionalized carbon black cathodes for lithium batteries with high power densities, Chem. Mater., 2015, 27, 3568-3571.10.1021/acs.chemmater.5b00990Search in Google Scholar
[5] Power A. C., Gorey B., Chandra S., Chapman J., Carbon nanomaterials and their application to electrochemical sensors: a review, Nanotechnol. Rev., 2018, 7, 19-41.10.1515/ntrev-2017-0160Search in Google Scholar
[6] Kameya Y., Hayashi T., Motosuke M., Stability of platinum nanoparticles supported on surface-treated carbon black, Appl. Catal. B-environ, 2016, 189, 219-225.10.1016/j.apcatb.2016.02.049Search in Google Scholar
[7] Yuan J. J., Hong R. Y., Wang Y. Q., Feng W. G., Low-temperature plasma preparation and application of carbon black nanoparticles, Chem. Eng. J., 2014, 253, 107-120.10.1016/j.cej.2014.05.043Search in Google Scholar
[8] Roy S., Petrova R. S., Mitra S., Effect of carbon nanotube (CNT) functionalization in epoxy-CNT composites, Nanotechnol. Rev., 2018, 7, 475-485.10.1515/ntrev-2018-0068Search in Google Scholar PubMed PubMed Central
[9] Yeo K. K., Oxidation and surface functional group analyses under ozone treatment of carbon black, Elastomers and Composites, 2005, 40, 188-195.Search in Google Scholar
[10] Jiang D., Wang C., Jiang Z., Zhou W., Zhang F., Influences of dispersants with different structures on the dispersibility of carbon black, Chinese J. Process Eng., 2015, 15, 153-158.Search in Google Scholar
[11] Xiao F., Bedane A. H., Zhao J. X., Mann M. D., Pignatello J. J., Thermal air oxidation changes surface and adsorptive properties of black carbon (char/biochar), Sci. Total. Environ., 2018, 618, 276-283.10.1016/j.scitotenv.2017.11.008Search in Google Scholar PubMed
[12] Morales-Lara F., Domingo-Garcia M., Lopez-Garzon R., Luz Godino-Salido M., Penas-Sanjuan A., Javier Lopez-Garzon F., Perez-Mendoza M., Melguizo M., Grafting the surface of carbon nanotubes and carbon black with the chemical properties of hyperbranched polyamines, Sci. Technol. Adv. Mat., 2016, 17, 541-553.10.1080/14686996.2016.1221728Search in Google Scholar PubMed PubMed Central
[13] Xia Y., Kuanjun F., Procedures of hydrophilic modification of carbon black surface, Chem. Ind. Eng. Pro., 2007, 26, 657-663.Search in Google Scholar
[14] Sun D. L., Hong R. Y., Wang F., Liu J. Y., Rajesh Kumar M., Synthesis and modification of carbon nanomaterials via AC arc and dielectric barrier discharge plasma, Chem. Eng. J., 2016, 283, 9-20.10.1016/j.cej.2015.07.023Search in Google Scholar
[15] Zhang Z., An Y., Nanotechnology for the oil and gas industry - an overview of recent progress, Nanotechnol. Rev., 2018, 7, 341-353.10.1515/ntrev-2018-0061Search in Google Scholar
[16] Raos G., Application of the Christensen-Lo model to the reinforcement of elastomers by fractal fillers, Macromol. Theor. Simul, 2003, 12, 17-23.10.1002/mats.200390002Search in Google Scholar
[17] Dannenberg E. M., Bound rubber and carbon-black reinforcement, Rubber Chem. Technol., 1986, 59, 512-524.10.5254/1.3538213Search in Google Scholar
[18] Bandaru P. R., Yamada H., Narayanan R., Hoefer M., The role of defects and dimensionality in influencing the charge, capacitance, and energy storage of graphene and 2D materials, Nanotechnol. Rev., 2017, 6, 421-433.10.1515/ntrev-2016-0099Search in Google Scholar
[19] Kim S. M., Kim K. J., Thiazole type accelerator effects on silane/silica filled natural rubber compound upon vulcanization and mechanical properties, Polym-Korea, 2012, 36, 235-244.10.7317/pk.2012.36.2.235Search in Google Scholar
[20] Zhang H., Xie J., An S., Qian X., Cheng H., Zhang F., Li X., A novel measurement of contact angle on cylinder-shaped lignocellulosic fiber for surface wettability evaluation, Colloid Surface. A, 2018, 540.10.1016/j.colsurfa.2017.12.054Search in Google Scholar
[21] Papirer E., Brendle E., Ozil F., Balard H., Comparison of the surface properties of graphite, carbon black and fullerene samples, measured by inverse gas chromatography, Carbon, 1999, 37, 1265-1274.10.1016/S0008-6223(98)00323-6Search in Google Scholar
[22] Mills R. H., Jara R., Gardner D. J., van Heiningen A., Inverse gas chromatography for determining the surface free energy and acid-base chemical characteristics of a water extracted hardwood (Acer rubrum), J. Wood. Chem. Technol., 2009, 29, 11-23.10.1080/02773810802596497Search in Google Scholar
[23] Lam C. N. C., Wu R., Li D., Hair M. L., Neumann A. W., Study of the advancing and receding contact angles: liquid sorption as a cause of contact angle hysteresis, Adv. Colloid Interfac, 2002, 96, 169-191.10.1016/S0001-8686(01)00080-XSearch in Google Scholar
[24] Adao M., Saramago B. J. V., Fernandes A. C., Estimation of the surface properties of styrene-acrylonitrile random copolymers from contact angle measurements, J. Colloid Interf. Sci, 1999, 217, 94-106.10.1006/jcis.1999.6279Search in Google Scholar
[25] Kozbial A., Li Z., Conaway C., McGinley R., Dhingra S., Vahdat V., Zhou F., D’Urso B., Liu H., Li L., Study on the surface energy of graphene by contact angle measurements, Langmuir., 2014, 30, 8598-8606.10.1021/la5018328Search in Google Scholar
[26] Chibowski E., Perea-Carpio R., Problems of contact angle and solid surface free energy determination, Adv. Colloid Interfac, 2002, 98, 245-264.10.1016/S0001-8686(01)00097-5Search in Google Scholar
[27] Voelkel A., Strzemiecka B., Adamska K., Milczewska K., Inverse gas chromatography as a source of physiochemical data, J. Chromatogr. A, 2009, 1216, 1551-1566.10.1016/j.chroma.2008.10.096Search in Google Scholar PubMed
[28] Mohammadi-Jam S., Waters K. E., Inverse gas chromatography applications: A review, Adv. Colloid Interf., 2014, 212, 21-44.10.1016/j.cis.2014.07.002Search in Google Scholar PubMed
[29] Menzel R., Bismarck A., Shaffer M. S. P., Deconvolution of the structural and chemical surface properties of carbon nanotubes by inverse gas chromatography, Carbon, 2012, 50, 3416-3421.10.1016/j.carbon.2012.02.094Search in Google Scholar
[30] Peng Y., Gardner D. J., Han Y., Cai Z., Tshabalala M. A., Influence of drying method on the surface energy of cellulose nanofibrils determined by inverse gas chromatography, J. Colloid Interf. Sci, 2013, 405, 85-95.10.1016/j.jcis.2013.05.033Search in Google Scholar PubMed
[31] Darmstadt H., Cao N. Z., Pantea D. M., Roy C., Summchen L., Roland U., Donnet J. B., Wang T. K., Peng C. H., Donnelly P. J., Surface activity and chemistry of thermal carbon blacks, Rubber Chem. Technol., 2000, 73, 293-309.10.5254/1.3547592Search in Google Scholar
[32] Castellano M., Falqui L., Costa G., Turturro A., Valenti B., Castello G., Investigation on elastomer-silica interactions by inverse gas chromatography and image analysis aided transmission electron microscopy, J. Macromol. Sci. Phys., 2002, B41, 451-471.10.1081/MB-120004347Search in Google Scholar
[33] Wang M. J., Tu H. R., Murphy L. J., Mahmud K., Carbon-silica dual phase filler, a new generation reinforcing agent for rubber: Part VIII. Surface characterization by IGC, Rubber Chem. Technol., 2000, 73, 666-677.10.5254/1.3547612Search in Google Scholar
[34] Gamble J. F., Leane M., Olusanmi D., Tobyn M., Supuk E., Khoo J., Naderi M., Surface energy analysis as a tool to probe the surface energy characteristics of micronized materials-A comparison with inverse gas chromatography, Int. J. Pharm., 2012, 422, 238-244.10.1016/j.ijpharm.2011.11.002Search in Google Scholar PubMed
[35] Li Z., Xu K., Pan Y., Recent development of supercapacitor electrode based on carbon materials, Nanotechnol. Rev., 2019, 8, 35-49.10.1515/ntrev-2019-0004Search in Google Scholar
© 2020 S. Zhang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Generalized locally-exact homogenization theory for evaluation of electric conductivity and resistance of multiphase materials
- Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide
- Characterization of mechanical properties of epoxy/nanohybrid composites by nanoindentation
- Graphene and CNT impact on heat transfer response of nanocomposite cylinders
- A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites
- Ultrasmall Fe3O4 nanoparticles induce S-phase arrest and inhibit cancer cells proliferation
- Effect of aging on properties and nanoscale precipitates of Cu-Ag-Cr alloy
- Effect of nano-strengthening on the properties and microstructure of recycled concrete
- Stabilizing effect of methylcellulose on the dispersion of multi-walled carbon nanotubes in cementitious composites
- Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites
- Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation
- Preparation and properties of 3D interconnected CNTs/Cu composites
- On factors affecting surface free energy of carbon black for reinforcing rubber
- Nano-silica modified phenolic resin film: manufacturing and properties
- Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete
- Halloysite nanotubes in polymer science: purification, characterization, modification and applications
- Cellulose hydrogel skeleton by extrusion 3D printing of solution
- Crack closure and flexural tensile capacity with SMA fibers randomly embedded on tensile side of mortar beams
- An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites
- Utilization of red mud for producing a high strength binder by composition optimization and nano strengthening
- One-pot synthesis of nano titanium dioxide in supercritical water
- Printability of photo-sensitive nanocomposites using two-photon polymerization
- In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries
- Effect of nano and micro conductive materials on conductive properties of carbon fiber reinforced concrete
- Tribological performance of nano-diamond composites-dispersed lubricants on commercial cylinder liner mating with CrN piston ring
- Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance
- Genetic mechanisms of deep-water massive sandstones in continental lake basins and their significance in micro–nano reservoir storage systems: A case study of the Yanchang formation in the Ordos Basin
- Effects of nanoparticles on engineering performance of cementitious composites reinforced with PVA fibers
- Band gap manipulation of viscoelastic functionally graded phononic crystal
- Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution
- Manipulating conductive network formation via 3D T-ZnO: A facile approach for a CNT-reinforced nanocomposite
- Microstructure and mechanical properties of WC–Ni multiphase ceramic materials with NiCl2·6H2O as a binder
- Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite
- Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function
- Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing
- Corrosion behaviour of multilayer CrN coatings deposited by hybrid HIPIMS after oxidation treatment
- Surface hydrophobicity and oleophilicity of hierarchical metal structures fabricated using ink-based selective laser melting of micro/nanoparticles
- Research on bond–slip performance between pultruded glass fiber-reinforced polymer tube and nano-CaCO3 concrete
- Antibacterial polymer nanofiber-coated and high elastin protein-expressing BMSCs incorporated polypropylene mesh for accelerating healing of female pelvic floor dysfunction
- Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films
- A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination
- Arrangement structure of carbon nanofiber with excellent spectral radiation characteristics
- Effect of different particle sizes of nano-SiO2 on the properties and microstructure of cement paste
- Superior Fe x N electrocatalyst derived from 1,1′-diacetylferrocene for oxygen reduction reaction in alkaline and acidic media
- Facile growth of aluminum oxide thin film by chemical liquid deposition and its application in devices
- Liquid crystallinity and thermal properties of polyhedral oligomeric silsesquioxane/side-chain azobenzene hybrid copolymer
- Laboratory experiment on the nano-TiO2 photocatalytic degradation effect of road surface oil pollution
- Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage
- Conversion of sub-µm calcium carbonate (calcite) particles to hollow hydroxyapatite agglomerates in K2HPO4 solutions
- Exact solutions of bending deflection for single-walled BNNTs based on the classical Euler–Bernoulli beam theory
- Effects of substrate properties and sputtering methods on self-formation of Ag particles on the Ag–Mo(Zr) alloy films
- Enhancing carbonation and chloride resistance of autoclaved concrete by incorporating nano-CaCO3
- Effect of SiO2 aerogels loading on photocatalytic degradation of nitrobenzene using composites with tetrapod-like ZnO
- Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles
- Hydration activity, crystal structural, and electronic properties studies of Ba-doped dicalcium silicate
- Microstructure and mechanical properties of brazing joint of silver-based composite filler metal
- Polymer nanocomposite sunlight spectrum down-converters made by open-air PLD
- Cryogenic milling and formation of nanostructured machined surface of AISI 4340
- Braided composite stent for peripheral vascular applications
- Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre–reinforced starch biopolymer composites
- Study on influencing factors of photocatalytic performance of CdS/TiO2 nanocomposite concrete
- Improving flexural and dielectric properties of carbon fiber epoxy composite laminates reinforced with carbon nanotubes interlayer using electrospray deposition
- Scalable fabrication of carbon materials based silicon rubber for highly stretchable e-textile sensor
- Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery
- Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries
- Synthesis of functionalized carbon nanotubes for fluorescent biosensors
- Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete
- Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage
- Microstructural evolution and properties of Cu–20 wt% Ag alloy wire by multi-pass continuous drawing
- Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene
- Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications
- Preparation and piezoresistivity of carbon nanotube-coated sand reinforced cement mortar
- Vibrational analysis of an irregular single-walled carbon nanotube incorporating initial stress effects
- Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials
- Single pulse laser removal of indium tin oxide film on glass and polyethylene terephthalate by nanosecond and femtosecond laser
- Mechanical reinforcement with enhanced electrical and heat conduction of epoxy resin by polyaniline and graphene nanoplatelets
- High-efficiency method for recycling lithium from spent LiFePO4 cathode
- Degradable tough chitosan dressing for skin wound recovery
- Static and dynamic analyses of auxetic hybrid FRC/CNTRC laminated plates
- Review articles
- Carbon nanomaterials enhanced cement-based composites: advances and challenges
- Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide
- Review on modeling and application of chemical mechanical polishing
- Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites
- Advances in modelling and analysis of nano structures: a review
- Mechanical properties of nanomaterials: A review
- New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics
- A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials
- Recent development and applications of nanomaterials for cancer immunotherapy
- Advances in biomaterials for adipose tissue reconstruction in plastic surgery
- Advances of graphene- and graphene oxide-modified cementitious materials
- Theories for triboelectric nanogenerators: A comprehensive review
- Nanotechnology of diamondoids for the fabrication of nanostructured systems
- Material advancement in technological development for the 5G wireless communications
- Nanoengineering in biomedicine: Current development and future perspectives
- Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview
- Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review
- Carbon nanotube–reinforced polymer composite for electromagnetic interference application: A review
- Functionalized layered double hydroxide applied to heavy metal ions absorption: A review
- A new classification method of nanotechnology for design integration in biomaterials
- Finite element analysis of natural fibers composites: A review
- Phase change materials for building construction: An overview of nano-/micro-encapsulation
- Recent advance in surface modification for regulating cell adhesion and behaviors
- Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions
- Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review
- Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications
- A review of passive methods in microchannel heat sink application through advanced geometric structure and nanofluids: Current advancements and challenges
- Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices
- Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging
- Synthesis of graphene: Potential carbon precursors and approaches
- A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE)
- Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water
- Analysis of functionally graded carbon nanotube-reinforced composite structures: A review
- Application of nanomaterials in ultra-high performance concrete: A review
- Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer
- Advanced nickel nanoparticles technology: From synthesis to applications
- Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable?
- Progress in construction of bio-inspired physico-antimicrobial surfaces
- From materials to devices using fused deposition modeling: A state-of-art review
- A review for modified Li composite anode: Principle, preparation and challenge
- Naturally or artificially constructed nanocellulose architectures for epoxy composites: A review
Articles in the same Issue
- Research Articles
- Generalized locally-exact homogenization theory for evaluation of electric conductivity and resistance of multiphase materials
- Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide
- Characterization of mechanical properties of epoxy/nanohybrid composites by nanoindentation
- Graphene and CNT impact on heat transfer response of nanocomposite cylinders
- A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites
- Ultrasmall Fe3O4 nanoparticles induce S-phase arrest and inhibit cancer cells proliferation
- Effect of aging on properties and nanoscale precipitates of Cu-Ag-Cr alloy
- Effect of nano-strengthening on the properties and microstructure of recycled concrete
- Stabilizing effect of methylcellulose on the dispersion of multi-walled carbon nanotubes in cementitious composites
- Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites
- Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation
- Preparation and properties of 3D interconnected CNTs/Cu composites
- On factors affecting surface free energy of carbon black for reinforcing rubber
- Nano-silica modified phenolic resin film: manufacturing and properties
- Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete
- Halloysite nanotubes in polymer science: purification, characterization, modification and applications
- Cellulose hydrogel skeleton by extrusion 3D printing of solution
- Crack closure and flexural tensile capacity with SMA fibers randomly embedded on tensile side of mortar beams
- An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites
- Utilization of red mud for producing a high strength binder by composition optimization and nano strengthening
- One-pot synthesis of nano titanium dioxide in supercritical water
- Printability of photo-sensitive nanocomposites using two-photon polymerization
- In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries
- Effect of nano and micro conductive materials on conductive properties of carbon fiber reinforced concrete
- Tribological performance of nano-diamond composites-dispersed lubricants on commercial cylinder liner mating with CrN piston ring
- Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance
- Genetic mechanisms of deep-water massive sandstones in continental lake basins and their significance in micro–nano reservoir storage systems: A case study of the Yanchang formation in the Ordos Basin
- Effects of nanoparticles on engineering performance of cementitious composites reinforced with PVA fibers
- Band gap manipulation of viscoelastic functionally graded phononic crystal
- Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution
- Manipulating conductive network formation via 3D T-ZnO: A facile approach for a CNT-reinforced nanocomposite
- Microstructure and mechanical properties of WC–Ni multiphase ceramic materials with NiCl2·6H2O as a binder
- Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite
- Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function
- Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing
- Corrosion behaviour of multilayer CrN coatings deposited by hybrid HIPIMS after oxidation treatment
- Surface hydrophobicity and oleophilicity of hierarchical metal structures fabricated using ink-based selective laser melting of micro/nanoparticles
- Research on bond–slip performance between pultruded glass fiber-reinforced polymer tube and nano-CaCO3 concrete
- Antibacterial polymer nanofiber-coated and high elastin protein-expressing BMSCs incorporated polypropylene mesh for accelerating healing of female pelvic floor dysfunction
- Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films
- A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination
- Arrangement structure of carbon nanofiber with excellent spectral radiation characteristics
- Effect of different particle sizes of nano-SiO2 on the properties and microstructure of cement paste
- Superior Fe x N electrocatalyst derived from 1,1′-diacetylferrocene for oxygen reduction reaction in alkaline and acidic media
- Facile growth of aluminum oxide thin film by chemical liquid deposition and its application in devices
- Liquid crystallinity and thermal properties of polyhedral oligomeric silsesquioxane/side-chain azobenzene hybrid copolymer
- Laboratory experiment on the nano-TiO2 photocatalytic degradation effect of road surface oil pollution
- Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage
- Conversion of sub-µm calcium carbonate (calcite) particles to hollow hydroxyapatite agglomerates in K2HPO4 solutions
- Exact solutions of bending deflection for single-walled BNNTs based on the classical Euler–Bernoulli beam theory
- Effects of substrate properties and sputtering methods on self-formation of Ag particles on the Ag–Mo(Zr) alloy films
- Enhancing carbonation and chloride resistance of autoclaved concrete by incorporating nano-CaCO3
- Effect of SiO2 aerogels loading on photocatalytic degradation of nitrobenzene using composites with tetrapod-like ZnO
- Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles
- Hydration activity, crystal structural, and electronic properties studies of Ba-doped dicalcium silicate
- Microstructure and mechanical properties of brazing joint of silver-based composite filler metal
- Polymer nanocomposite sunlight spectrum down-converters made by open-air PLD
- Cryogenic milling and formation of nanostructured machined surface of AISI 4340
- Braided composite stent for peripheral vascular applications
- Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre–reinforced starch biopolymer composites
- Study on influencing factors of photocatalytic performance of CdS/TiO2 nanocomposite concrete
- Improving flexural and dielectric properties of carbon fiber epoxy composite laminates reinforced with carbon nanotubes interlayer using electrospray deposition
- Scalable fabrication of carbon materials based silicon rubber for highly stretchable e-textile sensor
- Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery
- Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries
- Synthesis of functionalized carbon nanotubes for fluorescent biosensors
- Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete
- Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage
- Microstructural evolution and properties of Cu–20 wt% Ag alloy wire by multi-pass continuous drawing
- Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene
- Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications
- Preparation and piezoresistivity of carbon nanotube-coated sand reinforced cement mortar
- Vibrational analysis of an irregular single-walled carbon nanotube incorporating initial stress effects
- Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials
- Single pulse laser removal of indium tin oxide film on glass and polyethylene terephthalate by nanosecond and femtosecond laser
- Mechanical reinforcement with enhanced electrical and heat conduction of epoxy resin by polyaniline and graphene nanoplatelets
- High-efficiency method for recycling lithium from spent LiFePO4 cathode
- Degradable tough chitosan dressing for skin wound recovery
- Static and dynamic analyses of auxetic hybrid FRC/CNTRC laminated plates
- Review articles
- Carbon nanomaterials enhanced cement-based composites: advances and challenges
- Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide
- Review on modeling and application of chemical mechanical polishing
- Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites
- Advances in modelling and analysis of nano structures: a review
- Mechanical properties of nanomaterials: A review
- New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics
- A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials
- Recent development and applications of nanomaterials for cancer immunotherapy
- Advances in biomaterials for adipose tissue reconstruction in plastic surgery
- Advances of graphene- and graphene oxide-modified cementitious materials
- Theories for triboelectric nanogenerators: A comprehensive review
- Nanotechnology of diamondoids for the fabrication of nanostructured systems
- Material advancement in technological development for the 5G wireless communications
- Nanoengineering in biomedicine: Current development and future perspectives
- Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview
- Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review
- Carbon nanotube–reinforced polymer composite for electromagnetic interference application: A review
- Functionalized layered double hydroxide applied to heavy metal ions absorption: A review
- A new classification method of nanotechnology for design integration in biomaterials
- Finite element analysis of natural fibers composites: A review
- Phase change materials for building construction: An overview of nano-/micro-encapsulation
- Recent advance in surface modification for regulating cell adhesion and behaviors
- Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions
- Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review
- Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications
- A review of passive methods in microchannel heat sink application through advanced geometric structure and nanofluids: Current advancements and challenges
- Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices
- Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging
- Synthesis of graphene: Potential carbon precursors and approaches
- A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE)
- Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water
- Analysis of functionally graded carbon nanotube-reinforced composite structures: A review
- Application of nanomaterials in ultra-high performance concrete: A review
- Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer
- Advanced nickel nanoparticles technology: From synthesis to applications
- Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable?
- Progress in construction of bio-inspired physico-antimicrobial surfaces
- From materials to devices using fused deposition modeling: A state-of-art review
- A review for modified Li composite anode: Principle, preparation and challenge
- Naturally or artificially constructed nanocellulose architectures for epoxy composites: A review

