Abstract
The aim of this study was to determine the concentration of phthalic acid esters (PAEs) in firefighter protective clothing, made from two different material configurations varying in composition, used during live-fire enclosure simulations. Analysis involved six PAEs, with the total PAE concentration being dependent on the protective clothing material configuration and its associated composition. Before washing, a significantly higher Mconcentration of phthalates (548 µg g−1) was recorded in the membrane of material configuration A (composition: 50% meta-aramid, 25% para-aramid, and 25% polytetrafluoroethylene laminate) compared to the outer shell and the thermal barrier. In turn, material configuration B revealed a higher phthalate content (38 µg g−1) in the outer shell (58% para-aramid, 40% polybenzimidazole, and 2% antistatic fiber). The washing process after ten uses of the clothing showed a significant effect on reducing total phthalate content only for the membrane in material configuration A, which showed a reduction in total phthalate concentration from 548 to 275 µg g−1. Washing was more effective for material configuration A: 32 ± 23% for the outer shell, 48 ± 14% for the middle membrane, and 10 ± 10% for the thermal barrier.
1 Introduction
Research on the health effects of hazardous substances contained in textiles indicates risks of exposure associated with the use of clothing. To date, research has primarily focused on dermatitis caused by dyes and finishing resins. However, literature addressing carcinogenic, mutagenic, and reproductive toxicants in textile products is still limited. Such carcinogenic effects have been suggested for certain dyes, especially azo dyes, as well as for some antibacterial agents such as triclosan. Brominated flame retardants, phthalates, and the degradation products of highly fluorinated polymeric hydrophobic agents have also been linked to reproductive and developmental toxicity [1].
Until recently, relatively little attention has been paid to the impact of clothing use and maintenance on the dermal absorption of harmful chemicals. Morrison et al. [2] measured the absorption of two phthalates – diethyl phthalate (DEP) and di-n-butyl phthalate (DBP) – in individuals wearing clean clothing (cotton garments) and in those wearing clothing exposed to air for 6 h. The amounts of DEP and DBP absorbed while wearing fresh clothing were 0.017 and 0.007 μg kg−1/(μg m−3), respectively. Conversely, in individuals exposed to air, these amounts were 0.178 and 0.261 μg kg−1/(μg m−3), respectively. Fresh clothing provided protection, whereas clothing exposed to air increased dermal absorption of DEP and DBP by 3.3 and 6.5 times, respectively [2].
During washing, clothing can retain chemicals from cleaning solvents, which subsequently contribute to health risks for wearers. After washing, garments are dried either by air or mechanically. Air-dried clothing can absorb chemicals from the surrounding air during the drying process. During mechanical drying, certain chemicals may be thermally desorbed, while other chemicals, such as fabric softeners, may be absorbed by the clothing [1]. Gong et al. [3] found that the effectiveness of machine washing in removing phthalates from cotton jeans increases with the octanol–water partition coefficient of the phthalate (K ow is the partition coefficient for a two-phase system consisting of n-octanol and water). The median values ranged from very low (<5%) for di-(2-ethylhexyl) phthalate (DEHP) to very high (∼75%) for dimethyl phthalate (DMP) [1].
In 2003, the Danish Environmental Protection Agency (DEPA) reported measurements of DEHP in various types of textiles, including cotton, wool, linen, and viscose, with concentrations ranging from 2.4 to 7.7 µg g−1 [4]. Textiles were observed to contain the phthalates: di-2-ethylhexyl phthalate (DEHP), dibutyl phthalate (DBP), diisononylphthalate, diisodecyl phthalate, di-n-octyl phthalate (DNOP), and diisobutyl phthalate (DiBP). Phthalates were reported in several garments made from various types of fabrics, such as cotton, polyester, and spandex [5]. Phthalates occur in the textile industry, as they are associated with the coating of materials and screen printing. It should be noted that textile inks, printing pastes, and dyes used in the production of textiles contain phthalates. In addition, pre-production products and raw materials can be treated with various phthalates during the manufacturing process, which include synthetic leather, waterproof coatings, rubber, and raincoats. Other important factors for consideration are the packaging and storage of textile products, as contact with other materials may lead to the release of phthalates from, e.g., containers, labels, and packaging made of synthetic materials [6].
Tang et al. [7] assessed the content of 15 phthalates in various types of clothing, mostly made of cotton, although other garments contained mixtures of cotton, polyester, spandex, or nylon. For most clothing items, three phthalates exhibited the highest concentrations: di-2-ethylhexyl phthalate (DEHP), dibutyl phthalate (DBP), and DiBP, while the content of benzyl butyl phthalate (BBP) was the lowest [7].
The maximum limit for individual phthalates or total phthalates in the United States, Canada, the EU, Denmark, South Korea, China, and Taiwan is 0.1% by weight, with a slightly lower limit for selected phthalates in Denmark [8]. The adopted limit of 0.1% applies to an individual phthalate or the sum of any combination of phthalates in the final product. The limit pertains to clothing or related accessories, as well as textiles other than clothing that, under normal or reasonably foreseeable conditions of use, come into contact with the human skin to a similar extent as clothing.
Phthalates also contaminate firefighter protective clothing used during rescue and firefighting operations. They are present in the chemical residues deposited on the surface of protective clothing [9]. Protective clothing, as well as the skin on the hands, neck, and back, is contaminated with harmful substances that are combustion by-products encountered during firefighters’ duties [10]. During the deposition of chemicals on clothing, these substances can penetrate the material and subsequently pass through the successive layers of the clothing assembly. This can result in direct contact of the chemicals with the skin. The literature emphasizes that harmful substances are absorbed through the skin, a process facilitated by elevated skin temperature during firefighting situations [11].
The nature of residential fires has changed over the years. The contents of buildings and their constituent materials have evolved. This has contributed to an increasing diversity of chemicals present during fires. This change is largely due to the evolving nature of materials used in the finishing of homes and furnishings, which now contain a higher proportion of synthetic materials [12]. Materials found in modern buildings and used in furnishings are increasingly synthetic, leading to the production of numerous toxic by-products during combustion. Wildfires can also cause the deposition of various compounds from the polycyclic aromatic hydrocarbon (PAH) group on firefighter equipment and gear [13], as well as clothing and skin [10]. Cross-contamination of the skin with chemicals from personal protective equipment may also occur.
Lacey et al. conducted research on phthalates (phthalic acid ester [PAE]) and PAHs, which contaminate firefighter protective clothing [14]. Samples from worn firefighter protective apparel were subjected to analysis aimed at determining the content of specified compounds in the materials of the clothing. The results of the studies revealed significant quantities of phthalates, which affect the human hormonal system, posing a particular danger to young individuals who largely comprise the firefighter demographic [15,16]. The concentration of one of the analyzed phthalates, di-(2-ethylhexyl) phthalate (DEHP), reached up to 340 µg g−1 in the material of the hood and 220 µg g−1 in the sleeve. Lacey et al. [14] found that firefighters are exposed to high levels of DEHP, a probable human carcinogen, at levels significantly exceeding those recommended by the World Health Organization, which have been the most frequent focus of research to date.
Consequently, the exposure of firefighters to phthalate esters merits further investigation. In subsequent work, Alexander and Baxter [11] embarked on research designed to evaluate the presence of polybrominated diphenyl ether (PBDE) in firefighter protective clothing, including gloves and hoods. At least one congener of PBDE was detected in every sample of new and worn protective clothing. Exposure to PBDEs may lead to the development of cancerous diseases, as has been observed by Alexander and Baxter [11]. The researchers [11] compared the results of their studies on the content of PBDE with previous reports pertaining to the analysis of WHO guidelines and phthalate diesters. Their findings indicate that all three types of chemical substances were present in the clothing samples.
The aim of this study was to determine PAE content in firefighter protective clothing – specifically in the outer shell, moisture barrier membrane, and thermal barrier – used during live-fire training simulations. Samples were taken from clothing post-fire and post-washing. For comparison, analyses were also conducted of materials in their new, pre-fire condition. The effectiveness of the washing process was assessed in terms of reducing the concentration of PAE contaminants in firefighter protective clothing. The study investigated differences in washing effectiveness between the various materials used in clothing. Scanning electron microscopy (SEM) tests were carried out to evaluate the surface of firefighter protective clothing materials subjected to ten uses and washing. To assess the presence of phthalates in contaminated firefighter protective clothing after repeated use and to determine the effectiveness of their removal during washing, spectroscopic analysis was performed using Fourier transform infrared (FTIR) spectroscopy.
2 Experimental
2.1 Materials
The study involved two types of firefighter protective clothing, each consisting of a jacket and trousers. Both types of clothing were made from a three-layer material configuration: an outer shell, a middle moisture barrier membrane, and an inner thermal barrier layer. The two types of firefighter protective clothing, designated as A and B, differed in terms of the composition of the materials used (Table 1).
Characterization of the materials used in the two types of firefighter protective clothing under study
| Type of clothing | Composition | Mass per unit area (m2 g−1) | Thickness (mm) | Sample weight1 (g) |
|---|---|---|---|---|
| A | Outer shell: 98% meta-aramid, 2% antistatic fiber (twill weave) | 218.6 ± 2.5 | 0.50 ± 0.01 | 0.35 |
| Moisture membrane: 50% meta-aramid, 25% para-aramid, 25% polytetrafluoroethylene laminate | 176.5 ± 1.0 | 1.14 ± 0.02 | 0.28 | |
| Thermal barrier: lining: 50% aramid, 50% flame retardant (FR) viscose; felt: 85% meta-aramid, 15% para-aramid (plain weave) | 177.7 ± 1.6 | 0.96 ± 0.04 | 0.28 | |
| B | Outer shell: 58% para-aramid, 40% polybenzimidazole, 2% antistatic fiber (plain weave) | 200.2 ± 1.0 | 0.45 ± 0.01 | 0.32 |
| Moisture membrane: 25% meta-aramid, 25% para-aramid, 50% polytetrafluoroethylene laminate | 107.9 ± 1.9 | 0.61 ± 0.03 | 0.17 | |
| Thermal barrier: lining: 93% meta-aramid, 5% para-aramid, 2% antistatic fiber; felt: 67% meta-aramid, 33% para-aramid (plain weave) | 167.9 ± 1.7 | 0.84 ± 0.02 | 0.27 |
1Sample surface area: 0.0016 m2.
2.2 Use procedure for firefighter protective clothing
Prior to testing, firefighter protective clothing was subjected to preliminary use under controlled conditions in a fire training chamber. The clothing was deployed during training drills at the Firefighting Academy of Warsaw Training Base for Testing Grounds and Rescue Innovation in Nowy Dwór Mazowiecki. Two items of protective clothing were provided for use, including one item fabricated from a material configuration designated as A and one item from a material configuration designated as B (Figure 1).

Photographs of firefighter protective clothing type B after use in a live-fire training simulator (a – jacket, b – trousers).
The firefighter protective clothing was worn on ten occasions, exposing it each time to smoke and chemical emissions from fires, notably those involving chipboard and furniture materials. Each fire event lasted between 40 and 120 min. Following each use, the clothing was washed using a water-based process in accordance with the manufacturer’s recommendations.
2.3 Cleaning procedure for firefighter clothing after multiple uses
Water-based washing of firefighter protective clothing was conducted in accordance with the maintenance protocol outlined in the user manual supplied by the manufacturer. The process employed a specialized laundry machine, Touch Plus Control (Fagor, Poland). The washing temperature for clothing made from two types of material systems (configuration A and type B) was uniform, set at 60°C. The total duration of the washing cycle was approximately 50–60 min. Drying was conducted at a consistent temperature of 60°C using a TS4175 WW cabinet dryer (Electrolux, Sweden), with a drying time of 180 min.
The washing agents used in our procedure were identical to those used by the participating fire and rescue units. They consisted of a detergent specifically formulated for use with the washing equipment and, at the same time, firefighter protective clothing. All these specialized detergents were produced by a single company.
To minimize cross-contamination:
each garment was washed separately in the washing machine;
each garment underwent washing in the same machine;
the washing machine was cleaned between washing cycles for different garments by conducting a standard washing cycle;
directly prior to each washing cycle, an empty run was performed using water and washing agents.
2.4 Sampling procedure
Samples were cut from protective clothing items that had been worn ten times and exposed to harmful chemical substances, both before and after washing. For these two variants, three sets of samples were cut from the protective clothing element after use (before washing) and after the last tenth washing. A single set of samples consisted of three samples of the following layers: an outer shell, a middle moisture barrier membrane, and an inner thermal barrier layer. The dimensions of the samples were 0.02 m × 0.02 m. Additionally, tests on new protective clothing were conducted for an additional item of protective clothing. The total number of individual samples was 54 (18 for new clothing, 18 for clothing after use before washing, and 18 for clothing after washing). The study on clothing after use and washing was performed on separated clothing elements such as trouser legs (right and left components). The right-side elements were used for sampling from the worn (contaminated) protective clothing, while the left-side elements underwent a washing process according to the manufacturer’s instructions (Figure 1). Samples for analysis were cut from areas above the knee in trousers.
To prevent damage during washing, protective clothing elements were hemmed with an overlock machine before the washing process and then reinforced with a lockstitch for added strength.
2.5 Chemicals
The following reagents were used in the study: acetonitrile, dichloromethane (J. T. Baker, USA), MilliQ ultrapure water (Millipore, Germany), and a collection of certified PAE standards – EPA 506 Phthalate Esters Mix, which included a concentration of 1,000 µg mL−1 of a six-phthalate standard (Sigma-Aldrich Supelco, USA).
3 Methods
3.1 Determination of PAEs
3.1.1 Analytical procedure
The quantification of PAEs was achieved through gas chromatography coupled with mass spectrometry (GC/MS) using an Agilent 7890A gas chromatograph connected to a 5975 C mass spectrometer and an RTX-5 silMS column (Restek, USA), which was 30 m long, 0.25 mm in diameter, and had 0.25 µm film thickness. The oven temperature was initially set to 40°C for 2 min, then increased at a rate of 20°C min−1 to 300°C over 15 min, with the injection chamber also set at 300°C. Helium served as the carrier gas at a flow rate of 1 mL min−1, with an injection volume of 2 µL. Detection was conducted in selected ion monitoring (SIM) mode, monitoring specific m/z ratios of several phthalates.
3.1.2 Preparation of calibration curves, blank samples, and loss of PAEs
The methodology was validated within the measurement range of 0.125–5 µg mL−1. The overall precision for the assay of the six phthalates was established at 5.5%, while the expanded uncertainty for five of the phthalates did not exceed 25%, except BBP, which exceeded 30%. The correlation coefficients obtained, which varied according to PAE and material type, ranging from 0.993 to 0.999 (Table 2). Measurements were carried out in SIM. The corresponding m/z values (ratios of the mass of an ion to its charge) of the analyzed compounds were monitored.
Equations for calibration curves of PAEs and corresponding correlation coefficients (R 2)
| PAE | Retention time (min) | Corresponding m/z values | Material1 | Curve equation | Correlation coefficient2 |
|---|---|---|---|---|---|
| DMP | 9.764 | 77 | Outer shell | y = 1.3 × 105 x − 2.5 × 103 | R 2 = 0.997 |
| 163 | Membrane | y = 2.7 × 105 x − 6.4 × 103 | R 2 = 0.999 | ||
| Thermal barrier | y = 2.4 × 105 x + 3.0 × 104 | R 2 = 0.997 | |||
| DEP | 10.622 | 177 | Outer shell | y = 1.0 × 105 x − 1.0 × 104 | R 2 = 0.998 |
| 149 | Membrane | y = 2.3 × 105 x − 5.4 × 102 | R 2 = 0.999 | ||
| Thermal barrier | y = 2.1 × 105 x + 1.0 × 104 | R 2 = 0.993 | |||
| DBP | 12.606 | 149 | Outer shell | y = 7.0 × 104 x − 1.1 × 104 | R 2 = 0.996 |
| 150 | Membrane | y = 1.7 × 105 x + 1.9 × 104 | R 2 = 0.995 | ||
| Thermal barrier | y = 1.6 × 105 x + 2.2 × 104 | R 2 = 0.994 | |||
| BBP | 14.421 | 91 | Outer shell | y = 1.6 × 104 x + 1.5 × 102 | R 2 = 0.999 |
| 149 | Membrane | y = 3.6 × 104 x + 2.9 × 103 | R 2 = 0.997 | ||
| Thermal barrier | y = 4.6 × 104 x − 2.2 × 103 | R 2 = 0.997 | |||
| di-(2-ethylhexyl) phthalate | 15.151 | 149 | Outer shell | y = 3.0 × 104 x + 5.1 × 103 | R 2 = 0.998 |
| 167 | Membrane | y = 3.7 × 104 x + 4.5 × 103 | R 2 = 0.996 | ||
| Thermal barrier | y = 5.6 × 104 x − 1.4 × 103 | R 2 = 0.999 | |||
| DNOP | 15.958 | 149 | Outer shell | y = 2.2 × 104 x + 3.2 × 102 | R 2 = 0.996 |
| 279 | Membrane | y = 4.5 × 104 x + 6.5 × 102 | R 2 = 0.997 | ||
| Thermal barrier | y = 5.8 × 104 x + 2.9 × 103 | R 2 = 0.999 |
1The tests were performed on three samples of each type of material (sample size 0.02 m × 0.02 m). Three injections were performed for each sample. 2The results of the qualitative PAE determination were confirmed on the basis of the retention time of the analytes (retention time differences of up to 0.01 min were assumed), the monitoring of selected ions in SIM mode at an ionization constant of 70 eV, and comparison of the mass spectrum with a library of spectra. It was assumed that the superimposition of the spectrum of the analyte with the spectrum of the standard from the Wiley library confirmed the presence of a given PAE in the tested sample if the probability was at least 90%.
Analyses of blank samples were performed identically to those methods applied in the preparation of calibration curves. Blank samples were extracted from each of the three distinct layers of new, unused firefighter clothing (outer shell, membrane, and thermal layer), with five samples procured from each material type. It was observed that DBP and di-(2-ethylhexyl) phthalate (DEHP) were present in all material layers. Additionally, DEP was detected in the membrane layer. The surface area values for these phthalates were considered when determining their concentrations in material samples taken from worn firefighter protective clothing.
3.1.3 Losses
To compensate for potential PAE losses in the samples, the method of sample preparation and processing during chemical analysis mirrored that used in the preparation of calibration curves. Individual calibration curves were established for each material layer (outer shell, membrane, and thermal layer). Given that these calibration curves were constructed using protective clothing samples and that PAE analysis was uniformly conducted during both calibration and actual analysis phases, it is reasonable to assert that the analysis adequately accounted for PAE losses.
3.1.4 Sample preparation
The samples extracted from worn firefighter clothing for PAE analysis underwent preparation and analysis identical to that of the samples utilized for calibration curve preparation, with the exception that no PAE solutions were applied onto them.
3.2 SEM analysis
The surface morphology of the samples was examined using a Hitachi SU8010 (Hitachi, Japan) cold field emission scanning electron microscope (FE-SEM). Observations were conducted at an accelerating voltage of 5 kV with a magnification of ×50. To prepare the samples for surface analysis, they were vacuum-coated with gold to improve surface conductivity using a Quorum Technologies Q150ES sputtering device operated under a protective argon atmosphere at a pressure of 0.3 bar. Additionally, nitrogen was introduced to purge the sputtering chamber, facilitating a clean and stable environment for the coating process.
3.3 FTIR analysis
FTIR analysis was performed using a system consisting of an IRSpirit FTIR spectrophotometer equipped with a single-reflection attenuated total reflectance (QATR-S) accessory for direct measurement of solid samples and LabSolutions IR software used for instrument control and data analysis (Shimadzu Corporation Analytical & Measuring Instruments Division, Japan). FTIR spectra were recorded for each sample at three randomly selected locations in the wavenumber range from 4,000 to 500 cm−1.
For FTIR analysis, samples were cut from firefighter protective clothing of types A and B. Tests involved new membrane samples after ten uses (before the tenth washing cycle) and after the tenth washing cycle.
3.4 Statistical analyses
The resultant PAE concentration data were subjected to statistical analysis using Statistica version 10.0 software (StatSoft, Poland).
Statistical analysis of the study results was conducted to assess the significance of the impact of material configuration type on the total phthalate concentrations and the effectiveness of the washing process.
Measurable variables (phthalate concentration and washing effectiveness) were characterized using the mean, standard deviation, and median, as well as minimum and maximum values.
To examine the significance of differences between two groups (independent samples), the following tests were used:
t-test – when the conditions of normal distribution and homogeneity of variance were met,
Cochran-Cox test – when the condition of normal distribution was met but the condition of homogeneity of variance was not.
The statistical significance threshold was set at α = 0.05. Results were deemed statistically significant if the probability value was p ≤ 0.05.
Detailed statistical analysis values are presented in Supplementary Materials (Tables S4–S6).
4 Results and discussion
4.1 Phthalate content in contaminations of firefighter protective clothing
Chemical analysis of contaminations in firefighter protective clothing revealed phthalates (PAEs) in all material layers (outer fabric, membrane, and internal thermal barrier) after repeated use (Figures 2 and 3). Among the six compounds analyzed, the presence of BBP, bis(2-ethylhexyl) phthalate, DBP, and DNOP was pronounced; these substances are classified as category 3 or 1 endocrine-active substances.

Concentrations of individual PAEs in knee samples from three layers of firefighter protective clothing type A after ten uses during live-fire enclosure training and ten washing cycles (error bars indicate standard deviations).
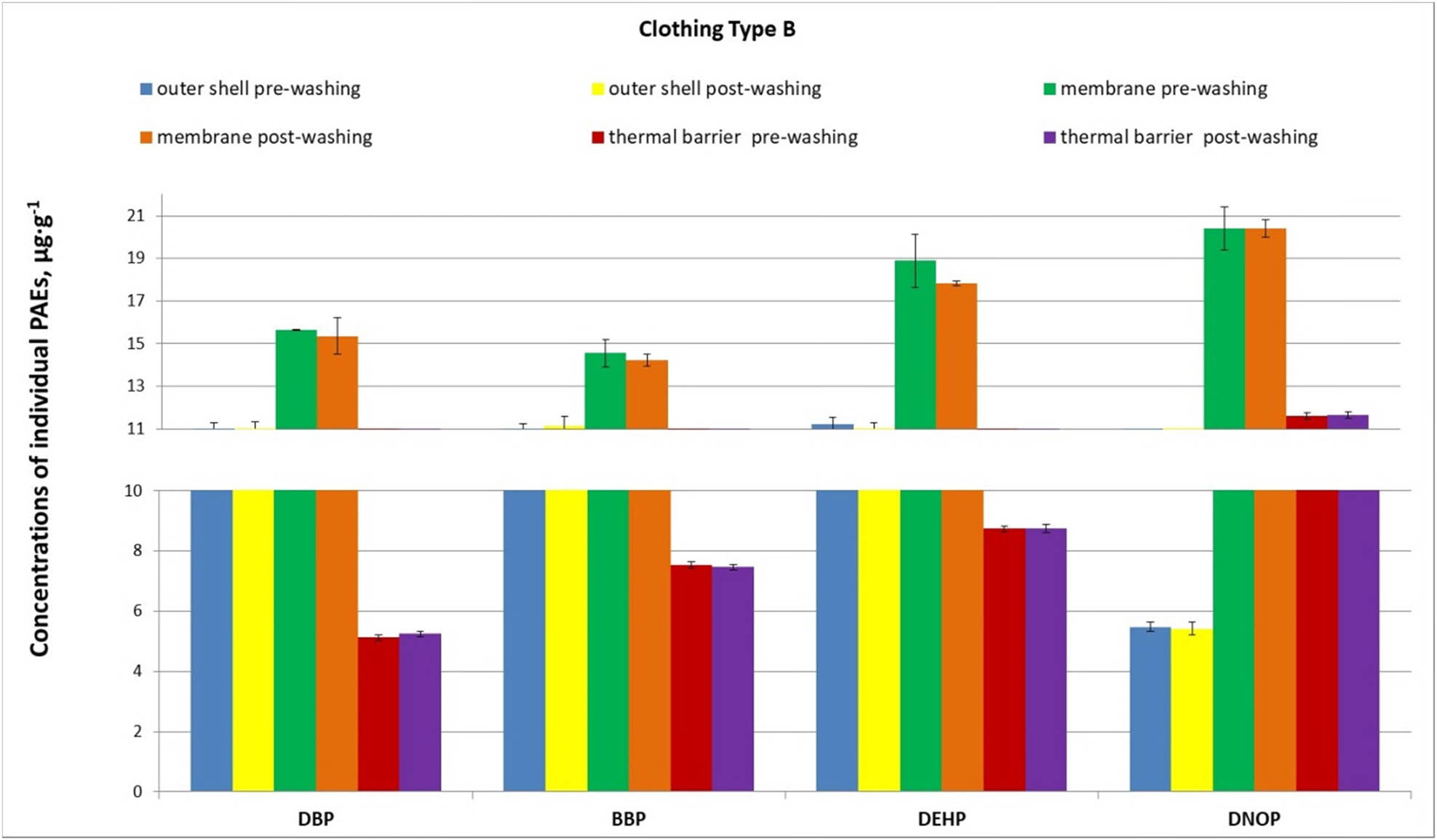
Concentrations of individual PAEs in knee samples from three layers of firefighter protective clothing type B after ten uses during live-fire enclosure training and ten washing cycles (error bars indicate standard deviations).
Analysis of the study results indicates that for firefighter protective clothing made from two different material configurations (A and B), the influence of the type of configuration used and the resultant material composition on the concentrations of individual phthalates varied by the material layer (fabric, outer, membrane, and thermal barrier). For the outer fabric and internal thermal barrier, higher phthalate concentrations were observed in configuration B (Figure 4). Conversely, for the middle membrane layer, a higher phthalate content was noted in configuration A (Figure 2).

Washing effectiveness for total PAEs across the three layers of firefighter protective clothing (outer shell, membrane, and thermal barrier).
Considering the function of the material, the highest concentrations of individual phthalates were determined in the middle layer of the material configuration – the moisture barrier membrane. Prior to washing, concentrations of individual phthalates in the membrane of material configuration A (clothing A) reached up to 313 µg g−1 for bis(2-ethylhexyl) phthalate (Figure 2), while in the membrane of configuration B, concentrations of the same phthalate were approximately 20 µg g−1 (Figure 3). Lower concentrations, reaching up to 10 µg g−1, were recorded for all other phthalates in the thermal barrier of material configuration A and in both the outer fabric and thermal barrier of configuration B.
The final, tenth water washing cycle of the protective clothing, which had been frequently used in training chambers, revealed that four or five of the six phthalates studied were still present, depending on the type of material configuration used. It was observed that protective clothing subjected to ten full cycles of use and washing exhibited varying phthalate concentrations based on the material configuration employed in its manufacture (Figures 2 and 3).
For the outer fabric, elevated phthalate concentrations were observed in the external material of configuration B (clothing B), with the content of BBP, bis(2-ethylhexyl) phthalate, and DBP being 11.1, 10.8, and 10.8 µg g−1, respectively (Figure 3).
For the middle layer, the vapor-permeable membrane, it was observed that, irrespective of the material configuration used, completing ten washing cycles did not result in significant changes in phthalate concentrations. Similarly, less favorable outcomes were recorded for material configuration A compared to pre-washing. Concentrations of bis(2-ethylhexyl) phthalate, DNOP, BBP, and DBP in the membrane from configuration A were several times higher (from 2 to 7 times) than those in the membrane from configuration B (Figures 2 and 3). The largest observed discrepancy, a sevenfold difference, was recorded for DNOP: 148.2 µg g−1 in the membrane of configuration A compared to 20.4 µg g−1 in the membrane of configuration B. Furthermore, it was observed that for the membrane of material configuration A, the concentrations of di-n-butyl and DNOPs were higher after washing than before. This suggested the occurrence of secondary membrane contamination.
Following the completion of the tenth washing cycle of firefighter protective clothing, the internal thermal barrier layer exhibited phthalate concentrations at levels comparable to those before washing. The differences were minimal, below 0.5 µg g−1, and this was observed in both material configurations A and B (Figures 2 and 3).
Alexander and Baxter [11] conducted studies on the chemical content of firefighter protective clothing after use in fires, specifically focusing on phthalates among other contaminants [11]. They used jackets (sleeves and hoods) and gloves from firefighter protective clothing for their research, extracting chemicals using methylene chloride and analyzing them using the EPA 8270 method for semi-volatile contaminants, including 20 PAHs and 6 phthalate esters. Their findings indicated that 22 chemical substances were detected in at least 1 sample of clothing. Notably, di-(2-ethylhexyl) phthalate (DEHP), a plasticizer added to polyvinyl chloride to enhance flexibility, was identified in every sample, with the highest concentrations found in hood material (340 µg g−1) and sleeve material (220 µg g−1), which were 52–875 times higher than any measured PAH. Similarly, the current study also found high DEHP levels in firefighter protective clothing materials, particularly in the middle layer (membrane) of clothing type A after ten uses and before the tenth wash, where DEHP concentrations reached nearly 320 µg g−1. These measurements suggest that firefighters were exposed to high levels of DEHP, a probable carcinogen to humans, significantly above most of the previously studied levels [11,15]. Therefore, firefighters’ exposure to phthalate esters warrants further investigation, as exposure to semi-volatile organic compounds could occur through inhalation or by deposition on equipment and clothing, which subsequently contacts the skin, posing a risk of dermal absorption. Given their highly lipophilic nature, Alexander and Baxter [11] suggested that phthalate diesters could be readily absorbed through the skin, especially under the elevated temperatures typical in firefighting scenarios.
Poutasse et al. [17] tested the developed markers for assessing chemical exposures of firefighters. They conducted concentration tests of harmful chemicals, including phthalates, to determine the effect of duty time, number of calls, and firefighter rank on exposure to chemicals. The results showed that concentrations of individual phthalates were usually higher in markers used during duty compared to those used off duty. This included phthalates analyzed in our study, such as DEHP and DBP, as well as others like DIBP, di-n-nonyl phthalate, and guaiacol. The highest concentration, similar to our findings, was recorded for DEHP, at approximately 550 nmol g−1 for markers used by firefighters off duty and about 950 nmol g−1 for markers used on duty. Comparing with our results, it was observed that DEHP was also present in protective clothing materials for firefighters in large quantities (11.23 µg g−1, outer shell clothing A before washing). Poutasse et al. [17] concluded that phthalate concentrations increased slightly with years spent in the fire service, which was also visible for PAHs. Therefore, prolonged firefighting service may increase exposure to chemicals.
Research has also been conducted on other chemicals released during fires. A study on the occupational hazards of firefighters in Great Britain related to carcinogenic substances released during fires was conducted by Stec et al. [18]. Samples for testing were taken from the skin surface (jaw, throat, and hands), personal protective equipment, and the work environment (office and fire station). A group of 16 PAH compounds (including benzo(a)pyrene, and 7,12-dimethylbenz(a)anthracene) were determined in materials before and after 60 min of exercises in a shipping container. PAH concentrations, similar to those of phthalates, were high, reaching a maximum of 2,000 mg m−2 for clothing and 6,000 mg m−2 for masks.
4.2 Influence of clothing type on total phthalate concentration
Studies have demonstrated a varied influence of material composition on the determined total concentration of the analyzed phthalate group in clothing materials worn in a live-fire training simulator. For configuration A, considerably higher phthalate concentrations were observed in the membrane (548 µg g−1; Table 3). For configuration B, the total PAE concentration was about eight times lower (69 µg g−1). Statistical analysis showed significant differences (p = 0.0120). Meanwhile, configuration B materials exhibited a higher phthalate level in the outer shell (38 µg g−1) compared to configuration A materials (p = 0.0001). The fabric configuration did not affect total phthalate content in the internal layer of protective clothing, i.e.`, in the thermal barrier, where, regardless of the fabric type and composition, comparable amounts were accumulated (32–33 µg g−1). In this case, statistical analysis did not show any significant differences (p = 0.6948).
Total PAE concentrations across the three layers of firefighter protective clothing after ten uses and ten washing cycles (mean with standard deviation)
| Protective clothing | Sample region | Total PAE concentrations, µg g−1 (µg of PAE per g of sample) | |||||
|---|---|---|---|---|---|---|---|
| Outer shell1 | Moisture membrane1 | Thermal barrier1 | |||||
| Pre-tenth wash | Post-tenth wash | Pre-tenth wash | Post-tenth wash | Pre-tenth wash | Post-tenth wash | ||
| A | Knee | 5.4 ± 1.9 | 3.4 ± 0.36 | 548 ± 92 | 276 ± 20 | 32 ± 5.9 | 28 ± 1.9 |
| B | Knee | 38 ± 1.3 | 38 ± 1.6 | 69 ± 2.9 | 68 ± 1.6 | 33 ± 0.41 | 33 ± 0.45 |
1Tests were performed on three samples of each type of material configuration (sample size 0.02 × 0.02 m).
It was observed that the process of washing firefighter protective clothing after use had minimal impact on the total phthalate content across nearly all the material layers (outer shell, membrane, and thermal barrier), regardless of the material configuration used (A or B). A notable exception occurred in the membrane of configuration A, where total phthalate concentration substantially decreased from 548 to 275 µg g−1 after ten washing cycles. For the remaining materials in both configurations, no significant reduction in phthalate concentration was observed post-washing (Table 3).
In the case of the outer shell of material configuration A, characterized by a twill weave, the total PAE concentration was about 7–8 times lower, both before and after washing, compared to the concentration determined for the outer shell of configuration B with a plain weave (Table 3). In the case of thermal barriers, the type of weave was the same (plain), but it was not important because total PAE concentrations were similar for material configurations A and B.
Research conducted by Rabajczyk et al. [19] on the sorption of another group of chemicals, 18 PAHs, in firefighter protective clothing materials showed the highest total concentration of 2.11 µg g−1 in samples taken from the armpit area of the jacket and from the buttock area of the trousers. The lowest total PAH concentration of 0.01 µg g−1 was found in gloves, the inner cuff of the forearm, and trousers (samples taken from the thigh). It was observed that the distribution of PAH concentrations was uneven in the case of the jacket and trousers made of aramid fibers. The total PAH concentrations determined by Rabajczyk et al. [19] were much lower than those in our case, which reached several hundred µg g−1. What was noticeable here was the influence of the type of chemicals accumulating in firefighter clothing, as well as the amount of exposure to chemicals.
The materials employed in the various configurations varied in composition (membrane and lining) or fiber type (outer fabric). The outer fabric of configuration B comprised 58% para-aramid, 40% polybenzimidazole (PBI), and 2% antistatic fiber, whereas the outer fabric of configuration A contained a significantly higher proportion of meta-aramid fibers (98% meta-aramid and 2% antistatic fibers; Table 1). The reduced aramid fiber content in the outer fabric of configuration B, alongside the presence of PBI fibers, may have contributed to greater absorption of hazardous chemical substances. The surface mass of both outer fabrics A and B was comparable, at 210 and 205 g m−2, respectively.
Among a plethora of aromatic polyamides, (para-)aramid fibers are particularly notable for their practical application and significance. Constructed from linear, stiff, and regularly arranged paraphenylene terephthalamide molecular chains, these fibers exhibit a highly structured lattice. The macromolecules of poly-(p-phenylene terephthalamide) are aligned linearly along the axis, forming a highly ordered structural layer interconnected by robust hydrogen bonds. It is the regularity of this semi-crystalline structure that imparts exceptional physico-mechanical properties to para-aramid fibers [20,21]. However, the high chemical stability of the aromatic rings, linked by robust amide bonds, ensures thermal resistance and durability. Meta-aramid fibers PMIA display superior flame resistance compared to para-aramid fibers. In addition, meta-aramid fibers show substantial resistance to a wide range of chemical agents. They withstand most high-concentration inorganic acids and maintain good resistance to alkaline conditions at room temperature. PBI is an organic fiber noted for its flame-retardant properties [20,21].
The membrane incorporated into configuration B also featured a composition with half the amount of meta-aramid and twice the amount of polytetrafluoroethylene (PTFE) laminate compared to other configurations (Table 1). The membrane in configuration B was composed of 25% meta-aramid, 25% para-aramid, and 50% PTFE laminate. In turn, the membrane in configuration A consisted of 50% meta-aramid, 25% para-aramid, and 25% PTFE laminate. The differing proportions of meta-aramid and PTFE laminate in these configurations may have led to a greater accumulation of phthalates in the membrane of configuration A.
The varying concentrations of meta-aramid fibers within the thermal barrier of both material configurations (A and B) did not influence the differential accumulation of phthalates in the clothing materials, nor did they affect the varied removal of chemical substances during the washing process. Similar quantities of phthalates accumulated in the thermal barriers of both configurations. The thermal barrier of configuration A comprised 50% aramid and 50% FR viscose in the lining and 85% meta-aramid with 15% para-aramid in the felt. Conversely, the thermal barrier of configuration B contained a lining of 93% meta-aramid, 5% para-aramid, and 2% antistatic fiber, with the felt consisting of 67% meta-aramid and 33% para-aramid. Both thermal barrier types had comparable surface weight of approximately 170 g m−2.
4.3 Effectiveness of washing in reducing PAE concentrations
The effectiveness of the washing process in reducing PAE concentrations was quantified for the three distinct layers of protective clothing (outer shell, membrane, and thermal barrier) using the following equation [15]:
where c 0 is the PAE concentration in pre-wash samples and c 1 is the PAE concentration in post-wash samples.
The effectiveness of washing was computed separately for each PAE, with consideration given to the type of clothing and the composition of the materials used.
Research demonstrated that, as a result of washing, phthalates were removed to a low or moderate extent from the various materials of firefighter protective clothing that had been subjected to multiple uses (Figure 4). For configuration A materials, washing effectiveness for samples collected from the knee area was 32% for the outer shell, 48% for the membrane, and 10% for the thermal barrier. On the other hand, washing effectiveness for configuration B materials showed substantially lower rates, with 0.7% for the outer shell, 2.3% for the middle membrane, and a negative 0.3% for the thermal barrier.
Under identical conditions of firefighter protective clothing use in a training chamber, a higher washing effectiveness was observed for configuration A materials compared to configuration B. Due to the large variation in results for the outer shell and thermal barrier, the differences in washing effectiveness for configurations A and B were not significant (p-values of 0.1448 and 0.1998, respectively). Statistical analysis confirmed the significance of differences between configurations A and B only for the membrane (p = 0.0051). The highest washing effectiveness was recorded for the membrane layer in both configurations A and B, ranging from 2% in configuration B to 48% in configuration A. This variability was also noted in the effectiveness of phthalate removal from the outer shell and thermal barrier in configurations A and B (Figure 4). Washing effectiveness for the outer shell varied from 1% in configuration B to 32% in configuration A (Figure 4). For the thermal barrier, the observed washing effectiveness was the lowest, ranging from −0.3% (configuration B) to 10% (configuration A). In one case, potentially due to secondary contamination of the thermal barrier in configuration B, a negative washing effectiveness (−0.3%) was recorded.
The authors attribute the variability in washing effectiveness for protective clothing material configurations A and B to the differing responses of these materials during the washing process.
The outer shell of configuration A (Figure 5b and h), after use in an exercise chamber by firefighters and ten washing cycles, showed a smaller degree of deterioration (number of torn fibers) compared to the outer shell in configuration B (Figure 6b and h), which was especially visible under ×1,000 magnification. In both configurations of firefighter protective clothing materials in new condition, an almost perfect membrane without any creases was observed. However, numerous creases appeared following use and the washing process. A loosening of the weave was also observed, characterized by larger pores than those present before the washing process (Figures 5d and j and 6d and j).

SEM images of firefighter protective clothing materials in configuration A: (a and g) new outer shell; (b and h) outer shell after ten uses and washing; (c and i) new membrane; (d and j) membrane after ten uses and washing; (e) new thermal barrier; and (f) thermal barrier after ten uses and washing. Magnification: ×50 (a–f) and ×1,000 (g–j).

SEM images of firefighter protective clothing materials in configuration B: (a and g) new outer shell, (b and h) outer shell after ten uses and washing, (c and i) new membrane, (d and j) membrane after ten uses and washing, (e) new thermal barrier, and (f) thermal barrier after ten uses and washing. Magnification: ×50 (a–f) and ×1,000 (g–j).
It was observed that degradation of the material structure did not contribute to higher washing effectiveness. The outer fabric, membrane, and thermal barrier of configuration B exhibited greater damage during use and washing, along with significantly lower effectiveness in phthalate removal.
Considering the foregoing, the authors posited that the composition and fabrication methods of materials used in both configurations A and B could influence the level of phthalate removal during washing. The study demonstrated that configuration A materials were more amenable to removing phthalate compounds accumulated on their surfaces, thereby facilitating higher washing effectiveness.
Figures 7 and 8 show representative FTIR spectra for membranes used in material configurations A and B. The FTIR spectra reveal characteristic absorption bands corresponding to the structural components of the membrane used, namely aramid polymers and PTFE. The obtained spectroscopic data were consistent with previously reported results, particularly those presented by Khandare [22] and Kobayashi et al. [23].

FTIR spectra for the membrane in material configuration A (N, new membrane; 10U, after ten uses; 10 W, after ten washing cycles).

FTIR spectra for the membrane in material configuration B (N, new membrane; 10U, after ten uses; 10 W, after ten washing cycles).
Comparative analysis of the FTIR spectra for the membrane in various use conditions indicates changes in band intensities and slight shifts in characteristic peaks. These variations can be interpreted as evidence of phthalate adsorption on the surface or within the structure of the membrane material. Notably, significant changes were observed in the spectral range of 2,810–3,010 cm−1.
For membrane A, two absorption bands at 2,970 and 2,980 cm−1, which are typically assigned in the literature to ═C–H stretching vibrations in aromatic rings (characteristic of aramid polymer structures) disappeared after ten uses (Figure 7). The disappearance of these bands may indicate partial coverage or chemical modification of the existing functional groups due to phthalate adsorption or the presence of other organic compounds in the working environment. In contrast, the appearance of a new band at 2,960 cm−1 in membrane B can be attributed to the presence of –CH3 or –CH2– groups, associated with the alkyl chains of phthalate compounds (Figure 8).
Although laundering leads to a slight decrease in the intensity of the aforementioned bands, it does not result in their complete removal. This suggests that phthalates may form relatively stable physicochemical interactions with the membrane material. This could be due to both surface adsorption and the diffusion of phthalates into the polymer structure at the molecular level, which significantly hampers their removal under standard washing conditions.
5 Limitations
The limitations of the study were due to the small size of protective clothing samples, which may not encompass all potential contamination scenarios during use. The research included one uniform material configuration A and another of material configuration B. The findings suggest variations in the deposition of contaminants on the clothing, contingent upon the type of materials used. It would be advantageous for future studies to expand the number of uniforms examined.
It should be acknowledged that testing phthalate esters (PAEs) concentrations in samples from the same side (outer or inner) of protective clothing before and after washing was not feasible. This limitation was due to the fact that outer-side samples were used to determine the PAE content before washing, while inner-side samples were tested post-washing. This approach was necessitated by the destructive nature of the chemical analytical process.
An additional constraint involved the assumption that contamination levels were equal on both sides of the clothing. This assumption influenced the estimation of washing effectiveness, which relied on the concentrations of contaminants in samples before and after washing. This assumption represents a limitation, given that in practice, firefighter clothing may exhibit uneven contamination due to the nature of the firefighters’ duties and the equipment employed.
6 Conclusions
The presented study on firefighter protective clothing made from two material configurations, differing in composition, and deployed during live-fire enclosure simulations, revealed a significant impact of material type on the total concentration of the analyzed group of phthalate esters. For configuration A materials, a markedly higher concentration of phthalates was observed in the membrane (548 µg g−1, comprising 50% meta-aramid, 25% para-aramid, and 25% poly(tetrafluoroethylene) laminate). In turn, configuration B materials were characterized by a higher phthalate content in the outer shell (38 µg g−1, consisting of 58% para-aramid, 40% PBI, and 2% antistatic fiber). The type of material configuration had no influence on the overall phthalate content in the internal layer of the protective clothing, i.e., the thermal barrier, where similar amounts accumulated regardless of material type and composition (32–33 µg g−1; configuration A: 50% aramid, 50% FR viscose; felt: 85% meta-aramid, 15% para-aramid; configuration B: lining: 93% meta-aramid, 5% para-aramid, 2% antistatic fiber; felt: 67% meta-aramid, 33% para-aramid).
Machine washing, a sophisticated cleaning technique that employs a machine with a customized program, removed only modest quantities of PAE contaminating the clothing. The washing process did not demonstrate a significant effect on reducing total phthalate content across all the material layers (external fabric, membrane, and thermal barrier), irrespective of the material configuration used (A, B). The exception was the membrane in material configuration A, where total PAE concentration decreased from 548 to 275 µg g−1. Nonetheless, the washing effectiveness of configuration A materials was higher, with 32 ± 23% for the outer shell, 48 ± 14% for the middle membrane, and 10 ± 10% for the thermal barrier. In contrast, washing effectiveness for configuration B materials was considerably lower, at 0.7 ± 1.1% for the outer shell, 2.3 ± 4.2% for the middle membrane, and −0.3 ± 0.2% for the thermal barrier. In the case of configuration B, secondary contamination of the materials occurred.
The presented study constitutes an important contribution to ongoing research aimed at evaluating the effectiveness of washing firefighter protective clothing and elucidating factors related to the accumulation of contaminants on clothing materials. Given the endocrine-disrupting effects of phthalate esters, it is crucial to examine the protective clothing worn by firefighters and evaluate the potential health risks associated with such contamination.
Acknowledgments
The authors wish to express their gratitude to the Firefighting Academy – Training Base for Testing Grounds and Rescue Innovation in Nowy Dwór Mazowiecki for their cooperation in providing protective clothing for use during live-fire enclosure simulations and assistance with washing the clothing after use.
-
Funding information: This task was completed on the basis of the results of research carried out within the scope of the sixth stage of the National Programme “Governmental Programme for Improvement of Safety and Working Conditions,” funded by state services of the Ministry of Family, Labour and Social Policy. Task no. 6.ZS.11 Entitled “Training materials supporting the safe use of protective clothing for firefighters to reduce the risk associated with the accumulation of harmful chemical substances.” The Central Institute for Labour Protection – National Research Institute is the Programme’s main coordinator.
-
Author contributions: S. Krzemińska: conceptualization, formal analysis, visualization, writing of the original draft, review and editing; M. Szewczyńska: methodology, investigation, review of writing, supervision; P. Kozikowski: investigation; A. Leniart: investigation; P. Miśkiewicz: writing of the original draft, visualization; M. Kuźmiński: investigation.
-
Conflict of interest: The authors declare no conflicts of interest.
References
[1] Licina, D., Morrison, G. C., Bekö, G., Weschler, C. H. J., Nazaroff, W. W. (2019). Clothing-mediated exposures to chemicals and particles. Environmental Science & Technology, 53(10), 5559–5575.10.1021/acs.est.9b00272Suche in Google Scholar PubMed
[2] Morrison, G. C., Weschler, C. J., Bekö, G., Koch, H. M., Salthammer, T., Schripp, T., et al. (2016). Role of clothing in both accelerating and impeding dermal absorption of airborne SVOCs. Journal of Exposure Science & Environmental Epidemiology, 26(1), 113–118.10.1038/jes.2015.42Suche in Google Scholar PubMed
[3] Gong, M., Weschler, C. J., Zhang, Y. (2016). Impact of clothing on dermal exposure to phthalates: observations and insights from sampling both skin and clothing. Environmental Science & Technology, 50(8), 4350–4357.10.1021/acs.est.6b00113Suche in Google Scholar PubMed
[4] Laursen, S. E., Hansen, J., Drøjdahl, A., Hansen, O. Chr, Pommer, K., Pedersen, E., et al. (2023). Survey of chemicals in consumer products, survey of chemical compounds in textile fabric. Danish Environmental Protection Agency (DEPA). Retrieved Apr 15, 2024 https://eng.mst.dk/media/tztbd2md/23.pdf.Suche in Google Scholar
[5] Antal, B., Kuki, A., Nagy, L., Nagy, T., Zsuga, M., Keki, S. (2016). Rapid detection of hazardous chemicals in textiles by direct analysis in real-time mass spectrometry (DART-MS). Analytical and Bioanalytical Chemistry, 408(19), 5189–5198.10.1007/s00216-016-9603-zSuche in Google Scholar PubMed
[6] Pinto, V. C. D., Peleg Mizrachi, M. (2025). The health impact of fast fashion: exploring toxic chemicals in clothing and textiles. Encyclopedia, 5(2), 84.10.3390/encyclopedia5020084Suche in Google Scholar
[7] Tang, Z., Chai, M., Wang, Y., Cheng, J. (2020). Phthalates in preschool children’s clothing manufactured in seven Asian countries: Occurrence, profiles and potential health risks. Journal of Hazardous Materials, 5(387), 121681.10.1016/j.jhazmat.2019.121681Suche in Google Scholar PubMed
[8] American Apparel Footwear Association (AAFA), (2019). Restricted Substances List (RSL), 20th edn. Washington. Retrieved Apr 03, 2024 https://www.aafaglobal.org/AAFA/Priorities/Brand_Protection/AAFA/Priority/Brand_Protection.aspx?hkey=30082999-9ee3-47d3-8c34-91bd284f88d6.Suche in Google Scholar
[9] Krzemińska, S., Szewczyńska, M. (2022). Hazard of chemical substances contamination of protective clothing for firefighters – a survey on use and maintenance. International Journal of Occupational Medicine and Environmental Health, 35(2), 235–248.10.13075/ijomeh.1896.01868Suche in Google Scholar PubMed PubMed Central
[10] Fent, K. W., Alexander, B., Roberts, J., Robertson, S., Toennis, C., Sammons, D., et al. (2017). Contamination of firefighter personal protective equipment and skin and the effectiveness of decontamination procedures. Journal of Occupational and Environmental Hygiene, 14(10), 801–814.10.1080/15459624.2017.1334904Suche in Google Scholar PubMed
[11] Alexander, B. M., Baxter, C. S. (2016). Flame-retardant contamination of firefighter personal protective clothing – a potential health risk for firefighters. Journal of Occupational and Environmental Hygiene, 13(9), D-148–D-155.10.1080/15459624.2016.1183016Suche in Google Scholar PubMed
[12] Giebułtowicz, J., Rużycka, M., Wroczyński, P., Purser, D. A., Stec, A. A. (2017). Analysis of fire deaths in Poland and influence of smoke toxicity. Forensic Science International, 277, 77–87.10.1016/j.forsciint.2017.05.018Suche in Google Scholar PubMed
[13] Groot, E., Caturay, A., Khan, Y., Copes, R. (2019). A systematic review of the health impacts of occupational exposure to wildland fires. International Journal of Occupational Medicine and Environmental Health, 32(2), 121–140.10.13075/ijomeh.1896.01326Suche in Google Scholar PubMed
[14] Lacey, S., Alexander, B. M., Baxter, C. S. (2014). Plasticizer contamination of firefighter personal protective clothing a potential factor in increased health risks in firefighters. Journal of Occupational and Environmental Hygiene, 11(5), D43–D48.10.1080/15459624.2013.877142Suche in Google Scholar PubMed
[15] Krzemińska, S., Szewczyńska, M. (2022). PAH contamination of firefighter protective clothing and cleaning effectiveness. Fire Safety Journal, 131, 103610.10.1016/j.firesaf.2022.103610Suche in Google Scholar
[16] Krzemińska, S., Szewczyńska, M., Kozikowski, P. (2024). Analysis of PAHs content in materials of firefighter protective clothing and cleaning effectiveness after use. Scientific Reports of Fire University, 1(89), 85–110.10.5604/01.3001.0054.4246Suche in Google Scholar
[17] Poutasse, C. M., Poston, W. S. C., Jahnke, S. A., Haddock, Ch. K., Tidwell, L. G., Hoffman, P. D., et al. (2020). Discovery of firefighter chemical exposures using military-style silicone dog tags. Environment International, 142, 105818.10.1016/j.envint.2020.105818Suche in Google Scholar PubMed PubMed Central
[18] Stec, A. A., Dickens, K. E., Salden, M., Hewitt, F. E., Watts, D. P., Houldsworth, P. E., et al. (2018). Occupational exposure to polycyclic aromatic hydrocarbons and elevated cancer incidence in firefighters. Scientific Reports, 8(2476), 1–8.10.1038/s41598-018-20616-6Suche in Google Scholar PubMed PubMed Central
[19] Rabajczyk, A., Gniazdowska, J., Stojek, P., Bąk, Ł. (2024). Sorption processes of selected PAHs on selected fire-resistant materials used in special firefighter clothing. Materials, 17(8), 1741.10.3390/ma17081741Suche in Google Scholar PubMed PubMed Central
[20] Ertekin, M., (2017). Aramid fibres. In M. Ö. Seydibeyoğlu, A. K. Mohanty, & M. Misra (Eds.). Fibre technology for fibre-reinforced composites. Woodhead Publishing Series in Composites Science and Engineering, Woodhead Publishing India Pvt Ltd, Delhi, (pp. 153–167).Suche in Google Scholar
[21] Czerwienko, D., Lemańska, K., Pastuszka, Ł. (2012). Technology of materials for firefighters’ clothing. [in Polish: Technologia materiałów na ubrania strażackie]. Bezpieczeństwo i Technika Pożarnicza/Safety and Firefighting Technology, 4, 119–129.Suche in Google Scholar
[22] Khandare, P. (2014). Qualitative analysis of aramide polymers by FT-IR spectroscopy. International Journal of Engineering Science Invention, 3(2), 2319.Suche in Google Scholar
[23] Kobayashi, M., Nishimura, F., Kim, J.-H., Yonezawa, S. (2023). Dyeable hydrophilic surface modification for PTFE substrates by surface fluorination. Membranes, 3(1), 57.10.3390/membranes13010057Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Study and restoration of the costume of the HuoLang (Peddler) in the Ming Dynasty of China
- Texture mapping of warp knitted shoe upper based on ARAP parameterization method
- Extraction and characterization of natural fibre from Ethiopian Typha latifolia leaf plant
- The effect of the difference in female body shapes on clothing fitting
- Structure and physical properties of BioPBS melt-blown nonwovens
- Optimized model formulation through product mix scheduling for profit maximization in the apparel industry
- Fabric pattern recognition using image processing and AHP method
- Optimal dimension design of high-temperature superconducting levitation weft insertion guideway
- Color analysis and performance optimization of 3D virtual simulation knitted fabrics
- Analyzing the effects of Covid-19 pandemic on Turkish women workers in clothing sector
- Closed-loop supply chain for recycling of waste clothing: A comparison of two different modes
- Personalized design of clothing pattern based on KE and IPSO-BP neural network
- 3D modeling of transport properties on the surface of a textronic structure produced using a physical vapor deposition process
- Optimization of particle swarm for force uniformity of personalized 3D printed insoles
- Development of auxetic shoulder straps for sport backpacks with improved thermal comfort
- Image recognition method of cashmere and wool based on SVM-RFE selection with three types of features
- Construction and analysis of yarn tension model in the process of electromagnetic weft insertion
- Influence of spacer fabric on functionality of laminates
- Design and development of a fibrous structure for the potential treatment of spinal cord injury using parametric modelling in Rhinoceros 3D®
- The effect of the process conditions and lubricant application on the quality of yarns produced by mechanical recycling of denim-like fabrics
- Textile fabrics abrasion resistance – The instrumental method for end point assessment
- CFD modeling of heat transfer through composites for protective gloves containing aerogel and Parylene C coatings supported by micro-CT and thermography
- Comparative study on the compressive performance of honeycomb structures fabricated by stereo lithography apparatus
- Effect of cyclic fastening–unfastening and interruption of current flowing through a snap fastener electrical connector on its resistance
- NIRS identification of cashmere and wool fibers based on spare representation and improved AdaBoost algorithm
- Biο-based surfactants derived frοm Mesembryanthemum crystallinum and Salsοla vermiculata: Pοtential applicatiοns in textile prοductiοn
- Predicted sewing thread consumption using neural network method based on the physical and structural parameters of knitted fabrics
- Research on user behavior of traditional Chinese medicine therapeutic smart clothing
- Effect of construction parameters on faux fur knitted fabric properties
- The use of innovative sewing machines to produce two prototypes of women’s skirts
- Numerical simulation of upper garment pieces-body under different ease allowances based on the finite element contact model
- The phenomenon of celebrity fashion Businesses and Their impact on mainstream fashion
- Marketing traditional textile dyeing in China: A dual-method approach of tie-dye using grounded theory and the Kano model
- Contamination of firefighter protective clothing by phthalates
- Sustainability and fast fashion: Understanding Turkish generation Z for developing strategy
- Digital tax systems and innovation in textile manufacturing
Artikel in diesem Heft
- Study and restoration of the costume of the HuoLang (Peddler) in the Ming Dynasty of China
- Texture mapping of warp knitted shoe upper based on ARAP parameterization method
- Extraction and characterization of natural fibre from Ethiopian Typha latifolia leaf plant
- The effect of the difference in female body shapes on clothing fitting
- Structure and physical properties of BioPBS melt-blown nonwovens
- Optimized model formulation through product mix scheduling for profit maximization in the apparel industry
- Fabric pattern recognition using image processing and AHP method
- Optimal dimension design of high-temperature superconducting levitation weft insertion guideway
- Color analysis and performance optimization of 3D virtual simulation knitted fabrics
- Analyzing the effects of Covid-19 pandemic on Turkish women workers in clothing sector
- Closed-loop supply chain for recycling of waste clothing: A comparison of two different modes
- Personalized design of clothing pattern based on KE and IPSO-BP neural network
- 3D modeling of transport properties on the surface of a textronic structure produced using a physical vapor deposition process
- Optimization of particle swarm for force uniformity of personalized 3D printed insoles
- Development of auxetic shoulder straps for sport backpacks with improved thermal comfort
- Image recognition method of cashmere and wool based on SVM-RFE selection with three types of features
- Construction and analysis of yarn tension model in the process of electromagnetic weft insertion
- Influence of spacer fabric on functionality of laminates
- Design and development of a fibrous structure for the potential treatment of spinal cord injury using parametric modelling in Rhinoceros 3D®
- The effect of the process conditions and lubricant application on the quality of yarns produced by mechanical recycling of denim-like fabrics
- Textile fabrics abrasion resistance – The instrumental method for end point assessment
- CFD modeling of heat transfer through composites for protective gloves containing aerogel and Parylene C coatings supported by micro-CT and thermography
- Comparative study on the compressive performance of honeycomb structures fabricated by stereo lithography apparatus
- Effect of cyclic fastening–unfastening and interruption of current flowing through a snap fastener electrical connector on its resistance
- NIRS identification of cashmere and wool fibers based on spare representation and improved AdaBoost algorithm
- Biο-based surfactants derived frοm Mesembryanthemum crystallinum and Salsοla vermiculata: Pοtential applicatiοns in textile prοductiοn
- Predicted sewing thread consumption using neural network method based on the physical and structural parameters of knitted fabrics
- Research on user behavior of traditional Chinese medicine therapeutic smart clothing
- Effect of construction parameters on faux fur knitted fabric properties
- The use of innovative sewing machines to produce two prototypes of women’s skirts
- Numerical simulation of upper garment pieces-body under different ease allowances based on the finite element contact model
- The phenomenon of celebrity fashion Businesses and Their impact on mainstream fashion
- Marketing traditional textile dyeing in China: A dual-method approach of tie-dye using grounded theory and the Kano model
- Contamination of firefighter protective clothing by phthalates
- Sustainability and fast fashion: Understanding Turkish generation Z for developing strategy
- Digital tax systems and innovation in textile manufacturing

