Abstract
The performance of wound dressing determines the effect of wound closure and recovery. Water absorption and bacteriostasis of wound dressings play an important role in wound recovery and healing. In this study, an optimized chitosan wound dressing-tough chitosan dressing (TCS) with high water absorption, high bacteriostasis, and degradability was developed. The chemical structure of chitosan remained stable during the process of optimized treatment, and an increase in mechanical properties was obtained for the dressing. After optimization, the water absorption and antibacterial properties of the chitosan dressing were greatly improved, which is significantly better than sodium alginate dressing. The authors believe that TCS dressing with high hygroscopicity and high bacteriostasis has great potential application value in the field of wound recovery and healing.
1 Introduction
Skin trauma is one of the most common injuries in humans [1], which is painful and would cause wound infections by breaking the integrity and protective function of the skin. The recovery of skin traumatic wounds affects the patient’s physical and mental condition. The implications of effective wound healing for both the patient and the economy are massive [2], and how to accelerate the healing of wounds was a focus question. Thus, the design of new wound materials is an urgent need for the development of modern medical technology [3,4].
In general, traditional dressings cannot provide a moist environment and play a bacteriostatic role in the wound [5]. Gauze, as a traditional wound dressing, could provide some protection for skin traumatic wounds, but their hydrophilicity was poor [6]. Hydrogel dressings possess excellent hydrophilicity and can provide a soothing and cooling effect to decrease the temperature of cutaneous wounds [7]. However, the exudate will lead to maceration and bacterial proliferation of the hydrogel dressings [8]. Alginate dressing has good biodegradability and hydrophilicity, which can stimulate macrophages to initiate an inflammatory response and accelerate wound healing [9]. However, the bacteriostatic effect of alginate dressing was poor [10]. Collagen dressings could greatly simulate the extracellular matrix, creating a physiological interface between the wound surface and the environment to promote wound healing [11,12]. However, allogeneic or heterogeneous collagen may cause immune rejection [13]. Nanosilver dressing is excellent in antibacterial wounds but may accumulate in the body by crossing the skin and mucous membrane barrier and cause damage to the human body [14]. The performance of wound dressing could be greatly improved by not only maintaining moisture at the wound site but also providing antibacterial effects to protect the wound from infection to promote wound healing [15].
To meet the needs of developing ideal wound dressings, nanotechnology-based materials have been attractive to researchers in the field of biomedical materials [16,17]. Because of the similarity in morphology and structure between the nanofibers and the natural extracellular matrix proteins, the nanofibers have certain promoting effects on the recovery of damaged tissues [18]. Particularly for wound healing, the nanofiber dressing prepared by electrospinning technology has excellent performance on promoting skin regeneration in wound treatment [19,20], because it could promote cell proliferation and migration [21]. The reinforced fiber with nanoparticles can effectively improve the mechanical properties and biological effects of the fiber surface, which is more conducive to cell growth [22].
As a natural polysaccharide substance, chitosan has various physiological functions such as biocompatibility, non-toxicity, and bacteriostasis [23,24]. Therefore, chitosan has been widely used in tissue engineering materials, medical fibers, antibacterial agents, drug sustained-release materials, and many other fields and other daily chemical industries [25,26]. The capacity of chitosan on promoting wound healing and hemostasis has been reported [27]. As a tissue engineering material, chitosan can also promote cell proliferation and tissue regeneration [28,29]. Its degradation product, chitosan oligosaccharides, also has excellent biological functions on tissue regeneration [30,31]. Among the dressing materials manufactured in recent years, chitosan dressing with biocompatibility has been considered as a dressing with promising applications [32,33]. However, chitosan materials have poor degradation performance under general conditions, which hinders their application [34].
To solve the disadvantages that traditional skin dressings cannot provide a moist environment, weak water absorption, and weak bacteriostasis ability, an optimized chitosan wound dressing was prepared in this study. The chitosan dressing was treated by the protonation reaction to obtain the TCS dressing with high water absorption, high bacteriostasis, and degradability. This optimized chitosan dressing could be considered as an excellent dressing candidate in practical applications.
2 Materials and methods
2.1 Preparation of chitosan dressing
The experiment used a protonation reaction in a non-aqueous system to make the chitosan carry positive ions. First, 100 g of chitosan (average MW = 9,000, commercial available) was immersed in 100 mL of ethanol, and 80 g of 75% acetic acid was slowly added dropwise, then reacted at room temperature for 3–4 h after stirring. After filtering and drying, optimized chitosan could be obtained. Dressings were fabricated using nonwoven technology [35]. Ordinary chitosan nonwoven dressing was used as a control. For ease of reference, the untreated chitosan dressing and treated chitosan dressing were named as UTCS and TCS dressings, respectively.
2.2 Scanning electron microscopy analysis
The surface of chitosan dressing was observed by electron microscope before and after optimization. Appropriate amount of flat dressing was cut, dried, and then sprayed with gold for observation under an electron microscope. By observing the morphology of the fiber and counting the diameter of the fiber, the effect of optimization treatment on the chitosan fiber was analyzed.
2.3 Fourier transform infrared analysis
Take the appropriate amount of UTCS and TCS for Fourier transform infrared (FTIR) spectroscopy to analyze the component. First, the dried dressing was cut and grind, and some potassium bromide was added. The mixture was then extruded into flakes and placed in a FTIR spectrometer for analysis. The experiment compared the obtained infrared spectrum curves and analyzed whether the optimization treatment would affect the composition of the chitosan dressing.
2.4 Mechanical analysis
In the experiment, the tensile mechanical analysis of the UTCS and TCS dressings was performed, and the commercialized alginate dressing was selected as a comparison to evaluate the mechanical properties of the samples. The mechanical testing device was used to analyze the tensile strength of the dressing, and five parallel samples were set in each group to reduce the experimental error.
2.5 Wettability
The contact angle instrument was used to measure the hydrophilicity of different chitosan dressing. The contact angle of the chitosan dressing was measured under wet conditions. The dressing samples were placed on the contact angle test bench to measure the wettability, and the situation of water droplets on the material was observed from a top view. In this experiment, five parallel samples were used for each group.
2.6 Swelling test
The swelling rate of UTCS, TCS, and commercial control dressings was measured in the swelling test experiment.
The three kinds of dressings were cut to the 1 × 1 cm size, and three parallel samples were set in each group. The weight (m 1) of each sample was recorded separately, then the sample was immersed in phosphate buffer saline (PBS), and the swelling experiment was performed at room temperature. The time points of 10, 20, 30 min, 1, 1.5, 2, 3, 4, 6, 12, 24, 48, 72, and 96 h were set in the experiment. At each time point, the excess PBS was discarded and the weight (m t) of each sample was recorded. The calculation method of swelling rate was:
2.7 Degradation
The PBS solution was used to analyze the degradation of the dressing in vitro. The weight of each sample was recorded at the beginning of the experiment (m 1), and then the samples were soaked in 3 mL of PBS and incubated at 37°C. The samples were taken out at the time point (1, 3, 5, 7, 10, and 15 days), the lyophilized weight of samples (m t) was recorded, and three parallel samples were set at each time point. The degradation rate was calculated by the following equation:
2.8 Antibacterial test
The modified chitosan powder (I) and commercial bacteriostatic powder (II) were formulated into the concentration of 32,000 μg/mL with 0.5% acetic acid solution as the original solution. The concentrations of 16,000, 8,000, 4,000, 2,000, 1,000, 500, 250, 125, 62.5, and 31.25 μg/mL were diluted in 96-well plates. The volume of solution in each well was 100 μL. Then another 100 μL of bacterial solution (OD 600 = 0.5) was added to each well. Only culture medium was added as a control group. Three strains of Staphylococcus aureus, Escherichia coli, and Candida albicans were inoculated separately, and each drug concentration was repeated thrice. After shaking culture for 18 h, the microplate reader read the absorbance at 600 nm.
Then, the antibacterial properties between chitosan powder and chitosan nonwoven dressing were tested. The bacteriostatic difference between the two groups was analyzed by counting the number of colonies formed after cultivation. The bacterial liquid without adding sample was treated in the same way and used as a negative control.
After that, the commercial sodium alginate (SA) wound dressing was then used as a control to evaluate the antibacterial properties of the chitosan dressing. The UTCS dressing (1), TCS dressing (2), and SA commercial wound dressing (3) were cut to the same size (1 × 0.5 cm). Then, the samples were immersed in the bacterial culture medium. The bacterial liquid without adding dressing was treated in the same way and used as a negative control. Three strains of S. aureus, E. coli, and C. albicans were used for the experiment, and each experiment was repeated thrice. The number of colony-forming unit was counted after overnight cultivation. Finally, the following equation was used to calculate the antibacterial rate %:
2.9 Statistical analysis
All data analyses were performed with GraphPad Prism. Differences between the experimental groups were analyzed and compared by t-test analysis of variance. Statistical significance was designated with *P < 0.05, **P < 0.01, and ***P < 0.001.
3 Results and discussion
3.1 Morphology of the dressing
In this study, chitosan powder was dispersed in ethanol by dropwise addition of acetic acid. In this process, the amino groups of chitosan were protonated and possessed positive charges in acidic solutions with improved hydrophilicity [36]. Then, positive charged chitosan powder was obtained after filtration. Then, TCS dressing was prepared by nonwoven technology.
To investigate the microstructure of the dressings, scanning electron microscopy (SEM) analysis was conducted [37]. The morphologies and diameter distributions of chitosan dressing before and after treatment are shown in Figure 1. As shown in SEM images, the fibers of both UTCS and TCS dressings were smooth, cylindrical, and randomly oriented (Figure 1a). There was no significant difference between UTCS and TCS on the macroscopic morphology (Figure 1b). The fiber diameter of UTCS dressing was 12.09 ± 0.5937 μm (Figure 1c). The average fiber diameter of TCS dressing was 13.36 ± 0.4523 μm (Figure 1c), which is very close to that of UTCS dressing with no statistical difference.
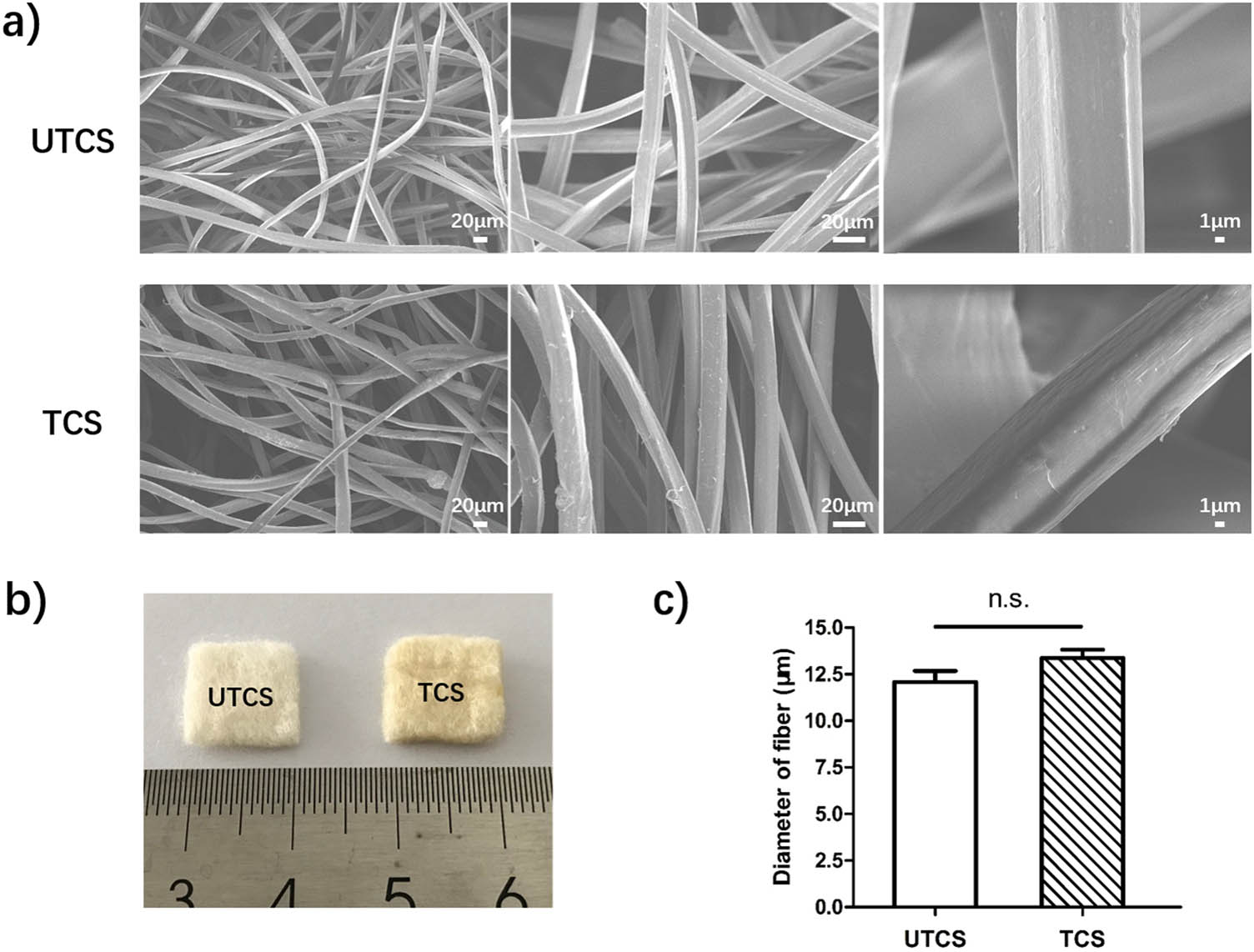
(a) Scanning electron micrograph of UTCS and TCS dressings; (b) macro photographs of UTCS and TCS dressings; (c) statistical analysis of the fiber diameter of two groups (n = 30). All data were mean ± SEM. The differences in each group were performed using two-tailed unpaired t-test. NS indicates P > 0.05.
3.2 FTIR analysis
To determine the composition of chitosan dressings, we characterized their chemical structures by FTIR analysis. The characteristic peaks of chitosan were all found in the FTIR spectra of both UTCS and TCS in Figure 2. The peak in absorbance at 3,450 and 2,867 cm−1 represents O–H group and C–H stretch, respectively. The peak at 1,071 cm−1 represents skeletal vibration involving bridge C–O stretch and 1,375 cm−1 represents asymmetric C–H bending of CH2 group [38]. This indicates that the optimization process did not change the original main ingredients of the chitosan dressing. It is also worth noting that the peaks indicated by arrows 1 and 2 appear only in the infrared curve of the TCS group, which may be caused by the protonation of amino groups and free H ions or residual acetic acid, respectively.
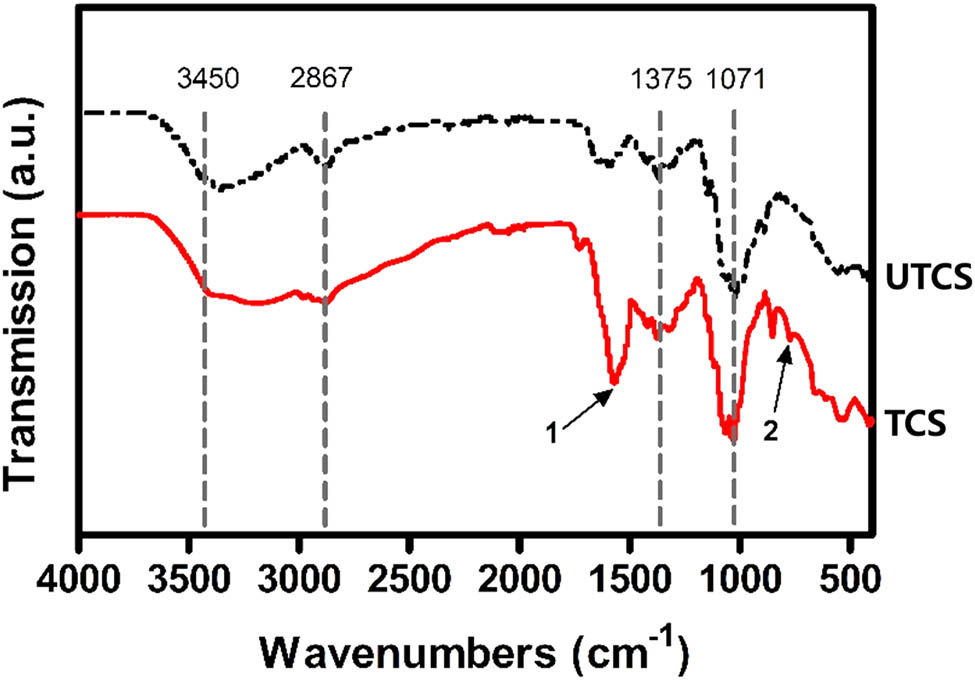
FTIR spectra of the chitosan dressing before and after optimization.
3.3 Mechanical tensile test
Excellent mechanical properties are required for developing desirable wound dressing to maintain integrity and protect the damaged area under external forces [39]. Therefore, tensile tests were performed to determine the stretchability of wound dressings of varied compositions. The results showed that the SA wound dressing ruptured easily at small loads strains of 2.351 N. The UTCS ruptured at 8.098 N. The Young’s modulus of UTCS was basically the same as the SA dressing (0.396 MPa), which was 0.369 MPa (Figure 3d). In contrast, TCS sustained loads of 21.5 N without rupture and had the highest mechanical strength (1.078 MPa) in comparison to UTCS (0.405 MPa) and SA (0.118 MPa) groups. Moreover, the young’s modulus of TCS (3.29 MPa) was significantly higher than UTCS (0.369 MPa) and SA (0.396 MPa). After the optimization, the mechanical properties of the chitosan dressing were significantly enhanced. Study had shown that the shear force is the main reason to destroy the structure of nanofibers [40]. It may be that after optimizing the treatment process, the chitosan fiber becomes very easy to absorb water. After the dressing was dried again, the dressing was tougher because the chitosan fibers became tighter and could bear greater shearing force. In general, the Young’s modulus of human skin is about 0.5–2 MPa [41]. Therefore, the Young’s modulus (3.29 MPa) of TCS dressing could meet the needs of the requirement of large motion when applied to wound sites.
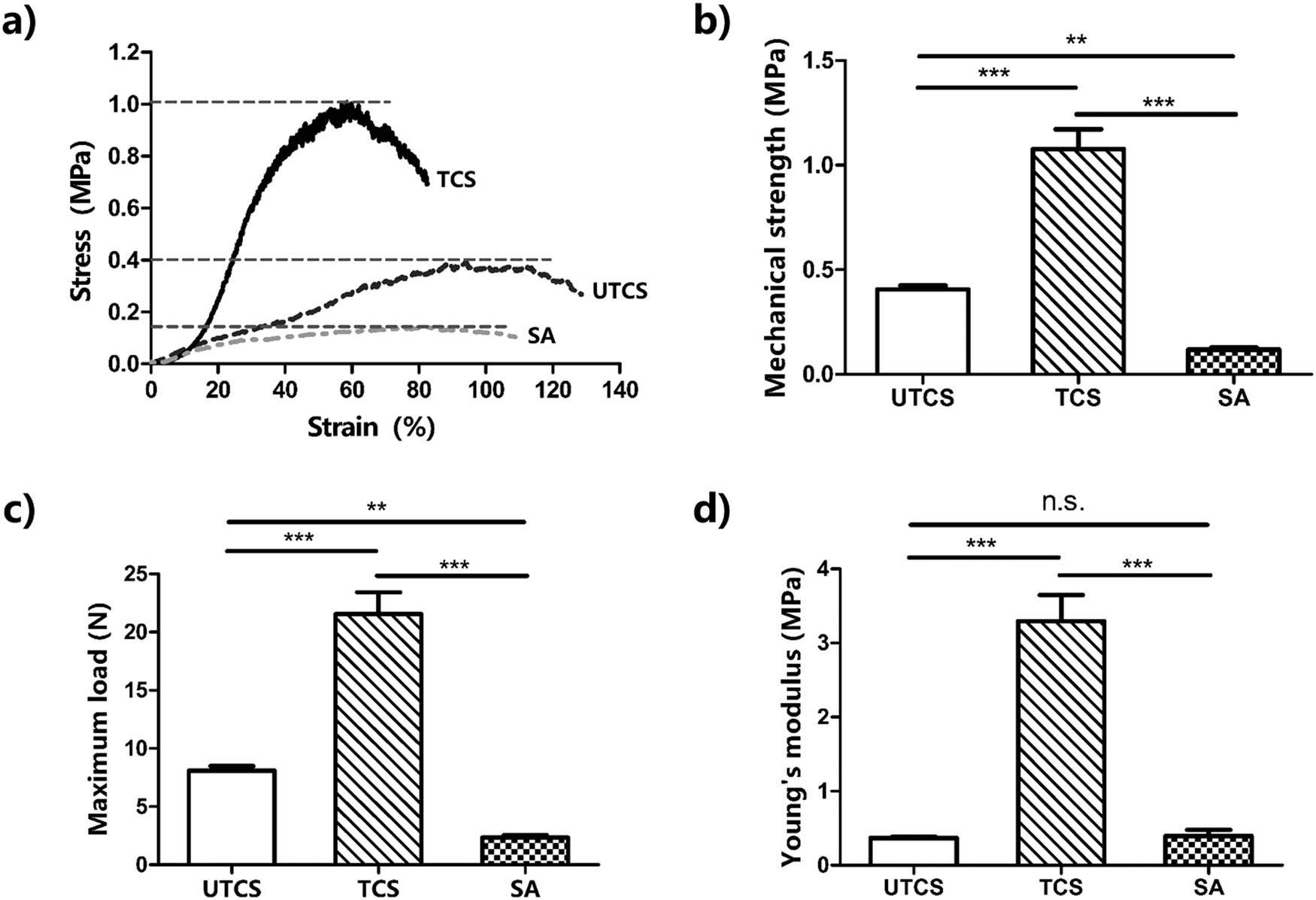
Mechanical test of the wound dressing: (a) the stress–strain curve of the dressing; (b) mechanical strength of dressing (n = 5); (c) maximum load of the dressing (n = 5); (d) Young’s modulus for each dressing (n = 5). All data were mean ± SEM. The differences in each group were performed using two-tailed unpaired t-test. NS indicates P > 0.05. **P < 0.01 and ***P < 0.001.
3.4 Wettability analysis
Because the surface wettability significantly influences the biocompatibility of dressings exposed to tissue, we determined the contact angle of the chitosan dressings and compared with that of SA dressing. Figure 4 shows the droplet from the side view in contact angle analysis. The water droplets rapidly infiltrated and spread out on the surface of UTCS, TCS, and SA dressings without showing the contact angle after dropped on the dressing. Both UTCS and TCS groups showed comparable hydrophilicity to SA dressing under wetting conditions, which is because of the fact that chitosan itself is a hydrophilic substance. These results suggest that TCS could absorb exudative tissue fluid at the wound site effectively.
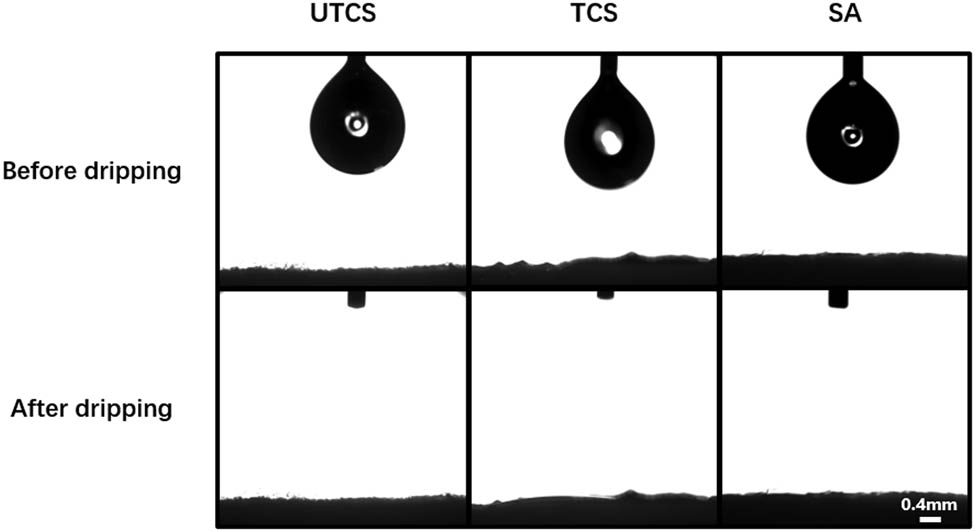
The contact angle of three dressings under wetting conditions before and after water drop. Scale bars represent 0.4 mm.
3.5 Swelling capacity and degradation
The capability of retaining and absorbing water is a significant parameter to evaluate its application in wound dressing field. It would prohibit the accumulation of exudates at the wound site and could absorb nutrition into the dressing [42]. Therefore, the ideal wound dressing should have excellent water retention ability and swelling rate to maintain a moist healing environment to promote wound healing [43].
Figure 5 shows the swelling ratio of wound dressing from different groups. The swelling rate of all groups increased significantly within 10 min. However, the UTCS group reached its maximum swelling rate after 10 min. In contrast, both the TCS and SA groups reached their maximum swelling after 48 h. The highest swelling ratio of 2,100% was observed in the TCS group, whereas UTCS dressing showed the least swelling ratio of 300%. The final swelling rate of SA dressing was between the other two groups (850%). Besides, after soaked in PBS for 2 h, the volume of TCS dressing was enlarged. The appearance of it became transparent and moist. The water was fully absorbed and wrapped by the TCS group. TCS dressing has a better ability to absorb the water which could reach twice the SA dressing because of the protonation of the chitosan in optimized dressing. This property was effective in cleaning a wound with a large amount of exudate.
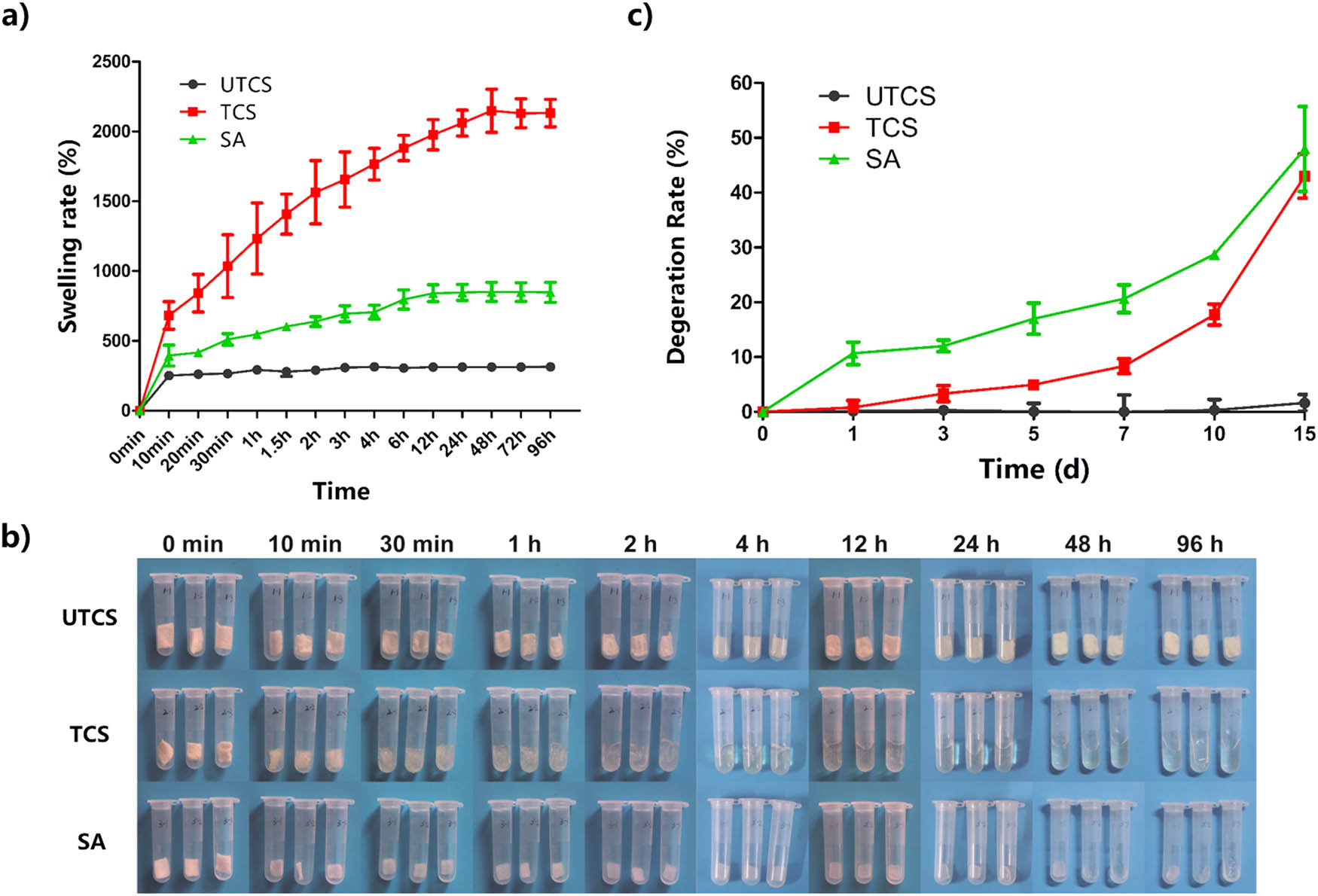
(a) Swelling curves of three different dressings; (b) swelling behavior of three dressings in PBS; (c) degradation of three dressings within 15 days. Each group had three parallel samples (n = 3). All data were mean ± SEM.
The degradation analysis of three wound dressings was performed by PBS (Figure 5). Soaking in PBS for 15 days, the dry weight degradation rate of UTCS, TCS, and SA was 1.64, 43.01, and 49.97%, respectively. Clearly, the UTCS group showed no degradation basically (<5%) at the end of the analysis, and the degradation rate of the TCS (>40%) was significantly higher than that of UTCS. These results were in line with the results of the swelling rate shown in Figure 5b. The increased degradation rate of TCS was because the chitosan becomes more soluble in water after protonation on the amino group of the chitosan. The TCS has the same degradation tendency (43.01%) as the commercial SA dressing (49.97%), and there is no significant difference in the final degradation rate between them. Therefore, the chitosan dressing (TCS) prepared in this experiment achieved excellent degradation performance, which could match the degree of wound healing better. This degradation characteristic makes the TCS dressing have the potential for clinical application.
3.6 Antibacterial experiment
Because of the lack of skin protection at the wound, the defected sites are easy to be infected by various bacteria from the outside; therefore, the antibacterial effect of the dressing on bacteria is very important for the recovery of the wound [44]. Optimum wound healing dressing that has inherent antibacterial properties will be more attractive to play the role of barrier to protect the wound tissue from external bacterial infection [45].
In this study, the antibacterial activities of all wound dressing were evaluated using E. coli, S. aureus, and C. albicans. In Figure 6a and b, the powder of TCS (I) exhibited an excellent antibacterial rate (>95%) for S. aureus, E. coli, and C. albicans, indicating its outstanding inherent antibacterial properties. The antibacterial rates of commercial bacteriostatic powder (II) for the three strains hovered around 80%. Obviously, the antibacterial effect of TCS dressing powder was better than that of commercial antibacterial powder. This is because chitosan itself has excellent antibacterial properties, and its positively charged amino group may damage the bacterial cell wall, resulting in the release of bacterial intracellular fluid, thereby playing a bactericidal role [1,46].
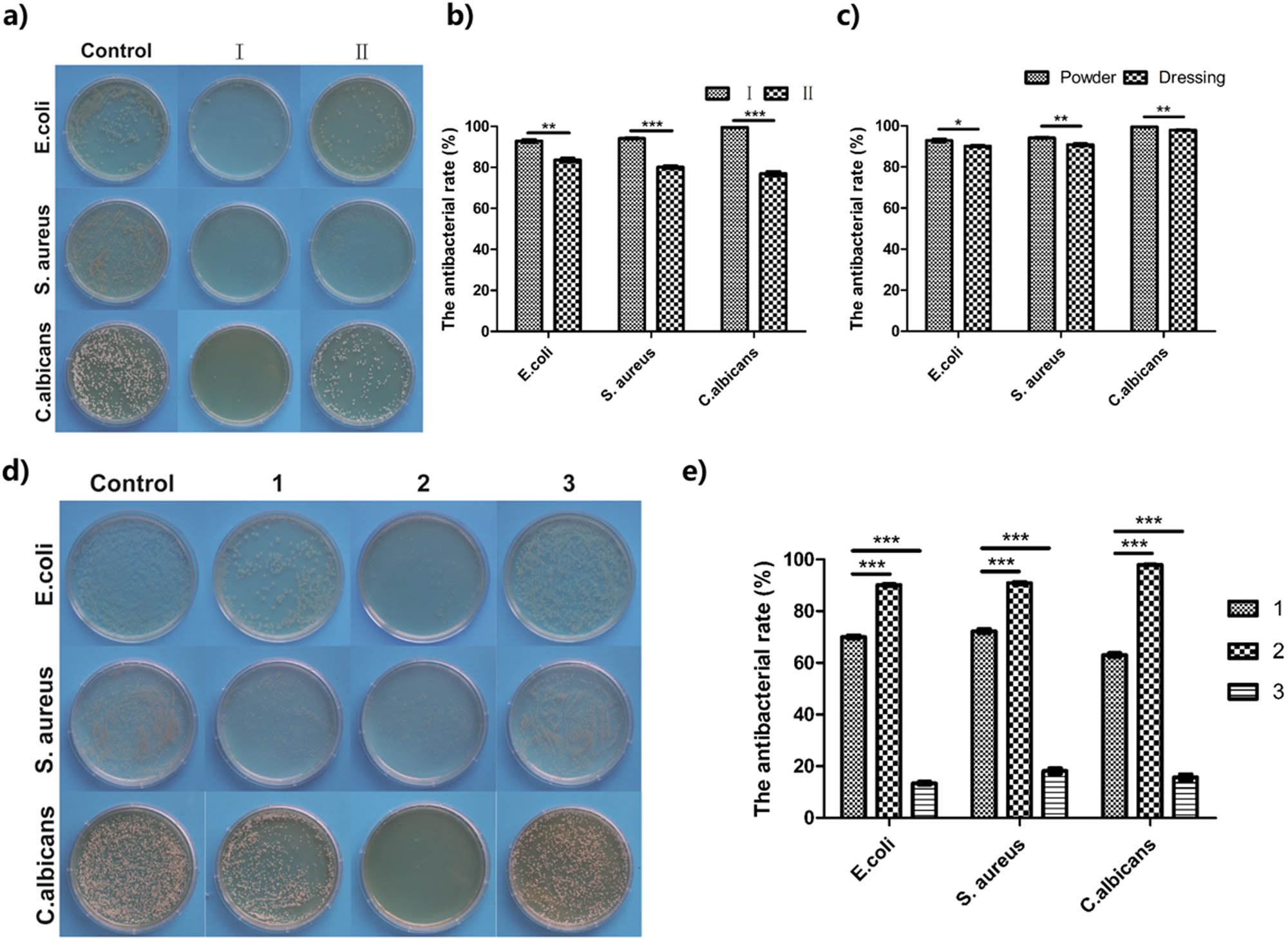
Antibacterial experiment of various dressings: (a and b) antibacterial experiment of chitosan in powder state, I: TCS powder, II: commercial antibacterial powder (n = 5); (c) antibacterial effect of TCS in different forms (n = 5); (d and e) antibacterial effect of various dressings on three bacterial species. 1: UTCS dressing; 2: TCS dressing; 3: SA wound dressing. n = 5 in each group. The differences between each group were performed using two-tailed unpaired t-test. *P < 0.05, **P < 0.01, and ***P < 0.001.
Figure 6c compares the antibacterial efficiency of TCS dressing in different forms. Regardless of the form of powder or complete excipients, their antibacterial rate for the three strains all reached 90% with no significant difference. Next, the UTCS, TCS, and SA dressings were compared for bacteriostatic effects (Figure 6d). From the perspective of the number of colonies of different bacterial species, the number of colonies on the optimized chitosan dressing group was greatly reduced, and the bacteriostatic rate was above 90%. Figure 6e shows that the antibacterial rate of the UTCS group was lower than that of the TCS group. This showed that the optimized treatment process could effectively improve the antibacterial ability of chitosan dressing. In contrast, in the SA group, the bacteriostatic rates of the three strains were all below 20%. This is because SA does not have inherent antimicrobial properties [47].
Chitosan itself has better antibacterial properties than SA materials, but the chitosan does not diffuse well in the bacterial solution before optimization. The optimized antibacterial effect of chitosan dressing can be attributed to the excellent water absorption and swelling of TCS dressing. Optimizing the chitosan dressing could absorb more culture fluid, which made it easier to spread in the bacterial solution, thus improving the bacteriostatic efficiency. Obviously, the TCS dressing was better than the other two groups. It is worth mentioning that the antibacterial effect of optimized chitosan against C. albicans was close to 100%, which had a significant advantage compared to the other two dressings. To sum up, this chitosan dressing with superior antibacterial properties has potential value as a clinical dressing.
4 Conclusion
To solve the poor degradability of chitosan dressing and improve the water absorption and antibacterial properties of chitosan dressing, this experiment prepared a chitosan dressing with degradable, ultra-high water absorption, high antibacterial properties, and excellent mechanical properties to meet the urgent needs of developing desirable wound materials. So as to meet the urgent needs of developing desirable wound materials, the water absorption of TCS dressing can reach 2,100% and the improved mechanical properties of TCS dressing were achieved. In terms of bacteriostasis, the bacteriostatic effect of TCS dressing is significantly better than the commercial SA dressing. The TCS dressing has certain application potential and value in the treatment of wound injury and healing process.
Acknowledgments
Y. Kong and X. Tang contributed equally to this work. The authors gratefully acknowledge the financial support of the National Key Research and Development Program of China (2018YFC1105600).
-
Conflict of interest: The authors declare no conflict of interest regarding the publication of this paper.
References
[1] Qu J, Zhao X, Liang YP, Zhang TL, Ma PX, Guo BL. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 2018;183:185–99.10.1016/j.biomaterials.2018.08.044Search in Google Scholar PubMed
[2] Rosenbaum AJ, Banerjee S, Rezak KM, Uhl RL. Advances in wound management. J Am Acad Orthop Sur. 2018;26(23):833–43.10.5435/JAAOS-D-17-00024Search in Google Scholar PubMed
[3] Annabi N, Rana D, Shirzaei Sani E, Portillo-Lara R, Gifford JL, Fares MM, et al. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials. 2017;139:229–43.10.1016/j.biomaterials.2017.05.011Search in Google Scholar PubMed
[4] Guo B, Ma PX. Conducting polymers for tissue engineering. Biomacromolecules. 2018;19(6):1764–82.10.1021/acs.biomac.8b00276Search in Google Scholar PubMed PubMed Central
[5] Ghomi ER, Khalili S, Khorasani SN, Neisiany RE, Ramakrishna S. Wound dressings: Current advances and future directions. J Appl Polym Sci. 2019;136(27):47738.10.1002/app.47738Search in Google Scholar
[6] Dhivya S, Padma VV, Santhini E. Wound dressings – a review. Biomedicine (Taipei). 2015;5(4):22.10.7603/s40681-015-0022-9Search in Google Scholar PubMed PubMed Central
[7] Koehler J, Brandl FP, Goepferich AM. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur Polym J. 2018;100:1–11.10.1016/j.eurpolymj.2017.12.046Search in Google Scholar
[8] Tao G, Cai R, Wang YJ, Liu LY, Zuo H, Zhao P, et al. Bioinspired design of AgNPs embedded silk sericin-based sponges for efficiently combating bacteria and promoting wound healing. Mater Des. 2019;180:107940.10.1016/j.matdes.2019.107940Search in Google Scholar
[9] Koga AY, Pereira AV, Lipinski LC, Oliveira MRP. Evaluation of wound healing effect of alginate films containing Aloe vera (Aloe barbadensis Miller) gel. J Biomater Appl. 2018;32(9):1212–21.10.1177/0885328218754615Search in Google Scholar PubMed
[10] Tang XX, Gu XY, Wang YL, Chen XL, Ling J, Yang YM. Stable antibacterial polysaccharide-based hydrogels as tissue adhesives for wound healing. Rsc Adv. 2020;10(29):17280–7.10.1039/D0RA02017FSearch in Google Scholar PubMed PubMed Central
[11] He Y, Hou Z, Wang J, Wang Z, Li X, Liu J, et al. Assessment of biological properties of recombinant collagen-hyaluronic acid composite scaffolds. Int J Biol Macromol. 2020;149:1275–84.10.1016/j.ijbiomac.2020.02.023Search in Google Scholar PubMed
[12] Muthukumar T, Sreekumar G, Sastry TP, Chamundeeswari M. Collagen as a potential biomaterial in biomedical applications. Rev Adv Mater Sci. 2018;53(1):29–39.10.1515/rams-2018-0002Search in Google Scholar
[13] Palchesko RN, Carrasquilla SD, Feinberg AW. Natural biomaterials for corneal tissue engineering, repair, and regeneration. Adv Healthc Mater. 2018;7(16):1701434.10.1002/adhm.201701434Search in Google Scholar PubMed
[14] Abdelsalam M, Al-Homidan I, Ebeid T, Abou-Emera O, Mostafa M, Abd El-Razik M, et al. Effect of silver nanoparticle administration on productive performance, blood parameters, antioxidative status, and silver residues in growing rabbits under hot climate. Animals-Basel. 2019;9(10):845.10.3390/ani9100845Search in Google Scholar PubMed PubMed Central
[15] Tao G, Wang YJ, Cai R, Chang HP, Song K, Zuo H, et al. Design and performance of sericin/poly(vinyl alcohol) hydrogel as a drug delivery carrier for potential wound dressing application. Mat Sci Eng C Mater. 2019;101:341–51.10.1016/j.msec.2019.03.111Search in Google Scholar PubMed
[16] Shi LX, Liu X, Wang WS, Jiang L, Wang ST. A Self-pumping dressing for draining excessive biofluid around wounds. Adv Mater. 2019;31(5):1804187.10.1002/adma.201804187Search in Google Scholar PubMed
[17] Alves P, Santos M, Mendes S, Miguel SP, de Sa KD, Cabral CSD, et al. Photocrosslinkable nanofibrous asymmetric membrane designed for wound dressing. Polymers-Basel. 2019;11(4):653.10.3390/polym11040653Search in Google Scholar PubMed PubMed Central
[18] Sun XM, Bai Y, Zhai H, Liu S, Zhang C, Xu YW, et al. Devising micro/nano-architectures in multi-channel nerve conduits towards a pro-regenerative matrix for the repair of spinal cord injury. Acta Biomater. 2019;86:194–206.10.1016/j.actbio.2018.12.032Search in Google Scholar PubMed
[19] Levengood SL, Erickson AE, Chang FC, Zhang M. Chitosan-poly(caprolactone) nanofibers for skin repair. J Mater Chem B. 2017;5(9):1822–33.10.1039/C6TB03223KSearch in Google Scholar PubMed PubMed Central
[20] Amini F, Semnani D, Karbasi S, Banitaba SN. A novel bilayer drug-loaded wound dressing of PVDF and PHB/Chitosan nanofibers applicable for post-surgical ulcers. Int J Polym Mater Po. 2019;68(13):772–7.10.1080/00914037.2018.1506982Search in Google Scholar
[21] Zhang XL, Huang C, Zhao Y, Jin XY. Preparation and characterization of nanoparticle reinforced alginate fibers with high porosity for potential wound dressing application. Rsc Adv. 2017;7(62):39349–58.10.1039/C7RA06103JSearch in Google Scholar
[22] Deng Y, Yang WZ, Shi D, Wu MJ, Xiong XL, Chen ZG, et al. Bioinspired and osteopromotive polydopamine nanoparticle-incorporated fibrous membranes for robust bone regeneration. NPG Asia Mater. 2019;11:39.10.1038/s41427-019-0139-5Search in Google Scholar
[23] Muxika A, Etxabide A, Uranga J, Guerrero P, de la Caba K. Chitosan as a bioactive polymer: Processing, properties and applications. Int J Biol Macromol. 2017;105:1358–68.10.1016/j.ijbiomac.2017.07.087Search in Google Scholar PubMed
[24] Guo SH, Fu DW, Utupova A, Sun DJ, Zhou M, Jin Z, et al. Applications of polymer-based nanoparticles in vaccine field. Nanotechnol Rev. 2019;8(1):143–55.10.1515/ntrev-2019-0014Search in Google Scholar
[25] Zhang L, Zhao WJ, Niu CM, Zhou YJ, Shi HY, Wang YL, et al. Genipin-cross-linked chitosan nerve conduits containing TNF-alpha inhibitors for peripheral nerve repair. Ann Biomed Eng. 2018;46(7):1013–25.10.1007/s10439-018-2011-0Search in Google Scholar PubMed
[26] Miao Y, Lu JW, Yin JH, Zhou CC, Guo YP, Zhou SM. Yb3+ -containing chitosan hydrogels induce B-16 melanoma cell anoikis via a Fak-dependent pathway. Nanotechnol Rev. 2019;8(1):645–60.10.1515/ntrev-2019-0056Search in Google Scholar
[27] Biranje SS, Madiwale PV, Patankar KC, Chhabra R, Dandekar-Jain P, Adivarekar RV. Hemostasis and anti-necrotic activity of wound-healing dressing containing chitosan nanoparticles. Int J Biol Macromol. 2019;121:936–46.10.1016/j.ijbiomac.2018.10.125Search in Google Scholar PubMed
[28] Re F, Sartore L, Moulisova V, Cantini M, Almici C, Bianchetti A, et al. 3D gelatin-chitosan hybrid hydrogels combined with human platelet lysate highly support human mesenchymal stem cell proliferation and osteogenic differentiation. J Tissue Eng. 2019;10:2041731419845852.10.1177/2041731419845852Search in Google Scholar PubMed PubMed Central
[29] Lau YT, Kwok LF, Tam KW, Chan YS, Shum DKY, Shea GKH. Genipin-treated chitosan nanofibers as a novel scaffold for nerve guidance channel design. Colloid Surf B. 2018;162:126–34.10.1016/j.colsurfb.2017.11.061Search in Google Scholar PubMed
[30] Zhao YH, Wang YJ, Gong JH, Yang L, Niu CM, Ni XJ, et al. Chitosan degradation products facilitate peripheral nerve regeneration by improving macrophage-constructed microenvironments. Biomaterials. 2017;134:64–77.10.1016/j.biomaterials.2017.02.026Search in Google Scholar PubMed
[31] Wang YJ, Zhao YH, Sun C, Hu W, Zhao J, Li GC, et al. Chitosan degradation products promote nerve regeneration by stimulating schwann cell proliferation via miR-27a/FOXO1 axis. Mol Neurobiol. 2016;53(1):28–39.10.1007/s12035-014-8968-2Search in Google Scholar PubMed
[32] Hamedi H, Moradi S, Hudson SM, Tonelli AE. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohyd Polym. 2018;199:445–60.10.1016/j.carbpol.2018.06.114Search in Google Scholar PubMed
[33] Patrulea V, Ostafe V, Borchard G, Jordan O. Chitosan as a starting material for wound healing applications. Eur J Pharm Biopharm. 2015;97:417–26.10.1016/j.ejpb.2015.08.004Search in Google Scholar PubMed
[34] Yan TT, Li CP, Ouyang QQ, Zhang DY, Zhong QK, Li PW, et al. Synthesis of gentamicin-grafted-chitosan with improved solubility and antibacterial activity. React Funct Polym. 2019;137:38–45.10.1016/j.reactfunctpolym.2019.01.013Search in Google Scholar
[35] Kiyak Y, Maze B, Pourdeyhimi B. Microfiber nonwovens as potential membranes. Sep Purif Rev. 2019;48(4):282–97.10.1080/15422119.2018.1479968Search in Google Scholar
[36] Zhang Y, Liu BL, Wang LJ, Deng YH, Zhou SY, Feng JW. Preparation, structure and properties of acid aqueous solution plasticized thermoplastic chitosan. Polymers-Basel. 2019;11(5):818.10.3390/polym11050818Search in Google Scholar PubMed PubMed Central
[37] Hashim H, Salleh MS, Omar MZ. Homogenous dispersion and interfacial bonding of carbon nanotube reinforced with aluminum matrix composite: A review. Rev Adv Mater Sci. 2019;58(1):295–303.10.1515/rams-2019-0035Search in Google Scholar
[38] Ho TT, Bremmell KE, Krasowska M, MacWilliams SV, Richard CJ, Stringer DN, et al. In situ ATR FTIR spectroscopic study of the formation and hydration of a fucoidan/chitosan polyelectrolyte multilayer. Langmuir. 2015;31(41):11249–59.10.1021/acs.langmuir.5b01812Search in Google Scholar PubMed
[39] Annabi N, Rana D, Sani ES, Portillo-Lara R, Gifford JL, Fares MM, et al. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials. 2017;139:229–43.10.1016/j.biomaterials.2017.05.011Search in Google Scholar PubMed
[40] Liu SD, Li DS, Yang Y, Jiang L. Fabrication, mechanical properties and failure mechanism of random and aligned nanofiber membrane with different parameters. Nanotechnol Rev. 2019;8(1):218–26.10.1515/ntrev-2019-0020Search in Google Scholar
[41] Fu RM, Tu LJ, Zhou YH, Fan L, Zhang FM, Wang ZG, et al. A tough and self-powered hydrogel for artificial skin. Chem Mater. 2019;31(23):9850–60.10.1021/acs.chemmater.9b04041Search in Google Scholar
[42] Sood N, Bhardwaj A, Mehta S, Mehta A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016;23(3):758–80.10.3109/10717544.2014.940091Search in Google Scholar PubMed
[43] Qu J, Zhao X, Liang YP, Xu YM, Ma PX, Guo BL. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem Eng J. 2019;362:548–60.10.1016/j.cej.2019.01.028Search in Google Scholar
[44] Zhao X, Wu H, Guo BL, Dong RN, Qiu YS, Ma PX. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials. 2017;122:34–47.10.1016/j.biomaterials.2017.01.011Search in Google Scholar PubMed
[45] Li X, Wu B, Chen H, Nan KH, Jin YY, Sun L, et al. Recent developments in smart antibacterial surfaces to inhibit biofilm formation and bacterial infections. J Mater Chem B. 2018;6(26):4274–92.10.1039/C8TB01245HSearch in Google Scholar PubMed
[46] Ali A, Ahmed S. A review on chitosan and its nanocomposites in drug delivery. Int J Biol Macromol. 2018;109:273–86.10.1016/j.ijbiomac.2017.12.078Search in Google Scholar PubMed
[47] Chen K, Wang FY, Liu SY, Wu XF, Xu LM, Zhang DK. In situ reduction of silver nanoparticles by sodium alginate to obtain silver-loaded composite wound dressing with enhanced mechanical and antimicrobial property. Int J Biol Macromol. 2020;148:501–9.10.1016/j.ijbiomac.2020.01.156Search in Google Scholar PubMed
© 2020 Yan Kong et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Generalized locally-exact homogenization theory for evaluation of electric conductivity and resistance of multiphase materials
- Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide
- Characterization of mechanical properties of epoxy/nanohybrid composites by nanoindentation
- Graphene and CNT impact on heat transfer response of nanocomposite cylinders
- A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites
- Ultrasmall Fe3O4 nanoparticles induce S-phase arrest and inhibit cancer cells proliferation
- Effect of aging on properties and nanoscale precipitates of Cu-Ag-Cr alloy
- Effect of nano-strengthening on the properties and microstructure of recycled concrete
- Stabilizing effect of methylcellulose on the dispersion of multi-walled carbon nanotubes in cementitious composites
- Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites
- Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation
- Preparation and properties of 3D interconnected CNTs/Cu composites
- On factors affecting surface free energy of carbon black for reinforcing rubber
- Nano-silica modified phenolic resin film: manufacturing and properties
- Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete
- Halloysite nanotubes in polymer science: purification, characterization, modification and applications
- Cellulose hydrogel skeleton by extrusion 3D printing of solution
- Crack closure and flexural tensile capacity with SMA fibers randomly embedded on tensile side of mortar beams
- An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites
- Utilization of red mud for producing a high strength binder by composition optimization and nano strengthening
- One-pot synthesis of nano titanium dioxide in supercritical water
- Printability of photo-sensitive nanocomposites using two-photon polymerization
- In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries
- Effect of nano and micro conductive materials on conductive properties of carbon fiber reinforced concrete
- Tribological performance of nano-diamond composites-dispersed lubricants on commercial cylinder liner mating with CrN piston ring
- Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance
- Genetic mechanisms of deep-water massive sandstones in continental lake basins and their significance in micro–nano reservoir storage systems: A case study of the Yanchang formation in the Ordos Basin
- Effects of nanoparticles on engineering performance of cementitious composites reinforced with PVA fibers
- Band gap manipulation of viscoelastic functionally graded phononic crystal
- Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution
- Manipulating conductive network formation via 3D T-ZnO: A facile approach for a CNT-reinforced nanocomposite
- Microstructure and mechanical properties of WC–Ni multiphase ceramic materials with NiCl2·6H2O as a binder
- Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite
- Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function
- Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing
- Corrosion behaviour of multilayer CrN coatings deposited by hybrid HIPIMS after oxidation treatment
- Surface hydrophobicity and oleophilicity of hierarchical metal structures fabricated using ink-based selective laser melting of micro/nanoparticles
- Research on bond–slip performance between pultruded glass fiber-reinforced polymer tube and nano-CaCO3 concrete
- Antibacterial polymer nanofiber-coated and high elastin protein-expressing BMSCs incorporated polypropylene mesh for accelerating healing of female pelvic floor dysfunction
- Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films
- A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination
- Arrangement structure of carbon nanofiber with excellent spectral radiation characteristics
- Effect of different particle sizes of nano-SiO2 on the properties and microstructure of cement paste
- Superior Fe x N electrocatalyst derived from 1,1′-diacetylferrocene for oxygen reduction reaction in alkaline and acidic media
- Facile growth of aluminum oxide thin film by chemical liquid deposition and its application in devices
- Liquid crystallinity and thermal properties of polyhedral oligomeric silsesquioxane/side-chain azobenzene hybrid copolymer
- Laboratory experiment on the nano-TiO2 photocatalytic degradation effect of road surface oil pollution
- Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage
- Conversion of sub-µm calcium carbonate (calcite) particles to hollow hydroxyapatite agglomerates in K2HPO4 solutions
- Exact solutions of bending deflection for single-walled BNNTs based on the classical Euler–Bernoulli beam theory
- Effects of substrate properties and sputtering methods on self-formation of Ag particles on the Ag–Mo(Zr) alloy films
- Enhancing carbonation and chloride resistance of autoclaved concrete by incorporating nano-CaCO3
- Effect of SiO2 aerogels loading on photocatalytic degradation of nitrobenzene using composites with tetrapod-like ZnO
- Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles
- Hydration activity, crystal structural, and electronic properties studies of Ba-doped dicalcium silicate
- Microstructure and mechanical properties of brazing joint of silver-based composite filler metal
- Polymer nanocomposite sunlight spectrum down-converters made by open-air PLD
- Cryogenic milling and formation of nanostructured machined surface of AISI 4340
- Braided composite stent for peripheral vascular applications
- Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre–reinforced starch biopolymer composites
- Study on influencing factors of photocatalytic performance of CdS/TiO2 nanocomposite concrete
- Improving flexural and dielectric properties of carbon fiber epoxy composite laminates reinforced with carbon nanotubes interlayer using electrospray deposition
- Scalable fabrication of carbon materials based silicon rubber for highly stretchable e-textile sensor
- Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery
- Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries
- Synthesis of functionalized carbon nanotubes for fluorescent biosensors
- Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete
- Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage
- Microstructural evolution and properties of Cu–20 wt% Ag alloy wire by multi-pass continuous drawing
- Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene
- Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications
- Preparation and piezoresistivity of carbon nanotube-coated sand reinforced cement mortar
- Vibrational analysis of an irregular single-walled carbon nanotube incorporating initial stress effects
- Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials
- Single pulse laser removal of indium tin oxide film on glass and polyethylene terephthalate by nanosecond and femtosecond laser
- Mechanical reinforcement with enhanced electrical and heat conduction of epoxy resin by polyaniline and graphene nanoplatelets
- High-efficiency method for recycling lithium from spent LiFePO4 cathode
- Degradable tough chitosan dressing for skin wound recovery
- Static and dynamic analyses of auxetic hybrid FRC/CNTRC laminated plates
- Review articles
- Carbon nanomaterials enhanced cement-based composites: advances and challenges
- Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide
- Review on modeling and application of chemical mechanical polishing
- Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites
- Advances in modelling and analysis of nano structures: a review
- Mechanical properties of nanomaterials: A review
- New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics
- A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials
- Recent development and applications of nanomaterials for cancer immunotherapy
- Advances in biomaterials for adipose tissue reconstruction in plastic surgery
- Advances of graphene- and graphene oxide-modified cementitious materials
- Theories for triboelectric nanogenerators: A comprehensive review
- Nanotechnology of diamondoids for the fabrication of nanostructured systems
- Material advancement in technological development for the 5G wireless communications
- Nanoengineering in biomedicine: Current development and future perspectives
- Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview
- Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review
- Carbon nanotube–reinforced polymer composite for electromagnetic interference application: A review
- Functionalized layered double hydroxide applied to heavy metal ions absorption: A review
- A new classification method of nanotechnology for design integration in biomaterials
- Finite element analysis of natural fibers composites: A review
- Phase change materials for building construction: An overview of nano-/micro-encapsulation
- Recent advance in surface modification for regulating cell adhesion and behaviors
- Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions
- Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review
- Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications
- A review of passive methods in microchannel heat sink application through advanced geometric structure and nanofluids: Current advancements and challenges
- Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices
- Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging
- Synthesis of graphene: Potential carbon precursors and approaches
- A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE)
- Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water
- Analysis of functionally graded carbon nanotube-reinforced composite structures: A review
- Application of nanomaterials in ultra-high performance concrete: A review
- Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer
- Advanced nickel nanoparticles technology: From synthesis to applications
- Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable?
- Progress in construction of bio-inspired physico-antimicrobial surfaces
- From materials to devices using fused deposition modeling: A state-of-art review
- A review for modified Li composite anode: Principle, preparation and challenge
- Naturally or artificially constructed nanocellulose architectures for epoxy composites: A review
Articles in the same Issue
- Research Articles
- Generalized locally-exact homogenization theory for evaluation of electric conductivity and resistance of multiphase materials
- Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide
- Characterization of mechanical properties of epoxy/nanohybrid composites by nanoindentation
- Graphene and CNT impact on heat transfer response of nanocomposite cylinders
- A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites
- Ultrasmall Fe3O4 nanoparticles induce S-phase arrest and inhibit cancer cells proliferation
- Effect of aging on properties and nanoscale precipitates of Cu-Ag-Cr alloy
- Effect of nano-strengthening on the properties and microstructure of recycled concrete
- Stabilizing effect of methylcellulose on the dispersion of multi-walled carbon nanotubes in cementitious composites
- Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites
- Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation
- Preparation and properties of 3D interconnected CNTs/Cu composites
- On factors affecting surface free energy of carbon black for reinforcing rubber
- Nano-silica modified phenolic resin film: manufacturing and properties
- Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete
- Halloysite nanotubes in polymer science: purification, characterization, modification and applications
- Cellulose hydrogel skeleton by extrusion 3D printing of solution
- Crack closure and flexural tensile capacity with SMA fibers randomly embedded on tensile side of mortar beams
- An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites
- Utilization of red mud for producing a high strength binder by composition optimization and nano strengthening
- One-pot synthesis of nano titanium dioxide in supercritical water
- Printability of photo-sensitive nanocomposites using two-photon polymerization
- In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries
- Effect of nano and micro conductive materials on conductive properties of carbon fiber reinforced concrete
- Tribological performance of nano-diamond composites-dispersed lubricants on commercial cylinder liner mating with CrN piston ring
- Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance
- Genetic mechanisms of deep-water massive sandstones in continental lake basins and their significance in micro–nano reservoir storage systems: A case study of the Yanchang formation in the Ordos Basin
- Effects of nanoparticles on engineering performance of cementitious composites reinforced with PVA fibers
- Band gap manipulation of viscoelastic functionally graded phononic crystal
- Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution
- Manipulating conductive network formation via 3D T-ZnO: A facile approach for a CNT-reinforced nanocomposite
- Microstructure and mechanical properties of WC–Ni multiphase ceramic materials with NiCl2·6H2O as a binder
- Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite
- Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function
- Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing
- Corrosion behaviour of multilayer CrN coatings deposited by hybrid HIPIMS after oxidation treatment
- Surface hydrophobicity and oleophilicity of hierarchical metal structures fabricated using ink-based selective laser melting of micro/nanoparticles
- Research on bond–slip performance between pultruded glass fiber-reinforced polymer tube and nano-CaCO3 concrete
- Antibacterial polymer nanofiber-coated and high elastin protein-expressing BMSCs incorporated polypropylene mesh for accelerating healing of female pelvic floor dysfunction
- Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films
- A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination
- Arrangement structure of carbon nanofiber with excellent spectral radiation characteristics
- Effect of different particle sizes of nano-SiO2 on the properties and microstructure of cement paste
- Superior Fe x N electrocatalyst derived from 1,1′-diacetylferrocene for oxygen reduction reaction in alkaline and acidic media
- Facile growth of aluminum oxide thin film by chemical liquid deposition and its application in devices
- Liquid crystallinity and thermal properties of polyhedral oligomeric silsesquioxane/side-chain azobenzene hybrid copolymer
- Laboratory experiment on the nano-TiO2 photocatalytic degradation effect of road surface oil pollution
- Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage
- Conversion of sub-µm calcium carbonate (calcite) particles to hollow hydroxyapatite agglomerates in K2HPO4 solutions
- Exact solutions of bending deflection for single-walled BNNTs based on the classical Euler–Bernoulli beam theory
- Effects of substrate properties and sputtering methods on self-formation of Ag particles on the Ag–Mo(Zr) alloy films
- Enhancing carbonation and chloride resistance of autoclaved concrete by incorporating nano-CaCO3
- Effect of SiO2 aerogels loading on photocatalytic degradation of nitrobenzene using composites with tetrapod-like ZnO
- Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles
- Hydration activity, crystal structural, and electronic properties studies of Ba-doped dicalcium silicate
- Microstructure and mechanical properties of brazing joint of silver-based composite filler metal
- Polymer nanocomposite sunlight spectrum down-converters made by open-air PLD
- Cryogenic milling and formation of nanostructured machined surface of AISI 4340
- Braided composite stent for peripheral vascular applications
- Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre–reinforced starch biopolymer composites
- Study on influencing factors of photocatalytic performance of CdS/TiO2 nanocomposite concrete
- Improving flexural and dielectric properties of carbon fiber epoxy composite laminates reinforced with carbon nanotubes interlayer using electrospray deposition
- Scalable fabrication of carbon materials based silicon rubber for highly stretchable e-textile sensor
- Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery
- Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries
- Synthesis of functionalized carbon nanotubes for fluorescent biosensors
- Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete
- Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage
- Microstructural evolution and properties of Cu–20 wt% Ag alloy wire by multi-pass continuous drawing
- Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene
- Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications
- Preparation and piezoresistivity of carbon nanotube-coated sand reinforced cement mortar
- Vibrational analysis of an irregular single-walled carbon nanotube incorporating initial stress effects
- Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials
- Single pulse laser removal of indium tin oxide film on glass and polyethylene terephthalate by nanosecond and femtosecond laser
- Mechanical reinforcement with enhanced electrical and heat conduction of epoxy resin by polyaniline and graphene nanoplatelets
- High-efficiency method for recycling lithium from spent LiFePO4 cathode
- Degradable tough chitosan dressing for skin wound recovery
- Static and dynamic analyses of auxetic hybrid FRC/CNTRC laminated plates
- Review articles
- Carbon nanomaterials enhanced cement-based composites: advances and challenges
- Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide
- Review on modeling and application of chemical mechanical polishing
- Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites
- Advances in modelling and analysis of nano structures: a review
- Mechanical properties of nanomaterials: A review
- New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics
- A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials
- Recent development and applications of nanomaterials for cancer immunotherapy
- Advances in biomaterials for adipose tissue reconstruction in plastic surgery
- Advances of graphene- and graphene oxide-modified cementitious materials
- Theories for triboelectric nanogenerators: A comprehensive review
- Nanotechnology of diamondoids for the fabrication of nanostructured systems
- Material advancement in technological development for the 5G wireless communications
- Nanoengineering in biomedicine: Current development and future perspectives
- Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview
- Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review
- Carbon nanotube–reinforced polymer composite for electromagnetic interference application: A review
- Functionalized layered double hydroxide applied to heavy metal ions absorption: A review
- A new classification method of nanotechnology for design integration in biomaterials
- Finite element analysis of natural fibers composites: A review
- Phase change materials for building construction: An overview of nano-/micro-encapsulation
- Recent advance in surface modification for regulating cell adhesion and behaviors
- Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions
- Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review
- Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications
- A review of passive methods in microchannel heat sink application through advanced geometric structure and nanofluids: Current advancements and challenges
- Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices
- Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging
- Synthesis of graphene: Potential carbon precursors and approaches
- A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE)
- Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water
- Analysis of functionally graded carbon nanotube-reinforced composite structures: A review
- Application of nanomaterials in ultra-high performance concrete: A review
- Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer
- Advanced nickel nanoparticles technology: From synthesis to applications
- Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable?
- Progress in construction of bio-inspired physico-antimicrobial surfaces
- From materials to devices using fused deposition modeling: A state-of-art review
- A review for modified Li composite anode: Principle, preparation and challenge
- Naturally or artificially constructed nanocellulose architectures for epoxy composites: A review

