Cotton fibres functionalized with plasmonic nanoparticles to promote the destruction of harmful molecules: an overview
-
Sami Boufi
, Luís Filipe Vieira Ferreira
Abstract
Self-decontaminating cotton fabrics were designed, produced and characterized aiming at the decomposition of harmful molecules namely chemical warfare agents (CWAs) by photocatalysis under day light or indoor illumination. This was achieved through the creation of a hybrid organic-inorganic nanostructured textile composed of a thin layer of TiO2 nanoparticles (NPs) generated in situ and chemically immobilised on the cellulose chains of cotton fibres. TiO2 NPs were converted into anatase by a hydrothermal procedure at low temperature around 100°C. The fabrics covered with TiO2 nanoparticles were examined in terms of their chemical composition, morphology, crystallinity, ageing, robustness and photocatalytic properties. In the whole preparation of the photocatalytic fabrics, only environment-friendly solvents (water or alcohol) were used. One of the important achievements in this work was providing fabrics with suitable photocatalytic activity under visible light. This was reached through plasmonic photocatalysis by generating noble metal nanoparticles (Au, Ag) and/ or their halides (AgBr, AgCl) neighbouring or topping the TiO2 NPs in the fabrics. The kinetics of degradation of the different systems were analysed and proved that the resulting fabrics could efficiently decompose, under visible light, organic dyes and dimethyl methylphosphonate (DMMP), a CWA simulant.
Graphical abstract
1 Introduction
Chemical weapons started to be used in large scale in World War I [1]. They were also largely employed by the Nazis in World War II and, more recently, in several localized conflicts in the Middle East, in spite of the numerous conventions prohibiting their use [2, 3, 4]. The decontamination and the protection of the civilian population from exposure to hazardous chemicals, such as Chemical Warfare Agents (CWAs), is a crucial challenge nowadays. The aim of the work here reviewed is to find effective solutions to improve the protection against CWAs.
The purpose of the CATALTEX project was to contribute for an efficient personal protection for people exposed to harmful molecules, bringing a new technological contribution for decontamination through their destruction. For this purpose, time and effort were spent to develop, to design and to elaborate new “smart” textiles endowed with self-decontaminating properties based on a photocatalytic nanotechnology. These functionalized fabrics can be easily implemented in any country having a valuable industrial level to depollute infected areas invaded by toxic molecules, as CWAs, or even toxic pollution in industrial or agricultural environments or merely household products in indoor air. First attempts have been based on fibres associated to activated carbon liners [5, 6, 7]. However, most of the resulting protective clothes made of such impermeable materials, have the drawback of being heavy, not breathable and expensive. Lighter materials endowing decontaminating properties can be generated, through the combination of fabrics, for instance cotton with nanoparticles, namely those of metal oxide nanoparticles (MONPs), which exhibit a high photocatalytic activity. “Smart textiles” based on functionalized cotton fabrics have been tested, aiming the destruction of harmful compounds, such as polluting molecules, dyes, or even CWAs by degrading them to harmless products namely water and carbon dioxide through a photocatalytic procedure under day-light or indoor illumination. This paper revisits some of the results obtained in the CATALTEX project, concerning the production of “smart textiles” endowing self-decontaminating properties capable of the total degradation of CWAs.
Photocatalysis can be achieved by the production of highly reactive ions or radicals generated in the neighbourhood of the oxide surface, induced by the visible or UV radiation [8, 9]. However, these highly reactive species have too short lifetimes to be harmful. These oxides had been the subject of an intense research since the seventies and its application in CWAs degradation has also been tackled, in several papers [10, 11]. Actually, these photocatalytic reactions are fundamentally based on the absorption, by the oxide, of photons having energy higher than its gap. This is accompanied by the promotion of electrons to the semi-conductor conduction band (e−) and the consequent generation of holes (electron vacancies, h+) in the valence band (electron-hole pairs). Mostly, oxides with shallow traps are used, where electrons can be easily promoted to higher energy levels through the action of visible or UV photons.The ensuing charge carriers h+ and e− are powerful oxidizing and reducing agents that react with water or atmospheric oxygen to generate highly active hydroxyl (OH•) and superoxide (O2) radicals, respectively. Both of these species are powerful oxidizing agents capable of breaking down organic compounds into CO2 and H2O. The sensitization of the oxide layer enables the photocatalytic power to persist even with visible light.
Nowadays, photocatalysis using MONPs is regarded as a promising way to decompose harmful gases. This is due to their high specific surface, large number of highly reactive edges, corner defect sites and unusual lattice planes. Among them, some oxides, namely, Titanium dioxide, TiO2, are recognized to have a strong photo-oxidation power with an effective ability for decomposing a wide range of warfare agents in the presence of UV radiation [12, 13, 14].
Different strategies published in the literature can be explored to generate TiO2 NPs on cotton fibres: in the presence or absence of molecular binders between the cotton surface and functionalized titania NPs, prepared ex situ, assisted by ultrasound radiation or UV light or embedded in different polymers, just to mention a few [15, 16, 17, 18, 19]. In the present case, a non-hydrolytic sol–gel process followed by a mild hydrothermal treatment was used. Cotton was functionalized with TiO2 nanolayer starting from a precursor: titanium tetrabutoxide (Ti(OBu)4 or TBT), presently the most cost effective precursor of TiO2 commercially available. Cotton fabrics are dipped into a solution of TBT in tert-butanol. TBT interacts with the cotton fibres through the cellulosic OH groups in the presence of acetic acid, leading to sixfold coordinated Ti. Through a hydrothermal treatment at low temperatures (from 80°C to 140°C), one can induce polycondensation leading to the nucleation and growth of anatase. Actually, this hydrothermal treatment enables the crystallization of the TiO2 layer into the anatase phase.
The optimization of the photocatalytic efficiency of the hybrid system Cotton-TiO2 is crucial because, in the amorphous state, the generated charges are promptly trapped before producing the highly reactive species at the surface [20, 21]. It is often reported in literature that a calcination treatment at a temperature over 400°C, necessary to achieve the crystallization of TiO2 into anatase, will greatly improve the photocatalytic properties of the oxide [22, 23]. However, at these temperatures the cotton would be burned. In this study, it was shown that hydrothermal treatment at lower temperatures can lead to crystallization of TiO2 NPs into the anatase phase [24]. Here, the charges generated under radiation on the anatase surface can be more easily exchanged to create highly active ions without being trapped. Actually, TiO2 has a good photocatalytic activity under ultraviolet radiation; however, due to its high gap (Eg = 3.2 eV) [25] its activity under visible light, lower than that under ultraviolet, will not be sufficient for self-decontamination.
To extend the photocatalytic activity of the functionalized fabrics Cotton-TiO2 to the visible domain, the usual method is doping the TiO2 with non-metals such as nitrogen, carbon or halogens, or transition metals, rare earths [26], or, even other semiconductors such as CdS [27]. Plasmonic photocatalysis can be a good strategy [28]. This consists in the inclusion of NPs of different nature such as Ag0 [29, 30], Au0 [31, 32], Cu0 [33], Pt0, Pd0 [34] or their halides such as AgBr [35] or AgCl [36] dispersed in the semi-conducting TiO2 layer. This allows two prominent effects to take place: the formation of a Schottky junction and of a localized surface plasmonic resonance (LSPR). The first one delays the recombination of electrons and holes, promoting the generation of the highly reactive species; the second one, extends the light absorption domain to the visible range. The case of silver NPs will be referred here [24]. In this case, visible radiation contributes to the photocatalysis, even if the photons have energy lower than the gap of the semiconductor. Hybrid Cotton-anatase samples prepared under a hydrothermal treatment temperature of 130°C have a gap of 3.2 eV [24], which impedes the excitation of the electrons located at the top of the valence band to the bottom of the conduction band. However, photons have energy enough for the excitation of the surface plasmon of silver. The excited electrons have enough energy to cross the junction to the conduction band of the semiconducting TiO2 NPs. Consequently, electrons resulting from theexcitation of surface plasmon of the metal NPs by visible light nourish the conduction band of the oxide, promoting its photocatalytic activity [37, 38].
The whole process was followed by several analytical tools and microscopies such as Raman spectroscopy [39], Field-Emission Scanning Electron Microscopy (FE-SEM) and Atomic Force Microscopy (AFM). Also X-ray photo-electron spectroscopy (XPS), a powerful technique for the analysis of the surface layer, as it can give extended information on the elements there present, as well as their oxidation state and their neighbourhood [40, 41], was extensively employed for characterizing the chemical composition of the cotton sample, before and after the NPs generation. UV-Vis absorption spectroscopy and time-resolved laser induced fluorescence (LIF) and phosphorescence (LIP) were mainly used to provide lifetimes and the nature of the interaction between the NPs and the substrate. Finally, the evaluation of the photocatalytic activity for the different hybrid systems was done following the discoloration of aqueous solutions of organic dyes, such as the Remazol Brilliant Blue R (RB) under a sunlight simulator equipped with selective filters. RB is a toxic anthraquinone-based dye used in the textile industry. Results obtained for Cotton-Ag-TiO2 and the comparison with Cotton-TiO2 will be presented here as a paradigmatic example. Some tests performed using CWAs simulant will be also referred here.
This paper reports the results obtained with the hybrid systems based on cotton/titania. Actually, instead of cotton, different types of natural textiles could be employed as silk, wool, line or even synthetic ones like polyester, acrylic or nylon as these fabrics dispose of reactive organic functions where NPs could be generated. Also ceramic NPs (inorganic non-metallic solids) can be applied to photo-catalysis and dyes photodegradation [42]. Magnetic NPs (e.g. Fe3O4, magnetite) are also of importance for the use in environmental remediation, such as water decontamination, biomedicine [42] and also as drug carriers for photodynamic therapy of cancer [43].
The textile treatment here used was already successfully applied to chitosan [44] and can be extended to other fabrics used, namely, in military or firemen uniforms, protection blankets or tent canvas. The originality of this work-relies mainly in the fact that the active photocatalytic layer is grown from the fabrics surface using the cellulose hydroxyl groups instead of being pre-synthesized and then just physically applied on the surface. This methodology intends to improve the fastness of the layer.
2 Materials and methods
2.1 Materials
Common gauze bandages (with a cellulose content exceeding 98%) were used as cotton fabrics models. All reagents of analytical grade were from Aldrich and used without further purification, including RB, AgNO3, NaBH4, glacial acetic acid and tert-butanol (99.7%) and Ti(OBu)4.
2.2 Sample preparation
To remove the adsorbed water, cotton samples were dried at 70°C for 4 h. Next, they were directly dipped into a 0.5, 1 or 1.5 (w/v)% solution of Ti(OBu)4 in a mixture of acetic acid and tert-butanol (10/90 wt%) for 12 h. After drying at 60°C for 2 h in an oven to liberate the solvent and next, samples were transferred into a 200 mL stainless steel autoclave, half-full of water, placed in an oven maintained at different hydrothermal treatment temperatures ranging from 80 to 140°C during 3 h. Samples were dried at the end of the preparation.
Samples containing TiO2 and Ag NPs, having sizes of the order of tens of nanometres, were produced by first generating Ag NPs on the cotton fabrics surface by selective reduction of Ag+ species and, then, by grafting, on the fabrics surface, the TBT following the non-hydrolytic solgel route described above. After a mild hydrothermal treatment (around 100°C), a nanostructured TiO2 anatase layer, firmly bound to the surface, was formed with uniform TiO2 NPs, 20-30 nm in size. The generation of metallic Ag NPs and/or crystalline TiO2 was proved by GSDR, XPS and Raman analysis. Using AgBr aqueous suspensions, instead of AgNO3 solutions, and following similar protocols, hybrid systems of Cotton-Ag-TiO2@AgBr could be formed.
In all preparations, green chemistry principles were followed.
2.3 Raman spectroscopy
A LabRAM Analytical Raman micro-spectrograph (Jobin-Yvon, Horiba group, France) using, as exciting radiation (λ = 632.8 nm), a He-Ne laser source and an air cooled CDD detector (acquisition time of 100 s) was used for recording Raman spectra.
2.4 X-ray photoelectron spectroscopy (XPS)
X-ray photoelectron spectra were acquired with a XSAM800 XPS dual anode spectrometer from KRATOS, irradiating the samples with non-monochromatic Mg Kα radiation (hν = 1253.6 eV). Details on operating conditions of the spectrometer, acquisition parameters, and data treatment were published elsewhere [12, 24].
2.5 Field-emission scanning electron microscopy (FE-SEM)
Images of Cotton-TiO2 were obtained with a Field-Emission Scanning Electron Microscopy (FE-SEM), a ZEISS SUPRA40, microscope completely controlled from a computer workstation. The electron source, a tungsten filament coated with a ZrO layer, is a hot cathode producing electrons by Schottky effect. Samples were attached on a carbon adhesive and coated with a 1 nm thin layer of Pt sputtered under an inert gas.
2.6 Ground state diffuse reflectance absorption spectroscopy (GSDR)
Ground-state absorption studies were performed using a homemade diffuse reflectance laser flash photolysis set-up. Details regarding the data treatment can also be found in the literature [45].
2.7 Time resolved laser induced luminescence studies (LIF and LIP)
The set-up for time resolved luminescence LIL (laser induced fluorescence, LIF, and laser induced phosphorescence, LIP) was also described in the literature [12]. Time-resolved emission spectra were performed in the nanosecond to second time range with a N2 laser (excitation wavelength = 337 nm, PTI model2000, ca 600 ps FWHM, about 1 mJ per pulse) and with the use of an ICCD with time gate capabilities (i-Star from Andor, model 720). Emission spectra were obtained at 77 K, due to the fact that, at room temperature, luminescence intensity is negligible for the anatase form of TiO2.
2.8 Photocatalytic tests
Photocatalytic tests were performed for the hybrid system Cotton-TiO2-Ag by immersing a piece of the modified fabric (5 cm × 5 cm) in beakers containing 50 mL of RB solution (10−4 M). The beaker was then irradiated by a Xenon lamp placed at 10 cm from the beaker top. A fixed amount of solution was withdrawn at regular time intervals and the concentration of RB was monitored by UV/Vis absorption measurements (Lambda 35 Perkin-Elmer Spectrophotometer). Prior to irradiation, beakers were kept in the dark for at least 1 h in order to reach adsorption-desorption equilibrium.
![Scheme 1 TiO2 functionalized cotton from concept to mechanism of action [30]. Reprinted (adapted) with permission from Elsevier](/document/doi/10.1515/ntrev-2019-0058/asset/graphic/j_ntrev-2019-0058_fig_001.jpg)
TiO2 functionalized cotton from concept to mechanism of action [30]. Reprinted (adapted) with permission from Elsevier
Tests using a SARIN simulant, dimethylmethylphosphonate (DMMP) were also performed in the Laboratory of Chemical Safety and Defence of the Portuguese Army in Lisbon (Portugal). The hybrid fabrics TiO2-Ag@AgBr was chosen to investigate its ability to degrade DMMP. The setting of the protocol for the experiments was established taking into account the illumination and the dark conditions, as well as the extraction methods. Basically, a drop is deposited on the textile and the extraction of the resulting products was accomplished, before and after illumination, using an appropriate solvent.
3 Results
The whole strategy and the different steps used in this study until the final elaboration of self-decontaminating textile can be summarized, as follows:
Immobilization and characterization of a thin layer of TiO2 NPs, chemically bound to the cotton fibres endowing photocatalytic activity;
Extending the photocatalytic activity to the visible light region through a plasmonic effect by including NPs of noble metals and/or their halides into the Cotton-TiO2 fabrics;
Characterization of the products obtained in each preparation step;
Implementing the fabrication of “smart textiles” using an environmentally friendly approach without the use of expensive chemicals;
Testing the ability of the hybrid systems to degrade CWA simulants, via photocatalytic oxidation, under day light and indoor illumination.
The idea of associating Ag NPs as heterojunction to TiO2 was motivated by the low photocatalytic efficiency of TiO2 functionalized cotton for the degradation of organic molecules under sunlight and visible light. This phenomenon is expected given the wide gap of TiO2 anatase, which is around 3.2 eV, making the visible range photons are not energetic enough to promote the excitation of the appended TiO2. The inclusion of Ag NPs in the form of tiny NPs well dispersed and effectively bound to the TiO2 layer alleviates this shortcoming benefiting from the Ag LSPR. However, the process of Ag generation should be well controlled to i) avoid saturation of the TiO2 layer; ii) control the size of Ag NPs with sizes lower than 20 nm and ensure effective dispersion within the immobilized TiO2 layer.
The concept and the mechanisms involved in the preparation of the final functionalized cotton (Cotton-Ag-TiO2) are presented on Scheme 1. The middle image is an AFM image of the hybrid system. Last diagram shows the energy levels of the valence and the conduction band of TiO2, where interband transitions are available by the absorption of ultraviolet radiation, resulting in a free electron and a hole. Before their recombination, electrons and holes can give rise to highly active species such as O•−2 and OH•. These radicals created from air, water molecules or aqueous species can efficiently destroy organic molecules under visible light, thanks to the plasmonic effect. The presence of the silver nanoparticle enables the formation of the Schottky junction and opens the possibility for the excitation of a LSPR.
To accomplish the aim of the project, characterization is needed at different stages of the “smart fabrics” preparation. For instance, crystallinity of the TiO2 NPs could be well established by Raman spectroscopy or even by X-ray diffraction. Most important vibrational modes of anatase phase Eg, B1g, A1g and Eg are located at 145, 397, 515 and 640 cm−1 respectively. Figure 1 presents a set of Raman spectra showing that the anatase peak appears for temperatures equal to or higher than 80°C at 153 cm−1 displaying, then, a red shift when compared to 145 cm−1, the corresponding value for the bulky anatase. This shift is here assigned to a phonon confinement effect [24, 46]. The anatase peak intensity evolution is compared to the cellulose region located at 1090 cm−1 in terms of the hydrothermal treatment temperature. One can, then, infer that anatase is created at quite low temperatures, much lower than that of the cotton calcination, which allows the anatase crystallization, without destruction of the organic material.
![Figure 1 Raman spectra of cotton-TiO2 samples showing the evolution of the characteristic peak of anatase (at 153 cm−1) as a function of the hydrothermal treatment temperature; cellulose peaks are located around 1098 cm−1 [24]; reprinted (modified) with permission from Royal Society of Chemistry](/document/doi/10.1515/ntrev-2019-0058/asset/graphic/j_ntrev-2019-0058_fig_002.jpg)
Raman spectra of cotton-TiO2 samples showing the evolution of the characteristic peak of anatase (at 153 cm−1) as a function of the hydrothermal treatment temperature; cellulose peaks are located around 1098 cm−1 [24]; reprinted (modified) with permission from Royal Society of Chemistry
The morphology of the cotton fibres and the degree of coverage of the TiO2 layers can be perceived in the image obtained by FE-SEM, presented in Figure 2. The image exhibits the cotton gauze surface after the hydrothermal treatment at 130°C. In the insert, with higher resolution, one can observe that the gauze is covered by a thin layer of TiO2 NPs of a few tens of nanometres of diameter.
![Figure 2 FE-SEM images of cotton fibres covered by a layer of TiO2 NPs after a hydrothermal temperature of 130°C; in the inserted window, a more resolved image shows a thin layer of TiO2 NPs covering the whole cotton surface [24]; reprinted (modified) with permission from Royal Society of Chemistry](/document/doi/10.1515/ntrev-2019-0058/asset/graphic/j_ntrev-2019-0058_fig_003.jpg)
FE-SEM images of cotton fibres covered by a layer of TiO2 NPs after a hydrothermal temperature of 130°C; in the inserted window, a more resolved image shows a thin layer of TiO2 NPs covering the whole cotton surface [24]; reprinted (modified) with permission from Royal Society of Chemistry
A scheme showing the formation of the cotton-anatase hybrid structures through the hydrothermal treatment from the initiator TBT is presented in Figure 3. The mechanisms leading to the formation of the cotton-anatase structures were described in a former publication [24].
![Figure 3 Schematic nucleation and growth of octahedral anatase crystals during the hydrothermal treatment, leading to Cotton-TiO2 hybrid structures [24]; reprinted (modified) with permission from Royal Society of Chemistry](/document/doi/10.1515/ntrev-2019-0058/asset/graphic/j_ntrev-2019-0058_fig_004.jpg)
Schematic nucleation and growth of octahedral anatase crystals during the hydrothermal treatment, leading to Cotton-TiO2 hybrid structures [24]; reprinted (modified) with permission from Royal Society of Chemistry
Lifetimes of the charges generated on the layer surface by radiation (visible or ultra-violet) are important factors to tailor the photocatalytic properties of the layer, as they can generate active ions responsible for the destruction of the organic molecules present around. GSDR spectra can reveal and measure the photo-physical properties of the layer. In some samples, TiO2 is most probably in an amorphous form and the luminescence spectra is difficult to obtain, even at 77 K. Figure 4a compares the absorption spectra of two samples: commercial TiO2 NPs and 1.5% TiO2 adsorbed onto cotton fibres treated by the hydrothermal method. The time resolved luminescence of TiO2 at 77 K is presented in Figure 4b. A half-life of about 1.5 μs was obtained for the emission of the adsorbed crystalline TiO2, at 77 K, peaking at approximately 530 nm. The results confirm that with suitable experimental conditions, crystalline forms of anatase can be obtained, covering the cellulose cotton fibres. Moreover, luminescence studies lead to lifetimes of the excited TiO2 (at 77 K) about 2 μs, which largely corroborates the photocatalytic properties of the cotton-TiO2 system.
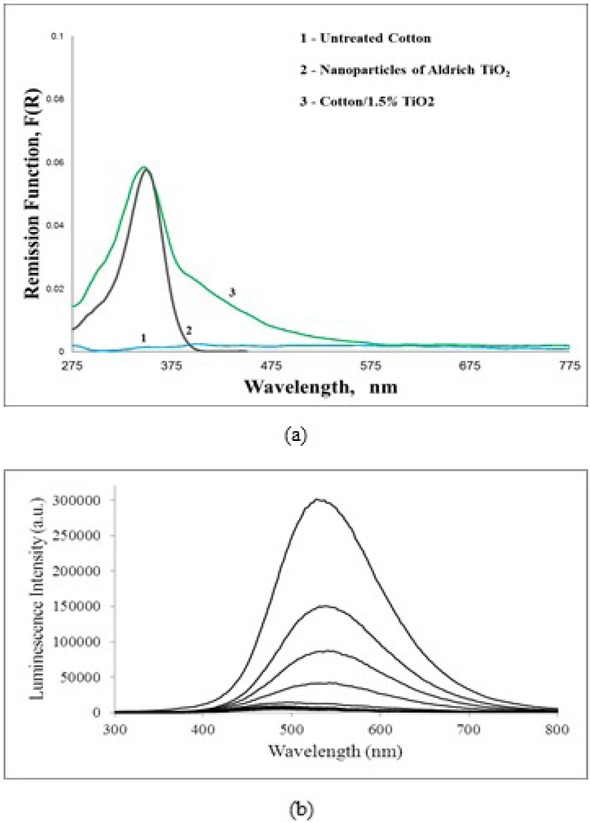
a) GSDR of untreated cotton, commercial TiO2 nanoparticles and Cotton impregnated with 1.5% TiO2; b) Time resolved Laser induced luminescence of cotton impregnated with 1.5% TiO2 at 77 K; the time interval between curves is 1 μs
These results show that hybrid organic-inorganic nanostructured layers, owning photocatalytic properties, can be produced on cotton at temperatures lower than 150°C, preventing any risk of thermal degradation or chain scission of the cellulose chains, while using environmentally friendly approaches and water or alcohol, as sole solvents.
XPS results have shown (Figure 5) the presence of carbon and oxygen typical of the cellulose, as well as the presence of aliphatic carbon existing just in the TBT, the TiO2 precursor. Figure 5a) shows that the titanium could be well-detected through its most intense region, the Ti 2p, exhibiting two different oxidation numbers: Ti4+ and a small fraction of Ti3+. A detailed qualitative and quantitative data analysis presented in Figure 5b) suggests that the transformation of the precursor into crystalline TiO2 NPs progresses from the interface cotton/titanate nanolayer to the extreme surface, justifying the relative increase of the Ti 2p signal with the increase of the thermal treatment temperature.
![Figure 5 a) XPS Ti 2p, O1s and C 1s regions, for Cotton-TiO2 samples submitted to hydrothermal treatment at different temperatures; b) XPS atomic ratios as a function of the hydrothermal temperature [24]; reprinted (modified) with permission from Royal Society of Chemistry](/document/doi/10.1515/ntrev-2019-0058/asset/graphic/j_ntrev-2019-0058_fig_006.jpg)
a) XPS Ti 2p, O1s and C 1s regions, for Cotton-TiO2 samples submitted to hydrothermal treatment at different temperatures; b) XPS atomic ratios as a function of the hydrothermal temperature [24]; reprinted (modified) with permission from Royal Society of Chemistry
TiO2 NPs show to be strongly bound to the cotton fibres and after some washing tests they could be reused. Ultrasonicated fabrics undergo partial loss of nanoparticles but surprisingly exhibit excellent photocatalytic properties likely due to a better distribution of the NPs on the fabrics. Cotton-TiO2, the first functionalized fabrics to be prepared in this study, presents a quite effective photoactivity under ultraviolet. However, its photoactivity in the visible domain is not sufficiently high for the aims here proposed. To enhance it, plasmonic photocatalysis can be the solution dispersing noble metal nanoparticles into the semiconductor photocatalysts, resulting in two effects: formation of a Schottky junction and of a localized surface plasmon resonance [47]. For this purpose, a second hybrid system was investigated, the hybrid system Cotton-Ag-TiO2. Ag NPs were generated on cotton fibres using diluted AgNO3 aqueous solutions, preceding the generation of the TiO2 layer [30].
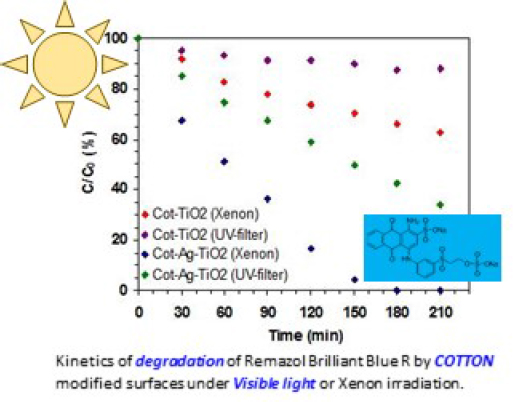
The photocatalytic activity of the two hybrid systems, Cotton-TiO2 and Cotton-Ag-TiO2, was compared following the discoloration of RB in water under a 40 WXenon lamp irradiation, as described above.
Figure 6 shows the evolution of RB concentration in an irradiated aqueous solution, where a piece of modified cotton (Cotton-TiO2 or Cotton-Ag-TiO2) was immersed. In spite of a larger rate of discoloration when the filter is absent, a measurable rate is obtained in the presence of the UV-filter, especially in the case of Cotton-Ag-TiO2 attesting the plasmonic effect. The Cotton-TiO2 sample, in the presence of a UV-filter induces a very limited discolouration of the solution, probably due to some adsorption of the dye on the piece of the cotton-TiO2 surface.
![Figure 6 Evolution of the RB concentration of an aqueous solution in presence of a piece of modified cotton, Cotton-TiO2 or Cotton-Ag-TiO2 immersed in the RB solution under a Xenon lamp, in the presence or in absence of a UV-filter [30]; reprinted with permission from Elsevier](/document/doi/10.1515/ntrev-2019-0058/asset/graphic/j_ntrev-2019-0058_fig_007.jpg)
Evolution of the RB concentration of an aqueous solution in presence of a piece of modified cotton, Cotton-TiO2 or Cotton-Ag-TiO2 immersed in the RB solution under a Xenon lamp, in the presence or in absence of a UV-filter [30]; reprinted with permission from Elsevier
Aiming to further enhance the photocatalytic efficiency, a third approach was used, where the TiO2 layer was coupled with a AgBr heterojunction instead of Ag NPs alone. Here again, the method adopted is ecofriendly, easy to process and the hydrothermal treatment was kept lower than 150°C. For this purpose, a layer of TiO2 was first generated as described above, followed by immersion of the cotton/TiO2 in a suspension of AgBr freshly prepared by mixing a solution of AgNO3 (5×10−3 M) with a solution of KBr (5×10−3 M). The cotton sample was then submitted to a hydrothermal treatment.
Among the different hybrid systems prepared, the TiO2-Ag@AgBr functionalized fabrics was also characterized by Raman, XRD and XPS. GSDR analysis revealed a strong absorption in the visible region brought by the LSPR of Ag nanocrystals generated at the AgBr surface. XPS evidenced the presence of Ag+, Ag0 and bromine, suggesting that Ag0 formed a shell around the deposited AgBr. The immobilized TiO2-Ag-AgBr heterostructured layer imparts a strong photocatalytic activity under visible light for the degradation of dyes in aqueous solution [35].
The tests performed using a SARIN simulant, DMMP, show that the fraction of DMMP degraded under visible light irradiation in the presence of functionalized cotton is much higher than that obtained with unmodified cotton [35].
4 Conclusions
Innovative approaches to functionalize cotton fabrics with covalently bonded nanostructured layers of TiO2 in the anatase crystalline form were used. Here, TiO2 NPs are generated in the cellulose hydroxyl groups instead of being pre-synthesized and spread onto the cotton fabrics. The methodology presented improves the fastness of the layer, contrarily to that where TiO2 NPs are just physically applied onto the surface. TiO2 NPs obtained can crystallize to anatase through mild conditions under temperatures compatible with the integrity of the fabrics. The method developed is in agreement with the Green Chemistry principles, easy to implement, reliable and cost-effective. TiO2 NPs show to be strongly bound to the cotton fibres and exhibit a high photocatalytic activity under ultraviolet. The degradation of dye molecules demonstrated the high photocatalytic activity of the hybrid system Cotton-TiO2 under UV-radiation.
In contrast, Cotton-TiO2 hybrid systems have a low activity under sunlight illumination. However, this can be successfully improved through the plasmonic effect, by the inclusion on the fabrics of silver NPs and/or their halides, among others. The hybrid Cotton-Ag-TiO2 enables the degradation of dyes under visible light. These hybrid systems were also ultrasonicated and no significant loss of photocatalytic properties was detected attesting the efficient binding of the monolayer to the cotton.
Samples of cotton fabrics decorated with TiO2 and AgBr NPs were also tested, exhibiting a strong photocatalytic activity under day light illumination. The hybrid cotton, where anatase was associated to NPs of silver and silver halides, showed a good efficiency for the degradation of a CWA simulant (DMPP).
These new plasmonic photocatalysts functionalized fabrics exhibit self-detoxification properties and seem to hold promises for application in chemical protective clothes and might offer new opportunities for the design of novel functional materials for toxic chemical protection, namely against chemical pollutants in water, household products in indoor air, pollution in factory or agronomic environment and, naturally, against CWAs. This methodology is foreseen to be easily scalable to industrial processing.
Acknowledgement
The authors would like to thank NATO for the Collaborative Linkage Grant awarded to the consortium of CATALTEX, a Multi-Year Project entitled “Self-Decontaminating Smart Textiles for Chemical Warfare Degradation” in the framework of “Science for Peace and Security” (SfP 984842). A.M. Ferraria also thanks the Fundação para a Ciência e a Tecnologia FCT for UID/NAN/50024/2013 project and grant SFRH/BPD/108338/2015.
Abbreviation List
- AFM
atomic force microscopy
- CWAs
chemical warfare agents
- DMMP
dimethyl-methylphosphonate
- FE-SEM
field-emission scanning electron microscopy
- FWHM
full width at half maximum
- GSDR
ground state diffuse reflectance absorption spectroscopy
- LIF
laser induced fluorescence
- LIL
laser induced luminescence
- LIP
laser induced phosphorescence
- LSPR
localized surface plasmon resonance
- MONPs
metal oxide nanoparticles
- NPs
nanoparticles
- RB
remazol brilliant blue R
- TBT or Ti(OBu)4
titanium tetrabutoxide
- XPS
X-ray photoelectron spectroscopy
References
[1] Chauhan S., Chauhan S., D’Cruz R., Faruqi S., Singh K.K., Varma S., Singh M., Karthik V., Chemical warfare agents, Environ. Toxicol. Pharmacol., 2008, 26, 113-122.10.1016/j.etap.2008.03.003Search in Google Scholar PubMed
[2] Lang W., Gehr W., La Convention européenne sur les armes chimiques et le droit international, Ann. Français de Droit Int., 1992, 38, 136-151.10.3406/afdi.1992.3067Search in Google Scholar
[3] Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on their Destruction - Disarmament, Chapter XXVI, Geneva, 3 September 1992, United Nations Treaty Collection, https://treaties.un.org/doc/Treaties/1997/04/19970429%2007-52%20PM/CTC-XXVI_03_ocred.pdfSearch in Google Scholar
[4] Üzümcü A., Working Together for a World Free of Chemical Weapons and Beyond, Nobel Peace Prize Lecture 2013, https://www.opcw.org/fileadmin/OPCW/Nobel/OPCW_Nobel_Lecture.p dfSearch in Google Scholar
[5] Schreuder-Gibson H.L., Truong Q., Walker J.E., Owens J.R., Wander J.D., Jones W.E., Chemical and Biological Protection and Detection in Fabrics for Protective Clothing, MRS Bull., 2003, 28, 574-578.10.1557/mrs2003.168Search in Google Scholar
[6] Bhati S., Mahur J., Dixit S., Chaubey O.N., Study on effect of chemical impregnation on the surface and porous characteristics of activated carbon fabric prepared from viscose rayon, Carbon Lett., 2014, 15, 45-49.10.5714/CL.2014.15.1.045Search in Google Scholar
[7] Rahman Bhuiyan M.A., Wang L., Shaid A., Shanks R.A., Ding J., Advances and applications of chemical protective clothing system (a review), J. Ind. Text., 2019, 491, 97-138.10.1177/1528083718779426Search in Google Scholar
[8] Kumar S.G., Devi L.G., Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics, J. Phys. Chem. A, 2011, 115, 13211-13241.10.1021/jp204364aSearch in Google Scholar PubMed
[9] Byrne C., Subramanian G., Pillai S.C., Recent advances in photo-catalysis for environmental applications, J. Environ. Chem. Eng., 2018, 6, 3531-3555.10.1016/j.jece.2017.07.080Search in Google Scholar
[10] Sundarrajan S., Chandrasekaran A.R., Ramakrishna S., An Update on Nanomaterials-Based Textiles for Protection and Decontamination, J. Am. Ceram. Soc., 2010, 93, 3955-3975.10.1111/j.1551-2916.2010.04117.xSearch in Google Scholar
[11] Jang Y.J., Kim K., Tsay O.G., Atwood D.A., Churchill D.G., Update 1 of: Destruction and Detection of Chemical Warfare Agents, Chem. Rev., 2015, 115, PR1-PR76.10.1021/acs.chemrev.5b00402Search in Google Scholar PubMed
[12] Errokh A., Ferraria A.M., Conceição D.S., Vieira Ferreira L.F., Botelho do Rego A.M., Rei Vilar M., Boufi S., Controlled growth of Cu2O nanoparticles bound to cotton fibres, Carbohydr. Polym., 2016, 141, 229-237.10.1016/j.carbpol.2016.01.019Search in Google Scholar PubMed
[13] Anpo M., Takeuchi M., The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation, J. Catal., 2003, 216, 505-516.10.1016/S0021-9517(02)00104-5Search in Google Scholar
[14] Uddin M.J., Cesano F., Bonino F., Bordiga S., Spoto G., Scarano D., Zecchina A., Photoactive TiO2 films on cellulose fibers: synthesis and characterization, J. Photochem. Photobiol: Chem., 2007, 189, 286-294.10.1016/j.jphotochem.2007.02.015Search in Google Scholar
[15] Hu R., Zhao Z., Zhou J., Fan T., Liu Y., Zhao T., Lu M., Ultrasound assisted surface micro-dissolution to embed nano TiO2 on cotton fabrics in ZnCl2 aqueous solution, Ultrason. Sonochem., 2019, 56, 160-166.10.1016/j.ultsonch.2019.04.006Search in Google Scholar PubMed
[16] Yu J., Pang Z., Zheng C., Zhou T., Zhang J., Zhou H., Wei Q., Cotton fabric finished by PANI/TiO2 with multifunctions of conductivity, anti-ultraviolet and photocatalysis activity, Appl. Surf. Sci., 2019, 470, 84-90.10.1016/j.apsusc.2018.11.112Search in Google Scholar
[17] Komeily-Nia Z., Montazer M., Heidarian P., Nasri-Nasrabadi B., Smart photoactive soft materials for environmental cleaning and energy production through incorporation of nanophotocatalyst on polymers and textiles, Polym. Adv. Technol., 2019, 30, 235-253.10.1002/pat.4480Search in Google Scholar
[18] Noman M.T., Ashraf M.A., Jamshaid H., Ali A., A Novel Green Stabilization of TiO2 Nanoparticles onto Cotton, Fiber. Polym., 2018, 19, 2268-2277.10.1007/s12221-018-8693-ySearch in Google Scholar
[19] Wei M., Wang S., Sun C., Zhou C., Ni J., Modification and Characterization of Nano-TiO2 for Efficient Fixation on Cotton Fibers, Fiber. Polym., 2018, 19, 2278-2283.10.1007/s12221-018-8594-0Search in Google Scholar
[20] Tao J., Batzill M., Role of Surface Structure on the Charge Trapping in TiO2 Photocatalysts, J. Phys. Chem. Lett., 2010, 21, 3200-3206.10.1021/jz1013246Search in Google Scholar
[21] Chiesa M., Paganini M.C., Livraghi S., Giamello E., Charge trapping in TiO2 polymorphs as seen by Electron Paramagnetic Resonance spectroscopy, Phys. Chem. Chem. Phys., 2013, 15, 9435-9447.10.1039/c3cp50658dSearch in Google Scholar PubMed
[22] Pillai S.C., Periyat P., George R., McCormack M.K., Hayden H., Colreavy J., Corr D., Hinder S.J., Synthesis of High-Temperature Stable Anatase TiO2 Photocatalyst, J. Phys. Chem. C, 2007, 111, 1605-1611.10.1021/jp065933hSearch in Google Scholar
[23] Wetchakun N., Phanichphant S., Effect of temperature on the degree of anatase-rutile transformation in titanium dioxide nanoparticles synthesized by the modified sol-gel method, Curr. Appl. Phys., 2008, 8, 343-346.10.1016/j.cap.2007.10.028Search in Google Scholar
[24] Abid M., Bouattour S., Conceição D.S., Ferraria A.M., Vieira Ferreira L.F., Botelho do Rego A.M., Rei Vilar M., Boufi S., Hybrid cotton-anatase prepared under mild conditions with high photo-catalytic activity under sunlight, RSC Adv., 2016, 6, 58957-58969.10.1039/C6RA10806GSearch in Google Scholar
[25] Luttrell T., Halpegamage S., Tao J., Kramer A., Sutter E., Batzilla M., Why is anatase a better photocatalyst than rutile? - Model studies on epitaxial TiO2 films, Sci. Rep., 2014, 4, 4043.10.1038/srep04043Search in Google Scholar PubMed PubMed Central
[26] Huang F., Yan A., Zhao H., Influences of Doping on Photocatalytic Properties of TiO2 Photocatalyst, In: Semiconductor Photocatalysis, Materials, Mechanisms and Applications, Cao W., (Ed.), Intech Open, 2016.10.5772/63234Search in Google Scholar
[27] Hamdi A., Ferreira D.P., Ferraria A.M., Conceição D.S., Vieira Ferreira L.F., Carapeto A.P., Boufi S., Bouattour S., Botelho do Rego A.M., TiO2-CdS Nanocomposites: Effect of CdS Oxidation on the Photocatalytic Activity, J. Nanomater., 2016, 6581691.10.1155/2016/6581691Search in Google Scholar
[28] Pelaez M., Nolan N.T., Pillai S.C., Seery M.K., Falaras P., Kontos A.G., Dunlop P.S.M., Hamilton J.W.J., Byrne J.A., O’Shea K., Entezari M.H., Dionysiou D.D., A review on the visible light active titanium dioxide photocatalysts for environmental applications, Appl. Catal. B, 2012, 125, 331-349.10.1016/j.apcatb.2012.05.036Search in Google Scholar
[29] Nyamukamba P., Mungondori H.H., Tichagwa L., Petrik L., Okoh O., The Effect of Ag Nanoparticles of Varying Morphology on the Photocatalytic Activity of Ag/TiO2 Nanocomposites, SF J. Nanochem. Nanotechnol., 2018, 1, 1009.Search in Google Scholar
[30] Abid A., Bouattour S., Ferraria A.M., Conceição D., Carapeto A.P., Vieira Ferreira L.F., Botelho do Rego A.M., Chehimi M.M., Rei Vilar M., Boufi S., Facile functionalization of cotton with nanostructured silver/titania for visible-light plasmonic photocatalysis, J. Colloid Interface Sci., 2017, 507, 83-94.10.1016/j.jcis.2017.07.109Search in Google Scholar PubMed
[31] Aliev S.A., Nikolaev N.E., Trofimov N.S., Chekhlov T.K., Properties of TiO2 films with gold nanoparticles, J. Phys. Conf. Series, 2016, 737, 012036.10.1088/1742-6596/737/1/012036Search in Google Scholar
[32] Abid M., Bouattour S., Ferraria A.M., Conceição D.S., Carapeto A.P., Vieira Ferreira L.F., Botelho do Rego A.M., Rei Vilar M., Boufi S., Functionalization of cotton fabrics with plasmonic photo-active nanostructured Au-TiO2 layer, Carbohydr. Polym., 2017, 176, 336-344.10.1016/j.carbpol.2017.08.090Search in Google Scholar PubMed
[33] Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740-2779.10.1039/C1CS15237HSearch in Google Scholar PubMed PubMed Central
[34] Reddy N.L., Rao V.N., Vijayakumar M., Santhosh R., Anandan S., Karthik M., Shankar M.V., Reddy K.R., Shetti N.P., Nadagouda M.N., Aminabhavi T.M., A review on frontiers in plasmonic nano-photocatalysts for hydrogen production, Int. J. Hydrogen Energy, 2019, 44, 10453-10472.10.1016/j.ijhydene.2019.02.120Search in Google Scholar
[35] Boufi S., Abid M., Bouattour S., Ferraria A.M., Conceição D.S., Vieira Ferreira L.F., Corbel G., Neto P.M., Lopes P.A., Rei Vilar M., Botelho do Rego A.M., Cotton Functionalized with Heterostructured TiO2-Ag-AgBr Layer for Solar Photocatalytic Degradation of Dyes and Toxic Organophosphates, Int. J. Bio. Macromol., 2019, 128, 902-910.10.1016/j.ijbiomac.2019.01.218Search in Google Scholar PubMed
[36] Tsapekos P., Alvarado-Morales M., Boscaro D., Mazarji M., Sartori L., Angelidaki I., TiO2-AgCl Based Nanoparticles for Photocatalytic Production of Phenolic Compounds from Lignocellulosic Residues, Energy Fuels, 2018, 32, 6813-6822.10.1021/acs.energyfuels.8b00572Search in Google Scholar
[37] Zhang X., Sui H., X. Su W.H., Cheng W., Wang X., Zhao B., Charge transfer process at the Ag/MPH/TiO2 interface by SERS: alignment of the Fermi level, Phys. Chem. Chem. Phys., 2016, 18, 30053-30060.10.1039/C6CP04370DSearch in Google Scholar PubMed
[38] Leong KH, Aziz AA, Sim LC, Saravanan P, Jang M, Bahnemann D. Mechanistic insights into plasmonic photocatalysts in utilizing visible light, Beilstein J. Nanotechnol., 2018, 9, 628-648.10.3762/bjnano.9.59Search in Google Scholar PubMed PubMed Central
[39] Ohsaka T., Izumi F., Fujiki Y., Raman spectrum of anatase, TiO2 J. Raman Spectrosc., 1978, 7, 321-324.10.1002/jrs.1250070606Search in Google Scholar
[40] Briggs D., Grant J.T. (Eds), Surface analysis by Auger and x-ray photoelectron Spectroscopy, IMPublications, Chichester, UK, 2003, 900, ISBN 1-901019-04-7.Search in Google Scholar
[41] Ferraria A.M., Carapeto A.P., Botelho do Rego A.M., X-ray photo-electron spectroscopy: silver salts revisited, Vacuum, 2012, 86, 1988-1991.10.1016/j.vacuum.2012.05.031Search in Google Scholar
[42] Thomas S., Harshita B.S.P., Mishra P., Talegaonkar S., Ceramic nanoparticles: fabrication methods and applications in drug delivery, Curr. Pharm. Des., 2015, 21, 6165-6188.10.2174/1381612821666151027153246Search in Google Scholar PubMed
[43] Penon O., Marín M.J., Amabilino D.B., Russell D.A., Pérez-García L., Iron oxide nanoparticles functionalized with novel hydrophobic and hydrophilic porphyrins as potential agents for photodynamic therapy, J. Colloid Interface Sci., 2016, 462, 154-165.10.1016/j.jcis.2015.09.060Search in Google Scholar PubMed
[44] Jbeli A., Hamden Z., Bouattour S., Ferraria A.M., Conceição D.S., Vieira Ferreira L.F., Chehimi M.M., Botelho do Rego A.M., Rei Vilar M., Boufi S., Chitosan-Ag-TiO2 films: An effective photocatalyst under visible light, Carbohydr. Polym., 2018, 19, 31-40.10.1016/j.carbpol.2018.06.122Search in Google Scholar PubMed
[45] Vieira Ferreira L.F., Ferreira Machado I.L., Surface photochemistry: Organic molecules within nanocavities of Calixarenes, Curr. Drug Discov. Technol., 2007, 4, 229-245.10.2174/157016307783220521Search in Google Scholar PubMed
[46] ZhangW.F., He Y.L., Zhang M.S., Yin Z., Chen Q., Raman scattering study on anatase TiO2 nanocrystals, J. Phys. D: Appl. Phys., 2000, 33, 912-916.10.1088/0022-3727/33/8/305Search in Google Scholar
[47] Zhang X., Chen Y.L., Liu R.S., Tsai D., Plasmonic Photocatalysis, Rep. Prog. Phys., 2013, 76, 046401.10.1088/0034-4885/76/4/046401Search in Google Scholar PubMed
© 2019 S. Boufi et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Research Articles
- Investigation of rare earth upconversion fluorescent nanoparticles in biomedical field
- Carbon Nanotubes Coated Paper as Current Collectors for Secondary Li-ion Batteries
- Insight into the working wavelength of hotspot effects generated by popular nanostructures
- Novel Lead-free biocompatible piezoelectric Hydroxyapatite (HA) – BCZT (Ba0.85Ca0.15Zr0.1Ti0.9O3) nanocrystal composites for bone regeneration
- Effect of defects on the motion of carbon nanotube thermal actuator
- Dynamic mechanical behavior of nano-ZnO reinforced dental composite
- Fabrication of Ag Np-coated wetlace nonwoven fabric based on amino-terminated hyperbranched polymer
- Fractal analysis of pore structures in graphene oxide-carbon nanotube based cementitious pastes under different ultrasonication
- Effect of PVA fiber on durability of cementitious composite containing nano-SiO2
- Cr effects on the electrical contact properties of the Al2O3-Cu/15W composites
- Experimental evaluation of self-expandable metallic tracheobronchial stents
- Experimental study on the existence of nano-scale pores and the evolution of organic matter in organic-rich shale
- Mechanical characterizations of braided composite stents made of helical polyethylene terephthalate strips and NiTi wires
- Mechanical properties of boron nitride sheet with randomly distributed vacancy defects
- Fabrication, mechanical properties and failure mechanism of random and aligned nanofiber membrane with different parameters
- Micro- structure and rheological properties of graphene oxide rubber asphalt
- First-principles calculations of mechanical and thermodynamic properties of tungsten-based alloy
- Adsorption performance of hydrophobic/hydrophilic silica aerogel for low concentration organic pollutant in aqueous solution
- Preparation of spherical aminopropyl-functionalized MCM-41 and its application in removal of Pb(II) ion from aqueous solution
- Electrical conductivity anisotropy of copper matrix composites reinforced with SiC whiskers
- Miniature on-fiber extrinsic Fabry-Perot interferometric vibration sensors based on micro-cantilever beam
- Electric-field assisted growth and mechanical bactericidal performance of ZnO nanoarrays with gradient morphologies
- Flexural behavior and mechanical model of aluminum alloy mortise-and-tenon T-joints for electric vehicle
- Synthesis of nano zirconium oxide and its application in dentistry
- Surface modification of nano-sized carbon black for reinforcement of rubber
- Temperature-dependent negative Poisson’s ratio of monolayer graphene: Prediction from molecular dynamics simulations
- Finite element nonlinear transient modelling of carbon nanotubes reinforced fiber/polymer composite spherical shells with a cutout
- Preparation of low-permittivity K2O–B2O3–SiO2–Al2O3 composites without the addition of glass
- Large amplitude vibration of doubly curved FG-GRC laminated panels in thermal environments
- Enhanced flexural properties of aramid fiber/epoxy composites by graphene oxide
- Correlation between electrochemical performance degradation and catalyst structural parameters on polymer electrolyte membrane fuel cell
- Materials characterization of advanced fillers for composites engineering applications
- Humic acid assisted stabilization of dispersed single-walled carbon nanotubes in cementitious composites
- Test on axial compression performance of nano-silica concrete-filled angle steel reinforced GFRP tubular column
- Multi-scale modeling of the lamellar unit of arterial media
- The multiscale enhancement of mechanical properties of 3D MWK composites via poly(oxypropylene) diamines and GO nanoparticles
- Mechanical properties of circular nano-silica concrete filled stainless steel tube stub columns after being exposed to freezing and thawing
- Arc erosion behavior of TiB2/Cu composites with single-scale and dual-scale TiB2 particles
- Yb3+-containing chitosan hydrogels induce B-16 melanoma cell anoikis via a Fak-dependent pathway
- Template-free synthesis of Se-nanorods-rGO nanocomposite for application in supercapacitors
- Effect of graphene oxide on chloride penetration resistance of recycled concrete
- Bending resistance of PVA fiber reinforced cementitious composites containing nano-SiO2
- Review Articles
- Recent development of Supercapacitor Electrode Based on Carbon Materials
- Mechanical contribution of vascular smooth muscle cells in the tunica media of artery
- Applications of polymer-based nanoparticles in vaccine field
- Toxicity of metallic nanoparticles in the central nervous system
- Parameter control and concentration analysis of graphene colloids prepared by electric spark discharge method
- A critique on multi-jet electrospinning: State of the art and future outlook
- Electrospun cellulose acetate nanofibers and Au@AgNPs for antimicrobial activity - A mini review
- Recent progress in supercapacitors based on the advanced carbon electrodes
- Recent progress in shape memory polymer composites: methods, properties, applications and prospects
- In situ capabilities of Small Angle X-ray Scattering
- Review of nano-phase effects in high strength and conductivity copper alloys
- Progress and challenges in p-type oxide-based thin film transistors
- Advanced materials for flexible solar cell applications
- Phenylboronic acid-decorated polymeric nanomaterials for advanced bio-application
- The effect of nano-SiO2 on concrete properties: a review
- A brief review for fluorinated carbon: synthesis, properties and applications
- A review on the mechanical properties for thin film and block structure characterised by using nanoscratch test
- Cotton fibres functionalized with plasmonic nanoparticles to promote the destruction of harmful molecules: an overview
Articles in the same Issue
- Research Articles
- Investigation of rare earth upconversion fluorescent nanoparticles in biomedical field
- Carbon Nanotubes Coated Paper as Current Collectors for Secondary Li-ion Batteries
- Insight into the working wavelength of hotspot effects generated by popular nanostructures
- Novel Lead-free biocompatible piezoelectric Hydroxyapatite (HA) – BCZT (Ba0.85Ca0.15Zr0.1Ti0.9O3) nanocrystal composites for bone regeneration
- Effect of defects on the motion of carbon nanotube thermal actuator
- Dynamic mechanical behavior of nano-ZnO reinforced dental composite
- Fabrication of Ag Np-coated wetlace nonwoven fabric based on amino-terminated hyperbranched polymer
- Fractal analysis of pore structures in graphene oxide-carbon nanotube based cementitious pastes under different ultrasonication
- Effect of PVA fiber on durability of cementitious composite containing nano-SiO2
- Cr effects on the electrical contact properties of the Al2O3-Cu/15W composites
- Experimental evaluation of self-expandable metallic tracheobronchial stents
- Experimental study on the existence of nano-scale pores and the evolution of organic matter in organic-rich shale
- Mechanical characterizations of braided composite stents made of helical polyethylene terephthalate strips and NiTi wires
- Mechanical properties of boron nitride sheet with randomly distributed vacancy defects
- Fabrication, mechanical properties and failure mechanism of random and aligned nanofiber membrane with different parameters
- Micro- structure and rheological properties of graphene oxide rubber asphalt
- First-principles calculations of mechanical and thermodynamic properties of tungsten-based alloy
- Adsorption performance of hydrophobic/hydrophilic silica aerogel for low concentration organic pollutant in aqueous solution
- Preparation of spherical aminopropyl-functionalized MCM-41 and its application in removal of Pb(II) ion from aqueous solution
- Electrical conductivity anisotropy of copper matrix composites reinforced with SiC whiskers
- Miniature on-fiber extrinsic Fabry-Perot interferometric vibration sensors based on micro-cantilever beam
- Electric-field assisted growth and mechanical bactericidal performance of ZnO nanoarrays with gradient morphologies
- Flexural behavior and mechanical model of aluminum alloy mortise-and-tenon T-joints for electric vehicle
- Synthesis of nano zirconium oxide and its application in dentistry
- Surface modification of nano-sized carbon black for reinforcement of rubber
- Temperature-dependent negative Poisson’s ratio of monolayer graphene: Prediction from molecular dynamics simulations
- Finite element nonlinear transient modelling of carbon nanotubes reinforced fiber/polymer composite spherical shells with a cutout
- Preparation of low-permittivity K2O–B2O3–SiO2–Al2O3 composites without the addition of glass
- Large amplitude vibration of doubly curved FG-GRC laminated panels in thermal environments
- Enhanced flexural properties of aramid fiber/epoxy composites by graphene oxide
- Correlation between electrochemical performance degradation and catalyst structural parameters on polymer electrolyte membrane fuel cell
- Materials characterization of advanced fillers for composites engineering applications
- Humic acid assisted stabilization of dispersed single-walled carbon nanotubes in cementitious composites
- Test on axial compression performance of nano-silica concrete-filled angle steel reinforced GFRP tubular column
- Multi-scale modeling of the lamellar unit of arterial media
- The multiscale enhancement of mechanical properties of 3D MWK composites via poly(oxypropylene) diamines and GO nanoparticles
- Mechanical properties of circular nano-silica concrete filled stainless steel tube stub columns after being exposed to freezing and thawing
- Arc erosion behavior of TiB2/Cu composites with single-scale and dual-scale TiB2 particles
- Yb3+-containing chitosan hydrogels induce B-16 melanoma cell anoikis via a Fak-dependent pathway
- Template-free synthesis of Se-nanorods-rGO nanocomposite for application in supercapacitors
- Effect of graphene oxide on chloride penetration resistance of recycled concrete
- Bending resistance of PVA fiber reinforced cementitious composites containing nano-SiO2
- Review Articles
- Recent development of Supercapacitor Electrode Based on Carbon Materials
- Mechanical contribution of vascular smooth muscle cells in the tunica media of artery
- Applications of polymer-based nanoparticles in vaccine field
- Toxicity of metallic nanoparticles in the central nervous system
- Parameter control and concentration analysis of graphene colloids prepared by electric spark discharge method
- A critique on multi-jet electrospinning: State of the art and future outlook
- Electrospun cellulose acetate nanofibers and Au@AgNPs for antimicrobial activity - A mini review
- Recent progress in supercapacitors based on the advanced carbon electrodes
- Recent progress in shape memory polymer composites: methods, properties, applications and prospects
- In situ capabilities of Small Angle X-ray Scattering
- Review of nano-phase effects in high strength and conductivity copper alloys
- Progress and challenges in p-type oxide-based thin film transistors
- Advanced materials for flexible solar cell applications
- Phenylboronic acid-decorated polymeric nanomaterials for advanced bio-application
- The effect of nano-SiO2 on concrete properties: a review
- A brief review for fluorinated carbon: synthesis, properties and applications
- A review on the mechanical properties for thin film and block structure characterised by using nanoscratch test
- Cotton fibres functionalized with plasmonic nanoparticles to promote the destruction of harmful molecules: an overview

