Abstract
Immunotherapy, which utilizes the patient’s own immune system to fight against cancer, further results in durable antitumor responses and reduces metastasis and recurrence, has become one of the most effective and important cancer therapies along with surgery, radiotherapy, and chemotherapy. Nanomaterials with the advantages of large specific surface, delivery function, and controllable surface chemistry are used to deliver antigens or adjuvants, or both, help to boost immune responses with the imaging function or just act as adjuvants themselves and modulate tumor microenvironment (TME). In this review, recent development and applications of nanomaterials for cancer immunotherapy including delivery systems based on nanomaterials, uniting imaging, self-adjuvants, targeting functions, artificial antigen presenting cells, and TME modulation are focused and discussed.
1 Introduction
Cancer immunotherapy has drawn a great attention due to the unique characteristics of the immune system targeting tumor cells without sacrificing healthy cells, and the feature of long-term control of cancer in the response population, leading to a suppression of cancer metastasis and recurrence, which is never seen in other therapies like surgery, radiotherapy, or chemotherapy [1,2]. Bacterial toxins, which were used for cancer treatment by William Coley in 1890, was a rudiment of cancer immunotherapy [3], then the Sipuleucel-T (APC 8015), which was the first dendritic vaccine-based cellular immunotherapy, and ipilimumab, which was the first checkpoint inhibitor and a fully monoclonal antibody, were approved by the US Food and Drug Administration (FDA) in 2010 and 2011, respectively [4,5]. Since then, the development of cancer immunotherapy has been dramatically induced and developed, but there are still many challenges of enhancing the therapeutic benefits and reducing side effects.
Nanomaterials with a size at least one dimension between 1 and 200 nm are being practically useful for cancer diagnosis, prevention, and treatment in novel radiotherapy, chemotherapy, photodynamic therapy, gene therapy, and immunotherapy [6,7,8]. For immunotherapy, multiple nanomaterial-based drug delivery systems including liposomes, polymeric micelles, gold nanoparticles, and carbon nanotube are being used to deliver antigens or adjuvants or both simultaneously [9,10,11,12]. Free antigens with relatively low efficiency in cross-presentation are normally poorly presented to major histocompatibility complex (MHC) class I molecules due to the micropinocytosis internalization mechanism, and they could be rapidly eliminated and degraded after being administered subcutaneously or intradermally [13,14,15]. However, the nanoparticles that are administered intradermally, intramuscularly, and intraperitoneally could enhance the antigen cross-presentation, resulting in significantly higher humoral and cellular immune responses [16,17,18]. Furthermore, nanomaterials with targeting ligands being preloaded on the surface are easy to be taken up by the targeting cells [19,20]. Additionally, nanomaterials could deliver both antigens and adjuvants at the same time to avoid the immune tolerance caused by the lack of “danger signals,” which are generated by specific adjuvants to dendrite cells (DCs) encountering antigens when delivering antigens and adjuvants separately [21,22]. The most commonly used nanomaterial-based delivery systems include liposome-based system, polymeric nanomaterial-based system, gold nanoparticle-based system, carbon-based system, and porous silicon-based system [23,24,25].
It would be an advantage if the nanomaterial-based delivery systems are equipped with additional imaging function to monitor the treatment effect or act as adjuvants themselves without co-delivering antigens and adjuvants to decrease the difficulty of delivery while increasing the immune response. It has been demonstrated that gold nanoparticles, Fe3O4-based nanoparticles, and upconversion nanoparticles are imaging functionalized for CT and photoacoustic imaging, magnetic resonance imaging (MRI), and upconversion luminescence, respectively [26,27]. Poly (d,l-lactide-co-glycolide) (PLGA), carbon nanotubes, acetylated dextran, and graphene-based nanomaterials have been proved to be the self-adjuvants functionalized to induce the uptake of antigens by antigen-presenting cells (APCs) and increase the activation of APCs, which further develops more effective antitumor immune response [28,29]. Nanomaterials can also play a more important role in regulating the immunosuppressive tumor microenvironment (TME) themselves.
In this review, we will focus on the recent development and applications of nanomaterials for cancer immunotherapy. Some perspectives of applying nanomaterials to immunotherapy will be offered through summarizing and discussing the recent development.
2 Rationale of the functions of nanomaterials for immunotherapy
Cancer immunotherapy is designed to generate a self-sustaining cycle of cancer immunity, which is called “cancer immunity cycle” (Figure 1) [30,31], enabling it to amplify and propagate without generating unrestrained autoimmune inflammatory responses [31]. There are seven steps being classified as two parts of systematic-based and local-based immunotherapies for the cancer immunity cycle: (1) neoantigens are created, released, and captured by DCs; (2) the captured antigens on MHCI and MHCII molecules are processed and presented to T cells; (3) the DCs prime and activate immature T cells in the draining lymph nodes; (4) the activated effector T cells traffic to the tumor site; (5) the activated effector T cells infiltrate into tumors; (6) the T cells recognize cancer cells through T-cell receptor; and (7) the effector T cells kill the target cancer cells by inducing the apoptotic pathways. The killing of cancer cells releases additional tumor-associated antigens (step 1) to repeat and enhance the immunity cycle afterward [13,30].

The cancer immunity cycle.
During the immunotherapy treatment, the cancer immunity cycle might be blocked at one or more steps, leading to insufficient therapeutic effect or even immune escape. In addition, the immunosuppressive TME could be generated to impede anti-tumor immune responses. The cancer-associated fibroblasts, tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and some cytokines, which constitute the immunosuppressive TME, could facilitate the growth of tumor cells and protect them from being eliminated [13,32,33,34].
In consideration of the limitations of cancer immunotherapy or cancer immunity cycle, nanomaterials are used to overcome the limitations and develop immunotherapies more effectively in terms nano-vaccine and TME modulation. The functions of nanomaterials are listed in Table 1. For the period of cancer vaccine functionalizing, nanomaterials could be used to deliver antigen or adjuvants, or both, act as self-adjuvants, conduct lymph node drainage, target DCs, and present antigens. For TME modulation period, nanomaterials could be used to target immune checkpoints, soluble mediators, TAMs, MDSCs, Tregs, and tumor-associated fibroblasts (TAFs) [35,36,37]. With the specific properties of antigens and/or adjuvants protection and delivery, large specific surface area, controllable surface chemistry and size, multifunction, and self-adjuvants, nanomaterials are being widely used in cancer immunotherapy by promoting the cancer immunity cycle [38]. The rationale of each function is summarized as follows:
Delivery of antigens: delivery of antigens or adjuvants is one of the most important functions applied at the first step of the immunity cycle. The tumor antigens are capable of generating the immune response, and adjuvants are capable of generating “danger signals” to assist to stimulate the maturation of DCs. In this way, antigens and adjuvants are the crucial parts to initiate or reinitiate the immunity cycle. But the antigens and adjuvants are easy to be degraded or failed to be taken up by DCs through the conventional delivery methods, which eventually leads to the failure of generating the immune response. To increase the delivery efficiency, various kinds of nanomaterials are used to encapsulate the antigens or adjuvants or both to protect them from degradation to generate the immune response.
Co-encapsulation and co-delivery: delivering the antigens and adjuvants separately might cause the immune tolerance because of the absence of “danger signals” generated by adjuvants. The nanomaterials that could co-encapsulate and co-delivery both the antigens and the adjuvants at the same time could not only protect the antigens and adjuvants but also increase the efficiency of DCs uptake.
Self-adjuvant: sometimes, the nanomaterials that are used for delivering the antigens could act as adjuvants themselves to generate the “danger signals” without co-delivering the specific adjuvants and then promote the antigen presentation, stimulating the immune response.
Lymph node drainage: the nanomaterials encapsulated with antigens or adjuvants or both are needed to drain into the lymph node, where they could be presented by resident DCs. In this way, the size of the nanoparticle becomes the critical factor, which affects whether it could drain into lymph node and be taken up by DCs. The nanoparticles with a size of 40–50 nm are optimal for effective draining into lymph node to generate the immune response eventually.
DCs uptake: similar to the lymph node drainage, the DCs uptake is also affected by the size of the nanoparticles, and it would decrease the uptake efficiency when the size of the nanoparticles exceeds 500 nm.
DCs targeting: the nanoparticles could be modified with specific ligands for DCs surface receptors to increase the efficiency of DCs uptake.
Antigen presentation: antigen presentation is a prerequisite to active the cytotoxic T lymphocyte (CTL). During the presentation process, soluble antigens are internalized by micropinocytosis, and they are cross-presented to MHCI with a low efficiency. In this way, the nanoparticles that encapsulate antigens could enter DCs through phagocytosis and present antigens with a higher efficiency.
Peptide vaccine delivery: peptide vaccines are generally composed of two or more small fragments of antigen proteins and adjuvants. When the peptide vaccines are administered subcutaneously freely, the small fragments of antigens and adjuvants could be degraded and eliminated rapidly. In this way, nanomaterials are used to encapsulate and deliver the peptide vaccine to improve the efficiency.
DNA and mRNA antigens delivery: DNA vaccines could mimic live infections compared with the conventional peptide vaccines. But the delivery efficiency is pretty low and the immunogenicity is weak when the DNA vaccines are administered as naked DNA, which could be degraded by nucleases. In this way, nanomaterials could be used to encapsulate and deliver the DNA-based vaccines.
TME modulation: when immune response is successfully generated and T cells are effectively infiltrated into tumor cells, the T cells are ready to kill tumor cells. But the suppression of TME still could inhibit the immune cycle, leading to the failure of immunotherapy. Suppression of TME modulation is mainly caused by tumor cells, TAFs, TAMs, MDSCs, Tregs, etc. In this way, nanomaterials could be used to modulate the immunosuppressive TME by targeting TAFs, TAMs, MDSCs, and Tregs and inhibiting the soluble mediators.
Targeting immune checkpoints: interactions between ligands and activated or inhibitory receptors could regulate T-cell activation or tolerance with multiple signals, including T cell receptor (TCR) recognition, CD28/B7 co-stimulation, and cytokine stimulation, and the co-stimulation signals are termed as immune checkpoints. Nanomaterials could be used to deliver the immune checkpoint modulators to tumors to complete the immunity cycle.
Targeting soluble mediators: many cytokines and chemokines play an important role in immunosuppressive TME. Targeting the soluble mediators using nanomaterials encapsulated with small size molecule drugs or siRNA is an effective way to modulate TME.
Targeting cellular mediators:
The function of nanomaterials for cancer immunotherapy
| Function period | ||
|---|---|---|
| Cancer vaccine | TME modulation | |
| Functions of nanomaterials for cancer immunotherapy | Delivery of antigens and/or adjuvants | Immune checkpoint |
| Self-adjuvants | Soluble mediators | |
| Lymph node drainage | Targeting TAMs | |
| DC uptake | Targeting MDSCs | |
| DC targeting | Targeting Tregs | |
| Antigen presentation artificial antigen presentation (aAPCs) | Targeting TAFs | |
| Peptide vaccine DNA and mRNA | ||
TAMs, which are immune cells, could be presented in a large amount in the TME. The immunoregulatory cytokines interleukin (IL)-12, IL-1b, IL-6, and tumor necrosis factor (TNF)-α, and TNF-β, which are produced by TAMs, could inhibit anti-cancer immune responses to promote the tumor growth. The nanomaterials that are surface-modified could be used to target and kill the TAMs.
Tregs are immunosuppressive T cells, which could control the severity of immune responses by inhibiting the activity of anti-tumor T-effector cells. Nanomaterials could be used to suppress or even kill the Tregs to generate tumor immunity.
MDSCs are tumor suppressor cells, which could control cancer inflammation by activating Tregs and suppressing other immune cells. Nanomaterials could deliver immunomodulators to eliminate MDSCs to improve cancer immunotherapy.
TAFs could mediate cancer sustaining and proliferative pathways and nanomaterials could easily deliver drugs into the tumor.
In this review, we will focus on the innovative researches and applications of nanomaterials in cancer immunotherapy. The most used nanomaterials and their pioneer works as well as some perspectives will be summarized and discussed.
3 Nanomaterials for cancer immunotherapy
Various kinds of nanomaterials including liposome-based, polymer-based, metallic-based and inorganic-based materials are being applied for cancer immunotherapy. The most used nanomaterials for cancer immunotherapy with various functions are listed in Table 2, with their advantages and disadvantages.
Nanomaterials for cancer immunotherapy
| Nanomaterials | Functions | Advantages | Disadvantages |
|---|---|---|---|
| PLGA [39,40,41] | Delivery and co-delivery system; peptide/DNA/mRNA and whole cell antigen delivery; target immune checkpoint, Tregs, TAFs, MDSCs; aAPCs | Multifunctional; versatile sizes, morphology and surface functionalization; high antigen loading; stronger cellular response and longer humoral response than liposome | Inflammation may be caused by degradation products; antigen degradation caused by encapsulation; difficult to produce monodisperse nanoparticle |
| Liposome [42,43,44,45] | Delivery and co-delivery system; target TAMs | Versatile chemistry; high biocompatibility; low immunogenicity; easy functionalization; antigen and adjuvant protection | Poor stability; poor immunogenic; poor drug loading efficiency |
| Gold nanoparticle [46,47,48] | Delivery system; imaging function; target Tregs | Versatile size; easy functionalization; biocompatible; additional imaging function | Nonbiodegradable; long-term toxicity |
| Fe3O4 nanoparticle [27,49,50] | Delivery system; imaging function; target immune checkpoint | Additional imaging function | Uptake efficiency strongly depends on the size of the nanoparticles |
| Carbon nanotube [51,52,53] | Delivery system; self-adjuvant; gene delivery; target Tregs | Additional self-adjuvant function; easy preparation and functionalization; biocompatible; bind macromolecules | Nonbiodegradable; low solubility; cytotoxicity; could lead to activation of the innate immune system and inflammation |
| Porous silicon [54,55,56] | Delivery system; self-adjuvant | Additional self-adjuvant function; easy preparation and functionalization; biocompatible; bind macromolecules | Nonbiodegradable; low solubility; cytotoxicity; could lead to activation of the innate immune system and inflammation |
| Iron dextran [57,58,59] | aAPCs | Biocompatible; enhance circulation time; innovative antigenic source | Stability |
| Quantum dot [57,60] | aAPCs | Biocompatible; enhance circulation time; innovative antigenic source | Stability |
3.1 Poly(d,l-lactide-co-glycolide)
PLGA, which is approved by the FDA, is one of the most applied and important polymeric-based nanomaterials due to its multifunction, biodegradation and protection of agents for delivery or co-delivery of agents, self-adjuvant, and applications for TME modulation. The behavior of PLGA nanoparticle could be changed dramatically when they are changed from micro-level to nano-level. The adsorption and adhesion of PLGA nanoparticles could be much stronger due to the great increase of the specific surface area. In this way, the drug absorption could be significantly improved. The preparation of PLGA nanoparticles includes emulsion or microemulsion polymerization, interfacial polymerization, and precipitation polymerization, which belong to “bottom-up” method starting with a monomer, emulsion solvent evaporation, emulsion diffusion, solvent displacement, slating-out, and spherical crystallization, which belong to “top-down” method starting with the preformed polymer [61].
3.1.1 Delivery and co-delivery of agent
Delivery or co-delivery of antigens or adjuvants or both is one of the most important functions of PLGA for cancer immunotherapy. The PLGA nanoparticles could encapsulate the agents that are necessary for initiating the immune cycle and deliver them for DCs uptake with the protection from being degraded. But the antigens might be degraded by co-encapsulation and inflammation might be caused by degradation products. The effects of antigen-loading methods on antigen exposure to the immune system and evaluating the resulting antigen-specific immune responses were investigated by Liu et al. through three classes of antigen adsorbed, antigen encapsulated, and antigen adsorbed/encapsulated PLGA hybrid nanoparticles as delivery systems, which were called “out,” “in,” and “both” nanoparticles, respectively [62]. The results indicated that the lysosomal escape and cross-presentation of antigens, which were exposed to “in” and “both” nanoparticles, were more efficient. Both adequate initial antigen exposure and long-term antigen persistence at the injection site for “both” nanoparticles were found to be more effective through in vivo experiments. Antigen-specific immune responses of “in” and “both” nanoparticles were higher than that of “out” nanoparticles. The study also revealed that antigen entrapment was an important factor in modulating immune response of the antigens delivered by PLGA nanoparticles.
The efficacies of full-length ovalbumin (OVA) with adjuvant α-galactosylceramide (α-GalCer) and OVA with toll-like receptor (TLR) ligands in nanoparticles were investigated by Dölen et al. [63]. They found that the OVA + α-GalCer nanoparticles were more effective than the OVA + TLR nanoparticles in generating and stimulating antigen-specific cytotoxic T lymphocytes without the need for CD4+ T cell. Furthermore, the co-encapsulation of both α-GalCer and antigen in the PLGA nanoparticles was essential for T-cell responses. Moreover, the growth of established tumors could even be delayed by OVA + α-GalCer nanoparticles. In this way, the encapsulation of antigen and α-GalCer could be a choice for designing PLGA-based nanoparticles for cancer immunotherapy.
3.1.2 Peptide, DNA/mRNA, and whole cell antigen delivery
Peptide, which is composed of one or more small fragments of antigen proteins and adjuvants, DNA/mRNA vaccines, and whole cell antigens all are relatively large in size, easy to be degraded, and eliminated rapidly when being delivered nakedly. They can be encapsulated and delivered by PLGA nanoparticles with protection to increase the delivery efficiency. Biodegradable PLGA nanoparticles were used to co-encapsulate anti-PD1 peptide (APP) and hollow gold nanoshell (HAuNs), which was termed as AA@PN, to construct a therapeutic strategy for immune checkpoint PD-1/PD-L1 blockade by Luo et al. [64]. They developed an administration strategy, which could maintain perdurable and controllable drug release by encapsulating the peptide for its sustained release, decreasing the frequency of administration and maintaining a constant drug concentration. The results showed that APP exhibited sustained release behavior from AA@PN within 40 days, which could be easily accelerated by near-infrared (NIR) laser (Figure 2).

Illustration of preparation and structure of AA@PN and its combined therapeutic modalities.
Amir Kalvanagh et al. [65] encapsulated pcDNA encoding interferon (IFN)-λ1 (pIFN-λ1) with PLGA nanoparticles, which were spherical in shape with a mean diameter of 380 ± 3 nm to protect them against DNase enzyme action and increase the efficiency of gene delivery. The results showed that the encapsulation efficiency and loading capacity of PLGA nanoparticles were 75 ± 5% and 0.83 ± 0.06, respectively. The PLGA nanoparticles, which were bioactive, could be engulfed by RAW264.7 cells, and the pIFN-λ1 released from the nanoparticles sustainably. The study indicated that PLGA nanoparticles were an alternative choice for DNA delivery, which could be an alternative delivery system for cancer immunotherapy.
3.1.3 Targeting immune checkpoint, Tregs, TAFs, and MDSCs
Targeting immune checkpoint, Tregs, TAFs, and MDSCs, which belong to TME modulation, are additional functions of PLGA nanomaterials for cancer immunotherapy. PLGA nanoparticles could target the molecules to modulate TME to help to complete the immune cycle. Indocyanine green, a photothermal agent, and imiquimod (R837), Toll-like-receptor-7 agonist, were coencapsulated with PLGA nanoparticles for checkpoint-blockade immunotherapy by Chen et al. [66]. They found that the tumor-associated antigens generated after photothermal tumor ablation showed vaccine-like functions, and in combination with checkpoint blockade using anti-cytotoxic T-lymphocyte antigen-4 (CTL4), the generated immunological responses could attract tumor cells (Figure 3). This PLGA-based nanoparticles with a strong immunological memory effect could be an alternative method to offer protection against tumor rechallenging after elimination of initial tumors.

The mechanism of anti-tumor immune responses induced by the PLGA-based immunotherapy with checkpoint blockade.
Zhao et al. focused on the TME modulation and studied the delivery of methyl-2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oate (CDDO-Me) by PLGA nanoparticles, and the results showed that the Tregs and MSDCs in a B16F10 melanoma model were dramatically decreased and TAFs were remodeled. The PLGA-CDDO-Me nanoparticles with lipid–calcium–phosphate (LCP) nanoparticles loaded with TRP2 peptide vaccine increased the antitumor efficacy than the bare TRP2 vaccine. The study further revealed that PLGA nanoparticles was an alternative drug delivery system for immune cells in the TME [67].
3.1.4 Artificial antigen-presenting cells
Biomimetic artificial antigen-presenting cells (aAPCs) could deliver the stimulatory signals including T-cell recognition signal and activation signal to activate and modulate the immune system. The PLGA-based nanoparticles could be synthesized for antigen presentation and T-cell activation ex vivo and in vivo. Meyer et al. [68] activated T cells in vivo using synthetic ellipsoidal PLGA aAPCs to demonstrate that nonspherical and anisotropic-shaped nanomaterials could take advantage of nonspecific cellular uptake. The results indicated that ellipsoidal PLGA-based aAPCs showed superior pharmacokinetic profiles over spherical aAPCs. Moreover, the live whole animal imaging analysis showed that ellipsoidal PLGA-based aAPCs remained in the periphery for longer periods of time than spherical aAPCs (Figure 4).

Nonspherical nano-ellipsoidal aAPCs have superior pharmacokinetics over nanospherical aAPCs: (a) spherical aAPCs and (b) ellipsoidal aAPCs.
3.2 Liposomes
Liposomes are being widely used as delivery systems for specific drugs, genes, antibodies, antigens, adjuvants, and targeting functions due to their properties of biocompatibility with low toxicity, prevention for pharmaceuticals, smaller size, and easily changed charge and surface chemistry. The size of the liposome nanoparticles mainly ranges from 25 nm to 2.5 µm. The liposome nanoparticles are composed of one or more bilayer membranes, which directly affect the amount of drug encapsulation along with the size of the nanoparticles. The liposome nanoparticles were prepared with mechanical dispersion method, solvent dispersion method, detergent removal method, etc., and the methods could be divided into four steps: drying down the lipids from organic solvent, then dispersing the lipid through aqueous media, purifying the resultant liposome, and last, analyzing the final product [69].
3.2.1 Delivery and co-delivery of agents
Liposomes are being widely used for cancer treatment due to their great biological compatibility with low cytotoxicity, offering protection for drugs from degradation or inactivation, easily changed size, charge, and chemistry of surface. The liposome nanoparticles could also deliver and co-deliver agents with the protection to increase the deliver efficiency. But the drug loading efficiency of the liposome nanoparticles is relatively low compared with that of the PLGA nanoparticles.
Immunoliposome co-delivery of bufalin and anti-CD40 was designed by Li et al. to induce therapeutic efficacy without systemic side effects [70]. The results demonstrated that bufalin liposomes with anti-CD40 antibody exhibited enhanced cytotoxicity compared with bufalin alone. The liposome delivery system exerted immune modulation through allowing anti-CD40 to be presented to APCs for antitumor effects. The prolonged release period at the tumor site could block the system toxicity.
As a new class of adjuvant for cancer treatment, cyclic di-GMP (c-di-GMP), a ligand of the stimulator of interferon genes (STING) signal pathway, is widely employed for cancer immunotherapy. But the delivery of c-di-GMP remains a technology problem. Nakamura et al. loaded the c-di-GMP into YSK05 lipid-containing liposomes to deliver it to the cytosol effectively [71]. The YSK05-liposomes (c-di-GMP/YSK05-Lip) could induce the production of type I interferon with the activation of natural killer (NK) cells, which could lead to an antitumor effect in a lung metastasis. The results represented the use of a potential new adjuvant system in immunotherapy.
Cruz et al. encapsulated a fragment of the TAT NY-ESO-1 with a T-helper peptide into liposome nanoparticles as a nano-vaccine targeting the Fcγ-receptor to improve the immunological response against tumors [72]. The results showed that the liposome nanoparticles encapsulated with the peptide adjuvants could induce the most potent immunological response, and the liposome-based targeted vaccine could be an effective method to activate and deliver antigens to DCs to improve the immunotherapeutic response.
3.2.2 Targeting TAMs
In the TME modulation period, the liposome nanoparticles could mainly target TAMs, which show much less targets compared with the PLGA nanomaterials. The lipid-based nanoparticles, which were functionalized with fusion peptide coencapsulating M2pep and α-peptide, were used as the siRNA delivery system for specific elimination of M2-like TAMs by Qian et al. [73]. The lipid-based delivery system resulted in decreasing tumor size and prolonging survival, and at the same time, the nanoparticles increased the expression of immunostimulative cytokines IL-12 and IFN-γ and decreased T cell infiltration in the TME.
Zhou et al. used epirubicin-loaded liposomes encapsulated with synthesized sialic acid–cholesterol conjugate (SA-CH) for cancer immunotherapy of targeting TAMs, which was termed as EPI-SAL. The results indicated that EPI-SAL could improve the delivery of EPI to TAMs, reduce the number of TAMs in tumor-bearing mice, provide the strongest antitumor activity, and prolong the lifespan of tumor-bearing mice with decreased systemic toxicity. The research also indicated that EPI-loaded liposomes encapsulated with SA-CH could be an effective treatment for targeting TAMs for cancer immunotherapy. The delivery system targeting TAMs is shown in Figure 5 [74].

The liposome-based delivery system targeting TAMs.
3.3 Gold nanoparticles
Gold nanoparticles have gained a lot of attention in cancer immunotherapy and imaging because they are biocompatible and bioinert, have versatile sizes and shapes, and are easily controlled by many synthetic methods. But the non-biodegradation and low-term toxicity become the limitations for their wide use. The size of the gold nanoparticles could be down to 1.5 nm and a wide array of solution are being used for preparation including HAuCl4 with citric acid in boiling water, tetraoctylammonium bromide as the phase transfer reagent and sodium borohydride as the reducing agent, tetrachloroauric acid solution, and TiO2 supporting solution [75].
3.3.1 Delivery system and imaging function
The functions of delivery agents and imaging are usually combined together for gold nanoparticles being employed for cancer immunotherapy. T cells could be marked by gold nanoparticles as a computer tomography (CT) contrast agent, which could allow to be examined the distribution, migration, and kinetics of T cells. The gold nanoparticles were used to design a composite-based immunostimulatory DNA hydrogel to enhance anti-tumor immune responses by Yata et al. [48]. The loaded hexapod-like structured DNA was released by laser irradiation, which efficiently stimulated immune cells to release proinflammatory cytokines. Then, EG7-OVA tumor-bearing mice, which received the gold nanoparticle hydrogel, were laser irradiated to increase the local temperature and the mRNA expression; the treatment effectively inhibited the growth of tumor and prolonged the survival period of the tumor-bearing mice.
Meir et al. designed a method for longitudinal and quantitative in vivo cell tracking using gold nanoparticles [76]. In the research, T cells were labeled with gold nanoparticles as a CT scanning agent, which were injected intravenously to the mice bearing human melanoma xenografts. The whole-body distribution of the targeted gold nanoparticles was observed through the CT scanning results, and the labeled cells could be clearly seen in the lungs for 48 h after injection (Figure 6). They also compared the gold nanoparticles for CT imaging with fluorescence imaging, and the results indicated that the novel method of cell tracking with gold nanoparticles was an effective tool for research and clinical application for cancer immunotherapy.

CT scanning of the whole-body distribution and migration of T cells labeled with gold nanoparticles. (a) 3D volume rendering CT image of the labeled T cells, which accumulated in the lungs for 48 h after injection; (b) 3D image of spleen; (c) 3D image of liver; and (d) 2D CT image of lungs.
3.3.2 Targeting Tregs
Gold nanoparticles could also be used for targeting Tregs of TME modulation, and like liposome nanoparticles, gold nanoparticles could mainly target Tregs. Yeste et al. used gold nanoparticles to administer acid methyl ester and a T-cell epitope to promote the generation of Tregs by DCs [77]. The results indicated that the DCs that were treated with gold nanoparticles could promote the differentiation of Tregs in vitro. Furthermore, the gold nanoparticles that were loaded with acid methyl ester and myelin oligodendrocyte glycoprotein peptide fragment (MOG) [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55] could expand the FoxP3+ Treg compartment and suppress the development of autoimmune encephalomyelitis, and hence, gold nanoparticles were proved to be a novel method for inducing functional Tregs in autoimmune disorders.
3.4 Fe3O4 nanoparticles
Fe3O4 nanoparticles have been approved by the FDA for clinical use due to their magnetic targeting function and T 2-weighted MRI. But, the individual Fe3O4 nanoparticles cannot be used for photothermal therapy because of their low molar extinction coefficient. Fe3O4 nanoparticles are prepared by arc discharge, laser ablation, mechanical grinding, microemulsions, nanoprecipitation, high temperature decomposition of organic precursors, etc. [78].
3.4.1 Delivery system and imaging function
Similar to gold nanoparticles, Fe3O4 nanoparticles also exhibit the combined functions of delivery system and imaging, which could assist to complete MRI, and the uptake efficiency is highly dependent on the size of the nanoparticles. Ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles with magnetic resonance properties could stay in blood pool for a longer period than large magnetic nanoparticles. In this way, Yuan et al. designed Fe3O4-based USPIO nanoparticles, which were subjected to caspase 3 (Casp 3)-instructed aggregation. The results indicated that the Fe3O4-based USPIO nanoparticles could enhance T 2 MRI of tumor apoptosis effectively [79]. Moreover, Li et al. synthesized Fe3O4 nanoparticles, which were water dispersible, colloidally stable, and biocompatible for imaging and cancer photothermal therapy. The Fe3O4 nanoparticles could target specificity to CD44 receptor; additionally, they could be a nanoplatform for MR/CT imaging of cancer cells in vitro and xenografted tumor in vivo. These results further indicated that Fe3O4 nanoparticles could be applied for multifunctional nanoplatform with drug delivery and imaging functions [80].
3.4.2 Targeting immune checkpoint
In the TME modulation, Fe3O4 nanoparticles could mainly target the immune checkpoint to deliver the modulators. A novel Fe3O4 nanoparticle-based multifunctional drug carrier was designed and studied by Ge et al. [81]. The carrier was fabricated by (1) mPEG-PLGA, which was applied for encapsulating the spherical super-particles (SPs), (2) imiquimod (R837), which was an immune adjuvant and could promote DCs and phagocytize tumor-associated antigen, and (3) Fe3O4 nanoparticles. Thus, the carrier was termed as Fe3O4-R837 SPs. The schematic illustration of Fe3O4-R837 SPs photothermal therapy with PD-L1 checkpoint blockade for cancer immunotherapy is shown in Figure 7. The carrier could destroy the tumors with NIR irradiation, and the antigens could induce strong antitumor immune responses. The method in combination with PD-L1 checkpoint blockade was proved to eliminate primary tumors, prevent metastasis, and inhibit the growth of tumor cells. The results further indicated that Fe3O4-based nanoparticles could be applied for cancer immunotherapy by targeting immune checkpoint in TME modulation.
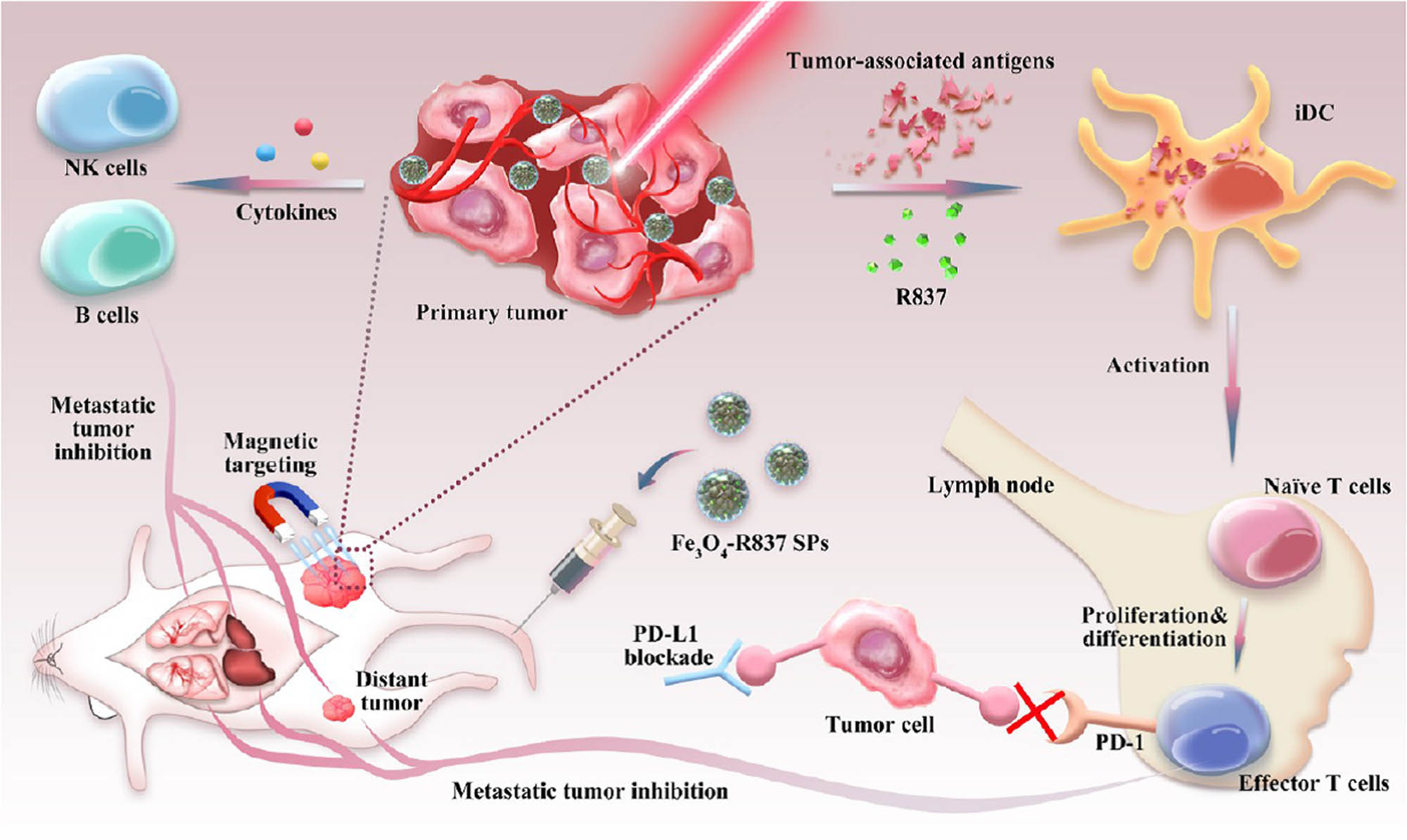
The schematic illustration of Fe3O4-R837 SPs photothermal therapy with PD-L1 checkpoint blockade for cancer immunotherapy.
3.5 Carbon nanotube
Carbon nanotubes are widely used for cancer immunotherapy due to their excellent properties of drug delivery, non-toxicity, nonimmunogenic, and diagnostics. In the recent research studies and applications of multifunctional carbon nanotubes, they are mostly used as a drug delivery system for antigens, adjuvants or gene, and adjuvants themselves. Carbon nanotubes were fabricated by arc discharge, template methods, chemical vapor deposition, electrolysis, flame synthesis, electron or ion beam irradiation, laser ablation, pyrolysis, solar approaches, etc. [82].
3.5.1 Delivery system and self-adjuvant
When carbon nanotubes deliver the agents to generate immune response, they could also act as self-adjuvants to generate the “danger signals” to promote the antigens presentation and immune responses, but the cytotoxicity becomes the limitation. Parra et al. assessed the immune response–amplifying properties of carbon nanotubes to haptens with azoxystrobin. Four kinds of functionalized CNT–BSA–AZc6 nanotubes in different sizes, which were 1–2 nm in diameter with 5–30 µm in length and 50–80 nm in diameter with 10–20 µm in length, were linked to BSA–AZc6 conjugate, which was fabricated with an azoxystrobin derivative bearing a carboxylated spacer arm (hapten AZc6) and bovine serum albumin (BSA). The results indicated that the IgG-type antibody responses were assessed in terms of the titer and affinity. Moreover, the CNT–BSA–AZc6 nanotubes could act as an adjuvant themselves to obtain IgG responses without any other additional adjuvants. They also found that the shorter immunogens would generate the stronger responses due to the better uptake by APCs when carbon nanotubes were less than 1 µm in length [28].
Jambhrunkar et al. developed pristine mesoporous carbon hollow spheres as protein carriers and adjuvants for generating the immune responses [83]. Invaginated mesostructured hollow carbon spheres (IMHCSs) have excellent properties, including high loading capacity, controllable ovalbumin release, safety profile, and high antigen delivery efficacy. They indicated that the novel designed IMHCSs were safer adjuvants than QuilA, which could better promote Th2-biased immune responses for nano-vaccine delivery.
3.5.2 Gene delivery
Relatively large size DNA, mRNA, shRNA, etc., could also be encapsulated with carbon nanotubes to be protected and delivered. Taghavi et al. designed a novel carbon nanotube-based delivery system for shRNA delivery, which was fabricated by a modified branched polyethylenimine (10 kDa), polyethylene glycol (PEG), single-walled carbon nanotubes (SWCNT), AS1411 aptamer, and a very low DOX content. The schematic illustration of shRNA and DOX co-delivery into gastric cancer cells through the delivery system is shown in Figure 8. The results indicated that the combination treatment of cancer therapy using the novel delivery system could inhibit the growth of gastric cancer cells and further revealed that the carbon nanotube-based delivery system could be applied for antitumor activity [84].

The schematic illustration of shRNA and DOX co-delivery into gastric cancer cells through the delivery system.
3.5.3 Targeting Tregs
Carbon nanotubes could also modulate TME by targeting Tregs. Sacchetti et al. were the first to focus on intratumoral immune cell targeting investigation to explore the ability of ligands against Treg-specific receptors to drive selective internalization of PEG-modified single-walled carbon nanotubes (PEG-SWCNTs) into Treg residing in the tumor microenvironment. The results indicated that Tregs targeting efficiency was dependent on incubation time, dose, number of ligands, and surface marker; moreover, the PEG-SWCNTs conjugated with anti-GITR antibody (DTA-1) against glucocorticoid-induced TNFR-related receptor (GITR) could be internalized through receptor-mediated endocytosis by intratumoral Tregs [85].
3.6 Porous silicon
Porous silicon nanomaterials have drawn a great attention for drug delivery system and self-adjuvant in cancer therapies due to their properties of controlled geometry, tunable nanoporous structure, high specific surface area, and versatile surface chemistry. The porous silicon-based delivery system could deliver the antigens to DCs directly, prolong the release, be functionalized with adjuvant molecules, and increase the cross-presentation of antigens. But similar to carbon nanotube, porous silicon is non-biodegradable and long-term cytotoxic. Similar to PLGA nanoparticles, the synthesis of porous silicon could also be divided into two parts: “bottom up” method and “top down” method. The “bottom up” method starts with a porous template, then the template is filled up and etched to obtain porous silicon nanoparticles. The “top down” method starts with bulk sized silicon, followed by electroless etching, electrochemical etching, etc. [86].
Fontana et al. designed three porous silicon-based nano-vaccines for cancer immunotherapy, including thermally oxidized porous silicon (TOPSi) encapsulated into acetalated dextran (AcDEX) (TOPSi@AcDEX), TOPSi encapsulated into spermine-modified AcDEX (SpAcDEX) (TOPSi@SpAcDEX), and TOPSi encapsulated into AcDEX and cancer cell membrane (CCM) (TOPSi@AcDEX@CCM). These results indicated that TOPSi@AcDEX could be encapsulated with vesicles derived from cancer cells to combine with the adjuvant properties of porous silicon; TOPSi@SpAcDEX could induce the expression of co-stimulatory signals in the immortal cell lines and blood monocytes; TOPSi@AcDEX@CCM could enhance the secretion of IFN-γ in peripheral blood mononuclear cell (PBMC) without inducing the secretion of IL-4. Overall, the three porous silicon-based nano-vaccines could be an alternative delivery system with promising adjuvant properties for the materials derived from patient’s tumor [87].
Mahony et al. investigated the immunization to the OVA by using mesoporous silica nanoparticle-based delivery system and self-adjuvant in mice. The research indicated that the amino-functionalized MCM-41 nanoparticles could act as a self-adjuvant and elicit immune responses without local or systemic side effects [88].
3.7 Iron dextran and quantum dots as aAPCs
Iron dextran and quantum dots are basically functionalized with aAPCs only in cancer immunotherapy. For quantum dots, there are mainly two approaches for the preparation: (1) formation of semiconductor nanoparticles through colloidal chemistry and (2) epitaxial growth and patterning in nanoscale [89].
Iron dextran nanoparticles are mainly used as aAPCs in cancer immunotherapy due to their extensive characterization and biocompatibility. Perica et al. synthesized iron dextran-based aAPCs to rapidly expand tumor-specific T cells by “enrichment” and “expansion” methods. The schematic of synthesis is shown in Figure 9. First, the iron dextran nanoparticles that were 50–100 nm in diameter were synthesized by coupling MHC-Ig dimer and CD28 antibodies. Then, the antigen-specific CD8+ T cells were bound to iron dextran aAPCs and proliferated in “enrichment” and “expansion” steps, respectively. The results indicated that strong T-cell responses were generated for both shared tumor antigens and computationally predicted neo-epitopes. The study further reported that the iron dextran-based aAPCs synthesized by “enrichment” and “expansion” could be an effective treatment for cancer immunotherapy [90].

The schematic of synthesis of iron dextran-based aAPCs.
Perica et al. also manufactured and compared two types of aAPCs fabricated by iron dextran paramagnetic particles, which were 50–100 nm in diameter, and avidin-coated quantum dot nanocrystals, which were around 30 nm. They reported that both iron dextran particles and quantum dot nanocrystals could enhance tumor retention in a subcutaneous mouse melanoma model, leading to effective T-cell stimulation and inhibition of tumor growth in vivo [57].
4 Conclusions and perspectives
In this review, we summarized and discussed the various nanomaterials, including PLGA nanomaterials, liposome nanoparticles, gold nanoparticles, Fe3O4 nanoparticles, carbon nanotubes, porous silicon-based nanomaterials, iron dextran, and quantum dots, with different functions and properties applied for cancer immunotherapy. Nanomaterials could overcome the limitations of conventional delivery systems and provide an excellent breadth of novel methods to generate specific immune responses for cancer immunotherapy. Nanomaterial-based immunotherapy could produce long-lasting and broader immune responses through the functions of delivery and co-delivery of antigens, adjuvants, genes, peptides, and whole cell antigens to APCs; self-adjuvant; and targeting TME modulation. Nanomaterials play a more important role in cancer immunotherapy, and cancer immunotherapy will be further developed by using the novel nanomaterials. Ultimately, the research and development will benefit the patients suffering from cancer.
Despite nanomaterials have a lot of advantages for cancer immunotherapy, there are still some disadvantages and problems needed to be further investigated. First, the safety and the systemic cytotoxicity caused by nanoparticles from cellular level to animal level are still not clarified enough. Second, even the nanomaterials could generally increase the efficiency of antigen delivery, DCs uptake, antigen presentation, or TME modulation, it is still not clear enough that which nanoparticle is more effective with less side effects for specific tumors like lung cancer or breast cancer. The interactions between nanomaterials and immune system are still needed to be studied and clarified. Third, the biggest advantage of nanoparticle is the small size, which is in nanoscale. However, the effects of nanoparticle size on the functions and efficiency are still needed to be studied. Besides, if the nanomaterial in particular size could be commercially manufactured still needs to be investigated. Last but not least, the relatively simple function equipped with nanoparticles exhibits low efficiency for cancer immunotherapy.
In future research, the author considers four aspects as the research focuses. (1) Developing the novel nanomaterials that are safer to use and have low toxicity from cellular level to animal level and high specificity. Sometimes, the immunotherapy with nanomaterials could fail to generate robust immune response or the generated immune response is irrelevant for the particular tumor or patient. So, the novel nanomaterials which could achieve the right signals to generate the specific immune response for the specific tumor or patient should be focused on. (2) The nanomaterials could be designed with multifunctions individually for specific patient. For example, we can try to combine two or more nanoparticles to supply each other to obtain the composite nanoparticles in reasonable size with comprehensive specific functions for the specific conditions and patients, (3) Immunodeficient mice xenografted with human cancer cell lines are normally used for in vivo cancer research, but they are not suitable for studying cancer immunotherapy. So, the further research studies should employ novel experiment methods, or evaluation criterion, which could connect the results of animal experiment with real human reactions, or even new animal model to better mimic pathophysiological conditions of humans. (4) Cancer immunotherapy is a super complicated science and engineering combined with oncology, immunology, material science and engineering, etc. The application of nanomaterials probably could not solve all problems and limitations during the treatment. The well-controllable artificial robots in macro- or nanoscale have the potential to fill the vacancy. For the next-generation cancer treatment, we believe that it is possible that the artificial robots encapsulated with specific antigens and adjuvants are perfectly controlled to deliver them to DCs or participate in antigen presentation to generate a robust immune response or even can participate in other steps of immune cycle for cancer treatment.
References
[1] Duan X, Chan C, Han W, Guo N, Weichselbaum RR, Lin W. Immunostimulatory nanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors. Nat Commun. 2019;10(1):1–15.10.1038/s41467-019-09221-xSearch in Google Scholar PubMed PubMed Central
[2] Peruzzi PP, Chiocca EA. Cancer immunotherapy: a vaccine from plant virus proteins. Nat Nanotechnol. 2016;11(3):214.10.1038/nnano.2015.306Search in Google Scholar PubMed
[3] Kirkwood JM, Tarhini AA, Panelli MC, Moschos SJ, Zarour HM, Butterfield LH, et al. Next generation of immunotherapy for melanoma. J Clin Oncol. 2008;26(20):3445–55.10.1200/JCO.2007.14.6423Search in Google Scholar PubMed
[4] Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–77.10.1007/978-3-7091-1300-4_4Search in Google Scholar
[5] Sondak VK, Smalley KS, Kudchadkar R, Grippon S, Kirkpatrick P. Ipilimumab. Nat Rev Drug Discovery. 2011;10(6):411.10.1038/nrd3463Search in Google Scholar PubMed
[6] Seth A, Oh DB, Lim YT. Nanomaterials for enhanced immunity as an innovative paradigm in nanomedicine. Nanomedicine. 2015;10(6):959–75.10.2217/nnm.14.200Search in Google Scholar PubMed
[7] Li W, Wei H, Li H, Gao J, Feng SS, Guo Y. Cancer nanoimmunotherapy using advanced pharmaceutical nanotechnology. Nanomedicine. 2014;9(16):2587–605.10.2217/nnm.14.127Search in Google Scholar PubMed
[8] Poilil SS, Moon MJ, Park R, Jeong YY. Bioactive nanoparticles for cancer immunotherapy. Int J Mol Sci. 2018;19(12):3877.10.3390/ijms19123877Search in Google Scholar PubMed PubMed Central
[9] Azadeh K, Matthew TS, Elizabeth SI, Lisa MM, Sarah MT, Spencer KT, et al. Combining activatable nanodelivery with immunotherapy in a murine breast cancer model. J Controlled Release. 2019;303:42–54.10.1016/j.jconrel.2019.04.008Search in Google Scholar PubMed PubMed Central
[10] Sun JJ, Chen YC, Huang YX, Zhao WC, Liu YH, Venkataramanan R, et al. Programmable co-delivery of the immune checkpoint inhibitor NLG919 and chemotherapeutic doxorubicin via a redox-responsive immunostimulatory polymeric prodrug carrier. Acta Pharmacol Sin. 2017;38(6):823–34.10.1038/aps.2017.44Search in Google Scholar PubMed PubMed Central
[11] Liang R, Xie J, Li J, Wang K, Liu L, Gao Y, et al. Liposomes-coated gold nanocages with antigens and adjuvants targeted delivery to dendritic cells for enhancing antitumor immune response. Biomaterials. 2017;149:41–50.10.1016/j.biomaterials.2017.09.029Search in Google Scholar PubMed
[12] Zhang C, Zhao Z, Zha JW, Wang GX, Zhu B. Single-walled carbon nanotubes as delivery vehicles enhance the immunoprotective effect of a DNA vaccine against spring viremia of carp virus in common carp. Fish Shellfish Immunol. 2017;71:191–201.10.1016/j.fsi.2017.10.012Search in Google Scholar PubMed
[13] Qian H, Liu B, Jiang X. Application of nanomaterials in cancer immunotherapy. Mater Today Chem. 2018;7:53–64.10.1016/j.mtchem.2018.01.001Search in Google Scholar
[14] Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–81.10.1038/nature13988Search in Google Scholar PubMed PubMed Central
[15] Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74.10.1126/science.aaa4971Search in Google Scholar PubMed
[16] Amoozgar Z, Goldberg MS. Targeting myeloid cells using nanoparticles to improve cancer immunotherapy. Adv Drug Delivery Rev. 2015;91:38–51.10.1016/j.addr.2014.09.007Search in Google Scholar PubMed
[17] Heße C, Kollenda S, Rotan O, Pastille E, Adamczyk A, Wenzek C, et al. A tumor-peptide based nanoparticle vaccine elicits efficient tumor growth control in anti-tumor immunotherapy. Mol Cancer Ther. 2019;18(6):1069–80.10.1158/1535-7163.MCT-18-0764Search in Google Scholar PubMed
[18] Granucci F, Prosperi D. Nanoparticles: “magic bullets” for targeting the immune system. Semin Immunol. 2017;34:1.10.1016/j.smim.2017.10.002Search in Google Scholar PubMed
[19] Sykes EA, Dai Q, Sarsons CD, Chen J, Rocheleau JV, Hwang DM, et al. Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proc Natl Acad Sci U S A. 2016;113(9):E1142–51.10.1073/pnas.1521265113Search in Google Scholar PubMed PubMed Central
[20] Song W, Tang Z, Zhang D, Zhang Y, Yu H, Li M, et al. Anti-tumor efficacy of c(RGDfK)-decorated polypeptide-based micelles co-loaded with docetaxel and cisplatin. Biomaterials. 2014;35(9):3005–14.10.1016/j.biomaterials.2013.12.018Search in Google Scholar PubMed
[21] Awate S, Babiuk LAB, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. 2013;4:114.10.3389/fimmu.2013.00114Search in Google Scholar PubMed PubMed Central
[22] Zubeldia JM, Ferrer M, Dávila I, Justicia JL. Adjuvants in allergen-specific immunotherapy: modulating and enhancing the immune response. J Investig Allergol Clin Immunol. 2019;29(2):103–11.10.18176/jiaci.0349Search in Google Scholar PubMed
[23] Yuba E, Harada A, Sakanishi Y, Watarai S, Kono K. A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials. 2013;34(12):3042–52.10.1016/j.biomaterials.2012.12.031Search in Google Scholar PubMed
[24] Broos S, Lundberg K, Akagi T, Kadowaki K, Akashi M, Greiff L, et al. Immunomodulatory nanoparticles as adjuvants and allergen-delivery system to human dendritic cells: implications for specific immunotherapy. Vaccine. 2010;28(31):5075–85.10.1016/j.vaccine.2010.05.004Search in Google Scholar PubMed
[25] Liu J, Zhang R, Xu ZP. Nanoparticle-based nanomedicines to promote cancer immunotherapy: recent advances and future directions. Small. 2019;15(32):1900262.10.1002/smll.201900262Search in Google Scholar PubMed
[26] Mieszawska AJ, Mulder WJ, Fayad ZA, Cormode DP. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol Pharm. 2013;10(3):831–47.10.1021/mp3005885Search in Google Scholar PubMed PubMed Central
[27] Zhang X, Wu F, Men K, Huang R, Zhou B, Zhang R, et al. Modified Fe3O4 magnetic nanoparticle delivery of CpG inhibits tumor growth and spontaneous pulmonary metastases to enhance immunotherapy. Nanoscale Res Lett. 2018;13(1):240.10.1186/s11671-018-2661-8Search in Google Scholar PubMed PubMed Central
[28] Parra J, Abad-Somovilla A, Mercader JV, Taton TA, Abad-Fuentes A. Carbon nanotube-protein carriers enhance size-dependent self-adjuvant antibody response to haptens. J Controlled Release. 2013;170(2):242–51.10.1016/j.jconrel.2013.05.019Search in Google Scholar PubMed
[29] Chen Y, Yuan F, Jiang X, Lv Q, Luo N, Gong C, et al. Discovery of a self-assembling and self-adjuvant lipopeptide as a saccharide-free peptide vaccine targeting EGFRvIII positive cutaneous melanoma. Biomater Sci. 2018;6(5):1120–8.10.1039/C8BM00017DSearch in Google Scholar
[30] Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10.10.1016/j.immuni.2013.07.012Search in Google Scholar PubMed
[31] Song W, Musetti SN, Huang L. Nanomaterials for cancer immunotherapy. J Biomater. 2017;148:16–30.10.1016/j.biomaterials.2017.09.017Search in Google Scholar PubMed PubMed Central
[32] Zhang Y, Xiong X, Huai Y, Dey A, Hossen MN, Roy RV, et al. Gold nanoparticles disrupt tumor microenvironment-endothelial cell cross talk to inhibit angiogenic phenotypes in vitro. J Bioconjugate Chem. 2019;30(6):1724–33.10.1021/acs.bioconjchem.9b00262Search in Google Scholar PubMed PubMed Central
[33] Runa F, Hamalian S, Meade K, Shisgal P, Gray PC, Kelber JA. Tumor microenvironment heterogeneity: challenges and opportunities. Curr Mol Biol Rep. 2017;3(4):218–29.10.1007/s40610-017-0073-7Search in Google Scholar PubMed PubMed Central
[34] Wang LY, Huo MF, Chen Y, Shi JL. Tumor microenvironment-enabled nanotherapy. Adv Healthcare Mater. 2018;7(8):1701156.10.1002/adhm.201701156Search in Google Scholar PubMed
[35] Kieler M, Unseld M, Bianconi D, Prager G. Challenges and perspectives for immunotherapy in adenocarcinoma of the pancreas: the cancer immunity cycle. J Pancreas. 2018;47(2):142–57.10.1097/MPA.0000000000000970Search in Google Scholar PubMed
[36] Gajiwala S, Torgeson A, Garrido-Laguna I, Kinsey C, LIoyd S. Combination immunotherapy and radiation therapy strategies for pancreatic cancer-targeting multiple steps in the cancer immunity cycle. J Gastrointest Oncol. 2018;9(6):1014.10.21037/jgo.2018.05.16Search in Google Scholar PubMed PubMed Central
[37] Aoki H, Ueha S, Shichino S, Ogiwara H, Hashimoto S, Kakimi K, et al. TCR repertoire analysis reveals mobilization of novel CD8+T cell clones into the Cancer-immunity cycle following anti-CD4 antibody administration. Front Immunol. 2019;9:3185.10.3389/fimmu.2018.03185Search in Google Scholar PubMed PubMed Central
[38] Mei YL, Wang RB, Jiang W, Bo Y, Zhang TF, Yu JL, et al. Recent progress in nanomaterials for nucleic acid delivery in cancer immunotherapy. Biomater Sci. 2019;7(7):2640–51.10.1039/C9BM00214FSearch in Google Scholar
[39] Demento SL, Cui WG, Criscione JM, Stern E, Tulipan J, Kaech SM, et al. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials. 2012;33(19):4957–64.10.1016/j.biomaterials.2012.03.041Search in Google Scholar PubMed PubMed Central
[40] Sima R, Marieke FF, Jan WK, Maryam A, Ferry O, Wim HE. Particulate systems based on poly(lactic-co-glycolic) acid (PLGA) for immunotherapy of cancer. Curr Pharm Des. 2015;21(29):4201–16.10.2174/1381612821666150901100247Search in Google Scholar PubMed
[41] Herrmann VL, Wieland DE, Legler DF, Wittmann V, Groettrup M. The STEAP1262-270 peptide encapsulated into PLGA microspheres elicits strong cytotoxic T cell immunity in HLA-A* 0201 transgenic mice-A new approach to immunotherapy against prostate carcinoma. Prostate. 2016;76(5):456–68.10.1002/pros.23136Search in Google Scholar PubMed
[42] Yoshizaki Y, Yuba E, Sakaguchi N, Koiwai K, Harada A, Kono K. Potentiation of pH-sensitive polymer-modified liposomes with cationic lipid inclusion as antigen delivery carriers for cancer immunotherapy. Biomaterials. 2014;35(28):8186–96.10.1016/j.biomaterials.2014.05.077Search in Google Scholar PubMed
[43] Yuba E, Tajima N, Yoshizaki Y, Harada A, Hayashi H, Kono K. Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy. Biomaterials. 2014;35(9):3091–101.10.1016/j.biomaterials.2013.12.024Search in Google Scholar PubMed
[44] Yuba E. Liposome-based immunity-inducing systems for cancer immunotherapy. Mol Immunol. 2018;98:8–12.10.1016/j.molimm.2017.11.001Search in Google Scholar PubMed
[45] Pujol-Autonell I, Mansilla MJ, Rodriguez-Fernandez S, Cano-Sarabia M, Navarro-Barriuso J, Ampudia RM, et al. Liposome-based immunotherapy against autoimmune diseases: therapeutic effect on multiple sclerosis. Nanomedicine. 2017;12(11):1231–42.10.2217/nnm-2016-0410Search in Google Scholar PubMed
[46] Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chem Rev. 2015;115(19):11109–46.10.1021/acs.chemrev.5b00109Search in Google Scholar PubMed PubMed Central
[47] Almeida JPM, Figueroa ER, Drezek RA. Gold nanoparticle mediated cancer immunotherapy. Nanomedicine. 2014;10(3):503–14.10.1016/j.nano.2013.09.011Search in Google Scholar PubMed PubMed Central
[48] Yata T, Takahashi Y, Tan M, Nakatsuji H, Ohtsuki S, Murakami T, et al. DNA nanotechnology-based composite-type gold nanoparticle-immunostimulatory DNA hydrogel for tumor photothermal immunotherapy. Biomaterials. 2017;146:136–45.10.1016/j.biomaterials.2017.09.014Search in Google Scholar PubMed
[49] Wu L, Zhang F, Wei Z, Li X, Zhao H, Lv H, et al. Magnetic delivery of Fe3O4@polydopamine nanoparticle-loaded natural killer cells suggest a promising anticancer treatment. Biomater Sci. 2018;6(10):2714–25.10.1039/C8BM00588ESearch in Google Scholar PubMed
[50] Al-Deen FN, Selomulya C, Ma C, Coppel RL. Superparamagnetic nanoparticle delivery of DNA vaccine, DNA vaccines. New York, NY: Humana Press; 2014. p. 181–94.10.1007/978-1-4939-0410-5_12Search in Google Scholar PubMed
[51] Zhou S, Hashida Y, Kawakami S, Mihara J, Umeyama T, Imahori H, et al. Preparation of immunostimulatory single-walled carbon nanotube/CpG DNA complexes and evaluation of their potential in cancer immunotherapy. Int J Pharm. 2014;471(1–2):214–23.10.1016/j.ijpharm.2014.05.037Search in Google Scholar PubMed
[52] Hassan HAFM, Smyth L, Wang JTW, Costa PM, Ratnasothy K, Diebold SS, et al. Dual stimulation of antigen presenting cells using carbon nanotube-based vaccine delivery system for cancer immunotherapy. Biomaterials. 2016;104:310–22.10.1016/j.biomaterials.2016.07.005Search in Google Scholar PubMed PubMed Central
[53] Hassan HAFM, Diebold SS, Smyth LA, Walters AA, Lombardi G, Al-Jamala KT. Application of carbon nanotubes in cancer vaccines: achievements, challenges and chances. J Controlled Release. 2019;297:79–90.10.1016/j.jconrel.2019.01.017Search in Google Scholar PubMed
[54] Shahbazi MA, Fernández TD, Mäkilä EM, Guével X, Mayorga C, Kaasalainen MH, et al. Surface chemistry dependent immunostimulative potential of porous silicon nanoplatforms. Biomaterials. 2014;35(33):9224–35.10.1016/j.biomaterials.2014.07.050Search in Google Scholar PubMed
[55] Santos HA, Mäkilä E, Airaksinen AJ, Bimbo LM, Hirvonen J. Porous silicon nanoparticles for nanomedicine: preparation and biomedical applications. Nanomedicine. 2014;9(4):535–54.10.2217/nnm.13.223Search in Google Scholar PubMed
[56] Savage DJ, Liu X, Curley SA, Ferrari M, Serda RE. Porous silicon advances in drug delivery and immunotherapy. Curr Opin Pharmacol. 2013;13(5):834–41.10.1016/j.coph.2013.06.006Search in Google Scholar PubMed PubMed Central
[57] Perica K, Medero ADL, Durai M, Chiu YL, Bieler JG, Sibener L, et al. Nanoscale artificial antigen presenting cells for T cell immunotherapy. Nanomedicine. 2014;10(1):119–29.10.1016/j.nano.2013.06.015Search in Google Scholar PubMed PubMed Central
[58] Li H, Shao S, Cai J, Burner D, Lu L, Chen Q, et al. Artificial human antigen-presenting cells are superior to dendritic cells at inducing cytotoxic T-cell responses. Immunology. 2017;152(3):462–71.10.1111/imm.12783Search in Google Scholar PubMed PubMed Central
[59] Garnier A, Hamieh M, Drouet A, Leprince J, Vivien D, Frébourg T, et al. Artificial antigen‐presenting cells expressing HLA class II molecules as an effective tool for amplifying human specific memory CD4+T cells. Immunol Cell Biol. 2016;94(7):662–72.10.1038/icb.2016.25Search in Google Scholar PubMed
[60] Fang RH, Zhang L. Nanoparticle-based modulation of the immune system. Annu Rev Chem Biomol Eng. 2016;7:305–26.10.1146/annurev-chembioeng-080615-034446Search in Google Scholar PubMed
[61] Astete CE, Sabliov CM. Synthesis and characterization of PLGA nanoparticles. J Biomater Sci Polym Ed. 2006;17(3):247–89.10.1163/156856206775997322Search in Google Scholar PubMed
[62] Liu LX, Ma PC, Wang H, Zhang C, Sun HF, Wang C, et al. Immune responses to vaccines delivered by encapsulation into and/or adsorption onto cationic lipid–PLGA hybrid nanoparticles. J Controlled Release. 2016;225:230–9.10.1016/j.jconrel.2016.01.050Search in Google Scholar PubMed
[63] Dölen Y, Kreutz M, Gileadi U, Tel J, Vasaturo A, van Dinther EAW, et al. Co-delivery of PLGA encapsulated invariant NKT cell agonist with antigenic protein induce strong T cell-mediated antitumor immune responses. OncoImmunology. 2016;5(1):e1068493.10.1080/2162402X.2015.1068493Search in Google Scholar PubMed PubMed Central
[64] Luo LH, Yang J, Zhu CQ, Jiang MS, Guo XM, Li W, et al. Sustained release of anti-PD-1 peptide for perdurable immunotherapy together with photothermal ablation against primary and distant tumors. J Controlled Release. 2018;278:87–99.10.1016/j.jconrel.2018.04.002Search in Google Scholar PubMed
[65] Amir Kalvanagh P, Ebtekara M, Kokhaei P, Soleimanjah H. Preparation and characterization of PLGA nanoparticles containing plasmid DNA encoding human IFN-lambda-1/IL-29. Iran J Pharm Res. 2019;18(1):156–67.Search in Google Scholar
[66] Chen Q, Xu LG, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193.10.1038/ncomms13193Search in Google Scholar PubMed PubMed Central
[67] Zhao Y, Huo MR, Xu ZH, Wang YH, Huang L. Nanoparticle delivery of CDDO-Me remodels the tumor microenvironment and enhances vaccine therapy for melanoma. Biomaterials. 2015;68:54–66.10.1016/j.biomaterials.2015.07.053Search in Google Scholar PubMed PubMed Central
[68] Meyer RA, Sunshine JC, Perica K, Kosmides AK, Aje K, Schneck JP, et al. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small. 2015;11(13):1519–25.10.1002/smll.201402369Search in Google Scholar PubMed PubMed Central
[69] Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102.10.1186/1556-276X-8-102Search in Google Scholar PubMed PubMed Central
[70] Li Y, Yuan JN, Yang Q, Cao W, Zhou XX, Xie YH, et al. Immunoliposome co-delivery of bufalin and anti-CD40 antibody adjuvant induces synergetic therapeutic efficacy against melanoma. Int J Nanomed. 2014;9:5683–700.10.2147/IJN.S73651Search in Google Scholar PubMed PubMed Central
[71] Nakamura T, Miyabe H, Hyodo M, Sato Y, Hayakawa Y, Harashima H. Liposomes loaded with a STING pathway ligand, cyclic di-GMP, enhance cancer immunotherapy against metastatic melanoma. J Controlled Release. 2015;216:149–57.10.1016/j.jconrel.2015.08.026Search in Google Scholar PubMed
[72] Cruz LJ, Rueda F, Simón L, Cordobilla B, Albericio F, Domingoet JC. Liposomes containing NYESO1/tetanus toxoid and adjuvant peptides targeted to human dendritic cells via the Fc receptor for cancer vaccines. Nanomedicine. 2014;9(4):435–49.10.2217/nnm.13.66Search in Google Scholar
[73] Qian Y, Qiao S, Dai YF, Xu GQ, Dai BL, Lu LS, et al. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophages. ACS Nano. 2017;11(9):9536–49.10.1021/acsnano.7b05465Search in Google Scholar PubMed
[74] Zhou S, Zhang T, Peng B, Luo X, Liu XR, Hu L, et al. Targeted delivery of epirubicin to tumor-associated macrophages by sialic acid–cholesterol conjugate modified liposomes with improved antitumor activity. Int J Pharm. 2017;523(1):203–16.10.1016/j.ijpharm.2017.03.034Search in Google Scholar PubMed
[75] Yeh YC, Creran B, Rotello VM. Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale. 2012;4(6):1871–80.10.1039/C1NR11188DSearch in Google Scholar
[76] Meir R, Shamalov K, Betzer O, Motiei M, Horovitz-Fried M, Yehuda R, et al. Nanomedicine for cancer immunotherapy: tracking cancer-specific T-cells in vivo with gold nanoparticles and CT imaging. ACS Nano. 2015;9(6):6363–72.10.1021/acsnano.5b01939Search in Google Scholar PubMed
[77] Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2012;109(28):11270–5.10.1073/pnas.1120611109Search in Google Scholar PubMed PubMed Central
[78] Sun J, Zhou SB, Hou P, Yang Y, Weng J, Li XH, et al. Synthesis and characterization of biocompatible Fe3O4 nanoparticles. J Biomed Mater Res Part A. 2007;80(2):333–41.10.1002/jbm.a.30909Search in Google Scholar PubMed
[79] Yuan Y, Ding ZL, Qian JC, Zhang J, Xu JY, Dong XJ, et al. Casp3/7-instructed intracellular aggregation of Fe3O4 nanoparticles enhances T2 MR imaging of tumor apoptosis. Nano Lett. 2016;16(4):2686–91.10.1021/acs.nanolett.6b00331Search in Google Scholar PubMed
[80] Li J, Hu Y, Yang J, Wei P, Sun WJ, Shen MW, et al. Hyaluronic acid-modified Fe3O4@Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomaterials. 2015;38:10–21.10.1016/j.biomaterials.2014.10.065Search in Google Scholar PubMed
[81] Ge R, Liu CW, Zhang X, Wang WJ, Li BX, Liu Y, et al. Photothermal-activatable Fe3O4 superparticle nanodrug carriers with PD-L1 immune checkpoint blockade for anti-metastatic cancer immunotherapy. ACS Appl Mater Interfaces. 2018;10(24):20342–55.10.1021/acsami.8b05876Search in Google Scholar PubMed
[82] Yan Y, Miao JW, Yang ZH, Xiao FX, Yang HB, Liu B, et al. Carbon nanotube catalysts: recent advances in synthesis, characterization and applications. Chem Soc Rev. 2015;44(10):3295–346.10.1039/C4CS00492BSearch in Google Scholar PubMed
[83] Jambhrunkar M, Yu MH, Zhang HW, Abbaraju P, Meka AK, Cavallaro A, et al. Pristine mesoporous carbon hollow spheres as safe adjuvants induce excellent Th2-biased immune response. Nano Res. 2018;11(1):370–82.10.1007/s12274-017-1640-1Search in Google Scholar
[84] Taghavi S, Nia AH, Abnous K, Ramezani M. Polyethylenimine-functionalized carbon nanotubes tagged with AS1411 aptamer for combination gene and drug delivery into human gastric cancer cells. Int J Pharm. 2017;516(1):301–12.10.1016/j.ijpharm.2016.11.027Search in Google Scholar PubMed
[85] Sacchetti C, Rapini N, Magrini A, Cirelli E, Bellucci S, Mattei M, et al. In vivo targeting of intratumor regulatory T cells using PEG-modified single-walled carbon nanotubes. Bioconjugate Chem. 2013;24(6):852–8.10.1021/bc400070qSearch in Google Scholar PubMed
[86] Ge M, Fang X, Rong J, Zhou C. Review of porous silicon preparation and its application for lithium-ion battery anodes. Nanotechnology. 2013;24(42):422001.10.1088/0957-4484/24/42/422001Search in Google Scholar PubMed
[87] Fontana F, Shahbazi MA, Liu DF, Zhang HB, Mäkilä E, Salonen J, et al. Multistaged nanovaccines based on porous silicon@acetalated dextran@cancer cell membrane for cancer immunotherapy. Adv Mater. 2017;29(7):1603239.10.1002/adma.201603239Search in Google Scholar PubMed
[88] Mahony D, Cavallaro AS, Stahr F, Mahony TJ, Qiao SZ, Mitter N. Mesoporous silica nanoparticles act as a self-adjuvant for ovalbumin model antigen in mice. Small. 2013;9(18):3138–46.10.1002/smll.201300012Search in Google Scholar PubMed
[89] Drbohlavova J, Adam V, Kizek R, Hubaleket J. Quantum dots-characterization, preparation and usage in biological systems. Int J Mol Sci. 2009;10(2):656–73.10.3390/ijms10020656Search in Google Scholar PubMed PubMed Central
[90] Perica K, Bieler JG, Schütz C, Varela JC, Douglass J, Skora A, et al. Enrichment and expansion with nanoscale artificial antigen presenting cells for adoptive immunotherapy. ACS Nano. 2015;9(7):6861–71.10.1186/2051-1426-2-S3-P34Search in Google Scholar
© 2020 Yao Huang and Jinhua Zeng, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Generalized locally-exact homogenization theory for evaluation of electric conductivity and resistance of multiphase materials
- Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide
- Characterization of mechanical properties of epoxy/nanohybrid composites by nanoindentation
- Graphene and CNT impact on heat transfer response of nanocomposite cylinders
- A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites
- Ultrasmall Fe3O4 nanoparticles induce S-phase arrest and inhibit cancer cells proliferation
- Effect of aging on properties and nanoscale precipitates of Cu-Ag-Cr alloy
- Effect of nano-strengthening on the properties and microstructure of recycled concrete
- Stabilizing effect of methylcellulose on the dispersion of multi-walled carbon nanotubes in cementitious composites
- Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites
- Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation
- Preparation and properties of 3D interconnected CNTs/Cu composites
- On factors affecting surface free energy of carbon black for reinforcing rubber
- Nano-silica modified phenolic resin film: manufacturing and properties
- Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete
- Halloysite nanotubes in polymer science: purification, characterization, modification and applications
- Cellulose hydrogel skeleton by extrusion 3D printing of solution
- Crack closure and flexural tensile capacity with SMA fibers randomly embedded on tensile side of mortar beams
- An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites
- Utilization of red mud for producing a high strength binder by composition optimization and nano strengthening
- One-pot synthesis of nano titanium dioxide in supercritical water
- Printability of photo-sensitive nanocomposites using two-photon polymerization
- In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries
- Effect of nano and micro conductive materials on conductive properties of carbon fiber reinforced concrete
- Tribological performance of nano-diamond composites-dispersed lubricants on commercial cylinder liner mating with CrN piston ring
- Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance
- Genetic mechanisms of deep-water massive sandstones in continental lake basins and their significance in micro–nano reservoir storage systems: A case study of the Yanchang formation in the Ordos Basin
- Effects of nanoparticles on engineering performance of cementitious composites reinforced with PVA fibers
- Band gap manipulation of viscoelastic functionally graded phononic crystal
- Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution
- Manipulating conductive network formation via 3D T-ZnO: A facile approach for a CNT-reinforced nanocomposite
- Microstructure and mechanical properties of WC–Ni multiphase ceramic materials with NiCl2·6H2O as a binder
- Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite
- Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function
- Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing
- Corrosion behaviour of multilayer CrN coatings deposited by hybrid HIPIMS after oxidation treatment
- Surface hydrophobicity and oleophilicity of hierarchical metal structures fabricated using ink-based selective laser melting of micro/nanoparticles
- Research on bond–slip performance between pultruded glass fiber-reinforced polymer tube and nano-CaCO3 concrete
- Antibacterial polymer nanofiber-coated and high elastin protein-expressing BMSCs incorporated polypropylene mesh for accelerating healing of female pelvic floor dysfunction
- Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films
- A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination
- Arrangement structure of carbon nanofiber with excellent spectral radiation characteristics
- Effect of different particle sizes of nano-SiO2 on the properties and microstructure of cement paste
- Superior Fe x N electrocatalyst derived from 1,1′-diacetylferrocene for oxygen reduction reaction in alkaline and acidic media
- Facile growth of aluminum oxide thin film by chemical liquid deposition and its application in devices
- Liquid crystallinity and thermal properties of polyhedral oligomeric silsesquioxane/side-chain azobenzene hybrid copolymer
- Laboratory experiment on the nano-TiO2 photocatalytic degradation effect of road surface oil pollution
- Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage
- Conversion of sub-µm calcium carbonate (calcite) particles to hollow hydroxyapatite agglomerates in K2HPO4 solutions
- Exact solutions of bending deflection for single-walled BNNTs based on the classical Euler–Bernoulli beam theory
- Effects of substrate properties and sputtering methods on self-formation of Ag particles on the Ag–Mo(Zr) alloy films
- Enhancing carbonation and chloride resistance of autoclaved concrete by incorporating nano-CaCO3
- Effect of SiO2 aerogels loading on photocatalytic degradation of nitrobenzene using composites with tetrapod-like ZnO
- Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles
- Hydration activity, crystal structural, and electronic properties studies of Ba-doped dicalcium silicate
- Microstructure and mechanical properties of brazing joint of silver-based composite filler metal
- Polymer nanocomposite sunlight spectrum down-converters made by open-air PLD
- Cryogenic milling and formation of nanostructured machined surface of AISI 4340
- Braided composite stent for peripheral vascular applications
- Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre–reinforced starch biopolymer composites
- Study on influencing factors of photocatalytic performance of CdS/TiO2 nanocomposite concrete
- Improving flexural and dielectric properties of carbon fiber epoxy composite laminates reinforced with carbon nanotubes interlayer using electrospray deposition
- Scalable fabrication of carbon materials based silicon rubber for highly stretchable e-textile sensor
- Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery
- Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries
- Synthesis of functionalized carbon nanotubes for fluorescent biosensors
- Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete
- Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage
- Microstructural evolution and properties of Cu–20 wt% Ag alloy wire by multi-pass continuous drawing
- Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene
- Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications
- Preparation and piezoresistivity of carbon nanotube-coated sand reinforced cement mortar
- Vibrational analysis of an irregular single-walled carbon nanotube incorporating initial stress effects
- Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials
- Single pulse laser removal of indium tin oxide film on glass and polyethylene terephthalate by nanosecond and femtosecond laser
- Mechanical reinforcement with enhanced electrical and heat conduction of epoxy resin by polyaniline and graphene nanoplatelets
- High-efficiency method for recycling lithium from spent LiFePO4 cathode
- Degradable tough chitosan dressing for skin wound recovery
- Static and dynamic analyses of auxetic hybrid FRC/CNTRC laminated plates
- Review articles
- Carbon nanomaterials enhanced cement-based composites: advances and challenges
- Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide
- Review on modeling and application of chemical mechanical polishing
- Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites
- Advances in modelling and analysis of nano structures: a review
- Mechanical properties of nanomaterials: A review
- New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics
- A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials
- Recent development and applications of nanomaterials for cancer immunotherapy
- Advances in biomaterials for adipose tissue reconstruction in plastic surgery
- Advances of graphene- and graphene oxide-modified cementitious materials
- Theories for triboelectric nanogenerators: A comprehensive review
- Nanotechnology of diamondoids for the fabrication of nanostructured systems
- Material advancement in technological development for the 5G wireless communications
- Nanoengineering in biomedicine: Current development and future perspectives
- Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview
- Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review
- Carbon nanotube–reinforced polymer composite for electromagnetic interference application: A review
- Functionalized layered double hydroxide applied to heavy metal ions absorption: A review
- A new classification method of nanotechnology for design integration in biomaterials
- Finite element analysis of natural fibers composites: A review
- Phase change materials for building construction: An overview of nano-/micro-encapsulation
- Recent advance in surface modification for regulating cell adhesion and behaviors
- Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions
- Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review
- Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications
- A review of passive methods in microchannel heat sink application through advanced geometric structure and nanofluids: Current advancements and challenges
- Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices
- Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging
- Synthesis of graphene: Potential carbon precursors and approaches
- A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE)
- Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water
- Analysis of functionally graded carbon nanotube-reinforced composite structures: A review
- Application of nanomaterials in ultra-high performance concrete: A review
- Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer
- Advanced nickel nanoparticles technology: From synthesis to applications
- Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable?
- Progress in construction of bio-inspired physico-antimicrobial surfaces
- From materials to devices using fused deposition modeling: A state-of-art review
- A review for modified Li composite anode: Principle, preparation and challenge
- Naturally or artificially constructed nanocellulose architectures for epoxy composites: A review
Articles in the same Issue
- Research Articles
- Generalized locally-exact homogenization theory for evaluation of electric conductivity and resistance of multiphase materials
- Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide
- Characterization of mechanical properties of epoxy/nanohybrid composites by nanoindentation
- Graphene and CNT impact on heat transfer response of nanocomposite cylinders
- A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites
- Ultrasmall Fe3O4 nanoparticles induce S-phase arrest and inhibit cancer cells proliferation
- Effect of aging on properties and nanoscale precipitates of Cu-Ag-Cr alloy
- Effect of nano-strengthening on the properties and microstructure of recycled concrete
- Stabilizing effect of methylcellulose on the dispersion of multi-walled carbon nanotubes in cementitious composites
- Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites
- Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation
- Preparation and properties of 3D interconnected CNTs/Cu composites
- On factors affecting surface free energy of carbon black for reinforcing rubber
- Nano-silica modified phenolic resin film: manufacturing and properties
- Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete
- Halloysite nanotubes in polymer science: purification, characterization, modification and applications
- Cellulose hydrogel skeleton by extrusion 3D printing of solution
- Crack closure and flexural tensile capacity with SMA fibers randomly embedded on tensile side of mortar beams
- An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites
- Utilization of red mud for producing a high strength binder by composition optimization and nano strengthening
- One-pot synthesis of nano titanium dioxide in supercritical water
- Printability of photo-sensitive nanocomposites using two-photon polymerization
- In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries
- Effect of nano and micro conductive materials on conductive properties of carbon fiber reinforced concrete
- Tribological performance of nano-diamond composites-dispersed lubricants on commercial cylinder liner mating with CrN piston ring
- Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance
- Genetic mechanisms of deep-water massive sandstones in continental lake basins and their significance in micro–nano reservoir storage systems: A case study of the Yanchang formation in the Ordos Basin
- Effects of nanoparticles on engineering performance of cementitious composites reinforced with PVA fibers
- Band gap manipulation of viscoelastic functionally graded phononic crystal
- Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution
- Manipulating conductive network formation via 3D T-ZnO: A facile approach for a CNT-reinforced nanocomposite
- Microstructure and mechanical properties of WC–Ni multiphase ceramic materials with NiCl2·6H2O as a binder
- Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite
- Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function
- Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing
- Corrosion behaviour of multilayer CrN coatings deposited by hybrid HIPIMS after oxidation treatment
- Surface hydrophobicity and oleophilicity of hierarchical metal structures fabricated using ink-based selective laser melting of micro/nanoparticles
- Research on bond–slip performance between pultruded glass fiber-reinforced polymer tube and nano-CaCO3 concrete
- Antibacterial polymer nanofiber-coated and high elastin protein-expressing BMSCs incorporated polypropylene mesh for accelerating healing of female pelvic floor dysfunction
- Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films
- A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination
- Arrangement structure of carbon nanofiber with excellent spectral radiation characteristics
- Effect of different particle sizes of nano-SiO2 on the properties and microstructure of cement paste
- Superior Fe x N electrocatalyst derived from 1,1′-diacetylferrocene for oxygen reduction reaction in alkaline and acidic media
- Facile growth of aluminum oxide thin film by chemical liquid deposition and its application in devices
- Liquid crystallinity and thermal properties of polyhedral oligomeric silsesquioxane/side-chain azobenzene hybrid copolymer
- Laboratory experiment on the nano-TiO2 photocatalytic degradation effect of road surface oil pollution
- Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage
- Conversion of sub-µm calcium carbonate (calcite) particles to hollow hydroxyapatite agglomerates in K2HPO4 solutions
- Exact solutions of bending deflection for single-walled BNNTs based on the classical Euler–Bernoulli beam theory
- Effects of substrate properties and sputtering methods on self-formation of Ag particles on the Ag–Mo(Zr) alloy films
- Enhancing carbonation and chloride resistance of autoclaved concrete by incorporating nano-CaCO3
- Effect of SiO2 aerogels loading on photocatalytic degradation of nitrobenzene using composites with tetrapod-like ZnO
- Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles
- Hydration activity, crystal structural, and electronic properties studies of Ba-doped dicalcium silicate
- Microstructure and mechanical properties of brazing joint of silver-based composite filler metal
- Polymer nanocomposite sunlight spectrum down-converters made by open-air PLD
- Cryogenic milling and formation of nanostructured machined surface of AISI 4340
- Braided composite stent for peripheral vascular applications
- Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre–reinforced starch biopolymer composites
- Study on influencing factors of photocatalytic performance of CdS/TiO2 nanocomposite concrete
- Improving flexural and dielectric properties of carbon fiber epoxy composite laminates reinforced with carbon nanotubes interlayer using electrospray deposition
- Scalable fabrication of carbon materials based silicon rubber for highly stretchable e-textile sensor
- Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery
- Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries
- Synthesis of functionalized carbon nanotubes for fluorescent biosensors
- Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete
- Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage
- Microstructural evolution and properties of Cu–20 wt% Ag alloy wire by multi-pass continuous drawing
- Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene
- Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications
- Preparation and piezoresistivity of carbon nanotube-coated sand reinforced cement mortar
- Vibrational analysis of an irregular single-walled carbon nanotube incorporating initial stress effects
- Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials
- Single pulse laser removal of indium tin oxide film on glass and polyethylene terephthalate by nanosecond and femtosecond laser
- Mechanical reinforcement with enhanced electrical and heat conduction of epoxy resin by polyaniline and graphene nanoplatelets
- High-efficiency method for recycling lithium from spent LiFePO4 cathode
- Degradable tough chitosan dressing for skin wound recovery
- Static and dynamic analyses of auxetic hybrid FRC/CNTRC laminated plates
- Review articles
- Carbon nanomaterials enhanced cement-based composites: advances and challenges
- Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide
- Review on modeling and application of chemical mechanical polishing
- Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites
- Advances in modelling and analysis of nano structures: a review
- Mechanical properties of nanomaterials: A review
- New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics
- A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials
- Recent development and applications of nanomaterials for cancer immunotherapy
- Advances in biomaterials for adipose tissue reconstruction in plastic surgery
- Advances of graphene- and graphene oxide-modified cementitious materials
- Theories for triboelectric nanogenerators: A comprehensive review
- Nanotechnology of diamondoids for the fabrication of nanostructured systems
- Material advancement in technological development for the 5G wireless communications
- Nanoengineering in biomedicine: Current development and future perspectives
- Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview
- Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review
- Carbon nanotube–reinforced polymer composite for electromagnetic interference application: A review
- Functionalized layered double hydroxide applied to heavy metal ions absorption: A review
- A new classification method of nanotechnology for design integration in biomaterials
- Finite element analysis of natural fibers composites: A review
- Phase change materials for building construction: An overview of nano-/micro-encapsulation
- Recent advance in surface modification for regulating cell adhesion and behaviors
- Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions
- Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review
- Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications
- A review of passive methods in microchannel heat sink application through advanced geometric structure and nanofluids: Current advancements and challenges
- Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices
- Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging
- Synthesis of graphene: Potential carbon precursors and approaches
- A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE)
- Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water
- Analysis of functionally graded carbon nanotube-reinforced composite structures: A review
- Application of nanomaterials in ultra-high performance concrete: A review
- Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer
- Advanced nickel nanoparticles technology: From synthesis to applications
- Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable?
- Progress in construction of bio-inspired physico-antimicrobial surfaces
- From materials to devices using fused deposition modeling: A state-of-art review
- A review for modified Li composite anode: Principle, preparation and challenge
- Naturally or artificially constructed nanocellulose architectures for epoxy composites: A review

