Abstract
C36H28Co1N6O4, triclinic, P1̄ (no. 2), a = 6.3166(4) Å, b = 8.5454(5) Å, c = 14.8075(9) Å, α = 105.157(2)°, β = 94.494(3)°, γ = 101.890(2)°, V = 747.5(2) Å3, Z = 1, Rgt(F) = 0.0332, wRref(F2) = 0.0790, T = 100(2) K.
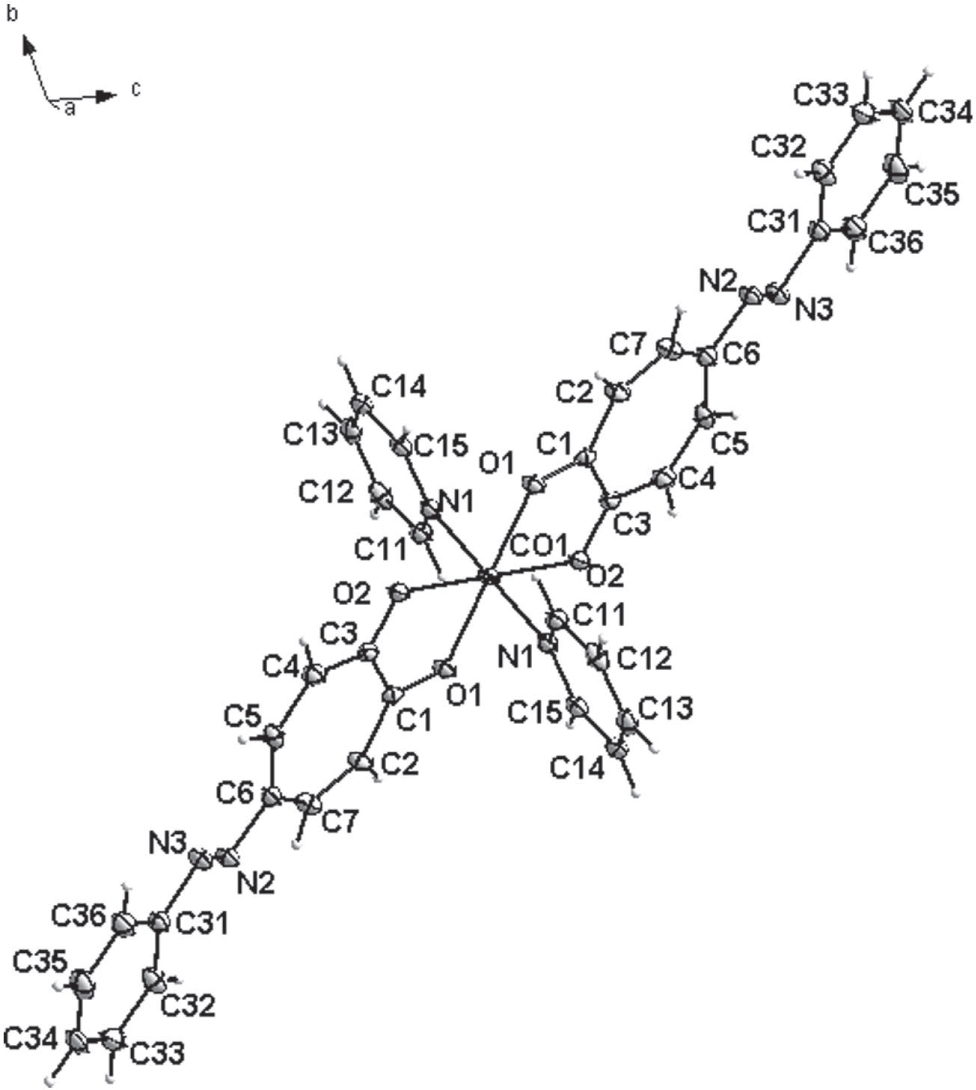
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Red columnar |
| Size: | 0.34 × 0.10 × 0.08 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 6.3 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| 2θmax, completeness: | 56.8°, >98% |
| N(hkl)measured, N(hkl)unique, Rint: | 10536, 3714, 0.0225 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3222 |
| N(param)refined: | 214 |
| Programs: | Bruker programs [11], SIR92 [12], SHELX [13, 14] , |
| WinGX [15], publCIF [16], PLATON [17] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.4184(2) | 0.24143(18) | 0.32614(10) | 0.0139(3) |
| C2 | 0.4405(3) | 0.10253(19) | 0.25390(11) | 0.0172(3) |
| H2 | 0.5755 | 0.0761 | 0.2603 | 0.021* |
| C3 | 0.2300(3) | 0.32071(18) | 0.32599(10) | 0.0148(3) |
| C4 | 0.0381(3) | 0.25923(19) | 0.25863(11) | 0.0186(3) |
| H4 | −0.0628 | 0.3252 | 0.2683 | 0.022* |
| C5 | −0.0268(3) | 0.12028(19) | 0.18166(11) | 0.0185(3) |
| H5 | −0.1658 | 0.1051 | 0.1500 | 0.022* |
| C6 | 0.0851(3) | −0.00141(19) | 0.14405(10) | 0.0170(3) |
| C7 | 0.2986(3) | −0.00094(19) | 0.17572(11) | 0.0185(3) |
| H7 | 0.3546 | −0.0838 | 0.1381 | 0.022* |
| C11 | 0.1193(3) | 0.34610(19) | 0.58620(11) | 0.0178(3) |
| H11 | 0.0795 | 0.4434 | 0.5826 | 0.021* |
| C12 | −0.0129(3) | 0.2394(2) | 0.62583(11) | 0.0209(3) |
| H12 | −0.1374 | 0.2657 | 0.6493 | 0.025* |
| C13 | 0.0431(3) | 0.0934(2) | 0.62988(11) | 0.0226(4) |
| H13 | −0.0428 | 0.0198 | 0.6563 | 0.027* |
| C14 | 0.2296(3) | 0.05830(19) | 0.59385(11) | 0.0212(3) |
| H14 | 0.2698 | −0.0401 | 0.5946 | 0.025* |
| C15 | 0.3546(3) | 0.17273(19) | 0.55673(10) | 0.0170(3) |
| H15 | 0.4807 | 0.1497 | 0.5334 | 0.020* |
| C31 | −0.3063(3) | −0.30783(19) | −0.03555(11) | 0.0187(3) |
| C32 | −0.1900(3) | −0.4247(2) | −0.07566(12) | 0.0228(3) |
| H32 | −0.0437 | −0.4099 | −0.0528 | 0.027* |
| C33 | −0.2936(3) | −0.5624(2) | −0.14954(12) | 0.0254(4) |
| H33 | −0.2167 | −0.6405 | −0.1763 | 0.030* |
| C34 | −0.5119(3) | −0.5849(2) | −0.18410(11) | 0.0257(4) |
| H34 | −0.5802 | −0.6775 | −0.2342 | 0.031* |
| C35 | −0.6273(3) | −0.4706(2) | −0.14437(12) | 0.0268(4) |
| H35 | −0.7735 | −0.4861 | −0.1675 | 0.032* |
| C36 | −0.5247(3) | −0.3312(2) | −0.06933(12) | 0.0236(4) |
| H36 | −0.6029 | −0.2543 | −0.0421 | 0.028* |
| N1 | 0.3022(2) | 0.31525(15) | 0.55284(9) | 0.0155(3) |
| N2 | −0.0128(2) | −0.14182(16) | 0.06543(9) | 0.0187(3) |
| N3 | −0.2147(2) | −0.16271(16) | 0.04200(9) | 0.0195(3) |
| O1 | 0.57008(17) | 0.30884(13) | 0.39636(7) | 0.0158(2) |
| O2 | 0.24615(18) | 0.45279(13) | 0.39276(7) | 0.0169(2) |
| Co1 | 0.5000 | 0.5000 | 0.5000 | 0.01385(9) |
Source of material
The reagents available commercially were used without further purification. 5-(Phenyldiazenyl)tropolone was synthesized according to the previously published method [3]. 5-(Phenyldiazenyl)tropolone (452 mg, 2.0 mmol) and pyridine (162 μL, 2.0 mmol) were added to a solution of Co(CH3COO)2⋅4H2O (249 mg, 1.0 mmol) in EtOH:H2O (40 ml, 1:1). The solution was refluxed (110 °C) for 7 days, filtered and allowed to crystallize. Crystals were obtained from the slow evaporation of the solution at room temperature (81% yield) within 2 weeks.
Experimental details
Methyl H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms, with C—H = 0.95 Å and Uiso(H) = 1.2 Ueq(C). The highest (0.39e Å−3) and lowest (−0.30e Å−3) residual electron density peaks were located respectively at 0.69 Å from the C1 and 0.58 Å from the Co1 atoms.
Discussion
Past investigations have highlighted the fact that the tropolone-type ligands (TropH) are very effective chelating agents and have a wide range of chemical applications [1], [2], [3], [4]. The complexation of these ligands to transition metal centres is however not a novel notion, with the tropolonato anion forming a five-membered chelating ring resulting in a smaller bite angle than that of the β-diketonato analogues [1, 5] . These ligands are ideally suited to the formation of structures with higher coordination numbers, which has been ascribed to the planarity and compactness of the ligand [6].
Tropolonato compounds have been noted to have useful chemical properties that can be exploited for a wide variety industrial purposes. Examples include the use of Rh(I) tropolonates in the asymmetric hydrosilation of acetophenone, the use of Th(IV) tropolonates for extraction of thorium from other rare earth elements and the Re(I) analogues for model radiopharmeceutical application [7], [8], [9].
In contrast, an in-depth literature study revealed limited examples of cobalt tropolonato complexes, with the only notable occurance highlighting the use of cobalt(III) bistropolonates ([Co(Trop)2(L)]+) as potential hypoxia-selective cytotoxins [10]. This observation regarding the limited solid-state crystallographic properties of cobalt(II) tropolonato compounds lead to the investigation of the crystal structure of [Co(ph-azotrop)2(py)2].
The asymmetric unit of the title crystal structure consists of half of the [Co(ph-azotrop)2(py)2] complex. The cobalt atom is located on an inversion centre and is coordinated to both phenyl-azotropolonato and pyridine with the other half of the molecule being generated by symmetry. The environment around the cobalt(II) metal centre has a distorted octahedral geometry with the pyridine ligands in the axial position. The Co—O distances are 2.063(1) Å and 2.077(1) Å respectively with a Co—N distance of 2.157(1) Å. The O—Co—O bite angle was determined to be 77.80(4)°. The molecules are held together by a head-to-tail π⋯π stacking interaction involving the phenyl-azotropolonato ligands, with a distance of 3.638 Å between best planes defined by the ligands. While free phenyl-azotropone [3] was found to be essentially planar, the cobalt(II) complex displays bending and twisting of the phenyl-azotropolonato back-bone. In the structure of the title compound an additional π⋯π interaction was observed between pyridine ligands with a distance of 2.617 Å, generating a three-dimensional supramolecular network.
Acknowledgement
Financial assistance from the University of the Free State is gratefully acknowledged. We also express our gratitude towards SASOL and the South African National Research Foundation (SA-NRF/THRIP) for financial support of this project. Part of this material is based on work supported by the SA-NRF/THRIP under grant No. GUN 2068915. Opinions, findings, conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of the SA-NRF.
References
1 Steyl, G.; Roodt, A.: Molecular and crystallographic study of tropolone type derivatives by ab initio Hartree–Fock calculations. S. Afr. J. Chem. 59 (2006) 21–27.Search in Google Scholar
2 Muetteries, E. L.; Wright, C. M.: Chelate chemistry. III. Chelates of high coordination number. J. Am. Chem. Soc. 87 (1965) 4706–4717.10.1021/ja00949a009Search in Google Scholar
3 Hill, T. N.; Mangwaela, M. S.; Steyl, G.: 5-(Phenyldiazenyl)tropolone. Acta Crystallogr. E68 (2012) o941.10.1107/S1600536812008677Search in Google Scholar
4 Hill, T. N.; Steyl, G.: (3,5,7-Tribromotropolonato-κ2O,O′)tris-(triphenylphosphine-κP)silver(I). Acta Crystallogr. E65 (2009) m191.10.1107/S1600536809000890Search in Google Scholar
5 Muetteries, E. L.; Wright, C. M.: Chelate chemistry. I. Tropolone and aminotroponimine derivatives of the main-group elements. J. Am. Chem. Soc. 86 (1964) 5132–5141.10.1021/ja01077a017Search in Google Scholar
6 Steyl, G.; Roodt, A.: Tropolone as neutral compound and ligand in palladium complexes. In: Models, Mysteries, and Magic of Molecules (Eds. J. C. A. Boeyens, J. F. Ogilvie) p. 325–340. Springer Verlag 2008.10.1007/978-1-4020-5941-4_15Search in Google Scholar
7 Brunner, H.; Knott, A.; Benn, R.; Rufinska, A.: Asymmetrische katalysen: XXVII. Rh-komplexe mit von tropolon abgeleiteten optisch aktiven liganden. J. Organomet. Chem. 295 (1985) 211.10.1016/0022-328X(85)80273-4Search in Google Scholar
8 Dyrssen, D.: Studies on the extraction of metal complexes. Acta Chem. Scand. 9 (1955) 1567–1574.10.3891/acta.chem.scand.09-1567Search in Google Scholar
9 Schutte, M.; Visser, H. G.; Roodt, A.: Aquatricarbonyl(3,5,7-tribromo tropolonato)rhenium(I) methanol solvate. Acta Crystallogr. E64 (2008) m1610–m1611.10.1107/S1600536808038737Search in Google Scholar
10 Ware, D. C.; Palmer, H. R.; Brothers, P. J.; Rickard, C. E.; Wilson, W. R.; Denny, W. A.: Bis-tropolonato derivatives of cobalt(III) complexes of bidentate aliphatic nitrogen mustards as potential hypoxia-selective cytotoxins. J. Inorg. Biochem. 68 (1997) 215–224.10.1016/S0162-0134(97)00090-1Search in Google Scholar
11 Bruker. APEX2, SAINT and SADABS. Brucker AXS Inc., Madison, Wisconsin, USA, (2012).Search in Google Scholar
12 Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M. C.; Polidori, G.; Camalli, M.: SIRPOW.92 – a program for automatic solution of crystal structures by direct methods optimized for powder data. J. Appl. Cryst. 27 (1994) 435–43610.1107/S0021889894000221Search in Google Scholar
13 Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
14 Schneider, T. R.; Sheldrick, G. M.: Substructure solution with SHELXD. Acta Crystallogr. D58 (2002) 1772–1779.10.1107/S0907444902011678Search in Google Scholar
15 Farrugia, L. J.: WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 32 (1999) 837–838.10.1107/S0021889899006020Search in Google Scholar
16 Westrip, S. P.: publCIF: software for editing, validating and formatting crystallographic information files. J. Appl. Cryst. 43 (2010) 920–925.10.1107/S0021889810022120Search in Google Scholar
17 Spek, A. L.: Structure validation in chemical crystallography. Acta Cryst. D65 (2009) 148–155.10.1107/S090744490804362XSearch in Google Scholar PubMed PubMed Central
©2017 Tania N. Hill et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16


