Abstract
Commercial LiFePO4 (LFP) electrode still cannot meet the demand of high energy density lithium-ion batteries as a result of its low theoretical specific capacity (170 mA h g−1). Instead of traditional electrochemical inert polyvinylidene fluoride (PVDF), the incorporation of multifunctional polymeric binder becomes a possible strategy to overcome the bottleneck of LFP cathode. Herein, a novel polyimide (PI) binder was synthesized through a facile hydrothermal polymerization route. The PI binder exhibits better connection between active particles with uniform dispersion than that of PVDF. The multifunctional PI binder not only shows well dispersion stability in the organic electrolyte, but also contributes to extra capacity because of the existence of electrochemical active carbonyl groups in the polymer chain. Besides, the high intrinsic ion conductivity of PI also results in promoted ion transfer kinetic. Consequently, the LFP cathode using PI binder (LFP–PI) shows larger capacity and better rate capability than LFP cathode with PVDF binder (LFP–PVDF). Meanwhile, the superior binding ability also endows LFP–PI with great cycling stability compared to the LFP–PVDF electrode.
1 Introduction
Lithium-ion batteries (LIBs) have covered most of the aspects of human civilization such as various mobile electronic devices, electric vehicles, and large-scale energy storage [1,2,3,4,5,6,7,8]. Compared with LiCoO2, LiMn2O4, and Li-rich layered cathode, LiFePO4 (LFP) has already dominated the most commercial applications for its excellent cycle stability, high safety, environmental friendliness, and low cost [9,10]. However, the poor electronic conductivity and low ion mobility result in inferior rate capability for the LFP cathode [11,12]. These shortcomings can be improved by the size reduction to the nanoscale, carbon/conductive polymer coating, and element-doping strategies [13,14,15,16,17]. The incorporation of carbon additives during the electrode fabrication process also can effectively promote the electronic conductivity. In addition, the high reversibility and moderate operating potential also make LFP more competitive compared to other cathodes. Therefore, there are still no alternatives available to completely replace the LFP cathode in terms of operational safety, stability, and manufacturing cost. Despite the significant progresses in the past few decades, the energy density of LFP-based batteries has almost reached its limit value because of its low theoretical specific capacity (170 mA h g−1), which seriously impeded the development of high-density LIBs. Therefore, the poor energy density has become a bottleneck restricting further development of LFP.
Generally, it is almost impossible to improve the specific capacity of LFP cathode through the material modification. Instead, reducing inactive components, increasing active mass loading, and using lightweight package during the electrode fabrication process seem to be the feasible strategies [18,19]. Among the critical electrode components, the current collector and conductive additive that supply continuous and uninterrupted electron transfer channel are almost certainly impossible to be replaced [20,21,22]. Therefore, optimizing the content and properties of polymeric binder functioning as the bridge between active particles becomes a feasible solution to enhance the energy density [23]. Currently, the commercial binders used in both cathode and graphite anode are dominated by polyvinylidene fluoride (PVDF) as a result of its superior electrochemical stability, mechanical properties, processing properties, oxidation, and corrosion resistance for the high energy C–F bond [24,25,26]. Nevertheless, the swelling in the electrolyte during long-time operation would result in the failure of binding property, which further causes the isolation and crack of active particles. The binding mechanism of PVDF relies on weak van der Waals force, which cannot withstand the large volume change of electrodes especially for silicon anode [27]. Notably, the poor ion conductivity of PVDF also leads to sluggish ion diffusion and large polarization, further limiting the rate capability. In addition, some other binders such as polytetrafluoroethylene, carboxyl methyl cellulose, polyacrylic acid, and styrene butadiene rubber have also been used in various energy storage systems [23,28]. However, their differences in solubility, dispersion, adhesion strength, conductivity, and electrochemical stability make them only suitable for specific solvent, electrolyte, or voltage window [29,30,31].
Recently, polyimide (PI) binder has become a promising candidate for LIBs because of its superior thermal/mechanical properties, strong adhesion strength, excellent film-forming ability, and high intrinsic ion conductivity [32]. Kim et al. reported that Si anode prepared with PI binder exhibited twice the capacity retention than that of Si anode with PVDF [27]. The outstanding mechanical stability of PI can effectively buffer the huge volume change of Si anode during repeated discharge/charge process [33,34]. Inspired by the excellent thermal and chemical stability of PI, Song et al. used a fluorinated PI as the binder of high-voltage Li-rich layered oxide cathode (FPI–LMNC) [35]. Because of the strong interaction between –CF3 groups and surface of LMNC, the formed protective layer effectively suppressed the decomposition of active materials at high voltage (up to 4.7 V). Thus FPI–LMNC showed a significantly enhanced cycling stability than that of LMNC electrode prepared with PVDF. Moreover, PI as a widely used engineering plastic with abundant resources and structural diversity also possesses the potential of large-scale application in energy storage [36,37]. Therefore, the exploration of PI binders holds enormous promise for the enhancement of energy density for high-safety LFP cathode. Besides, PI has been mainly synthesized by thermal amination process involving toxic organic solvents and high-temperature procedures [38]. By comparison, the hydrothermal polymerization is an environment-friendly synthetic route with cost-effectiveness as described in our previous work [37,39,40], avoiding the adoption of toxic solvents and harsh conditions.
Herein, a multifunctional PI binder was synthesized through a facile hydrothermal polymerization route and subsequently used in the fabrication process of LFP electrode. The morphology, dispersion, chemical stability in organic electrolyte, and structural stability during long cycles of LFP electrodes fabricated with PVDF (LFP–PVDF) and PI (LFP–PI) binders were investigated and compared in detail. The PI binder not only serves as the effective bridges between active LFP particles but also boosts the uniform distribution of particles. Besides, the high intrinsic ion conductivity of PI can accelerate ion transfer between active LFP particles. More importantly, the active carbonyl groups existing in the PI chain can contribute to the extra capacity based on the reversible enolization reaction. Therefore, LFP–PI exhibits larger capacity and better rate capability compared with LFP–PVDF. Besides, the superior chemical stability of PI binder in organic electrolyte also endows LFP–PI electrode with great cycling stability. This work confirms that the design of multifunctional polymeric binder can break the performance bottleneck of LFP electrode and offers a new view for the development of high energy density LIBs.
2 Experimental
2.1 Materials
LFP powder was purchased from Guangdong Canrd New Energy Technology Co., Ltd. PVDF was purchased from Solvay Co., Ltd. PI was synthesized through a hydrothermal polymerization method using 3,3′,4,4′-benzophenonetetracarboxylic dianhydride (BTDA) and 1-(4-aminophenyl)-2,3-dihydro-1,3,3-trimethyl-1H-inden-5-amine (DAPI) as monomers. In a typical synthesis, equimolar BTDA (1 g) and DAPI were firstly dispersed in 60 mL deionized water under vigorous stirring at 80°C for 2 h. Then the obtained monomer salt was washed by deionized water to remove the unreacted monomer followed by drying process at 60°C. The as-prepared monomer salt powder was transferred to a Teflon-lined autoclave and kept at 200°C for 12 h. After being cooled to room temperature, the precipitate was collected by vacuum filtration and washed with deionized water. Finally, the PI was obtained after drying at 80°C overnight under vacuum condition.
2.2 Characterization
The morphology was characterized by SU 8010 field emission scanning electron microscope. Powder X-ray diffraction patterns were recorded at room temperature with a D8 Advance diffractometer. Fourier transform infrared spectra (FT-IR) were conducted on a Nexus 470 spectrometer. Thermogravimetric analysis (TGA) was performed on a Mettler Toledo (Switzerland) at a heating rate of 20°C/min in a nitrogen atmosphere.
2.3 Electrochemical measurements
The working electrodes consisted of LFP (80 wt%), conductive carbon SP (10 wt%), and binders (10 wt%) in N-methyl pyrrolidone (NMP). The LFP cathodes were prepared with two different binders of PVDF (LFP–PVDF) and PI (LFP–PI), respectively. Meanwhile, the LFP cathodes were also fabricated using the mixture of PVDF and PI as binders with different PI contents of 30, 50, and 70 wt%, namely LFP–PI-0.3, LFP–PI-0.5, and LFP–PI-0.7. The electrolyte was 1 mol/L LiPF6 in 1:1:1 (v/v/v) mixture of ethylene carbonate (EC)/dimethyl carbonate (DMC)/ethyl methyl carbonate (EMC). Coin type half-cells (CR-2032) were assembled under an argon-filled glovebox with lithium foil as the counter electrode and Celgard 2400 porous membrane as the separator. Cyclic voltammetry (CV) was performed on CHI 760 electrochemical workstation within the potential window of 2.5–4.2 V (vs Li/Li+). Charge–discharge tests were performed on a CT2001A battery testing system between 2.5 and 3.7 V (vs Li/Li+). Electrochemical impedance spectroscopy (EIS) tests were conducted in a frequency range from 0.1 Hz to 100 kHz with AC amplitude of 5 mV by CHI 760 electrochemical workstation.
3 Results and discussion
Figure 1a and b shows the molecular structures and FT-IR spectra of commercial PVDF and the as-prepared PI. PVDF exhibits the typical characteristic peaks at 1,400 and 1,180 cm−1, representing the presence of C–F bonds. As for PI binder, the peaks located at 1,780 and 1,720 cm−1 are assigned to the asymmetric and symmetric stretching vibration peaks of C–O moieties, respectively. Another strong peak at around 1,373 cm−1 corresponds to the absorption peak of C–N. The FT-IR results confirm the successful synthesis of the targeted PI [41]. The morphologies of PVDF and PI were investigated as shown in Figure 1c–f. The PVDF presents uniform spherical morphology with the average particle size of 200–300 nm (Figure 1c and e). In contrast, the PI appears irregular particles with distinct ridges on the surface, which may be ascribed to the poor symmetry of its molecular structure (Figure 1d and f). The higher degree of disorder with a rougher surface for PI can provide a larger specific surface, thus leading to better binding ability for active materials compared to the regular spherical particles of PVDF.

(a) Chain structures and (b) FT-IR spectra of PVDF and PI binders; SEM images of (c and e) PVDF and (d and f) PI binders.
Figure 2a shows the XRD patterns of PVDF and PI binders. The PVDF shows sharp diffraction peaks because of the regular arrangement of polymer chains because of its symmetrical molecular structure. In contrast, only a bulging peak at about 17° can be observed for PI, which could be explained by the random arrangement of polymer chains with poor crystallinity because of its branch chain existed in the diamine units. TGA curves were also performed to investigate the thermal stability of two binders (Figure 2b). The PI shows high decomposition temperature above 500°C, with a weight loss of only 50 wt% even if the temperature reaches up to 800°C. However, PVDF starts to decompose at about 420°C and quickly degrades to the residual weight of only 30 wt% at 500°C. When the temperature rises to 800°C, complete decomposition almost occurred. The TGA results indicate that PI has a better thermal stability than PVDF, which is mainly because of the enhanced structural stability supported by the strong p–π conjugation resulted from the large amounts of rigid aromatic rings [34,41]. The higher thermal stability of PI binder ensures a wide temperature range, thereby promoting the safety and electrochemical performance of LIBs when used in extremely high temperature environment [42].

(a) XRD patterns and (b) TGA curves of PVDF and PI binders.
The morphology and binding stability of fresh LFP–PVDF and LFP–PI electrode films were further analyzed as shown in Figure 3. Active LFP particles in the LFP–PVDF electrode appear obvious agglomeration and local LFP particles aggregated into micron-sized bulk (Figure 3a and b). Especially, partial LFP particles and conductive carbon are disconnected without PVDF binding (Figure 3c), which may be ascribed to the uneven dispersion during the fabrication process of electrode slurry. LFP–PI electrodes exhibit even distribution of active particles without partial aggregation (Figure 3d and e). In addition, obvious PI binders can be observed between active particles and conductive carbon (Figure 3f). The uniform distribution and better connection of active materials can promote the full infiltration of electrolyte and provide continuous Li-ions diffusion path. Besides, the better binding stability of PI also can effectively avoid re-aggregation of particles during charge–discharge process. The above results reveal that the as-prepared PI can also serve as an effective binder for LFP electrode.
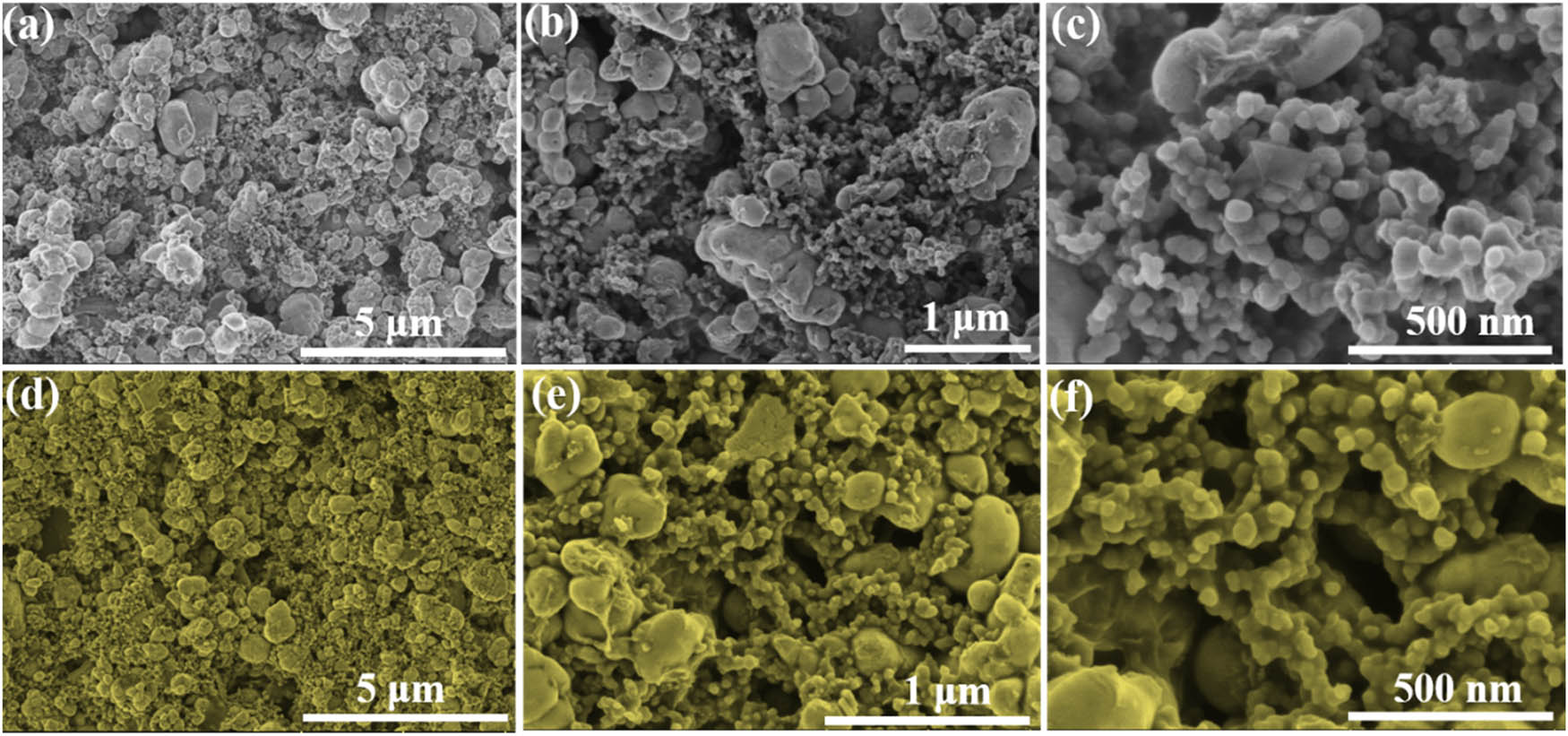
SEM images of fresh (a–c) LFP–PVDF and (d–f) LFP–PI electrode films.
To systematically investigate the influence of different binders on the electrochemical performance of LFP electrode, the coin-type cells with LFP–PVDF and LFP–PI as working electrodes were assembled. Figure 4a shows the typical CV curves of LFP–PVDF and LFP–PI within the same voltage range of 2.5–4.2 V (vs Li/Li+) at 1.0 mV S−1. LFP–PI and LFP–PVDF show sharp redox peaks at 3.24/3.78 and 3.26/3.66 V, respectively, which are assigned to the typical Li-ions insertion/extraction behavior in LFP electrode [43]. It is worth mentioning that LFP–PI shows significantly larger peak current than that of LFP–PVDF, which can be explained by the higher electrochemical activity of LFP–PI electrode because of the highly intrinsic ion conductivity of PI binder and the uniform distribution of active materials [44]. However, LFP–PI exhibits larger peak potential difference (0.54 V) than that of LFP–PVDF (0.40 V), which may be because of the necessary activation process of PI binder during the initial cycles. Figure 4b shows the rate capabilities of LFP–PVDF and LFP-P. Although they deliver similar reversible capacity at a small rate of 0.2C (1C = 170 mA g−1), LFP–PI shows significantly larger capacity retention of 52.6% than that of LFP–PVDF (23.8%) when the rate increases from 0.2C to 5C, suggesting a much better rate capability. On one hand, the high ion conductivity of PI binder can accelerate the ion transfer between adjacent LFP grains. Besides, the electrochemical activity of PI binder also contributes to extra capacity based on the reversible enolization reaction of carbonyl groups existing in the polymer skeleton [45]. On the other hand, the PVDF binder with electrochemically inert chain structure and poor ion conductivity is unfavorable for the electrochemical performance. Although the LFP–PVDF shows an expected cycle stability with almost no capacity decay after 200 cycles at 5C, it delivers significantly lower capacity than that of LFP–PI at the same rate (Figure 4c). It should be noted that the capacity of LFP–PI gradually increases in the first few cycles because of the active process of PI binders, and the specific capacity after stabilization is 40% higher than that of LFP–PVDF. Besides, the LFP–PI also exhibits superior cycling stability with the capacity retention as high as 98% after 200 cycles compared to the stabilized capacity after active process. To further explore the influence of different binders on the reaction kinetics of LFP electrodes, the Li-ion diffusion coefficients (

(a) CV curves at 1.0 mV S−1, (b) rate capabilities, (c) cycle performances at 5C, and (d) Li-ion diffusion coefficients (
To verify the stability of PI binder in organic electrolyte, the PI and PVDF powders are immersed into the 1 M LiPF6 in EC/DMC/EMC electrolyte as shown in Figure 5a. PVDF quickly settled to the bottom of the electrolyte when it was immersed, suggesting a poorer dispersibility. In contrast, the PI binder exhibits uniform dispersion and forms yellow suspension without obvious sedimentation at the beginning of immersing. As the time increased to 24 h, the electrolyte gradually appeared delaminated with a slight sedimentation, and the amount of PI sedimentation did not continue to increase significantly until the immersing time was extended to 48 h. The morphology changes of PI and PVDF powders after immersion in the electrolyte for 48 h were further investigated as shown in Figure 5b and c. Spherical-like PVDF particles with smooth surface cracked into irregular agglomerate after swelling in the organic electrolyte [42]. However, the PI almost maintains the original morphology with similar particle size after immersing in the electrolyte compared to that of the pristine PI powder (Figure 1f). The above results indicate that the PI binder possesses superior dispersion and structural stability in organic electrolyte. SEM images of the two electrode films after cycling test were also analyzed to verify the superior stability of PI binders (Figure 5d and e). Obviously, LFP–PI electrode shows similar morphology with the fresh electrode (Figure 3d). LFP–PVDF electrode shows manifest re-aggregation during cycling, which can be attributed to the failure morphology of PVDF binder in the electrolyte, leading to the significant reduction of its adhesive ability.

(a) Digital photographs of PVDF and PI powders after immersing in the electrolyte for different times; SEM images of (b) PVDF and (c) PI powders after immersing in electrolyte for 48 h; SEM images of (d) LFP–PVDF and (e) LFP–PI electrode films after cycling test.
Although the PI binder exhibits excellent stability in the organic electrolyte and contributes to additional capacity based on the reversible reaction of carbonyl groups, there is still a long way to completely replace the commercial PVDF binder. For example, the physical properties such as adhesion strength, ductility, tensile, and electrolyte uptake of PI are necessary to be deeply investigated to optimize the binder content and electrode manufacturing process. The physical/chemical changes of PI binder during the charge–discharge process should be carefully monitored through theoretical and experimental methods. Besides, the influence of molecular weight and chain structure on the adhesive performance also needs further exploration in terms of the structural diversity of PI. Therefore, the implementation of PI-PVDF hybrid binder by combining the advantages of each provides a possible alternative to develop high energy density LIBs. Figure 6 shows the electrochemical performances of three PI-PVDF hybrid binders with different PI content (30, 50, and 70 wt%) accounting for the total binder content (10 wt%) in the electrode components. When the rate increased from 0.2C to 5C, the LFP–PI electrodes show gradually promoted capacity retention of 27.1% (LFP–PI-0.3), 30.8% (LFP–PI-0.5), and 50.4% (LFP–PI-0.7) with the increase in PI content (Figure 6a). The reversible capacities of LFP–PI-0.5 and LFP–PI-0.7 are much higher than that of LFP–PI-0.3 at a small rate of 0.2C, further verifying that the incorporation of PI with high ion conductivity and electrochemical activity in PVDF binder during the electrode fabrication process can elevate the energy density of LFP electrode. Moreover, all the LFP–PI-0.3, LFP–PI-0.5, and LFP–PI-0.7 electrodes exhibit superior cycling stability during 300 cycles at 1C after the first few cycles of activation with the stabilized reversible capacities of 128, 134, and 141 mA h g−1, respectively (Figure 6b). The above results suggest that the PI binder can not only contribute extra capacity and accelerate the electrochemical reaction kinetics of the LFP electrode, but also maintain good bonding stability during long cycling.

(a) Rate capabilities and (b) cycling performances at 1C of LFP–PI-0.3, LFP–PI-0.5, and LFP–PI-0.7 electrodes.
4 Conclusion
A multifunctional PI was successfully synthesized through a facile hydrothermal polymerization method and served as a binder for commercial LFP cathode. Compared to the traditional PVDF binder, the PI binder serves as the effective bridges between active particles with stable distribution while boosting ion diffusion through the electrode. The high dispersion stability of PI in NMP can effectively prevent the re-aggregation of active particles during the repeated charge–discharge processes. Moreover, the active carbonyl groups existing in the PI skeleton also can contribute to an extra capacity based on the reversible enolization reaction. The multifunction of PI can synergistically improve the capacity and rate performance of the LFP electrode. Thus LFP–PI electrode shows larger capacity and better rate capability compared with LFP–PVDF electrode. Meanwhile, the PI also can maintain well dispersion stability in the organic electrolyte, endowing LFP–PI electrode with great cycling stability compared to the LFP–PVDF electrode. This work offers a new view for developing high-safety and high energy density LFP batteries.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51902349), Hubei Provincial Natural Science Foundation of China (2019CFB260), and Fundamental Research Funds for Central Universities (CZP19001 and CZQ19003).
-
Conflict of interest: The authors declare no conflicts of interest regarding the publication of this paper.
References
[1] Lin DC, Liu YY, Cui Y. Reviving the lithium metal anode for high-energy batteries. Nat Nanotechnol. 2017;12(3):194–206.10.1038/nnano.2017.16Search in Google Scholar PubMed
[2] Liao CY, Zhang Q, Zhai TY, Li HQ, Zhou HS. Development and perspective of the insertion anode Li3VO4 for lithium-ion batteries. Energy Storage Mater. 2017;7:17–31.10.1016/j.ensm.2016.11.009Search in Google Scholar
[3] Han XY, Li R, Qiu SQ, Zhang XF, Zhang Q, Yang YK. Sonochemistry-enabled uniform coupling of SnO2 nanocrystals with graphene sheets as anode materials for lithium-ion batteries. RSC Adv. 2019;9(11):5942–7.10.1039/C9RA00554DSearch in Google Scholar
[4] Pan YS, Xu K, Wu CL. Recent progress in supercapacitors based on the advanced carbon electrodes. Nanotechnol Rev. 2019;8(1):299–314.10.1515/ntrev-2019-0029Search in Google Scholar
[5] Jiang YL, He CE, Qiu SQ, Zhang JL, Wang XG, Yang YK. Scalable mechanochemical coupling of homogeneous Co3O4 nanocrystals onto in situ exfoliated graphene sheets for asymmetric supercapacitors. Chem Eng J. 2020;397:125503.10.1016/j.cej.2020.125503Search in Google Scholar
[6] Kumari N, Patel SR, Gohel JV. Current progress and future prospective of perovskite solar cells: a comprehensive review. Rev Adv Mater Sci. 2018;53(2):161–86.10.1515/rams-2018-0012Search in Google Scholar
[7] Orlova TS, Shpeizman VV, Glebova NV, Nechitailov AA, Spitsyn AA, Ponomarev DA, et al. Environmentally friendly monolithic highly-porous biocarbons as binder-free supercapacitor electrodes. Rev Adv Mater Sci. 2018;55(1):50–60.10.1515/rams-2018-0027Search in Google Scholar
[8] Li ZH, Xu K, Pan YS. Recent development of supercapacitor electrode based on carbon materials. Nanotechnol Rev. 2019;8(1):35–49.10.1515/ntrev-2019-0004Search in Google Scholar
[9] Wang JJ, Sun XL. Olivine LiFePO4: the remaining challenges for future energy storage. Energy Environ Sci. 2015;8(4):1110–38.10.1039/C4EE04016CSearch in Google Scholar
[10] Wang JJ, Sun XL. Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium-ion batteries. Energy Environ Sci. 2012;5(1):5163–85.10.1039/C1EE01263KSearch in Google Scholar
[11] Yu F, Zhang LL, Li YC, An YX, Zhu MY, Dai B. Mechanism studies of LiFePO4 cathode material: lithiation/delithiation process, electrochemical modification and synthetic reaction. RSC Adv. 2014;4(97):54576–602.10.1039/C4RA10899JSearch in Google Scholar
[12] Ohmer N, Fenk B, Samuelis D, Chen CC, Maier J, Weigand M, et al. Phase evolution in single-crystalline LiFePO4 followed by in situ scanning X-ray microscopy of a micrometre-sized battery. Nat Commun. 2015;6:6045–51.10.1038/ncomms7045Search in Google Scholar PubMed
[13] Wang JJ, Yang JL, Tang YJ, Liu J, Zhang Y, Liang GX, et al. Size-dependent surface phase change of lithium iron phosphate during carbon coating. Nat Commun. 2014;5:3415–22.10.1038/ncomms4415Search in Google Scholar PubMed
[14] Liang YC, Wen KC, Mao YW, Liu ZP, Zhu GL, Yang F, et al. Shape and size control of LiFePO4 for high-performance lithium-ion batteries. ChemElectroChem. 2015;2(9):1227–37.10.1002/celc.201500114Search in Google Scholar
[15] Ha SH, Lee YJ. Core-shell LiFePO4/carbon-coated reduced graphene oxide hybrids for high-power lithium-ion battery cathodes. Chemistry. 2015;21(5):2132–8.10.1002/chem.201404952Search in Google Scholar PubMed
[16] Kapaev RR, Novikova SA, Chekannikov AA, Gryzlov DY, Kulova TL, Skundin AM, et al. Effect of carbon sources and synthesis conditions on the LiFePO4/C cathode properties. Rev Adv Mater Sci. 2018;57(2):183–92.10.1515/rams-2018-0063Search in Google Scholar
[17] Ghouri ZK, Motlak M, Afaq S, Barakat NA, Abdala A. Template-free synthesis of Se-nanorods-rGO nanocomposite for application in supercapacitors. Nanotechnol Rev. 2019;8(1):661–70.10.1515/ntrev-2019-0057Search in Google Scholar
[18] Zou F, Manthiram A. A review of the design of advanced binders for high-performance batteries. Adv Energy Mater. 2020;10(45):2002508.10.1002/aenm.202002508Search in Google Scholar
[19] Mantia FL, Huggins RA, Cui Y. Oxidation processes on conducting carbon additives for lithium-ion batteries. J Appl Electrochem. 2013;43(1):1–7.10.1007/s10800-012-0499-9Search in Google Scholar
[20] Ye YS, Chou LY, Liu YY, Wang HS, Lee HK, Huang WX, et al. Ultralight and fire-extinguishing current collectors for high-energy and high-safety lithium-ion batteries. Nat Energy. 2020;5(10):786–93.10.1038/s41560-020-00702-8Search in Google Scholar
[21] Ventrapragada LK, Creager SE, Rao AM, Podila R. Carbon nanotubes coated paper as current collectors for secondary li-ion batteries. Nanotechnol Rev. 2019;8(1):18–23.10.1515/ntrev-2019-0002Search in Google Scholar
[22] Pasha BA, Kaleemulla M. Processing and characterization of aluminum metal matrix composites: an overview. Rev Adv Mater Sci. 2018;56(1):79–90.10.1515/rams-2018-0039Search in Google Scholar
[23] Chen H, Ling M, Hencz L, Ling HY, Li GR, Lin Z, et al. Exploring chemical, mechanical, and electrical functionalities of binders for advanced energy-storage devices. Chem Rev. 2018;118(18):8936–82.10.1021/acs.chemrev.8b00241Search in Google Scholar PubMed
[24] Hwang SS, Sohn M, Park HI, Choi JM, Cho CG, Kim H. Effect of the heat treatment on the dimensional stability of Si electrodes with PVDF binder. Electrochim Acta. 2016;211:356–63.10.1016/j.electacta.2016.05.183Search in Google Scholar
[25] Xu JT, Chou SL, Gu QF, Liu HK, Dou SX. The effect of different binders on electrochemical properties of LiNi1/3Mn1/3Co1/3O2 cathode material in lithium ion batteries. J Power Sources. 2013;225:172–8.10.1016/j.jpowsour.2012.10.033Search in Google Scholar
[26] Liu YF, Jiang LY, Wang HN, Wang H, Jiao W, Chen GZ, et al. A brief review for fluorinated carbon: synthesis, properties and applications. Nanotechnol Rev. 2019;8(1):573–86.10.1515/ntrev-2019-0051Search in Google Scholar
[27] Kim JS, Choi WC, Cho KY, Byun DJ, Lim JC, Lee JK. Effect of polyimide binder on electrochemical characteristics of surface-modified silicon anode for lithium ion batteries. J Power Sources. 2013;244:521–6.10.1016/j.jpowsour.2013.02.049Search in Google Scholar
[28] Thompson L, Azadmanjiri J, Nikzad M, Sbarski I, Wang J, Yu AM. Cellulose nanocrystals: production, functionalization and advanced applications. Rev Adv Mater Sci. 2019;58(1):1–16.10.1515/rams-2019-0001Search in Google Scholar
[29] Lee JH, Paik U, Hackley VA, Choi YM. Effect of poly(acrylic acid) on adhesion strength and electrochemical performance of natural graphite negative electrode for lithium-ion batteries. J Power Sources. 2006;161(1):612–6.10.1016/j.jpowsour.2006.03.087Search in Google Scholar
[30] Li J, Le DB, Ferguson PP, Dahn JR. Lithium polyacrylate as a binder for tin–cobalt–carbon negative electrodes in lithium-ion batteries. Electrochim Acta. 2010;55(8):2991–5.10.1016/j.electacta.2010.01.011Search in Google Scholar
[31] Liu TF, Tong CJ, Wang B, Liu LM, Zhang SQ, Lin Z, et al. Trifunctional electrode additive for high active material content and volumetric lithium-ion electrode densities. Adv Energy Mater. 2019;9(10):1803390.10.1002/aenm.201803390Search in Google Scholar
[32] Uchida S, Mihashi M, Yamagata M, Ishikawa M. Electrochemical properties of non-nano-silicon negative electrodes prepared with a polyimide binder. J Power Sources. 2015;273:118–22.10.1016/j.jpowsour.2014.09.096Search in Google Scholar
[33] Choi J, Kim K, Jeong J, Cho KY, Ryou MH, Lee YM. Highly adhesive and soluble copolyimide binder: Improving the long-term cycle life of silicon anodes in lithium-ion batteries. ACS Appl Mater Interfaces. 2015;7(27):14851–8.10.1021/acsami.5b03364Search in Google Scholar PubMed
[34] Yao DH, Yang Y, Deng YH, Wang CY. Flexible polyimides through one-pot synthesis as water-soluble binders for silicon anodes in lithium ion batteries. J Power Sources. 2018;379:26–32.10.1016/j.jpowsour.2017.12.086Search in Google Scholar
[35] Pham HQ, Kim G, Jung HM, Song SW. Fluorinated polyimide as a novel high-voltage binder for high-capacity cathode of lithium-ion batteries. Adv Funct Mater. 2018;28(2):1704690.10.1002/adfm.201704690Search in Google Scholar
[36] Zhang XF, Cui X, Lu CH, Li H, Zhang Q, He CE, et al. Conjugated polyimide-coated carbon nanofiber aerogels in a redox electrolyte for binder-free supercapacitors. Chem Eng J. 2020;401:126031.10.1016/j.cej.2020.126031Search in Google Scholar
[37] Zhang Q, Lin GY, He Y, Cui X, Yang YK. Chain engineering-tailored microstructures and lithium storage performance of hydrothermally-synthesized linear polyimides. Mater Today Chem. 2020;17:100341.10.1016/j.mtchem.2020.100341Search in Google Scholar
[38] Baumgartner B, Bojdys MJ, Unterlass MM. Geomimetics for green polymer synthesis: highly ordered polyimides via hydrothermal techniques. Polym Chem. 2014;5(12):3771–6.10.1039/C4PY00263FSearch in Google Scholar
[39] He Y, Li H, Zhang Q, He CE, Zhang XF, Yang YK. Homogeneous coating of carbon nanotubes with tailored N-doped carbon layers for improved electrochemical energy storage. RSC Adv. 2019;9(70):40933–9.10.1039/C9RA06289KSearch in Google Scholar PubMed PubMed Central
[40] Liu X, Qiu SQ, Mei P, Zhang Q, Yang YK. Chain structure-dependent electrochemical performance of polyimide cathode materials for lithium-ion batteries. J Mater Sci. 2021;56:3900–10.10.1007/s10853-020-05510-9Search in Google Scholar
[41] Song ZP, Zhan H, Zhou YH. Polyimides: promising energy-storage materials. Angew Chem Int Ed. 2010;49(45):8444–8.10.1002/anie.201002439Search in Google Scholar PubMed
[42] Qian GN, Wang L, Shang YM, He XM, Tang SF, Liu M, et al. Polyimide binder: a facile way to improve safety of lithium ion batteries. Electrochim Acta. 2016;187:113–8.10.1016/j.electacta.2015.11.019Search in Google Scholar
[43] Wang JJ, Chen-Wiegart YK, Wang J. In operando tracking phase transformation evolution of lithium iron phosphate with hard X-ray microscopy. Nat Commun. 2014;5:4570–9.10.1038/ncomms5570Search in Google Scholar PubMed
[44] Cho JH, Park JH, Lee MH, Song HK, Lee SY. A polymer electrolyte-skinned active material strategy toward high-voltage lithium ion batteries: a polyimide-coated LiNi0.5Mn1.5O4 spinel cathode material case. Energy Environ Sci. 2012;5(5):7124–31.10.1039/c2ee03389eSearch in Google Scholar
[45] Wang H, Yao CJ, Nie HJ, Wang KZ, Zhong YW, Chen PW, et al. Recent progress in carbonyl-based organic polymers as promising electrode materials for lithium-ion batteries (LIBs). J Mater Chem A. 2020;8(24):11906–22.10.1039/D0TA03321ASearch in Google Scholar
[46] Zhang Q, He Y, Mei P, Cui X, Yang YK, Lin ZQ. Multi-functional PEDOT-engineered sodium titanate nanowires for sodium-ion batteries with synchronous improvements in rate capability and structural stability. J Mater Chem A. 2019;7:19241–7.10.1039/C9TA04406JSearch in Google Scholar
© 2020 Qing Zhang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Generalized locally-exact homogenization theory for evaluation of electric conductivity and resistance of multiphase materials
- Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide
- Characterization of mechanical properties of epoxy/nanohybrid composites by nanoindentation
- Graphene and CNT impact on heat transfer response of nanocomposite cylinders
- A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites
- Ultrasmall Fe3O4 nanoparticles induce S-phase arrest and inhibit cancer cells proliferation
- Effect of aging on properties and nanoscale precipitates of Cu-Ag-Cr alloy
- Effect of nano-strengthening on the properties and microstructure of recycled concrete
- Stabilizing effect of methylcellulose on the dispersion of multi-walled carbon nanotubes in cementitious composites
- Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites
- Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation
- Preparation and properties of 3D interconnected CNTs/Cu composites
- On factors affecting surface free energy of carbon black for reinforcing rubber
- Nano-silica modified phenolic resin film: manufacturing and properties
- Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete
- Halloysite nanotubes in polymer science: purification, characterization, modification and applications
- Cellulose hydrogel skeleton by extrusion 3D printing of solution
- Crack closure and flexural tensile capacity with SMA fibers randomly embedded on tensile side of mortar beams
- An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites
- Utilization of red mud for producing a high strength binder by composition optimization and nano strengthening
- One-pot synthesis of nano titanium dioxide in supercritical water
- Printability of photo-sensitive nanocomposites using two-photon polymerization
- In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries
- Effect of nano and micro conductive materials on conductive properties of carbon fiber reinforced concrete
- Tribological performance of nano-diamond composites-dispersed lubricants on commercial cylinder liner mating with CrN piston ring
- Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance
- Genetic mechanisms of deep-water massive sandstones in continental lake basins and their significance in micro–nano reservoir storage systems: A case study of the Yanchang formation in the Ordos Basin
- Effects of nanoparticles on engineering performance of cementitious composites reinforced with PVA fibers
- Band gap manipulation of viscoelastic functionally graded phononic crystal
- Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution
- Manipulating conductive network formation via 3D T-ZnO: A facile approach for a CNT-reinforced nanocomposite
- Microstructure and mechanical properties of WC–Ni multiphase ceramic materials with NiCl2·6H2O as a binder
- Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite
- Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function
- Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing
- Corrosion behaviour of multilayer CrN coatings deposited by hybrid HIPIMS after oxidation treatment
- Surface hydrophobicity and oleophilicity of hierarchical metal structures fabricated using ink-based selective laser melting of micro/nanoparticles
- Research on bond–slip performance between pultruded glass fiber-reinforced polymer tube and nano-CaCO3 concrete
- Antibacterial polymer nanofiber-coated and high elastin protein-expressing BMSCs incorporated polypropylene mesh for accelerating healing of female pelvic floor dysfunction
- Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films
- A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination
- Arrangement structure of carbon nanofiber with excellent spectral radiation characteristics
- Effect of different particle sizes of nano-SiO2 on the properties and microstructure of cement paste
- Superior Fe x N electrocatalyst derived from 1,1′-diacetylferrocene for oxygen reduction reaction in alkaline and acidic media
- Facile growth of aluminum oxide thin film by chemical liquid deposition and its application in devices
- Liquid crystallinity and thermal properties of polyhedral oligomeric silsesquioxane/side-chain azobenzene hybrid copolymer
- Laboratory experiment on the nano-TiO2 photocatalytic degradation effect of road surface oil pollution
- Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage
- Conversion of sub-µm calcium carbonate (calcite) particles to hollow hydroxyapatite agglomerates in K2HPO4 solutions
- Exact solutions of bending deflection for single-walled BNNTs based on the classical Euler–Bernoulli beam theory
- Effects of substrate properties and sputtering methods on self-formation of Ag particles on the Ag–Mo(Zr) alloy films
- Enhancing carbonation and chloride resistance of autoclaved concrete by incorporating nano-CaCO3
- Effect of SiO2 aerogels loading on photocatalytic degradation of nitrobenzene using composites with tetrapod-like ZnO
- Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles
- Hydration activity, crystal structural, and electronic properties studies of Ba-doped dicalcium silicate
- Microstructure and mechanical properties of brazing joint of silver-based composite filler metal
- Polymer nanocomposite sunlight spectrum down-converters made by open-air PLD
- Cryogenic milling and formation of nanostructured machined surface of AISI 4340
- Braided composite stent for peripheral vascular applications
- Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre–reinforced starch biopolymer composites
- Study on influencing factors of photocatalytic performance of CdS/TiO2 nanocomposite concrete
- Improving flexural and dielectric properties of carbon fiber epoxy composite laminates reinforced with carbon nanotubes interlayer using electrospray deposition
- Scalable fabrication of carbon materials based silicon rubber for highly stretchable e-textile sensor
- Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery
- Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries
- Synthesis of functionalized carbon nanotubes for fluorescent biosensors
- Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete
- Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage
- Microstructural evolution and properties of Cu–20 wt% Ag alloy wire by multi-pass continuous drawing
- Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene
- Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications
- Preparation and piezoresistivity of carbon nanotube-coated sand reinforced cement mortar
- Vibrational analysis of an irregular single-walled carbon nanotube incorporating initial stress effects
- Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials
- Single pulse laser removal of indium tin oxide film on glass and polyethylene terephthalate by nanosecond and femtosecond laser
- Mechanical reinforcement with enhanced electrical and heat conduction of epoxy resin by polyaniline and graphene nanoplatelets
- High-efficiency method for recycling lithium from spent LiFePO4 cathode
- Degradable tough chitosan dressing for skin wound recovery
- Static and dynamic analyses of auxetic hybrid FRC/CNTRC laminated plates
- Review articles
- Carbon nanomaterials enhanced cement-based composites: advances and challenges
- Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide
- Review on modeling and application of chemical mechanical polishing
- Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites
- Advances in modelling and analysis of nano structures: a review
- Mechanical properties of nanomaterials: A review
- New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics
- A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials
- Recent development and applications of nanomaterials for cancer immunotherapy
- Advances in biomaterials for adipose tissue reconstruction in plastic surgery
- Advances of graphene- and graphene oxide-modified cementitious materials
- Theories for triboelectric nanogenerators: A comprehensive review
- Nanotechnology of diamondoids for the fabrication of nanostructured systems
- Material advancement in technological development for the 5G wireless communications
- Nanoengineering in biomedicine: Current development and future perspectives
- Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview
- Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review
- Carbon nanotube–reinforced polymer composite for electromagnetic interference application: A review
- Functionalized layered double hydroxide applied to heavy metal ions absorption: A review
- A new classification method of nanotechnology for design integration in biomaterials
- Finite element analysis of natural fibers composites: A review
- Phase change materials for building construction: An overview of nano-/micro-encapsulation
- Recent advance in surface modification for regulating cell adhesion and behaviors
- Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions
- Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review
- Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications
- A review of passive methods in microchannel heat sink application through advanced geometric structure and nanofluids: Current advancements and challenges
- Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices
- Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging
- Synthesis of graphene: Potential carbon precursors and approaches
- A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE)
- Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water
- Analysis of functionally graded carbon nanotube-reinforced composite structures: A review
- Application of nanomaterials in ultra-high performance concrete: A review
- Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer
- Advanced nickel nanoparticles technology: From synthesis to applications
- Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable?
- Progress in construction of bio-inspired physico-antimicrobial surfaces
- From materials to devices using fused deposition modeling: A state-of-art review
- A review for modified Li composite anode: Principle, preparation and challenge
- Naturally or artificially constructed nanocellulose architectures for epoxy composites: A review
Articles in the same Issue
- Research Articles
- Generalized locally-exact homogenization theory for evaluation of electric conductivity and resistance of multiphase materials
- Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide
- Characterization of mechanical properties of epoxy/nanohybrid composites by nanoindentation
- Graphene and CNT impact on heat transfer response of nanocomposite cylinders
- A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites
- Ultrasmall Fe3O4 nanoparticles induce S-phase arrest and inhibit cancer cells proliferation
- Effect of aging on properties and nanoscale precipitates of Cu-Ag-Cr alloy
- Effect of nano-strengthening on the properties and microstructure of recycled concrete
- Stabilizing effect of methylcellulose on the dispersion of multi-walled carbon nanotubes in cementitious composites
- Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites
- Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation
- Preparation and properties of 3D interconnected CNTs/Cu composites
- On factors affecting surface free energy of carbon black for reinforcing rubber
- Nano-silica modified phenolic resin film: manufacturing and properties
- Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete
- Halloysite nanotubes in polymer science: purification, characterization, modification and applications
- Cellulose hydrogel skeleton by extrusion 3D printing of solution
- Crack closure and flexural tensile capacity with SMA fibers randomly embedded on tensile side of mortar beams
- An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites
- Utilization of red mud for producing a high strength binder by composition optimization and nano strengthening
- One-pot synthesis of nano titanium dioxide in supercritical water
- Printability of photo-sensitive nanocomposites using two-photon polymerization
- In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries
- Effect of nano and micro conductive materials on conductive properties of carbon fiber reinforced concrete
- Tribological performance of nano-diamond composites-dispersed lubricants on commercial cylinder liner mating with CrN piston ring
- Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance
- Genetic mechanisms of deep-water massive sandstones in continental lake basins and their significance in micro–nano reservoir storage systems: A case study of the Yanchang formation in the Ordos Basin
- Effects of nanoparticles on engineering performance of cementitious composites reinforced with PVA fibers
- Band gap manipulation of viscoelastic functionally graded phononic crystal
- Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution
- Manipulating conductive network formation via 3D T-ZnO: A facile approach for a CNT-reinforced nanocomposite
- Microstructure and mechanical properties of WC–Ni multiphase ceramic materials with NiCl2·6H2O as a binder
- Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite
- Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function
- Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing
- Corrosion behaviour of multilayer CrN coatings deposited by hybrid HIPIMS after oxidation treatment
- Surface hydrophobicity and oleophilicity of hierarchical metal structures fabricated using ink-based selective laser melting of micro/nanoparticles
- Research on bond–slip performance between pultruded glass fiber-reinforced polymer tube and nano-CaCO3 concrete
- Antibacterial polymer nanofiber-coated and high elastin protein-expressing BMSCs incorporated polypropylene mesh for accelerating healing of female pelvic floor dysfunction
- Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films
- A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination
- Arrangement structure of carbon nanofiber with excellent spectral radiation characteristics
- Effect of different particle sizes of nano-SiO2 on the properties and microstructure of cement paste
- Superior Fe x N electrocatalyst derived from 1,1′-diacetylferrocene for oxygen reduction reaction in alkaline and acidic media
- Facile growth of aluminum oxide thin film by chemical liquid deposition and its application in devices
- Liquid crystallinity and thermal properties of polyhedral oligomeric silsesquioxane/side-chain azobenzene hybrid copolymer
- Laboratory experiment on the nano-TiO2 photocatalytic degradation effect of road surface oil pollution
- Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage
- Conversion of sub-µm calcium carbonate (calcite) particles to hollow hydroxyapatite agglomerates in K2HPO4 solutions
- Exact solutions of bending deflection for single-walled BNNTs based on the classical Euler–Bernoulli beam theory
- Effects of substrate properties and sputtering methods on self-formation of Ag particles on the Ag–Mo(Zr) alloy films
- Enhancing carbonation and chloride resistance of autoclaved concrete by incorporating nano-CaCO3
- Effect of SiO2 aerogels loading on photocatalytic degradation of nitrobenzene using composites with tetrapod-like ZnO
- Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles
- Hydration activity, crystal structural, and electronic properties studies of Ba-doped dicalcium silicate
- Microstructure and mechanical properties of brazing joint of silver-based composite filler metal
- Polymer nanocomposite sunlight spectrum down-converters made by open-air PLD
- Cryogenic milling and formation of nanostructured machined surface of AISI 4340
- Braided composite stent for peripheral vascular applications
- Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre–reinforced starch biopolymer composites
- Study on influencing factors of photocatalytic performance of CdS/TiO2 nanocomposite concrete
- Improving flexural and dielectric properties of carbon fiber epoxy composite laminates reinforced with carbon nanotubes interlayer using electrospray deposition
- Scalable fabrication of carbon materials based silicon rubber for highly stretchable e-textile sensor
- Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery
- Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries
- Synthesis of functionalized carbon nanotubes for fluorescent biosensors
- Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete
- Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage
- Microstructural evolution and properties of Cu–20 wt% Ag alloy wire by multi-pass continuous drawing
- Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene
- Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications
- Preparation and piezoresistivity of carbon nanotube-coated sand reinforced cement mortar
- Vibrational analysis of an irregular single-walled carbon nanotube incorporating initial stress effects
- Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials
- Single pulse laser removal of indium tin oxide film on glass and polyethylene terephthalate by nanosecond and femtosecond laser
- Mechanical reinforcement with enhanced electrical and heat conduction of epoxy resin by polyaniline and graphene nanoplatelets
- High-efficiency method for recycling lithium from spent LiFePO4 cathode
- Degradable tough chitosan dressing for skin wound recovery
- Static and dynamic analyses of auxetic hybrid FRC/CNTRC laminated plates
- Review articles
- Carbon nanomaterials enhanced cement-based composites: advances and challenges
- Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide
- Review on modeling and application of chemical mechanical polishing
- Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites
- Advances in modelling and analysis of nano structures: a review
- Mechanical properties of nanomaterials: A review
- New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics
- A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials
- Recent development and applications of nanomaterials for cancer immunotherapy
- Advances in biomaterials for adipose tissue reconstruction in plastic surgery
- Advances of graphene- and graphene oxide-modified cementitious materials
- Theories for triboelectric nanogenerators: A comprehensive review
- Nanotechnology of diamondoids for the fabrication of nanostructured systems
- Material advancement in technological development for the 5G wireless communications
- Nanoengineering in biomedicine: Current development and future perspectives
- Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview
- Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review
- Carbon nanotube–reinforced polymer composite for electromagnetic interference application: A review
- Functionalized layered double hydroxide applied to heavy metal ions absorption: A review
- A new classification method of nanotechnology for design integration in biomaterials
- Finite element analysis of natural fibers composites: A review
- Phase change materials for building construction: An overview of nano-/micro-encapsulation
- Recent advance in surface modification for regulating cell adhesion and behaviors
- Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions
- Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review
- Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications
- A review of passive methods in microchannel heat sink application through advanced geometric structure and nanofluids: Current advancements and challenges
- Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices
- Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging
- Synthesis of graphene: Potential carbon precursors and approaches
- A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE)
- Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water
- Analysis of functionally graded carbon nanotube-reinforced composite structures: A review
- Application of nanomaterials in ultra-high performance concrete: A review
- Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer
- Advanced nickel nanoparticles technology: From synthesis to applications
- Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable?
- Progress in construction of bio-inspired physico-antimicrobial surfaces
- From materials to devices using fused deposition modeling: A state-of-art review
- A review for modified Li composite anode: Principle, preparation and challenge
- Naturally or artificially constructed nanocellulose architectures for epoxy composites: A review

