Abstract
Propolis is a natural bee product with phenolic components and flavonoid content. As propolis is hydrophobic, it is poorly absorbed by the body, querying the use of other technologies. This review focuses on the biosynthesis, characterization, and evaluation of some biological activities of nanoparticles (AgNPs and SeNPs). The nanoparticles were generated utilizing bee propolis extract, taking into account the benefits of green nanoparticle synthesis. Due to the smaller size, nano-propolis is more easily absorbed by the body. Nano-propolis has the potential to improve efficacy in the realms of medicine and biology. Green chemistry approach to nanoparticle synthesis offers several advantages, including process scaling, economic feasibility, and a safe technique to make nanoparticles. Bioreduced AgNPs can be employed as a therapeutic agent to treat a variety of human ailments. After deeply studying and reviewing different research studies, it was evaluated that the natural nanoparticles have the potential to be effective in the treatment of bacterial and fungal infections.
Graphical abstract
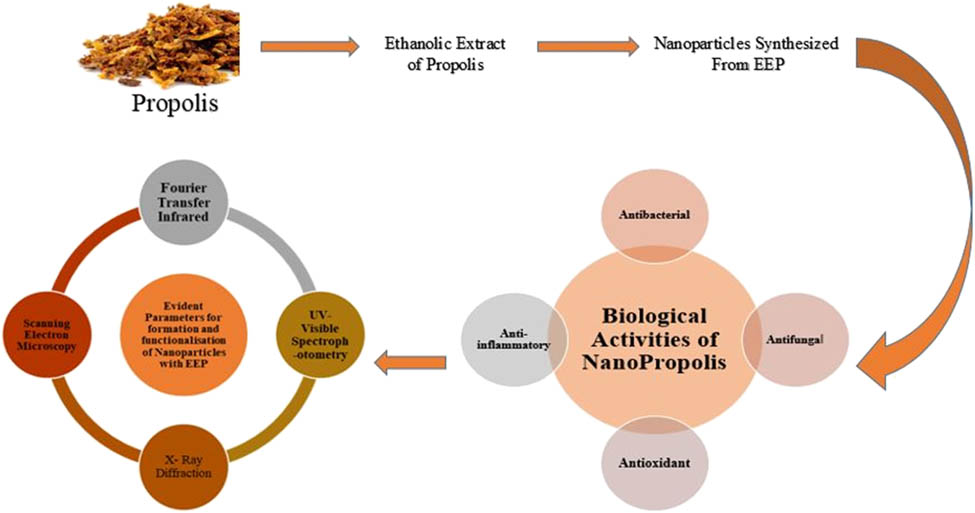
1 Introduction
Nanotechnology is the science and technology of small, precise objects that are between 1 and 100 nm in size [1]. The term “nano” is derived from a Greek word that means “dwarf,” and it is frequently used in conjunction with other words such as “nanometer,” “nanobots,” and “nanotechnology” [1,2]. Nanomaterials may undergo novel chemical and physical changes in their structure as a result of their smaller size, indicating increased reactivity and solubility [1,3]. When compared to transition metal nanoparticles such as iron, nickel, and cobalt, noble metals such as ruthenium, rhodium, palladium, silver, osmium, iridium, platinum, and gold are the most widely used in nanotechnology because of their relatively low cytotoxicity for biological or environmental conditions [4,5,6].
The risk for environmental contamination is heightened with the development of new chemical or physical techniques, as the chemical procedures involved in the production of nanoparticles generate a substantial number of harmful byproducts. As a result, green chemistry is required, which comprises a clean, non-toxic, and environmentally acceptable approach of nanoparticle synthesis [7,8]. Green synthesis refers to the production of nanoparticles via a process involving a natural molecule as a reducing agent and metal salts [9]. Because of the natural coating of the organic molecules, biosynthesis of selenium nanoparticles (SeNPs) is safe, affordable, eco-friendly, more stable, and do not aggregate over time [10,11,12,13,14]. Due to the presence of reducing, stabilizing properties, and eco-friendly characteristics, the use of propolis is preferred for the biosynthesis of nanoparticles [4]. Chemical and physical methods of producing nanoparticles are not commercially feasible and culminate in the generation of unsafe nanoparticles and environmental pollution. The use of green nanotechnology is an impeccable solution for combating these adverse affects. Selenium, an essential trace element [10,15,16,17,18,19] is mostly preferred for the biosynthesis of nanoparticles as it evinces different properties as given in Table 1. The presence of free selenium exerts harmful effects on cells, despite its physiological relevance. As a result, researchers in this sector are focusing on Se synthesis in nano molecules. Selenium in nano form has demonstrated remarkable biological activity while causing no toxicity [10,16].
Different biological activities of selenium which makes it most preferable for the biosynthesis of nanoparticles
| No. | Biological activities | References |
|---|---|---|
| 1 | Antioxidant | [10,45] |
| 2 | Anti-inflammatory | [10,46] |
| 3 | Antimutagenic | [10,47] |
| 4 | Anticarcinogenic | [10,48,49] |
| 5 | Chemopreventive | [10,50] |
| 6 | Antiviral | [10,51] |
| 7 | Antibacterial | [10,52] |
| 8 | Antifungal | [10,53,54] |
Silver nanoparticles (AgNPs) are now the most widely utilized nanomaterial in consumer products [3]. AgNPs are the most common and important of all types because they have been shown to have different properties like antibacterial properties against a broad variety of human pathogens [20,21] antifungal, antiviral, catalytic [20,22], antioxidant, anticancer, anti-inflammatory, hepatoprotective, and larvicidal effects [23], as given in Table 2. AgNPs are well-known among noble metal NPs for their extensive medical applications [21]. Drug delivery, food processing, agriculture, textile manufacturing, water treatment, redox catalysis, green house construction, and medicine have all benefited from AgNPs [24,25,26,27,28,29,30,31,32].
Properties exhibited by AgNPs
| No. | Biological activities | References |
|---|---|---|
| 1 | Antimicrobial | [5,55,56,57,58,59] |
| 2 | Antibacterial | [5,22,60,61,62,63] |
| 3 | DNA sequencing | [5,64] |
| 4 | Antifungal | [22,65,66] |
| 5 | Antiviral | [22,67,68,69] |
| 6 | Catalytic | [22,70,71,72] |
This article focuses on the green synthesis of nanoparticles using bee product propolis, as well as their most significant applications in medical sciences. Propolis is a honeybee product, a resinous mixture with a complex chemical profile that has been utilized in folk medicine since antiquity [20,33], and it is influenced by various factors like climate zones, seasons, vegetation, and environmental circumstances [20,34,35]. Propolis have many advantages such as antibacterial, antifungal, hepatoprotective, antiproliferative, antimicrobial, antioxidative, anti-inflammatory, antiviral, immunomodulatory, and regenerative activities [1,10,35,36,37,38,39,40,41,42] as given in Figure 1.
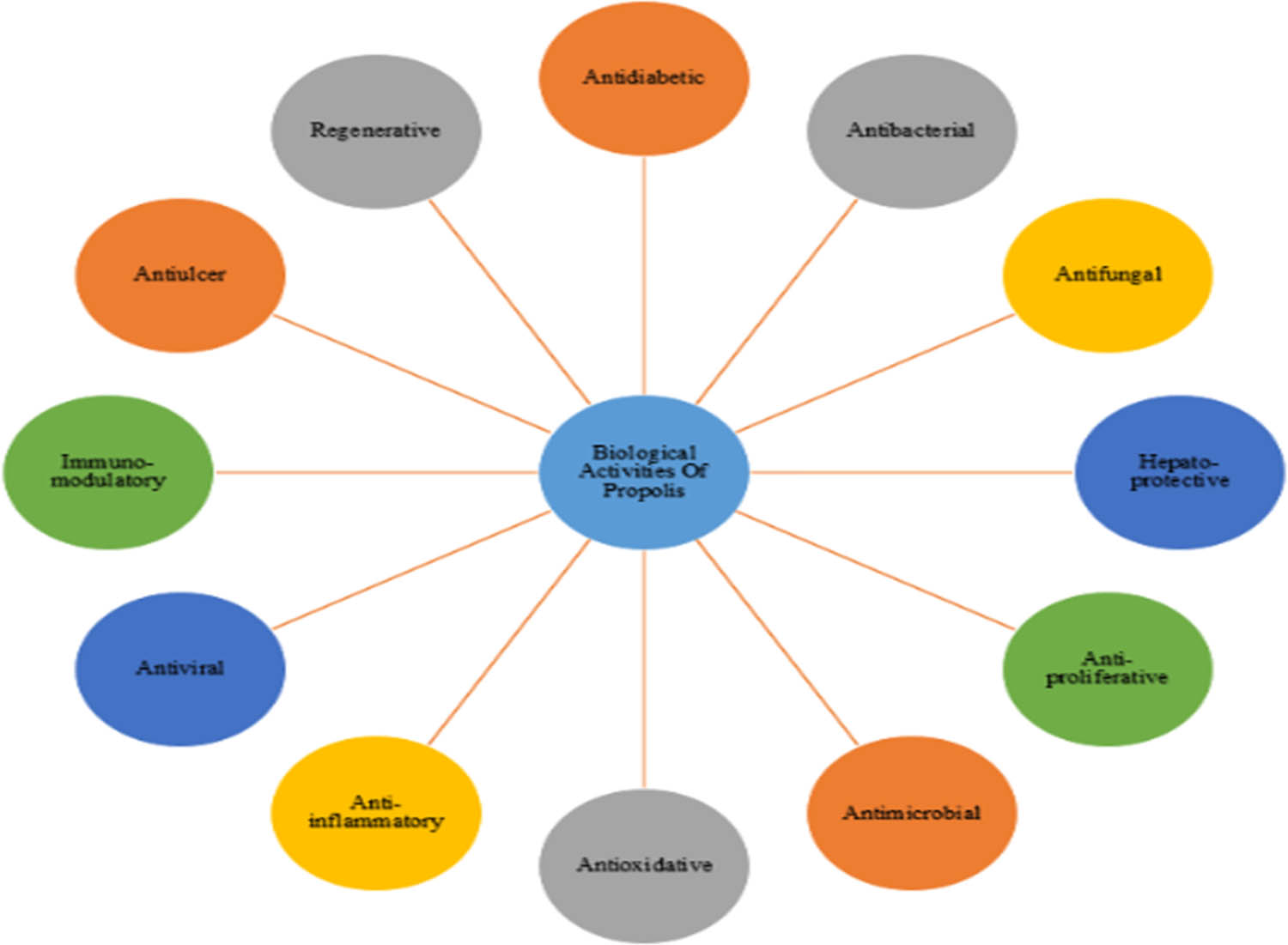
Biological properties of propolis.
Polyphenols and flavonoids have been identified as active components of propolis so far. These chemicals have anti-atherosclerotic, anti-inflammatory, and anti-angiogenic properties, as well as cardioprotective, vasoprotective, antioxidant, anti-atherosclerotic, anti-inflammatory, and anti-angiogenic properties [35]. But low bioavailability, low solubility, and low absorption of free form of propolis restrict all its advantages [1]. To reap all of the benefits of free-form propolis, nanopropolis can be produced using a variety of approaches [1]. Nano-propolis is a nano-sized (1–100 nm in diameter) propolis particles that have been linked together to improve its effectiveness without altering its characteristics. To procure nano-propolis, various nanoencapsulation technologies can be employed [1,43]. Because of the smaller size, nano-propolis can be readily absorbed by body [1]. The reduction power of propolis is dependent on the existence of several active groups with reducing activity, and this is one of the modern strategies for producing nanoparticles [10]. When compared to propolis, nano-propolis is expected to provide better antibacterial action [1]. Various properties of nanopropolis have been given in Table 3.
Biological activities exhibited by nanopropolis
| No. | Biological activity | References |
|---|---|---|
| 1 | Antibacterial | [1,3,43] |
| 2 | Antifungal | [3] |
| 3 | Anti-diabetic | [44] |
The hypoglycaemic impact of nano powder propolis was studied in ref. [44]. Streptozotocin-induced diabetic rats were categorized into two groups: the diabetic control group and the group that received nano powder propolis (0.9 mL). After the rats had been fed with nano-propolis for 4 weeks, an oral glucose tolerance test was performed, and blood sugar, blood lipid levels, and body weights were measured after a 16 h fast. Author of ref. [44] discovered that nano-propolis was efficacious in the treatment of diabetes by lowering blood sugar levels and regenerating damaged β-cells in streptozotocin-induced diabetic mice.
2 Methods used in various studies for the preparation of nanopropolis and nanoparticles (Ag and Se)
2.1 Preparation of nanopropolis
To improve the handling properties of nano- and micropropolis, author of ref. [43] used encapsulation methods to create nano- and micropropolis via casein micelles. Encapsulation is a process that traps a substance or mixture of coated components in a system. A coated material is known as the active or core material, and the coating material is known as the shell, wall material, carrier, or encapsulant. Thus, nanopropolis coated with casein micelle would be produced using a high-pressure ball mill homogenizer to produce nanosized particles [73,74]. Encapsulation via casein micelle with a homogenizer was done for the Indonesian Propolis following sonication and then separation by micro and ultrafiltration system, synthesizing micro and nano-particles [1,43].
2.1.1 Encapsulation via casein micelle with a homogenizer
The pH of cow milk obtained from a market store is adjusted to 6.4 by incubating it at 30°C for 1 h with hydrochloride acid of 1 N. After adding rennet and agitating it for 15 min at 30°C, the casein was aggregated. To increase the particle size, the aliquot was incubated at the same temperature for 15 min. Filtration was used to separate the casein and other proteins. After inactivating the rennet with hot water (70°C) for 5 min, the casein and water were separated by filtration. Using 12% SDS PAGE, the molecular weight of casein was examined [43].
2.1.2 Synthesis of nanopropolis
5 g casein was taken and diluted in 50 mL of 10 mM phosphate buffer with pH = 10. In ethanol, 5 mL propolis was added while stirring. Every 5 min, 1 mL of 10% CaCl2 was added to the mixture. The pH of the mixture was adjusted to 7 using 0.1 N HCl or 0.1 N NaOH. For 5 min, the mixture was also sonicated. Microfiltration (Whatmann paper No. 42) was used to separate the mixture. Cut off the permate at 10 kDa and ultrafiltrate it. Phosphate buffer was used to dilute the retentante of micro and ultra filtration. The total of polyphenols and total flavonoids were assessed in the ultrafiltration permeate, which contained unencapsulated propolis [43].
2.2 Methods used in experiments for preparation of polymeric nanoparticles
2.2.1 Preparation of NIPAAM/VP/PEG-A copolymeric nanoparticles
NIPAAM + VP + PEG-A + MBA were dissolved in water in molar ratios of 90:5:5, they took 90 mg NIPAAM, 5 μL of freshly distilled VP, 500 μL of PEG-A (1% w/v), 10 mL of water. To cross link polymer chains, 30 μL of MBA was used. Polymerization was done using 30 μL of APS, 20 μL of TEMED, 20 μL of FAS (0.5% w/v) in N2 atmosphere at 30°C. After that dialysis for 2–3 min was done in aqueous medium for co-polymeric nanoparticles containing unreacted monomers as given in Figure 2. Pure co-polymeric nanoparticles in aqueous medium were employed for lyophilization to obtain dry powder of co-polymeric nanoparticles. To remove any inhibitors, NIPAAM was recrystallized using hexane, VP was freshly distilled before use, and PEG-A was washed three times with n-hexane [76].
![Figure 2
Procedure studied in the preparation of co-polymeric nanoparticles. NIPAAM – N-isopropylacrylamide; VP – N-vinyl-2-pyrrolidone; PEG-A – poly(ethyleneglycol) monoacrylate; MBA – N,N′-methylene bis-acrylamide; APS – ammonium persulfate; TEMED – tetramethyl ethylene diamine; FAS – ferrous ammonium sulfate [76].](/document/doi/10.1515/gps-2022-0059/asset/graphic/j_gps-2022-0059_fig_002.jpg)
Procedure studied in the preparation of co-polymeric nanoparticles. NIPAAM – N-isopropylacrylamide; VP – N-vinyl-2-pyrrolidone; PEG-A – poly(ethyleneglycol) monoacrylate; MBA – N,N′-methylene bis-acrylamide; APS – ammonium persulfate; TEMED – tetramethyl ethylene diamine; FAS – ferrous ammonium sulfate [76].
2.2.2 Preparation of propolis loaded polymeric nanoparticles with polycaprolactone-pluronic polymeric matrix
Nanoprecipitation with poly-caprolactone and pluronic combination is another approach for the synthesis of propolis loaded polymeric nanoparticles. The preformed polymer interfacial deposition method, also known as nanoprecipitation preformed polymer, was used to create colloidal suspensions of nanoparticles. The organic and aqueous phase components were weighed and placed in separate containers, then subjected to sonication until finished evaporation. The organic phase (500 μL) was then added into the aqueous phase (50 mL) with vortexing for 1 min following the freeze drying of nanoparticles. Polymeric nanoparticle suspensions with loaded propolis were submitted to the assays for characterization [75].
2.2.3 Extraction of propolis
Various authors prepared propolis extract by dissolving dry propolis. 35 g of dried propolis powder was dissolved in 250 mL of deionized water. The extraction was carried out by placing the flask on an orbital shaker at room temperature for 72 h and agitating it at 100 rpm. The aqueous solution was filtered with Whatman filter paper No. 1 after extraction. Crude extract was prepared by concentrating the extract under reduced pressure [9].
2.2.4 Loading of propolis
Loading of the propolis in the polymeric nanoparticles was done using a post-polymerization method. For this, in 10 mL of distilled water, 100 mg of the lyophilized powder was added and stirred to reconstitute the micelles. In Chloroform, free propolis was dissolved (CHCl3; 10 mg‧mL−1). With constant vortexing and mild sonication, the propolis solution in CHCl3 was slowly added to the polymeric solution. By physical entrapment, propolis was directly loaded into the hydrophobic core of the nanoparticles. Then, these propolis-loaded nanoparticles were lyophilized to dry powder for subsequent use [75].
2.3 Biosynthesis of SeNPs
Propolis extract has potential application in green synthesis of Se NPs due to the presence of natural reducing and stabilizing biocompounds in its composition, such as chalcones, flavones, phosphoric acid, acetic acid, butanol, butanoic acid, butyl ester, hydroxyl and keto waxes, ketones, terpenoids, steroids, and sugars. The reduction of selenium ion to was mostly mediated by metabolites from propolis extracts, specifically alcohol and phenolic compounds [77].
2.3.1 From ethanol extract of propolis
For the synthesis of SeNPs, three main methodologies can be applied, including physical methods which involves pyrolysis, physical vapor deposition, crushing, lithography, grinding, attrition, and ball milling [10,78,79], chemical methods including solvothermal, sol gel, thermal decomposition, microwave-assisted synthesis, ultrasonic-assisted, and electrochemical methods [80,81,82,83] and biosynthetic processes [10,18]. The most prevalent method for preparing SeNPs is reduction, which includes chemical reduction [18,84], γ-radiolytic reduction [85], and bacterial reduction [86].
Main drawback of these methods is that they have long synthesis time, requires high temperature, have high production cost, low yield, and also involves some hazardous chemicals affecting human health and the environment as they might be genotoxic, cytotoxic, or carcinogenic. As a result green chemistry methods have been developed and have become popularized which involve the use of biological systems such as microorganisms (bacteria, fungi, algae, and yeast) and plant extracts [87]. Use of biogenic synthesis of SeNPs is more advantageous as it is economical, non-toxic, uses non-hazardous materials, produces stable nanomaterials, also have natural organic covering to avoid the nanoparticles aggregation. SeNPs have exceptional applications such as significant biocidal role, capability to provide protection against free radicals, least toxicological effects, and prominent biological activities.
To 30 mM sodium selenite solution, 10 mL of freshly prepared ethanol extract was added along with 40 mM ascorbic acid. Mixture was placed at room temperature for 24 h until the light brown color changed to orange color. Centrifugation was done for newly biosynthesized SeNPs at 10,000 rpm for 30 min at 4°C. Washing was done for the separated nanoparticles thrice with double distilled water and then washed by using ethanol. After that SeNPs were dried overnight and their powdered form was used for further characterization and analysis [88]. Cycloheximide, a common fungicide, was employed as a positive control, and ethanol was utilized as a negative control. Schematic representation is given in Figure 3.
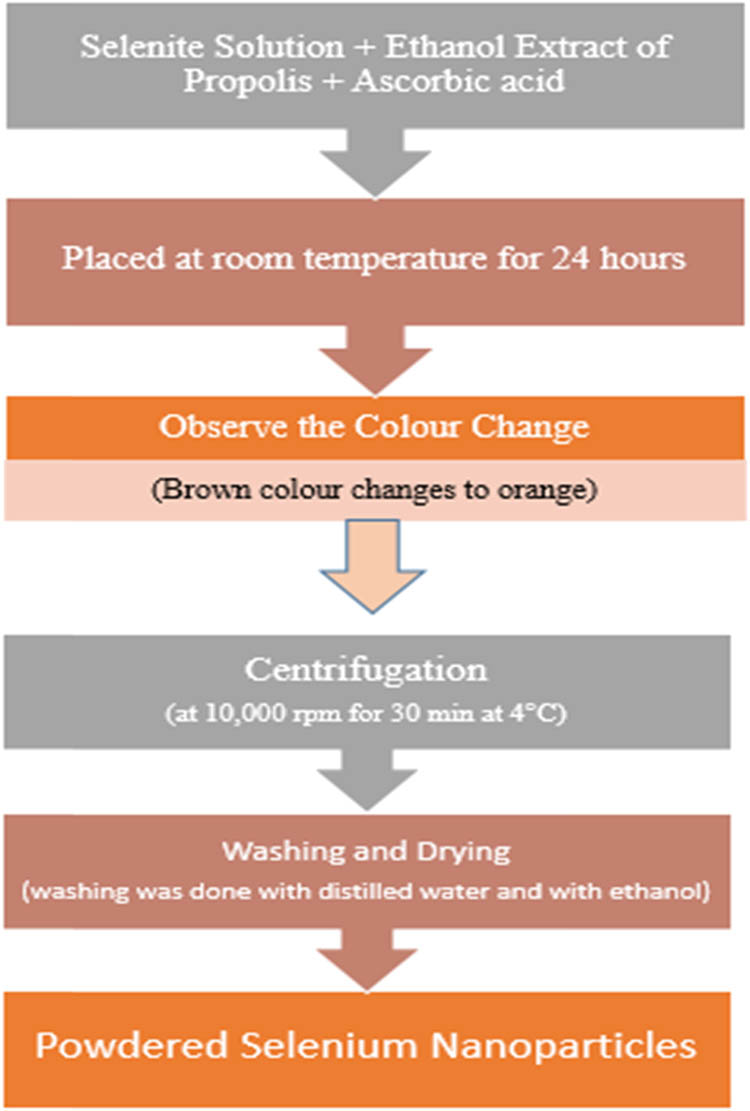
Schematic representation for the synthesis of selenium nanoparticles from ethanolic extract of propolis.
2.3.2 Other techniques for fabrication of SeNPs with propolis
It has been revealed in many studies that there are also some other techniques such as microwave irradiation, UV radiation, hydrothermal, ultrasonication, self-assembling, and conventional heating for the fabrication of SeNPs with propolis extract [77]. Initially propolis extract was added to the prepared selenium salt solution and this combination was used as sample for the different methods. In hydrothermal technique, colloidal solution was autoclaved at 121°C for 15 min with 1.5 atm. By using 800 W microwave device, the mixture solution was heated for 30 s in microwave radiation technique. In the ultrasonication method, the probe of the equipment was engrossed in the colloidal solution and further by using a laboratory ultrasound device it was treated with a frequency and power of 20 kHz and 300 W, respectively. From a 6 W UV lamp, UV light of 365 nm was used for the exposure of mixture solution at room temperature for a maximum of 1 h. Colloidal solution was kept overnight at room temperature (33°C) in the self-assembling technique [77].
2.3.3 From watery extract of propolis
150 mL of sodium selenite solution was poured gently drop by drop to a conical flask containing 300 mL of propolis watery extract solution. To avoid the effect of light on the nanoparticle composition process, a sheet of aluminum foil was placed over the flask. By adding drops of 1 N NaOH to the mixture, the pH was adjusted to 8, and it was agitated for 6 h at 37–40°C. Centrifugation at 12,000 rpm for 30 min in 20°C separated the produced nanoparticles [10].
2.4 Methods used in various studies for the preparation of AgNPs
Due to the formation of AgNPs in the reaction mixture, the color changed from light yellow to dark brown during the incubation period. This color change was due to their specific properties (surface plasmon resonance). UV-Vis spectroscopy, X-ray diffraction (XRD) pattern, transmission electron microscopy (TEM), scanning electron microscope (SEM) with energy dispersive X-ray (EDX), atomic force microscopy, dynamic light scattering, and thermal gravity-differential thermal analysis were all used to validate the synthesis of AgNPs [7]. AgNPs can be prepared by using the green synthesis method as shown in Figure 4 and by using silver nitrate and sodium hydroxide with propolis extract.
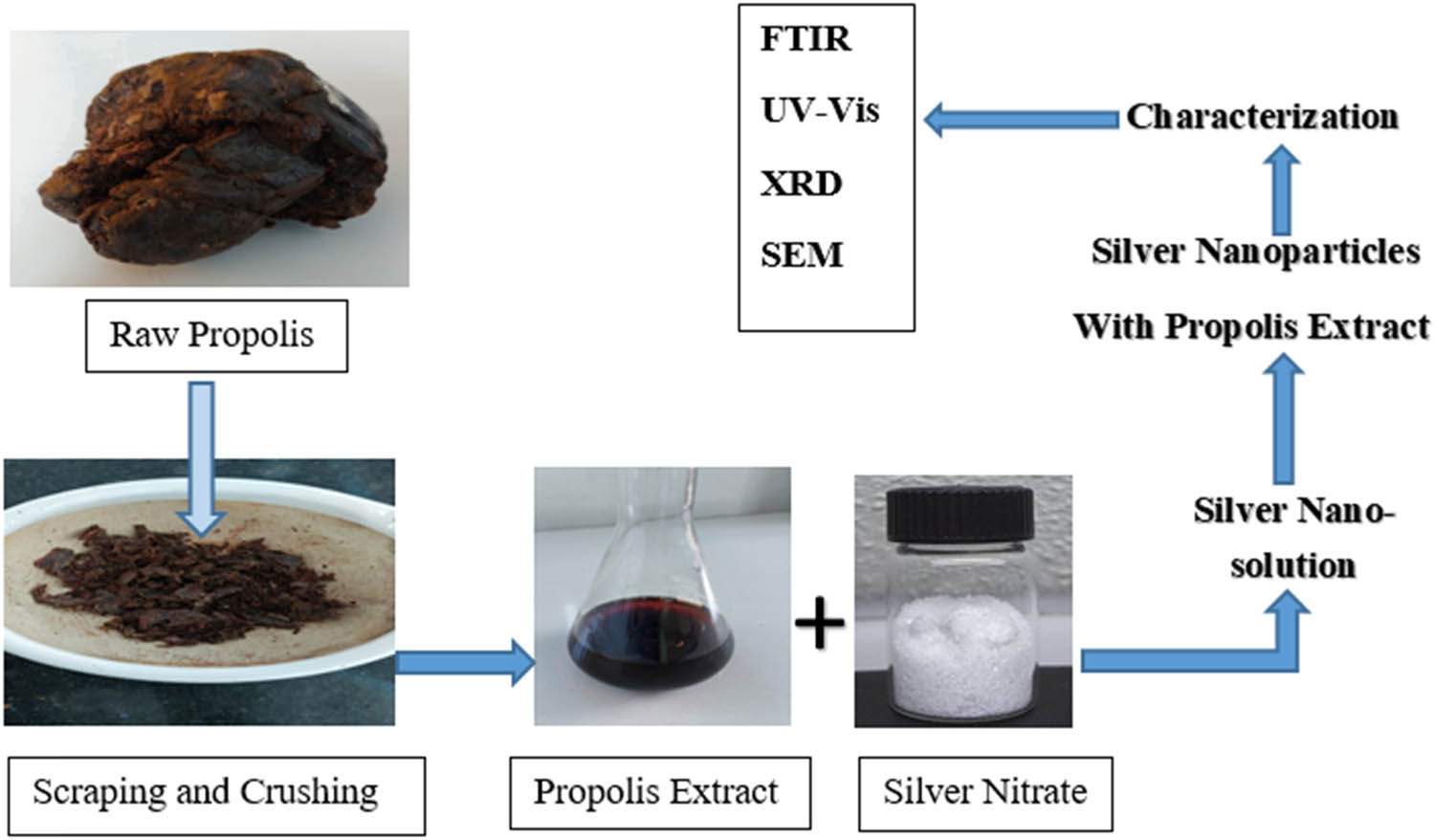
Schematic procedure for the biosynthesis of silver nanoparticles with propolis.
Green synthesis method was used for the preparation of propolis AgNPs [9]. 1 mL of 10% propolis extract was mixed with 9 mL of 5 mM silver nitrate (AgNO3) at room temperature using a magnetic stirrer to make AgNPs [9,20,89]. Color change had been used to visually assess the reaction, and the UV-Vis spectrum was regularly evaluated. For 30 min, the colloidal solution of the produced nanoparticles was centrifuged at 10,000 rpm. The final AgNPs were separated and dried after the process was repeated three times [20].
Propolis extract, AgNO3 (Dynamics, Brazil), and sodium hydroxide (Vetec, Brazil) were employed in the production of AgNPs containing propolis (AgNP-P). Propolis extracts were resuspended in water and stirred on the Quimis® Q261M23 plate, Brazil. This solution’s pH (0.1 M NaOH) was adjusted to 10.6, which is required for flavonoids to have electrons accessible for the silver reduction process 14. After adding AgNO3 and waiting for 30 min, the colloidal suspension was stored away from light. The experiments were carried out at a temperature of 32 ± 2°C [4].
3 Characterization of nanopropolis and nanoparticles (Ag and Se)
3.1 Characterization of nanopropolis
3.1.1 Determination of total encapsulated polyphenols or flavonoids
where A – total polyphenols or flavonoids added initially, and B – total unencapsulated polyphenols or flavonoids [43].
3.1.2 Particle size analysis
The diameters of the nano/micro particles were measured using a laser light scattering granularity analyzer (DelsaTM Nano, Germany) Beckman Coulter. A sample was taken and triplicate analyses were performed [43].
3.1.3 TEM analysis
TEM was used to study the microstructure of particles which was operated at 80 kV. The sample was obtained by placing one preparation drop on a collodion support on grids.
3.2 Characterization of SeNPs
Nanoparticles property was determined by evaluating the characteristics of nanoparticles such as size, structure, and shape [3,5,18,21,57,88,90]. Different methods to characterize the biosynthesized nanoparticles are given in Figure 5.
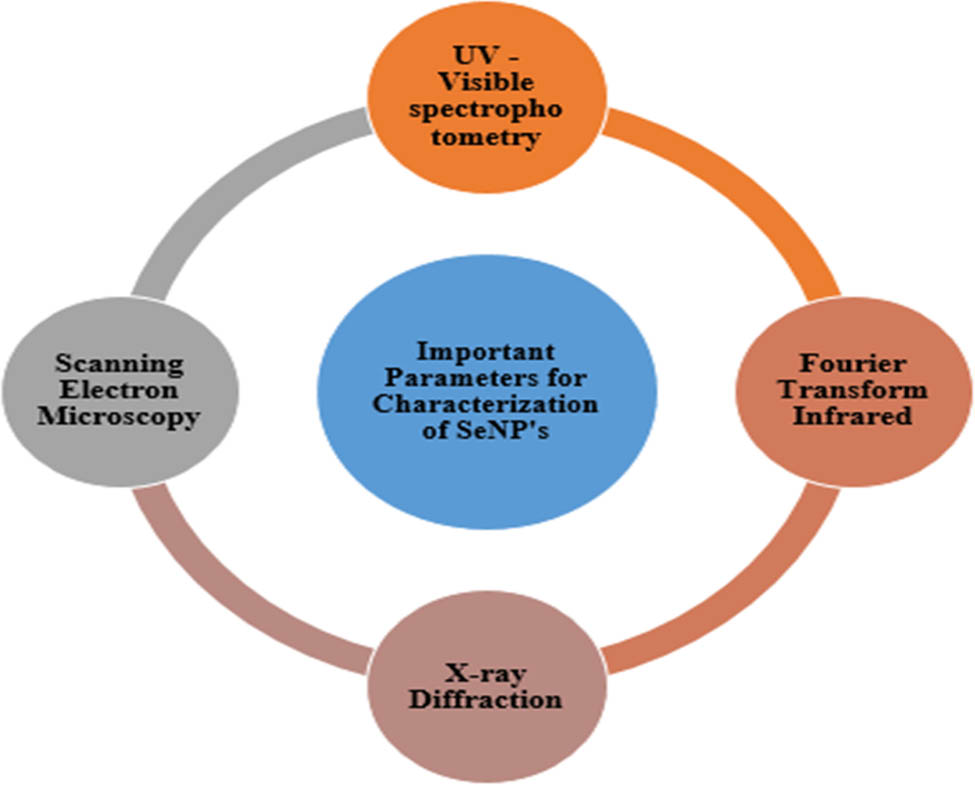
Characterization of biosynthesized nanoparticles.
3.2.1 UV-Vis spectrum analysis
For the identification and characterization of propolis, best used technique was UV-Vis spectrum. By using Genesys 10S UV-Vis spectrophotometer, the wavelength of biosynthesized SeNPs from 200–800 nm was recorded and analyzed for the maximum absorption peaks [48]. Shimadzu UV-1600, Japan, was used by authors of ref. [10] to study the spectrum with wave range of 190–1,100 nm, scan speed of sampling interval was 0.5 min.
3.2.2 Fourier transform infrared spectroscopy analysis (FT-IR)
Shimadzu – 00463 model spectrophotometer was used to obtain FT-IR spectrum. It was obtained in the spectral region of 4,000–600 cm−1 with a resolution of 4 cm−1 and 64 co-added scans [88]. FT-IR spectroscopy (ABB/spectrolab/MB3000/UK) was used to examine the produced SeNP. The FTIR characterization was done using the conventional KBr pellet technique, which measures infrared intensity vs wavelength (wave number) of light from 400 to 4,000 cm−1 [10]. Organic functional group responsible for stabilization of nanoparticles, nature of associated functional groups, structural features of biological extracts with nanoparticles and surface chemistry was confirmed by using FT-IR spectrum [10,88].
3.2.3 XRD analysis
XRD was used to investigate the composition of the synthesized particles [10], and also for the structure of nanoparticles and phase identification [88]. Bruker D8 Advanced Eco X-ray diffractometer having Bragg-Brentano focusing geometry was used to take the XRD readings and then this XRD data were analyzed for the identification of the crystalline phases by using X’pert high score software [88].
3.2.4 SEM analysis
To illustrate the morphological characteristics and size of biosynthesized SeNPs, scanning electron microscope (Hitachi, Model SU3500) with ultrahigh resolution field emission was used [88]. To analyze the morphology and form of the particles, samples were mildly pressed into pellets at 0.5 ton-load to achieve the best picture under SEM [10,91].
3.2.5 Antimicrobial assay
3.2.5.1 Antibacterial assay – resazurin microtiter plate method
To check the minimum inhibition concentration resazurin microtiter plate method was used. By this method, antibacterial activity of the biosynthesized SeNP’s against pathogenic gram-positive bacterial strains (Staphylococcus aureus, Bacillus cereus, and Streptococcus mutans) and gram-negative bacterial strains (Escherichia coli, Salmonella typhi, and Pseudomonas aeruginosa) was studied. In sterile water, resazurin solution (10%) was prepared and was stored as a stock solution at 20°C. When required it was used by diluting to 1:10 with sterile water. 100 μL of sterilized Luria Bertani (LB) broth, along with 30 µL of 0.1% resazurin solution, 100 μL of serially diluted biosynthesized SeNPs (1,000, 500, 250, 125, 62.5, 31.25, 15.25, and 7.81 µg‧mL−1) were added in each well of the microtiter plate. Consequently, inoculation was done for 100 μL of each bacterial culture, and to prevent the sample from drying, 200 μL of deionized water was added. The control wells were prepared with culture media, resazurin dye, and bacterial suspension. Broth and dye were added in color blank wells. Sealed plates were incubated for 24 h at 37°C. It was observed that after incubation, the color changed from blue to pink, whereas no growth of organisms was noticed in blue colored wells, only pink and orange colored wells exhibited positive results with growth of organisms [88].
3.2.5.2 Antifungal assay
Antifungal assay of biosynthesized SeNPs from propolis extract was studied by using well diffusion method. Pathogenic fungi such as Aspergillus niger, Aspergillus flavon, and Candida albicans were used. With solidified potato dextrose agar media, fungal inoculums of Aspergillus niger, Aspergillus flavon, and Candida albicans were thoroughly disseminated on sterilized Petri plates. Using sterile cork borer, 5 wells with 5.5 diameter were drilled out on each agar plate. Various sample concentrations of 50 μL were filled in these wells. Incubation of culture plate was done at 25°C for 72 h. For all the three pathogenic fungi, the zone of inhibition was measured in diameter (mm) surrounding the wells [88].
3.3 Characterization of AgNPs
The synthesis of AgNPs was monitored using a Jasco V530 double beam UV-Vis spectrophotometer to record the UV-Vis spectra. The scanning speed was 1,000 nm‧min−1 [20].
Bruker Vertex 70 (USA) was used for the FTIR measurements with a scanning range of 4,000–310 cm−1, and the materials were placed in KBr discs for spectral acquisition [20].
Delsa nano submicron particle size analyzer (Beckman Coulter, USA) was used for the determination of particle size and zeta potential of the synthesized AgNPs [20].
To determine the presence of elemental silver in AgNPs Quanta 200 environmental scanning electron microscope (ESEM) with EDX silicon-drift detector was used [20]. For the determination of surface morphology and size, TEM (Hitachi High-Tech HT7700 transmission electron microscope, Japan) was used [20].
3.3.1 Analysis of photocatalytic activity of synthesized AgNPs
Under sunlight irradiation, the degradation of malachite green was observed to determine the photocatalytic activity of the synthesized AgNPs. In 50 mL of malachite green solution (10 mg‧L−1), 10 mg AgNPs were added, and the mixture was magnetically swirled for 45 min in the dark. After that, the colloidal suspension was exposed to sunlight at room temperature with steady stirring under constant irradiation. The color shift was tracked over time, and the samples were obtained and centrifuged every 30 min. In the region of 400–800 nm, the supernatant was scanned. Meanwhile, a control containing only the dye was tested at the same conditions, as was a sample comprising AgNPs suspended in dye solution, constantly swirled in the dark [20].
3.3.2 Phagocytosis activity assay
Phagocytosis activity assay was performed to check the effectiveness of nanoparticles in stimulation of phagocytic cells. Suliman’s protocol (with some modifications) was used to assess macrophage phagocytic activity. Fresh human blood sample was isolated in tubes containing EDTA. 200
3.3.3 Biochemical assay
3.3.3.1 In-vitro antimicrobial assay
Agar diffusion method was used to evaluate the antimicrobial activity, for fungi, Mueller-Hinton medium (HiMedia, Germany) was used, and for bacteria, Mueller-Hinton agar (Oxoid, UK) was used [20]. Microorganisms used for antimicrobial assay were Staphylococcus aureus (S. aureus) ATCC 25923, Pseudomonas aeruginosa (P. aeruginosa) ATCC 27853, and Candida parapsilosis (C. parapsilosis) ATCC 22019 [20]. Inhibition zones in mm were measured after the samples (extract and AgNPs) were applied and incubated at 24°C for fungi and 37°C for bacteria for 24 h [20,92]. Ciprofloxacin (5 µg‧disc−1) and nystatin (100 µg‧disc−1) were used as positive controls. All experiments were carried out in triplicate, and the findings were expressed as mean ± standard deviation [20].
3.3.3.2 Determination of antioxidant activity
DPPH free radical scavenging assay and lipoxygenase inhibition assay were used to assess the antioxidant activity of the synthesized AgNPs. IC50 values were calculated for samples [20].
3.3.3.3 Determination of antibacterial activity
Author of ref. [4] studied the antibacterial activity against Staphylococcus aureus (CCCD-S007), Staphylococcus epidermidis (CCCD-S010), Escherichia coli (ATCC-25922), and Pseudomonas aeruginosa (ATCC-27853). M-11 Protocol of Clinical and Laboratory Standards Institute 30 was employed to determine the antibacterial activity. Minimum inhibitory concentration (MIC) was studied by broth microdilution. A 50% (v/v) Tween® 20 (Dynamics, Brazil) was used to dissolve the samples, which had an initial concentration of 33.3 mg‧mL−1. The microorganisms were added to all samples using the McFarland 0.5% scale, at a concentration of 1.5 × 106 CFU. The microplates were then conditioned in a bacteriological oven at 36°C for 24 h. 2,3,5-Triphenyltetrazolium chloride (1.0%) solution was added and allowed to react for 3 h to allow visualization. The growth control consisted of culture medium and bacteria, the negative control was Tween® 20 at the concentration used, the sterility control consisted only of culture medium, and the positive control was chlorhexidine. For MIC, culture medium used was brain heart infusion [4].
Aliquots from the MIC plate and above were aliquoted, seeded in Petri dishes, and transferred to a bacteriological oven under the same conditions as before to obtain the minimum bactericidal concentration (MBC). The microbe was still alive and well, as evidenced by colony expansion. The culture medium used for MBC was Mueller-Hinton (Both KSVI, Brazil) [4].
4 Analysis of results and discussions of various experiments for the characterization of nanopropolis and nanoparticles (Ag and Se)
4.1 Characterization of nanopropolis
4.1.1 Result for the determination of total encapsulated polyphenols and flavonoids
High flavonoid and moderate polyphenol capacities were exhibited by nanopropolis [1,43]. It was reported by authors of ref. [7] that encapsulation efficiency of total polyphenols and total flavonoids was 67% and 94%, respectively.
4.1.2 Particle size analysis
Particle size analyzer was used to determine the size of particles, variation was reported in nanoparticle size between 252 and 530 nm [43].
4.2 Results for the characterization of SeNPs
4.2.1 UV-vis spectrum analysis
In the range of 200–800 nm, the absorption spectrum was recorded for biosynthesized SeNPs. For selenium in the samples, a high absorption peak was recorded between 250 and 280 nm [88].
4.2.2 FT-IR analysis
FT-IR analysis confirmed the presence of different functional groups like alcohol and polyphenols. It was reported that the presence of these functional groups was mainly involved in the reduction of selenium ion to SeNPs. Peak values were observed between 2,950 and 2,850 cm−1 which were due to the presence of organic polymer lignin. It was evident from different research papers that absorption peak was present between 1,750 and 1,470 cm−1 which corresponded to the carboxyl group peak, also at 1,030 and 730 cm−1, another peak was observed which corresponded to phenolic group [88].
4.2.3 XRD analysis
The diffraction intensity was observed from 2
4.2.4 SEM analysis
For the assessment of the physical dimensions, morphology, and shape of the nanoparticles, widely used technique is SEM. Researchers revealed that the structure and size of biosynthesized nanoparticles get influenced by the concentration of polyphenol in propolis extract. Biosynthesized SeNPs by propolis extract were found to have spherical shape with a smooth surface and size range between 30 and 36 nm [10].
4.3 Results for the characterization of AgNPs from different research approaches
4.3.1 Determination of MIC and MBC
It was noticed that S. aureus and P. aeruginosa were amenable to AgNPs effect. Gram-positive bacteria (S. aureus) exhibited highest antibacterial activity that was most probably due to the differences in molecular makeup of cell walls [29,93]. AgNPs could be a source of reactive oxygen species (ROS), which can damage bacteria proteins and DNA, causing cell membrane permeability to change and bacterial membrane destruction [20,90]. MIC values for antibacterial activity of propolis was reported to be 4,162, 8,325, and 16,650 μg‧mL−1 for S. aureus, S. epidermidis, and P. aeruginosa, respectively. Different results had been reported in the literature: some show that AgNPs are more effective against Gram-positive bacteria than Gram-negative bacteria [20,56], while others lead to the opposite conclusion [20,24] given in Table 4. Furthermore, Gram-negative bacteria had an exterior lipidic membrane with a negative surface charge. The AgNPs linked with propolis (AgNP-P) share the same surface charge, which can cause electrostatic repulsion between them, making it difficult to fix and penetrate AgNPs into bacterial cells and necessitating a greater concentration to kill this type of bacteria.
Antibacterial activities of AgNPs [20]
| No. | Used microorganisms | Antibacterial activity diameter of inhibition zones (mm) |
|---|---|---|
| 1 | S. aureus | 10 ± 0.1 |
| 2 | P. aeruginosa | 2 ± 0 |
| 3 | C. parapsilosis | 9 ± 0.2 |
4.3.2 Visual and UV-Vis analysis
AgNPs’ biosynthesis was confirmed by observing the change in color of the reaction mixture which is due to the excitation of surface plasmon vibrations in AgNPs [7,20,94]. The color of the reaction mixture changed from white-yellowish to brown [20,94]. After continuous stirring, the UV-Vis spectra of the mixture extract, silver nitrate, was recorded in the 350–600 nm wavelength region, revealing the surface plasmon resonance [20]. Due to the formation of small spherical nanoparticles, an absorption band at 480 nm was recorded. AgNPs derived from aqueous propolis extract were active at a shorter wavelength [20]. Many other studies revealed the presence of peak at 420 nm [7], and in the propolis samples from Tamilnadu, absorption spectra was observed between 260 and 290 nm [95].
4.3.3 FT-IR analysis
FT-IR analysis was performed to determine the biomolecules identified in the propolis extract that are responsible for the reduction, capping, and stabilization of AgNPs. From the propolis extract the functional groups were recorded at different wavelengths [20]. The carboxylic group was detected in the FT-IR spectrum of the propolis aqueous extract without AgNO3 with an intense peak at 1,716 and 1,100 cm−1 [7]. The stretching vibrations of OH from phenolic compounds were observed at 3,403 cm−1 and stretching vibration of C–H were recorded at 2,919 and 2,849 cm−1. 1,635 cm−1 mainly corresponded to stretching vibration of C═C and C═O groups from flavonoids and asymmetric bending vibration of N–H from amino acids. Due to stretching C═C of aromatic ring, bending vibration of C–H from CH3, CH2, and the stretching vibration of aromatics from flavonoids and aromatic rings’ peak were recorded at 1,514 and 1,449 cm−1, respectively. 1,264 cm−1 corresponded to C–O group of polyols (hydroxyflavonoids), 1,082 cm−1 due to C–O stretching ester group, 815 cm−1 due to aromatic ring vibration, and 698 cm−1 corresponded to phenyl group [20,96]. The majority of the FT-IR bands in propolis aqueous extract were due to triterpenoids, flavonoids, furanoids, sugars, coumarins, quinines, tannins, phenols, and acids [7]. Some peaks in the FT-IR spectra of AgNPs shifted or vanished as a result, indicating that particular polyphenols, flavonoids, or amino acids are involved in the formation of nanoparticles [20].
4.3.4 Particle size and TEM analysis
The majority of AgNPs were spherical in shape [7,20] and ranged in size from 10 to 50 nm, with an average size of about 15 nm [20], 9–30 nm [7]. The TEM pictures revealed a weak aggregation tendency, which could be due to the extract’s contribution to AgNPs’ stabilization [20]. In a study, it was reported that the size of nanoparticles with ethanolic extract of propolis at pH = 10.62 was 50 nm and at same pH for water extract of propolis, it was 20 nm [97]. It was revealed in the field emission SEM analysis that the size of synthesized AgNPs was 62, 41, and 82 nm for the samples from different regions of Tamilnadu [95].
4.3.5 Determination of zeta potential
AgNPs coated with polyphenolic chemicals found in propolis extract had a negative zeta potential of −21.36 mV. This suggested that the suspension of colloidal nanoparticles was stable [20,24]. The AgNPs’ negatively charged surface reduces particle agglomeration by preventing particle rejection [20,57].
4.3.6 Determination of elemental silver in AgNPs
The existence of elemental silver was confirmed by the EDX spectra of AgNPs, which showed a peak pattern at 3 keV in the silver region [7,20,93]. Apart from silver, the most prominent elements in the chemical composition of AgNPs were carbon, which made up 53.26%, oxygen – 14.06%, and nitrogen – which made up 03.12%, showing the presence of compounds connected to nanoparticles [20]. After 4 h, a spectroscopic signal indicating the production of AgNPs was seen around 480 nm. In the extract and silver nitrate spectra, this peak does not appear. AgNPs are polydispersed, as seen by the spreading contour of the peak [5,295]. Atomic % reported via EDX spectrum of AgNPs was 91.23, 1.71, 0.66, 0.53, 0.96, and 4.91 for elements C-K, Na-K, Mg-K, Si-K, Ca-K, and Ag-L, respectively [95].
4.3.7 Analysis of photocatalytic activity
Malachite green (colorant) degradation was analyzed to determine the photocatalytic activity of the synthesized AgNPs. Malachite green, as well as its reduced form, can be found in both aquatic and terrestrial systems, posing a risk to human health [20,98]. Initially, the photocatalytic degradation was analyzed by color change, a color shift from dark blue to light blue was noticed. Thereafter, the diminution in color was measured spectrophotometrically by recording the absorbance at 617 nm. It could be possible to get a significant decline in absorbance over time, indicating a decrease in dye concentration owing to degradation. The control containing merely the dye did not change color when exposed to sunshine, while the color and absorbance of the sample mixed in the dark dropped.
Dye degradation was calculated by the formula:
where C o is the initial concentration of the malachite green, and C t is the concentration of the malachite green after each period of exposure time to sunlight [20,81]. It was illustrated that the obtained AgNPs were highly photocatalytically active under light radiation as when the sample was exposed to sunlight, dye degradation obtained was 90%, for the control degradation it was 5%, and 30% for the sample mixed in dark. Photons of visible light hitting AgNPs are absorbed via the surface plasmon resonance phenomenon, which causes electrons to be excited to a higher energy state during sunlight exposure.
4.3.8 Determination of phagocytosis activity assay
Phagocytosis is a process in which phagocytic cells engulf, destroy, and digest bacteria or foreign substances [9,99]. Significant increase was noticed in the activity of phagocytic cells. This increased activity of phagocytic cells to engulf bacteria could be due to certain chemical components present in the AgNPs of propolis which acted as immune modulators [9].
5 Conclusion
It was observed that due to the presence of natural reducing and stabilizing compounds in propolis extract, they had potential application in green synthesis of nanoparticles. A significant number of chemical components found in propolis have a synergistic influence in the formation of AgNPs. Nanopropolis exhibited high flavonoid content. UV-Vis spectra, XRD, FT-IR, and SEM images all verified the production of nanoparticles and their functionalization with ethanol extract of bee propolis. These SeNPs had shown substantial antioxidant action against bacterial and fungal strains. So they could be utilized to destroy pathogens in order to prevent diseases. In this review, applicability of propolis for biosynthesis of nanoparticles and its biomedical applications has been explored. Moreover, the role of propolis extract as stabilizer and reducer was confirmed by the biogenic synthesis of AgNPs. The findings of this review represents a promising nanoproduct with its pharmaceutical and biomedical applications.
6 Research possibilities
It was evident from different studies that alcohol and phenolic compounds found in propolis extracts were primarily responsible for the reduction of selenium ion to SeNPs [88]. The biosynthesized SeNPs from propolis extract could be a powerful antioxidant and antibacterial agent used to cure diseases caused by bacterial and fungal strains [3,88]. Still more research is needed to enhance propolis-based nanoparticles synthesis, their efficiency, particle size control, and use in medicine and healthcare [88]. Further, biosynthesized nanoparticles from propolis extract can also be checked for antitumoral, regenerative properties and its therapeutic efficacy.
Acknowledgment
Throughout the writing of this review article I have received a great deal of support and assistance. I would like to thank my supervisor, Dr. Priyanka Kumari, whose expertise was invaluable in formulating this review article.
-
Funding information: Authors state no funding involved.
-
Author contributions: Priyanka Kumari and Bindiya Barsola have made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data from research papers. Priyanka Kumari has given final approval of the version to be published. Bindiya Barsola have been involved in drafting the manuscript or revising it critically for important intellectual content.
-
Conflict of interest: Authors state no conflict of interest.
References
[1] Tatli Seven P, Seven I, Gul Baykalir B, Iflazoglu Mutlu S, Salem AZ. Nanotechnology and nano-propolis in animal production and health: An overview. Ital J Anim Sci. 2018;17(4):921–30. 10.1080/1828051X.2018.1448726.Suche in Google Scholar
[2] Chakravarthi V, Balaji S. Applications of nanotechnology in veterinary medicine. Vet World. 2010;3(10):477–80. 10.5455/vetworld.2010.Suche in Google Scholar
[3] Afrouzan H, Amirinia C, Mirhadi SA, Ebadollahi A, Vaseji N, Tahmasbi G. Evaluation of antimicrobial activity of propolis and nanopropolis against Staphylococcus aureus and Candida albicans. Afr J Microbiol Res. 2012;6(2):421–5. 10.5897/AJMR.Suche in Google Scholar
[4] Barbosa VT, Souza JK, Alvino V, Meneghetti MR, Florez‐Rodriguez PP, Moreira RE, et al. Biogenic synthesis of silver nanoparticles using Brazilian propolis. Biotechnol Prog. 2019;35(6):e2888. 10.1002/btpr.2888.Suche in Google Scholar PubMed
[5] Kagithoju S, Godishala V, Nanna RS. Eco-friendly and green synthesis of silver nanoparticles using leaf extract of Strychnos potatorum Linn. F. and their bactericidal activities. 3 Biotech. 2015;5(5):709–14. 10.1007/s13205-014-0272-3.Suche in Google Scholar PubMed PubMed Central
[6] Leela A, Vivekanandan M. Tapping the unexploited plant resources for the synthesis of silver nanoparticles. Afr J Biotechnol. 2008;7(17):3162–5. http://www.academicjournals.org/AJBSuche in Google Scholar
[7] Priyadarshini JF, Sivakumari K, Selvaraj R, Ashok K, Jayaprakash P, Rajesh S. Green synthesis of silver nanoparticles from propolis. Res J Life Sci Bioinform Pharm Chem Sci. 2018;4:23–36. 10.26479/2018.0404.02.Suche in Google Scholar
[8] De Marco S, Piccioni M, Pagiotti R, Pietrella D. Antibiofilm and antioxidant activity of propolis and bud poplar resins versus Pseudomonas aeruginosa. eCAM. 2017;2017:1–11. 10.1155/2017/5163575.Suche in Google Scholar PubMed PubMed Central
[9] Taqi ZJ, Abdul-Wahed HE, AL-Saadi HK, Jabir MS. Potential activity of silver nanoparticles synthesized by Iraqi propolis on phagocytosis. AIP Conf. Proc. 2020;2213(1):020104. 10.1063/5.0000155.Suche in Google Scholar
[10] Wali AT. Biosynthesis, characterization and bioactivity of selenium nanoparticles synthesized by propolis. Iraqi J Vet Med. 2019;43(1):197–209. 10.30539/iraqijvm.v43i1.490.Suche in Google Scholar
[11] Nancharaiah YV, Lens PN. Selenium biomineralization for biotechnological applications. Trends biotechnol. 2015;33(6):323–30. 10.1016/j.tibtech.2015.03.004.Suche in Google Scholar PubMed
[12] Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275(1):177–82. 10.1016/j.jcis.2004.02.012.Suche in Google Scholar PubMed
[13] Dubey SP, Lahtinen M, Sillanpää M. Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf A Physicochem Eng Asp. 2010;364(1–3):34–41. 10.1016/j.colsurfa.2010.04.023.Suche in Google Scholar
[14] Ndwandwe BK, Malinga SP, Kayitesi E, Dlamini BC. Advances in green synthesis of selenium nanoparticles and their application in food packaging. Int J Food Sci. 2020;56(6):2640–50. 10.1111/ijfs.14916.Suche in Google Scholar
[15] Lu J, Holmgren A. Selenoproteins. J Biol Chem. 2009;284(2):723–7. 10.1074/jbc.R800045200.Suche in Google Scholar PubMed
[16] Hadrup N, Loeschner K, Mandrup K, Ravn-Haren G, Frandsen HL, Larsen EH, et al. Subacute oral toxicity investigation of selenium nanoparticles and selenite in rats. Drug Chem Toxicol. 2019;42(1):76–83. 10.1080/01480545.2018.1491589.Suche in Google Scholar PubMed
[17] Levander OA. Clinical consequences of low selenium intake and its relationship to vitamin E. Ann N Y Acad Sci. 1982;393(1):70–82. 10.1111/j.1749-6632.1982.tb31233.x.Suche in Google Scholar PubMed
[18] Dwivedi C, Shah CP, Singh K, Kumar M, Bajaj PN. An organic acid-induced synthesis and characterization of selenium nanoparticles. J Nanotechnol. 2011;2011:1–6. 10.1155/2011/651971.Suche in Google Scholar
[19] Cao XB, Xie Y, Zhang SY, Li FQ. Ultra‐Thin Trigonal Selenium Nanoribbons Developed from Series‐Wound Beads. Adv Mater. 2004;16(7):649–53. 10.1002/adma.200306317.Suche in Google Scholar
[20] Corciova A, Mircea C, Burlec AF, Cioanca O, Tuchilus C, Fifere A, et al. Antioxidant, antimicrobial and photocatalytic activities of silver nanoparticles obtained by bee propolis extract assisted biosynthesis. Farmacia. 2019;67(3):482–9. 10.31925/farmacia.2019.3.16.Suche in Google Scholar
[21] Kumar DA, Palanichamy V, Roopan SM. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim Acta A Mol Biomol Spectrosc. 2014;127:168–71. 10.1016/j.saa.2014.02.058.Suche in Google Scholar PubMed
[22] Khatoon N, Mazumder JA, Sardar M. Biotechnological applications of green synthesized silver nanoparticles. J Nanosci Curr Res. 2017;2(1):107.10.4172/2572-0813.1000107Suche in Google Scholar
[23] Heydari R. Biological applications of biosynthesized silver nanoparticles through the utilization of plant extracts. Herb Med J. 2017;2(2):87–95. 10.22087/hmj.v2i2.618.Suche in Google Scholar
[24] Patil S, Chaudhari G, Paradeshi J, Mahajan R, Chaudhari BL. Instant green synthesis of silver-based herbo-metallic colloidal nanosuspension in Terminalia bellirica fruit aqueous extract for catalytic and antibacterial applications. 3 Biotech. 2017;7(1):36. 10.1007/s13205-016-0589-1.Suche in Google Scholar PubMed PubMed Central
[25] Jagtap UB, Bapat VA. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind Crop Prod. 2013;46:132–7. 10.1016/j.indcrop.2013.01.019.Suche in Google Scholar
[26] Chaloupka K, Malam Y, Seifalian AM. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28(11):580–8. 10.1016/j.tibtech.2010.07.006.Suche in Google Scholar PubMed
[27] Prow TW, Grice JE, Lin LL, Faye R, Butler M, Becker W, et al. Nanoparticles and microparticles for skin drug delivery. Adv Drug Deliv Rev. 2011;63(6):470–91. 10.1016/j.addr.2011.01.012.Suche in Google Scholar PubMed
[28] Chaudhry Q, Castle L. Food applications of nanotechnologies: an overview of opportunities and challenges for developing countries. Trends Food Sci Technol. 2011;22(11):595–603. 10.1016/j.tifs.2011.01.001.Suche in Google Scholar
[29] Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar DS. Nanoparticulate material delivery to plants. Plant Sci. 2010;179(3):154–63. 10.1016/j.plantsci.2010.04.012.Suche in Google Scholar
[30] Kelly FM, Johnston JH. Colored and functional silver nanoparticle− wool fiber composites. ACS Appl Mater Interfaces. 2011;3(4):1083–92. 10.1021/am101224v.Suche in Google Scholar PubMed
[31] Dankovich TA, Gray DG. Bactericidal paper impregnated with silver nanoparticles for point-of-use water treatment. Env Sci Technol. 2011;45(5):1992–8. 10.1021/es103302t.Suche in Google Scholar PubMed
[32] Küünal S, Kutti S, Rauwel P, Wragg D, Hussainova I, Rauwel E. New methodology for the antifungal testing of surfactant-free silver metal nanoparticles for applications in green housing. Key Eng Mater. 2016;674:133–8. 10.4028/www.scientific.net/KEM.674.133.Suche in Google Scholar
[33] Kim DM, Lee GD, Aum SH, Kim HJ. Preparation of propolis nanofood and application to human cancer. Biol Pharm Bull. 2008;31(9):1704–10. 10.1248/bpb.31.1704.Suche in Google Scholar
[34] Silva-Carvalho R, Baltazar F, Almeida-Aguiar C. Propolis: a complex natural product with a plethora of biological activities that can be explored for drug development. eCAM. 2015;2015:1–29. 10.1155/2015/206439.Suche in Google Scholar
[35] Daleprane JB, Abdalla DS. Emerging roles of propolis: antioxidant, cardioprotective, and antiangiogenic actions. eCAM. 2013;2013:1–8. 10.1155/2013/175135.Suche in Google Scholar
[36] Ghisalberti EL. Propolis: a review. Bee world. 1979;60(2):59–84. 10.1080/0005772X.1979.11097738.Suche in Google Scholar
[37] Bankova V. Chemical diversity of propolis and the problem of standardization. J Ethnopharmacol. 2005;100(1–2):114–7. 10.1016/j.jep.2005.05.004.Suche in Google Scholar
[38] Banskota AH, Nagaoka T, Sumioka LY, Tezuka Y, Awale S, Midorikawa K, et al. Antiproliferative activity of the Netherlands propolis and its active principles in cancer cell lines. Ethnopharmacol. 2002;80(1):67–73. 10.1016/s0378-8741(02)00022-3.Suche in Google Scholar
[39] Marcucci MC. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26(2):83–99. 10.1051/apido:19950202.Suche in Google Scholar
[40] Athikomkulchai S, Awale S, Ruangrungsi N, Ruchirawat S, Kadota S. Chemical constituents of Thai propolis. Fitoterapia. 2013;88:96–100. 10.1016/j.fitote.2013.04.008.Suche in Google Scholar PubMed
[41] Bueno-Silva B, Alencar SM, Koo H, Ikegaki M, Silva GV, Napimoga MH, et al. Anti-inflammatory and antimicrobial evaluation of neovestitol and vestitol isolated from Brazilian red propolis. J Agric Food Chem. 2013;61(19):4546–50. 10.1021/jf305468f.Suche in Google Scholar PubMed
[42] Sforcin JM. Propolis and the immune system: a review. J Ethnopharmacol. 2007;113(1):1–14. 10.1016/j.jep.2007.05.012.Suche in Google Scholar
[43] Sahlan M, Supardi T. Encapsulation of Indonesian propolis by casein micelle. Int J Pharma Bio Sci. 2013;4(1):297–305.Suche in Google Scholar
[44] Chung NK, Cho YC, Ha CS, Kim HS. Hypoglycemic effects of nano powder propolis on streptozotocin-induced diabetic rats. Korean J Vet Serv. 2010;33(2):199–206.Suche in Google Scholar
[45] Dkhil MA, Zrieq R, Al-Quraishy S, Abdel Moneim AE. Selenium nanoparticles attenuate oxidative stress and testicular damage in streptozotocin-induced diabetic rats. Molecules. 2016;21(11):1517. 10.3390/molecules21111517.Suche in Google Scholar
[46] Aaseth J, Alexander J, Bjørklund G, Hestad K, Dusek P, Roos PM, et al. Treatment strategies in Alzheimer’s disease: a review with focus on selenium supplementation. Biometals. 2016;29(5):827–39. 10.1007/s10534-016-9959-8.Suche in Google Scholar
[47] Peng F, Guo X, Li Z, Li C, Wang C, Lv W, et al. Antimutagenic effects of selenium-enriched polysaccharides from pyracantha fortuneana through suppression of cytochrome P450 1A subfamily in the mouse liver. Molecules. 2016;21(12):1731. 10.3390/molecules21121731.Suche in Google Scholar
[48] Hassan CE, Webster TJ. The effect of red-allotrope selenium nanoparticles on head and neck squamous cell viability and growth. Int J Nanomed. 2016;11:3641–54. 10.2147/IJN.S105173.Suche in Google Scholar
[49] Stolzoff M, Webster TJ. Reducing bone cancer cell functions using selenium nanocomposites. J Biomed Mater Res. 2016;104(2):476–82. 10.1002/jbm.a.35583.Suche in Google Scholar
[50] Maiyo F, Singh M. Selenium nanoparticles: Potential in cancer gene and drug delivery. Nanomedicine. 2017;12(9):1075–89. 10.2217/nnm-2017-0024.Suche in Google Scholar
[51] Rayman MP. The importance of selenium to human health. lancet. 2000;356(9225):233–41. 10.1016/S0140-6736 (00)02490-9.Suche in Google Scholar
[52] Wang Q, Larese-Casanova P, Webster TJ. Inhibition of various gram-positive and gram-negative bacteria growth on selenium nanoparticle coated paper towels. Int J Nanomed. 2015;10:2885–94. 10.2147/IJN.S78466.Suche in Google Scholar PubMed PubMed Central
[53] Shakibaie M, Mohazab NS, Mousavi SAA. Antifungal activity of selenium nanoparticles synthesized by Bacillus species Msh-1 against Aspergillus fumigatus and Candida albicans. Jundishapur J Microbiol. 2015;8(9):e26381. 10.5812/jjm.26381.Suche in Google Scholar PubMed PubMed Central
[54] Guisbiers G, Wang Q, Khachatryan E, Mimun LC, Mendoza-Cruz R, Larese-Casanova P, et al. Inhibition of E. coli and S. aureus with selenium nanoparticles synthesized by pulsed laser ablation in deionized water. Int J Nanomed. 2016;11:3731–6. 10.2147/IJN.S106289.Suche in Google Scholar PubMed PubMed Central
[55] Wijnhoven SW, Peijnenburg WJ, Herberts CA, Hagens WI, Oomen AG, Heugens EH, et al. Nano-silver–a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology. 2009;3(2):109–38. 10.1080/17435390902725914.Suche in Google Scholar
[56] Khalil MM, Ismail EH, El-Baghdady KZ, Mohamed D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab J Chem. 2014;7(6):1131–9. 10.1016/j.arabjc.2013.04.007.Suche in Google Scholar
[57] Padalia H, Moteriya P, Chanda S. Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arab J Chem. 2015;8(5):732–41. 10.1016/j.arabjc.2014.11.015.Suche in Google Scholar
[58] Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Env Microbiol. 2007;73(6):1712–20. 10.1128/AEM.02218-06.Suche in Google Scholar PubMed PubMed Central
[59] Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomed Nanomed – Nanotechnol. 2007;3(1):95–101. 10.1016/j.nano.2006.12.001.Suche in Google Scholar PubMed
[60] Baker C, Pradhan A, Pakstis L, Pochan DJ, Shah SI. Synthesis and antibacterial properties of silver nanoparticles. J Nanosci Nanotechnol. 2015;5(2):244–9. 10.1166/jnn.2005.034.Suche in Google Scholar PubMed
[61] Shahverdi AR, Minaeian S, Shahverdi HR, Jamalifar H, Nohi AA. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: a novel biological approach. Process Biochem. 2007;42(5):919–23. 10.1016/j.procbio.2007.02.005.Suche in Google Scholar
[62] Sharma VK, Yngard RA, Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci. 2009;145(1–2):83–96. 10.1016/j.cis.2008.09.002.Suche in Google Scholar PubMed
[63] Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ. Metal oxide nanoparticles as bactericidal agents. Langmuir. 2002;18(17):6679–86. 10.1021/la0202374.Suche in Google Scholar
[64] Cao Y, Jin R, Mirkin CA. DNA-modified core− shell Ag/Au nanoparticles. J Am Chem Soc. 2001;123(32):7961–2. 10.1021/ja011342n.Suche in Google Scholar PubMed
[65] Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomed Nanomed-Nanotechnol. 2007;3(1):95–101. 10.1016/j.nano.2006.12.001.Suche in Google Scholar PubMed
[66] Panáček A, Kolář M, Večeřová R, Prucek R, Soukupova J, Kryštof V, et al. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials. 2009;30(31):6333–40. 10.1016/j.biomaterials.2009.07.065.Suche in Google Scholar PubMed
[67] Sun RWY, Chen R, Chung NPY, Ho CM, Lin CLS, Che CM. Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. ChemComm. 2005;40:5059–61. 10.1039/B510984A.Suche in Google Scholar
[68] Elechiguerra JL, Burt JL, Morones JR, Bragado AC, Gao X, Lara HH, et al. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnol. 2005;3(1):6. 10.1186/1477-3155-3-6.Suche in Google Scholar PubMed PubMed Central
[69] Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnol. 2010;8(1):1–10. 10.1186/1477-3155-8-1.Suche in Google Scholar PubMed PubMed Central
[70] Suvith VS, Philip D. Catalytic degradation of methylene blue using biosynthesized gold and silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc. 2014;118:526–32. 10.1016/j.saa.2013.09.016.Suche in Google Scholar PubMed
[71] Kumar P, Govindaraju M, Senthamilselvi S, Premkumar K. Photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Ulva lactuca. Colloids Surf B. 2013;103:658–61. 10.1016/j.colsurfb.2012.11.022.Suche in Google Scholar PubMed
[72] Jiang ZJ, Liu CY, Sun LW. Catalytic properties of silver nanoparticles supported on silica spheres. J Phys Chem. 2005;109(5):1730–5. 10.1021/jp046032g.Suche in Google Scholar PubMed
[73] Madene A, Jacquot M, Scher J, Desobry S. Flavour encapsulation and controlled release–a review. Int J Food Sci. 2006;41(1):1–21. 10.1111/j.1365-2621.2005.00980.x.Suche in Google Scholar
[74] Hamdi D, Wijanarko A, Hermansyah H, Asih SC, Sahlan M. Production of nanopropolis using high pressure ball mill homogenizer. IOP Conf Ser Earth Env Sci. 2019;217(1):012014. 10.1088/1755-1315/217/1/012014.Suche in Google Scholar
[75] do Nascimento TG, da Silva PF, Azevedo LF, da Rocha LG, de Moraes Porto IC, Lima E, et al. Polymeric nanoparticles of Brazilian red propolis extract: preparation, characterization, antioxidant and leishmanicidal activity. Nanoscale res lett. 2016;11(1):301. 10.1186/s11671-016-1517-3.Suche in Google Scholar PubMed PubMed Central
[76] Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A, et al. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnology. 2007;5(1):1–18. 10.1186/1477-3155-5-3.Suche in Google Scholar PubMed PubMed Central
[77] Hatami R, Javadi A, Jafarizadeh-Malmiri H. Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization. Green Process Synth. 2020;9(1):685–92. 10.1515/gps-2020-0065.Suche in Google Scholar
[78] Zhang J, Wang H, Yan X, Zhang L. Comparison of short-term toxicity between Nano-Se and selenite in mice. Life Sci. 2005;76(10):1099–109. 10.1016/j.lfs.2004.08.015.Suche in Google Scholar PubMed
[79] Noah NM, Ndangili PM. Green synthesis of nanomaterials from sustainable materials for biosensors and drug delivery. Sens Int. 2022;3:100166. 10.1016/j.sintl.2022.100166.Suche in Google Scholar
[80] Iranifam M, Fathinia M, Rad TS, Hanifehpour Y, Khataee AR, Joo SW. A novel selenium nanoparticles-enhanced chemiluminescence system for determination of dinitrobutylphenol. Talanta. 2013;107:263–9. 10.1016/j.talanta.2012.12.043.Suche in Google Scholar PubMed
[81] Latha D, Arulvasu C, Prabu P, Narayanan V. Photocatalytic activity of biosynthesized silver nanoparticle from leaf extract of Justicia adhatoda. Mech Mater Sci Eng J. 2017;9(1)):1–6. 10.2412/mmse.81.72.41.Suche in Google Scholar
[82] Tan Y, Wang Y, Jiang L, Zhu D. Thiosalicylic acid-functionalized silver nanoparticles synthesized in one-phase system. J Colloid Interf Sci. 2002;249(2):336–45. 10.1006/jcis.2001.8166.Suche in Google Scholar PubMed
[83] Petit C, Lixon P, Pileni MP. In situ synthesis of silver nanocluster in AOT reverse micelles. J Phys Chem. 1993;97(49):12974–83. 10.1021/j100151a054.Suche in Google Scholar
[84] Wing-WaháYam V. High-yield synthesis of selenium nanowires in water at room temperature. ChemComm. 2006;9:1006–8. 10.1039/b515025f.Suche in Google Scholar
[85] Zhu Y, Qian Y, Huang H, Zhang M. Preparation of nanometer-size selenium powders of uniform particle size by γ-irradiation. Mater Lett. 1996;28(1–3):119–22. 10.1016/0167-577X(96)00046-8.Suche in Google Scholar
[86] Oremland RS, Herbel MJ, Blum JS, Langley S, Beveridge TJ, Ajayan PM, et al. Structural and spectral features of selenium nanospheres produced by Se-respiring bacteria. Appl Env Microbiol. 2004;70(1):52–60. 10.1128/AEM.70.1.52-60.2004.Suche in Google Scholar PubMed PubMed Central
[87] Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Peng Q, et al. Nano-selenium and its nanomedicine applications: a critical review. Int J Nanomed. 2018;13:2107–28. 10.2147/IJN.S157541.Suche in Google Scholar PubMed PubMed Central
[88] Shubharani R, Mahesh M, Murthy V. Biosynthesis and Characterization, Antioxidant and Antimicrobial Activities of Selenium Nanoparticles from Ethanol Extract of Bee Propolis. J Nanomed Nanotechnol. 2019;10(1):1–7. 10.4172/2157-7439.1000522.Suche in Google Scholar
[89] Alsaedi II, Taqi ZJ, Hussien AMA, Sulaiman GM, Jabir MS. Graphene nanoparticles induces apoptosis in MCF-7 cells through mitochondrial damage and NF-KB pathway. Mater Res Exp. 2019;6(9):095413. 10.1088/2053-1591/ab33af.Suche in Google Scholar
[90] Nayagam V, Gabriel M, Palanisamy K. Green synthesis of silver nanoparticles mediated by Coccinia grandis and Phyllanthus emblica: a comparative comprehension. Appl Nanosci. 2018;8(3):205–19. 10.1007/s13204-018-0739-3.Suche in Google Scholar
[91] Wang Z, Fang C, Megharaj M. Characterization of iron–polyphenol nanoparticles synthesized by three plant extracts and their fenton oxidation of azo dye. ACS Sustain. Chem Eng. 2014;2(4):1022–5. 10.1021/sc500021n.Suche in Google Scholar
[92] Ioannou E, Poiata A, Hancianu M, Tzakou O. Chemical composition and in vitro antimicrobial activity of the essential oils of flower heads and leaves of Santolina rosmarinifolia L. from Romania. Nat Prod Res. 2007;21(1):18–23. 10.1080/14786410600921706.Suche in Google Scholar PubMed
[93] Kumar TVR, Murthy JSR, Rao MN, Bhargava Y. Evaluation of silver nanoparticles synthetic potential of Couroupita guianensis Aubl, flower buds extract and their synergistic antibacterial activity. 3 Biotech. 2016;6(1):92. 10.1007/s13205-016-0407-9.Suche in Google Scholar PubMed PubMed Central
[94] Shetty P, Supraja N, Garud M, Prasad TNVKV. Synthesis, characterization and antimicrobial activity of Alstonia scholaris bark-extract-mediated silver nanoparticles. J Nanostructure Chem. 2014;4(4):161–70. 10.1007/s40097-014-0132-z.Suche in Google Scholar
[95] Jayanthi B. Biosynthesis of Silver Nanoparticles using stingless bee propolis and their biomedical applications. PhD, Post Graduate and Research Department of Chemistry Ethiraj College for Women (Autonomous) Chennai; 2017.Suche in Google Scholar
[96] Oliveira RN, Mancini MC, Oliveira FCSD, Passos TM, Quilty B, Thiré RMDSM, et al. FTIR analysis and quantification of phenols and flavonoids of five commercially available plant extracts used in wound healing. Rev Mater. 2016;21:767–79. 10.1590/S1517-707620160003.0072.Suche in Google Scholar
[97] Roy N, Mondal S, Laskar RA, Basu S, Mandal D, Begum NA. Biogenic synthesis of Au and Ag nanoparticles by Indian propolis and its constituents. Colloids Surf B. 2010;76(1):317–25. 10.1016/j.colsurfb.2009.11.011.Suche in Google Scholar PubMed
[98] Cha CJ, Doerge DR, Cerniglia CE. Biotransformation of malachite green by the fungus Cunninghamella elegans. Appl. Environ. Microbio. 2001;67(9):4358–60. 10.1128/AEM.67.9.4358-4360.2001.Suche in Google Scholar PubMed PubMed Central
[99] Jabir MS, Sulaiman GM, Taqi ZJ, Li D. Iraqi propolis increases degradation of IL-1β and NLRC4 by autophagy following Pseudomonas aeruginosa infection. Microbes Infect. 2018;20(2):89–100. 10.1016/j.micinf.2017.10.007.Suche in Google Scholar PubMed
© 2022 Bindiya Barsola and Priyanka Kumari, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Artikel in diesem Heft
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

