Abstract
C18H24ClO5P, triclinic, P1̅ (no. 2), a = 11.5140(14) Å, b = 11.5358(15) Å, c = 15.582(2) Å, α = 76.106(2)°, β = 77.556(2)°, γ = 74.783(3)°, V = 1912.8(4)Å3, Z = 4, Rgt(F) = 0.0481, wRref(F2) = 0.1313, T = 173(2) K.
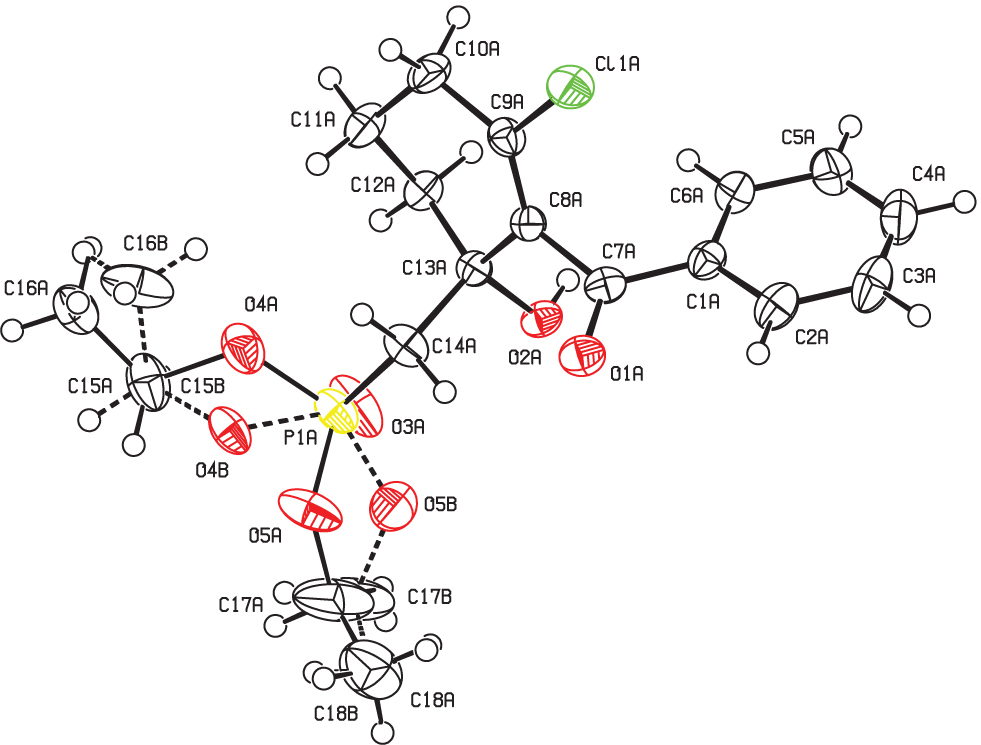
The asymmetric unit of the title crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless prism |
| Size: | 0.40 × 0.30 × 0.15 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 3.1 cm−1 |
| Diffractometer, scan mode: | Bruker SMART, φ and ω |
| 2θmax, completeness: | 50°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 10499, 6692, 0.026 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5210 |
| N(param)refined: | 497 |
| Programs: | Bruker programs [1, 3] , SADABS [2], Platon [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Cl1 | 1.03058(7) | 0.81968(8) | 0.30756(6) | 0.0453(2) |
| O1 | 0.78757(19) | 1.0592(2) | 0.20686(13) | 0.0431(5) |
| O2 | 0.60668(17) | 1.03214(17) | 0.41117(13) | 0.0289(4) |
| H1 | 0.620(3) | 1.025(3) | 0.462(2) | 0.039(9)* |
| O3 | 0.35600(18) | 0.9756(2) | 0.42174(13) | 0.0390(5) |
| O4 | 0.42857(19) | 0.7713(2) | 0.37031(15) | 0.0441(5) |
| O5 | 0.37216(19) | 0.9643(2) | 0.25892(13) | 0.0451(6) |
| P1 | 0.42617(7) | 0.91247(7) | 0.34881(5) | 0.03158(19) |
| C1 | 0.8757(2) | 1.1090(2) | 0.31327(18) | 0.0281(6) |
| C2 | 0.9190(3) | 1.2041(3) | 0.2512(2) | 0.0367(7) |
| H2 | 0.9081 | 1.2184 | 0.1905 | 0.044* |
| C3 | 0.9775(3) | 1.2776(3) | 0.2773(2) | 0.0429(8) |
| H3 | 1.0070 | 1.3416 | 0.2346 | 0.051* |
| C4 | 0.9926(3) | 1.2573(3) | 0.3658(2) | 0.0424(8) |
| H4 | 1.0331 | 1.3072 | 0.3838 | 0.051* |
| C5 | 0.9492(3) | 1.1648(3) | 0.4279(2) | 0.0380(7) |
| H5 | 0.9591 | 1.1520 | 0.4887 | 0.046* |
| C6 | 0.8909(2) | 1.0899(3) | 0.40233(18) | 0.0311(6) |
| H6 | 0.8616 | 1.0261 | 0.4454 | 0.037* |
| C7 | 0.8161(2) | 1.0308(3) | 0.28143(17) | 0.0285(6) |
| C8 | 0.7910(2) | 0.9139(2) | 0.34242(17) | 0.0261(6) |
| C9 | 0.8790(2) | 0.8147(3) | 0.35722(19) | 0.0313(6) |
| C10 | 0.8641(3) | 0.6936(3) | 0.4145(2) | 0.0410(7) |
| H10A | 0.9005 | 0.6797 | 0.4692 | 0.049* |
| H10B | 0.9078 | 0.6274 | 0.3812 | 0.049* |
| C11 | 0.7299(3) | 0.6895(3) | 0.4407(2) | 0.0413(7) |
| H11A | 0.7198 | 0.6215 | 0.4924 | 0.050* |
| H11B | 0.7011 | 0.6736 | 0.3901 | 0.050* |
| C12 | 0.6526(3) | 0.8103(3) | 0.46569(18) | 0.0338(6) |
| H12A | 0.5670 | 0.8027 | 0.4871 | 0.041* |
| H12B | 0.6830 | 0.8274 | 0.5151 | 0.041* |
| C13 | 0.6572(2) | 0.9165(2) | 0.38572(17) | 0.0263(6) |
| C14 | 0.5851(2) | 0.9142(3) | 0.31363(17) | 0.0290(6) |
| H14A | 0.6256 | 0.8408 | 0.2872 | 0.035* |
| H14B | 0.5917 | 0.9868 | 0.2654 | 0.035* |
| C15 | 0.3125(4) | 0.7355(4) | 0.4116(3) | 0.0727(12) |
| H15A | 0.2930 | 0.7442 | 0.4751 | 0.087* |
| H15B | 0.2462 | 0.7908 | 0.3804 | 0.087* |
| C16 | 0.3190(4) | 0.6118(4) | 0.4066(3) | 0.0748(13) |
| H16A | 0.3441 | 0.6018 | 0.3441 | 0.112* |
| H16B | 0.2387 | 0.5924 | 0.4296 | 0.112* |
| H16C | 0.3786 | 0.5564 | 0.4427 | 0.112* |
| C17 | 0.2574(3) | 1.0520(4) | 0.2536(2) | 0.0527(9) |
| H17A | 0.2503 | 1.1146 | 0.2893 | 0.063* |
| H17B | 0.1891 | 1.0101 | 0.2784 | 0.063* |
| C18 | 0.2510(4) | 1.1109(4) | 0.1596(2) | 0.0668(12) |
| H18A | 0.3192 | 1.1517 | 0.1352 | 0.100* |
| H18B | 0.1738 | 1.1716 | 0.1559 | 0.100* |
| H18C | 0.2559 | 1.0489 | 0.1249 | 0.100* |
| Cl1A | 1.32032(7) | 0.72983(7) | 0.15285(6) | 0.0423(2) |
| O1A | 1.12185(18) | 0.50762(19) | 0.28351(13) | 0.0371(5) |
| O2A | 1.05677(18) | 0.48866(17) | 0.08935(13) | 0.0290(4) |
| H1A | 1.105(3) | 0.478(3) | 0.043(3) | 0.056(11)* |
| O3A | 0.78414(19) | 0.5752(2) | 0.06055(16) | 0.0533(7) |
| O4Aa | 0.7399(3) | 0.7731(2) | 0.1049(3) | 0.0454(11) |
| O4Bb | 0.6684(3) | 0.7317(3) | 0.1629(2) | 0.0370(15) |
| P1A | 0.78278(7) | 0.62847(7) | 0.13530(5) | 0.0347(2) |
| C1A | 1.3059(2) | 0.4317(2) | 0.19283(18) | 0.0295(6) |
| C2A | 1.3536(3) | 0.3493(3) | 0.2649(2) | 0.0397(7) |
| H2A | 1.3099 | 0.3500 | 0.3240 | 0.048* |
| C3A | 1.4640(3) | 0.2666(3) | 0.2510(2) | 0.0489(9) |
| H3A | 1.4964 | 0.2113 | 0.3005 | 0.059* |
| C4A | 1.5268(3) | 0.2646(3) | 0.1651(3) | 0.0502(9) |
| H4A | 1.6028 | 0.2081 | 0.1557 | 0.060* |
| C5A | 1.4802(3) | 0.3442(3) | 0.0926(2) | 0.0440(8) |
| H5A | 1.5231 | 0.3411 | 0.0336 | 0.053* |
| O5Aa | 0.6929(3) | 0.5995(3) | 0.22647(18) | 0.0511(12) |
| O5Bb | 0.7742(4) | 0.5107(4) | 0.2121(3) | 0.060(2) |
| C6A | 1.3702(3) | 0.4287(3) | 0.1062(2) | 0.0349(7) |
| H6A | 1.3389 | 0.4845 | 0.0565 | 0.042* |
| C7A | 1.1878(2) | 0.5194(2) | 0.21040(18) | 0.0277(6) |
| C8A | 1.1467(2) | 0.6242(2) | 0.13589(17) | 0.0245(6) |
| C9A | 1.1967(2) | 0.7207(3) | 0.10722(19) | 0.0294(6) |
| C10A | 1.1584(3) | 0.8301(3) | 0.0367(2) | 0.0373(7) |
| H10C | 1.2228 | 0.8305 | −0.0169 | 0.045* |
| H10D | 1.1489 | 0.9056 | 0.0595 | 0.045* |
| C11A | 1.0387(3) | 0.8293(3) | 0.0105(2) | 0.0379(7) |
| H11C | 1.0300 | 0.8839 | −0.0485 | 0.045* |
| H11D | 0.9697 | 0.8610 | 0.0551 | 0.045* |
| C12A | 1.0341(3) | 0.6995(2) | 0.00547(18) | 0.0292(6) |
| H12C | 0.9579 | 0.7023 | −0.0154 | 0.035* |
| H12D | 1.1037 | 0.6676 | −0.0387 | 0.035* |
| C13A | 1.0395(2) | 0.6133(2) | 0.09632(17) | 0.0250(6) |
| C14A | 0.9216(2) | 0.6385(3) | 0.16439(19) | 0.0336(7) |
| H14C | 0.9104 | 0.7218 | 0.1759 | 0.040* |
| H14D | 0.9338 | 0.5802 | 0.2214 | 0.040* |
| C15Aa | 0.6118(3) | 0.8303(3) | 0.0940(2) | 0.0491(9) |
| H15Ca | 0.5566 | 0.8097 | 0.1509 | 0.059* |
| H15Da | 0.5894 | 0.8000 | 0.0469 | 0.059* |
| C15Bb | 0.6118(3) | 0.8303(3) | 0.0940(2) | 0.0491(9) |
| H15Eb | 0.5257 | 0.8642 | 0.1179 | 0.059* |
| H15Fb | 0.6135 | 0.7976 | 0.0405 | 0.059* |
| C16Aa | 0.6003(5) | 0.9663(3) | 0.0682(4) | 0.0441(14) |
| H16Da | 0.5158 | 1.0068 | 0.0611 | 0.066* |
| H16Ea | 0.6545 | 0.9858 | 0.0116 | 0.066* |
| H16Fa | 0.6230 | 0.9952 | 0.1152 | 0.066* |
| C16Bb | 0.6830(8) | 0.9282(7) | 0.0697(5) | 0.059(3) |
| H16Gb | 0.6475 | 0.9946 | 0.0240 | 0.088* |
| H16Hb | 0.7679 | 0.8938 | 0.0461 | 0.088* |
| H16Ib | 0.6802 | 0.9604 | 0.1231 | 0.088* |
| C17Aa | 0.6375(10) | 0.4924(10) | 0.2437(3) | 0.082(2) |
| H17Ca | 0.6844 | 0.4347 | 0.2041 | 0.098* |
| H17Da | 0.5526 | 0.5191 | 0.2315 | 0.098* |
| C17Bb | 0.6575(13) | 0.4704(19) | 0.2400(3) | 0.082(2) |
| H17Eb | 0.6604 | 0.4014 | 0.2115 | 0.098* |
| H17Fb | 0.5894 | 0.5386 | 0.2216 | 0.098* |
| C18Aa | 0.6389(4) | 0.4309(4) | 0.3399(2) | 0.0753(13) |
| H18Da | 0.5982 | 0.3623 | 0.3538 | 0.113* |
| H18Ea | 0.5960 | 0.4899 | 0.3785 | 0.113* |
| H18Fa | 0.7235 | 0.4002 | 0.3504 | 0.113* |
| C18Bb | 0.6389(4) | 0.4309(4) | 0.3399(2) | 0.0753(13) |
| H18Gb | 0.5972 | 0.3628 | 0.3574 | 0.113* |
| H18Hb | 0.5892 | 0.4997 | 0.3674 | 0.113* |
| H18Ib | 0.7182 | 0.4043 | 0.3603 | 0.113* |
aOccupancy: 0.604(4); bOccupancy: 0.396(4).
Source of material
To a stirred solution of n-butyl lithium (1.6 M solution in hexane; 5.4 mL, 8.7 mmol) in THF (30 mL) at −65 °C was added dropwise with stirring a solution of diethyl methylphosphonate (1.22 g, 8.0 mmol) in THF (1 mL). The mixture was stirred at −65 °C for 30 min, and a solution of 2-benzoyl-3-chlorocyclohex-2-enone (1.89 g, 8.0 mmol) in THF (5 mL) was added. After 15 min at −65 °C, the mixture was allowed to warm up to room temperature and was then stirred for 1 h at this temperature. The mixture was quenched with saturated aqueous ammonium chloride and extracted with chloroform (3 × 30 mL). The chloroform extract was washed with water (2 × 20 mL), dried (MgS04), and filtered, and the solvent was removed under reduced pressure. The residue was purified by column chromatography to afford the title compound as a colorless solid (1.71 g, 55%), 88–90 °C (1:3 EtOAc-Pet. ether, v/v). Infrared spectrum: υmax: (ATR) 1265 (P = O), 1580 (C = C), 1658 (C = O) and 3267 (OH); 1H-NMR (300 MHz, CDCl3) 1.29 (3H, t, J 7.2 Hz, 1 × CH3 of POEt), 1.31 (3H, t, J 7.2 Hz, 1 × CH3 of POEt), 1.78 (1H, m, 1 × H of CH2), 2.01–2.18 (2H, m, 1 × H of 2 × CH2), 2.21 (1H, d, J 15.9 Hz, 1 × H of CH2P), 2.27 (1H, d, J 15.9 Hz, 1 × H of CH2P), 2.36–2.64 (3H, m, 1 × H of CH2 and CH2), 4.06 (4H, m, 2 × CH2 of POEt), 4.54 (1H, br s, OH), 7.45 (2H, dt, J 1.5 and 7.1 Hz, 3′-H and 5′-H), 7.56 (1H, tt, J 1.2 and 7.2 Hz, 4′-H) and 7.99 (2H, dd, J 1.2 and 8.4 Hz, 2′-H and 6′-H); 13C-NMR (75.0 MHz, CDCl3) 16.3 (dd, JCP 2.7 and 6.2Hz, CH3 of POEt), 19.7 (s, CH2), 33.0 (s, CH2), 35.5 (s, CH2), 34.7 (d, JCP 135.3 Hz, CH2P), 35.6 (s, CH2), 61.5 (d, JCP 7.0 Hz, 1 × CH2 of POEt), 62.3 (d, JCP 6.1 Hz, 1 × CH2 of POEt), 72.0 (d, JCP 2.7 Hz, C-1), 128.5 (s, C-3’ and C-5’), 129.8 (s, C-2’ and C-6’), 133.5 (s, C-4′), 133.6 (d, JCP 3.1 Hz, C-3), 136.7 (s, C-1′), 138.5 (d, JCP 16.7 Hz, C-2) and 196.7 (s, C = O); 31P-NMR (CDCl3) 29.40; HRMS (EI): found: 386.1049. C18H24O5ClP requires’ 386.1050. Crystals were obtained by recrystallization from 3:2 ethyl acetate-petroleum ether mixture.
Experimental details
Hydrogen atoms were located from the difference map then positioned geometrically and allowed to ride on their respective parent atoms. Hydrogen atoms involved in hydrogen bonding were located from the difference map and refined freely. Two ethoxy groups made up of atoms O4A, C15A and C16A and O5A, C17A and C18A were found to be disordered. These were refined over two positions with the final occupancies over the two positions being 0.604(4) and 0.396(4), respectively.
Discussion
Owing to the importance of cycloalkenones as chemical units in a large number of naturally occurring substances [5], development of new synthetic methods leading to substituted derivatives is currently one focal point in synthetic organic chemistry [6].
Lithiated diethyl alkylphosphonates react with 3-chlorocyclohex-2-en-1-ones substituted with a benzoyl group at the 2-position to afford the 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol. This compound could be purified by column chromatography and its melting point determined without any signs of decomposition. The related adduct of trimethylsilylmagnesium bromide, on the other hand, previously fragmented spontaneously in situ to yield a diene [7], [8], [9].
The crystal structure contains two molecules in the asymmetric unit − one ordered and the other disordered around the ethoxy groups (cf. the figure). O-H⋯O = P intermolecular hydrogen bonding occurs between adjacent molecules forming a hydrogen bonded pair of centrosymetrically related molecules for both crystallographically independent molecules (graph set descriptor: R22(12)). Ordered molecules form hydrogen bonds exclusively to the other ordered molecules whereas disordered molecules form hydrogen bonds to the other disordered molecules.
Bond lengths and angles are all in the expected ranges.
Acknowledgement
The authors are grateful to the University of South Africa for financial assistance and M.A. Fernandes of Wits University for X-ray data.
References
1 Bruker SAINT+. Version 6.02 (includes XPREP and SADABS), Bruker AXS Inc., Madison, Wisconsin, USA, (1999).Suche in Google Scholar
2 Sheldrick, G. M.: SADABS. University of Göttingen, Germany, (1996).Suche in Google Scholar
3 Bruker. SHELXTL. Version 5.1. (includes XS, XL, XP, XSHELL) Bruker AXS Inc., Madison, Wisconsin, USA, (1999).Suche in Google Scholar
4 Spek, A.: Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 36 (2003) 7–13.10.1107/S0021889802022112Suche in Google Scholar
5 Kim, J. H.; Lim, H. J.; Cheon, S. H.: A facile synthesis of (6S,1′S)-(+)-hernandulcin and (6S,1′R)-(+)-epihernandulcin. Tetrahedron 59 (2003) 7501–7507.10.1002/chin.200403179Suche in Google Scholar
6 Mphahlele, M. J.; Modro T. A.: Reaction of phosphonate-stabilized carbanions with cyclic enones bearing a β-leaving group. J. Org. Chem. 60 (1995) 8236–8240.10.1021/jo00130a024Suche in Google Scholar
7 Modro, A. M.; Modro, T. A.; Mphahlele, M. J.; Perlikowska, W.; Pienaar, A.; Sales, M.; van Rooyen, P. H.: Reaction of carbanions generated from arylmethylphosphonates with cyclic enones. regio- and stereoselectivity of addition. Can. J. Chem. 76 (1998) 1344–1352.10.1139/v98-180Suche in Google Scholar
8 Mphahlele, M. J.; Pienaar, A.; Modro, T. A.: Reactions of phosphorus-stabilized carbanions with cyclic enones. aromatization of the substitution and addition products. Perkin Trans. (1996) 1455–1460.10.1039/p29960001455Suche in Google Scholar
9 Tamura, Y.; Wada, A.; Sasho, M.; Kita, Y.: Reactions of 2-benzoyl-3-chloro-2-cyclohexen-1-ones with some organometallic compounds. Chem. Pharm. Bull. 31 (1983) 52–56.10.1248/cpb.31.52Suche in Google Scholar
©2017 Marole M. Maluleka et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16

