Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
-
Ousman Brahim Mahamat
, Fadoua Asraoui
, Ayoub Farihi
, Mohamed Bouhrim
, Ibtissam Boussaoudi
, Soumaya El Ismaili
, Rashed N. Herqash
, Abdelaaty A. Shahat
, Brahim Boy Otchom
and Younes Saoud
Abstract
Azadirachta indica, commonly known as neem or Mim in Chad, is recognized for its significant biological activities and is used for medicinal purposes. This study quantifies phenolic content, analyzes chemical composition via high-performance liquid chromatography–mass spectrometry, and evaluates the antioxidant activity of Mim leaf extracts with ethanol (ELE) and water (WLE). The analysis identified bioactive compounds with strong radical scavenging activity and conducted in silico studies by molecular docking with AutoDock Tools. Crystal structures were sourced from the Protein Data Bank, and the Swiss absorption, distribution, metabolism, and excretion platform analyzed the pharmacokinetic properties. Results indicated that WLE had a higher phenolic content (264.7 ± 0.03 µg gallic acid equivalent [GAE]/mg) compared to ELE (135.3 ± 0.05 µg GAE/mg). Flavonoid content was greater in ELE (138.33 ± 0.002 µg catechin equivalent [CE]/mg) than in WLE (83.38 ± 0.002 µg CE/mg). Major compounds identified through high-performance liquid chromatography coupled to mass spectrometry included diethyl phthalate in ELE (92.31%) and butylated hydroxytoluene (BHT) in WLE (28.12%). Antioxidant activities measured by 2,2-di-phenyl-1-picrylhydrazyl, 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid), and ferric-reducing power assays showed promising results for both extracts. BHT demonstrated a better affinity for glutathione reductase and lipoxygenases, while vanillin showed a strong affinity for cyclooxygenase. Most compounds exhibited high intestinal absorption and are not P-glycoprotein substrates, indicating potential for oral medication. Finally, Mim extracts contain diverse compounds that contribute to their notable antioxidant activity.
1 Introduction
Phytochemical compounds and their chemical analogs have provided abundant clinically useful drugs in the treatment of chronic and acute diseases. Still, research is continuing to search for newer therapeutic agents from medicinal plants [1]. Azadirachta indica (family of Meliaceae), known as Mim, Neem, Nim tree, and Indian Lilac, was first discovered in India about 4,500 years ago. It is known to grow to approximately 15–20 m high and can live for about 200 years [2]. Usually, the Mim tree is found in dry, tropical, or subtropical locations [2]; however, it can also be found along the sandy riverbanks in Australia [3]. This plant has been used to cure multiple acute and chronic diseases in different parts of Asia and Africa. It is one of the first medicinal plants mentioned in Siddha medicine, which is the oldest medical system known to mankind. Neem is also one of the most respected trees among Indians [4]. It has also been used as a tonic and to eradicate bugs from beds, books, grain bins, cupboards, and closets. In Indonesia, neem leaves are used as a diuretic for treating diabetes, headache, and heartburn and for stimulating the appetite [5]. Some studies have been conducted into the Mim plant to investigate the phytochemicals characteristics and have demonstrated that Mim is a good source of phenolic acid substances including tannic and salicylic acid, with major flavonoids being hyper-side (glycoside), kaempferol, quercetin, and vitamins [6,7], terpenoids and tetraterpenoids or limonoids [8,9,10], anti-cancerous molecules [2,11], and anti-inflammatory and glycoprotein substances [12]. Various parts of A. indica (called in India the divine tree), such as flowers, leaves, seeds, and bark, have been used to treat both acute and chronic human diseases like pyrexia, headache, ulcers, respiratory disorders, cancer, and diabetes [13,14]. It is one of the primary sources of many therapeutic agents [15]. The tree is popular for its pharmacological attributes [16,17] while the extracts were reported as anti-microbicidal and antibacterial [18,19,20,21,22], chronic inflammation of oral cavity [20], anti-inflammatory [21,22], anti-dermal complications [23], antipyretic and analgesic [24], hepatoprotective [25,26,27,28], hypolipidemic or dyslipidemia [28,29], antihyperglycemic/hypoglycaemic [30], anti-insecticidal [31,32,33,34,35], neuroprotective [33], cardioprotective [34], and antiproliferative or anti-tumour [35] and used as antioxidant or anti-oxidative stress [7,30,36,37,38,39]. The plant has been used against malaria and treating type 2 diabetes in Nigeria and South Africa [36], India, and other parts of Asia [5]. Oxidative stress occurs when there is an imbalance between oxidants and antioxidants in favor of the oxidants. This leads to a disruption of redox signaling, control, and/or molecular damage [37,40]. Oxidative stress due to free radicals causes damage to protein, lipid membranes, and nucleic acids, which ultimately causes cell death in both types of diabetes mellitus [12]. A study has shown that leaf extract of Mim potentially normalizes lipid peroxidation, superoxide anions, and hydroxyl radicals in high-fat diet-induced rats [41,42]. Oxidative stress is known to be the root cause of several diseases [43,44]. In today’s pharmaceutical research, synthetic drugs are commonly used for treatment. However, these drugs often come with side effects. On the other hand, treatments based on medicinal plants have proven to be beneficial without any negative impact or secondary influences [36]. The plants used for medicine and food, such as fruits and vegetables, have a positive impact on various aspects of life. These include the relief of physical ailments, decreased reliance on synthetic antibiotics, and increased life expectancy [45,46]. These plants have antioxidant properties due to the presence of phenolic and terpenoid compounds in the leaves, stalks, fruits, and seeds [47,48,49]. Antioxidants, due to their ability to neutralize free radicals and prevent cell damage, are commonly used as food supplements and have been studied for their potential to inhibit diseases such as heart disease and cancer [47]. The antioxidant activity of extracts is linked to their redox properties, which allow them to act as reducing agents. This function is typically associated with the presence of reductants, which exhibit antioxidant effects by breaking the chain of free radicals. They do this by donating a hydrogen atom or by preventing the formation of peroxides [50,51]. Reducing agents can reduce lipid peroxides and related oxidants through redox reactions and are also referred to as oxygen scavengers [52]. The available methods to quantify antioxidant activity can be classified based on the mechanism of action by which the applied compounds stop chain-breaking reactions. They can be divided into two groups: hydrogen-atom transfer (HAT) (HAT reactions) and single electron transfer (SET) (compound reduction reactions through electron transfer from an antioxidant) [53]. Among the SET methods, the most used are 2,2-di-phenyl-1-picrylhydrazyl (DPPH radical scavenging capacity assay), ferric-reducing (FRAP) assay, Trolox equivalent antioxidant capacity (or 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) [ABTS]) assay, copper reduction assay, and reducing power assay [54].
The Human Genome Project’s completion has led to the identification of new drug targets [55]. Advanced techniques like protein purification, X-ray crystallography, and nuclear magnetic resonance (NMR) spectroscopy have provided detailed insights into protein and protein–ligand complex structures [56]. These recent advancements enable computational methods to be integrated into all facets of drug discovery today [57]. Molecular docking methods are widely used in modern drug design to explore the conformation of ligands within the binding sites of macromolecular targets. This approach also estimates the binding free energy between the ligand and receptor by evaluating critical phenomena involved in the intermolecular recognition process [58]. High-performance liquid chromatography coupled to mass spectrometry (HPLC–MS) detection is a separation technique that can be applied to analyze compounds of different properties from low up to very high molecular mass substances. This chromatographic separation involves passing a mixture dissolved in a mobile phase through another material called the stationary phase, and both mobile and stationary phases are immiscible [59]. An integrated combination of affinity HPLC–MS to isolate compounds and multi-target molecular docking provides a powerful approach for mining active components [60] present in the Mim plant, such as antioxidants. The Mim plant has also been traditionally used as medicine in Chadian culture to treat various ailments. Mim leaf water extracts are used to treat general fever, headache, skin infections, kidney and urinary problems, respiratory issues, and malaria. Various other parts of the plant are also used as medicine. However, in Chad, there has not been systematic documentation of bioactive compounds of the Mim plant identified by liquid chromatography technique and evaluated in vitro for their antioxidant properties. Additionally, few studies have been conducted on Mim from Chad, combining the active compounds with an in silico study. This study aims to prepare leaf extracts of Mim to identify bioactive compounds using HPLC–MS, evaluate phenolic contents, determine the antioxidant activity, and perform an in silico study.
2 Materials and methods
2.1 Chemicals and reagents
DPPH, ABTS, FRAP reagents, and butylated hydroxytoluene (BHT) were purchased from Sigma (St. Louis, USA). Folin–Ciocalteu phenol reagents and standards such as gallic acid and ascorbic acid were obtained from Merck Life Science (Darmstadt, Germany). All other chemicals were of analytical grade and obtained from Sigma Aldrich (Seeize, Germany). The absorbance was measured using a ultraviolet (UV)–visible (VIS) spectrophotometer (Macylab, China).
2.2 Sample collection
The leaf sample of Mim was collected in September 2021 from N’Djamena, Republic of Chad (Figure 1). Plant identification was carried out by Toumaï University herbarium (N’Djamena) and assessments by foresters from the National Federation Associations of Healers and Practitioners of Medicine (Fédération Nationale des Associations des praticiens de la Medicine au Tchad [FENAPMT], N’Djamena) and then deposited in Toumaï University herbarium, and the Biology, Ecology and Health Laboratory herbarium (Morocco), with a voucher specimen code GA-LBES/Ech02.

(a) Neem tree, (b) leaves, and (c) seed (photos from the author, Republic of Chad).
2.3 Sample preparation and extraction procedure
The dry leaves of Mim (150 g) were ground using a grinder from Japan. Once the leaves were thoroughly dried, they were powdered and stored in a glass bottle in a dark place for further extraction. Ethanol extracts were obtained using a Soxhlet extractor. The ethanol was then evaporated using a rotary evaporator. Water extracts were obtained by freeze-drying at −25°C for 1 day. Finally, the extracts were stored in a refrigerator (4°C) in airtight bottles until further analysis. The extracts were subsequently weighed to calculate the yield of the extraction for the solvents used. The yield of extracts is calculated using a weight ratio of samples as follows:
where W de is the weight of the dry extract after solvents have been removed and W dp is the weight of the dry plant material or plant powder before extraction.
2.4 Total phenol content determination
The Folin–Ciocalteu reagent method is an electron transfer-based assay and gives reducing capacity, which has normally been expressed as phenolic contents [53]. Total phenolic content (TPC) was assayed using the Folin–Ciocalteu reagent following the method based on the hetero-polyphosphoric tungstate molybdate reagent to react with tyrosine to give a blue color proportionate to the protein content [61,62]. TPC in the Mim leaf extracts was determined by the Folin-Ciocalteu method as described by Prior et al. [53] and Singleton et al. [62] with a moderate modification as reported by the authors Matić et al. and Ainsworth & Gillespie, respectively [50,63]. Briefly, 500 µL of the diluted extracts, 1,500 µL of the Folin–Ciocalteu reagent, and 1,200 µL of 7.5% saturated aqueous sodium carbonate (Na2CO3) were mixed; the mixture was then thoroughly homogenized and incubated for 45 min at 40°C. After incubation, the absorbance of each sample was measured at 765 nm using the UV/Vis spectrophotometer against distilled water as blank. The TPC was calculated by using the gallic acid calibration curve. All assays were performed in triplicate. The equation curve of the gallic acid equivalent (GAE) for polyphenols is Y = ax + b, Y = 0.0081x – 0.0567R 2 = 0.996. The TPC was expressed as µg of GAE per 100 mg of dry weight (DW) (µg GAE/100 mg of DW).
2.5 Total flavonoid content (TFC) determination
The TFC of Mim leaf extracts was determined using the aluminum chloride colorimetric assay, according to the protocol by Prior et al. [53] and Singleton et al. [62] with a moderate modification as reported by the authors Matić et al. and Ainsworth & Gillespie, respectively [50,63]. Briefly, 200 µL of each extract was mixed with 2,000 µL of distilled water and subsequently with 150 µL of sodium nitrite solution (5% NaNO2) and added water up to the mark that was 5% sodium nitrate; the mixture was incubated for 6 min at room temperature. Thereafter, 150 µL of aluminum trichloride solution (10% AlCl3) was added and then allowed to stand for 6 min; afterward, 2 mL of sodium hydroxide solution (4% NaOH) was added to the mixture, and then 200 µL of distilled water was added. Then, the mixture was thoroughly mixed and allowed to stand for 30 min at room temperature. The absorbance was measured at 510 nm versus the reagent blank containing ethanol instead of the sample, using the ultraviolet spectrophotometer. The (+)-catechin standard was used as a standard compound for the quantification of total flavonoids. All trials were performed in triplicate. The equation curve of (+)-catechin equivalent (CE) for flavonoids is Y = ax + b, Y = 0.0012x – 0.07R 2 = 0.9961. The TFC was expressed as µg of (+)-catechin equivalent per 100 mg of DW (µg CE/100 mg of DW) (Table 1).
Polyphenols and flavonoid contents of the ethanol and water of Mim leaf extract
| Plant extracts | Polyphenols (µg GAE/mg) | Flavonoids (µg CE/mg) |
|---|---|---|
| Ethanol | 135.30 ± 0.05 | 138.33 ± 0.00 |
| Water | 264.70 ± 0.03 | 83.38 ± 0.00 |
Data are expressed as mean ± SD (n = 3).
µg GAE/mg extract: Micrograms of gallic acid equivalent per milligram of extract; µg CE/mg extract: Micrograms of (+)-catechin equivalent per milligram of extract.
The total polyphenols and flavonoids content were calculated by the following equation:
where X is the polyphenols or flavonoids content, µg/mg plant extract, A is the mean absorbance of plant extract solution, and a and b are the constants obtained from the equation curve.
2.6 HPLC–MS analysis
Separations were performed using a Thermo Scientific™ Dionex™ Ultimate™ 3000 XRS LC system with a Thermo Scientific™ Hypersil™ Gold aQ, 100 × 2.1 mm ID, 1.9 μm particle HPLC column. The flow rate was 200 μL/min using the following chromatographic gradient: mobile phase: A: 80% formic acid 0.3% (pH 2.5) and 20% (v/v) of methanol B: methanol + 0.1% formic acid. The gradient was programmed as follows: 100:0 (solvent A:solvent B) for 10 min, from 100:0 to 80:20 in 3 min, remaining at 80:20 for 5 min, to 60:40 in 8 min, remaining at 60:40 for 3 min, to 100:0 in 1 min. The flow rate was 1.0 mL/min, and the oven temperature was set at 30°C. Mass spectrometer method: samples were analyzed using a TSQ Endura triple-quadrupole mass spectrometer in a Full Scan mode. Ionization was performed using the new Ion Max NG ion source. In heated electrospray ionization (ESI) mode H, with a vaporizer temperature of 300°C and a capillary temperature of 350°C. Sheath and auxiliary gas flows were 45 and 10 (arbitrary units), respectively, with an ionization voltage of 3,000 volts in both positive and negative ion modes. Collision gas pressure was set at 1.5 mTorr throughout the experiments.
2.7 Antioxidant activity evaluation
2.7.1 Free radical scavenging activity (RSA) by DPPH assay
The antioxidant activity was determined according to the protocol described by Saleh Al-Hashemi and Hossain [51]. Briefly, 10 mg of each extract (ethanol and water) was taken in a test tube and dissolved with 10 mL of methanol. Then, different concentrations, including 200, 100, 80, 60, 40, and 20 µg/mL, were prepared by using a serial dilution technique. Half a milliliter of a 0.004% methanol solution of DPPH solution was added to all test tubes, shaken gently by hand, and kept in a dark place for one and a half hours. The absorbance (Abs.) of all concentrations of samples was measured by using UV-visible spectroscopy at a wavelength of 517 nm with a blank containing DPPH and methanol. The butylated hydroxytoluene (BHT) was used as a positive control. Finally, the antioxidant activity of extracts was expressed as the IC50 (µg/mL). To get the value of IC50, first, the percentage of the % RSA of the DPPH assay was calculated following the formula:
Then, the IC50 of the DPPH RSA was calculated using linear regression based on the remaining DPPH radicals (Y = ax + b) against the sample concentration using the formula:
2.7.2 Free RSA by ABTS assay
ABTS RSA was determined according to the method of Elouafy et al. [64]. The ABTS stock solution was prepared by reacting an aqueous solution of ABTS (10 mg in 10 mL of distilled water) with an aqueous solution of potassium persulfate (9.46 mg in 0.5 mL of distilled water). This mixture was allowed to react in the dark at room temperature for 12–16 h. Afterward, the ABTS + stock solution was diluted with methanol until it reached an absorbance of 0.7 ± 0.02 at 734 nm. A volume of 75 µL of the test sample was then mixed with 925 µL of the diluted ABTS + solution. Ascorbic acid (10 µg/mL) was used as a standard in ABTS + assay for the preparation of the calibration curve. The antioxidant activity of extracts in the ABTS assay was expressed as the IC50 (µg/mL). To get the value of IC50, the percentage of the % RSA of ABTS assay was calculated following the formula:
where A0 is the absorbance of the control and A1 is the absorbance of the sample.
Then, the IC50 of ABTS RSA was calculated from the line regression of the remaining ABTS% RSA (Y = ax + b) against the sample concentration using the same formula of equation (4).
2.7.3 Free RSA by FRAP assay
The FRAP assay of extracts was determined according to the method reported by Parusnath et al. [65] with minor modifications. Briefly, 1 mL of the tested extract solution (10 mg/mL) at various concentrations was mixed with 0.5 mL of potassium ferricyanide (1%, w/v) and 0.5 mL of phosphate buffer (0.2 M, pH 6.6). This mixture was incubated for 20 min at 50°C, after which 0.5 mL of trichloroacetic acid (10%) was added. Following centrifugation for 20 min at 3,000 rpm, 0.5 mL of the upper layer was collected and mixed with 0.5 mL of distilled water and 500 µL of FeCl3 (0.1%). Ascorbic acid (10 mg/mL) served as a standard for the calibration curve, and the absorbance was measured at 700 nm using a spectrophotometer. The results were expressed as milligrams of ascorbic acid equivalents per gram of dry weight (mg AAE/g DW). All tests were conducted in triplicate.
2.8 Molecular docking study
The main phytochemical compounds present in the ethanolic and aqueous extracts of Mim were obtained in 3D SDF format from the PubChem database. The molecular docking of these ligands as potential inhibitors of the glutathione reductase (GR), lipoxygenase (LOX), and cyclooxygenase (COX) enzymes was evaluated. The crystal structure of each enzyme was prepared by removing water molecules and adding polar hydrogens and Kollman charges using AutoDock Tools.
2.8.1 Protein preparation
The crystal structures of the proteins GR (PDB: 3DK9), LOX (PDB: 1YGE), and COX (PDB: 6Y3C) were obtained from the Protein Data Bank (PDB) at www.rcsb.org. Each structure was prepared by removing water molecules and adding polar hydrogens and Kollman charges using AutoDock Tools (ADT; version 1.5.7). A grid of 40 × 40 × 40 Å with a point spacing of 0.375 Å was created for analysis. We created a grid (Å) to cover the outer regions and active sites of the proteins, centered on their coordinates. The macromolecules were saved in PDB format for analysis [66].
2.8.2 Ligand preparation
We found key phytochemicals in the ethanol and water extracts of Mim, listed with standard inhibitors like Zileuton (CID: 60490), Xanthene (CID: 7107), and Celecoxib (CID: 2662). These compounds are available in 3D SDF format from the PubChem database at https://pubchem.ncbi.nlm.nih.gov/. We tested their potential as inhibitors of GR, LOX, and COX using molecular docking. First, we changed the compounds to PDB format using PyMOL (version 2.5.3) [66] and then to PDBQT format using AutoDock Tools (ADT; version 1.5.7, The Scripps Research Institute) [67].
2.9 Absorption, distribution, metabolism, and excretion (ADME) studies
Understanding how substances behave in the body is important. This includes their ADME. These steps show how a substance moves from being taken into being eliminated. We now use computer tools to predict these ADME properties of molecules [68]. These tools help us see how well a molecule can pass through cell membranes and interact with transporters and enzymes involved in absorption and excretion and how stable it is when metabolized. For our analysis, we used the Swiss ADME platform (available online: www.swissadme.ch) [66]. This platform helps us examine the properties of phytochemicals in Mim’s ethanol and aqueous extracts. It allows us to evaluate their potential as treatments and understand their pharmacokinetic characteristics, giving us a clear picture of their ADME profile.
2.10 Statistical analysis
Analysis of data and graphs was made using Microsoft Excel Worksheet software 2021, Version 2108 (Build 14332.20517). The data were expressed as mean ± standard deviation (SD) for the triplicate experiments (n = 3). Differences were considered significant when p < 0.05. Linear regression analysis was used to calculate the IC50 value.
3 Results
3.1 Extraction yield
The extractive yield is almost equal between the solvents. Ethanol and water extractive yields are presented in Figure 2 (respectively 9.0 and 9.8%, w/w).
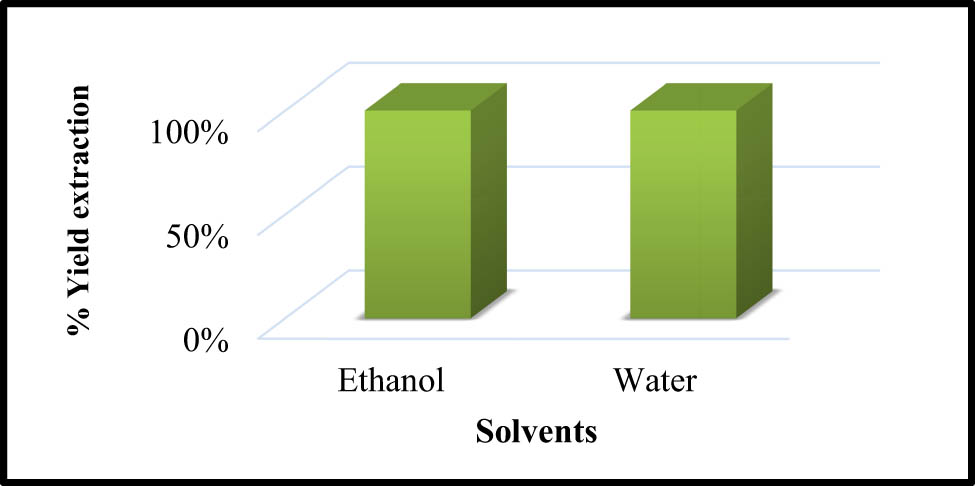
The percentage of extractive yield of Mim leaf extract using ethanol and water.
3.2 Phenolic and flavonoid contents
The Folin–Ciocalteu reagent method showed the presence of polyphenols in both ethanol and water extracts of the Mim leaf. The aluminum chloride colorimetric method also showed the presence of flavonoid compounds in both ethanol and water extracts. The TPC is reported as a GAE curve, with the equation Y = 0.0081x – 0.0567 and R 2 = 0.996, which is greater than 0.995. The results indicated that the highest TPC was found in water extracts, with a value of 264.7 ± 0.03 µg GAE/mg of extract (equivalent to 0.2647 ± 0.03 mg/mg). In comparison, the ethanol extracts showed a TPC of 135.3 ± 0.05 µg GAE/mg of extract (equivalent to 0.1353 mg/mg) (Table 1). The TFC is reported as a (+)-CE curve, represented by the equation Y = 0.0012x – 0.07, with R 2 = 0.9961, also greater than 0.995. The highest TFC was found in ethanol extracts, measuring 138.33 ± 0.002 µg CE/mg of extract (equivalent to 0.13833 mg/mg), while water extracts showed a lower value of 83.38 ± 0.002 µg CE/mg of extract (equivalent to 0.8338 mg/mg) (Table 1).
3.3 HPLC–MS analysis results
The HPLC–MS analysis of ethanol leaf extracts (ELE) (Figure 3) revealed the presence of phenolic compounds such as eugenol (0.30%) (Table 2) and terpenoids with notable antioxidant properties, including phytol (1.68%), germacrene B, and vomifoliol (Table 2). The ELE also contains a major bioactive compound with strong antibacterial properties, which is the diethyl phthalate (92.31%) (Table 2). The HPLC analysis of water leaf extracts (WLE) (Figure 4) also showed the presence of several major phenolic compounds with significant antioxidant properties, including BHT (found in the Mim leaf as a natural antioxidant) (28.12%), benzoic acid (5.61%), 2-methoxy-4-vinylphenol (3.45%), eugenol (2.12%), syringol (2,6-dimethoxyphenol) (a derivate of syringic acid) (1.58%), vanillin (1.17%), 1,2-benzenediol (0.62%), and phenylacetic acid (0.46%) (Table 3). It also revealed the presence of terpenoids, including 3-buten-2-ol,2-methyl-((2E)-2-penten-2-ol (0.51%). Furthermore, the WLE revealed the presence of bioactive molecules, including 2,3-dihydro-benzofuran, hydromorphone, 1 hexadecanol, cis-9-octadecen-1-ol, and stearyl alcohol (Table 3).
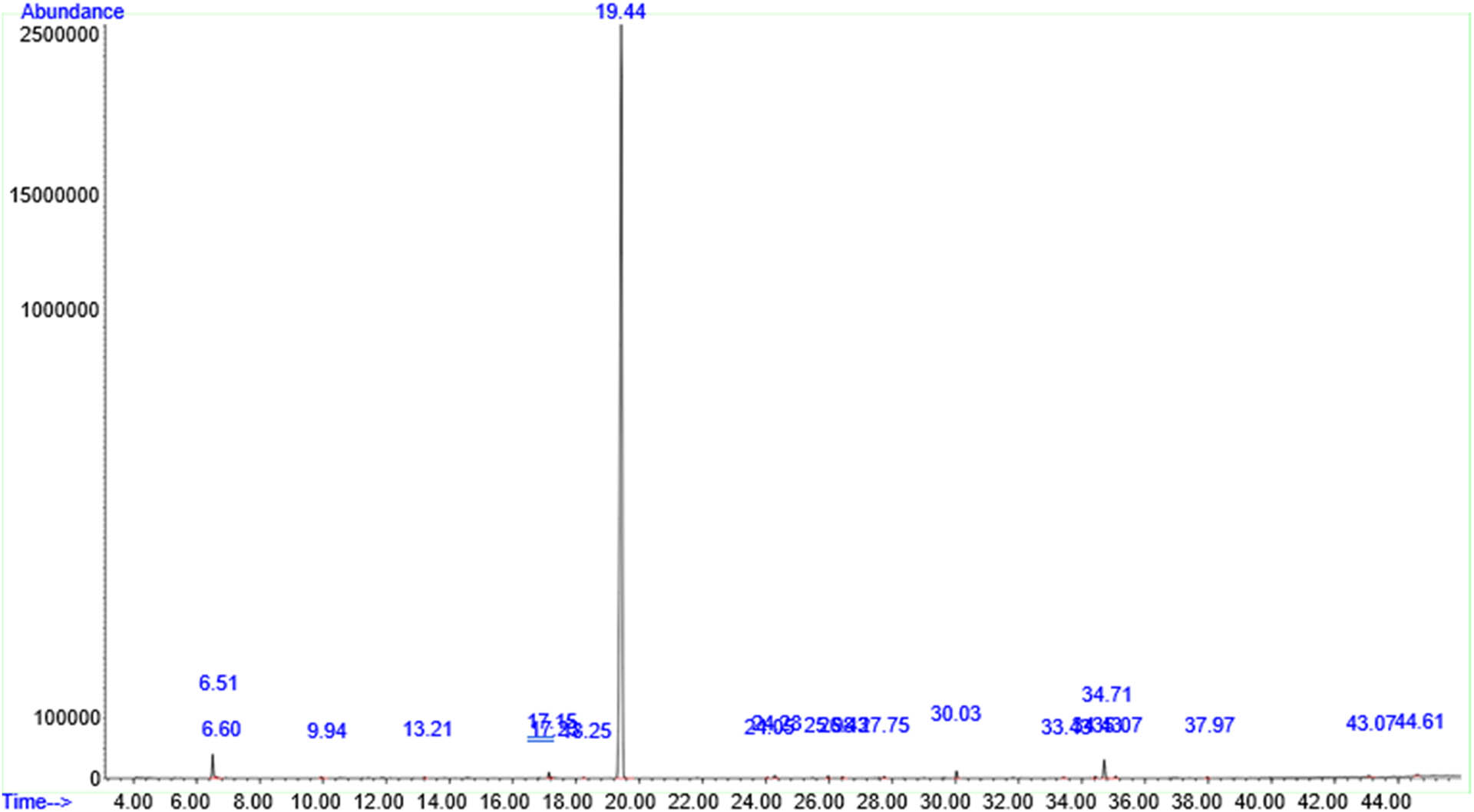
A typical HPLC/MS chromatogram depicting the chemical constituents in the ELE of Mim.
Compounds identified through HPLC–MS analysis of Mim ELE
| Peak number | Compound name | RT (min) | Area | Similarity (%) | Percentage (%) | Molecular weight (g/mol) | Molecular formula | Chemical class and property | Reference of the chemical class and property |
|---|---|---|---|---|---|---|---|---|---|
| 1 | N,N-Dimethylaniline | 6.51 | 2,532,471 | 97 | 2 | 121.18 | C8H11N | Aromatic amine, an intermediate in the manufacture of dyes | [69] |
| 2 | Undecane | 6.60 | 308,478 | 35 | 0.24 | 156,308 | C11H24 | Alkane hydrocarbon, anti-allergic, anti-inflammatory, antimicrobial | [70] |
| 3 | Indole | 9.94 | 239,052 | 4 | 0.19 | 117.151 | C8H7N | Aromatic heterocyclic organic compound, anti-cancerous, antioxidant | [71] |
| 4 | Undecanol-3 | 13.21 | 136,665 | 25 | 0.11 | 172.31 | C11H24O | Fatty secondary alcohol, flavoring ingredient in foods | [72] |
| 5 | Lauric acid methyl ester (dodecanoic acid) | 17.15 | 611,264 | 95 | 0.48 | 200.322 | C12H24O2 | Saturated fatty acid, antioxidant, enhance the sperm quality | [73] |
| 6 | Methyl o-(bromochloroacetyl) benzoate | 17.24 | 145,278 | 40 | 0.11 | 291.52 | C10H8BrClO3 | Organic compound, insect repellent | [74] |
| 7 | Germacrene B | 18.25 | 117,883 | 64 | 0.09 | 204.35 | C15H24 | Volatile organic hydrocarbon and achiral sesquiterpene, antimycobacterial, antiplasmodial | [75,76] |
| 8 | Diethyl phthalate (1,2-benzenedicarboxylic acid, diethyl ester, or anozol) | 19.44 | 1.17 × 1008 | 97 | 92.31 | 222.24 | C12H14O4 | Anhydride organic compound, antimicrobial | [77] |
| 9 | Myristic acid (tetradecanoic acid) | 24.05 | 267,413 | 83 | 0.10 | 228.37 | C16H28O2 | Saturated fatty acid, antioxidant, antimicrobial | [78] |
| 10 | Eugenol (2-methoxy-4-(prop-2-en-1-yl) phenol) | 24.29 | 384,094 | 49 | 0.30 | 164.204 | C10H12O2 | Monoterpene and phenolic compound, antimicrobial, antifungal | [79] |
| 11 | Vomifoliol ((4S)-4-hydroxy-4-[(E,3R)-3-hydroxybut-1-enyl]-3,5,5-trimethylcyclohex-2-en-1-one) | 25.97 | 335,291 | 59 | 0.27 | 224.30 | C13H20O3 | Sesquiterpene, neuroprotective | [80] |
| 12 | Docosanedioic acid, dimethyl ester | 26.43 | 192,181 | 47 | 0.15 | 398.62 | C24H46O4 | Dicarboxylic fatty acid, antifungal | [81] |
| 13 | 6-Methyl-2-heptanone | 27.74 | 210,368 | 43 | 0.17 | 128.2120 | C8H16O | Volatile organic compound, antifungal | [82] |
| 14 | Palmitic acid ME (hexadecanoic acid) | 30.03 | 802,770 | 98 | 0.63 | 270.5 | C17H34O2 | Saturated fatty acid, antioxidant, nematicide, pesticide | [83,84] |
| 15 | 2H-Benzocyclohepten-2-one,3,4,4a,5,6,7,8,9-octahydro-4a-methyl-, (S)- | 33.43 | 181,058 | 49 | 0.14 | 178.27 | C12H18O | Phenol organic compound | PubChem CID 10678901 |
| 16 | Isopulegol (5-methyl-2-(prop-1-en-2-yl) cyclohexan-1-ol) | 34.43 | 239,307 | 35 | 0.19 | 154.25 | C10H18O | Monoterpene alcohol, antibacterial, drug against clinical complications of Parkinson disease | [85,86] |
| 17 | Phytol (2-hexadecen-1-ol, 3,7,11,15-tetramethyl- (2E,7R,11R)-) | 34.71 | 2,126,480 | 86 | 1.68 | 296.5 | C20H40O | Acyclic diterpenoid, antioxidant, antimicrobial, anticancer, antiradical | [87] |
| 18 | Stearic acid ME (octadecanoic acid) | 35.07 | 267,413 | 87 | 0.21 | 284,47 | C18H36O2 | Saturated fatty acid, cholesterol-neutral | [88] |
| 19 | Stearyl alcohol (octadecan-1-ol) | 37.97 | 150,679 | 22 | 0.12 | 270.49 | C18H38O | Saturated fatty acid, skin cleanser of dry and sensitive skin | [89] |
| Total: 99.98% |
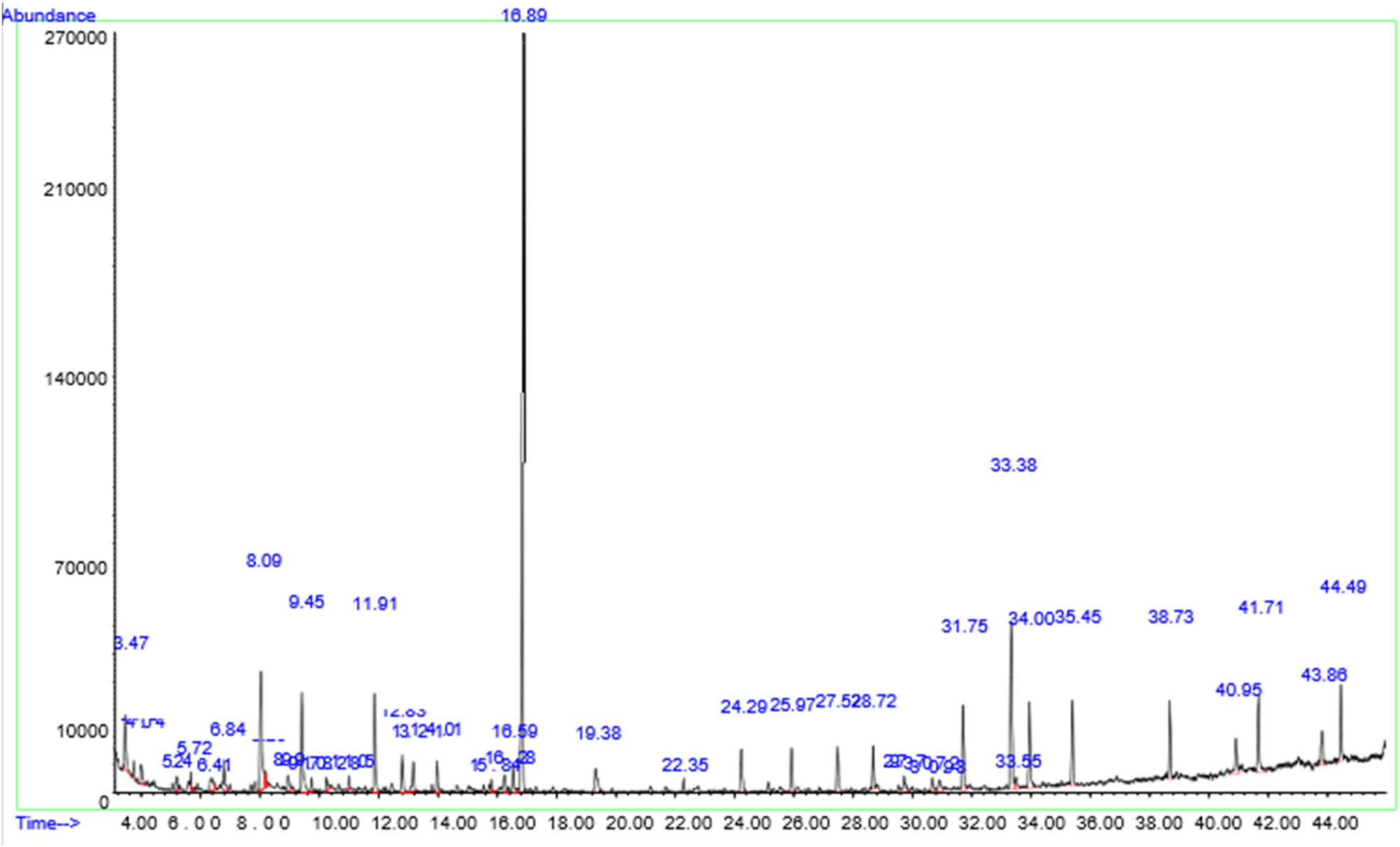
A typical HPLC/MS chromatogram depicting the chemical constituents in the WLE of Mim.
Compounds identified through HPLC–MS analysis of Mim WLE
| Peak number | Compound name | RT (min) | Area | Similarity (%) | Percentage (%) | Molecular weight (g/mol) | Molecular formula | Chemical class and property | Reference of the chemical class and the property |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ascorbic acid (3-oxo-l-gulofuranolactone) | 3.47 | 791,040 | 9 | 2.40 | 176.12 | C6H8O6 | Organic compound, vitamin, antioxidant, against chronic diseases | [90,91,92] |
| 2 | 2-Octene, 3,7-dimethyl | 3.78 | 164,615 | 35 | 0.50 | 140.26 | C10H20 | Volatile lipophilic compound, allylic diol | [93] |
| 3 | Butanoic acid, 3-hydroxy-3-methyl | 4.04 | 362,334 | 50 | 1.10 | 132.16 | C5H10O3 | Hydroxy monocarboxylic acid, indicator of biotin deficiency | PubChem CID 69362 |
| 4 | 3-Buten-2-ol, 2-methyl- ((2E)-2-penten-2-ol) | 5.25 | 168,785 | 9 | 0.51 | 86.13 | C5H10O | Terpenoid | [94] |
| 5 | 2,3,3-Trimethyloctane | 5.72 | 174,358 | 40 | 0.53 | 156.31 | C11H24 | Alkane | PubChem CID 537321 |
| 6 | Propanoic acid or propionic acid | 6.40 | 309,857 | 9 | 0.94 | 74.08 | C3H6O2 | Short-chain fatty acid, mediator that connects nutrition, gut microbiota, and overall physiology | [95] |
| 7 | 1,2-Ethanediamine, N,N,N′,N′-tetramethyl- | 6.83 | 492,981 | 45 | 1.50 | 116.20 | C6H16N2 | Mono alkylamine, chelator and a catalyst | PubChem CID 8037 |
| 8 | Benzoic acid (phenolic acid) | 8.09 | 1,846,634 | 95 | 5.61 | 122.12 | C6H5COOH | Phenyl compound, antioxidant, antidiabetic, improves the gut functions | [96,97] |
| 9 | 2-Phenyl-3-oxetanone (tropic acid β-lactone) | 8.21 | 249,605 | 9 | 0.76 | 148.16 | C9H8O2 | Alkyne oxetane, anticancerous | [98,99] |
| 10 | 1,2-Benzenediol (pyrocatechol or catechol) | 8.99 | 203,617 | 80 | 0.62 | 110,11 | C6H4(OH)2 | Organic phenol, antioxidant, antiviral, antimicrobial | [100,101,102] |
| 11 | 2,3-Dihydro-benzofuran (coumaran) | 9.44 | 1,499,269 | 86 | 4.55 | 120.15 | C8H8O | Heterocyclic compound, anticancerous | [103,104] |
| 12 | Alpha-aminomethyleneglutaconic anhydride ((3E)-3-(amino methylene)-2H-pyran-2,6(3H)-dione) | 9.78 | 124,379 | 47 | 0.38 | 139.11 | C6H5NO3 | Amine, vinylogous carbamate | [105] |
| 13 | Phenylacetic acid (α-toluic acid or also benzeneacetic acid) | 10.28 | 151,400 | 43 | 0.46 | 136.15 | C8H8O2 | Phenolic acid, antimicrobial, anticancer, antitumour | [106] |
| 14 | Tetradecane | 11.04 | 93,121 | 72 | 0.28 | 198.39 | C14H30 | Alkane hydrocarbon, volatile component, a pheromone | [107] |
| 15 | 2-Methoxy-4-vinylphenol | 11.90 | 1,136,399 | 94 | 3.45 | 150.17 | C9H10O2 | Phenol compound, a food additive | [108,109] |
| 16 | 2,6-Dimethoxyphenol (Syringol) | 12.83 | 521,877 | 90 | 1.58 | 154.25 | C8H10O3 | Phenol compound, a smoke flavor in foods | [110] |
| 17 | Thiazole, 5-methyl- | 13.21 | 439,991 | 22 | 1.34 | 99.15 | C4H5NS | Heterocyclic compound, antioxidant, analgesic, antibacterial, anticancer | [111,112] |
| 18 | Vanillin (4-hydroxy-3-methoxybenzaldehyde) | 14.01 | 38,6637 | 97 | 1.17 | 152.15 | C8H8O3 | Phenolic aldehyde, anti-inflammatory agent, a food additive, antioxidant, anticancer | [113] |
| 19 | Cyclotetradecane | 15.84 | 118,957 | 80 | 0.36 | 196.37 | C14H28 | Cycloalkane | [114] |
| 20 | Carbofuran | 16.28 | 269,346 | 7 | 0.82 | 222.25 | C11H16O | Carbamate, pesticide | [115] |
| 21 | 1,3,5-Hexatriene | 16.58 | 352,842 | 64 | 1.07 | 80.13 | C6H8 | Alkene | — |
| 22 | Butylated hydroxytoluene (BHT) or 4-methyl-2,6-ditertiarybutylphenol | 16.90 | 926,1354 | 98 | 28.12 | 220.36 | C15H24O | Phenolic compound, antioxidant | [116,117] |
| 23 | 1,2-Benzenedicarboxylic acid, mono-butyl ester | 19.38 | 511,699 | 53 | 1.55 | 222.23 | C12H14O4 | Carboxylic acid, Allelopathic, antimicrobial, insecticidal, antiproliferative | [118,119] |
| 24 | 1-Tridecene | 22.36 | 159,129 | 10 | 0.48 | 182.35 | C13H26 | Volatile organic compound and acyclic olefin and aliphatic unsaturated hydrocarbon | PubChem CID 17095 |
| 25 | Eugenol (1-hydroxy-2-methoxy-4-prop-2-enylbenzene) | 24.29 | 698,237 | 53 | 2.12 | 164.204 | C10H12O2 | Phenylpropane and phenolic organic compound, antioxidant | [120,121] |
| 26 | 2-Cyclohexen-1-one, 4-hydroxy-3,5,6-trimethyl-4-(3-oxo-1-butenyl)- | 25.97 | 634,640 | 64 | 1.93 | 222.28 | C13H18O3 | Volatile organic compound | PubChem CID 5371378 |
| 27 | Hydromorphone—TMS2 (5 alpha-époxy-3-hydroxy-17-méthyl morphinan-6-one) | 27.52 | 607,128 | 43 | 1.84 | 285,33 | C17H19NO3 | Hydrogenated ketone and heterocyclic, analgesic | [122] |
| 28 | 1-Hexadecanol (palmityl alcohol) | 28.73 | 510,992 | 91 | 1.55 | 242.44 | C16H34O | Fatty alcohol, anti-inflammatory, nematicide, pesticide, antioxidant, hypocholesterolaemia activities | [123,124] |
| 29 | Ionol ((4,7-megastigmadien-9-ol or 4-(2,6,6-trimethyl-2-cyclohexenyl)-3-buten-2-ol | 29.77 | 292,658 | 7 | 0.89 | 194.31 | C13H22O | Volatile organic compound, sesquiterpenoid | PubChem CID 11264038 |
| 30 | 3-Cyclohexen-1-ol, 5-(2-butenylidene)-4,6,6-trimethyl | 30.73 | 156,515 | 30 | 0.48 | 190.28 | C13H18O | Organic compound | — |
| 31 | 10-Bromo-7-hydroxy-11-iodolaurene | 30.98 | 245,446 | 47 | 0.75 | 421.11 | C15H18BrIO | Hydroxytoluene or aromatic alcohol, antioxidant, fragrance | PubChem CID 23425301 |
| 32 | 3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetra-siloxane | 31.75 | 1,091,024 | 43 | 3.31 | 577.2 | C18H52O7Si7 | Organosilicon compound, with medicinal applications and also used as polymer fire retardants | [125,126] |
| 33 | cis-9-Octadecen-1-ol (oleyl alcohol) | 33.38 | 2,191,988 | 32 | 6.66 | 268.48 | C18H36O | Unsaturated fatty alcohol, absorption enhancer in pulmonary protein delivery | [127] |
| 34 | 1-Tridecyne | 33.55 | 254,535 | 47 | 0.77 | 180.33 | C13H24 | Alkyne | [128] |
| 35 | Stearyl alcohol (octadecanol-1) | 34.00 | 1,196,073 | 95 | 3.63 | 270.49 | C18H38O | Saturated fatty acid, emulsifier, emollient and thickener in skin creams, lotions | [89] |
| 36 | Cyclo-dodeca-siloxane, tetra-cosamethyl | 35.45 | 1,052,571 | 70 | 3.20 | 889.8 | C24H72O12Si12 | Organic compound, antibacterial, antioxidant | [129] |
| 37 | Oxazepam—TMS2 (SÉRESTA) (7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-2H-1,4-benzodiazepin-2-one) | 41.71 | 1,172,600 | 43 | 2.87 | 286.71 | C15H11ClN2O2 | Secondary alcohol, tranquilizer, anticonvulsant | [130] |
| 38 | Hexane-dioic acid, mono (2-ethylhexyl) ester | 40.95 | 614,042 | 49 | 1.86 | 258.35 | C14H26O4 | Carboxylic acid, antimicrobial, acaricide | [131,132] |
| 39 | Bistrimethylsilyl N-acetyl eicosasphinga-4,11-dienine | 38.73 | 946,352 | 43 | 3.56 | 511.38 | C28H57NO3Si2 | Secondary metabolite | [133] |
| 40 | 1,2-Benzenedicarboxylic acid, diisooctyl ester (diisooctyl phthalate) | 43.86 | 475,532 | 49 | 1.44 | 390.6 | C24H38O4 | Phthalic acid ester or fatty acid ester/Terpene, anti-inflammatroy antibacterial, antifungal | [134] |
| 41 | 1,1,1,5,7,7,7-Heptamethyl-3,3-bis(trimethylsiloxy) tetra-siloxane | 44.49 | 1,005,609 | 35 | 3.05 | 443.96 | C13H39O5Si6 | Organic compound, antibacterial against cariogenic oral pathogens | [135] |
| Total: 98.49% |
3.4 Antioxidant activity
The antioxidant properties of Mim leaf for both extracts (ELE and WLE) were evaluated using the DPPH, ABTS, and FRAP assays. The results were quantified as Mean IC50 values (µg/mL) ± SD and are shown in Figure 5. The extracts demonstrated significant antioxidant activity, with notable differences observed between the types of extracts and the assays employed. When comparing the three assays with the extracts (Figure 5), the ABTS scavenging activity exhibited the highest importance based on IC50 values, followed by FRAP, and then DPPH assays. For the ethanol extracts, the IC50 value for the ABTS assay was 2.01 ± 0.005 µg/mL (compared to the standard positive control ascorbic acid IC50 = 4.61 ± 0.005 µg/mL) followed by FRAP (EC50 = 5.84 ± 0.05 µg/mL), and DPPH assay with IC50 = 6.27 ± 0.69 µg/mL (compared to the standard positive control, BHT with IC50 = 5.81 ± 0.65 µg/mL). In contrast, the water extracts recorded the highest IC50 value of 5.00 ± 0.06 µg/mL with FRAP assay, followed by ABTS assay (IC50 = 5.51 ± 0.15 µg/mL) and then DPPH assay IC50 = 6.38 ± 0.30 µg/mL) (Figure 5).
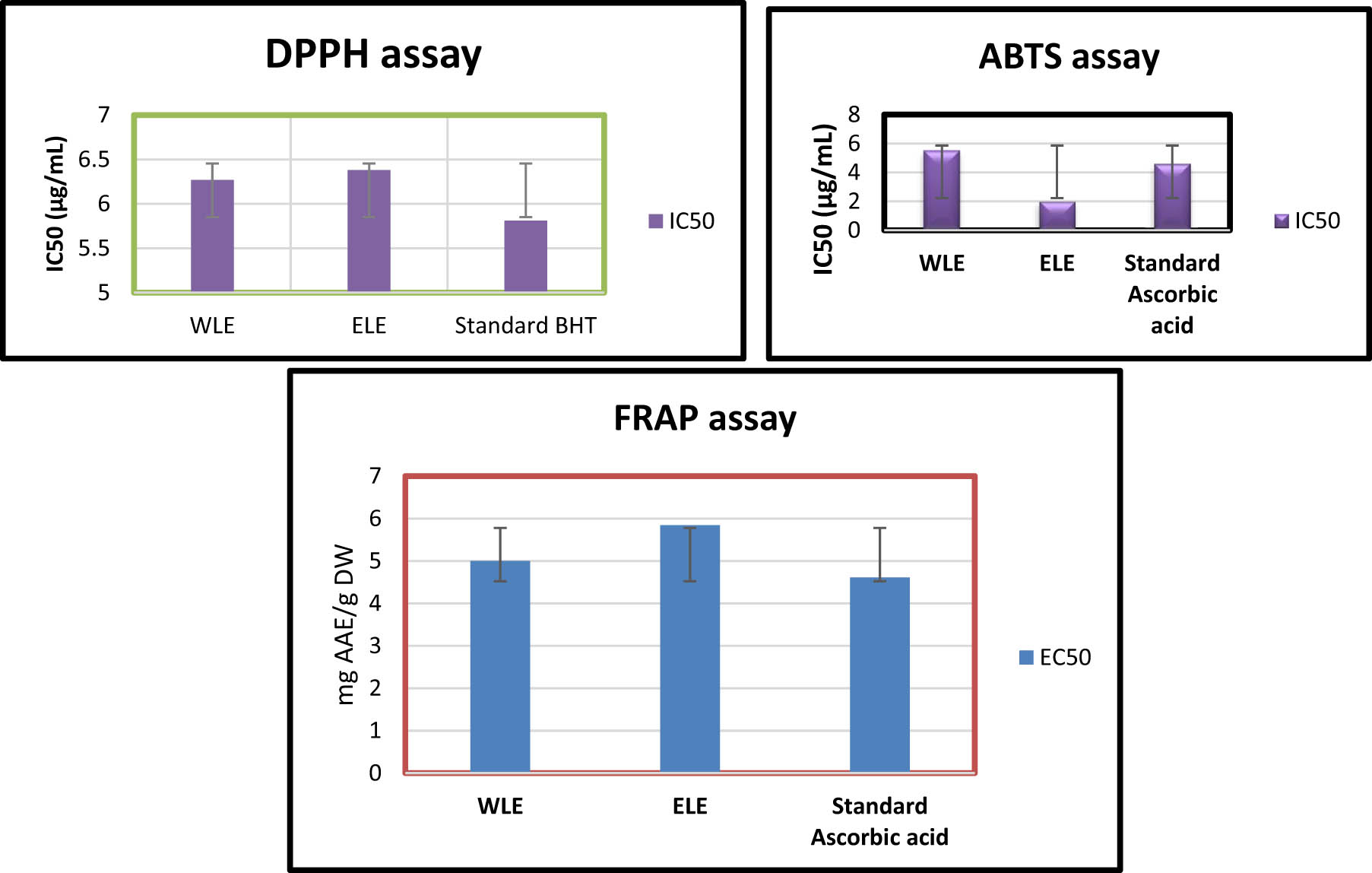
DPPH, ABTS, and FRAP RSA of Mim (A. indica) extracts WLE and ELE. BHT and ascorbic acid are used as standards. IC50 and EC50 represent the concentrations of the sample providing 50% inhibition. Mean IC50 (µg/mL) values ± SD were presented in this figure. The ±SD was represented by the vertical error bars.
3.5 Molecular docking
To understand the impact of Mim extracts on antioxidant activities, we performed molecular docking of the bioactive components present in these extracts with the corresponding molecular receptors. These compounds were tested against four proteins: GR (PDB: 3DK9), COX (PDB: 6Y3C), and LOX (PDB: 1YGE). Each enzyme with its standard inhibitor is presented as follows: Xanthine for GR, Zileuton for LOX, and Celecoxib for COX.
The results of these tests provide insights into the potential therapeutic effects of Mim extracts in managing oxidative stress and inflammatory conditions. The results indicated that all the molecules displayed a weak or comparable affinity for GR when compared to the standard inhibitor, Xanthene (−8.2 kcal/mol) (Table 4). Among them, BHT was noteworthy for its relatively strong affinity of −7.9 kcal/mol, effectively interacting with several amino acid residues of the enzyme. Specifically, it exhibited a pi-alkyl binding interaction with Phe 78, a donor–donor binding interaction with His 82, and a pi-sigma type interaction with Tyr 85 (Figure 6e), compared to xanthene, which interacts with only one amino acid, Val 61 (Figure 6f).
Molecular binding affinities (kcal/mol) indicate how phytochemicals in ethanol and water extracts of Mimulus interact with GR (PDB: 3DK9), COX (PDB: 6Y3C), and LOX (PDB: 1YGE)
| GR (PDB: 3DK9) | LOX (PDB: 1YGE) | COX (PDB: 6Y3C) | |
|---|---|---|---|
| Compounds | Affinity (kcal/mol) | Affinity (kcal/mol) | Affinity (kcal/mol) |
| Xanthene (inhibitor standard) | −8.2 | — | — |
| Zileuton (inhibitor standard) | — | −7.0 | — |
| Celecoxib (inhibitor standard) | — | — | −8.1 |
| Diethyl phthalate | −6.7 | −6.2 | −5.9 |
| Phytol | −5.9 | −6.7 | −6.6 |
| Benzoic acid | −6.7 | −5.7 | −5.7 |
| 2,3-Dihydro-benzofuran | −6.4 | −4.5 | −5.9 |
| Phenylacetic acid | −6.7 | −6.1 | −5.8 |
| 2,6-Dimethoxyphenol | −5.6 | −5.3 | −5.8 |
| Vanillin | −6.0 | −5.6 | −7.9 |
| BHT | −7.9 | −7.1 | −6.0 |
| Eugenol | −6.7 | −6.2 | −6.1 |
| Oleyl alcohol | −5.5 | −5.6 | −5.1 |
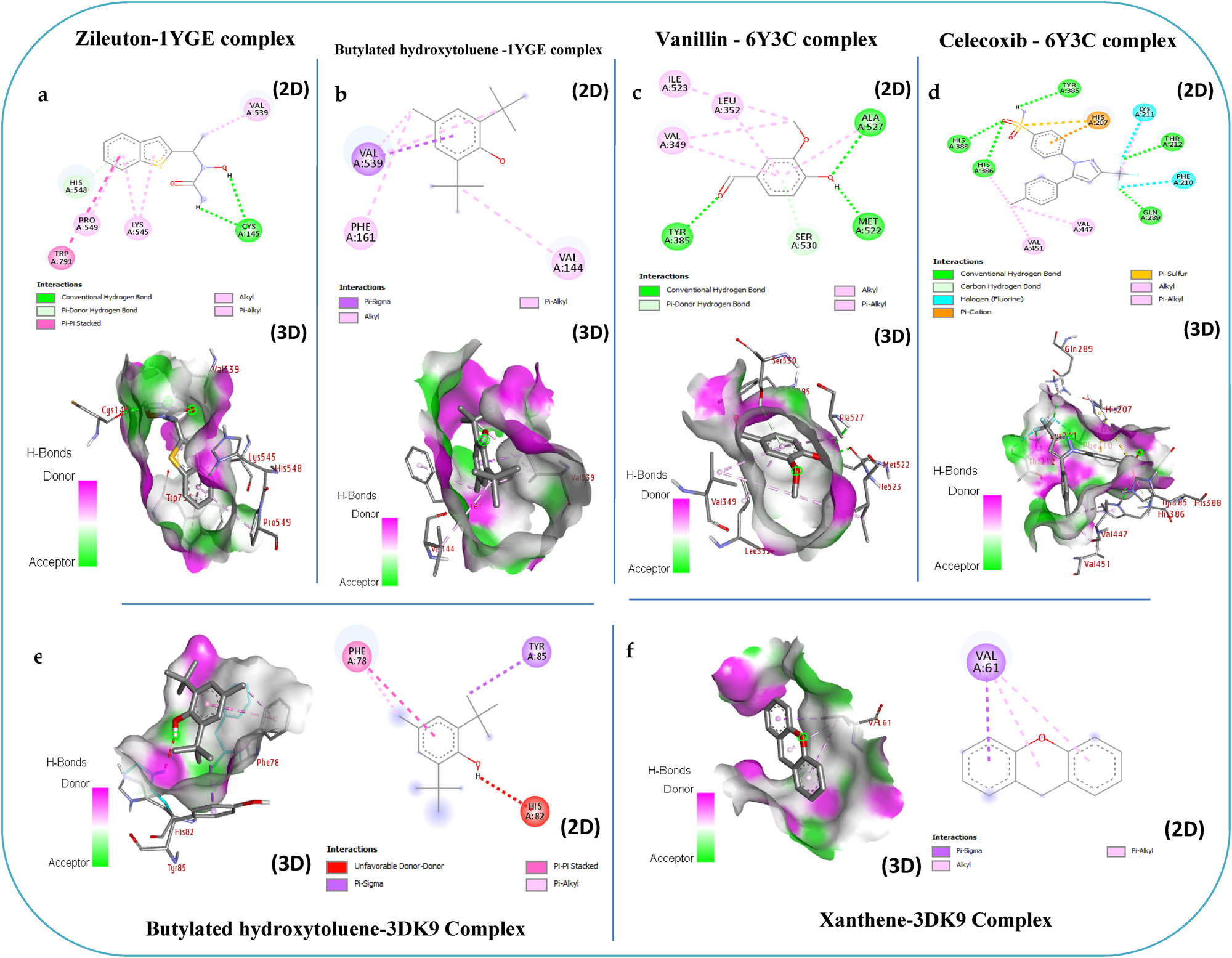
Binding interactions in 2D and 3D of the phytochemicals identified in Mim extracts with Lipoxygenase (PDB: 1YGE) (a), Cyclooxygenase (PDB: 6Y3C) (c), and Glutathione Reductase (PDB: 3DK9) (e), are compared with the standard inhibitors Zileuton (b), Celecoxib (d), and Xanthene (f).
The study found that all the tested molecules have a weak or similar affinity for LOX compared to Zileuton, which is the standard inhibitor with an affinity of −7.0 kcal/mol (Table 5). However, butylhydroxytoluene is different; it shows a stronger affinity of −7.1 kcal/mol by effectively interacting with several amino acid residues in the enzyme. More specifically, it demonstrates pi-alkyl, alkyl, and pi-sigma binding interactions with Val 144, Phe 358, and Val 539 (Figure 6b), compared to Zileuton, which interacts effectively with several amino acid residues of the enzyme (Figure 6a). Moreover, all the molecules exhibit weak or close affinity for COX compared to celecoxib, a standard inhibitor (−8.1 kcal/mol) (Table 4). Vanillin has a strong affinity for the enzyme, measuring −7.9 kcal/mol. It effectively interacts with various amino acid residues. It forms hydrogen bonds with Tyr 385, Met 522, and Ala 527. Vanillin also engages in alkyl and pi-alkyl interactions with Val 349, Leu 352, and Ile 523. Additionally, it forms a pi-donor hydrogen bond with Ser 530 (Figure 6c), compared to celecoxib interacts effectively with several amino acid residues of the enzyme (Figure 6d).
In-silico analysis of the pharmacokinetic properties (ADME) of phytochemicals found in the ethanol and water extracts of Mim
| Physicochemical properties | Lipophilicity | Drug likeness | Pharmacokinetics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | MW g/mol | HBA | HBD | TPSA Å2 | Rotatable bonds | MlogP | WlogP | Lipinski’s | Verber’s | BBB permeation | GI absorption | CYP1A2 inhibitor |
| Diethyl phthalate | 222.2 | 4 | 0 | 52.6 | 6 | 2.3 | 2.0 | 0 | 0 | Yes | High | Yes |
| Phytol | 296.5 | 1 | 1 | 20.2 | 13 | 5.2 | 6.3 | 1 | 1 | No | Low | No |
| Benzoic acid | 122.1 | 2 | 1 | 37.3 | 1 | 1.6 | 1.3 | 0 | 0 | Yes | High | No |
| 2,3-Dihydro-benzofuran | 120.1 | 1 | 0 | 9.2 | 0 | 1.7 | 1.6 | 0 | 0 | Yes | High | Yes |
| Phenylacetic acid | 136.1 | 2 | 1 | 37.3 | 2 | 1.6 | 1.3 | 0 | 0 | Yes | High | No |
| 2-Methoxy-4-vinylphenol | 150.1 | 2 | 1 | 29.4 | 2 | 1.7 | 1.9 | 0 | 0 | Yes | High | Yes |
| 2,6-Dimethoxyphenol | 154.1 | 3 | 1 | 38.6 | 2 | 0.8 | 1.4 | 0 | 0 | Yes | High | No |
| Vanillin | 152.1 | 3 | 1 | 46.5 | 2 | 0.5 | 1.2 | 0 | 0 | Yes | High | No |
| BHT | 152.1 | 3 | 1 | 46.5 | 2 | 0.5 | 1.2 | 0 | 0 | Yes | High | No |
| Eugenol | 164.2 | 2 | 1 | 29.4 | 3 | 2.0 | 2.1 | 0 | 0 | Yes | High | Yes |
| Oleyl alcohol | 268.4 | 1 | 1 | 20.2 | 15 | 4.7 | 6.0 | 1 | 1 | No | High | Yes |
3.6 ADME analysis
Using computer models for early drug tests helps quickly choose drug candidates and streamline development. These studies lower the chances of side effects, reduce drug development failures, and improve clinical success. The ADME study found that all plant compounds from Mim’s ethanol and water extracts met Lipinski’s and Veber’s criteria for oral drug development, except for Phytol and Oleyl alcohol, which exceed the limit of 10 rotatable bonds set by Veber’s rule (Table 5). Our investigation showed that diethyl phthalate (TPSA = 20.2 Å2, WlogP = 4.4), benzoic acid (TPSA = 37.3 Å2, WlogP = 1.3), 2,3-dihydro-benzofuran (TPSA = 9.2 Å2, WlogP = 1.6), phenylacetic acid (TPSA = 37.3 Å2, WlogP = 1.3), 2-methoxy-4-vinylphenol (TPSA = 29.4 Å2, WlogP = 1.9), 2,6-dimethoxyphenol (TPSA = 38.6 Å2, WlogP = 1.4), vanillin (TPSA = 46.5 Å2, WlogP = 1.2), BHT (TPSA = 46.5 Å2, WlogP = 1.2), and eugenol (TPSA = 29.4 Å2, WlogP = 2.1)) (Table 5), and all these compounds can cross the blood-brain barrier (BBB) (Figure 7). All the compounds analyzed were identified as nonsubstrates of P-glycoprotein (PGP–), except for Phytol, which is classified as a substrate (PGP+). With the exception of phytol, all the molecules demonstrated high intestinal absorption (Table 5). Our analysis also indicated that, apart from diethyl phthalate, 2,3-dihydrobenzofuran, 2-methoxy-4-vinylphenol, eugenol, and oleyl alcohol, none of the studied compounds acted as inhibitors or substrates of CYP450 enzymes, particularly CYP1A2 (Table 5).
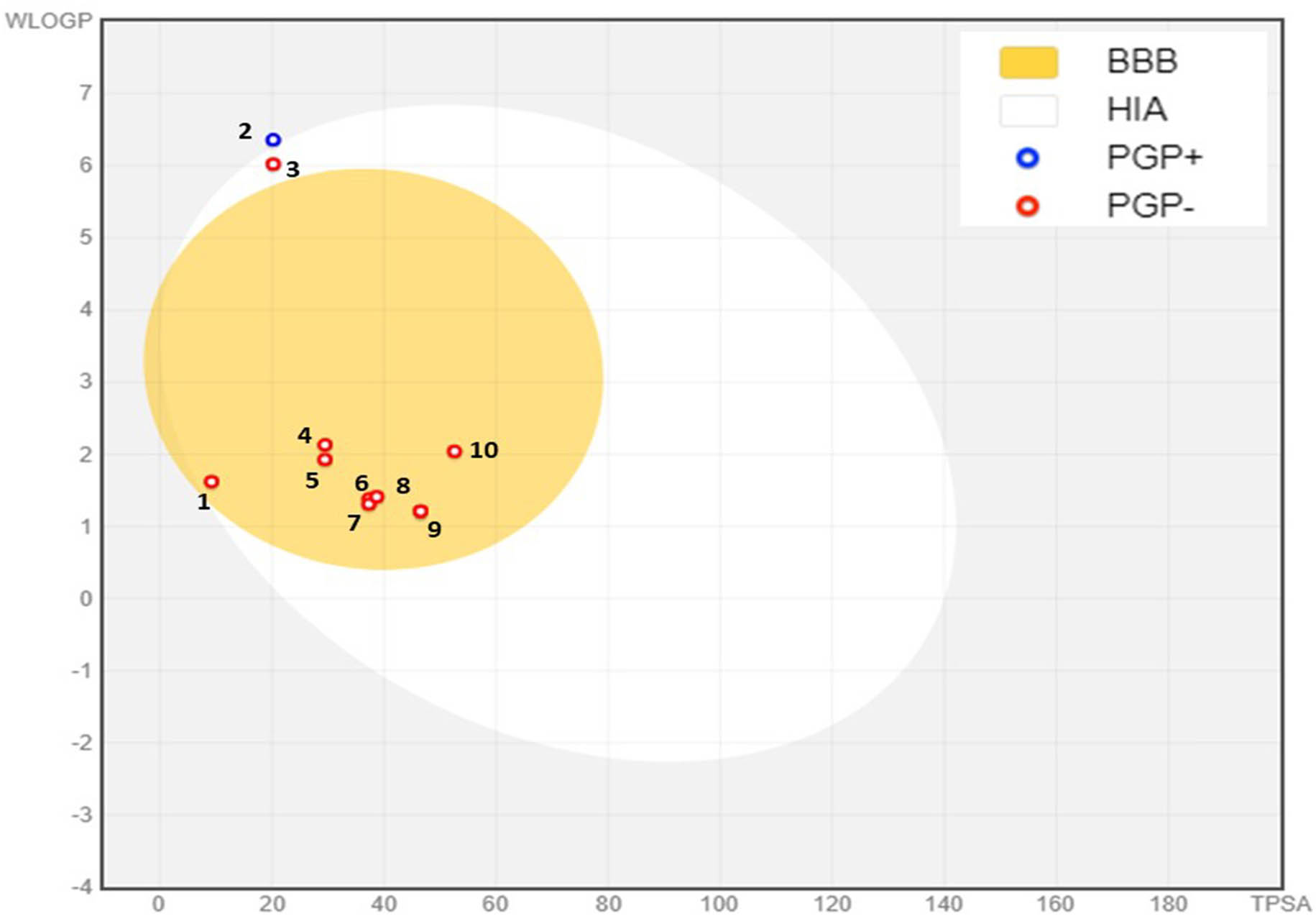
BOILED-Egg model of BBB permeability and GI absorption of phytochemicals identified in the ethanol and aqueous extracts of Mim. (1) 2,3-Dihydro-benzofuran, (2) phytol, (3) oleyl alcohol, (4) eugenol, (5) 2-methoxy-4-vinylphenol, (6) benzoic acid, (7) phenylacetic acid, (8) 2,6-dimethoxyphenol, (9) vanillin, and (10) diethyl phthalate. PGP–: non-substrate of P-glycoprotein, PGP+: P-glycoprotein substrate.
Figure 8 displays the bioavailability radars for the identified compounds, offering a clear visual representation of their potential for oral bioavailability. The pink area on the radar signifies the region where the entire graphical representation of a molecule must fall to be considered drug-like. The bioavailability radars for diethyl phthalate, 2,3-dihydrobenzofuran, and 2,6-dimethoxyphenol are situated within the pink zone, indicating their potential as drug candidates (Figure 8).
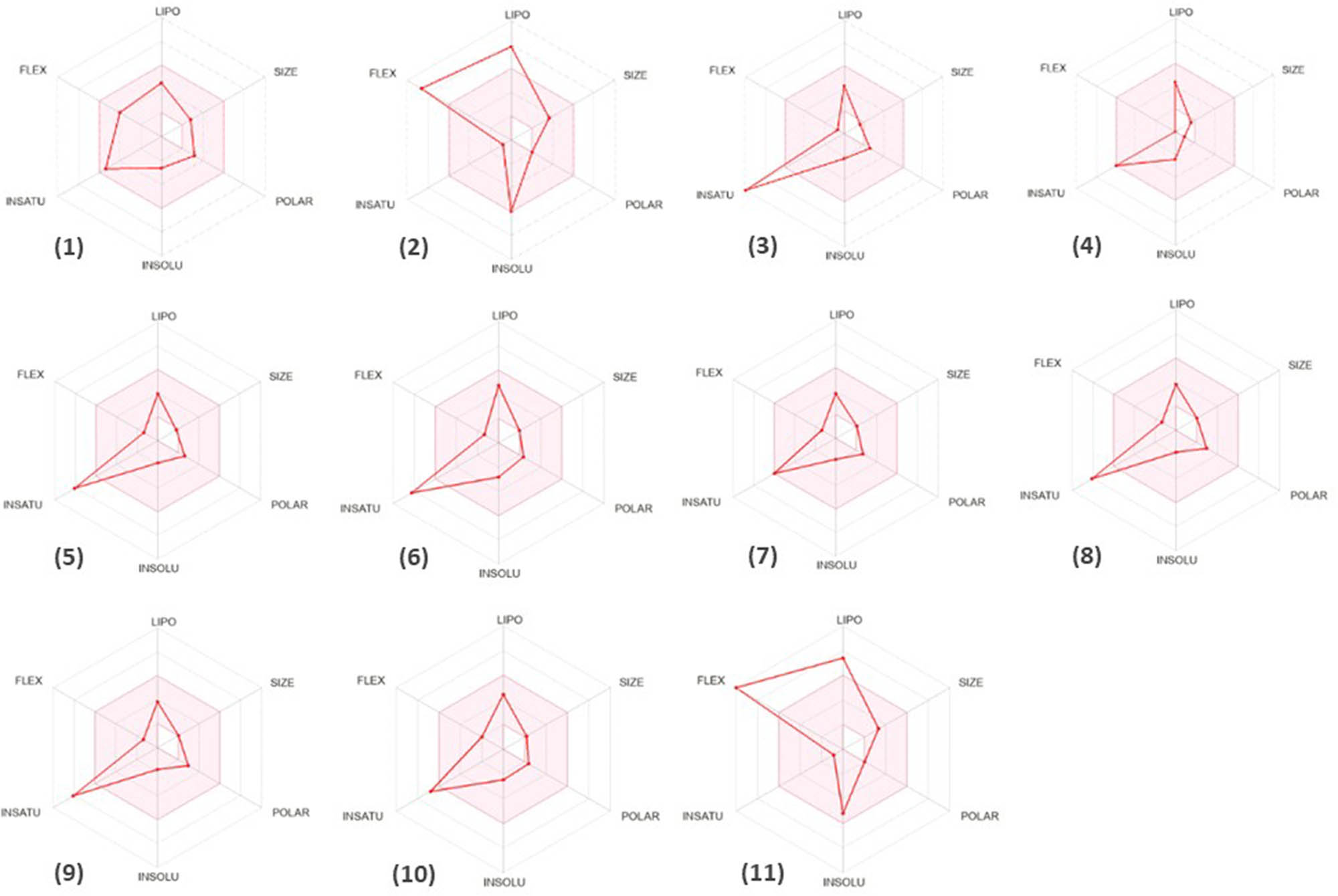
The bioavailability radar shows the phytochemicals found in the ethanol and water extracts of Mim. (1) Diethyl phthalate, (2) phytol, (3) benzoic acid, (4) 2,3-dihydro-benzofuran, (5) phenylacetic acid, (6) 2-methoxy-4-vinylphenol, (7) 2,6-dimethoxyphenol, (8) vanillin, (9) BHT, (10) eugenol, and (11) oleyl alcohol.
4 Discussion
Ethanol and water extracts yield showed, respectively, 9.0% (w/w) and 9.8% (w/w), this result can be explained by that the degree of polarity of the two solvents is almost the same because both are polar protic, and also their dipoles moment is nearly as follows: ethanol: dipole moment 1.69 and water: dipole moment 1.85. The amount obtained 9% (ethanol) and 9.8% (water) has to be the average and the median when compared with previous work, while Hashim et al. [136] found a maximum percentage of yield from Soxhlet extraction using ethanol with the value of 21.5%, suggesting that ethanol enhanced the extract production. They concluded that the maximum yield of ethanol was achieved at 90°C using both the Soxhlet extraction and immersion methods. Additionally, they noted that factors such as the particle size of neem leaf powder and the extraction temperature should be considered to improve results. Similarly, the authors [136] observed that using water as a solvent resulted in a lower extract yield of 9.8% (w/w). However, water can still be considered a viable alternative if an environmentally friendly solution is preferred. In parallel, Liauw et al. [137] found that the amount of yield of ethanol crude oil extract from Soxhlet was higher (41.11%) compared with ethanol extract obtained in this study (9%, w/w). Our results for the yield of water extract are similar to those reported by Rahmani et al. [7], who found that the extraction yields using different methods were as follows: decoction at 11% ± 0.1 w/w, percolation at 12% ± 0.3, and maceration at 16% ± 0.91. They noted that the statistical analysis indicated significant differences in extraction efficiency among the methods, with the maceration method demonstrating the highest efficiency. They suggested that the cause can be attributed to the effect of methanol and the extraction time on the extraction of active substances from the leaves [7]. Sithisarn et al. [138] have found that the freeze-drying method yielded the highest crude extract at 51.50% (w/w). They also obtained relatively close to our results of yield when the maceration, percolation, and Soxhlet extraction with various concentrations of ethanol (20–95%) gave a high yield of crude extracts (10–25%, w/w), but the yield of the spray-dried crude extract was low (3.04%, w/w) [139]. The yield of decoction extract (8.15%, w/w) was moderately lower than the ones obtained in this study for ethanol and water extracts (9 and 9.8%, w/w, respectively). This difference can be attributed to the greater efficiency of Soxhlet extraction compared to traditional extraction methods. In Soxhlet extraction, the same solvent is recycled with each new extraction, which helps to reduce solvent consumption. The authors [140] found that several factors affect how efficiently we can extract compounds. For example, the extraction efficiency of phenolic compounds from plants depends on their chemical properties, the extraction method, the length of time, storage conditions, and any interfering substances present [140]. The solvent used for extraction is also very important [141]. Its properties, such as boiling point, dielectric constant, and polarity, affect both the amount of oil extracted and the level of bioactive compounds [142]. While higher temperatures might increase the yield, they can also harm the stability of antioxidant components and cause membrane damage when temperatures go above 40°C [143].
The TPC was the highest with the water extracts (264.7 ± 0.03 µg GAE/mg of extracts) (= 0.2647 ± 0.03 mg/mg) while the ethanol extracts showed a value of 135.3 ± 0.05 µg GAE/mg of extracts (= 0.1353 mg/mg) (Table 1). The TFC was the highest (138.33 ± 0.002 µg CE/mg of extracts) (= 0.13833 mg/mg) for ethanol extracts, while the water extracts showed the lower value (83.38 ± 0.002 µg CE/mg of extracts) (= 0.8338 mg/mg) (Table 1). This difference observed between the concentrations of total polyphenols and flavonoids can be attributed to the selectivity of the solvent used for extraction. Ethanol, a polar solvent, is effective at extracting a wide range of polyphenols, including flavonoids, as well as other compounds like phenolic acids and tannins. It is particularly efficient in extracting flavonoids, which tend to be more soluble in organic solvents like ethanol. In contrast, water is a very polar solvent that is better suited for extracting highly polar compounds, such as hydrolyzable tannins, phenolic acids, certain glycosylated flavonoids, and water-soluble components such as anthocyanins, lipids and fatty acids, polysaccharides, saponins, vitamins, and minerals [144]. As a result, in our ethanolic extract, flavonoids may have been extracted more effectively than other types of polyphenols, leading to a slightly higher concentration of flavonoids compared to total polyphenols. While this difference is minor and typical, if the discrepancy is significant, it may be worthwhile to review the extraction conditions and methods used. It is also important to note that in certain foods or environments, flavonoids can make up the majority of polyphenols. For example, in red fruits, anthocyanins (a type of flavonoid) are dominant; in tea, catechins (another type of flavonoid) are the primary polyphenols; and in coffee, however, phenolic acids are more prevalent than flavonoids [145,146]. Thus, in some cases, flavonoids are indeed the most abundant polyphenols.
Comparing these results with other works on the same species, the authors [51] discovered that among the six crude extracts, the water extract contained the lowest amount of phenolic compounds, measuring 0.42 mg/100 g of dry powder sample. The methanol extract followed with 1.27 mg/100 g of dry powder. In contrast, the ethyl acetate extract had the highest concentration of phenolic compounds, at 3.58 mg/100 g of dry powder sample. Furthermore, Khamis Al-Jadidi and Hossain [147] obtained higher values when they found that the total phenolic of different leaves’ crude extracts was obtained through the maceration method using solvents of varying polarities, resulting in yields ranging from 20.80 to 107.29 mg of extract per 100 g of powdered plant material. The TFC in these crude extracts varied from 61.50 to 529.50 mg/100 g of the powdered samples [147].
During the HPLC–MS analysis, the ELE of the Mim plant showed the presence of a major compound, which is the diethyl phthalate (1,2-benzenedicarboxylic acid, diethyl ester or anozol) (92.31%). Diethyl phthalate is an anhydride organic compound reported with significant antimicrobial and antioxidant properties [77]. The ethanolic extract of the leaves revealed a limited number of compounds with antioxidant properties, with a significant concentration of diethyl phthalate, which accounted for 92% of the extract. This finding is based on the HPLC–MS analysis performed. Additionally, the ethanol extract demonstrated a high antioxidant activity in the ABTS assay, with an IC50 value of 2.01 ± 0.005 µg/mL, compared to the standard IC50 of 4.61 ± 0.005 µg/mL. Moreover, it is important to note that flavonoid and phenolic compounds were present in the ethanol extracts, which are believed to contribute to the antioxidant activity observed in the Mim leaf. Phenolic compounds are known for their strong antioxidant, antibacterial, and anti-inflammatory properties, which can help reduce the risk of chronic diseases [146]. On the other hand, the HPLC–MS analysis of WLE revealed the presence of various promising phenolic compounds, including BHT (28.12%) detected as a natural antioxidant detected in this studied species, benzoic acid (5.61%), 2-methoxy-4-vinylphenol (3.45%), eugenol (2.12%), syringol (2,6-dimethoxyphenol, a derivative of syringic acid) (1.58%), vanillin (1.17%), 1,2-benzenediol (0.62%), and phenylacetic acid (0.46%). Other compounds, such as 2,3-dihydro-benzofuran, hydromorphone, 1-hexadecanol, cis-9-octadecen-1-ol, and stearyl alcohol, were also detected and have diverse biological activities [148,149]. 2,3-Dihydro-benzofuran is reported as an anticancer agent [104]; hydromorphone has antiallergic and anti-inflammatory properties [150]; and 1-hexadecanol is reported to have pesticide properties [124]. cis-9-Octadecen-1-ol is a fatty acid reported to be an absorption enhancer in pulmonary protein delivery [127]. Stearyl alcohol serves as a non-ionic surfactant, emulsifier, emollient, and thickener in skin creams, lotions, and various other cosmetic products [89].
The phenolic compound, namely BHT, is detected in this study as a natural antioxidant from the Chadian Mim plant. BHT is a commonly synthesized antioxidant used in food, which is hardly observed in natural sources [151]. The detection of BHT in this study showed that it is also a natural antioxidant. It is also detected in the Litchi (Litchi chinensis Sonn.) fruit by Jiang et al. [152] through ESI in mass spectroscopy analysis and NMR spectra detection.
The water Mim leaf extracts in our study contained 5.61% benzoic acid (phenolic acid), which has been reported to possess strong antioxidant properties [96]. This compound was also found in a study conducted by Cesa et al. [153], where it was identified as the most abundant compound in pure Mim oil, with a concentration of 12.6 ± 1.35 µg/mL by HPLC-photo diode array analysis. T-cinnamic acid, with a quantity of 1.9 ± 0.2 µg/mL, was also reported as abundant. Additionally, benzoic acid was detected in the Mim leaf extract in a study by Boukeloua et al. [6] on the chemical and biological profiles of Algerian A. indica leaf extract, with a quantity of 56.82 ± 0.04 µg/g extract. Vanillin (1.17%) detected in our study in the WLE was also identified by Boukeloua et al. [6] in the Mim leaf from Algeria by LC–MS/MS analysis, with a quantity of 123.89 ± 0.03 µg/g extract. Vanillin is a phenolic aldehyde compound with high antioxidant activity [154], antimicrobial [155], and antigenotoxicity agent [156].
The third major phenolic compound detected (3.45%) in the current study is the 2-methoxy-4-vinylphenol [108], which has been reported to exhibit strong antioxidant activity and serves as an antimicrobial preservative in food and beverages [108]. Other important minor phenolic compounds, such as eugenol, phenylacetic acid, 1,2-benzenediol, and syringol (2,6-dimethoxyphenol, the derivate of syringic acid, a promising phenolic compound with high antioxidant activity), have also been reported to possess strong antioxidant activity [110,121]. In a study conducted by Rahmani et al. [7], many phenolic compounds were detected in the WLE of Mim obtained by percolation and then analyzed by HPLC. These phenolic compounds are flavonoids (0.34%), quercetin (5.05%), phenolic acid (10.75%), syringic acid (acid 4-hydroxy-3,5-diméthoxybenzoïc), nimbolide (49.20%), 2′,3′-dehydrosalannol (9.01%), and other unknown flavonoids (0.27%). Alternatively, Hossain et al. [157] evaluated the antioxidant properties of Mim crude leaf extracts (chloroform, butanol, ethyl acetate, hexane, and methanol) and found by GC–MS analysis revealed that most identified compounds include normal hydrocarbons, phenolic compounds, terpenoids, alkaloids, and glycosides. The antioxidant activity primarily relies on phenolic compounds, alkaloids, terpenoids, and their derivatives [157]. In the ethanol and water Mim extracts, compounds discovered through HPLC–MS analysis were found to be similar to those identified by several authors [157,158,159] through GC–MS analysis. In the ethyl acetate crude extract, Hossain et al. [157] found phenolic compounds such as phytol (detected also in this study in both extracts) and 1-tridecene. Phytol is a type of alcohol made from chlorophyll. It is often used as a food additive and in medicine [87,160] and acts as a precursor for synthetic vitamin E and vitamin K [161,162]. Suttiarporn and Choommongko [163] have used a microwave-assisted extraction method to extract a promising anticancer compound called nimbolide from 5g of dried Mim leaves using ethanol as a solvent. The extracted nimbolide was purified using silica gel thin-layer chromatography, resulting in 0.0336 g of nimbolide with a yield of 0.67% and a purity of over 98% using ethyl acetate/hexane in a three-hour process. The authors [138] performed thin-layer chromatography (TLC) on the Mim leaf extract made by decoction. The results showed spots that matched compounds like quercetin and rutin flavonoids, which have antioxidant properties. Quercetin had an effective concentration (EC50) value of 2.29 μg/mL, while rutin had an EC50 value of 34.67 μg/mL. In a 2011 study by Mahmoud et al. [164], researchers analyzed an ethyl acetate extract using HPLC. They found a main component called nimonol. They purified nimonol and confirmed its structure with NMR analysis. This main component exhibited lower antifungal activity compared to the mixture of fractions eluted by HPLC. In the study by Vergallo et al. [165], an ethanolic extract of Mim leaf was obtained using percolation with 70% ethanol. This extract underwent multiple liquid–liquid extractions using three organic solvents (in order of safety: n-butanol, ethyl acetate, and dichloromethane) with increasing solvent polarity. Flavonoid compounds in the Mim leaf extract were identified using TLC after solubilizing the extract in distilled water. HPLC analysis revealed the presence of stragalin, quercitrin, iso quercitrin, nicotiflorin, and rutin. These flavonoids demonstrated antioxidant and antihyperglycemic/hypoglycemic properties [165]. In a study conducted by Ghimeray et al. [166], samples of Mim’s leaf, seed, and bark were extracted using 80% methanol. These samples were then divided into fractions using hexane, ethyl acetate, and n-butanol. The resulting dried samples underwent analysis using HPLC to measure their azadirachtin and nimbin contents, which were then compared with standard chromatograms. The study revealed that the methanolic seed extract contained the highest azadirachtin content, followed by the hexane extract. The bark’s hexane fraction also contained azadirachtin, while the leaf had a small amount in the water fraction. Moreover, the highest nimbin content was found in the bark’s hexane fraction, followed by the methanolic extracts and the butanol fraction. The leaf’s hexane fraction also exhibited significant nimbin content. As a result, the study concluded that the leaf and bark, which had higher phenolic and flavonoid contents, displayed effective antioxidant activity [166].
Our findings indicate that Mim leaf extracts can regulate hyperactivity and reduce free radical activity caused by chemicals produced in the body. The antioxidant activity of the water and ethanol extracts of Mim leaves, as measured by the DPPH assay, was significantly higher compared to previous studies. In those earlier works, the authors [51] reported that the butanol extract exhibited the highest antioxidant activity for the DPPH assay, while the hexane extract showed the lowest activity, followed by the aqueous crude extracts. The authors [157] evaluated the antioxidant activity of different crude extracts by the DPPH method and found that all the crude extracts used were able to decolorize with DPPH and have significant antioxidant activity; among them, the chloroform crude extracts showed the highest antioxidant activity with a value of 88 µg/mL. The results suggest that extracts from the leaf of the Siamese tree (Mim or neem) have strong antioxidant potential. They concluded that their study supports the traditional use of the young leaves and flowers of the Mim as a bitter tonic for promoting good health [157]. Similarly, Sithisarn et al. [167] studied the antioxidant activity of the Siamese tree and obtained that leaf extract effectively scavenged free radicals in the DPPH test, achieving 50% activity at 26.5 µg/mL. They found that the total antioxidant activity of this extract was 0.959 mM of standard trolox. At 100 µg/mL, the leaf aqueous extracts significantly decreased malondialdehyde levels by 46% by the thiobarbituric acid reactive substances (TBARS) method [167]. The authors [138] also evaluated the antioxidant activity of Mim leaf extracts using methods like percolation, decoction, maceration, Soxhlet extraction, spray drying, and freeze drying. They concluded that decoction and spray-dried extraction methods gave the most active extract for the DPPH scavenging activity, respectively, with values IC50: 31.41 mg/mL and IC50: 36.28 mg/mL. Maceration, percolation, and Soxhlet extraction with various concentrations of ethanol (20–95%) showed lower antioxidant activity (IC50: 122–265 mg/mL). However, the freeze-dried extract gave medium antioxidant activity (IC50: 74.19 mg/mL) [138]. The authors [7] have also evaluated the RSA of Mim using three different extracts through the DPPH method. Among the extracts, using an 80% methanol solution for 24 h with the percolation method produced the highest RSA. This extract is the most effective. In an in vitro study by Darbar et al. [25], the aqueous Mim leaf extracts obtained by percolation have a positive impact on the liver damage caused by aceclofenac (ACE) (a nonsteroidal anti-inflammatory drug) in rats. In the experiment, Mim leaf extract at 250 and 500 mg/kg significantly reduced liver damage caused by ACE. Blood levels of liver enzymes aspertate aminotransferase, alanine aminotransferase, alkaline phosphatase, and ã-glutamyl transpeptidase decreased (p < 0.05), and liver TBARS levels also dropped (p < 0.01). Additionally, Mim extract restored key liver enzymes, such as SOD (p < 0.05), CAT (p < 0.05), and glutathione peroxidase (GPx) (p < 0.01), and increased GSH content (p < 0.05). These results indicate that Mim leaves can protect the liver and provide antioxidant support against ACE-induced damage [25].
Concerning the molecular docking results, BHT and vanillin show promise as therapeutic agents due to their interactions with key enzymes involved in oxidative stress and inflammation. BHT acts as an inhibitor of GR and LOX. It interacts effectively with GR to maintain cellular redox balance. It has a slightly higher affinity for LOX compared to standard inhibitors, suggesting its potential to reduce oxidative stress and related diseases [168,169,170]. Conversely, vanillin exhibits favorable binding interactions with COX, which plays a crucial role in inflammation by mediating the biosynthesis of prostaglandins. Its stable binding profile, including hydrogen bonds and alkyl interactions, positions vanillin as a potential COX inhibitor. This suggests that vanillin could offer both antioxidant and anti-inflammatory benefits. Given the presence of both BHT and vanillin in extracts of Mim, these extracts could be valuable for their combined antioxidant and anti-inflammatory properties [171]. These molecular docking results help explain the observed antioxidant activity of the extracts in in vitro studies. Overall, the combination of in-silico predictions and in vitro findings underscores the value of the extracts of Mim as effective sources of antioxidant agents.
The profile of ethanol extracts showed promising molecules such as the diethyl phthalate, which exhibits important antibacterial and antioxidant properties [77]. The results are further supported by the in-silico antioxidant activity assessed for all analyzed compounds, including diethyl phthalate. These compounds exhibited a close affinity for GR, the enzyme responsible for converting oxidized glutathione into its reduced form. The reduced glutathione (GSH) functions as an antioxidant, reacting with free radicals and organic peroxides. Additionally, GSH plays a crucial role in detoxification through glutathione S-transferase-catalyzed reactions, and in the antioxidant system via GPx-catalyzed reactions [169,172].
For compounds intended for oral drug development, it is essential to adhere to Lipinski’s and Veber’s rules. Generally, compounds should not violate more than one of these rules: (1) They can have a maximum of 10 hydrogen bond acceptors (nitrogen or oxygen atoms). (2) Their octanol–water partition coefficient (logP or MlogP) must be less than 5. (3) Their molecular mass should be less than 500 Da. (4) They can have no more than five hydrogen bond donors [173]. The phytochemical compounds found in the ethanolic and water extracts from Mim meet the criteria set by Lipinski and Veber. This shows their potential as candidates for developing oral drugs. Among these compounds, diethyl phthalate, benzoic acid, 2,3-dihydro-benzofuran, phenylacetic acid, 2-methoxy-4-vinylphenol, 2,6-dimethoxyphenol, vanillin, BHT, and eugenol can cross the BBB. This penetrative capacity is attributed to their low polarity and moderate lipophilicity, which facilitate effective distribution throughout the brain tissue [173,174]. Moreover, the analyzed compounds were not found to be substrates of PGP, suggesting that they might avoid early elimination by PGP, thereby maintaining more stable and prolonged therapeutic levels in the body [175]. A molecule that is easily absorbed in the intestine has clear advantages. It improves bioavailability, effectiveness, ease of use, and tolerance. This makes it a strong choice for developing oral medications [176]. Most of the examined molecules showed high intestinal absorption and did not inhibit or serve as substrates for CYP1A2 enzymes. This indicates a reduced likelihood of affecting drug metabolism, thereby improving the safety profile of these phytochemical compounds [177]. The bioavailability radars of diethyl phthalate, 2,3-dihydrobenzofuran, and 2,6-dimethoxyphenol fall within the pink zone, which is essential for evaluating a molecule’s potential as a therapeutic agent, as it indicates the likelihood of effective absorption and distribution throughout the body [175]. Hence, extracts of Mim, which include these promising compounds, are recommended for further exploration as oral medications due to their advantageous pharmacokinetic profiles and therapeutic potential.
5 Conclusion
The leaf extracts of Mim plants, both ethanol and water extracts, exhibited high levels of phenolic and flavonoid compounds in this study. The phenolic content was measured at 264.7 ± 0.03 µg GAE/mg for the WLE and 135.3 ± 0.05 µg GAE/mg for the ELE. In terms of flavonoid content, the ELE showed 138.33 ± 0.002 µg CE/mg, while the WLE had 83.38 ± 0.002 µg CE/mg. Both extracts demonstrated significant antioxidant activity by effectively scavenging free radicals. In the DPPH assay, the IC50 values were observed to be 5.00 ± 0.06 µg/mL for WLE and 5.84 ± 0.05 µg/mL for ELE. In the ABTS assay, the IC50 values were 2.01 ± 0.005 µg/mL for WLE and 5.51 ± 0.15 µg/mL for ELE. For the FRAP assay, the IC50 values were 6.38 ± 0.30 µg/mL for WLE and 6.27 ± 0.69 µg/mL for ELE. The HPLC–MS analysis of WLE revealed the presence of important phenolic compounds with significant antioxidant properties and potential pharmacological values. The study identifies several important compounds, including BHT, which is recognized for the first time as a natural antioxidant in the freeze-dried extract of Mim leaves. Other compounds found in the extract include benzoic acid, 2-methoxy-4-vinylphenol, eugenol, syringol (2,6-dimethoxyphenol, a derivative of syringic acid), vanillin, 1,2-benzenediol, and phenylacetic acid. Furthermore, terpenoid compounds with strong antioxidant properties were also identified, such as phytol, germacrene B, and vomifoliol. The study also highlights other active molecules that have significant antioxidant properties and potential pharmacological value. The in silico analysis of the compounds from Mim leaves demonstrated diverse interactions with essential enzymes involved in antioxidant processes. These compounds also show favorable characteristics for the development of oral medications. The extracts from Mim leaves hold great potential for creating innovative natural therapies to combat oxidative stress. Traditionally, in the Republic of Chad, Mim leaf extract has been used to treat headaches and fevers, and it contains bioactive compounds that could be harnessed for various medical applications. This experimental work provides valuable scientific insights and data about the bioactive molecules in Mim leaf extracts, benefiting both the scientific community in the Republic of Chad and the global community.
Acknowledgments
The authors thank Professor El Hassan Sakar from the Laboratory of Biology, Ecology, and Health at the Faculty of Sciences of Tetouan, along with his collaborators, for their assistance with the HPLC–MS analysis. Additionally, we are grateful to Professor Ahmed Ibn Mansour from the Laboratory of Applied Organic Chemistry in the Department of Chemistry at the Faculty of Sciences of Tetouan for his technical support regarding the HPLC–MS analysis results. Furthermore, we sincerely appreciate Ms. Khadidja Brahim Mahamat from the Toumaï University herbarium in N’Djamena, as well as the team at the FENAPMT for their help in the collection and identification of the species studied. The authors extend their gratitude to King Saud University for supporting this work through the Ongoing Research Funding program (ORF-2025-1057).
-
Funding information: This research did not receive any external funding. This work was supported by the Ongoing Research Funding program (ORF-2025-1057), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: Conceptualization, O.B.M., Y.S., and B.B.O.; data curation, O.B.M. and A.F.; formal analysis, O.B.M. and A.F.; funding acquisition, R.H. and A.S.; investigation, O.B.M., M.B., Y.S., and B.B.O.; methodology, O.B.M., F.A., A.F., M.B., and Y.S.; resources, O.B.M. and Y.S.; software, O.B.M. and A.F.; supervision, Y.S. and B.B.O.; validation, Y.S., B.B.O., and M.B.; visualization, F.A., A.F., M.B., I.B., S.E., and B.B.O.; writing – original draft, O.B.M., R.H., A.S., and Y.S.; and writing – review and editing, M.B., Y.S., and B.B.O. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The authors declare no conflicts of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
[1] Ahmad Khan MS, Ahmad I. Herbal medicine: current trends and future prospects. New look to phytomedicine: Advancements in herbal products as novel drug leads. Amsterdam, Netherlands: Elsevier Inc; 2018. p. 3–13. 10.1016/B978-0-12-814619-4.00001-X.Search in Google Scholar
[2] Patel SM, Nagulapalli Venkata KC, Bhattacharyya P, Sethi G, Bishayee A. Potential of neem (Azadirachta indica A. Juss) for prevention and treatment of oncologic diseases. SemCancer Biol. 2016;40–41:100–15. 10.1016/j.semcancer.2016.03.002.Search in Google Scholar PubMed
[3] Csurhes S. Pest plant risk assessment Neem tree Azadirachta indica. Department of Agriculture and Fisheries Biosecurity Queensland. State of Queensland, Australia: 2016.Search in Google Scholar
[4] Gupta CS, Prasad S, Tyagi AK, Kunnumakkara AB, Aggarwal BB. Phytomedicine Neem (Azadirachta indica): An indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14–20. 10.1016/j.phymed.2017.07.001.Search in Google Scholar PubMed
[5] Sujarwo W, Keim AP, Caneva G, Toniolo C, Nicoletti M. Ethnobotanical uses of neem (Azadirachta indica A.Juss.; Meliaceae) leaves in Bali (Indonesia) and the Indian subcontinent in relation with historical background and phytochemical properties. J Ethnopharmacology. 2016;189:186–93. 10.1016/j.jep.2016.05.014.Search in Google Scholar PubMed
[6] Boukeloua A, Leila S, Bendif H, Elkadeem AM, Boga M, Serralheiro ML, et al. A comprehensive study on chemical and biological profiles of Algerian Azadirachta indica (A. Juss). Phytochem Lett. 2024;61:89–96. 10.1016/j.phytol.2024.03.017.Search in Google Scholar
[7] Rahmani F, Mohammad K, Elhami H, Khanzadeh F. Characterization of phenolic compounds present in Neem leaf extracts using HPLC and determination of their antioxidant activity. J Food Res. 2013;24(1):103–17, https://www.researchgate.net/publication/270795602_Characterization_of_phenolic_compounds_present_in_Neem_leaf_extracts_using_HPLC_and_determination_of_their_antioxidant_activity%0Ahttps://sid.ir/paper/148507/en.Search in Google Scholar
[8] Garg HS, Biukuni DS. 2’,3’-Dehydrosalannol, a tetranortriterpenoid from Azadirachta indica leaves. Phytochemistry. 1985;24(4):866–7.10.1016/S0031-9422(00)84913-4Search in Google Scholar
[9] Srivastava P, Yadav N, Lella R, Schneider A, Jones A, Marlowe T, et al. Neem oil limonoids induce p53-independent apoptosis and autophagy. Carcinogenesis. 2012;33(11):2199–207.10.1093/carcin/bgs269Search in Google Scholar PubMed PubMed Central
[10] Farjaminezhad R, Garoosi G. Establishment of green analytical method for ultrasound-assisted extraction of azadirachtin, mevalonic acid and squalene from cell suspension culture of Azadirachta indica using response surface methodology. Ind Crop Products. 2020;144:111946. 10.1016/j.indcrop.2019.111946.Search in Google Scholar
[11] Nagini S, Nivetha R, Palrasu M, Mishra R. Nimbolide, a neem limonoid, is a promising candidate for the anticancer drug arsenal. J Med Chem. 2021;64(7):3560–77.10.1021/acs.jmedchem.0c02239Search in Google Scholar PubMed
[12] Sarkar S, Singh RP, Bhattacharya G. Exploring the role of Azadirachta indica (neem) and its active compounds in the regulation of biological pathways: an update on molecular approach. 3 Biotech. 2021;11(4):1–12. 10.1007/s13205-021-02745-4.Search in Google Scholar PubMed PubMed Central
[13] Arulkumar R, Karthikan S, Gopalasatheeskumar K, Arulkumaran G. Neem (Azadirachta indica): A miraculous medicinal plant from India. Int J Univers Pharm Bio Sci. 2019;8(4):48–59, https://www.researchgate.net/publication/335571014_neem_azadirachta_indica_a_miraculous_medicinal_plant_from_india.Search in Google Scholar
[14] Saleem S, Muhammad G, Hussain MA, Bukhari SNA. A comprehensive review of phytochemical profile, bioactives for pharmaceuticals, and pharmacological attributes of Azadirachta indica. Phytother Res. 2018;32(7):1241–72.10.1002/ptr.6076Search in Google Scholar PubMed
[15] Maragathavalli S, Brindha S, Kaviyarasi NS B, Annadurai B, Gangwar SK. Antimicrobial activity in leaf extract of neem (Azadirachta indica Linn.). Int J Sci Nat. 2012;3(1):110–3, https://www.researchgate.net/publication/284377658_Antimicrobial_activity_in_leaf_extract_of_neem.Search in Google Scholar
[16] Abbas G, Ali M, Hamaed A, Al-Sibani M, Hussain H, Al-Harrasi A. Azadirachta indica: The medicinal properties of the global problems-solving tree. Biodiversity and biomedicine: Our future. Amsterdam, Netherlands: Elsevier; 2020. p. 305–16. 10.1016/B978-0-12-819541-3.00017-7.Search in Google Scholar
[17] Rahmani AH, Almatroudi A, Alrumaihi F, Khan AA. Pharmacological and therapeutic potential of neem (Azadirachta indica). Pharmacogn Rev. 2018;12(24):250–5, www.phcogrev.com www.phcog.net.10.4103/phrev.phrev_8_18Search in Google Scholar
[18] Quelemes PV, Perfeito MLG, Guimarães MA, Dos Santos RC, Lima DF, Nascimento C, et al. Effect of neem (Azadirachta indica A. Juss) leaf extract on resistant Staphylococcus aureus biofilm formation and Schistosoma mansoni worms. J Ethnopharmacol. 2015;175:287–94. 10.1016/j.jep.2015.09.026.Search in Google Scholar PubMed
[19] Wylie MR, Windham IH, Blum FC, Wu H, Merrell DS. In vitro antibacterial activity of nimbolide against Helicobacter pylori. J Ethnopharmacol. 2022;285:114828. 10.1016/j.jep.2021.114828.Search in Google Scholar PubMed PubMed Central
[20] Kalaskar AR, Bhowate RR, Kalaskar RR, Ghonmode S. Novel neem leaves extract mouthwash therapy for oral lichen planus. J Herb Med. 2021;26:100408. 10.1016/j.hermed.2020.100408.Search in Google Scholar
[21] Kumar S, Agrawal D, Patnaik J. Analgesic effect of neem (azadirachta indica) seed oil on albino rats. Int J Pharma Bio Sci. 2012;3(2):222–5, https://www.semanticscholar.org/paper/ANALGESIC-EFFECT-OF-NEEM-%28AZADIRACHTA-INDICA%29-SEED-Kumar-Agrawal/e9f4baa1ee452cbc4e567a8605da067063e581a7.Search in Google Scholar
[22] Naik M, Agrawal D, Behera R, Bhattacharya A, Dehury S, Kumar S. Study of anti-inflammatory effect of neem seed oil (Azadirachta indica) on infected albino rats. J Health Res Rev. 2014;1(3):66.10.4103/2394-2010.153880Search in Google Scholar
[23] Ferdoush J, Misbahuddin M. Effect of ethanol extract of leaves of Azadirachta indica on palmar arsenical keratosis: A single-blind trial. Bangladesh J Pharmacol. 2014;9(3):279–83.10.3329/bjp.v9i3.19488Search in Google Scholar
[24] Kumar S, Kumar Vandana U, Agrawal D, Hansa J. Analgesic, anti-inflammatory and anti-pyretic effects of Azadirachta indica (Neem) leaf extract in albino rats. Int J Sci Res. 2013;4:2319–7064, www.ijsr.net.Search in Google Scholar
[25] Darbar S, Bose A, Bhaumik UK, Roy B, Chatterjee N, Pal TK. Antioxidant and hepatoprotective effect of Azadirachta indica leaf extract on aceclofenac induced hepatotoxicity in rats. J Pharm Res. 2009;8(2):116–21, https://www.semanticscholar.org/paper/Antioxidant-and-Hepatoprotective-Effect-of-indica-Soumendra-Anirbandeep/8cd90f9611b0aacd12e6a6243292096109c7b0a1.Search in Google Scholar
[26] Ikechukwu EN. Evaluation of antilipid peroxidation and hypolipidemic potentials of Azadirachta indica leaf aqueous extract in paracetamol-induced hepatotoxicity in Wistar rats. Int J Inf Res Rev. 2017;4(2):3615–9.Search in Google Scholar
[27] Seriana I, Akmal M, Darusman D, Wahyuni S, Khairan K, Sugito S. Neem leaf (Azadirachta indica A. Juss) ethanolic extract on the liver and kidney function of rats. Sci World J. 2021;2021:7970424.10.1155/2021/7970424Search in Google Scholar PubMed PubMed Central
[28] Bottenberg H, Singh BB. Effect of neem leaf extract applied using the ‘broom’ method, on cowpea pests and yield. Int J Pest Manag. 1996;42(3):207–9.10.1080/09670879609371995Search in Google Scholar
[29] Yarmohammadi F, Mehri S, Najafi N, Amoli SS, Hosseinzadeh H. The protective effect of Azadirachta indica (neem) against metabolic syndrome: A review. Iran J Basic Med Sci. 2021;24(4):280–92.Search in Google Scholar
[30] Chattopadhyay RR. Possible mechanism of antihyperglycemic effect of Azadirachta indica leaf extract: Part V. J Ethnopharmacology. 1999;67(3):373–6.10.1016/S0378-8741(99)00094-XSearch in Google Scholar PubMed
[31] Bhattacharyya N, Chutia M, Sarma S. Neem (Azadirachta indica A. Juss), A potent Biotesticide and Medicinal Plant: A review. J Plant Sci. 2007;2(3):251–9, https://scialert.net/fulltext/?doi=jps.2007.251.259.10.3923/jps.2007.251.259Search in Google Scholar
[32] Li L, Song X, Yin Z, Jia R, Zou Y. Insecticidal activities and mechanism of extracts from neem leaves against Oxya chinensis. Arquivo Brasileiro Med Vet Zootecnia. 2019;71(1):1–10.10.1590/1678-4162-8958Search in Google Scholar
[33] Sandhir R, Khurana M, Singhal NK. Potential benefits of phytochemicals from Azadirachta indica against neurological disorders. Neurochem Int. 2021;146:105023. 10.1016/j.neuint.2021.105023.Search in Google Scholar PubMed
[34] Peer PA, Trivedi PC, Nigade PB, Ghaisas MM, Deshpande AD. Cardioprotective effect of Azadirachta indica A. Juss. on isoprenaline induced myocardial infarction in rats. Int J Cardiol. 2008;126(1):123–6.10.1016/j.ijcard.2007.01.108Search in Google Scholar PubMed
[35] Santos KS, Barbosa AM, Freitas V, Muniz AVCS, Mendonça MC, Calhelha RC, et al. Antiproliferative activity of neem leaf extracts obtained by a sequential pressurized liquid extraction. Pharmaceuticals. 2018;11(76):2–10.10.3390/ph11030076Search in Google Scholar PubMed PubMed Central
[36] Sanni O, Erukainure OL, Chukwuma CI, Koorbanally NA, Ibeji CU, Islam MS. Azadirachta indica inhibits key enzyme linked to type 2 diabetes in vitro, abates oxidative hepatic injury and enhances muscle glucose uptake ex vivo. Biomed Pharmacother. 2019;109:734–43. 10.1016/j.biopha.2018.10.171.Search in Google Scholar PubMed
[37] Sies H. Oxidative stress: Concept and some practical aspects. Antioxidants. 2020;9(9):1–6.10.3390/antiox9090852Search in Google Scholar PubMed PubMed Central
[38] Shrivastava A, Chaturvedi U, Sonkar R, Khanna AK, Saxena JK, Bhatia G. Antioxidant effect of Azadirachta indica on high fat diet induced diabetic charles foster rats. Appl Biochem Biotechnol. 2012;167(2):229–36.10.1007/s12010-012-9681-0Search in Google Scholar PubMed
[39] Vaibhav K, Shrivastava P, Khan A, Javed H, Tabassum R, Ahmed ME, et al. Azadirachta indica mitigates behavioral impairments, oxidative damage, histological alterations and apoptosis in focal cerebral ischemia-reperfusion model of rats. Neurol Sci. 2013;34(8):1321–30.10.1007/s10072-012-1238-zSearch in Google Scholar PubMed
[40] Gambini J, Stromsnes K. Oxidative stress and inflammation: From mechanisms to therapeutic approaches. Biomedicines. 2022;10(4):753. 10.3390/biomedicines10040753.Search in Google Scholar PubMed PubMed Central
[41] Reddy VP. Oxidative stress in health and disease. Biomedicines. 2023;11(11):2925. 10.3390/biomedicines11112925.Search in Google Scholar PubMed PubMed Central
[42] Nollet LML, Gutierrez-Uribe JA. Phenolic compounds in food characterization and analysis. Taylor, Leo F, Nollet ML, Gutierrez-Uribe JA, editors. London, New York: Boca Raton; 2017. p. 450. 10.1201/9781315120157.Search in Google Scholar
[43] Li Q, Tu Y, Zhu C, Luo W, Huang W, Liu W, et al. Cholinesterase, β-amyloid aggregation inhibitory and antioxidant capacities of Chinese medicinal plants. Ind Crop Products. 2017;108:512–9. 10.1016/j.indcrop.2017.07.001.Search in Google Scholar
[44] Dai J, Mumper RJ. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313–52.10.3390/molecules15107313Search in Google Scholar PubMed PubMed Central
[45] Yu M, Gouvinhas I, Rocha J, Barros AI. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci Rep. 2021;11(1):1–14. 10.1038/s41598-021-89437-4.Search in Google Scholar PubMed PubMed Central
[46] Pichersky E, Raguso RA. Why do plants produce so many terpenoid compounds? N Phytologist. 2018;220(3):692–702.10.1111/nph.14178Search in Google Scholar PubMed
[47] Rao VR. Antioxidant agents. Advances in structure and activity relationship of coumarin derivatives. Warangal, India: Elsevier Inc; 2016. p. 137–50. 10.1016/B978-0-12-803797-3.00007-2.Search in Google Scholar
[48] Siddeeg A, Alkehayez NM, Abu-hiamed HA, Al-sanea EA, Al-farga AM. Mode of action and determination of antioxidant activity in the dietary sources: An overview. Saudi J Biol Sci. 2021;28(3):1633–44. 10.1016/j.sjbs.2020.11.064.Search in Google Scholar PubMed PubMed Central
[49] Munteanu IG, Apetrei C. Analytical methods used in determining antioxidant activity: a review. Int J Mol Sci. 2021;22(3380):1–30. 10.3390/ijms22073380.Search in Google Scholar PubMed PubMed Central
[50] Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc. 2007;2(4):875–7.10.1038/nprot.2007.102Search in Google Scholar PubMed
[51] Saleh Al-Hashemi ZS, Hossain MA. Biological activities of different neem leaf crude extracts used locally in Ayurvedic medicine. Pac Sci Rev A: Nat Sci Eng. 2016;18(2):128–31. 10.1016/j.psra.2016.09.013.Search in Google Scholar
[52] Shahidi F, Zhong Y. Measurement of antioxidant activity. J Funct Foods. 2015;18:757–81. 10.1016/j.jff.2015.01.047.Search in Google Scholar
[53] Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53(10):4290–302.10.1021/jf0502698Search in Google Scholar PubMed
[54] Chaves N, Santiago A, Carlos J. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants. 2020;9(76):1–15, www.mdpi.com/journal/antioxidants.10.3390/antiox9010076Search in Google Scholar PubMed PubMed Central
[55] Heilbron K, Mozaffari SV, Vacic V, Yue P, Wang W, Shi J, et al. Advancing drug discovery using the power of the human genome. J Pathol. 2021;254(4):418–29.10.1002/path.5664Search in Google Scholar PubMed PubMed Central
[56] Meng X-Y, Zhang H-X, Mezei M, Cui M. Over view on molecular docking: a powerful approach for structure based drug discovery. Curr Comput Drug Des. 2011;7(2):146–57.10.2174/157340911795677602Search in Google Scholar PubMed PubMed Central
[57] Jorgensen WL. The many roles of computation in drug discovery. Science. 2004;303(5665):1813–8.10.1126/science.1096361Search in Google Scholar PubMed
[58] Ferreira LG, Dos Santos RN, Oliva G, Andricopulo AD. Molecular docking and structure-based drug design strategies. Molecules. 2015;20:13384–421.10.3390/molecules200713384Search in Google Scholar PubMed PubMed Central
[59] Lozano-Sánchez J, Borrás-Linares I, Sass-Kiss A, Segura-Carretero A. Chromatographic technique: high-performance liquid chromatography (HPLC). Modern techniques for food authentication. Amsterdam, Netherlands: Academic press, Elsevier Inc.; 2018. p. 459–526.10.1016/B978-0-12-814264-6.00013-XSearch in Google Scholar
[60] Li G, Fang Y, Ma Y, Dawa Y, Wang Q, Gan J, et al. Screening and isolation of potential anti-inflammatory compounds from Saxifraga atrata via affinity ultrafiltration-HPLC and multi-target molecular docking analyses. Nutrients. 2022;14(12):2405. 10.3390/nu14122405.Search in Google Scholar PubMed PubMed Central
[61] Dewanto V, Wu X, Adom KK, Liu ARH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. Agric Food Chem. 2002;50(10):3010–4.10.1021/jf0115589Search in Google Scholar PubMed
[62] Singleton VL, Orthofer R, Lamuela-Raventos ARM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. J Agric Food Chem. 1999;50(10):152–78.10.1016/S0076-6879(99)99017-1Search in Google Scholar
[63] Matić P, Sabljić M, Jakobek L. Validation of spectrophotometric methods for the determination of total polyphenol and total flavonoid content. J AOAC Int. 2017;100(6):1795–803.10.5740/jaoacint.17-0066Search in Google Scholar PubMed
[64] Elouafy Y, El Yadini A, Mortada S, Hnini M, Harhar H, Khalid A, et al. Antioxidant, antimicrobial, and α-glucosidase inhibitory activities of saponin extracts from walnut (Juglans regia L.) leaves. Asian Pac J Trop Biomed. 2023;13(2):60–9.10.4103/2221-1691.369610Search in Google Scholar
[65] Parusnath M, Naidoo Y, Singh M, Rihan H, Dewir YH. Phytochemical composition of Combretum molle (R. Br. ex G. Don.) Engl. & Diels Leaf and Stem Extracts. Plants. 2023;12(1702):1–19.10.3390/plants12081702Search in Google Scholar PubMed PubMed Central
[66] Farihi A, Bouhrim M, Chigr F, Elbouzidi A, Bencheikh N, Zrouri H, et al. Exploring medicinal herbs’ therapeutic potential and molecular docking analysis for compounds as potential inhibitors of human acetylcholinesterase in Alzheimer’s disease treatment. Medicina (Lithuania). 2023;59(10):1812. 10.3390/medicina59101812.Search in Google Scholar PubMed PubMed Central
[67] Shaweta S, Akhil S, Utsav G. Molecular Docking studies on the Anti-fungal activity of Allium sativum (Garlic) against Mucormycosis (black fungus) by BIOVIA discovery studio visualizer 21.1.0.0. Ann Antivir Antiretrovirals. 2021;5(1):028–32.10.17352/aaa.000013Search in Google Scholar
[68] Srivastava V, Yadav A, Sarkar P. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier Connect, the company’ s public news and information. Mater Today: Proc. 2020;49(2022):2999–3007.Search in Google Scholar
[69] IARC International Agency for Research on Cancer. Occupational Exposures of Hairdressers and Barbers and Personal Use of Hair Colourants; Some Hair Dyes, Cosmetic Colourants, Industrial Dyestuffs and Aromatic Amines. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 57 [Internet]. Lyon, France: World Health Organization, Geneva; 1993. p. 437. https://www.ncbi.nlm.nih.gov/books/NBK493268/.Search in Google Scholar
[70] Choi D, Kang W, Park T. Anti-allergic and anti-inflammatory effects of undecane on mast cells and keratinocytes. Molecules. 2020;25(7):1554, https://www.mdpi.com/1420-3049/25/7/1554.10.3390/molecules25071554Search in Google Scholar PubMed PubMed Central
[71] Konus M, Çetin D, Kızılkan ND, Yılmaz C, Fidan C, Algso M, et al. Synthesis and biological activity of new indole based derivatives as potent anticancer, antioxidant and antimicrobial agents. J Mol Structure. 2022;1263:133168. 10.1016/j.molstruc.2022.133168.Search in Google Scholar
[72] Burdock GA. Encyclopedia of food and color additives. 1st edn. New York: Taylor & Francis Group, LLC; 1996. p. 101.Search in Google Scholar
[73] Syamimi N, Ain S, Aiman M, Maznan F, Syaffinaz N, Mohamad N, et al. Lauric acid improves hormonal profiles, antioxidant properties, sperm quality and histomorphometric changes in testis and epididymis of streptozotocin-induced diabetic infertility rats. Toxicol Appl Pharmacology. 2023;470:116558. 10.1016/j.taap.2023.116558.Search in Google Scholar PubMed
[74] Strickland J, Larson NR, Feldlaufer M, Zhang A. Characterizing repellencies of methyl benzoate and its analogs against the Common Bed Bug, Cimex lectularius. Insects. 2022;13(11):1060, https://www.mdpi.com/2075-4450/13/11/1060.10.3390/insects13111060Search in Google Scholar PubMed PubMed Central
[75] Rodrigues AI, Castilho PC, Costa MC, Gonza HG. Synergistic antimycobacterial activities of sesquiterpene lactones from Laurus spp. J Antimicrob Chemother. 2007;59:548–52.10.1093/jac/dkl523Search in Google Scholar PubMed
[76] Garzo SP, Rodrı AD, Sa JA. Sesquiterpenoid metabolites with antiplasmodial activity from a Caribbean Gorgonian Coral, Eunicea sp. J Nat Products. 2005;68(9):1354–9.10.1021/np0501684Search in Google Scholar PubMed
[77] Premjanu N, Jaynthy C. Antimicrobial activity of diethyl phthalate: An in-silico approach. Asian J Pharm Clin Res. 2014;7(4):4–5, https://innovareacademics.in/journals/index.php/ajpcr/article/download/1446/1147/.Search in Google Scholar
[78] Gupta AD, Bansal VK, Babu V, Maithil N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). J Genet Eng Biotechnol. 2013;11(1):25–31. 10.1016/j.jgeb.2012.12.001.Search in Google Scholar
[79] Marchese A, Barbieri R, Coppo E, Orhan IE, Nabavi SF, Izadi M, et al. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit Rev Microbiol. 2017;43(6):668–89. 10.1080/1040841X.2017.1295225.Search in Google Scholar PubMed
[80] Tan MA, Jesus S, Grecebio BG, Alejandro JD, An SSA. Neuroprotective effects of vomifoliol, isolated from Tarenna obtusifolia Merr. (Rubiaceae), against amyloid‑beta1‑42‑treated neuroblastoma SH‑SY5Y cells. 3 Biotech. 2020;10(10):1–6. 10.1007/s13205-020-02421-z.Search in Google Scholar PubMed PubMed Central
[81] Kannaian N, Prakash U, Sripriya NS, Raj DD. Antioxidant potency and GC-MS composition of Origanum majorana Linn. Pak J Pharm Sci. 2019;32(5):2117–22, https://www.researchgate.net/publication/337870119.Search in Google Scholar
[82] Zhang D, Qiang R, Zhao J, Zhang J, Cheng J, Zhao D. Mechanism of a volatile organic compound (6-Methyl-2-Heptanone) emitted from Bacillus subtilis ZD01 Against Alternaria solani in Potato. Front Microbiol. 2022;12(808337):1–12.10.3389/fmicb.2021.808337Search in Google Scholar PubMed PubMed Central
[83] Bharathy V, Uthayakumari F. Bioactive components in leaves of Jatropha tanjorensis J. L. Ellis & Saroja by GC-MS analysis. Int J PharmTech Res. 2013;5(4):1839–43, https://sphinxsai.com/2013/OD/PharmOD13/pdfphamOD2013/PT=50(1839-1843)OD13.pdf.Search in Google Scholar
[84] Ghareeb RY, Shams El-Din NG, Maghraby DM, Ibrahim DS, Abdel-Megeed A, Abdelsalam NR. Nematicidal activity of seaweed‑synthesized silver nanoparticles and extracts against Meloidogyne incognita on tomato plants. Sci Rep. 2022;12(3841):1–16. 10.1038/s41598-022-06600-1.Search in Google Scholar PubMed PubMed Central
[85] DaSilva LYS, Alves DS, Guedes A, Ramos B, Pessoa RT, Jardelino L, et al. Evaluation of the inhibitory effect of isopulegol (ISO) and its β-cyclodextrin inclusion complex (ISO/β-CD) on the reversal of Staphylococcus aureus efflux pump activity. Polym Bull. 2024;81:9933–46.10.1007/s00289-024-05191-3Search in Google Scholar
[86] Kang B, Li N, Liu S, Qi S, Mu S. Protective effect of isopulegol in alleviating neuroinflammation in lipopolysaccharide-induced BV-2 cells and in Parkinson Disease model induced with MPTP. J Environ Pathol Toxicol Oncol. 2021;40(3):75–85, https://pubmed.ncbi.nlm.nih.gov/34587406/.10.1615/JEnvironPatholToxicolOncol.2021038944Search in Google Scholar PubMed
[87] Islam MT, Ali ES, Uddin SJ, Shaw S, Islam A, Ahmed I, et al. Phytol: A review of biomedical activities. Food Chem Toxicol. 2018;121:82–94.10.1016/j.fct.2018.08.032Search in Google Scholar PubMed
[88] Ding EL, Hutfless SM, Ding X, Girotra S. Chocolate and prevention of cardiovascular disease: a systematic review. Nutr Metab. 2006;3(2):1–12, http://www.nutritionandmetabolism.com/content/3/1/2.10.1186/1743-7075-3-2Search in Google Scholar PubMed PubMed Central
[89] Gupta S, Shah HB, Bhardwaj P, Holani A, Singh C, Yadav S, et al. Cetyl alcohol, stearyl alcohol and colloidal oatmeal-based gentle skin cleanser in management of dry and sensitive skin: a cross-sectional study. Int J Res Dermatol. 2023;6(6):353–61, https://www.ijord.com/index.php/ijord/article/view/1757.10.18203/issn.2455-4529.IntJResDermatol20233179Search in Google Scholar
[90] Nowak D, Goslinski M, Wojtowicz E, Przygonski K. Antioxidant properties and phenolic compounds of vitamin C-rich juices. J Food Sci. 2018;83(8):2237–46.10.1111/1750-3841.14284Search in Google Scholar PubMed
[91] Berretta M, Quagliariello V, Maurea N. Multiple effects of ascorbic acid against chronic diseases: Updated evidence from preclinical and clinical studies. Antioxidants. 2020;9(12):1182, https://pubmed.ncbi.nlm.nih.gov/33256059/.10.3390/antiox9121182Search in Google Scholar PubMed PubMed Central
[92] Njus D, Kelley PM, Tu Y, Schlegel HB. Free radical biology and medicine ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic Biol Med. 2020;159:37–43. 10.1016/j.freeradbiomed.2020.07.013.Search in Google Scholar PubMed
[93] Onken J, Berger RG. Biotransformation of citronellol by the basidiomycete Cystoderma carcharias in an aerated-membrane bioreactor. Appl Microbiol Biotechnol. 1999;51:158–63, https://link.springer.com/article/10.1007/s002530051376.10.1007/s002530051376Search in Google Scholar PubMed
[94] Dewick PM. The biosynthesis of C5–C25 terpenoid compounds. Nat Product Rep. 2002;19(2):181–222, https://pubs.rsc.org/en/content/articlelanding/2002/np/b002685i.10.1039/b002685iSearch in Google Scholar PubMed
[95] Al-lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta. 2010;1801(11):1175–83. 10.1016/j.bbalip.2010.07.007.Search in Google Scholar PubMed
[96] Jaradat N, Khasati A, Hawi M, Hawash M, Shekfeh S, Qneibi M, et al. Antidiabetic, antioxidant, and anti-obesity effects of phenylthio-ethyl benzoate derivatives, and molecular docking study regarding α-amylase enzyme. Sci Rep. 2022;12(1):1–9. 10.1038/s41598-022-07188-2.Search in Google Scholar PubMed PubMed Central
[97] Yang Q, Chen D, Yu B, He J. Benzoic acid used as food and feed additives can. BioMed Res Int. 2019;2019(1):5721585.10.1155/2019/5721585Search in Google Scholar PubMed PubMed Central
[98] Kawasaki M, Shirai T, Yatsuzuka K, Shirai R. Hydrolytic dynamic kinetic resolution of racemic 3-phenyl-2-oxetanone to chiral tropic acid. RSC Adv. 2024;14:6121–6, https://pubs.acs.org/10.1021/acs.jmedchem.3c01101.10.1039/D3RA08594ESearch in Google Scholar
[99] Rojas JJ, Bull JA. Oxetanes in drug discovery campaigns. J Med Chem. 2023;66:12697–709, https://pubs.acs.org/10.1021/acs.jmedchem.3c01101.10.1021/acs.jmedchem.3c01101Search in Google Scholar PubMed PubMed Central
[100] Berberian V, Allen CCR, Sharma ND, Boyd DR, Hardacre C. A comparative study of the synthesis of 3-substituted catechols using an enzymatic and a chemoenzymatic method. Adv Synth Catal. 2007;349:727–39.10.1002/adsc.200600437Search in Google Scholar
[101] Li R, Narita R, Ouda R, Kimura C, Nishimura H. Structure-dependent antiviral activity of catechol derivatives in pyroligneous acid against the encephalomycarditis virus. RSC Adv. 2018;8:35888–96.10.1039/C8RA07096BSearch in Google Scholar
[102] Jeong E, Jeon J, Lee C, Lee H. Antimicrobial activity of catechol isolated from Diospyros kaki Thunb. roots and its derivatives toward intestinal bacteria. Food Chem. 2009;115(3):1006–10. 10.1016/j.foodchem.2009.01.021.Search in Google Scholar
[103] Di Micco S, Spatafora C, Cardullo N, Riccio R, Fischer K, Pergola C, et al. 2,3-Dihydrobenzofuran privileged structures as new bioinspired lead compounds for the design of mPGES-1 inhibitors. Bioorg Med Chem. 2016;24(4):820–6. 10.1016/j.bmc.2016.01.002.Search in Google Scholar PubMed
[104] Farhat J, Alzyoud L, Alwahsh M, Al-omari B. Structure – activity relationship of benzofuran derivatives with potential anticancer activity. Cancers. 2022;14(2192):1–22. 10.3390/cancers14092196.Search in Google Scholar PubMed PubMed Central
[105] Tsai L, Silverton JV, Lingh HT. Reinvestigation of the reaction of coumalyl chloride with ammonia and amines. a-Aminomethyleneglutaconic anhydride: structure and properties. J Org Chem. 1978;43(23):4415–20, https://pubs.acs.org/doi/10.1021/jo00417a004.10.1021/jo00417a004Search in Google Scholar
[106] Samsonowicz M. Molecular structure of phenyl- and phenoxyacetic acids – spectroscopic and theoretical study. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2014;118:1086–97. 10.1016/j.saa.2013.09.127.Search in Google Scholar PubMed
[107] Francke W, Schulz S. Pheromones. In: Barton D, Nakanishi K, Meth-Cohn O, Mori K, editors. Comprehensive natural products chemistry. Germany: Elsevier Inc.; 1999. p. 197–261. 10.1016/B978-0-08-091283-7.00052-7.Search in Google Scholar
[108] Bauersachs E, Walser V, Reglitz K, Dawid C, Steinhaus M. Peracetic acid residues in orange juice can lead to a 5-vinylguaiacol-in duced clove-like off-flavor via Baeyer-Villiger oxidation of hesperidin. Food Chem. 2024;440:138252. 10.1016/j.foodchem.2023.138252.Search in Google Scholar PubMed
[109] Rubab M, Chelliah R, Saravanakumar K. Bioactive Potential of 2-Methoxy-4-vinylphenol and Benzofuran from Brassica oleracea L. var. capitate f, rubra (Red Cabbage) on Oxidative and Microbiological Stability of Beef Meat. Foods. 2020;9:568, www.mdpi.com/journal/foods.10.3390/foods9050568Search in Google Scholar PubMed PubMed Central
[110] Maga JA. Contribution of phenolic compounds to smoke flavor. In: Ho C-T, Lee CY, Huang MT, editors. Phenolic Compounds in Food and Their Effects on Health I. ACS Symposium Series. Washington, DC: American Chemical Society; 1992. p. 171–9. 10.1021/bk-1992-0506.ch013.Search in Google Scholar
[111] Singh IP, Gupta S, Kumar S. Thiazole compounds as antiviral agents: An update. Med Chem. 2020;16(1):4–23.10.2174/1573406415666190614101253Search in Google Scholar PubMed
[112] Ali SH, Sayed AR. Review of the synthesis and biological activity of thiazoles. Synth Commun. 2021;51(5):670–700. 10.1080/00397911.2020.1854787.Search in Google Scholar
[113] Olatunde A, Mohammed A, Auwal M, Tajuddeen N, Nasir M. Vanillin: A food additive with multiple biological activities. Eur J Med Chem Rep. 2022;5:100055. 10.1016/j.ejmcr.2022.100055.Search in Google Scholar
[114] Balzani V, Bergamini G, Ceroni P, Fritz V. Electronic spectroscopy of metal complexes with dendritic ligands. Coord Chem Rev. 2007;251:525–35.10.1016/j.ccr.2006.04.007Search in Google Scholar
[115] Khan A, Zaman T, Mohammad T, Tanjima F, Hasan F, Naz T, et al. Carbofuran affects cellular autophagy and developmental senescence through the impairment of Nrf2 signalling. J Cell Mol Med. 2022;26(1):35–47, https://pubmed.ncbi.nlm.nih.gov/34240810/.10.1111/jcmm.16774Search in Google Scholar PubMed PubMed Central
[116] Altemimi A, Lakhssassi N, Baharlouei A, Watson DG. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6(4):42.10.3390/plants6040042Search in Google Scholar PubMed PubMed Central
[117] Lourenço SC, Mold M, Alves VD. Antioxidants of natural plant origins: From sources to food industry applications. Molecules. 2019;24(22):4132, https://pubmed.ncbi.nlm.nih.gov/31731614/.10.3390/molecules24224132Search in Google Scholar PubMed PubMed Central
[118] Kumar A, Kaur S, Dhiman S, Singh PP, Bhatia G, Thakur S, et al. Targeting Akt/NF-κ B/p53 pathway and apoptosis inducing potential of 1,2-benzenedicarboxylic acid, bis (2-methyl propyl) ester isolated from Onosma bracteata Wall. against human osteosarcoma (MG-63) cells. Molecules. 2022;27(11):3478, https://www.mdpi.com/1420-3049/27/11/3478.10.3390/molecules27113478Search in Google Scholar PubMed PubMed Central
[119] Huang L, Zhu X, Zhou S, Cheng Z, Shi K, Zhang C. Phthalic acid esters: Natural sources and biological activities. Toxins. 2021;13(7):495, https://pubmed.ncbi.nlm.nih.gov/34357967/.10.3390/toxins13070495Search in Google Scholar PubMed PubMed Central
[120] Nisar MF, Khadim M, Rafiq M, Chen J, Yang Y, Wan CC. Pharmacological properties and health benefits of Eugenol: A comprehensive review. Oxid Med Cell Longev. 2021;2021(1):2497354.10.1155/2021/2497354Search in Google Scholar PubMed PubMed Central
[121] Gulcin I. Antioxidant activity of Eugenol: A structure activity relationship study. J Med Food. 2011;14(9):975–85.10.1089/jmf.2010.0197Search in Google Scholar PubMed
[122] Sarhill N, Walsh D, Nelson KA. Hydromorphone: pharmacology and clinical applications in cancer patients. Support Care Cancer. 2001;9:84–96.10.1007/s005200000183Search in Google Scholar PubMed
[123] Madhavan SA, Priyadharshini R, Sripriya R. In-vitro pharmacological and Gc-Ms analysis of bioactive compounds presents in Bytteneria herbaceae. Int J Zool Entomol Lett. 2021;1(1):20–6, https://www.zoologicaljournal.com/archives/2021.v1.i1.A.4.Search in Google Scholar
[124] Rehman YU, Iqbal A, Ali G, Alotaibi G, Ahmed A, Ayaz M. Phytochemical analysis, radical scavenging and glioblastoma U87 cells toxicity studies of stem bark of buckthorn (Rhamnus pentapomica R. Parker). BMC complementary medicine and therapies. BMC Complement Med Ther. 2024;24(1):12. 10.1186/s12906-023-04309-w.Search in Google Scholar PubMed PubMed Central
[125] Franz AK, Wilson SO. Organosilicon molecules with medicinal applications. J Med Chem. 2013;56(2):388–405, https://pubs.acs.org/doi/10.1021/jm3010114.10.1021/jm3010114Search in Google Scholar PubMed
[126] Han Z, Fina A, Camino G. Organosilicon compounds as polymer fire retardants. In Polymer green flame retardants. Amsterdam, Netherlands: Elsevier B.V; 2014. p. 389–418. 10.1016/B978-0-444-53808-6.00012-3.Search in Google Scholar
[127] Hussain A, Arnold JJ, Khan MA, Ahsan F. Absorption enhancers in pulmonary protein delivery. J Controlled Rel. 2004;94:15–24.10.1016/j.jconrel.2003.10.001Search in Google Scholar PubMed
[128] Thermo Fisher Chemicals. 1-Tridecyne, 97%. Thermo scientific chemicals; 2024. https://www.thermofisher.com/order/catalog/product/L02481.03 (Accessed on 2024-06-29).Search in Google Scholar
[129] Pradhan S, Dubey RC. GC–MS analysis and molecular docking of bioactive compounds of Camellia sinensis and Camellia assamica. Arch Microbiol. 2021;203(5):2501–10. 10.1007/s00203-021-02209-6.Search in Google Scholar PubMed
[130] Vardanyan RS, Hruby VJ. Anxiolytics (Tranquilizers). In: Vardanyan RS, Hruby VJ, editors. Synthesis of essential drugs. Amsterdam, Netherlands: Elsevier Science; 2006. p. 69–82. 10.1016/B978-0-444-52166-8.X5000-6.Search in Google Scholar
[131] Karthik Y, Krishnappa IMKS, Devappa R, Goud CA, Wani KRMA, Alkafafy M, et al. Antiproliferative activity of antimicrobial peptides and bioactive compounds from the mangrove Glutamicibacter mysorens. Front Microbiol. 2023;14(1096826):1–20.10.3389/fmicb.2023.1096826Search in Google Scholar PubMed PubMed Central
[132] Hassan A, Adebote DA, Amupitan JO, Okonkwo EM. Antifeedant activity of the chemical constituents of Detarium microcarpum Guill&Perr. (Ceasalpinaceae). Aust J Basic Appl Sci. 2010;4(8):3238–43, https://www.ajbasweb.com/old/ajbas/2010/3238-3243.pdf.Search in Google Scholar
[133] Bakri MM, Al-Rajhi AMH, Abada E, Salem OMA, Shater AR, Mahmoud MS, et al. Mycostimulator of chitinolytic activity: Thermodynamic studies and its activity against human and food-borne microbial pathogens. BioResources. 2022;17(3):4378–94.10.15376/biores.17.3.4378-4394Search in Google Scholar
[134] Joghee S, Kalarikkal SP, Sundaram GM, Kumar TD, Chidambaram SB. Chemical profiling and in-vitro anti-inflammatory activity of bioactive fraction (s) from Trichodesma indicum (L.) R.Br. against LPS induced inflammation in RAW 264.7 murine macrophage cells. J Ethnopharmacol. 2021;279:114235. 10.1016/j.jep.2021.114235.Search in Google Scholar PubMed
[135] Kazemipoor M, Tehrani PF, Zandi H, Yazdi RG. Chemical composition and antibacterial activity of Berberis vulgaris (barberry) against bacteria associated with caries. Clin Exp Dent Res. 2021;7(4):601–8, https://pmc.ncbi.nlm.nih.gov/articles/PMC8404507/.10.1002/cre2.379Search in Google Scholar PubMed PubMed Central
[136] Hashim N, Abdullah S, Hassan LS, Ghazali SR, Jalil R. A study of neem leaves: Identification of method and solvent in extraction. Mater Today: Proc. 2021;42:217–21. 10.1016/j.matpr.2020.11.726.Search in Google Scholar
[137] Liauw MY, Natan FA, Widiyanti P, Ikasari D, Indraswati N, Soetaredjo FE. Extraction of Neem oil (Azadirachta indica A. Juss) using n-hexane and ethanol: studies of oil quality, kinetic and thermodynamic. ARPN J Eng Appl Sci. 2008;3(3):49–54, www.arpnjournals.com.Search in Google Scholar
[138] Sithisarn P, Supabphol R, Gritsanapan W. Comparison of free radical scavenging activity of Siamese neem tree (Azadirachta indica A. Juss var. siamensis Valeton) leaf extracts prepared by different methods of extraction. Med Princ Pract. 2006;15(3):219–22.10.1159/000092185Search in Google Scholar PubMed
[139] Subramanian S, Salleh AS, Bachmann RT, Hossain MS. Simultaneous extraction and separation of oil and azadirachtin from seeds and leaves of Azadirachta indica using binary solvent extraction. Nat Product Sci. 2019;25(2):150–6.10.20307/nps.2019.25.2.150Search in Google Scholar
[140] Akhbari M, Hamedi S, Aghamiri Z. sadat. Optimization of total phenol and anthocyanin extraction from the peels of eggplant (Solanum melongena L.) and biological activity of the extracts. J Food Meas Charact. 2019;13(4):3183–97. 10.1007/s11694-019-00241-1.Search in Google Scholar
[141] Eikani MH, Golmohammad F, Rowshanzamir S. Subcritical water extraction of essential oils from coriander seeds (Coriandrum sativum L.). J Food Eng. 2007;80(2):735–40.10.1016/j.jfoodeng.2006.05.015Search in Google Scholar
[142] Daud NM, Putra NR, Jamaludin R, Md Norodin NS, Sarkawi NS, Hamzah MHS, et al. Valorisation of plant seed as natural bioactive compounds by various extraction methods: A review. Trends Food Sci Technol. 2022;119:201–14. 10.1016/j.tifs.2021.12.010.Search in Google Scholar
[143] Sarkar A, Ghosh U. Classical single factor optimisation of parameters for phenolic antioxidant extraction from Tamarind Seed (Tamarindus indica). Plant Sci Today. 2016;3(3):258.10.14719/pst.2016.3.3.242Search in Google Scholar
[144] Lee J, Thilini J, Jayakody M, Kim J, Jeong J, Choi K, et al. The influence of solvent choice on the extraction of bioactive compounds from Asteraceae: A comparative review. Foods. 2024;13(3151):1–21. 10.3390/foods13193151.Search in Google Scholar PubMed PubMed Central
[145] Dias MC, Pinto DC, Silva AM. Plant flavonoids: Chemical characteristics and biological activity. Molecules. 2021;26:5377.10.3390/molecules26175377Search in Google Scholar PubMed PubMed Central
[146] Ciupei D, Colişar A, Leopold L, Stănilă A, Diaconeasa ZM. Polyphenols: From classification to therapeutic potential and bioavailability. Foods. 2024;13:4131. 10.3390/foods13244131.Search in Google Scholar PubMed PubMed Central
[147] Khamis Al-Jadidi HS, Hossain MA. Studies on total phenolics, total flavonoids and antimicrobial activity from the leaves crude extracts of neem traditionally used for the treatment of cough and nausea. Beni-Suef Univ J Basic Appl Sci. 2015;4(2):93–8. 10.1016/j.bjbas.2015.05.001.Search in Google Scholar
[148] Nevagi RJ, Dighe SN, Dighe SN. Biological and medicinal signi fi cance of benzofuran. Eur J Med Chem. 2015;97:561–81.10.1016/j.ejmech.2014.10.085Search in Google Scholar PubMed
[149] Miao Y, Hu Y, Yang J, Liu T, Sun J. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019;9:27510–40.10.1039/C9RA04917GSearch in Google Scholar PubMed PubMed Central
[150] Murray A, Hagen NA, Neurology F. Hydromorphone. J Pain Symptom Manag. 2005;29(5):57–66.10.1016/j.jpainsymman.2005.01.007Search in Google Scholar PubMed
[151] Babu B, Wu JT. Production of natural butylated hydroxytoluene as an antioxidant by freshwater phytoplankton. J Phycology. 2008;44(6):1447–54.10.1111/j.1529-8817.2008.00596.xSearch in Google Scholar PubMed
[152] Jiang G, Lin S, Wen L, Jiang Y, Zhao M, Chen F, et al. Identification of a novel phenolic compound in litchi (Litchi chinensis Sonn.) pericarp and bioactivity evaluation. Food Chem. 2013;136(2):563–8. 10.1016/j.foodchem.2012.08.089.Search in Google Scholar PubMed
[153] Cesa S, Sisto F, Zengin G, Scaccabarozzi D, Kokolakis AK, Scaltrito MM, et al. Phytochemical analyses and pharmacological screening of Neem oil. South Afr J Botany. 2019;120:331–7. 10.1016/j.sajb.2018.10.019.Search in Google Scholar
[154] Tai A, Sawano T, Yazama F, Ito H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim Biophys Acta. 2011;1810(2):170–7. 10.1016/j.bbagen.2010.11.004.Search in Google Scholar PubMed
[155] Chen P, Liu Y, Li C, Hua S, Sun C, Huang L. Antibacterial mechanism of vanillin against Escherichia coli. Heliyon. 2023;9(9):e19280. 10.1016/j.heliyon.2023.e19280.Search in Google Scholar PubMed PubMed Central
[156] Santos JH, Graf U, Luiza M, Helena H, De Andrade R. The synergistic effects of vanillin on recombination predominate over its antimutagenic action in relation to MMC-induced lesions in somatic cells of Drosophila melanogaster. Mutat Res. 1999;444(2):355–65.10.1016/S1383-5718(99)00101-1Search in Google Scholar
[157] Hossain MA, Al-Toubi WAS, Weli AM, Al-Riyami QA, Al-Sabahi JN. Identification and characterization of chemical compounds in different crude extracts from leaves of Omani neem. J Taibah Univ Sci. 2013;7(4):181–8. 10.1016/j.jtusci.2013.05.003.Search in Google Scholar
[158] Balasubramanian S, Ganesh D, Surya Narayana VVS. GC-MS analysis of phytocomponents in the methanolic extract of Azadirachta indica (neem). Int J Pharma Bio Sci. 2014;5(4):P258–62.Search in Google Scholar
[159] Dineshkumar G, Rajakumar R. GC-MS evaluation of bioactive molecules from the methanolic leaf extract of Azadirachta indica (A. Juss). Asian J Pharm Sci Technol. 2015;5(2):64–9, www.ajpst.com.Search in Google Scholar
[160] Moraes D, De Oliveira RN. Phytol, a diterpene alcohol from chlorophyll, as a drug against neglected tropical disease Schistosomiasis mansoni. PLOS Negl Trop Dis. 2014;8(1):1–12, www.plosntds.org.10.1371/journal.pntd.0002617Search in Google Scholar PubMed PubMed Central
[161] Taj T, Sultana RS, Chakraborty M, Phytol A. Phytoconstituent, its chemistry and pharmacological actions. GIS Sci J. 2021;8(1):395–406, https://www.researchgate.net/publication/348351025.10.1007/978-981-16-7637-6_35Search in Google Scholar
[162] Chikati R. Molecular Evaluation of RNase from Aspergillus niger and Phytol of Nymphaea pubescens as Cytoskeletol targeting elements in Cancer. In International Conference on Epidemiology and Evolutionary Genetics. Orlando: OMICS Group Conferences; 2013. p. 4172. 10.4172/2161-1165.S1.003.Search in Google Scholar
[163] Suttiarporn P, Choommongko V. Microwave-assisted improved extraction and purification of anticancer Nimbolide from Azadirachta indica (Neem) leaves. Molecules. 2020;25:2913, www.mdpi.com/journal/molecules.10.3390/molecules25122913Search in Google Scholar PubMed PubMed Central
[164] Mahmoud DA, Hassanein NM, Youssef KA, Abou Zeid MA. Antifungal activity of different Neem leaf extracts and the Nimonol against some important human pathogens. Braz J Microbiol. 2011;42:1007–16.10.1590/S1517-83822011000300021Search in Google Scholar
[165] Vergallo C, Panzarini E, Dini L. High performance liquid chromatographic profiling of antioxidant and antidiabetic flavonoids purified from Azadirachta indica (neem) leaf ethanolic extract. Pure Appl Chem. 2019;91(10):1631–40.10.1515/pac-2018-1221Search in Google Scholar
[166] Ghimeray AK, Jin CW, Ghimire BK, Dong HC. Antioxidant activity and quantitative estimation of azadirachtin and nimbin in Azadirachta Indica A. Juss grown in foothills of Nepal. Afr J Biotechnol. 2009;8(13):3084–91, https://www.researchgate.net/publication/267822353%0AAntioxidant.Search in Google Scholar
[167] Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99(1):109–12.10.1016/j.jep.2005.02.008Search in Google Scholar PubMed
[168] Güller P. The in vitro and in silico inhibition mechanism of glutathione reductase by resorcinol derivatives: A molecular docking study. J Mol Struct. 2021;1228:129790. 10.1016/j.molstruc.2020.129790.Search in Google Scholar
[169] Güller P, Karaman M, Güller U, Aksoy M, Küfrevioğlu Öİ. A study on the effects of inhibition mechanism of curcumin, quercetin, and resveratrol on human glutathione reductase through in vitro and in silico approaches. J Biomol Struct Dyn. 2021;39(5):1744–53.10.1080/07391102.2020.1738962Search in Google Scholar PubMed
[170] Filipsky T, Riha M, Macakova K, Anzenbacherova E, Karlickova J, Mladenka P. Antioxidant effects of coumarins include direct radical scavenging, metal chelation and inhibition of ROS-producing enzymes. Curr Top Med Chem. 2015;15(5):415–31.10.2174/1568026615666150206152233Search in Google Scholar PubMed
[171] Ioana G, Cical P, Babes PA, Calniceanu H, Popa A, Ciavoi G, et al. Anti-inflammatory and antioxidant properties of carvacrol and magnolol, in periodontal disease and diabetes mellitus. Molecules. 2021;26(6899):1–29. 10.3390/molecules26226899.Search in Google Scholar PubMed PubMed Central
[172] Hagey P, Healy EF. Quantitative assay for glutathione reductase. Austin, USA: St. Edward’s University; 2014. p. 1–14. https://www.slideserve.com/glenda/development-of-a-quantitative-assay-for-glutathione-reductase.Search in Google Scholar
[173] Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Delivery Rev. 2012;64:4–17. 10.1016/j.addr.2012.09.019.Search in Google Scholar
[174] Daina A, Zoete V. A boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem. 2016;11:1117–21.10.1002/cmdc.201600182Search in Google Scholar PubMed PubMed Central
[175] Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:1–13. 10.1038/srep42717.Search in Google Scholar PubMed PubMed Central
[176] Stillhart C, Vučićević K, Augustijns P, Basit AW, Batchelor H, Flanagan TR, et al. Impact of gastrointestinal physiology on drug absorption in special populations – An UNGAP review. Eur J Pharm Sci. 2020;147:105280. 10.1016/j.ejps.2020.105280.Search in Google Scholar PubMed
[177] Taşçıoğlu N, Saatçi Ç, Emekli R, Tuncel G, Eşel E, Dundar M. Investigation of cytochrome p450 CYP1A2, CYP2D6, CYP2E1 and CYP3A4 gene expressions and polymorphisms in alcohol withdrawal. Klinik Psikiyatri Derg. 2021;24(3):298–306.10.5505/kpd.2021.60938Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Phytochemical investigation and evaluation of antioxidant and antidiabetic activities in aqueous extracts of Cedrus atlantica
- Influence of B4C addition on the tribological properties of bronze matrix brake pad materials
- Discovery of the bacterial HslV protease activators as lead molecules with novel mode of action
- Characterization of volatile flavor compounds of cigar with different aging conditions by headspace–gas chromatography–ion mobility spectrometry
- Effective remediation of organic pollutant using Musa acuminata peel extract-assisted iron oxide nanoparticles
- Analysis and health risk assessment of toxic elements in traditional herbal tea infusions
- Cadmium exposure in marine crabs from Jiaxing City, China: Insights into health risk assessment
- Green-synthesized silver nanoparticles of Cinnamomum zeylanicum and their biological activities
- Tetraclinis articulata (Vahl) Mast., Mentha pulegium L., and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation
- Exploration of plant alkaloids as potential inhibitors of HIV–CD4 binding: Insight into comprehensive in silico approaches
- Recovery of phenylethyl alcohol from aqueous solution by batch adsorption
- Electrochemical approach for monitoring the catalytic action of immobilized catalase
- Green synthesis of ZIF-8 for selective adsorption of dyes in water purification
- Optimization of the conditions for the preparation of povidone iodine using the response surface methodology
- A case study on the influence of soil amendment on ginger oil’s physicochemical properties, mineral contents, microbial load, and HPLC determination of its vitamin level
- Removal of antiviral favipiravir from wastewater using biochar produced from hazelnut shells
- Effect of biochar and soil amendment on bacterial community composition in the root soil and fruit of tomato under greenhouse conditions
- Bioremediation of malachite green dye using Sargassum wightii seaweed and its biological and physicochemical characterization
- Evaluation of natural compounds as folate biosynthesis inhibitors in Mycobacterium leprae using docking, ADMET analysis, and molecular dynamics simulation
- Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
- Elevational gradients shape total phenolic content and bioactive potential of sweet marjoram (Origanum majorana L.): A comparative study across altitudinal zones
- Study on the CO2 absorption performance of deep eutectic solvents formed by superbase DBN and weak acid diethylene glycol
- Preparation and wastewater treatment performance of zeolite-modified ecological concrete
- Multifunctional chitosan nanoparticles: Zn2+ adsorption, antimicrobial activity, and promotion of aquatic health
- Comparative analysis of nutritional composition and bioactive properties of Chlorella vulgaris and Arthrospira platensis: Implications for functional foods and dietary supplements
- Growth kinetics and mechanical characterization of boride layers formed on Ti6Al4V
- Enhancement of water absorption properties of potassium polyacrylate-based hydrogels in CaCl2-rich soils using potassium di- and tri-carboxylate salts
- Electrochemical and microbiological effects of dumpsite leachates on soil and air quality
- Modeling benzene physicochemical properties using Zagreb upsilon indices
- Characterization and ecological risk assessment of toxic metals in mangrove sediments near Langen Village in Tieshan Bay of Beibu Gulf, China
- Protective effect of Helicteres isora, an efficient candidate on hepatorenal toxicity and management of diabetes in animal models
- Valorization of Juglans regia L. (Walnut) green husk from Jordan: Analysis of fatty acids, phenolics, antioxidant, and cytotoxic activities
- Molecular docking and dynamics simulations of bioactive terpenes from Catharanthus roseus essential oil targeting breast cancer
- Selection of a dam site by using AHP and VIKOR: The Sakarya Basin
- Characterization and modeling of kidney bean shell biochar as adsorbent for caffeine removal from aquatic environments
- The effects of short-term and long-term 2100 MHz radiofrequency radiation on adult rat auditory brainstem response
- Biochemical insights into the anthelmintic and anti-inflammatory potential of sea cucumber extract: In vitro and in silico approaches
- Resveratrol-derived MDM2 inhibitors: Synthesis, characterization, and biological evaluation against MDM2 and HCT-116 cells
- Phytochemical constituents, in vitro antibacterial activity, and computational studies of Sudanese Musa acuminate Colla fruit peel hydro-ethanol extract
- Chemical composition of essential oils reviewed from the height of Cajuput (Melaleuca leucadendron) plantations in Buru Island and Seram Island, Maluku, Indonesia
- Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
- Stability studies of titanium–carboxylate complexes: A multi-method computational approach
- Efficient adsorption performance of an alginate-based dental material for uranium(vi) removal
- Synthesis and characterization of the Co(ii), Ni(ii), and Cu(ii) complexes with a 1,2,4-triazine derivative ligand
- Evaluation of the impact of music on antioxidant mechanisms and survival in salt-stressed goldfish
- Optimization and validation of UPLC method for dapagliflozin and candesartan cilexetil in an on-demand formulation: Analytical quality by design approach
- Biomass-based cellulose hydroxyapatite nanocomposites for the efficient sequestration of dyes: Kinetics, response surface methodology optimization, and reusability
- Multifunctional nitrogen and boron co-doped carbon dots: A fluorescent probe for Hg2+ and biothiol detection with bioimaging and antifungal applications
- Separation of sulphonamides on a C12-diol mixed-mode HPLC column and investigation of their retention mechanism
- Characterization and antioxidant activity of pectin from lemon peels
- Fast PFAS determination in honey by direct probe electrospray ionization tandem mass spectrometry: A health risk assessment insight
- Correlation study between GC–MS analysis of cigarette aroma compounds and sensory evaluation
- Synthesis, biological evaluation, and molecular docking studies of substituted chromone-2-carboxamide derivatives as anti-breast cancer agents
- The influence of feed space velocity and pressure on the cold flow properties of diesel fuel
- Acid etching behavior and mechanism in acid solution of iron components in basalt fibers
- Protective effect of green synthesized nanoceria on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rat
- Evaluation of the antianxiety activity of green zinc nanoparticles mediated by Boswellia thurifera in albino mice by following the plus maze and light and dark exploration tests
- Yeast as an efficient and eco-friendly bifunctional porogen for biomass-derived nitrogen-doped carbon catalysts in the oxygen reduction reaction
- Novel descriptors for the prediction of molecular properties
- 10.1515/chem-2025-0198
- Turmeric oil-fortified nutraceutical-SNEDDS: An approach to boost therapeutic effectiveness of dapagliflozin during treatment of diabetic patients
- Special Issue on Advancing Sustainable Chemistry for a Greener Future
- One-pot fabrication of highly porous morphology of ferric oxide-ferric oxychloride/poly-O-chloroaniline nanocomposite seeded on poly-1H pyrrole: Photocathode for green hydrogen generation from natural and artificial seawater
- High-efficiency photocathode for green hydrogen generation from sanitation water using bismuthyl chloride/poly-o-chlorobenzeneamine nanocomposite
- Special Issue on Phytochemicals, Biological and Toxicological Analysis of Plants
- Comparative analysis of fruit quality parameters and volatile compounds in commercially grown citrus cultivars
- Total phenolic, flavonoid, flavonol, and tannin contents as well as antioxidant and antiparasitic activities of aqueous methanol extract of Alhagi graecorum plant used in traditional medicine: Collected in Riyadh, Saudi Arabia
- Study on the pharmacological effects and active compounds of Apocynum venetum L.
- Chemical profile of Senna italica and Senna velutina seed and their pharmacological properties
- Essential oils from Brazilian plants: A literature analysis of anti-inflammatory and antimalarial properties and in silico validation
- Toxicological effects of green tea catechin extract on rat liver: Delineating safe and harmful doses
- Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies
Articles in the same Issue
- Research Articles
- Phytochemical investigation and evaluation of antioxidant and antidiabetic activities in aqueous extracts of Cedrus atlantica
- Influence of B4C addition on the tribological properties of bronze matrix brake pad materials
- Discovery of the bacterial HslV protease activators as lead molecules with novel mode of action
- Characterization of volatile flavor compounds of cigar with different aging conditions by headspace–gas chromatography–ion mobility spectrometry
- Effective remediation of organic pollutant using Musa acuminata peel extract-assisted iron oxide nanoparticles
- Analysis and health risk assessment of toxic elements in traditional herbal tea infusions
- Cadmium exposure in marine crabs from Jiaxing City, China: Insights into health risk assessment
- Green-synthesized silver nanoparticles of Cinnamomum zeylanicum and their biological activities
- Tetraclinis articulata (Vahl) Mast., Mentha pulegium L., and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation
- Exploration of plant alkaloids as potential inhibitors of HIV–CD4 binding: Insight into comprehensive in silico approaches
- Recovery of phenylethyl alcohol from aqueous solution by batch adsorption
- Electrochemical approach for monitoring the catalytic action of immobilized catalase
- Green synthesis of ZIF-8 for selective adsorption of dyes in water purification
- Optimization of the conditions for the preparation of povidone iodine using the response surface methodology
- A case study on the influence of soil amendment on ginger oil’s physicochemical properties, mineral contents, microbial load, and HPLC determination of its vitamin level
- Removal of antiviral favipiravir from wastewater using biochar produced from hazelnut shells
- Effect of biochar and soil amendment on bacterial community composition in the root soil and fruit of tomato under greenhouse conditions
- Bioremediation of malachite green dye using Sargassum wightii seaweed and its biological and physicochemical characterization
- Evaluation of natural compounds as folate biosynthesis inhibitors in Mycobacterium leprae using docking, ADMET analysis, and molecular dynamics simulation
- Novel insecticidal properties of bioactive zoochemicals extracted from sea urchin Salmacis virgulata
- Elevational gradients shape total phenolic content and bioactive potential of sweet marjoram (Origanum majorana L.): A comparative study across altitudinal zones
- Study on the CO2 absorption performance of deep eutectic solvents formed by superbase DBN and weak acid diethylene glycol
- Preparation and wastewater treatment performance of zeolite-modified ecological concrete
- Multifunctional chitosan nanoparticles: Zn2+ adsorption, antimicrobial activity, and promotion of aquatic health
- Comparative analysis of nutritional composition and bioactive properties of Chlorella vulgaris and Arthrospira platensis: Implications for functional foods and dietary supplements
- Growth kinetics and mechanical characterization of boride layers formed on Ti6Al4V
- Enhancement of water absorption properties of potassium polyacrylate-based hydrogels in CaCl2-rich soils using potassium di- and tri-carboxylate salts
- Electrochemical and microbiological effects of dumpsite leachates on soil and air quality
- Modeling benzene physicochemical properties using Zagreb upsilon indices
- Characterization and ecological risk assessment of toxic metals in mangrove sediments near Langen Village in Tieshan Bay of Beibu Gulf, China
- Protective effect of Helicteres isora, an efficient candidate on hepatorenal toxicity and management of diabetes in animal models
- Valorization of Juglans regia L. (Walnut) green husk from Jordan: Analysis of fatty acids, phenolics, antioxidant, and cytotoxic activities
- Molecular docking and dynamics simulations of bioactive terpenes from Catharanthus roseus essential oil targeting breast cancer
- Selection of a dam site by using AHP and VIKOR: The Sakarya Basin
- Characterization and modeling of kidney bean shell biochar as adsorbent for caffeine removal from aquatic environments
- The effects of short-term and long-term 2100 MHz radiofrequency radiation on adult rat auditory brainstem response
- Biochemical insights into the anthelmintic and anti-inflammatory potential of sea cucumber extract: In vitro and in silico approaches
- Resveratrol-derived MDM2 inhibitors: Synthesis, characterization, and biological evaluation against MDM2 and HCT-116 cells
- Phytochemical constituents, in vitro antibacterial activity, and computational studies of Sudanese Musa acuminate Colla fruit peel hydro-ethanol extract
- Chemical composition of essential oils reviewed from the height of Cajuput (Melaleuca leucadendron) plantations in Buru Island and Seram Island, Maluku, Indonesia
- Phytochemical analysis and antioxidant activity of Azadirachta indica A. Juss from the Republic of Chad: in vitro and in silico studies
- Stability studies of titanium–carboxylate complexes: A multi-method computational approach
- Efficient adsorption performance of an alginate-based dental material for uranium(vi) removal
- Synthesis and characterization of the Co(ii), Ni(ii), and Cu(ii) complexes with a 1,2,4-triazine derivative ligand
- Evaluation of the impact of music on antioxidant mechanisms and survival in salt-stressed goldfish
- Optimization and validation of UPLC method for dapagliflozin and candesartan cilexetil in an on-demand formulation: Analytical quality by design approach
- Biomass-based cellulose hydroxyapatite nanocomposites for the efficient sequestration of dyes: Kinetics, response surface methodology optimization, and reusability
- Multifunctional nitrogen and boron co-doped carbon dots: A fluorescent probe for Hg2+ and biothiol detection with bioimaging and antifungal applications
- Separation of sulphonamides on a C12-diol mixed-mode HPLC column and investigation of their retention mechanism
- Characterization and antioxidant activity of pectin from lemon peels
- Fast PFAS determination in honey by direct probe electrospray ionization tandem mass spectrometry: A health risk assessment insight
- Correlation study between GC–MS analysis of cigarette aroma compounds and sensory evaluation
- Synthesis, biological evaluation, and molecular docking studies of substituted chromone-2-carboxamide derivatives as anti-breast cancer agents
- The influence of feed space velocity and pressure on the cold flow properties of diesel fuel
- Acid etching behavior and mechanism in acid solution of iron components in basalt fibers
- Protective effect of green synthesized nanoceria on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rat
- Evaluation of the antianxiety activity of green zinc nanoparticles mediated by Boswellia thurifera in albino mice by following the plus maze and light and dark exploration tests
- Yeast as an efficient and eco-friendly bifunctional porogen for biomass-derived nitrogen-doped carbon catalysts in the oxygen reduction reaction
- Novel descriptors for the prediction of molecular properties
- 10.1515/chem-2025-0198
- Turmeric oil-fortified nutraceutical-SNEDDS: An approach to boost therapeutic effectiveness of dapagliflozin during treatment of diabetic patients
- Special Issue on Advancing Sustainable Chemistry for a Greener Future
- One-pot fabrication of highly porous morphology of ferric oxide-ferric oxychloride/poly-O-chloroaniline nanocomposite seeded on poly-1H pyrrole: Photocathode for green hydrogen generation from natural and artificial seawater
- High-efficiency photocathode for green hydrogen generation from sanitation water using bismuthyl chloride/poly-o-chlorobenzeneamine nanocomposite
- Special Issue on Phytochemicals, Biological and Toxicological Analysis of Plants
- Comparative analysis of fruit quality parameters and volatile compounds in commercially grown citrus cultivars
- Total phenolic, flavonoid, flavonol, and tannin contents as well as antioxidant and antiparasitic activities of aqueous methanol extract of Alhagi graecorum plant used in traditional medicine: Collected in Riyadh, Saudi Arabia
- Study on the pharmacological effects and active compounds of Apocynum venetum L.
- Chemical profile of Senna italica and Senna velutina seed and their pharmacological properties
- Essential oils from Brazilian plants: A literature analysis of anti-inflammatory and antimalarial properties and in silico validation
- Toxicological effects of green tea catechin extract on rat liver: Delineating safe and harmful doses
- Unlocking the potential of Trigonella foenum-graecum L. plant leaf extracts against diabetes-associated hypertension: A proof of concept by in silico studies

