Abstract
C67H47N7O3Zn, monoclinic, P21/c (no. 14), a = 11.8802(4) Å, b = 20.2309(6) Å, c = 22.3328(6) Å, β = 101.649(3)°, V = 5257.1(3) Å3, Z = 4, Rgt(F) = 0.0609, wRref(F2) = 0.1588, T = 298 K.
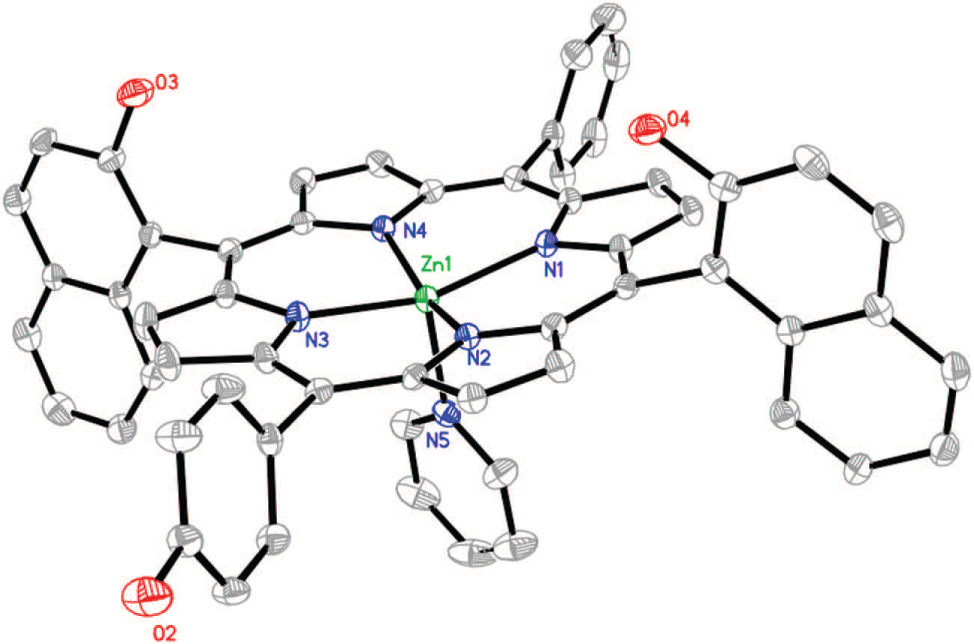
The asymmetric unit of the title crystal structure is shown in the figure. Hydrogen atoms are omitted for clarity. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Red block |
| Size: | 0.08 × 0.06 × 0.04 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 5.3 cm−1 |
| Diffractometer, scan mode: | Bruker SMART, φ and ω |
| 2θmax, completeness: | 50°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 18274, 9239, 0.047 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5932 |
| N(param)refined: | 706 |
| Programs: | SHELX [7], Bruker programs [8] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Zn1 | 0.28970(4) | 0.20869(2) | 0.34416(2) | 0.02434(16) |
| N1 | 0.1432(3) | 0.25980(15) | 0.35462(15) | 0.0235(8) |
| N2 | 0.3819(3) | 0.26131(15) | 0.41738(14) | 0.0225(7) |
| N3 | 0.4223(3) | 0.13985(15) | 0.35772(15) | 0.0269(8) |
| N4 | 0.1837(3) | 0.13898(15) | 0.29387(15) | 0.0239(8) |
| N5 | 0.3264(3) | 0.26243(17) | 0.26753(16) | 0.0294(8) |
| N6 | 0.0791(4) | 0.3849(2) | 0.1554(2) | 0.0618(13) |
| O2 | 1.0135(3) | 0.21671(17) | 0.58032(17) | 0.0552(10) |
| H2 | 1.0291 | 0.1848 | 0.6032 | 0.083* |
| O3 | 0.2654(3) | −0.07399(15) | 0.33739(17) | 0.0538(10) |
| H3 | 0.2651 | −0.0367 | 0.3517 | 0.081* |
| O4 | 0.1661(3) | 0.29550(14) | 0.54036(16) | 0.0426(8) |
| H4 | 0.1180 | 0.2852 | 0.5604 | 0.064* |
| C1 | 0.1372(3) | 0.31266(19) | 0.39204(18) | 0.0248(9) |
| C2 | 0.0229(4) | 0.3386(2) | 0.38058(19) | 0.0297(10) |
| H2A | −0.0024 | 0.3750 | 0.3997 | 0.036* |
| C3 | −0.0409(4) | 0.3010(2) | 0.33738(19) | 0.0301(10) |
| H3A | −0.1186 | 0.3066 | 0.3205 | 0.036* |
| C4 | 0.0335(3) | 0.25002(19) | 0.32191(18) | 0.0250(9) |
| C5 | −0.0015(3) | 0.1983(2) | 0.28026(18) | 0.0264(10) |
| C6 | −0.1268(4) | 0.1966(2) | 0.2491(2) | 0.0303(10) |
| C7 | −0.1597(4) | 0.2136(2) | 0.1877(2) | 0.0371(11) |
| H7 | −0.1041 | 0.2257 | 0.1658 | 0.045* |
| C8 | −0.2742(4) | 0.2127(2) | 0.1586(2) | 0.0466(14) |
| H8 | −0.2950 | 0.2247 | 0.1176 | 0.056* |
| C9 | −0.3582(5) | 0.1937(2) | 0.1905(3) | 0.0566(17) |
| H9 | −0.4351 | 0.1928 | 0.1710 | 0.068* |
| C10 | −0.3266(4) | 0.1765(3) | 0.2510(3) | 0.0591(16) |
| H10 | −0.3824 | 0.1636 | 0.2724 | 0.071* |
| C11 | −0.2113(4) | 0.1781(2) | 0.2807(2) | 0.0450(13) |
| H11 | −0.1910 | 0.1666 | 0.3218 | 0.054* |
| C12 | 0.0693(3) | 0.14677(19) | 0.26828(18) | 0.0259(10) |
| C13 | 0.0313(4) | 0.0912(2) | 0.22868(19) | 0.0300(10) |
| H13 | −0.0420 | 0.0851 | 0.2052 | 0.036* |
| C14 | 0.1206(4) | 0.0500(2) | 0.2319(2) | 0.0310(10) |
| H14 | 0.1207 | 0.0098 | 0.2117 | 0.037* |
| C15 | 0.2170(3) | 0.08000(19) | 0.27296(18) | 0.0254(9) |
| C16 | 0.3259(4) | 0.0517(2) | 0.28916(19) | 0.0281(10) |
| C17 | 0.3415(3) | −0.0145(2) | 0.2608(2) | 0.0291(10) |
| C18 | 0.3837(3) | −0.0183(2) | 0.20582(19) | 0.0269(10) |
| C19 | 0.4279(4) | 0.0381(2) | 0.1795(2) | 0.0338(11) |
| H19 | 0.4281 | 0.0791 | 0.1984 | 0.041* |
| C20 | 0.4693(4) | 0.0327(2) | 0.1277(2) | 0.0415(12) |
| H20 | 0.4985 | 0.0700 | 0.1120 | 0.050* |
| C21 | 0.4694(4) | −0.0275(3) | 0.0972(2) | 0.0503(14) |
| H21 | 0.4979 | −0.0304 | 0.0615 | 0.060* |
| C22 | 0.4267(4) | −0.0822(2) | 0.1208(2) | 0.0407(12) |
| H22 | 0.4248 | −0.1222 | 0.1001 | 0.049* |
| C23 | 0.3855(4) | −0.0796(2) | 0.17555(19) | 0.0281(10) |
| C24 | 0.3470(4) | −0.1368(2) | 0.2019(2) | 0.0379(12) |
| H24 | 0.3467 | −0.1773 | 0.1823 | 0.045* |
| C25 | 0.3102(4) | −0.1331(2) | 0.2562(2) | 0.0395(12) |
| H25 | 0.2873 | −0.1711 | 0.2739 | 0.047* |
| C26 | 0.3072(4) | −0.0716(2) | 0.2849(2) | 0.0332(11) |
| C27 | 0.4211(4) | 0.07902(19) | 0.3296(2) | 0.0290(10) |
| C28 | 0.5308(4) | 0.0479(2) | 0.3480(2) | 0.0396(12) |
| H28 | 0.5523 | 0.0070 | 0.3349 | 0.048* |
| C29 | 0.5981(4) | 0.0894(2) | 0.3882(2) | 0.0409(12) |
| H29 | 0.6738 | 0.0820 | 0.4080 | 0.049* |
| C30 | 0.5291(3) | 0.1469(2) | 0.3942(2) | 0.0292(10) |
| C31 | 0.5654(3) | 0.20023(19) | 0.43381(18) | 0.0250(9) |
| C32 | 0.6867(3) | 0.19960(19) | 0.47056(19) | 0.0275(10) |
| C33 | 0.7173(4) | 0.1629(2) | 0.5234(2) | 0.0427(12) |
| H33 | 0.6629 | 0.1354 | 0.5353 | 0.051* |
| C34 | 0.8270(4) | 0.1659(2) | 0.5594(2) | 0.0460(13) |
| H34 | 0.8460 | 0.1398 | 0.5942 | 0.055* |
| C35 | 0.9078(4) | 0.2076(2) | 0.5434(2) | 0.0343(11) |
| C36 | 0.8798(4) | 0.2436(2) | 0.4901(2) | 0.0348(11) |
| H36 | 0.9349 | 0.2702 | 0.4778 | 0.042* |
| C37 | 0.7699(4) | 0.2402(2) | 0.4545(2) | 0.0339(11) |
| H37 | 0.7516 | 0.2657 | 0.4192 | 0.041* |
| C38 | 0.4943(3) | 0.25169(18) | 0.44582(18) | 0.0241(9) |
| C39 | 0.5268(3) | 0.29985(19) | 0.49340(19) | 0.0281(10) |
| H39 | 0.5990 | 0.3045 | 0.5185 | 0.034* |
| C40 | 0.4335(3) | 0.3369(2) | 0.49504(19) | 0.0295(10) |
| H40 | 0.4288 | 0.3717 | 0.5216 | 0.035* |
| C41 | 0.3422(3) | 0.31235(19) | 0.44768(19) | 0.0255(10) |
| C42 | 0.2296(3) | 0.33701(19) | 0.43668(18) | 0.0252(9) |
| C43 | 0.1996(3) | 0.3860(2) | 0.48109(19) | 0.0274(10) |
| C44 | 0.1642(4) | 0.3630(2) | 0.53182(19) | 0.0307(10) |
| C45 | 0.1311(4) | 0.4060(2) | 0.5746(2) | 0.0391(12) |
| H45 | 0.1082 | 0.3888 | 0.6089 | 0.047* |
| C46 | 0.1325(4) | 0.4725(2) | 0.5660(2) | 0.0414(12) |
| H46 | 0.1102 | 0.5005 | 0.5945 | 0.050* |
| C47 | 0.1679(4) | 0.4997(2) | 0.5139(2) | 0.0312(10) |
| C48 | 0.1675(4) | 0.5682(2) | 0.5031(2) | 0.0398(12) |
| H48 | 0.1453 | 0.5971 | 0.5310 | 0.048* |
| C49 | 0.1992(4) | 0.5928(2) | 0.4523(2) | 0.0402(12) |
| H49 | 0.1966 | 0.6381 | 0.4451 | 0.048* |
| C51 | 0.2357(4) | 0.4837(2) | 0.4190(2) | 0.0341(11) |
| H51 | 0.2581 | 0.4560 | 0.3903 | 0.041* |
| C52 | 0.2016(3) | 0.45571(19) | 0.47124(19) | 0.0267(10) |
| C53 | 0.1163(6) | 0.4340(4) | 0.2524(3) | 0.079(2) |
| H53 | 0.1084 | 0.4325 | 0.2930 | 0.095* |
| C54 | 0.0724(4) | 0.3859(3) | 0.2157(2) | 0.0471(13) |
| H54 | 0.0355 | 0.3513 | 0.2312 | 0.056* |
| C56 | 0.1277(5) | 0.4384(3) | 0.1319(3) | 0.0619(16) |
| H56 | 0.1290 | 0.4408 | 0.0905 | 0.074* |
| C57 | 0.1743(6) | 0.4884(3) | 0.1712(5) | 0.097(3) |
| H57 | 0.2067 | 0.5243 | 0.1549 | 0.117* |
| C58 | 0.1759(6) | 0.4886(3) | 0.2324(4) | 0.078(2) |
| H58 | 0.2125 | 0.5211 | 0.2587 | 0.094* |
| C59 | 0.1326(5) | 0.0551(3) | 0.4127(2) | 0.108(3) |
| H59 | 0.0712 | 0.0363 | 0.3855 | 0.130* |
| C60 | 0.1164(6) | 0.1126(3) | 0.4439(3) | 0.152(4) |
| H60 | 0.0443 | 0.1324 | 0.4376 | 0.182* |
| C61 | 0.2081(8) | 0.1407(2) | 0.4845(3) | 0.098(3) |
| H61 | 0.1972 | 0.1791 | 0.5054 | 0.118* |
| C62 | 0.3159(7) | 0.1112(3) | 0.4939(2) | 0.152(5) |
| H62 | 0.3772 | 0.1299 | 0.5211 | 0.183* |
| C63 | 0.3320(5) | 0.0536(3) | 0.4627(3) | 0.132(4) |
| H63 | 0.4042 | 0.0339 | 0.4690 | 0.158* |
| N7 | 0.2404(6) | 0.0256(2) | 0.4221(2) | 0.103(2) |
| C65 | 0.2358(4) | 0.5501(2) | 0.4109(2) | 0.0369(11) |
| H65 | 0.2606 | 0.5675 | 0.3772 | 0.044* |
| C67 | 0.3138(4) | 0.2350(3) | 0.2118(2) | 0.0418(12) |
| H67 | 0.2884 | 0.1915 | 0.2067 | 0.050* |
| C68 | 0.3369(5) | 0.2686(3) | 0.1615(2) | 0.0585(15) |
| H68 | 0.3287 | 0.2480 | 0.1236 | 0.070* |
| C69 | 0.3725(5) | 0.3339(3) | 0.1693(3) | 0.0644(18) |
| H69 | 0.3878 | 0.3580 | 0.1364 | 0.077* |
| C70 | 0.3848(5) | 0.3624(3) | 0.2253(3) | 0.0574(16) |
| H70 | 0.4093 | 0.4059 | 0.2314 | 0.069* |
| C71 | 0.3602(4) | 0.3254(2) | 0.2729(2) | 0.0425(12) |
| H71 | 0.3676 | 0.3456 | 0.3109 | 0.051* |
Source of material
Dichloromethane was freshly distilled from CaH2 under a dry nitrogen atmosphere. Dipyrromethane [1], 2-methoxy-1-naphthylboronic acid [2] and n-hexadecyloxyl benzaldehyde [3] were prepared according to the published procedures. All other reagents and solvents were used as received. The complex ZnDNP was prepared according to the published procedures [4]. Suitable crystals of the title compound were obtained by slow diffusion of hexane into a solution of 1 in CHCl3/pyridine (100:1, v/v). The target compound ZnDNP was characterized by mass spectrometry, NMR spectroscopy, and electronic absorption spectroscopies in addition to elemental analysis. Crystal of ZnDNP was grown by liquid diffusion of hexane into a solution of ZnDNP in CHCl3/pyridine (100:1 v/v). 1H-NMR (CDCl3, 400 MHz, 293 K): δ 8.865 (m, 2H), 8.809 (m, 2H), 8.675 (m, 4H), 8.235 (d, 2H, J = 4.4 Hz), 8.205 (d, 2H, J = 4.4 Hz), 8.163 (d, 2H, J = 4.8 Hz), 8.043 (d, 2H, J = 4.8 Hz), 7.995 (M, 2H), 7.685 (m, 3H), 7.616 (d, 2H, J = 4.8 Hz), 7.306 (m, 2H), 7.107 (m, 2H), 6.972 (m, 2H), 6.785 (d, 2H, J = 8.0 Hz); 13C-NMR (CDCl3, 100 MHz, 293 K): δ 154.48, 150.84, 138.07, 135.64, 132.72, 131.20, 131.10, 130.41, 128.39, 127.64, 126.44, 123.03, 122.00, 117.55, 113.41, 110.93; UV-vis (CHCl3): λmax (log ϵ) 423 (5.62), 547 (4.43), 580 (3.80). MALDI-TOF-MS m/z calcd. for C52H34N4O3Zn: (M+) 826.2; Found: 826.5. Anal. Calcd. for C52H34N4O3Zn: C, 75.29; H, 3.50; N, 6.58. Found: C, 75.14; H, 3.45; N, 6.33.
Experimental details
The hydroxy H atoms were located in difference Fourier maps and their positions were refined with O—H bond-length restraints of 0.84(1) Å and with Uiso(H) = 1.2Ueq(O). The remaining H atoms were positioned in geometrically idealized positions and constrained to ride on their parent atoms, with C—H = 0.93 (aryl), and with Uiso(H) = 1.5Ueq(C).
Discussion
In the title structure the Zn center is five-coordinated by four pyrrole nitrogen atoms of porphyrin and one pyridine nitrogen atom, forming slightly distorted rectangular pyramid coordination geometry. The bond distance of Zn—N(pyridine) (2.145(3) Å) is longer than the mean Zn–N(porphyrin) bond distance (2.073(3) Å), both are similar to the corresponding reported values for other pyridine–Zn–porphyrin systems [5]. Due to the axial coordination of pyridine ligand, the Zn center deviates from the N4 mean plane (0.34 Å) and points to the axial pyridine ligand. As expected, the two naphthyl rings are almost perpendicular to the plane defined by the four nitrogen atoms of the porphyrin with a dihedral angle of 89.61° and 79.54°. This indicates a highly restricted rotational freedom of the 2-hydroxynaphthyl group due to the large steric hindrance effects. Notably, another pyridine molecule also binds to the porphyrin host via hydrogen bonding of N(pyridine)–HO(2-hydroxynaphthyl). Furthermore, a one-dimensional (1D) supramolecular double-chain structure can be observed in the crystal of the title compound ZnDNP, the linkages include: 1) hydrogen bondings of O(2-hydroxynaphthyl)⋯HO(4-hydroxyphenyl) and N(pyridine)⋯HO(2-hydroxylnaphthyl), with distances of 1.970(6) and 2.080(5) Å respectively. Here, the hydroxyl of naphthyl are both, hydrogen bonding donor and acceptor. 2) π-π stacking interactions of pyridine-porphyrin and pyridine-pyridine, with inter-ring distances of 3.249 and 3.359 Å, respectively. The above observations imply that the porphyrin ZnDNP can be a receptor with multiple binding sites and multiform intermolecular interactions [6].
Acknowledgement
This work was supported by the Key Scientific Research Projects of Colleges and Universities, Henan Province (Nos. 16A150001, 16A430012).
References
1 Laha, J. K.; Dhanalekshmi, S.; Taniguchi, M.; Ambroise, A.; Lindsey, J. S.: A scalable synthesis of meso-substituted dipyrromethanes. Org. Process Res. Dev. 7 (2003) 799–812.10.1021/op034083qSearch in Google Scholar
2 Nakanishi, T.; Miyashita, N.; Michinobu, T.; Wakayama, Y.; Tsuruoka, T.; Ariga, K.; Kurth, D. G.: Perfectly straight nanowires of fullerenes bearing long alkyl chains on graphite. J. Am. Chem. Soc. 128 (2006) 6328–6329.10.1021/ja061450fSearch in Google Scholar PubMed
3 Markov, A. V.; Ramírez-López, P.; Biedermannová, L.; Rulísek, L.; Dufková, L.; Kotora, M.; Zhu, F. J.; Kocovský, P.: On the mechanism of asymmetric allylation of aldehydes with allyltrichlorosilanes catalyzed by QUINOX, a chiral isoquinoline N-oxide. J. Am. Chem. Soc. 130 (2008) 5341–5348.10.1021/ja711338qSearch in Google Scholar PubMed
4 Yang, L. G.; Zhou, Y.; Zhu, M. L.; Zhao, L. Y.; Wei, L. Y.; Bian, Y. Z.: Stereochemistry and solid-state structure of an intrinsically chiral meso-patterned porphyrin: case study by NMR and single-crystal X-ray diffraction analysis. J. Org. Chem. 78 (2013) 9949–9955.10.1021/jo401825kSearch in Google Scholar PubMed
5 Senge, M. O.: Database of tetrapyrrole crystal structure determination, In The Porphyrin Handbook, Kadish, K. M.; Smith, K. M.; Guilard, R. Eds.; Academic Press: San Diego, CA, 2000; Vol. 10, p. 8.Search in Google Scholar
6 Wu, X.; Starnes, S. D.: L-nipecotic acid-porphyrin derivative: a chiral host with introverted functionality for chiral recognition. Org. Lett. 14 (2012) 3652–3655.10.1021/ol301499wSearch in Google Scholar PubMed
7 Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
8 Brucker. APEX2, SAINT and SADABS. Brucker AXS Inc., Madison, Wisconsin, USA, (2009).Search in Google Scholar
©2017 Yang Liguo et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16


