Abstract
C21H16N2O2, triclinic, P1̅ (no. 2), a = 8.5971(3) Å, b = 9.8586(3) Å, c = 10.6199(4) Å, α = 95.747(2)°, β = 104.384(2)°, γ = 101.768(2)°, V = 842.69(5) Å3, Z = 2, Rgt(F) = 0.0482, wRref(F2) = 0.1377, T = 293(2).
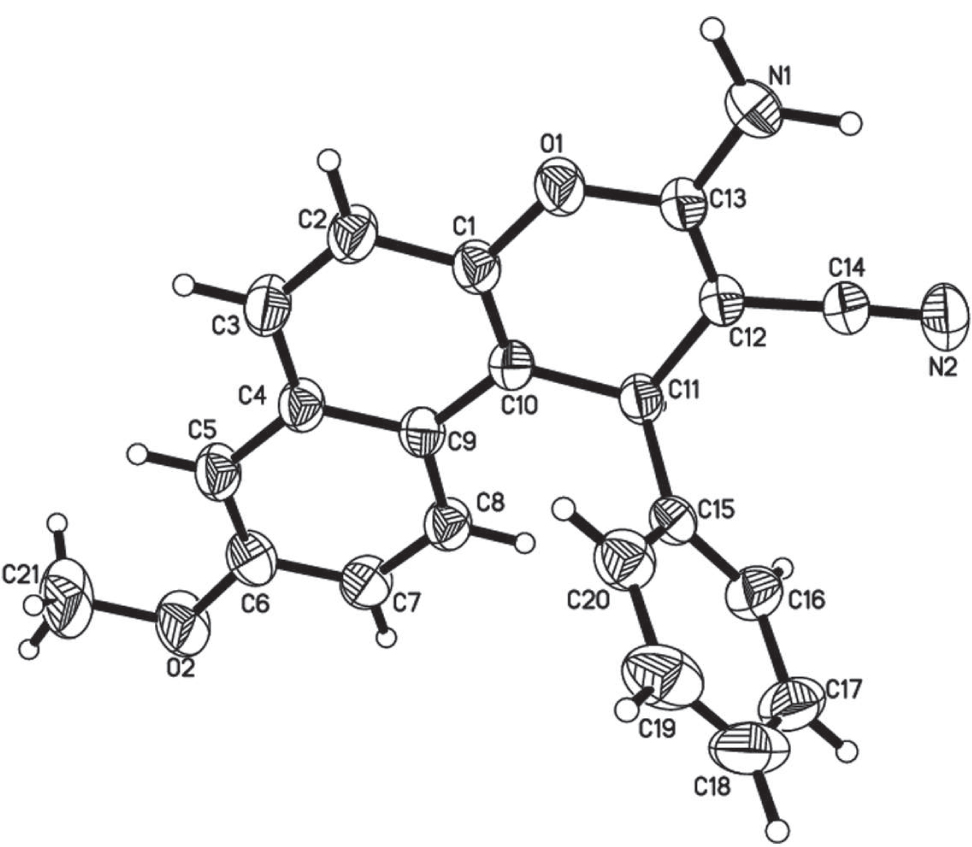
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.13 × 0.12 × 0.11 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 6.8 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| 2θmax, completeness: | 133.4°, >98% |
| N(hkl)measured, N(hkl)unique, Rint: | 8461, 2941, 0.062 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1905 |
| N(param)refined: | 263 |
| Programs: | SHELX [25, 26] , Bruker programs [27] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.83623(18) | 0.40865(14) | 0.54328(13) | 0.0528(4) |
| O2 | 0.3608(2) | 0.87430(18) | 0.12590(15) | 0.0774(6) |
| N1 | 0.9375(3) | 0.3536(2) | 0.7395(2) | 0.0589(6) |
| N2 | 0.8629(3) | 0.6056(2) | 0.97655(19) | 0.0767(7) |
| C1 | 0.7481(2) | 0.4836(2) | 0.45874(18) | 0.0439(5) |
| C2 | 0.7163(3) | 0.4301(2) | 0.32587(19) | 0.0529(6) |
| H2A | 0.7503 | 0.3482 | 0.3004 | 0.063* |
| C3 | 0.6361(3) | 0.4967(2) | 0.23322(19) | 0.0535(6) |
| H3A | 0.6170 | 0.4623 | 0.1425 | 0.064* |
| C4 | 0.5809(2) | 0.6164(2) | 0.27006(18) | 0.0451(5) |
| C5 | 0.4962(3) | 0.6851(2) | 0.17349(19) | 0.0518(6) |
| H5A | 0.4773 | 0.6514 | 0.0827 | 0.062* |
| C6 | 0.4422(3) | 0.7989(2) | 0.2102(2) | 0.0543(6) |
| C7 | 0.4666(3) | 0.8474(2) | 0.3442(2) | 0.0540(6) |
| H7A | 0.4250 | 0.9250 | 0.3693 | 0.065* |
| C8 | 0.5494(3) | 0.7842(2) | 0.4383(2) | 0.0488(5) |
| H8A | 0.5667 | 0.8199 | 0.5285 | 0.059* |
| C9 | 0.6105(2) | 0.6666(2) | 0.40522(17) | 0.0411(5) |
| C10 | 0.7004(2) | 0.59975(19) | 0.50236(17) | 0.0399(5) |
| C11 | 0.7463(2) | 0.65795(19) | 0.64729(17) | 0.0400(5) |
| H11A | 0.6430 | 0.6680 | 0.6705 | 0.048* |
| C12 | 0.8185(2) | 0.5554(2) | 0.72769(17) | 0.0429(5) |
| C13 | 0.8640(2) | 0.4439(2) | 0.67517(18) | 0.0441(5) |
| C14 | 0.8440(3) | 0.5814(2) | 0.8652(2) | 0.0513(6) |
| C15 | 0.8674(2) | 0.80160(19) | 0.68330(18) | 0.0416(5) |
| C16 | 0.8578(3) | 0.9016(2) | 0.7802(2) | 0.0562(6) |
| H16A | 0.7704 | 0.8831 | 0.8207 | 0.067* |
| C17 | 0.9744(4) | 1.0285(2) | 0.8190(3) | 0.0724(8) |
| H17A | 0.9669 | 1.0961 | 0.8861 | 0.087* |
| C18 | 1.0997(3) | 1.0564(3) | 0.7613(3) | 0.0791(9) |
| H18A | 1.1796 | 1.1434 | 0.7882 | 0.095* |
| C19 | 1.1104(3) | 0.9591(3) | 0.6644(3) | 0.0756(8) |
| H19A | 1.1975 | 0.9792 | 0.6239 | 0.091* |
| C20 | 0.9949(3) | 0.8315(2) | 0.6250(2) | 0.0572(6) |
| H20A | 1.0034 | 0.7644 | 0.5578 | 0.069* |
| C21 | 0.3207(4) | 0.8236(3) | −0.0114(2) | 0.1002(11) |
| H21A | 0.2560 | 0.8819 | −0.0608 | 0.150* |
| H21B | 0.2557 | 0.7263 | −0.0299 | 0.150* |
| H21C | 0.4227 | 0.8278 | −0.0379 | 0.150* |
| H2N1 | 0.960(4) | 0.284(3) | 0.692(3) | 0.099(10)* |
| H1N1 | 0.983(3) | 0.377(3) | 0.836(3) | 0.091(9)* |
Source of material
A mixture of 6-methoxy-2-naphthol (0.01 mol), malononitrile (0.01 mol), benzaldehyde (0.01 mol), absolute ethanol (30 mL) and piperidine (0.5 mL) was heated under reflux for 1 h. After a complete precipitation occurred, the solid product was collected by filtration, washed by methanol and recrystallized from ethanol to give the title compound as colourless crystals (yield 88% M.p.: 510–511 K (Lit. M.p.: 519–520 K [1]).
Experimental details
H atoms were placed in calculated positions and were included in the refinement using the riding model approximation, with Uiso(H) = 1.2Ueq(C). The H atoms of the methyl group were allowed to rotate with a fixed angle around the C—C bond to best fit the experimental electron density (HFIX 137 in the SHELX program suite [25]), with Uiso(H) = 1.5Ueq(C).
Discussion
Benzochromene derivatives represent an important class of compounds in heterocyclic chemistry and medicinal chemistry. In recent years, they have attracted considerable attention due to their antimicrobial [2, 3] , antileishmanial [4], [5], [6], [7], [8], [9], anticancer [10], [11], [12], [13], [14], [15], antiproliferative [22], antioxidant [16], [17], [18], hypertensive [14], antitumor [19], [20], [21] effects and activities, as well as proving potentially useful in the treatment of Alzheimer’s disease [23] and schizophrenia disorder [24].
In the title compound C21H16N2O2, the asymmetric unit contains one independent molecule. Molecules are packed in the crystal structure forming a centrosymmetric dimer classical intermolecular hydrogen bond N1—H1N1⋯N2i. The H⋯A distance is 2.07(3) Å, and the angle is 161(2)°. Symmetry code: (i) − x + 2, − y + 1, − z + 2.
Acknowledgement
The project was financially supported by King Saud University, Vice Deanship of Research Chairs.
References
1 Eid, F. A.; Abd El-Wahab, A. H. F.; Khafagy, M. M.; El-Hag Ali, G. A. M.: Synthesis and antimicrobial evaluation of naphtho[2,1-b]pyrano[2,3-d]pyrimidine and pyrano[3,2-e][1,2,4]-triazolo[1,5-c]pyrimidine derivatives. Acta Pharm. 54 (2004) 13–26.Search in Google Scholar
2 Qian-Zhu, L.; Xiao-Yan, N.; Jie, L.: Novel coumarin and 4H-chromene derivatives containing 4,5-dihydropyrazole moiety: synthesis and antibacterial activity. Lett. Drug. Des. Discov. 8 (2011) 558–561.10.2174/157018011795906857Search in Google Scholar
3 Xin-Hua, L.; Jin-Xin, L.; Lin-Shan, B.; Guo-Lin, L.; Chu-Xiou, P.: Novel dihydropyrazole derivatives linked with 4H-chromene: microwave-promoted synthesis and antibacterial activity. Lett. Drug. Des. Discov. 7 (2010) 487–490.10.2174/157017810791824847Search in Google Scholar
4 Foroumadi, A.; Emami, S.; Sorkhi, M.; Nakhjiri, M.; Nazarian, Z.; Heydar, S.; Ardestani, S.; Poorrajab, F.; Shafiee, A.: Chromene-based synthetic chalcones as potent antileishmanial agents: synthesis and biological activity. Chem. Biol. Drug. Des. 75 (2010) 590–596.10.1111/j.1747-0285.2010.00959.xSearch in Google Scholar
5 Narender, T.; Shweta Gupta, S.: A convenient and biogenetic type synthesis of few naturally occurring chromeno dihydrochalcones and their in vitro antileishmanial activity. Bioorg. Med. Chem. Lett. 14 (2009) 3913–3916.10.1016/j.bmcl.2004.05.071Search in Google Scholar
6 El-Agrody, A. M.; El-Hakium, M. H.; Abd El-Latif, M. S.; Fekry, A. H.; Sayed, S. M.; El-Gareab, K. A.: Synthesis of pyrano[2,3-d]pyrimidine and pyrano[3,2-e][1,2,4]triazolo[2,3-c]pyrimidine derivatives with promising antimicrobial activities. Acta Pharm. 50 (2000) 111–120.Search in Google Scholar
7 Bedair, A. H.; Emam, H. A.; El-Hady, N. A.; Ahmed, K. A. R.; El-Agrody, A. M.: Synthesis and antimicrobial activities of novel naphtho[2,1-b]pyran, pyrano[3,2-d]pyrimidine and pyrano[3,2-e][1,2,4]triazolo-[2,3-c]pyrimidine derivatives. Farmaco 56 (2001) 965–973.10.1016/S0014-827X(01)01168-5Search in Google Scholar
8 Khafagy, M. M.; Abd El-Wahab, A. H. F.; Eid, F. A.; El-Agrody, A. M.: Synthesis of halogen derivatives of benzo[h]chromene and benzo[a]anthracene with promising antimicrobial activities. Farmaco 57 (2002) 715–722.10.1016/S0014-827X(02)01263-6Search in Google Scholar
9 El-Agrody, A. M.; Sabry, N. M.; Motlaq, S. S.: Synthesis of some new 2-substituted 12H-chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine, 3-ethoxycarbonyl-12H-chromeno[3,2-e]-[3,2-e][1,2,4]τriazolo[1,5-c]pyrimidine-2-one, ethyl 2-formylamino/acetylamino-4H-chromene-3-carboxylate and some of their antimicrobial activities. J. Chem. Res. 35 (2011) 77–83.10.3184/174751911X12964930076728Search in Google Scholar
10 Halawa, A. H.; Fouda, A. M.; Al-Dies, A. M.; El-Agrody, A. M.: Synthesis, biological evaluation and molecular docking studies of 4Hbenzo[h]chromenes, 7H-benzo[h]-chromeno[2,3-d]pyrimidines as antitumor agents. Lett. Drug. Des. Discov. 13 (2016) 77–88.10.2174/1570180812666150611185830Search in Google Scholar
11 El-Agrody, A. M.; Halawa, A. H.; Fouda, A. M.; Al-Dies, A. M.: The anti-proliferative activity of novel 4H-benzo[h]chromenes, 7H-benzo[h]chromeno[2,3-d]pyrimidines and the structure-activity relationships of the 2-, 3-positions and fused rings at the 2, 3-positions. J. Saudi Chem. Soc. 21 (2017) 82–90.10.1016/j.jscs.2016.03.002Search in Google Scholar
12 El-Agrody, A. M.; Fouda, A. M.; Al-Dies, A.-A. M.: Studies on the synthesis, in-vitro antitumor activity of 4H-benzochromene, 7Hbenzo[h]chromeno[2,3-d]pyrimidine derivatives and Structure activity relationships of the 2-,3- and 2,3-positions. Med. Chem. Res. 23 (2014) 3187–3199.10.1007/s00044-013-0904-xSearch in Google Scholar
13 Sabry, N. M.; Mohamed, H. M.; Khattab, E. S. A. E. H.; Motlaq, S. S.; El-Agrody, A. M.: Synthesis of 4Hchromene, coumarin, 12H-chromeno[2,3-d]pyrimidine derivatives and some of their antimicrobial and cytotoxicity activities. Eur. J. Med. Chem. 46 (2011) 765–772.10.1016/j.ejmech.2010.12.015Search in Google Scholar PubMed
14 Rampa, A.; Bisi, A.; Belluti, F.; Gobbi, S.; Piazzi, L.; Valenti, P.; Zampiron, A.; Caputo, A.; Varani, K.; Borea, P. A.; Carrara, M.: Homopterocarpanes as bridged triarylethylene analogues:Synthesis and antagonistic effects in human MCF-7 breast cancer cells. Farmaco 60 (2005) 135–147.10.1016/j.farmac.2004.09.006Search in Google Scholar PubMed
15 Magedov, I. V.; Manpadi, M.; Evdokimov, N. M.; Elias, E. M.; Rozhkova, E.; Ogasawara, M. A.; Bettale, J. D.; Przheval’skii, N. M.; Rogelj, S.; Kornienko, A.: Antiproliferative and apoptosis inducing properties of pyrano[3,2-c]pyridones accessible by a onestep multicomponent synthesis. Bioorg. Med Chem. Lett. 17 (2007) 3872–3876.10.1016/j.bmcl.2007.05.004Search in Google Scholar PubMed PubMed Central
16 Singh, O. M.; Devi, N. S.; Thokchom, D. S.; Sharma, G. J.: Novel 3-alkanoyl/aroyl/ hetero-aroyl-2H-chromene-2-thiones: synthesis and evaluation of their antioxidant activities. Eur. J. Med. Chem. 45 (2010) 2250–2257.10.1016/j.ejmech.2010.01.070Search in Google Scholar PubMed
17 Vukovic, N.; Sukdolak, S.; Solujic, S.; Niciforovic, N.: Substituted imino and amino derivatives of 4-hydroxycoumarins as novel antioxidant, antibacterial and antifungal agents: synthesis and in vitro assessments. Food Chemistry 120 (2010) 1011–1018.10.1016/j.foodchem.2009.11.040Search in Google Scholar
18 Tandon, V. K.; Vaish, M.; Jain, S.; Bhakuni, D. S.; Srimal, R. C.: Synthesis, carbon-13-NMR and hypotensive action of 2,3-dihydro-2, 2-dimethyl-4H-naphtho[1, 2-b]pyran-4-one. Indian J. Pharm. Sci. 53 (1991) 22–23.Search in Google Scholar
19 Mahmoodi, M.; Aliabadi, A.; Emami, S.; Safavi, M.; Rajabalian, S.; Mohagheghi, M. A.; Khoshzaban, A.; Samzadeh-Kermani, A.; Lamei, N.; Shafiee, A.; Foroumadi, A.: Synthesis and in-vitro cytotoxicity of poly-functionalized 4-(2-arylthiazol-4-yl)-4H-chromenes. Arch. Pharm. Chem. 343 (2010) 411–416.10.1002/ardp.200900198Search in Google Scholar PubMed
20 Endo, S.; Matsunaga, T.; Kuwata, K.; Zhao, H.-T.; El-Kabbani, O.; Kitade, Y.; Hara, A.: Chromene-3-carboxamide derivatives discovered from virtual screening as potent inhibitors of the tumor maker, AKR1B10. Bioorg. Med. Chem. 18 (2010) 2485–2490.10.1016/j.bmc.2010.02.050Search in Google Scholar PubMed
21 Tseng, T.-H.; Chuang, S.-K.; Hu, C.-C.; Chang, C.-F.; Huang, Y.-C.; Lin, C.-W.; Lee, Y.-J.: The synthesis of morusin as a potent antitumor agent. Tetrahedron 66 (2010) 1335–1340.10.1016/j.tet.2009.12.002Search in Google Scholar
22 El-Agrody, A. M.; Fouda, A. M.; Khattab, E. S. A. E. H.: Synthesis, antitumor activity of 2-amino-4H-benzo[h]chromene derivatives and structure-activity relationships of the 3- and 4-positions. Med. Chem. Res. 22 (2013) 6105–6120.10.1007/s00044-013-0602-8Search in Google Scholar
23 Bruhlmann, C.; Ooms, F.; Carrupt, P.; Testa, B.; Catto, M.; Leonetti, F.; Altomare, C.; Cartti, A.: Coumarins derivatives as dual inhibitors of acetylcholinesterase and monoamine oxidase. J. Med. Chem. 44 (2001) 3195–1398.10.1021/jm010894dSearch in Google Scholar PubMed
24 Kesten, S. R.; Heffner, T. G.; Johnson, S. J.; Pugsley, T. A.; Wright, J. L.; Wise, D. L.: Design, Synthesis, and Evaluation of Chromen-2-ones as Potent and Selective Human Dopamine D4 Antagonists. J. Med. Chem. 42 (1999) 3718–3725.10.1021/jm990266kSearch in Google Scholar PubMed
25 Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
26 Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
27 Bruker. APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA, (2009).Search in Google Scholar
©2017 Rawda M. Okasha et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16

