Novel Lead-free biocompatible piezoelectric Hydroxyapatite (HA) – BCZT (Ba0.85Ca0.15Zr0.1Ti0.9O3) nanocrystal composites for bone regeneration
-
Chelli Sai Manohar
, Sai Pavan Prashanth Sadhu
Abstract
BCZT (Ba0.85Ca0.15Zr0.1Ti0.9O3) is a recent class of lead-free ferroelectric material associated with high piezoelectric coefficient, making it suitable to inspire hydroxyapatite (HA)-BCZT ceramics for bone materials. Nano-crystalline hydroxyapatite (HA) synthesized using the hydrothermal route was characterized via FT-IR, Raman spectroscopy, X-ray powder diffraction (XRD), and Scanning Electron Microscopy (SEM). We also rationalized its formation as a function of operating conditions such as dwell time and temperature in this route. The nano-crystalline BCZT powder was synthesized via a sol-gel technique and its structural and morphological characterization were carried out using Raman Spectroscopy, XRD and Transmission Electron Microscopy (TEM). These investigations facilitated the optimization of HA-BCZT compositions and their electrical poling conditions to achieve enhanced piezoelectric effect. The composites (HA-BCZT) sintered at 1350∘C exhibited promising piezoelectric properties. We report the enhanced piezoelectric coefficient (d33) of 7±1 pC/N for 50% HA-BCZT which is significant as compared to that reported in the literature for ~98% BT (barium titanate) -HA composites. We highlight the role of Simulated Body Fluid (SBF) on the intriguing phase-change of Tricalcium Phosphate (TCP) obtained at this sintering temperature, to hydroxyapatite for its essential contribution to promote bone growth. We theoretically support the confirmed in vitro biocompatibility of these composites.

Graphical abstract: Novel lead-free biocompatible piezoelectric HA-BCZT nanocrystal composites for accelerated Bone regeneration
1 Introduction
The intrinsic electrical properties associated with the human bone play a key role in bone healing/remodeling [1, 2]. Hydroxyapatite (HA) that is present roughly about 70% in the human bone [3], is the commonly used bone repair material [4, 5]. There are innumerable reports documenting the use of HA as porous scaffolds for bone substitution [6, 7]. However, the piezoelectricity associated with human bone tissue in vivo is ascribed to its composition that produces biological electricity due to the ionic displacement under a deformation force [8, 9, 10]. Since the OH groups in HA were found to be responsible for the reported piezoelectric activity, the retention of these polar groups is essential for observing spontaneous polarization [11, 12]. The available literature confirms that the reorientation of polar groups in HA leads to the cell growth in HA implants [13].
Given the restrictions for the use of PZT ceramics owing to their toxicity from the Lead, there has been a tremendous research on lead-free alternatives. Recent studies on the use of lead free HA-Barium Titanate (BT) composites have assumed prime importance in bone implants. The studies have confirmed the improved osteoblast growth owing to incorporation of piezoelectricity by the BT in the hydroxyapatite [14, 15]. However, a more promising class of lead-free ceramics has been the BCZT solid solutions that have no toxic elements [16, 17, 18, 19, 20, 21]. Further, as recorded in the Table 1, as per the prescribed standards of the EPA, USA, we note the stringent maximum containment levels permissible for the different elements. The chosen composition of (Ba0.85Ca0.15Zr0.1Ti0.9O3) makes it therefore non-toxic. Also unlike Lead that is both carcinogenic and genotoxic [22], there has been no reported carcinogenicity and genotoxicity observed from Ba, Ca, Zr and Ti, except the benign Baritosis obtained via Ba consumption [23]. BCZT (Ba0.85Ca0.15Zr0.1Ti0.9O3) is reported to possess a piezoelectric coefficient as high as 563 pC/N which is roughly three times that of BT reported to possess 190-260 pC/N [24]. Further, the electrical properties of BCZT are strongly linked to its crystal structure and microstructure that have been evaluated in detail [25, 26, 27]. It also has a well resolved morphotropic phase boundary, higher domain density and a large strain [28]. Most importantly, they have been demonstrated to be bio-compatible as a polymeric support for the osteogenic differentiation of stem cells within the bone-marrow [29]. All of these properties warrant a lead-free BCZT in the enhancement of piezoelectric activity of novel HA-BCZT composites for the bone formation in the complex physiological environment.
The elemental comparison of the toxicity factors as provided by the National Primary Drinking Water Regulation Table, EPA, USA 2010
| Metal | MCL (ppm) | Carcinogenicity | Genotoxicity |
|---|---|---|---|
| Pb | 0.015 | Yes (2B) | Yes |
| Ba | 2.00 | No (Baritosis - benign) | No |
| Ca | None | No | No |
| Zr | 25.0 | No | No |
| Ti | 0.10 | No | No |
MCL: Maximum Containment Levels
Therefore, to mimic the bone’s inherent electrical properties, various HA-BCZT nano-piezocomposites were fabricated in the ratios of 10, 20, 30, 40 and 50% by weight BCZT. The nano crystallinity plays a key role in controlling the desired properties of the material. For, the morphological effects on the surface and the internal volume of the crystallites are relatively easy to form and stabilize in this phase [14]. Therefore, to achieve the maximum piezoelectricity in these composites with the desired microstructure, the sintering and the poling conditions were optimized. Further, the temperature profile of HA and the role of simulated body fluid in its phase transformation were evaluated. We also verified their potential in bone regeneration by determining the biocompatibility through in vitro studies and provided a theoretical rationale for the observations.
The details of the systematic studies carried out on various aspects of the composites under investigation are elucidated in the following sections.
2 Materials and methods
2.1 Preparation of hydroxyapatite at nanoscale
The synthesis of nanostructured hydroxyapatite was carried out by the hydrothermal method similar to that reported by Li et al. [30]. The following chemical reagents with analytical purity were dissolved in 45 ml of deionized water (DI): 0.60 g of L-glutamic acid (Sigma), 0.38 g of dibasic sodium phosphate (Na2HPO4·2H2O), 0.07 g of sodium hydroxide (NaOH), 0.05–0.15 g of urea and 0.28 g of calcium nitrate tetrahydrate [Ca(NO3)2·4H2O]. The solution was then sealed and subjected to hydrothermal process at 160∘C in a 50 ml Teflon container with a dwell time of 24 h which yielded a colorless precipitate. Thus obtained precipitate was filtered and dried for 24 h in a hot air oven at 80∘C and thereafter characterized using XRD, FT-IR, Raman Spectroscopy and SEM. Further, to evaluate the role of temperature and dwell time in the formation of HA, we studied these reaction conditions for the appropriate modification in the aforementioned method prescribed by Li et al. The effect of temperature was determined by heating the individual reactant mixtures separately at 100, 120, 140 and 160∘C keeping the dwell time constant at 24 h. Subsequently, the effect of dwell time was studied by heating the reaction mixture in the furnace for 4, 8, 16, and 24 h maintaining the temperature constant at 160∘C.
2.2 Synthesis of nano-crystalline BCZT powder
The nano-crystalline BCZT was synthesized using the citrate based sol-gel technique [20, 21]. To begin with, titanium isopropoxide [Ti[OCH(CH3)2]4, Sigma Aldrich], (6 ml) was stirred in acetyl acetone [Merck Chemicals] (3 ml) for 10 mins to which a solution of citric acid (CA) [Sigma] (2.62 g) dissolved in DI water was added with stirring for 30 minutes to form solution ‘a’. Calculated quantities of barium nitrate (3.314 g) [Ba(NO3)2,Merck], calcium nitrate (0.519 g) [Ca(NO3)2·4H2O,Merck], zirconium nitrate (0.345 g) [ZrO(NO3)2H2O, Merck], and citric acid (1.5 g) were dis-solved in DI water separately and stirred for 10 mins to obtain solution ‘b.’ The mixture of solutions ‘a’ and ‘b’ was stirred for 30 mins at room temperature under a pH of ~7-8 maintained by adding ammonia solution with constant stirring. It was heated to form a yellow viscous gel at 150∘C and raised to 200∘C till the gel turned into a dark brownish residue which was ground with mortar and pestle to homogenize the fine powder. To remove the organic sub-stituent, the as-prepared powder was calcined at 450∘C for 5 h. After grinding, this powder was further heat-treated at 850∘C for 5 h to obtain the BCZT powder. This fine powder was structurally and morphologically characterized using XRD and TEM.
2.3 Fabrication of HA-BCZT composites
The mixture of hydroxyapatite and BCZT powders in different % by weight were ground using agate mortar and pestle as tabulated (Table 3). The mixed powder was pressed into discs in a 15 mm die at 25 MPa for 2 mins. Discs were also produced from 100% hydroxyapatite powder as a control for the study. The discs of HA-BCZT composites were sintered at temperatures ranging from 800-1350∘C for 2 h whereas the pristine HA and BCZT discs were sintered separately at 800∘C and 1400∘C for 2 h respectively. These sintered discs were poled at the electric fields between 4-16 kV cm−1 at 95∘C for a duration of 60 mins [3], to yield the optimum piezoelectric activity.
The comparison of the crystallinity and phase purity of hydroxyapatite, the shift in the various Raman Harmonic modes, and the EDX elemental composition for the different synthetic conditions using hydrothermal route
| Sample | Scores | Alternative | CS | Harmonic Modes (PO34−) | Elemental Ratio | ||||
|---|---|---|---|---|---|---|---|---|---|
| (H:A) | Phase | (nm) | ν1 | ν2 | ν4 | P | Ca | Ca:P | |
| HT_4 | 80:59 | Monetite | 34.57 | 430 | 590 | 961 | 36 | 38 | 1.05 |
| HT_8 | 67:61 | Monetite | 55.31 | 424 | 598 | 960 | 34 | 31 | 0.91 |
| HT_16 | 76:42 | OBTP | 47.93 | 430 | 589 | 960 | 29 | 25 | 0.86 |
| HT_24 | 75:23 | Apatite | 21.27 | 429 | 589 | 960 | 32 | 48 | 1.50 |
| HT_100 | 27:75 | Monetite | 45.92 | 423 | 594 | 989 | 28 | 18 | 0.64 |
| HT_120 | 33:77 | Monetite | 55.11 | 423 | 594 | 989 | 28 | 21 | 0.75 |
| HT_140 | 62:61 | Monetite | 34.57 | 432 | 594 | 965 | 30 | 29 | 0.97 |
| HT_160 | 71:15 | Monetite | 21.27 | 430 | 590 | 961 | 32 | 48 | 1.50 |
*HT: Hydrothermal; H: Hydroxyapatite; A: Alternate Phase, CS: Crystallite Size
The different ratios of the HA-BCZT nanocomposites prepared with their various sintering conditions with the corresponding d33 values obtained
| Ratio | Sintering | Actual Amounts | d33 | |
|---|---|---|---|---|
| (HA-BCZT) | (∘C) | HA(mg) | BCZT(mg) | (pC/N) |
| 0:100 | 1400 | 0 | 500 | 304 |
| 100:0 | 800 | 500 | 0 | - |
| 90:10 | 800 | 450 | 50 | - |
| 80:20 | 800 | 400 | 100 | - |
| 70:30 | 800 | 350 | 150 | - |
| 60:40 | 800 | 300 | 200 | - |
| 50:50 | 1350 | 250 | 250 | 7 |
2.4 Characterization of materials
X-ray powder diffraction was undertaken using the Pananalytical, X-Pert-3 diffractometer at a scan speed 2∘/min with Cu-Kα radiation (λ = 1.54 Å) in the 2θ range of 10∘ to 80∘. The crystallite size (Xc) was determined using the Scherrer equation. FT-IR spectroscopy was performed to identify the functional groups using Thermofischer Nicolet iS10 by KBr pellet technique recorded from 4000-400 cm−1 in transmission mode. A Thermofischer DSR Raman microscope equipped with a 780 nm laser was used for Raman spectroscopic studies. The DTA/TG studies were undertaken using the Q600 V20.9 Build 20. The crystallite size and morphology of the synthesized powders and composites were assessed using SEM (JEOL JSM-IT300). The piezoelectric coefficient d33 of the poled HA-BCZT pellets was measured using an APC d33 meter.
These well characterized composites were then evaluated for their biocompatibility and in vitro phase transformations using the following studies.
2.5 Cell adhesion and proliferation studies
We determined the impact of piezoelectricity associated with HA-BCZT composites on the growth of bone cells. In this regard, cells were cultured on unpoled discs of the composite as well as on 100% HA discs as control. MC3T3 cells that are mature osteoblastic phenotypes cultured in MEM-α were used to assess the cellular response to the materials. In preparation for cell culture as carried out by Baxter et al. [33], the triplicate of composite discs made of HA-BCZT in the ratios ranging from [100:0 - 50:50] were autoclaved at 120∘C under 15 MPa and soaked in complete culture medium overnight. Discs were then allowed to dry in air for 1 h in a 24-well plate in sterile conditions before cells were seeded at 20,000 cells/cm2 in 25 μl of complete culture medium. The culture plates were incubated for 2 h at 37∘C with 5% CO2 in order to allow cells to adhere to the ceramic surface before 1 ml of complete culture medium was added to each well. Cells were then incubated for 3 days; the culture medium changed on the second day.
2.6 Cellular metabolic activity
The metabolic activity of the cells was measured using an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay kit (CGD-1). The culture medium was removed from the wells and replaced with 500 μl of serum-free medium. After adding 50 μl of MTT solution to each well, the culture plates were incubated at 37∘C with 5% CO2 for 3 h. The medium was then removed and 200 μl of MTT solvent (DMSO) was added to each well, ensuring all visible crystals formed were dissolved. These solvents were then transferred to a fresh 24-well plate and a colorimetric measurement was carried out using Spectra Max M5 fluorescence spectrometer at 570 nm with a 630 nm reference wavelength.
2.7 Simulated body fluid immersion
To determine the role of body fluids on the phase transformations of HA, we undertook this following study. The HA powder that was calcined at 1300∘C for 3h was soaked for different durations in a solution of FBS Fetal Bovine Serum as a natural substitute for SBF [34]. The sample, soaked for 14 days, was taken out of the FBS, centrifuged and washed with acetone and distilled water. Samples were dried overnight in an oven at 120∘C and the phase transformation in the sample morphology was analysed by FT-IR.
2.8 Computational Simulations
2.8.1 Quantum Espresso
The hexagonal bravais-lattice of the HA crystal was built and energy optimized to its least energy conformation over 1000 iterations with the PAW pseudopotential in Quantum espresso [35]. The system was optimized for 144 kohn-sham states with a kinetic energy cut-off at 30.0 Ry and charge density cut-off at 120.0 Ry through 8 iterations of plain mixing to yield the least energy conformation. The corresponding electron density structure was then visualized in the VESTA software for the different symmetric planes.
2.8.2 Molecular docking Studies
The various proteins that are expressed in the bone formation [36], were extracted from the protein data bank [37], and suitably cleansed of their heteroatoms and solvent. The Heinz team generates the all-atom Interface Force Fields (IFF) for the simulation of nanostructures with high accuracy, inclusive of metals, minerals, and oxides. The 001HAcrystal lattice was obtained from their ready-to-use model of the HA unit cell with the corresponding cleavage plane. These were suitably docked using HEX 8.0.0 software where the algorithm was set to an output search for the top 100 energy cluster poses, with Shape only correlation type, 3D Fast Fourier Transform mode, Grid dimension of 0.6, a Distance range of 40, and the translation step of 0.8 [38].
3 Results and Discussion
3.1 Characterization of HA and BCZT
3.1.1 FT-IR spectroscopy
The primary peaks signifying the formation of HA are observed in the IR spectra (Supplementary data, Figure S1). As recorded in Figure S1(c), we confirm the phosphate peaks, the asymmetric P-O stretching and the presence of the key OH group. We note the primary peaks of 3423 and 1636 cm−1 for the adsorbed water and 3567 cm−1 corresponding to the stretching vibration of lattice OH- ions. We also note the 668 cm−1 OH deformation mode with a minor deviation from the expected 633 cm−1 that could be attributed to the defects in the crystal formation. The different PO34− peaks are observed at 472, 562, 603, 961, 1036, and 1091 cm−1.We note the key 961cm−1 peak corresponding to the asymmetric P-O stretching and the triple degenerate bending vibrations of phosphate in 603, 562 and 472 cm−1. This data are accordingly tabulated (Supplementary data, Table ST1).
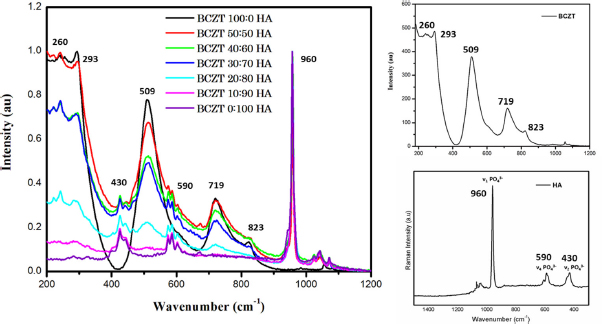
Raman spectroscopy of the HA-BCZT composites of different rations, the Raman spectra of the pristine BCZT and Hydroxyapatite with their active Raman modes
Further, we also employed the IR spectroscopy for studying the formation of HA as a function of the temperature and dwell time in the hydrothermal route. Figure S1(a) in the supplementary data captures the IR spectra obtained for the effect of dwell time, while the role of temperature is plotted in supplementary data, Figure S1(b). The observed formation of the crucial peak at 3567 cm−1 corresponding to the stretching vibration of lattice OH leads to the optimization of the reaction conditions namely, the temperature at 160∘C and dwell time of 24h in the hydrothermal treatment.
3.1.2 Raman spectroscopy
As observed in Figure 1, we obtain the Raman fingerprint reported for the pristine hydroxyapatite sample [39]. We observe the strong active ν1 Raman mode of PO3− at 960 4 cm−1 along with the weak ν2 Raman phosphate modes at 431 and 449 cm−1, and ν4 Raman active phosphate modes at 591 and 609 cm−1. To confirm the structure of BCZT, we note the Raman active A1g octahedral mode at 823 cm−1
alluding to the asymmetric Zr-Ti lattice in the synthesized nano-crystalline BCZT. Further, we note the presence of the sharp band corresponding to the B1/E (TO3 + LO2) modes at 293 cm−1, the strong intense A1 (TO3) mode at 509 cm−1, and the intense asymmetric band consisting of the A1(LO3)/E(LO4) modes at 719 cm−1. The confirmation of the cubic and tetragonal phases are reflected in the presence of the peaks observed at 509 cm−1, and 293 and 719 cm−1 respectively. The cluster of peaks in the 180–300 cm−1 range is attributed to the asymmetric phonon vibrations in the Ti–O bonds, while the observed peaks around 509 and 719 cm−1 reflect the Ba-O phonon vibrations [40]. Further, the normalized Raman spectra of the composites depicted in the Figure 1 demonstrate the increasing intensity of the BCZT peaks in the corresponding HA-BCZT composites with the growing overlap of the peaks corresponding to the individual samples. Thus, Raman spectroscopy affirmatively confirms the formation of the composites.
Further, the effect of the synthesis conditions on the formation of these modes is also monitored using Raman spectroscopy. Table 2 provides the corresponding data pertaining to the Raman spectra (Supplementary data, Figure S2). Here we note that the weak ‘ν2’ Raman phosphate mode is almost uniform across all the conditions and evolves into the signature peak at the optimal conditions of 24 h in 160∘C.

SEM images and the corresponding EDX report of the composition of the Nano-crystalline hydroxyapatite for the various dwell times in hydrothermal synthesis at 10 μm resolution
3.1.3 Morphology
Figure 2 provides the snapshot of the growth of the nano-crystalline hydroxyapatite with the dwell time in the hydrothermal route where the temperature is maintained constant at 160∘C, while the growth with respect to temperature keeping dwell time constant at 24 h is captured in the supplementary data (Figure S2). We observe the formation of sheets at the beginning that slowly morph into the rods, flowers and a combination of both. However, as recorded in Table 2, we have the comparison of the EDX data as obtained for the different hydrothermal synthetic routes where we observe that the sample prepared at 160∘C for the dwell time of 24 h yielded the closest desirable Ca:P ratio (1.5). We thus note the important role of dwell time and temperature in the formation of the nano-crystalline HA.
To rationalize these Ca:P ratios, the SEM images of the nano-crystalline hydroxyapatite synthesized under different reaction conditions were recorded (Supplementary data, Figure S3-S5). It can be reasoned that the Ca:P ratio plays a crucial role in the resulting morphological features of the composites. This is aptly supported by the recorded EDX for the two kinds of the structural morphologies evident in the structures – namely the flower and the rod shapes. Here we note that the local domains as circled (Supplementary data, Figure S4-S5) have unique ratios dependent on the rod and the flower morphologies. In general, it is evident that the rod morphology has more of Ca packed rather than the elemental composition of P.
3.1.4 X-ray powder diffraction studies
The X-ray powder diffraction studies were used to probe the phase purity of the BCZT nano-crystalline powder. The XRD pattern in Figure 3 confirms it to be a polycrystalline tetragonal perovskite structure with space group P4mm. No visible significant peaks of secondary phases were found. Using the Scherrer formula, we determined the crystallite size of BCZT to be close to 44nm. The X-ray powder diffraction peaks obtained for the HA sample by the hydrothermal route are significantly similar to the standard reference in JCPDS for hydroxyapatite (98-028-9993) with the major peak of (211) establishing the formation of HA (Figure 3). Bragg peaks that are encountered in the pattern could be indexed to a space group of P63. The evaluated crystallinity and phase purity of the sample demonstrated to be a hexagonal structure with no noticeable secondary phases.
For understanding the formation of the nano-crystalline hydroxyapatite, the XRD studies provide a significant picture. As recorded in Table 2, we have a detailed visualization of the formation in the crystal given the ratio
of the Hydroxyapatite phase to the alternative phase recorded at each condition.
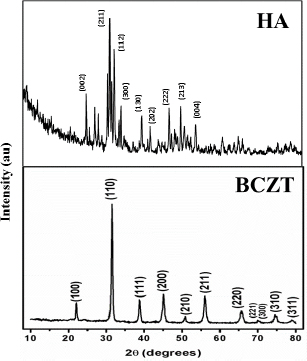
X-ray powder diffraction pattern of HA and BCZT (Ba0.85Ca0.15Zr0.1Ti0.9O3)
Firstly, we observe the formation of hydroxyapatite phase as early as 4 h itself, albeit in addition to the presence of other phases. We however note the decrease in this contamination of secondary phases to achieve the unequivocal hydroxyapatite phase relatively, with the increasing dwell time up to 24 h. Alternatively, as the function of increasing temperature in the hydrothermal synthesis, we notice the gradual growth in the hydroxyapatite phase and significant loss of the monetite phase only at the optimal temperature of 160∘C. Here we also observe that the average crystalline size varies widely with the conditions, forming the smallest nano-crystalline HA only at the optimal conditions of 24 h at 160∘C. The corresponding comparison of the various XRD patterns for the formation of the nano-crystalline HA in the different reaction conditions of hydrothermal route are captured in Figure 4.
3.1.5 Piezoelectricity in composites
While the pristine samples of HA exhibited no observable piezoelectricity, the various HA-BCZT composites were optimized for their sintering and poling conditions to maximize the piezoelectric activity as recorded in Table 3.

The comparison of the X-Ray Diffraction patterns for the formation of the nano-crystalline HA from the different reaction conditions in the hydrothermal route with respect to pure HA
The TGA curve of the synthesized nano-hydroxyapatite that was dried at 80∘C is captured in Figure 5. The net mass-loss during the heat treatment is calculated to be about 8.71%. The observed mass loss is relatively pronounced between 25-450∘C that is attributed to adsorbed water.

The normalized TG and DTA curves of the nano-hydroxyapatite powder dried at 80∘C recorded at a heating rate of 10∘C/min in air

The relative cell proliferation observed for the various HA-BCZT nanocomposites
The decomposition of carbonate into CO2 gas occurred from 600-800∘C [41]. From 800 to 1300∘C, a slight decrease in TGA curves shows the decomposition of Ca10(PO4)6 to CaO and Ca3(PO4)2 [42]. Therefore, we undertook the sintering of the HA at 800 to optimize the piezoelectricity. However, the BCZT has been reported to demonstrate the optimal density at lower crystallite size while retaining its piezoelectricity between 1300-1400∘C [43]. Therefore, on the formation of the HA-BCZT composites of different ratios, we optimized the sintering conditions in this range of 800∘C to 1400∘C. While the pristine HA exhibited no observable piezoelectricity, BCZT demonstrated a significant value of 304 pC/N when sintered at 1400∘C. We obtained a significant piezoelectric coefficient ‘d33’ for the pellet of 50 %w BCZT sintered at 1350∘C for 2 h, and poled at 10 kV for 2 h at 95∘C to yield 7±1 pCN−1. This translates into an important finding as the prior reports required ~98% BT to achieve 6.8 pCN−1 possessing minimal HA for bone incorporation [14].
3.1.6 Biocompatibility
For the use of the novel HA-BCZT nanocrystal composites as potential bone implants, we first evaluated their bio-compatibly in the growth of bone cells. In this study, we observed the minimal cell growth in the control HA sample that could be attributed to the absence of the intrinsic piezoelectric activity. However, HA-BCZT composites of different ratios demonstrated greater bone cell growth when compared to the control of the pristine HA sample as recorded in Figure 6. The cell proliferation tends to be maximum at 10% BCZT while it tapers down to 50% BCZT; a trend observed in the HA:BT composites too, that can be rationalized to their structural asymmetry in the composites that plays a key role on the piezoelectricity. Interestingly, while there is no poling to generate the desired piezoelectric phase in the material, we allude the observed increase in the bone cell proliferation to the piezoelectricity. This is attributed to the ‘inherent piezoelectric domains’ already present in the matrix. Further, as reported in the HA-BT samples, the effect of poling has no influence on the cell growth already achieved [33]. We thus demonstrate the bio-compatibility of our composites and their potential use as novel composites for accelerated bone regenerating material.

(a) TEM image of Ba0.85Ca0.15Zr0.1Ti0.9O3; (b) SEM image of cross-section of 50:50 HA-BCZT at 2 μm (c) SEM image of the 50:50 HA-BCZT surface at 1 μm with inset – EDX spectrum; (d) Elemental ratios observed in the HA-BCZT composite as determined by the EDX studies
To understand this phenomena, we examined the cross-section of the 50:50 HA-BCZT composite that yielded maximum piezoelectric coefficient using TEM and SEM as captured in Figure 7. The TEM image of the pristine BCZT is provided in the Figure 7(a), while the SEM image of the fractured surface of the HA-BCZT composite in Figure 7(b) depicts the uniform stacking of the multiple layers as a highly connected network conducive for the co-operative piezoelectric effect. Further, the SEM image in Figure 7(c) shows the indistinguishable grain boundaries of HA and BCZT packed efficiently with non-negligible porosity in the composite conducive for cell growth. The EDX studies for this composite demonstrate the composition of HA-BCZT to be 50:50 as noted in Figure 6(d).
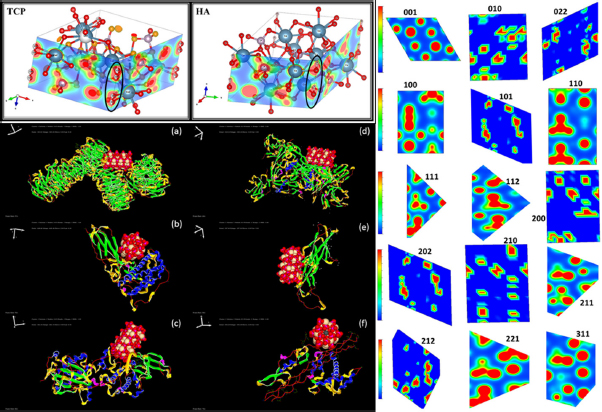
(clockwise) The electron density of the TCP versus HA, the electron density map of the different symmetric planes of the optimized HA crystal, and the best docking poses of the interaction of the HA 001 plane (ball model) with the various proteins (ribbon model) noted in the different stages of the bone cell growth
3.2 Computational Studies
For corroborating the present findings, we carried out the computational simulations. As evident from the Figure 8, we notice that the inherent symmetric electron density dipole is created across the OH in HA as against the symmetric O-O bond in TCP along the lattice edge that leads to spontaneous polarization in HA unlike TCP. This asymmetry compounded with that present in BCZT plays a key role in the consequent piezoelectricity. Therefore, when the composites were optimized for greater piezoelectricity, we observed greater bone cell proliferation. Further, to justify this observation, we simulated the electron densities of 15 different lattice planes of the least energy conformation of the HA crystal as recorded in the Figure 8 obtained by VESTA. Given that the (001) wulff surface is calculated to have the least surface energy and consequently optimal surface interaction [44], we notice its maximum polarity for interactions that were evaluated consequently with the proteins in the various stages of the cell growth.

The FT-IR spectra of the pristine TCP sample versus the soaked TCP sample in SBF after 14 days
3.3 Molecular docking studies
In this regard, we pursued docking simulations given the available literature on the expression patterns of bone related proteins in the successive stages of proliferation, bone matrix formation/maturation, and mineralization [36]. We pursued the protein ligand docking simulations using HEX 8.0.0 software with the HA and the aforementioned proteins. The key proteins expressed in the bone formation as reported, include Histone H3-H4 and Ribosomal protein S6 in the first stage, while greater expression of TGF-β1, Fibronectin and Osteonectin in later stages. The molecular docking studies demonstrate the favourable interaction of these different proteins, primarily
through the hydrogen bonding in their helical loops to the polar phosphate and hydroxyl groups present on the HA surface (Figure 8). The docking scores with respect to each of the proteins’ best docked pose is tabulated (Table 4) suggesting the impactful contribution of the scaffold in the different phases.
The binding scores obtained from the molecular docking studies for the interaction of HA 001 crystal plane with the various proteins in the different stages of the cell growth
| Protein | Hex Docking Score (au) |
|---|---|
| Ribosomal protein S6 | −402.16 |
| TGF-β1 | −528.24 |
| Fibronectin | −597.4 |
| SPARC | −593.58 |
This highlights the need for the presence of the OH groups in the HA (absent in TCP) to drive the bone cell growth and in turn validates the hypothesis of the role of piezoelectricity on bone growth.
![Figure S1 FT-IR spectra of the synthesized nano-hydroxyapatite prepared by the various conditions in the hydrothermal route: (a) Role of dwell time, (b) Effect of temperature and (c) The optimized ideal conditions as comparable to the reference reported in literature [S1]](/document/doi/10.1515/ntrev-2019-0006/asset/graphic/j_ntrev-2019-0006_fig_011.jpg)
FT-IR spectra of the synthesized nano-hydroxyapatite prepared by the various conditions in the hydrothermal route: (a) Role of dwell time, (b) Effect of temperature and (c) The optimized ideal conditions as comparable to the reference reported in literature [S1]
3.4 Effect of sintering temperature on the phase transformation of HA
Given the high sintering conditions used in the fabrication of the nanocrystal composites, we studied the chemical stability of HA under these conditions. In this regard, we pursued the role of temperature on hydroxyapatite. On increasing the sintering temperature, we observe the generation of more secondary phases (Supplementary data, Figure S6) which correspond to TCP. As a confirmation of the same,we also pursued the FT-IR studies. The FT-IR spectra (Supplementary data, Figure S7) demonstrate visibly that as the temperature increases, the phase transformation is evident. The hydroxyapatite (HA) transforms to TCP when it is subjected to the sintering temperature of 1300∘C as evidenced by the reduction in intensity of the OH peaks at 3571 and 630 cm−1.

The comparison of the Raman spectra obtained for the formation of nano-hydroxyapatite generated in the different operating conditions of hydrothermal route: (a) Role of dwell time, (b) Effect of temperature and (c) Standard raman reference of HA
3.5 Role of simulated body fluid
The HA nano-crystalline powder was sintered at 1300∘C to obtain dense structure. Unfortunately, HA transforms to TCP under these conditions as elucidated above. Therefore, the purpose of the following investigation is to see whether TCP gets converted back to HA under the influence of body fluid given the importance of the polar OH group in the piezoelectric effect. 100 mg of sintered TCP powder was soaked in 1 ml of Fetal Bovine Serum (FBS) for different durations. The one sample, that was soaked for 14 days, was separated using centrifuge and filtered, and heated up to 80∘C for 2 h. This was then evaluated for its functional transformation using the IR spectroscopy for the presence of any hydroxyl group. We observe the generation of the 3571 and 630 cm−1 peaks corresponding to the OH stretching and vibrational mode confirming the transformation of TCP to HA (Figure 9). Thus we have a rational understanding that TCP gets converted to HA with prolonged exposure to the simulated body fluids. This ensures that our high sintering temperatures that led to conversion of the phase of hydroxyapatite to TCP in HA-BCZT composites confirms the reclamation of the HA phase when implanted in the body.

S3 SEM images and corresponding EDX report of the composition of the nano-hydroxyapatite for the temperature profile of hydrothermal synthesis at 10μm resolution

SEM images and corresponding EDX report of the composition of the nano-hydroxyapatite in the rod and flower composition at 16h dwell time of hydrothermal synthesis at 10μm resolution

SEM images and corresponding EDX report of the composition of the nano-hydroxyapatite in the rod and flower composition at 8h dwell time of hydrothermal synthesis at 10μm resolution

XRD spectra of the hydroxyapatite with increasing sintering temperature

FT-IR spectrum of the hydroxyapatite with increasing sintering temperature
4 Conclusion
The study reiterates the relevance of piezoelectricity in bone regeneration. We evaluated the optimum conditions to generate a significant piezoelectricity (d33) using lead free BCZT in Hydroxyapatite for assisting in bone regeneration. We report the attainment of the enhanced d33 of up to 7±1 pC/N at 50% BCZT as against the optimal values obtained with respect to ~98% BT composite. The morphology of the pristine HA, BCZT and their composites were evaluated to rationalize their observed properties. However, given the phase change at higher temperature with loss of OH− which is noted to be key for the piezoelectricity in HA, we evaluated the role of body fluid that helps in regeneration of this polar group for the formation of the desired HA phase. We further demonstrated their biocompatibility and positive impact on bone cell growth, theoretically and experimentally, opening up a new class of piezo-composites for bone regeneration.
Conflict of Interest
Conflict of Interests: “There are no conflicts to declare”.
Acknowledgement
We are indebted to Bhagawan Sri Sathya Sai Baba the founding chancellor, SSSIHL for his vision and inspiration to serve the society. We are thankful to the administration and the Central Research Instruments Facility (CRIF), SSSIHL for the timely hand with the various resources in the relevant studies.
Supplementary information
Comparison of the assigned FT-IR peaks for the various functional groups of the synthesized hydroxyapatite versus the reference HA sample’s values from literature [S1]
| IR-HA, Literature (cm−1) | IR-HA, Experiment (cm−1) | ATTRIBUTE |
|---|---|---|
| 3432, 1642 | 3423, 1636 | Adsorbed water |
| 3571 | 3567 | Stretching vibration of lattice OH- ions |
| 633 | 668* | O-H deformation mode |
| 470, 568, 602, | 472,562,603, | Phosphate group |
| 964, 1041, 1093 | 961,1036, 1091 | |
| 964 | 961 | Asymmetric P-O stretching |
| 633, 602, 568 | 668,603,562 | Triple degenerate bending vibration of Phosphate group |
Bibliography
[1] Singh S, Saha S. 1984 Electrical Properties of Bone: A Review. Clin. Orthop. Relat. Res. 1984, 186, 249-27110.1097/00003086-198406000-00042Search in Google Scholar
[2] Lind M. Growth factor stimulation of bone healing: effects on osteoblasts, osteomies, and implants fixation Acta Orthop. Scand. 1998, 69, 1-3710.1080/17453674.1998.11744808Search in Google Scholar
[3] Lang SB. Review of ferroelectric hydroxyapatite and its application to biomedicine. Phase Transitions 2016, 1594, 1–1710.1080/01411594.2016.1182166Search in Google Scholar
[4] Shen X, Zhang Y, Gu Y, Xu Y, Liu Y, Li B, Chen L. Sequential and sustained release of SDF-1 and BMP-2 from silk fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration Biomaterials 2016, 106, 205-21610.1016/j.biomaterials.2016.08.023Search in Google Scholar PubMed
[5] Xin-Bo X, Xin-Ye N, Ya-Yun L, Cen-Cen C, Ji-Zhao Z, Xie-Rong Z. A Novel Strategy for Preparation of Si-HA Coatings on C/C Composites by Chemical Liquid Vaporization Deposition/Hydrothermal Treatments Sci. Rep. 2016, 6:31309, 1-910.1038/srep31309Search in Google Scholar PubMed PubMed Central
[6] Sun F, Zhou H, Lee J. Various preparation methods of highly porous hydroxyapatite/polymer nanoscale biocomposites for bone regeneration Acta Biomater. 2011, 7, 3813–382810.1016/j.actbio.2011.07.002Search in Google Scholar PubMed
[7] Feng P, Niu M, Gao C, Peng S, Shuai C. A novel two-step sintering for nano-hydroxyapatite scaffolds for bone tissue engineering Sci. Rep. 2014, 4:5599, 1-1010.1038/srep05599Search in Google Scholar PubMed PubMed Central
[8] Fukada E, Yasuda I. On the piezoelectric effect of bone J. Phys. Soc. Japan 1957, 12, 1158–116210.1143/JPSJ.12.1158Search in Google Scholar
[9] Facade E, Yasuda I. Piezoelectric effects in collagen J Appl Physiol 1964, 3, 117-12110.1143/JJAP.3.117Search in Google Scholar
[10] Braden M, Bairstow AG, Beider I, Ritter BG. Electrical and piezo-electrical properties of dental hard tissues Nature 1966, 212 1565–156610.1038/2121565a0Search in Google Scholar PubMed
[11] Lang SB, Tofail SM, Kholkin AL, Wojtaś M, Gregor M, Gandhi AA, Wang Y, Bauer S, Krause M, Plecenik A. Ferroelectric polarization in nanocrystalline hydroxyapatite thin films on silicon Sci. Rep 2013, 3:2215, 1-610.1038/srep02215Search in Google Scholar PubMed PubMed Central
[12] Prakasam M, Albino M, Lebraud E, Maglione M, Elissalde C, Largeteau A. Hydroxyapatite-barium titanate piezocomposites with enhanced electrical properties Journal of the American Ceramic Society 2017, 100:6, 2621–263110.1111/jace.14801Search in Google Scholar
[13] Yamashita K, Oikawa N,Umegaki T. Acceleration and deceleration of bone-like crystal growth on ceramic hydroxyapatite by electric poling Chem. Mater. 1996, 8, 2697–70010.1021/cm9602858Search in Google Scholar
[14] Tang Y, Wu C, Wu Z, Hu L, Zhang W, Zhao K. Fabrication and in vitro biological properties of piezoelectric bioceramics for bone regeneration Sci. Rep. 2017, 7:43360, 1-1210.1038/srep43360Search in Google Scholar PubMed PubMed Central
[15] Liu B, Chen L, Shao C, Zhang F, Zhou K, Cao J, Zhang D. Improved osteoblasts growth on osteomimetic hydroxyapatite/BaTiO3 composites with aligned lamellar porous structure Mater. Sci. Eng. C. 2016, 61, 8–1410.1016/j.msec.2015.12.009Search in Google Scholar PubMed
[16] Ehmke MC, Ehrlich SN, Blendell JE, Bowman KJ. Phase coexistence and ferroelastic texture in high strain (1-x) Ba(Zr02Ti0.8)O3– x(Ba07Ca0.3)TiO3 piezoceramics J. Appl. Phys. 2012, 111:124110, 1-710.1063/1.4730342Search in Google Scholar
[17] Keeble DS, Benabdallah F, Thomas PA, Maglione M, Kreisel J. Revised structural phase diagram of (Ba07Ca03TiO3)-(BaZr02Ti08O3) Appl. Phys. Lett. 2013, 102: 092903, 1-5Search in Google Scholar
[18] Damjanovic D, Biancoli A, Batooli L, Vahabzadeh A, Trodahl J. Elastic, dielectric, and piezoelectric anomalies and Raman spectroscopy of 0.5Ba(Ti08Zr0.2)O3-0.5(Ba07Ca0.3)TiO3Appl. Phys. Lett 2012, 100: 192907, 1-410.1063/1.4714703Search in Google Scholar
[19] Tian Y, Chao X, Wei L, Liang P, Yang Z. Phase transition behavior and electrical properties of lead-free (Ba1−xCax(Zr01Ti0.9)O3 piezoelectric ceramics J. Appl. Phys. 2013, 113: 184107, 1-710.1063/1.4804173Search in Google Scholar
[20] Baek C, Yun JH, Wang JE, Jeong CK, Lee KJ, Park KI, Kim DK. A flexible energy harvester based on a lead-free and piezoelectric BCTZ nanoparticle–polymer composite Nanoscale 2016, 8, 17632–1763810.1039/C6NR05784ESearch in Google Scholar PubMed
[21] Liu W, Ren X. Large Piezoelectric Effect in Pb-Free Ceramics Phys. Rev. Lett. 2009, 103: 257602, 1-410.1103/PhysRevLett.103.257602Search in Google Scholar PubMed
[22] García-Lestón J, Méndez J, Pásaro E, Laffon B. Genotoxic effects of lead: An updated review Environ. Int. 2010, 36, 623–63610.1016/j.envint.2010.04.011Search in Google Scholar PubMed
[23] Doig AT. Baritosis: a benign pneumoconiosis. Thorax 1976, 31, 30–3910.1136/thx.31.1.30Search in Google Scholar PubMed PubMed Central
[24] Simon-Seveyrat L, Hajjaji A, Emziane Y, Guiffard B, Guyomar D. Re-investigation of synthesis of BaTiO3by conventional solid-state reaction and oxalate coprecipitation route for piezoelectric applications Ceram. Int. 2007, 33, 35–4010.1016/j.ceramint.2005.07.019Search in Google Scholar
[25] Bao H, Zhou C, Xue D, Gao J, Ren X. A modified lead-free piezoelectric BZT-xBCT system with higher Tc J. Phys. D: Appl. Phys 2010, 43: 465401, 1-410.1088/0022-3727/43/46/465401Search in Google Scholar
[26] Bai W, Shen B, Fu F, Zhai J. Dielectric, ferroelectric, and piezoelectric properties of textured BZT – BCT lead-free thick film by screen printing Mater. Lett. 2012, 83, 20–2210.1016/j.matlet.2012.05.114Search in Google Scholar
[27] Tang X, Chan HL. Effect of grain size on the electrical properties of Ba, Ca, Zr, TiO3 relaxor ferroelectric ceramics Journal of Applied Physics 2005, 034109, 0–610.1063/1.1849817Search in Google Scholar
[28] Bharathi P, Varma KBR. Grain and the concomitant ferroelectric domain size dependent physical derived from oxalate precursor route Journal of Applied Physics 2016, 116:16407, 1-10Search in Google Scholar
[29] Scarisoreanu ND, Craciun F, Ion V, Birjega R, Bercea A, Dinca V, Dinescu M, Sima LE, Icriverzi M, Roseanu A, Gruionu L, Gruionu G. Lead-Free Piezoelectric (Ba,Ca)(Zr,Ti)O3 Thin Films for Biocompatible and Flexible Devices ACS Appl. Mater. Interfaces 2017, 9, 266–27810.1021/acsami.6b14774Search in Google Scholar PubMed
[30] Li C, Ge X, Li G, Gao Q, Ding R. A facile hydrothermal method for synthesis of submillimeter-long octacalcium phosphate and hydroxyapatite as drug carriers with sustained release behaviours. Adv. Powder Technol. 2014, 25, 1661–166610.1016/j.apt.2014.06.001Search in Google Scholar
[31] Bhimireddi R, Ponraj B, Varma KBR. Structural, Optical, and Piezoelectric Response of Lead-Free Ba0.95Mg0.05Zr0.1Ti0.9O3 Nanocrystalline Powder J. Am. Ceram. Soc. 2016, 99, 896–90410.1111/jace.14018Search in Google Scholar
[32] Wang M, Zuo R, Qi S, Liu L. Synthesis and characterization of sol–gel derived (Ba, Ca)(Ti, Zr) O3 nanoparticles J. Mater. Sci. Mater. Electron. 2012, 23, 753–75710.1007/s10854-011-0484-9Search in Google Scholar
[33] Baxter FR, Turner IG, Bowen CR, Gittings JP, Chaudhuri JB. An in vitro study of electrically active hydroxyapatite-barium titanate ceramics using Saos-2 cells J. Mater. Sci. Mater. Med. 2009, 20, 1697–170810.1007/s10856-009-3734-0Search in Google Scholar
[34] Gopi D, Kanimozhi K, Kavitha L. Opuntia ficus indica peel derived pectin mediated hydroxyapatite nanoparticles: Synthesis, spectral characterization, biological and antimicrobial activities Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 2015, 141, 135–14310.1016/j.saa.2015.01.039Search in Google Scholar
[35] Giannozzi P, Baroni S, Bonini N, Calandra M, Car R, Cavazzoni C, Ceresoli D, Chiarotti GL, Cococcioni M, Dabo I, Dal Corso A, De Gironcoli S, Fabris S, Fratesi G, Gebauer R, Gerstmann U, Gougoussis C, Kokalj A, Lazzeri M, Martin-Samos L, Marzari N, Mauri F, Mazzarello R, Paolini S, Pasquarello A, Paulatto L, Sbraccia C, Scandolo S, Sclauzero G, Seitsonen AP, Smogunov A,Umari P and Wentzcovitch RM. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials J. Phys. Condens. Matter 2009, 21.10.1088/0953-8984/21/39/395502Search in Google Scholar
[36] Choi JY, Lee BH, Song KB, Park RW, Kim IS, Sohn KY, Jo JS, Ryoo HM. Expression patterns of bone-related proteins during osteoblastic differentiation in MC3T3-E1 cells J. Cell. Biochem. 1996, 61, 609–1810.1002/(SICI)1097-4644(19960616)61:4<609::AID-JCB15>3.0.CO;2-ASearch in Google Scholar
[37] Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res 2000, 28, 235–4210.1093/nar/28.1.235Search in Google Scholar
[38] Ritchie DW. Evaluation of protein docking predictions using Hex 3.1 in CAPRI rounds 1 and 2 Proteins Struct. Funct. Genet. 2003, 52, 98–10610.1002/prot.10379Search in Google Scholar
[39] Cui X, Liang T, Liu C, Yuan Y, Qian J. Correlation of particle properties with cytotoxicity and cellular uptake of hydroxyapatite nanoparticles in human gastric cancer cells Mater. Sci. Eng. C 2016, 67, 453–6010.1016/j.msec.2016.05.034Search in Google Scholar
[40] Chang MC, Yu SC. Raman study for (Ba1−xCaxTiO3 and Ba(Ti1−yCayO3 crystalline ceramics J. Mater. Sci. Lett. 2000, 19, 1323–132510.1023/A:1006732628003Search in Google Scholar
[41] Ganguly R, Siruguri V, Gopalakrishnan IK, Yakhmi JV. Stability of the layered Sr3Ti2O7 structure in La1. 2 (Sr1-xCax) 1.8 Mn2O7 Journal of Physics: Condensed Matter 2000, 12, 1683-168910.1088/0953-8984/12/8/311Search in Google Scholar
[42] Costescu A, Pasuk I, Ungureanu F, Dinischiotua A, Costachea M, Huneau F, Galaupc S, Le coustumer P, Predoi D. Physico-chemical properties of nano-sized hexagonal Hydroxyapatite powder synthesized by sol-gel. Digest Journal of Nanomaterials and Biostructures. 2010, 5, 989-1000Search in Google Scholar
[43] Reyes-Montero A, Pardo L, López-Juárez R, González AM, Rea- López SO, Cruz MP, Villafuerte-Castrejón ME. Sub-10 μm grain size, Ba1−xCaxTi0.9Zr0.1O3 (x = 0.10 and x = 0.15) piezoceramics processed using a reduced thermal treatment. Smart Mater. Struct. 2015, 24, 065033.10.1088/0964-1726/24/6/065033Search in Google Scholar
[44] Chiatti F, Delle Piane M, Ugliengo P, Corno M. 2016 Water at hydroxyapatite surfaces: the effect of coverage and surface termination as investigated by all-electron B3LYP-D* simulations Theor. Chem. Acc. 2016, 135, 1–1510.1007/s00214-016-1818-8Search in Google Scholar
Bibliography
[S1] Arsad et al. 2nd International Conference on Biotechnology and Food Science. (2011)Search in Google Scholar
© 2019 C. SaiManohar et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Research Articles
- Investigation of rare earth upconversion fluorescent nanoparticles in biomedical field
- Carbon Nanotubes Coated Paper as Current Collectors for Secondary Li-ion Batteries
- Insight into the working wavelength of hotspot effects generated by popular nanostructures
- Novel Lead-free biocompatible piezoelectric Hydroxyapatite (HA) – BCZT (Ba0.85Ca0.15Zr0.1Ti0.9O3) nanocrystal composites for bone regeneration
- Effect of defects on the motion of carbon nanotube thermal actuator
- Dynamic mechanical behavior of nano-ZnO reinforced dental composite
- Fabrication of Ag Np-coated wetlace nonwoven fabric based on amino-terminated hyperbranched polymer
- Fractal analysis of pore structures in graphene oxide-carbon nanotube based cementitious pastes under different ultrasonication
- Effect of PVA fiber on durability of cementitious composite containing nano-SiO2
- Cr effects on the electrical contact properties of the Al2O3-Cu/15W composites
- Experimental evaluation of self-expandable metallic tracheobronchial stents
- Experimental study on the existence of nano-scale pores and the evolution of organic matter in organic-rich shale
- Mechanical characterizations of braided composite stents made of helical polyethylene terephthalate strips and NiTi wires
- Mechanical properties of boron nitride sheet with randomly distributed vacancy defects
- Fabrication, mechanical properties and failure mechanism of random and aligned nanofiber membrane with different parameters
- Micro- structure and rheological properties of graphene oxide rubber asphalt
- First-principles calculations of mechanical and thermodynamic properties of tungsten-based alloy
- Adsorption performance of hydrophobic/hydrophilic silica aerogel for low concentration organic pollutant in aqueous solution
- Preparation of spherical aminopropyl-functionalized MCM-41 and its application in removal of Pb(II) ion from aqueous solution
- Electrical conductivity anisotropy of copper matrix composites reinforced with SiC whiskers
- Miniature on-fiber extrinsic Fabry-Perot interferometric vibration sensors based on micro-cantilever beam
- Electric-field assisted growth and mechanical bactericidal performance of ZnO nanoarrays with gradient morphologies
- Flexural behavior and mechanical model of aluminum alloy mortise-and-tenon T-joints for electric vehicle
- Synthesis of nano zirconium oxide and its application in dentistry
- Surface modification of nano-sized carbon black for reinforcement of rubber
- Temperature-dependent negative Poisson’s ratio of monolayer graphene: Prediction from molecular dynamics simulations
- Finite element nonlinear transient modelling of carbon nanotubes reinforced fiber/polymer composite spherical shells with a cutout
- Preparation of low-permittivity K2O–B2O3–SiO2–Al2O3 composites without the addition of glass
- Large amplitude vibration of doubly curved FG-GRC laminated panels in thermal environments
- Enhanced flexural properties of aramid fiber/epoxy composites by graphene oxide
- Correlation between electrochemical performance degradation and catalyst structural parameters on polymer electrolyte membrane fuel cell
- Materials characterization of advanced fillers for composites engineering applications
- Humic acid assisted stabilization of dispersed single-walled carbon nanotubes in cementitious composites
- Test on axial compression performance of nano-silica concrete-filled angle steel reinforced GFRP tubular column
- Multi-scale modeling of the lamellar unit of arterial media
- The multiscale enhancement of mechanical properties of 3D MWK composites via poly(oxypropylene) diamines and GO nanoparticles
- Mechanical properties of circular nano-silica concrete filled stainless steel tube stub columns after being exposed to freezing and thawing
- Arc erosion behavior of TiB2/Cu composites with single-scale and dual-scale TiB2 particles
- Yb3+-containing chitosan hydrogels induce B-16 melanoma cell anoikis via a Fak-dependent pathway
- Template-free synthesis of Se-nanorods-rGO nanocomposite for application in supercapacitors
- Effect of graphene oxide on chloride penetration resistance of recycled concrete
- Bending resistance of PVA fiber reinforced cementitious composites containing nano-SiO2
- Review Articles
- Recent development of Supercapacitor Electrode Based on Carbon Materials
- Mechanical contribution of vascular smooth muscle cells in the tunica media of artery
- Applications of polymer-based nanoparticles in vaccine field
- Toxicity of metallic nanoparticles in the central nervous system
- Parameter control and concentration analysis of graphene colloids prepared by electric spark discharge method
- A critique on multi-jet electrospinning: State of the art and future outlook
- Electrospun cellulose acetate nanofibers and Au@AgNPs for antimicrobial activity - A mini review
- Recent progress in supercapacitors based on the advanced carbon electrodes
- Recent progress in shape memory polymer composites: methods, properties, applications and prospects
- In situ capabilities of Small Angle X-ray Scattering
- Review of nano-phase effects in high strength and conductivity copper alloys
- Progress and challenges in p-type oxide-based thin film transistors
- Advanced materials for flexible solar cell applications
- Phenylboronic acid-decorated polymeric nanomaterials for advanced bio-application
- The effect of nano-SiO2 on concrete properties: a review
- A brief review for fluorinated carbon: synthesis, properties and applications
- A review on the mechanical properties for thin film and block structure characterised by using nanoscratch test
- Cotton fibres functionalized with plasmonic nanoparticles to promote the destruction of harmful molecules: an overview
Articles in the same Issue
- Research Articles
- Investigation of rare earth upconversion fluorescent nanoparticles in biomedical field
- Carbon Nanotubes Coated Paper as Current Collectors for Secondary Li-ion Batteries
- Insight into the working wavelength of hotspot effects generated by popular nanostructures
- Novel Lead-free biocompatible piezoelectric Hydroxyapatite (HA) – BCZT (Ba0.85Ca0.15Zr0.1Ti0.9O3) nanocrystal composites for bone regeneration
- Effect of defects on the motion of carbon nanotube thermal actuator
- Dynamic mechanical behavior of nano-ZnO reinforced dental composite
- Fabrication of Ag Np-coated wetlace nonwoven fabric based on amino-terminated hyperbranched polymer
- Fractal analysis of pore structures in graphene oxide-carbon nanotube based cementitious pastes under different ultrasonication
- Effect of PVA fiber on durability of cementitious composite containing nano-SiO2
- Cr effects on the electrical contact properties of the Al2O3-Cu/15W composites
- Experimental evaluation of self-expandable metallic tracheobronchial stents
- Experimental study on the existence of nano-scale pores and the evolution of organic matter in organic-rich shale
- Mechanical characterizations of braided composite stents made of helical polyethylene terephthalate strips and NiTi wires
- Mechanical properties of boron nitride sheet with randomly distributed vacancy defects
- Fabrication, mechanical properties and failure mechanism of random and aligned nanofiber membrane with different parameters
- Micro- structure and rheological properties of graphene oxide rubber asphalt
- First-principles calculations of mechanical and thermodynamic properties of tungsten-based alloy
- Adsorption performance of hydrophobic/hydrophilic silica aerogel for low concentration organic pollutant in aqueous solution
- Preparation of spherical aminopropyl-functionalized MCM-41 and its application in removal of Pb(II) ion from aqueous solution
- Electrical conductivity anisotropy of copper matrix composites reinforced with SiC whiskers
- Miniature on-fiber extrinsic Fabry-Perot interferometric vibration sensors based on micro-cantilever beam
- Electric-field assisted growth and mechanical bactericidal performance of ZnO nanoarrays with gradient morphologies
- Flexural behavior and mechanical model of aluminum alloy mortise-and-tenon T-joints for electric vehicle
- Synthesis of nano zirconium oxide and its application in dentistry
- Surface modification of nano-sized carbon black for reinforcement of rubber
- Temperature-dependent negative Poisson’s ratio of monolayer graphene: Prediction from molecular dynamics simulations
- Finite element nonlinear transient modelling of carbon nanotubes reinforced fiber/polymer composite spherical shells with a cutout
- Preparation of low-permittivity K2O–B2O3–SiO2–Al2O3 composites without the addition of glass
- Large amplitude vibration of doubly curved FG-GRC laminated panels in thermal environments
- Enhanced flexural properties of aramid fiber/epoxy composites by graphene oxide
- Correlation between electrochemical performance degradation and catalyst structural parameters on polymer electrolyte membrane fuel cell
- Materials characterization of advanced fillers for composites engineering applications
- Humic acid assisted stabilization of dispersed single-walled carbon nanotubes in cementitious composites
- Test on axial compression performance of nano-silica concrete-filled angle steel reinforced GFRP tubular column
- Multi-scale modeling of the lamellar unit of arterial media
- The multiscale enhancement of mechanical properties of 3D MWK composites via poly(oxypropylene) diamines and GO nanoparticles
- Mechanical properties of circular nano-silica concrete filled stainless steel tube stub columns after being exposed to freezing and thawing
- Arc erosion behavior of TiB2/Cu composites with single-scale and dual-scale TiB2 particles
- Yb3+-containing chitosan hydrogels induce B-16 melanoma cell anoikis via a Fak-dependent pathway
- Template-free synthesis of Se-nanorods-rGO nanocomposite for application in supercapacitors
- Effect of graphene oxide on chloride penetration resistance of recycled concrete
- Bending resistance of PVA fiber reinforced cementitious composites containing nano-SiO2
- Review Articles
- Recent development of Supercapacitor Electrode Based on Carbon Materials
- Mechanical contribution of vascular smooth muscle cells in the tunica media of artery
- Applications of polymer-based nanoparticles in vaccine field
- Toxicity of metallic nanoparticles in the central nervous system
- Parameter control and concentration analysis of graphene colloids prepared by electric spark discharge method
- A critique on multi-jet electrospinning: State of the art and future outlook
- Electrospun cellulose acetate nanofibers and Au@AgNPs for antimicrobial activity - A mini review
- Recent progress in supercapacitors based on the advanced carbon electrodes
- Recent progress in shape memory polymer composites: methods, properties, applications and prospects
- In situ capabilities of Small Angle X-ray Scattering
- Review of nano-phase effects in high strength and conductivity copper alloys
- Progress and challenges in p-type oxide-based thin film transistors
- Advanced materials for flexible solar cell applications
- Phenylboronic acid-decorated polymeric nanomaterials for advanced bio-application
- The effect of nano-SiO2 on concrete properties: a review
- A brief review for fluorinated carbon: synthesis, properties and applications
- A review on the mechanical properties for thin film and block structure characterised by using nanoscratch test
- Cotton fibres functionalized with plasmonic nanoparticles to promote the destruction of harmful molecules: an overview

