Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
-
Moustafa Sherif Moustafa
, Afaf Abd El-Hameed
Abstract
The development of efficient methods for the synthesis of polyfunctional N-heterocycles is an important area of research in organic and medicinal chemistry. Pyrazolo[1,5-a]pyrimidine derivatives are purine analogous of biomedical importance and have been extremely studied for their broad spectrum of biological activities. Recently, they have attracted great interest in materials science owing to their photophysical properties. 3(5)-Aminopyrazoles are extensively utilized in the synthesis of condensed heterocyclic systems, particularly pyrazolo[1,5-a]pyrimidines via the reaction with 1,3-biselectrophilic reagents. However, the information available in the literature provides little in the way of reasoning their cyclization, particularly the initial attack either by the exocyclic amino group or endocyclic nitrogen. Unfortunately, the relative nucleophilicity of exo- and endocyclic nitrogen atoms in 1-unsubstituted 3(5)-aminopyrazoles is not clear and contradicting. It has been found that other factors can modulate the regioselectivity rather than basicity or steric hindrance for both active sites. The reported studies in the structure–activity relationship revealed that pyrazolo[1,5-a]pyrimidines having a substitution at fifth, sixth, and seventh positions possess potent biological activities, especially those with an amino group at the seventh position. We here developed a regioselective, high yield synthesis of 7-amino-5-arylpyrazolo[1,5-a]pyrimidine-3,6-dicarbonitriles by the reaction of N-(5-amino-4-cyano-1H-pyrazole-3-yl)-benzamide with various cinnamonitriles and enaminones in pyridine at 120°C under controlled microwave heating conditions. All structures of newly synthesized compounds were established by analytical and spectral data as well as single-crystal diffraction and rationalized for their formation.
1 Introduction
Heterocyclic compounds containing pyrazole scaffold signify a wide spectrum of pharmacological activities and constitute a common nucleus in a variety of biologically active compounds [1,2,3,4,5,6]. Pyrazolo[1,5-a]pyrimidines are potential molecules of interest [7] and have been reported to possess anticancer [8,9], antimicrobial [10,11], antiviral [12], antifungal [13], anti-inflammatory [14], as well as large range of activities [15,16,17,18,19]. Examples of pyrazolo[1,5-a]pyrimidine’s marketed drugs are illustrated in Figure 1.
![Figure 1
Pyrazolo[1,5-a]pyrimidines marketed drugs.](/document/doi/10.1515/gps-2022-0009/asset/graphic/j_gps-2022-0009_fig_001.jpg)
Pyrazolo[1,5-a]pyrimidines marketed drugs.
In this regard, the nature of substituents at fifth, sixth, and seventh positions displayed a crucial role in their biological potency. It is worth mentioning that the presence of an amino group at the seventh position had an additional advantage to form hydrogen bonds with the hinger region of the receptor [20].
The nucleophilicity and reactivity of different active centers in 5-aminopyrazole scaffolds have received considerable interest over the years. Concerning nitrogen centers, several published articles argue that in some synthetic protocols ring nitrogen is the most nucleophilic center while the opposite is observed in others [21,22]. Many different factors such as the nature of the ring substituent, temperature, pressure, solvent, catalyst type as well as the type of reaction controlling either the kinetic or thermodynamic can be utilized to modulate the selectivity of several transformations.
It has been reported that the reaction of 4-unsubstituted 3(5)-aminopyrazoles with bidentate reagents relies on the participation of the exocyclic amino group with ring nitrogen forming pyrazolo[1,5-a]pyrimidines, pyrazolo[5,1-c]-1,2,4-triazines, pyrazolo[1,5-a]-1,3,5-triazines or the nucleophilic carbon located at C4 of the pyrazole ring leading to pyrazolo[3,4-b]pyridines [23,24,25]. Whereas, for C4-substituted 3(5)-aminopyrazoles, considerable attention is required for the synthesis of bicyclic fused pyrazoloazines. Cyclization is affected by the electronic nature, either electron-donating or electron-attracting, of C-4. For example, electron-donating moieties augment the reactivity of the ring nitrogen favoring its initial attack by the bidentate reagent. However, electron-attracting substituents favor the initial attack by the less sterically hindered NH2 group.
It has been reported that the nature of the solvents plays a crucial role in such regioselective cyclocondensation. Owing to the amphoteric nature of 3(5)-aminopyrazoles, a basic solvent generates a heterocyclic anion resulting from the deprotonation of acidic-pyrole-like ring nitrogen, which enhances its reactivity. However, the acidic medium favors the exocyclic attack via protonating of the ring affording a cationic nucleus [26,27].
Arguably, a thorough investigation to establish the chemistry and regioselectivity of 3(5)-aminopyrazoles leading to complex heterocyclic scaffolds has not been established. Nevertheless, in-depth studies will be beneficial to elucidate their reactivity, their behavior and versatility in different environments, leading to the formation of a diversity of azoloazines in order to develop efficient synthetic methodologies.
Interestingly, a wide range of chemical transformations has now been performed under controlled microwave heating conditions. As compared to conventional heating reactions, microwaves couple directly with the molecules of the entire reaction mixture. Importantly, microwave heating possesses several advantages such as spectacular acceleration of many reactions, higher yields, mild reaction conditions, and shorter reaction times [28,29]. Moreover, several reports postulate the existence of a microwave effect as a specific radiation effect rather than the thermal one to rationalize for rate acceleration, changes in the reactivity as well as selectivity [30,31]. Our aim was to use a variety of factors to control the regioselectivity in such reactions leading to 7-aminopyrazolo[1,5-a]pyrimidines.
2 Materials and methods
Aldehydes, malononitrile, dimethylformamide dimethylacetal, and ketones were of commercial grade, and Pyridine was of analytically pure grade; all were purchased from Aldrich and Merck companies. 1H NMR (600 MHz) and 13C NMR (150 MHz) spectra were recorded using a Bruker DPX instrument (δ ppm). Mass spectra were determined by using a VG Auto spec QMS 30 and MSg (AEI) spectrometer in the EI (70 eV) mode. Melting points were recorded in a Gallen Kamp melting point apparatus and are uncorrected. X-ray crystallography was performed by using a Rigaku Rapid II and Bruker X8 Prospector single crystal X-ray diffractometer. All reactions were monitored by TLC with toluene/acetone 10:5, 10:6 as an eluent and were carried out until the starting materials were completely consumed.
2.1 General procedure for the synthesis of 7-aminopyrazolo[1,5-a]pyrimidine derivatives 4a–f and 7-arylpyrazolo[1,5-a]derivatives 7a–d
A solution of compounds 1 (1 mmol), 2 (1 mmol), or 5 (1 mmol) in pyridine (10 mL) was heated under reflux in a Milestone Microwave Lab station at 120°C for 20 min. The solvent was removed under reduced pressure, and the solid formed was isolated by filtration and recrystallized utilizing an appropriate solvent (Table 1).
Description of synthesized products 4a–f and 7a–d
| Compound no. | Solvent of recrystallization | Color of the product | Melting point (°C) |
|---|---|---|---|
| 4a | Ethanol | Orange crystals | 308–310 [33] |
| 4b | Ethanol-dioxane | Reddish brown crystals | >360 |
| 4c | Ethanol | Yellow crystals | 318–320 |
| 4d | Ethanol | Brown crystals | 310–312 |
| 4e | Ethanol | Yellow crystals | 288–290 |
| 4f | Ethanol | Brown crystals | 259–260 |
| 7a | Ethanol-dioxane | Brown crystals | 238–240 |
| 7b | Ethanol | Yellow crystals | 268–270 |
| 7c | Ethanol | Reddish brown crystals | 270–272 |
| 7d | Ethanol | Orange crystals | 168–170 |
2.2 7-Amino-5,2-diphenylpyrazolo[1,5-a]pyrimidine-3,6-dicarbonitrile (4a)
Yield: 0.309 g (92%); R f = 0.57 (toluene/acetone 10:5); 1H NMR (600 MHz, DMSO-d 6) δ 6.97 (1H, t, J = 6–6 Hz, Ar–H); 7.31 (2H, t, J = 7.8 Hz Ar–H); 7.56–7.59 (m, 3H, Ar–H); 7.84,7.85 (2H, dd, J = 3.3, 4.2 Hz, Ar–H); 7.95 (2H, d, J = 7.8 Hz, Ar–H); 9.02 (br, s, 2H, NH2, D2O exchangeable); 9.51 (s, 1H, NH, D2O exchangeable). 13C NMR (150 MHz, DMSO-d 6) δ = 62.76; 66.32; 70.20; 75.69; 113.32; 115.88; 118.19; 121.27; 128.38; 128.65; 128.67; 130.59; 136.67; 140.35; 149.48; 150.85; 155.63; 162.10. Analysis results for C20H13N7: C, 68.37; H, 3.73; N, 27.90; found: C, 68.22; H, 3.82; N, 27.88; EIMS (m/z): 351.01 [M+].
2.3 7-Amino-5-(4-nitrophenyl)-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3,6-dicarbonitrile (4b)
Yield: 0.356 g (90%); R f = 0.52 (toluene/acetone 10:5); 1H NMR (600 MHz, DMSO-d 6) δ 6.97 (1H, t, J = 6.6 Hz, Ar–H); 7.31 (2H, d, J = 1.2 Hz, Ar–H); 7.94 (2H, J = 7.8 Hz, Ar–H); 8.09 (2H, d, J = 9 Hz, Ar–H); 8.39 (2H, d, J = 8.4 Hz); 9.12 (br, s, 2H, NH2); 9.54 (s, 1H, NH). 13C NMR (150 MHz, DMSO-d 6) δ 66.34; 70.71; 76.11; 113.12; 115.49; 118.24; 121.36; 123.57; 128.68; 130.19; 140.28; 142.57; 148.48; 149.33; 150.72; 155.71; 159.99. Analysis results for C20H12N8O2: C, 60.60; H, 3.05; N, 28.27; found: C, 60.63; H, 3.12; N, 28.33; EIMS (m/z): 396.03 [M+].
2.4 7-Amino-5-(4-chlorophenyl)-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3,6-dicarbonitrile (4c)
Yield: 0.354 g (92%); R f = 0.75 (toluene/acetone 10:5); 1H NMR (600 MHz, DMSO-d 6) δ 6.97 (1H, t, J = 7.2 Hz, Ar–H); 7.31 (2H, t, J = 7.2 Hz, Ar–H); 7.64 (2H, d, J = 8.4 Hz, Ar–H); 7.87 (2H, d, J = 8.4 Hz, Ar–H); 7.95 (2H, d, J = 8.4 Hz, Ar–H); 9.03 (2H, br, s, NH2); 9.52 (1H, s, NH). 13C NMR (150 MHz, DMSO-d 6) δ 70.35; 75.68; 113.26; 115.78; 121.31; 128.52; 128.68; 130.53; 135.45; 140.33; 149.43; 150.79U; 155.65; 160.82. Analysis results for C20H12CLN7: C, 62.26; H, 3.14; Cl, 9.19; N, 25.41; found: C, 62.32; H, 3.18; Cl, 9.12; N, 25.58; EIMS (m/z) 385.12 [M+].
2.5 7-Amino-5-(4-methoxyphenyl)-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3,6-dicarbonitrile (4d)
Yield: 0.354 g (93%); 1H NMR (600 MHz, DMSO-d 6) δ 3.52 (3H, s, OCH3); 6.97 (1H, t, J = 7.2 Hz, Ar–H); 7.02–7.13 (2H, m, Ar–H); 7.03 (2H, t, J = 7.8 Hz, Ar–H); 7.85–7.88 (2H, m, Ar–H); 7.95 (2H, t, J = 6.6 Hz, Ar–H); 8.3 (2H, br, s, NH2); 9.48 (1H, s, NH); 13C NMR (150 MHz, DMSO-d 6) δ 55.39; 60.11; 76.8; 113.42; 113.76; 114.77; 115.13; 116.06; 116.17; 121.22; 124.07; 128.41; 128.65; 128.78; 130.42; 133.31; 140.38; 149.57, 150.83; 155.62; 160.39; 161.25; 161.37; 164.31. Analysis results for C21H15N6O: C, 66.13; H, 3.96; N, 25.71; found: C, 66.22; H, 4.01; N, 25.75; EIMS (m/z) 381.11 [M+]
2.6 7-Amino-5-(2-chlorophenyl)-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3,6-dicarbonitrile (4e)
Yield: 0.351 g (91%); 1H NMR (600 MHz, DMSO-d 6) δ 6.98 (1H, t, J = 7.8 Hz, Ar–H); 7.32 (2H, t, J = 7.8 Hz, Ar–H); 7.51 (1H, t, J = 7.2 Hz, Ar–H); 7.54 (2H, d, J = 1.8 Hz, Ar–H); 7.53 (2H, d, Ar–H); 7.565–7.592 (2H, m, Ar–H); 7.64–7.65 (1H, m, Ar–H); 7.95 (d, 2H, J = 7.8, Ar–H); 9.14 (2H, br, s, NH2); 9.565 (1H, s, NH), 13C NMR (150 MHz, DMSO-d 6) δ 66.35; 70.62; 78.16; 113.10; 114.66; 116.09; 118.24; 121.40; 127.41; 128.41; 128.58; 128.72; 129.56; 130.42; 131.07; 131.45; 136.24; 140.31; 148.62; 150.79; 155.64; 161.44. Analysis results for C20H12ClN7: C, 62.26; H, 3.14; Cl, 9.19; N, 25.41; found: C, 62.24; H, 3.21; Cl, 9.23; N, 25.39; EIMS (m/z): 385.03 [M+].
2.7 7-Amino-5-(3-bromophenyl-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3,6-dicarbonitrile (4f)
Yield: 0.386 g (90%); (toluene/acetone 10:5); 1H NMR (600 MHz, DMSO-d 6) δ 6.97 (1H, t, J = 7.2 Hz, Ar–H); 7.04 (2H, t, J = 7.2 Hz, Ar–H); 7.52, 7.54 (2H, dd, J = 4.2 and 7.8 Hz, Ar–H); 7.77 (1H, t, J = 1.2 Hz, Ar–H); 7.79 (1H, d, J = 1.2 Hz, Ar–H); 7.84 (1H, t, J = 6.6 Hz, Ar–H); 7.94 (2H, d, J = 7.8 Hz, Ar–H); 7.98 (1H, t, J = 1.8 Hz, Ar–H); 9.15 (2H, br, s, NH2); 9.53 (1H, S, NH). Analysis results for C20H12BrN4: C, 55.83; H, 2.81; Br, 18.57; N, 22.79; found: C, 55.88; H, 2.92; Br, 18.66; N, 22.52; EIMS (m/z): 429.01 [M+].
2.8 7-Phenyl-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile (7a)
Yield: 0.286 g (92%); 1H NMR (600 MHz, DMSO-d 6) δ 6.94 (1H, t, J = 7.2 Hz, Ar–H); 7.26 (2H, t, J = 7.8 Hz, Ar–H); 7.34 (1H, d, J = 4.8 Hz, Ar–H); 7.60–7.62 (3H, m, Ar–H); 7.63 (3H, t, J = 2.4 Hz, Ar–H); 7.64–7.67 (3H, m, Ar–H); 8.12 (1H, d, J = 1.8 Hz, C5–H); 8.13 (1H, d, J = 1.2 Hz, Ar–H); 8.65 (1H, J = 4.8 Hz, C6-H); 9.59 (1H, s, NH); 13C NMR (150 MHz, DMSO-d 6) δ 66.3 67.89; 109.24; 113.47; 117.95; 121.39; 128.42; 128.6; 129.44; 129.74; 131.51; 140.48; 145.9; 151.78; 152.22; 155.91. Analysis results for C19H13N5: C, 73.30; H, 4.21; N, 22.49; found: C, 73.22; H, 4.31; N, 22.42; EIMS (m/z): 311.17 [M+].
2.9 7-(2-Nitrophenyl)-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile (7b)
Yield: 0.320 g (90%); 1H NMR (600 MHz, DMSO-d 6) δ 6.90–6.93 (1H, m, Ar–H); 7.17–7.20 (2H, m, Ar–H); 7.39–7.41 (2H, m, Ar–H); 7.46 (1H, J = 4.8 Hz, Ar–H); 7.84–7.86 (1H, m, Ar–H); 7.94–7.97 (2H, m, Ar–H); 8.03–8.05 (1H, m, Ar–H); 8.39, 8.41 (1H, dd, J = 1.2, 1.2 Hz, C5–H); 8.79 (1H, J = 4.8 Hz, C6–H); 9.56 (1H, s, Ar–H); 13C NMR (150 MHz, DMSO-d 6) δ 68.12; 109.70; 113.06; 117.91; 117.99; 121.66; 124.63; 124.81; 128.49; 132.71; 134.98; 140.03; 144.57; 147.51; 150.51; 152.92; 156.09. Analysis results for C19H12N6O2: C, 64.04; H, 3.39; N, 23.58; found: C, 64.12; H, 4.01; N, 23.67; EIMS (m/z) 356.02 [M+].
2.10 7-(4-Methylphenyl)-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile (7c)
Yield: 0.289 g (89%); (toluene/acetone 10:6); 1H NMR (400 MHz, DMSO-d 6) δ 2.38 (3H, s, CH3); 6.94–7.69 (9H, m, Ar–H); 8.10 (1H, d, J = 1.2 Hz, C5–H); 8.66 (1H, d, J = 4.8 Hz, C6–H); 9.59 (1H, s, NH). Analysis results for C20H15N5: C, 73.83; H, 4.65; N 21.52; found: C, 73.88; H, 4.62; N, 2155.
2.11 7-(4-Methoxyphenyl)-2-(phenylamino)pyrazolo[15-a]pyrimidine-3-carbonitrile (7d)
Yield: 0.307 g (88%); (toluene/acetone 10:6); 1H NMR (400 MHz, DMSO-d 6) δ 3.34 (3H, s, OCH3); 6.92–7.57 (9H, m, Ar–H); 8.13 (1H, d, J = 1.2 Hz, C5–H); 8.65 (1H, d, J = 4.8 Hz, C6–H); 9.85 (1H, s, NH). Analysis results for C20H15N5O: C, 70.37; H, 4.43; N, 20.52; found: C, 70.44; H, 4.42; N, 20.57.
3 Results and discussion
The reaction of N-(5-amino-4-cyano-1H-pyrazole-3-yl)-benzamide 1 with a variety of electrophiles was reported to afford different isomeric pyrazolo[1,5-a]pyrimidine derivatives. Thus, in an early report [32], the reaction of 1 with tetracyano ethylene in dry ethyl acetate or methylene chloride at ambient temperature for 48 h or with 2-(dicyanomethylene) indan-1, 3-dione (CNIND) in dry pyridine and heating under reflux at 100°C for 3 h afforded mainly the corresponding 5-aminopyrazolo [1,5-a]pyrimidine via the initial attachment of ring nitrogen followed by cyclization of the formed 1:1 adduct with an exocyclic amino group, which was subsequently supported by other authors. Soliman et al. [33] have similarly reported that reaction of 1 with various halo reagents, active methylenes, and ketene dithioacetals under phase transfer conditions proceeds via the initial attack of the endocyclic ring nitrogen at the electrophile active site followed by cyclization of the cyclic intermediate with the exocyclic amino group. However, they revealed the initial attack by the exocyclic amino group upon reacting 1 with acetyl chloride, ethoxymethylene, malononitrile, and Lawson’s reagent followed by cyclization with the ring nitrogen. A similar pathway was reported by Ahmed et al. [34] and Dozhenko et al. [35]. Elnagdi et al. [36,37] examined the regio-orientation in the reaction of 5-aminopyrazoles with benzylidene malononitrile and enaminones utilizing (15N, 1H) HMBC to establish a more conclusive structure elucidation as the structure assigned for such reactions was mainly based on 1H NMR and IR spectra. They concluded that the reaction with benzylidene malononitrile proceeds initially by the nucleophilic attack of the exocyclic amino group; however, the initial attack of the ring nitrogen occurs with enaminones. Moreover, under such basic reaction conditions, the cyano group at the C-4 pyrazole ring remained inactive. In contrast, Hebishy and co-workers [38] recently reported the reaction of N-(5-amino-4-cyano1H-pyrazol-3-yl)-benzamide with arylidinemalononitrile afforded the corresponding 5-aminopyrazolo[1,5-a]pyrimidine derivatives. Recently, the catalyst-free Biginelli-type reaction of 5-amino-3-arylpyrazole-4-carbonitriles with ylidene-1,3-dicarbonyl compounds by refluxing in DMF has been reported by Dotsenko and co-workers [39]. The authors confirmed the formation of the corresponding 4,7-dihydropyrazolo[1,5-a]pyrimidine-3-carbonitriles via the intermediacy of the aza-Michael adduct resulting from the initial attack of the ring nitrogen and subsequent cyclo-condensation with the exocyclic amino group. The reaction of 1 with dimedone catalyzed by tin(ii) chloride dehydrate, which acts as a Lewis acid, and a low melting solvent was recently reported to afford the corresponding pyrazolo[5,4–b]quinolones via the nucleophilic attack of the exocyclic amino group on the carbonyl carbon of dimedone followed by cyclization of the formed imino form on to the cyano group and hydrolysis [40]. A similar phenomenon was reported by Schmidtke and co-authors [41].
To shed further light on this unresolved issue and in continuation of our studies in which we utilize microwave heating [42,43,44,45,46,47,48], we report herein the results of our investigation on the reaction of N-(5-amino-4-cyano-1H-pyrazole-3-yl)-benzamide 1 with various electrophiles under controlled microwave heating conditions. Our aim was to modulate several factors controlling regioselectivity in such reactions.
The reaction of 5-aminopyrazole derivative 1 with benzylidene malononitrile 2a in pyridine under microwave irradiation (120°C, 20 min) was found to afford selectively the corresponding 7-aminopyrazolo[1,5-a]-pyrimidine derivative 4a rather than isomeric 5-aminopyrazole 3a. The mass spectrum of 4a showed a molecular ion peak m/z = 351.01 (100%). The IR spectra (KBr) showed an amino group, two cyano groups, and C═N absorption bands at λ max = 3,441, 3,360, 2,179, 2,200, and 1,631 cm−1. 1H NMR spectra revealed a broad singlet at δ = 9.02 ppm integrated for two protons, which was assigned to the NH2 group at C-7. If the reaction product was isomeric 5-amino derivatives, such amino groups will appear at a higher field shift. This low downfield shift could be rationalized for the anisotropic effect of the adjacent pyrazole ring nitrogen. In addition, it reveals signals at δ = 6.97 (1H, t, J = 6.6 Hz, Ar–H), 7.31 (2H, t, J = 7.8 Hz, Ar–H), 7.56–7.59 (m, 3H, Ar–H), 7.84–7.85 (2H, dd, J = 3.3, 4.2 Hz, Ar–H), 7.95 (2H, d, J = 7.8 Hz, Ar–H), 9.02 (1H, s, aniline-NH, D2O exchangeable). Its 13C NMR spectrum showed characteristic signals at δ = 75.69 (pyrazole C-3), 115.88 (CN at C-6), 118.19 (CN at C-3), 121.27–140-35 (aromatic carbons), 149.48 (C-4), 155.63 (C-5), 162.1 (C-7). Furthermore, the structure of 4a was unambiguously confirmed by single-crystal X-ray diffraction [41] (Figure 2).

ORTEB diagram of compound 4a (X-ray).
With these results in hand, we investigated the scope of such reactions with a variety of substituted cinnamonitrile derivatives. Thus, the reaction of 1 with 2b–f under the same experimental conditions afforded the corresponding 7-aminopyrazolo [1,5-a]pyrimidine derivatives 4b–f, respectively, in excellent yields. The structures of 4b–f were established from their mass spectra, 1H NMR, and 13C NMR spectra, which showed a similar pattern as compound 4a as well as elemental analysis. Further confirmation was achieved by single-crystal X-ray diffraction of 4d [42] (Figure 3, Scheme 1). These results were in contrast to that reported for the formation of 5-aminopyrazolo[1,5-a]pyrimidines from the reaction of 1 with arylidenemalononitriles [37]

ORTEB diagram of compound 4d (X-ray).

The reaction of 5-aminopyrazole derivatives 1 with benzylidene malononitrile derivatives 2.
Another example of regioselectivity in the reaction of 5-aminopyrazoles with electrophilic reagents was reported [41] involving its reaction with (E)-3-(dimethylamino)-1-arylprop-2-en-1-one derivatives, which afforded the corresponding 5-arylpyrazolo[1,5-a]pyrimidines formed via the initial attack by the ring nitrogen. It is worth mentioning that such cyclocondensation is not favored in our case. Thus, the reaction of 1 with (E)-3-(dimethylamino)-1-phenylprop-2-en-1-one 5a afforded the corresponding 7-arylpyrazolo[1,5-a]pyrimidine 7a. The possible formation of 5-arylpyrazolo[1,5-a]pyrimidine 6a was ruled out based on analytical and spectral data. The mass spectra showed a molecular ion peak m/z = 311.17 (43%). The 1H NMR revealed a downfield doublet at δ = 8.65 ppm and a doublet at δ = 8.13 ppm, corresponding to C6–H and C5–H and signals at δ = 6.94 (1H, t, J = 7.2 Hz, Ar–H), 7.26 (2H, t, J = 7.8 Hz, Ar–H), 7.34 (1H, d, J = 4.8 Hz, Ar–H), 7.60–7.62 (3H, m, Ar–H), 7.63 (3H, t, J = 2.4 HZ, Ar–H), 7.64–7.67 (3H, m, Ar–H), 8.13 (1H, d, J = 1.2, Ar–H), 9.59 (1H, s, aniline–NH). If the reaction product was 6a, the signal assigned for C6–H will appear at a higher field shift. The 13C NMR spectrum revealed C6 and C7-carbons at δ = 109.24 and 151.78, which are difficult to rationalize for if the product was 6a in addition to the characteristic signals at δ = 117.95 (CN at C-3), 113.47–140.48 (aromatic carbons), 145.9 (C-4), and 152.22 (C-2).
Moreover, the structure of 7a was confirmed by single-crystal X-ray diffraction (Figure 4) [43].

ORTEB diagram of compound 6a (X-ray).
Similarly, compound 1 reacted with 5b under the same experimental conditions to afford the newly synthesized 7-arylpyrazolo[1,5-a]pyrimidines 7b–d (Scheme 2). The structure proposed for the reaction products was established based on a similar 1H NMR and 13NMR pattern as 7a

The reaction of 5-aminopyrazole derivatives 1 with enaminone derivatives 5.
A proposed mechanism to account for the formation of 4a–g and 7a–d was demonstrated in Scheme 3. An initial attack of the exocyclic amino group on the activated double bond system in 2a–g afforded 1:1 adduct 10 followed by cyclization via the addition of the ring NH to the cyano group and subsequent aromatization. The same applies for the formation of 7a, which proceeds via dimethylamine elimination from the reaction of the exocyclic amino group with 5a forming intermediate 13 and subsequent cyclization via the attack of the lone pair at the carbonyl group of ring nitrogen with water loss.

Mechanism of the formation of 4af and 7a–d.
4 Conclusion
In conclusion, we can reveal that ring nitrogen is the more basic center and the exocyclic amino group is the less hindered. An attack by ring nitrogen is favored with less sterically hindered electrophiles; however, the attack with the exocyclic amino group predominates with bulky electrophiles. However, no firm conclusion on which to determine the preferred tautomer form of the final product has been arrived. Here, we do believe that the nature of the substituents of the aminopyrazole scaffold plays a crucial role in such regioselectivity. The presence of a cyano group at C-4 in compound 1 reduces the donating ability of the ring nitrogen. Moreover, for the titled compound, the chains of dimers formed by pairs of ring N–H····N hydrogen bonds render it less active. We concluded that unambiguous assignment of the regioselectivity in such reactions requires advanced techniques, particularly X-ray diffraction crystallography as insufficient spectral data could be a mess resulting in incorrect conclusions.
Acknowledgments
Kamal U. Sadek is grateful to the Alexander von Humboldt Foundation for donating a milestone START Microwave Labstation. Saleh Al-Mousawi and Moustafa Sherif Moustafa are very grateful to the Kuwait Foundation for the Advancement of Science, project number PR1714SC02, and Kuwait University for general facilities projects GS01/03, GS01/05, and SG0308.
-
Funding information: This study was funded by project no. PR1714SC02.
-
Author contributions: Moustafa S. Moustafa: visualization and methodology; Ahmed M. Nour-Eldeen: formal analysis and data curation; Saleh M. Al-Mousawi: methodology, formal analysis; Afaf A. El-Hameed: writing – review and editing; Michael Magdy: writing – review and editing; Kamal U. Sadek: supervision and writing – original draft.
-
Conflict of interest: The authors state no conflict of interest.
Appendix
Characterization data for compounds 4a, 4b, 4c, 4d, 4e, 4f, 7a, 7b, 7c, and 7d.
A1 7-Amino-5,2-diphenylpyrazolo[1,5-a]pyrimidine-3,6-dicarbonitrile (4a)
Orange crystals; mp: 308–310°C; yield: 0.309 g (92%); R
f = 0.57 (toluene/acetone 10:5); 1H NMR (600 MHz, DMSO-d
6) δ 6.97 (1H, t, J = 6–6 Hz, Ar–H), 7.31 (2H, t, J = 7.8 Hz, 7.56–7.59) (m, 3H, Ar–H), 7.84, 7.85 (2H, dd, J = 3.3, 4.2 Hz, Ar–H), 7.95 (2H, d, J = 7.8 Hz, Ar–H), 9.02 (br, s, 2H, NH2, D2O exchangeable), 9.51 (S, 1H, NH, D2O exchangeable); 13C NMR (150 MHz, DMSO-d
6) δ = 62.76, 66.32, 70.20, 75.69, 113.32, 115.88, 118.19, 121.27, 128.38, 128.65, 128.67, 130.59, 136.67, 140.35, 149.48, 150.85, 155.63, 162.10. Analysis results for C20H13N7: C, 68.37; H, 3.73; N, 27.90; found: C, 68.22; H, 3.82; N, 27.88; EIMS (m/z): 351.01 [M+].
A2 7-Amino-5-(4-nitrophenyl)-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3,6-dicarbonitrile (4b)
Reddish brown crystals; mp: >360°C; yield: 0.356 g (90%); R
f = 0.52 (toluene/acetone 10:5); 1H NMR (600 MHz, DMSO-d
6) δ 6.97 (1H, t, J = 6.6 Hz, Ar–H), 7.31 (2H, d, J = 1.2 Hz, Ar–H), 7.94 (2H, J = 7.8 Hz, Ar–H), 8.09 (2H, d, J = 9 Hz, Ar–H), 8.39 (2H, d, J = 8.4 Hz), 9.12 (br, s, 2H, NH2), 9.54 (s, 1H, NH), 13C NMR (150 MHz, DMSO-d
6) δ 66.34, 70.71, 76.11, 113.12, 115.49, 118.24, 121.36, 123.57, 128.68, 130.19, 140.28, 142.57, 148.48, 149.33, 150.72, 155.71, 159.99. Analysis results for C20H12N8O2: C, 60.60; H, 3.05; N, 28.27; found: C, 60.63; H, 3.12; N, 28.33; EIMS (m/z): 396.03 [M+].
A3 7-Amino-5-(4-chlorophenyl)-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3,6-dicarbonitrile (4c)
Yellow crystals; mp: 318–320°C; yield: 0.354 g (92%); R
f = 0.75 (toluene/acetone 10:5); 1H NMR (600 MHz, DMSO-d
6) δ 6.97 (1H, t, J = 7.2 Hz, Ar–H), 7.31 (2H, t, J = 7.2 Hz, Ar–H), 7.64 (2H, d, J = 8.4 Hz, Ar–H), 7.87 (2H, d, J = 8.4 Hz, Ar–H), 7.95 (2H, d, J = 8.4 Hz, Ar–H), 9.03 (2H, br, s, NH2), 9.52 (1H, s, NH); 13C NMR (150 MHz, DMSO-d
6) δ 70.35, 75.68, 113.26, 115.78, 121.31, 128.52, 128.68, 130.53, 135.45, 140.33, 149.43, 150.79, 155.65, 160.82. Analysis results for C20H12CLN7: C, 62.26; H, 3.14; Cl, 9.19; N, 25.41; found: C, 62.32; H, 3.18; Cl, 9.12; N, 25.58; EIMS (m/z) 385.12 [M+]
A4 7-Amino-5-(4-methoxyphenyl)-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3,6-dicarbonitrile (4d)
Brown crystals; mp: 310–312°C; yield: 0.354 g (93%); 1H NMR (600 MHz, DMSO-d
6) δ 3.52 (3H, s, OCH3), 6.97 (1H, t, J = 7.2 Hz, Ar–H), 7.02–7.13 (2H, m, Ar–H), 7.03 (2H, t, J = 7.8 Hz, Ar–H), 7.85–7.88 (2H, m, Ar–H), 7.95 (2H, t, J = 6.6 Hz, Ar–H), 8.3 (2H, br, s, NH2), 9.48 (1H, s, NH); 13C NMR (150 MHz, DMSO-d
6) δ 55.39, 60.11, 76.8, 113.42, 113.76, 114.77, 115.13, 116.06, 116.17, 121.22, 124.07, 128.41, 128.65, 128.78, 130.42, 133.31, 140.38, 149.57, 150.83, 155.62, 160.39, 161.25, 161.37, 164.31. Analysis results for C21H15N6O: C, 66.13; H, 3.96; N, 25.71; found: C, 66.22; H, 4.01; N, 25.75; EIMS (m/z) 381.11 [M+].
A5 7-Amino-5-(2-chlorophenyl)-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3,6-dicarbonitrile (4e)
Yellow crystals; mp: 288–290°C; yield: 0.351 g (91%); 1H NMR (600 MHz, DMSO-d
6) δ 6.98 (1H, t, J = 7.8 Hz, Ar–H), 7.32 (2H, t, J = 7.8 Hz, Ar–H), 7.51 (1H, t, J = 7.2 Hz, Ar–H), 7.54 (2H, d, J = 1.8 Hz, Ar–H), 7.53 (2H, d, Ar–H), 7.565–7.592 (2H, m, Ar–H), 7.64–7.65 (1H, m, Ar–H), 7.95 (d, 2H, J = 7.8, Ar–H), 9.14 (2H, br, s, NH2), 9.565 (1H, s, NH), 13C NMR (150 MHz, DMSO-d
6) δ 66.35, 70.62, 78.16, 113.10, 114.66, 116.09, 118.24, 121.40, 127.41, 128.41, 128.58, 128.72, 129.56, 130.42, 131.07, 131.45, 136.24, 140.31, 148.62, 150.79, 155.64, 161.44. Analysis results for C20H12ClN7: C, 62.26; H, 3.14; Cl, 9.19; N, 25.41; found: C, 62.24; H, 3.21; Cl, 9.23; N, 25.39; EIMS (m/z): 385.03 [M+].
A6 7-Amino-5-(3-bromophenyl-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3,6-dicarbonitrile (4f)
Brown crystals; mp: 259–260°C; yield: 0.386 g (90%); (toluene/acetone 10:5); 1H NMR (600 MHz, DMSO-d
6) δ 6.97 (1H, t, J = 7.2 Hz, Ar–H), 7.04 (2H, t, J = 7.2 Hz, Ar–H); 7.52, 7.54 (2H, dd, J = 4.2 and 7.8 Hz, Ar–H), 7.77 (1H, t, J = 1.2 Hz, Ar–H), 7.79 (1H, d, J = 1.2 Hz, Ar–H), 7.84 (1H, t, J = 6.6 Hz, Ar–H), 7.94 (2H, d, J = 7.8 Hz, Ar–H), 7.98 (1H, t, J = 1.8 Hz, Ar–H), 9.15 (2H, br, s, NH2), 9.53 (1H, S, NH). Analysis results for C20H12BrN4: C, 55.83; H, 2.81; Br, 18.57; N, 22.79; found: C, 55.88; H, 2.92; Br, 18.66; N, 22.52; EIMS (m/z): 429.01 [M+].
A7 7-Phenyl-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile (7a)
Brown crystals; mp: 238–240°C; yield: 0.286 g (92%); 1H NMR (600 MHz, DMSO-d
6) δ 6.94 (1H, t, J = 7.2 Hz, Ar–H); 7.26 (2H, t, J = 7.8 Hz, Ar–H), 7.34 (1H, d, J = 4.8 Hz, Ar–H), 7.60–7.62 (3H, m, Ar–H), 7.63 (3H, t, J = 2.4 Hz, Ar–H), 7.64–7.67 (3H, m, Ar–H), 8.12 (1H, d, J = 1.8 Hz, C5–H), 8.13 (1H, d, J = 1.2 Hz, Ar–H), 8.65 (1H, J = 4.8 Hz, C6–H), 9.59 (1H, s, NH); 13C NMR (150 MHz, DMSO-d
6) δ 66.3–67.89, 109.24, 113.47, 117.95, 121.39, 128.42, 128.6, 129.44, 129.74, 131.51, 140.48, 145.9, 151.78, 152.22, 155.91. Analysis results for C19H13N5: C, 73.30; H, 4.21; N, 22.49; found: C, 73.22; H, 4.31; N, 22.42; EIMS (m/z): 311.17 [M+].
A8 7-(2-Nitrophenyl)-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile (7b)
Yellow crystals; mp: 268–270°C; yield: 0.320 g (90%); 1H NMR (600 MHz, DMSO-d
6) δ 6.90–6.93 (1H, m, Ar–H), 7.17–7.20 (2H, m, Ar–H), 7.39–7.41 (2H, m, Ar–H); 7.46 (1H, J = 4.8 Hz, Ar–H), 7.84–7.86 (1H, m, Ar–H), 7.94–7.97 (2H, m, Ar–H), 8.03–8.05 (1H, m, Ar–H), 8.39, 8.41 (1H, dd, J = 1.2, 1.2 Hz, C5–H), 8.79 (1H, J = 4.8 Hz, C6–H), 9.56 (1H, s, Ar–H), 13C NMR (150 MHz, DMSO-d
6) δ 68.12, 109.70, 113.06, 117.91, 117.99, 121.66, 124.63, 124.81, 128.49, 132.71, 134.98, 140.03, 144.57, 147.51, 150.51, 152.92, 156.09. Analysis performed for C19H12N6O2: C, 64.04; H, 3.39; N, 23.58; found: C, 64.12; H, 4.01; N, 23.67; EIMS (m/z) 356.02 [M+].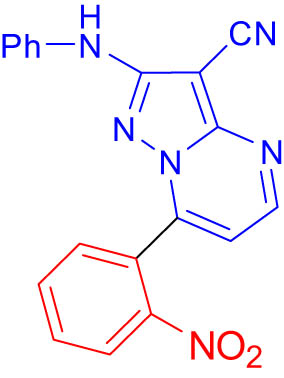
A9 7-(4-Methylphenyl)-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile (7c)
Reddish brown crystals; mp: 270–272°C; yield: 0.289 g (89%); (toluene/acetone 10:6), 1H NMR (400 MHz, DMSO-d
6) δ 2.38 (3H, s, CH3), 6.94–7.69 (9H, m, Ar–H), 8.10 (1H, d, J = 1.2 Hz, C5–H), 8.66 (1H, d, J = 4.8 Hz, C6–H), 9.59 (1H, s, NH). Analysis performed for C20H15N5: C, 73.83; H, 4.65; N, 21.52; found: C, 73.88; H, 4.62; N, 2155.
A10 7-(4-Methoxyphenyl)-2-(phenylamino)pyrazolo [1,5-a]pyrimidine-3-carbonitrile (7d)
Orange crystals; mp: 168–170°C; yield: 0.307 g (88%); (toluene/acetone 10:6); 1H NMR (400 MHz, DMSO-d
6) δ 3.34 (3H, s, OCH3), 6.92–7.57 (9H, m, Ar–H), 8.13 (1H, d, J = 1.2 Hz, C5–H), 8.65 (1H, d, J = 4.8 Hz, C6–H), 9.85 (1H, s, NH). Analysis results for C20H15N5O: C, 70.37; H, 4.43; N, 20.52; found: C, 70.44; H, 4.42; N, 20.57.
References
[1] Elguero J, Goya P, Jagerovic N. Targets in heterocyclic systems. 6th edn. Rome, Italy: Societa Chimica Italiana; 2002.Suche in Google Scholar
[2] Szaba C, Fischer J, Kis-varga A, Gyires K, derivatives J. New celecoxib as anti-inflammatory agents. J Med Chem. 2008;51:142–7.10.1021/jm070821fSuche in Google Scholar
[3] Tanitame A, Oyamada Y, Ofuji K, Terauchi H, Mawasaki M, Wachi M, et al. Synthesis and antibacterial activity of a novel series of DNA gyrase inhibitors: 5-[(E)-2-arylvinyl] pyrazoles. Bioorg Med Chem Lett. 2005;15:4299–303.10.1016/j.bmcl.2005.06.103Suche in Google Scholar
[4] Tanitame A, Oyamade Y, Ofuji K, Fujimoto M, Suzuki K, Meda T, et al. Synthesis and antibacterial activity of novel and potent DNA gyrase inhibitors with azole ring. Bioorg Med Chem. 2004;12:5515–24.10.1016/j.bmc.2004.08.010Suche in Google Scholar
[5] Dugi K, Mark M, Himmels Bach F. Preparation of improvement of insulin. PCT Int Appl. 2009; WO 2009139340.Suche in Google Scholar
[6] Dugi K, Mark M, Himmels Bach F. Pharmaceutical composition comprising a pyrazole-o-glucoside derivative. PCT Int Appl. 2009;WO 2009022009.Suche in Google Scholar
[7] Gregg BT, Tymoshenko DO, Razzano DA, Johnson MR. Pyrazolo[1,5-a]pyrimidines. identification of the privileged structure and combinatorial synthesis of 3-(Hetero) arylpyrazolo[1,5-a]pyrimidine-6-carboxamides. J Comb Chem. 2007;9:507–12.10.1021/cc0700039Suche in Google Scholar
[8] Kamal A, Fuazil S, Hussaini SMA, Ramaiah MG, Balakrishna M, Patel N, et al. Synthesis and mechanistic aspects of 2-anilinonicotinyl-pyrazolo[1,5-a]pyrimidine conjugates that regulate cell Synthesis and mechanistic aspects of 2-anilinonicotinyl-pyrazolo[1,5-a]pyrimidine conjugates that regulate cell proliferation in MCF-7 cells via oestrogen signalling. Bioorg Med Chem Lett. 2016;26:2077–83.10.1016/j.bmcl.2016.02.072Suche in Google Scholar
[9] Le J, Zhau YF, Zhao KI, Yman XY, Cong P. Synthesis and anti-tumor activities of novel pyrazolo[1,5-a] pyrimidines. Arch Pharm. 2006;339:593–7.10.1002/ardp.200600098Suche in Google Scholar
[10] Bondok S, Fadaly F, Metwally MA. Synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazole derivatives containing benzothiazole moiety. Eur J Med Chem. 2010;45:3692–701.10.1016/j.ejmech.2010.05.018Suche in Google Scholar
[11] El-Gaby MSA, Atalla AA, Gaber AM, Abd Al Wahab KA. Synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazole derivatives containing benzothiazole moiety. Farmaco. 2000;55(9–10):596–602.10.1016/S0014-827X(00)00079-3Suche in Google Scholar
[12] Hwang JY, Windisch MP, Jo S, Kim K, Kon S, Kim HC, et al. Discovery and characterization of a novel 7-aminopyrazolo[1,5-a] pyrimidine analogue as a potent hepatitis C virus inhibitor. Bioorg Med Chem Lett. 2012;22:7297–301.10.1016/j.bmcl.2012.10.123Suche in Google Scholar PubMed
[13] Zhang J, Peng-Ju Bai, Yu-Bin, Wang P, Wang T, Gau JM, et al. Synthesis of pyrazolo[1,5-a] pyrimidine derivatives and their antifungal activities against phytopathogenic fungi in vitro. Mol Divers. 2016;20:887–96.10.1007/s11030-016-9690-ySuche in Google Scholar PubMed
[14] Kaping S, Sunn M, Singha LI, Vishwakarma J. Ultrasound assisted synthesis of pyrazolo[1,5-a] pyrimidine-antipyrine hybrids and their anti-inflammatory and anti-cancer activities. Eur J Chem. 2020;11:68–79.10.5155/eurjchem.11.1.68-79.1942Suche in Google Scholar
[15] Suzuki M, Iwasaki H, Sakashita M, Kitahara M, Sakoda R. Synthesis and biological evaluations of condensed pyridine and condensed pyrimidine-based HMG-CoA reductase inhibitors. Bioorg Med Chem Lett. 2001;11:1285–8.10.1016/S0960-894X(01)00203-7Suche in Google Scholar
[16] Almansa C, de Arriba AF, Cavalcanti FL, Gomez LA, Miralles A, Merlos M, et al. Synthesis and SAR of a new series of cox-2-selective inhibitors: pyrazolo[1,5-a] pyrimidines. J Med Chem. 2001;44:350–61.10.1021/jm0009383Suche in Google Scholar
[17] Berger DM, Torres N, Dutia M, Powell D, Gopalsamy G, Levin JL, et al. Non-hinge-binding pyrazolo[1,5-a] pyrimidines as potent β-Raf kinase inhibitors. Bioorg Med Chem Lett. 2009;6519–23.10.1016/j.bmcl.2009.10.049Suche in Google Scholar
[18] Drizin I, Holladay MK, Yi L, Zhang HQ, Gopalakrishnan S, Gopalakrishnan M, et al. Structure-activity studies for a novel series of tricyclic dihydropyrimidines as KATP channel openers (KCOs). Bioorg Med Chem Lett. 2002;12:1481–4.10.1016/S0960-894X(02)00207-XSuche in Google Scholar
[19] Williamson DS, Parratt MJ, Bower JF, Moore JD, Richardson CM, Dokurno P, et al. Structure-guided design of pyrazolo[1,5-a] pyrimidines as inhibitors of human cyclin-dependent kinase 2. Bioorg Med Chem. 2005;15:863–7.10.1016/j.bmcl.2004.12.073Suche in Google Scholar PubMed
[20] Shaaban MR, Saleh TS, Mayhoub AS, Farag AM. Single step synthesis of new fused pyrimidine derivatives and their evaluation as potent Aurora-A kinase inhibitors. Eur J Med Chem. 2011;46:3690–5.10.1016/j.ejmech.2011.05.033Suche in Google Scholar PubMed
[21] Aggrawal R, Singh G, Kaushik P, Kaushik D, Paliwal D, Kumar A. Molecular docking design and one-pot expeditious synthesis of novel 2,5-diarylpyrazolo[1,5-a]pyrimidine-7-amines as anti-inflammatory agents. Eur J Med Chem. 2015;10:326–33.10.1016/j.ejmech.2015.06.011Suche in Google Scholar PubMed
[22] Abdallah AEM, Elgemei GEH. Design, synthesis, docking, and antimicrobial evaluation of some novel pyrazolo[1,5-a]pyrimidines and their corresponding cycloalkane ring-fused derivatives as purine analogies. Drug Des Devel Ther. 2018;12:1785–98.10.2147/DDDT.S159310Suche in Google Scholar PubMed PubMed Central
[23] Elnagdi MH, Taha NH, El-All FAMA, Abdel-Motaleb RM, Mahmoud FF. Studies on condensed pyrazoles: synthesis of new methyl and amino pyrazolo[1,5-a]pyrimidines and pyrazolo[5,1-c][1,2,4]triazines. Collect Czechoslov Chem Commun. 1989;54:1082–91.10.1135/cccc19891082Suche in Google Scholar
[24] Shaabani A, Nazeri MT, Afshari R. 5-Aminopyrazoles: Potent reagents in organic and medicinal synthesis. Mol Divers. 2019;23:751–807.10.1007/s11030-018-9902-8Suche in Google Scholar
[25] Aggawal R, Kumar S. 5-Aminopyrazoles as precursors in design and synthesis of fused pyrazoloazines. Beilstein J Org Chem. 2018;14:203–42.10.3762/bjoc.14.15Suche in Google Scholar
[26] Murlykina MV, Morozova AD, Zviagin IM, Sakhno YI, Desenko SM, Chebanov VA. Aminoazole-based diversity-oriented synthesis of heterocycles. Front Chem. 2016;6:1–43.10.3389/fchem.2018.00527Suche in Google Scholar
[27] Secrieru A, ONeill PM, Cristiano MLS. Revisiting the structure and chemistry of 3(5)-substituted pyrazoles. Molecules. 2020;25:42–5.10.3390/molecules25010042Suche in Google Scholar
[28] Kappe CO. Controlled microwave heating in modern organic synthesis. Angew Chem Int Ed. 2004;43:6250–84.10.1002/anie.200400655Suche in Google Scholar
[29] Jacob J. Microwave assisted reactions in organic synthesis: a review of recent advances. Int J Chem. 2012;29:29–43.Suche in Google Scholar
[30] Perreux L, Loupy A. A tentative rationalization of microwave effects in organic synthesis according to the reaction medium. Tetrahedron. 2001;57:9199–223.10.1016/S0040-4020(01)00905-XSuche in Google Scholar
[31] De la Hoz A, Diaz-Ortiz A, Moreno A. Review on non-thermal effects of microwave irradiation in organic synthesis. J Microw Power Electromagn Energy. 2016;41:45–66.10.1080/08327823.2006.11688549Suche in Google Scholar
[32] Mohamed NK, Ibrahim YR, Hassan AA, Mourad AEE. Synthesis of new pyarazolo[1,5-a]pyrimidine derivatives via CT-complexation. Arch Pharm (Weinh). 1993;326:245–7.10.1002/ardp.19933260414Suche in Google Scholar
[33] Soliman AMM, El-Aleem MA, El-ramaily AA, Sultan AA, Abdel-Ghany H. Synthesis of some novel imidazopyrazole and pyrazolopyrimidine derivatives. J Heterocycl Chem. 2014;51:1476–81.10.1002/jhet.1701Suche in Google Scholar
[34] Ahmed SA, Abdelhamid AO, El-Ghandour AA, Mohamed MA, Mohamed BM. Synthesis of some new pyrazolo[1,5-a]pyrimidines. J Chem Res. 2008;2008:26–31.10.3184/030823408X287096Suche in Google Scholar
[35] Lim FPL, Luna GA, Dozhenko V. One-pot, three-component aminotriazine annulation onto 5-aminopyrazole-4-carbonitriles under microwave irradiation. Tetrahedron Lett. 2015;56:521–4.10.1016/j.tetlet.2014.12.010Suche in Google Scholar
[36] Anwar HF, Fleita DH, Kolshorn H, Meier H, Elnagdi MH. 2H-Pyrazol-3-ylamines as precursors for the synthesis of polyfunctionally substituted pyrazolo[1,5-a] pyrimidines. Arkivoc. 2006;XV:133–44.10.3998/ark.5550190.0007.f16Suche in Google Scholar
[37] El Kholy A, Al–Qalaf F, Elnagdi MH. Regio-orientation in condensation of aminopyrazoles with 1,3-difunctional reagents: synthesis of new pyrazolo[1,5-a] pyrimidines; pyrazolo[3,4-d] pyridazines and 2,4-dihydropyrano[2,3-c] pyrazoles. Arkivoc. 2008;xiv:124–31.10.3998/ark.5550190.0009.e14Suche in Google Scholar
[38] Hebishy AMS, Salama HT, Egemei GH. New route to the synthesis of Benzamide-Based 5-aminopyrazoles and their fused heterocycles showing remarkable antiavian influenza virus activity. ACS Omega. 2020;5:25104–12.10.1021/acsomega.0c02675Suche in Google Scholar PubMed PubMed Central
[39] Kolosov MA, Beloborodov DA, Orlov VD, Dotsenko VV. Catalyst free Biginelli-type synthesis of new functionalized 4,7-dihydropyrazolo[1,5-a]pyrimidines. N J Chem. 2016;40:7573–9.10.1039/C6NJ00336BSuche in Google Scholar
[40] Marjani AP, Khalafy J, Salami F, Mohammadlou M. Tin(ii) chloride catalysed synthesis of new pyrazolo[5,4-b]quinolones under solvent-free conditions. Synthesis. 2015;47:A–E.10.1055/s-0034-1380189Suche in Google Scholar
[41] Makarov VA, Braun H, Richter M, Riabova OB, Kirchmair J, Kazakova ES, et al. Pyrazolopyrimidines: potent inhibitors targeting the capsid of rhino- and enteroviruses. ChemMedChem. 2015;10:1629–34.10.1002/cmdc.201500304Suche in Google Scholar PubMed PubMed Central
[42] Mekheimer RA, Hayllah AM, Moustafa MS, Al-Mousawi SA, Abd Elmonem M, Mostafa SA, et al. Microwave-assisted reactions: efficient and versatile one-step synthesis of 8-substituted xanthines and substituted pyrimidopteridine-2,4,6,8-tetraones under controlled microwave heating. Green Process Synth. 2021;10:1–7.10.1515/gps-2021-0014Suche in Google Scholar
[43] Sadek KU, Mekheimer RA, Mohamed TM, Moustafa MS, Elnagdi MH. Regioselectivity in the multicomponent reaction of 5-aminopyrazoles, cyclic 1,3-diketones and dimethylformamide dimethylacetal under controlled microwave heating. Beilstein J Org Chem. 2012;B:18–24.10.3762/bjoc.8.3Suche in Google Scholar PubMed PubMed Central
[44] Moustafa MS, Mekheimer RA, Al Mousawi S, Abd-Elmonem M, El-Zorba H, Hameed A, et al. Microwave-assisted efficient one-pot synthesis of N2-(tetrazol-5-yl)-6-aryl/heteroaryl-5,6-dihydro-1,3,5-triazine-2,4-diamines. Beilstein J Org Chem. 2020;16:1706–12.10.3762/bjoc.16.142Suche in Google Scholar PubMed PubMed Central
[45] Dyab AKF, Sadek KU. Microwave assisted one-pot green synthesis of cinnoline derivatives inside natural sporopollenin microcapsules. RSC Adv. 2018;8:23241–51.10.1039/C8RA04195DSuche in Google Scholar
[46] Sadek KU, Shaker RM, Elrady MA, Elnagdi MH. A novel method for the synthesis of polysubstituted diaminobenzonitrile derivatives using controlled microwave heating. Tertrahedron Lett. 2010;51:6319–21.10.1016/j.tetlet.2010.09.114Suche in Google Scholar
[47] Hameed AA, Ahmed EK, Fattah AAA, Andrade CKZ, Sadek KU. Green and efficient synthesis of polyfunctionally substituted cinnolines under controlled microwave irradiation. Res Chem Intermed. 2017;43:5523–33.10.1007/s11164-017-2944-1Suche in Google Scholar
[48] El Latif FMA, Barsy MA, Aref AM, Sadek KU. Microwave-assisted reactions: part 2: one-pot synthesis of pyrimido[1,2-a] pyrimidines. Green Chem. 2002;4:196–8.10.1039/b110723mSuche in Google Scholar
© 2022 Moustafa Sherif Moustafa et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Artikel in diesem Heft
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

