Abstract
Over the last decade, nickel nanoparticles (NiNPs) have been investigated for various potential applications due to their superior ferromagnetic properties such as magneto-crystalline anisotropy, high coercive forces, and chemical stability. Therefore, there has been a tremendous enhancement in the synthesis techniques, proposed reaction mechanisms, and applications of NiNPs. This paper presents a recent overview of the synthesis, reaction mechanisms, and applications of NiNPs. NiNPs in the size range of 1–100 nm are synthesized by various methods for research and commercial applications. The synthesis techniques are classified into three main types, namely, top-down, bottom-up, and hybrids of top-down and bottom-up protocols including solvothermal, physical, and chemical approaches. The detailed reaction mechanisms in the formation of NiNPs, especially for biosynthesis techniques, are extensively described. Trends in NiNP applications in fields such as biomedical, catalysis, supercapacitors, and dye-sensitized solar cells are explored. The basic advantages and role of NiNPs as a catalyst for various reactions are illustrated here.
Graphical abstract
1 Introduction
In the last decade, nanotechnology has broadened the scope for researchers, producers, and consumers in almost all sectors by enabling the engineering of functional systems at the nanoscale level mostly in the form of nanoparticles [1]. Nanoparticles are the raw materials used in nanotechnology [2]. NiNPs have gained much attention due to their unique magnetic, chemical, and physical properties as well as their potential applications in various technological fields such as catalysis [3], battery manufacture [4], novel ink for nanotube-printing [5], incorporation in textile [6], enhanced pseudo-capacitance [7], field-modulated gratings and optical switches [8], direct immobilization of biomolecules through magnetic force of NiNPs [9], and adsorption of yellow dyes [10]. In comparison to other magnetic nanoparticles, Ni nanoparticles possess great potential as a catalyst in reactions and propellant and sintering additive in coatings, plastics, and fibres [11]. Due to its relative abundance in the earth’s crust, Ni is more cost-effective than most of the metals in use as a catalyst [12]. The electrical conductivity of Ni enables its use in several applications [13]. NiNPs can be used as nanofluids in high purity, ultra-high purity, passivated, coated, and distributed forms [14].
Review articles on nickel abound in literature such as machinability of nickel-based superalloys [15], overview of nanostructured metal oxides and pure nickel oxides electrodes for supercapacitors [16], nickel-based materials for supercapacitors [17], on the performance of commercially available corrosion-resistant nickel alloys [18], recent advances in the stabilization of nickel and nickel oxide nanoparticles [19], and a brief overview on grain growth of bulk electrodeposited nanocrystalline nickel and nickel–iron alloys [7]. However, these reviews did not focus on NiNPs; they discussed nickel oxides and alloys. Hence, there is the need for thorough analyses of synthesis, reaction mechanisms, and applications of NiNPs.
The synthesis of Ni nanoparticles has often been associated with various challenges. These may include the difficulty of reducing Ni(ii) to Ni(0) at room temperature [20]. Most synthetic protocols involve the use of distilled water to wash the Ni nanoparticles, but Ni nanoparticles are easily oxidized in air to form NiO, Ni2O3, Ni(OH)2, or NiOOH, making the synthesis difficult [21]. Hou et al. reported that, in comparison to other magnetic metals such as Co, Pt, and Fe, there are few reports on the synthesis of monodispersed Ni nanoparticles [22]. Similar observation elsewhere constitutes a challenge in the fabrication of Ni nanoparticles [23]. In compliance with Chen and Hsieh [24], pure Ni nanoparticles in a nonmagnetic environment undergo oxidation. Despite the high electrocatalytic performance exhibited by Ni nanoparticles, its poor stability poses a real challenge [25]. The magnetic properties of Ni nanoparticles interfere with magnets of microwave systems during synthesis using the microwave. This makes it difficult to obtain a good yield of the Ni nanoparticles [26]. NiNPs tend to cluster on formation, due to their magnetic properties, and hence, to obtain monodispersed Ni nanoparticles will require special techniques [27]. NiNPs must be characterized immediately after production to avoid conversion to Ni compounds like oxides and hydroxides. These factors must be taken into consideration in planning the synthesis of Ni nanoparticles [28].
Global rising of environmental carbon costs have made it necessary for scientific research and industrial enterprise to invest in energy-saving and environmentally friendly alternative synthesis of Ni nanoparticles by plant-mediated pathways. Green plant production of Ni Nanoparticles is the use of plant parts, plant extracts, plant products, and organisms, leading to environmentally friendly products that are harmless to the ecosystem. The green production of metallic nanoparticles employing plant extracts as a reducing agent is considered economically viable and environmentally friendly.
The present study was designed to analyse recent synthesis, reaction mechanisms, and applications of Ni nanoparticles as well as the specific advantages of using Ni as a catalyst. The objectives of this article are based on highlighting recent techniques in the synthesis of Ni nanoparticles, ranging from green chemical methods to physical methods; in addition, to discuss the reaction mechanisms leading to the formation of Ni nanoparticles, as proposed and reported in recent literature. Furthermore, the scope of this study will be limited to addressing the applications of Ni nanoparticles in various fields of nanotechnology, science, and biomedical sciences as well as to discuss the specific advantages of Ni nanoparticles as catalysts in reactions, as compared to other magnetic nanoparticles.
2 Techniques for the synthesis of NiNPs
Top-down synthesis methods and bottom-up synthesis protocols have traditionally been adopted for the synthesis of Ni nanoparticles. Top-down synthesis protocols include breaking down bulk materials into nanoscale materials. The most widely used top-down nanoparticle synthesis methods are mechanical milling, laser ablation, nanolithography, thermal decomposition, and sputtering [29]. Bottom-up protocols include the utilization of metallic oxides and metallic salts as precursors for the reactions. These salts or oxides are eventually reduced to metallic nanoparticles using an appropriate solvent and reducing agent. In these techniques, the disperse mode, shape, and dimensions of the nanoparticles can be manipulated by varying the concentration of the precursor and the reducing agent, the pH, temperature, heating time, and the type and choice of the stabilizing agent [30]. Several bottom-up approaches for the fabrication of Ni nanosized materials have been reported. These are spatting, solvent-gel, gas evaporation, coprecipitation techniques, and plant-mediated synthesis [21]. Similarly, Ni nanoparticles have been fabricated in nonaqueous and aqueous media by chemical reaction, sonication [31], radiolytic [32], and chemical reduction and freeze-drying reactions [33].
2.1 Top-down methods for the fabrication of NiNPs
2.1.1 Mechanical milling
Mechanical milling is the most common method of generating diverse nanoparticles among the different top-down methods. Mechanical milling is used during synthesis for milling as well as post-annealing of nanoparticles where many elements are milled in an inert atmosphere. Mechanical milling is influenced by plastic deformation, contributing to a specific shape, whereas fracture leads to a decrease in the particle size while cold welding increases the particle size [34].
2.1.2 Laser ablation
Laser ablation synthesis is a method of production of nanoparticles from different solvents. Nanoparticles are generated by irradiation of a metal immersed in a liquid solution by a laser beam which condenses the plasma plume to produce the nanoparticles. Laser ablation synthesis is a reliable technique as well as an alternative to a chemical metal reduction in the synthesis of nanoparticles. This method provides a stable synthesis of nanoparticles in organic solvents and water without requiring stabilizing or capping agent [35].
2.1.3 Thermal decomposition
In this method, heat generated by endothermic chemical decomposition breaks the bonds in the compound. Decomposition temperature is the specific temperature at which an element chemically decomposes. Hence, the nanoparticles are generated by decomposing the metal at its decomposition temperature, thereby undergoing a chemical reaction producing the nanoparticles [36]. Figure 1 shows a scheme of thermal decomposition techniques to produce NiNPs [37].
2.1.4 Nanolithography
Nanolithography is a technique to produce nanomaterials having a minimum of one dimension in the range of 1–100 nm. These techniques include nanoimprint, optical, multiphoton, electron-beam, and scanning probe nanolithography. Nanolithography is a method of printing the necessary shape or structure on a substrate that is susceptible to light and extracts part of the material selectively to obtain the required shape and structure. Nanolithography enables the production of a single nanoparticle to a cluster of nanoparticles with the desired shape and size [38]. The disadvantages of these techniques are the requirements of complex equipment and the associated cost. Figure 1 shows an illustration of the nanolithography technique to produce nanoparticles [39].
2.1.5 Sputtering technique
In the sputtering technique, the deposition of nanoparticles on a surface occurs by the ejecting particles as they collide with ions. Sputtering is the deposition of a thin layer of nanoparticles followed by annealing [40]. The shape and size of the nanoparticles are determined by the substrate type, thickness of the layer, duration of annealing, and operating temperature.
Table 1 shows the list of some NiNPs synthesized from these methods. These techniques are used to create nanosized materials using chemical, electrochemical, and mechanical grinding protocols [1]. Top-down methods include mechanical grinding of coarse particles to nanosized particles, electrochemical deposition of nanoparticles from a metal electrode [2], laser ablation [3], and sputtering [4]. Selected top-down techniques for the fabrication of Ni nanoparticles are presented in Table 1.
Top-down techniques for the fabrication of Ni nanoparticles
| Top-down technique | Application of Ni nanoparticles | Reference |
|---|---|---|
| Lithography | Biomedical | Fu et al. [45] |
| Chemical etching | Catalyst | Heilmann et al. [46] |
| Laser ablation | Heterogeneous catalyst | Marzun et al. [32] |
| Mechanical milling | Material softening | Liu et al. [47] |
| Ball milling | Catalyst for CO2 hydrogenation | Ochirkhuyag et al. [48] |
| Sputtering | Magnetic biocatalyst | Bussamara et al. [49] |
| Robust catalytic reactions | Catalyst | Li et al. [50] |
| Plasma processing | electrocatalyst | Kim et al. [51] |
2.2 Bottom-up synthesis protocols in fabricating Ni nanomaterials
2.2.1 Sol–gel technique
This technique involves the sol – a colloidal solution of solids suspended in a liquid phase and the gel – a solid macromolecule submerged in a solvent. Most of the metallic nanoparticles can be synthesized by this method and it is the most preferred bottom-up method due to its simplicity. This is a wet-chemical process in which the precursor is a chemical solution containing an integrated system of discrete particles [52]. Metal oxides and salts are the typically used precursors in the sol–gel process. The precursor is then dispersed in a host liquid either by sonication, shaking, or stirring and the resultant system contains a liquid and a solid phase. Various methods such as filtration, sedimentation, and centrifugation are used to recover the nanoparticles. Figure 2 shows an illustration of the sol–gel technique [53].
2.2.2 Spinning fabrication
In this technique, the synthesis of nanoparticles is carried out by a spinning disc reactor. The rotating disc contains an inner chamber where the physical parameters such as temperature are controlled. The reactor is filled with inert gases or nitrogen to remove oxygen and avoid chemical reactions. The liquid precursor and water are pumped into the chamber and the disc is rotated at a different speed. The spinning allows the atoms or molecules to fuse together and are precipitated, collected, and dried [54]. The characteristics of the synthesized nanoparticles are determined by various operating parameters such as the disc rotation speed, liquid flow rate, location of feed, disc surface, and liquid/precursor ratio. Figure 2 shows an illustration of a spinning disc reactor [55].
2.2.3 Chemical vapour deposition (CVD) technique
In this technique, a thin film of gaseous reactants is deposited onto a substrate. The combination of gas molecules occurs in the reaction chamber at ambient temperature. The heated substrate then undergoes a chemical reaction with the combined gas. A thin film of products is deposited on the substrate surface which is recovered and used. In CVD, the influencing factor is the substrate temperature [56]. CVD advantages include the production of uniform, highly pure, hard, and strong nanoparticles. The disadvantages of CVD are highly toxic gaseous by-products and the prerequisite of unique equipment.
2.2.4 Pyrolysis technique
Pyrolysis is the most employed technique in industries for large scale production of the nanoparticle. The precursor is burned in a flame. The liquid or vapour precursor is fed into the furnace through a small hole where it burns at high pressure. The nanoparticles are then recovered from the combustion or by-product gases [57]. In some furnaces, laser and plasma are used rather than a flame to produce high temperature enabling easy evaporation. Pyrolysis employs simple methodology, high yield with economic efficiency as well as a continuous operation.
2.2.5 Biosynthesis
These techniques are used in the synthesis of nanoparticles that are nontoxic and biodegradable. Biosynthesis is a renewable and environmentally friendly approach. Plant extracts, bacteria, and fungi, together with the precursors, are used to produce nanoparticles rather than conventional chemicals for bio-reduction and capping purposes [59]. The nanoparticles synthesized by this approach are unique and possess enhanced properties that satisfy the requirements of biomedical applications. Figure 2 shows an illustration of the biosynthesis of NiNPs [58]. Table 2 shows some selected bottom-up techniques for the synthesis of Ni nanoparticles.
Selected bottom-up techniques for Ni nanoparticles production
| Bottom-up technique | Application of Ni nanoparticles | Reference |
|---|---|---|
| Chemical vapour deposition | Antimicrobial | Chaudhary et al. [60] |
| Sol–gel process | Superparamagnetic material | Li et al. [61] |
| Laser pyrolysis | Advanced functionalized material | Mckeown et al. [62] |
| Spray-pyrolysis | Water splitting | Li et al. [63] |
| Atomic condensation | Electrocatalyst | Fadil et al. [64] |
| Molecular condensation | Antibacterial activity | Bhattacharjee et al. [65] |
| Aerosol process | Enhanced electrocatalytic activity | Khalid et al. [66] |
2.2.6 Comparison of the advantages and disadvantages of top-down and bottom-up synthesis protocols
Top-down techniques have been successfully employed in nanotechnology for the fabrication of nanomaterials. Among the top-down protocols, nanolithography provides opportunity for fabrication of smaller nanomaterials. The techniques can easily be upgraded for large scale production. However, bottom-up techniques which rely on self-assembly of nanomaterials are difficult to upgrade for large scale production. While top-down techniques are well-established over the decades, bottom-up protocols offer unlimited possibilities in the design and fabrication of nanomaterials. Both top-down and bottom-up techniques have their advantages and disadvantages as presented in Table 3.
Comparison of top-down and bottom-up protocols
| Top-down protocols | Bottom-up protocols | |
|---|---|---|
| Advantages | Techniques are suitable for large scale production | Low cost of production |
| Protocols are well-established | High precision in designing size and shape of particles | |
| Techniques provide surface control and precision | Production of wide range of particle size and shape | |
| Disadvantages | High cost of production | Highly specific; cannot be easily generalized |
| Stronger resistance as feature becomes smaller | Difficulty in large scale production | |
| Defects become pronounced with decrease in size | Unsuitable for integrated devices |
3 Green plant-mediated production of Ni nanoparticles
Global rising of environmental carbon costs have made it necessary for scientific research and industrial enterprise to invest in energy-saving and environmentally friendly alternative [67] synthesis of Ni nanoparticles by plant-mediated pathways. Green plant production of Ni nanoparticles is the use of plant parts, plant extracts, plant products, and organisms, leading to environmentally friendly products that are harmless to the ecosystem. The green production of metallic nanoparticles employing plant extracts as a reducing agent is considered economically viable and environmentally friendly. These techniques have the advantage of being eco-friendly, low cost, and mostly single-step reactions [68]. Angajala and coworkers [68] reported that most plants are rich in secondary metabolites and responsible for the reduction of metal salts or metal oxides to metallic nanoparticles. Plant metabolites are widely available, free of contamination, and cost-effective and the process of reduction is a one-step reaction. Green production of nanoparticles can be easily upgraded to large scale production. Metal nanoparticles produced this way have characteristically been found to be of distinct sizes [69] and morphologies [70]. The organic functional groups in plant metabolites play an active role in the production of nanoparticles, as reducing, capping, and stabilizing agents [71]. The nanomaterials fabricated using plant metabolites have high polydispersity, well-defined size, and thermodynamic stability [72]. Green-based synthesis methods such as leaf extract, stem bark root as well as plant secretions are considered viable substitutes to physical and chemical protocols for the synthesis of metallic nanomaterials [73].
3.1 Synthesis of Ni nanoparticles using leaf extracts as reducing agent
The production of Ni nanoparticles using aqueous leaf extract of Ocimum sanctum has been reported by Pandian and coworkers [74]. They concluded that the green synthesis of nanoparticles provides more advancement in pharmaceutical and biomedical applications than chemical and physical methods due to its cost-effectiveness and eco-friendliness. Similarly, Nouneh and coworkers [75] reported the synthesis of spherical NiNPs deposited on indium tin oxide surface using a wet-chemical method. They claimed that this was the first in situ attachment of Ni nanoparticles on indium tin oxide substrate. Sudhasree and coworkers [76] published Ni nanoparticle synthesis by green and chemical routes. They compared the biological activity and toxicology of Ni nanoparticles synthesized by the two routes. They concluded that the green-synthesized Ni nanoparticles showed reduced size and better monodispersity compared to the chemically synthesized nanoparticles. The green-synthesized Ni nanoparticles were found to possess reliable antioxidant and antibacterial activity as well as being nontoxic to animal cells, as compared to chemically synthesized Ni nanoparticles.
Ni nanoparticle synthesis with Azadirachta and Psidium guajava leaves has been documented by Mariam and coworkers [77]. They discovered that the Ni mesh value was higher than the bulk value. They also stated that the Ni nanoparticles in HT29 cell lines were spherical and toxic. Chen and coworkers [78] reported the use of Medicago sativa, commonly called alfalfa plant extract, to synthesize Ni nanoparticles. They concluded that the flavonoids and reducing sugars in the extract were responsible for the bio-reduction of Ni. They further reported that the biosynthesis approach is more cost-effective and is a promising alternative to conventional methods of Ni nanoparticle synthesis. Mamuru and coworkers [79] documented the production of Ni nanoparticles by Annona squamosa (sugar apple) plant extract. Their study reveals that aryl amine in the phytochemicals of the leave extract was responsible for the reduction of Ni oxide to Ni nanoparticles. Angajala and coworkers [68] documented the fabrication of Ni nanoparticles using Aegle marmelos Correa plant leaf extract as a reducing, stabilizing, and capping agent. They also compared the as-synthesized Ni nanoparticles with Aegle marmelos Correa crude leaf extract for their in vitro anti-inflammatory, larvicidal mosquito effectiveness against three blood eating parasites. The results showed that the Ni nanoparticles possess an enhanced anti-inflammatory and larvicidal activity when compared to the crude leaf extract.
3.2 Synthesis of Ni nanoparticles using starch and plant secretion as reducing agent
Fardood and coworkers [80] fabricated NiO nanoparticles using Arabic gum by the solvent–gel technique. They reported the first green synthesis of NiO nanoparticles with Arabic gum gel as a bio-polymeric template. They claimed that the method can be employed for the fabrication of transition metallic nanoparticles from their oxides and other materials with low production cost. Similarly, several other reports on the synthesis of Ni nanoparticles employing green agents include those of Yu and Qiu [81] who fabricated nanosized nickel material using starch. They reported the synthesis of unique core–shell-structured Ni nanoparticles from starch and the metal salt by carbonization in flowing hydrogen. This apparently provides a new approach to the synthesis of carbon-encapsulated metal nanoparticles.
3.3 Synthesis of Ni nanoparticles using microorganisms as reducing agent
Dias and coworkers [82] synthesized Ni nanoparticles using strains of Aspergillus terreus immobilized in polyurethane foam. They demonstrate the feasibility of replacing synthetic adsorbents with bio-absorbents in the treatment of industrial waste. Furthermore, the bio-absorbents can be recycled and are cheaper than synthetic resins. Various reports in the literature show that algae, both living and dead, can reduce noble metals to metal nanoparticles [83]. This provides an opportunity for utilizing organisms [84] as an environmentally green approach to synthesize metal nanoparticles [85]. Figure 3 is an illustration of the pathway for the synthesis of metal nanoparticles from microorganisms [86].
![Figure 3
Illustration of metal nanoparticles synthesis procedure from microorganisms [86].](/document/doi/10.1515/ntrev-2020-0109/asset/graphic/j_ntrev-2020-0109_fig_003.jpg)
Illustration of metal nanoparticles synthesis procedure from microorganisms [86].
Based on these reports, green synthesis using plant parts has significantly improved over the past decade. Extracts of new plant species are constantly added to the literature as reducing agents in the fabrication of Ni nanoparticles. Green synthetic protocols are environmentally friendly, with low-cost starting materials [87]. Selected reports in which green reducing agents were used in the synthesis of Ni nanoparticles are summarized in Table 4.
Green reducing agents in the synthesis of Ni nanoparticles with shapes and sizes
| Green reducing agent | Shapes | Sizes (nm) | Reference |
|---|---|---|---|
| Ocimum sactum leaf extract | Spherical | 12–36 | Jeyaraj Pandian et al. [74] |
| Aegle marmelos leaf extract | Triangular | 80–100 | Angajala et al. [68] |
| Azadirachta indica and Psidium guajava leaf extract | Cubic | 22–44 | Mariam et al. [77] |
| Medicago sativa leaf extract | Cubic | 1–10 | Chen et al. [78] |
| Annona squamosa leaf extract | — | — | Mamuru et al. [79] |
| Acacia senegal latex secretion | Cubic | 34 | Fardood et al. [80] |
| Starch | Ellipsoidal | 30–50 | Yu and Qiu [81] |
| Microalgae | Colloidal | 3 | Song et al. [88] |
| Microalgae Chlorella vulgaris | Crystalline | 200–500 | Gong et al. [89] |
The sizes reflected in Table 4 show that all the nanoparticles produced via plant-assisted are within the size range 1–100 nm, except for Annona squamosa and Chlorella vulgaris. Therefore, they can be employed as catalyst in appropriate reactions. The green-synthesized Ni nanoparticles were found to possess reliable antioxidant and antibacterial activity as well as being nontoxic to animal cells, as compared to chemically synthesized Ni nanoparticles. Ni nanoparticles synthesized using leaf extract of Aegle marmelos Correa as reducing agent possess an enhanced anti-inflammatory and larvicidal activity when compared to the crude leaf extract. NiNPs synthesized by Azadirachta indica and Psidium guajava were further evaluated for the cytotoxic activity using HT-29 colon cancer cell lines and the results showed significant changes in the cell morphology such as swelling of cells and cell breakage, when treated with the synthesized NiO and Ni nanoparticles.
Biosorption studies using Aspergillus terreus to generate Ni nanoparticles showed that due to its affinity for binding simultaneously to a mixture of three heavy metals in solution, this strain of A. terreus can be considered as a good candidate for application at an industrial level for removing iron, chromium, and NiNPs. Extracts of new plant species are constantly added to literature as reducing agents in the fabrication of Ni nanoparticles. Similarly, more organisms are reported for the synthesis of Ni nanoparticles. This provides an opportunity for utilizing both plant extracts and organisms as an environmentally green approach to synthesize metal nanoparticles.
4 Proposed reaction mechanisms of Ni nanoparticle formation in plant-mediated green synthesis
The mechanism of binding and subsequent conversion of metallic ions to metal nanoparticles has been extensively discussed in various literature [90]. Plant metabolites such as polyphenols, terpenoids, phenolic acids, alkaloids, proteins, and sugars are important in the bio-reduction of metal ions to form nanoparticles. The functional groups involved in the reduction can be identified from the FT-IR bands of the plant extract, taken before and after the reduction. Shankar and coworkers [91] reported that terpenoids are sometimes associated with nanoparticle formation in green synthesis. Terpenoids contain various polymers, photosynthesized in most plants containing isoprene units with five-carbon, having strong antioxidant activity [90]. These can reduce metals to metal nanoparticles. Therefore, Ni nanoparticles can be synthesized by this approach.
4.1 Mechanisms of Ni nanoparticle formation involving terpenoids
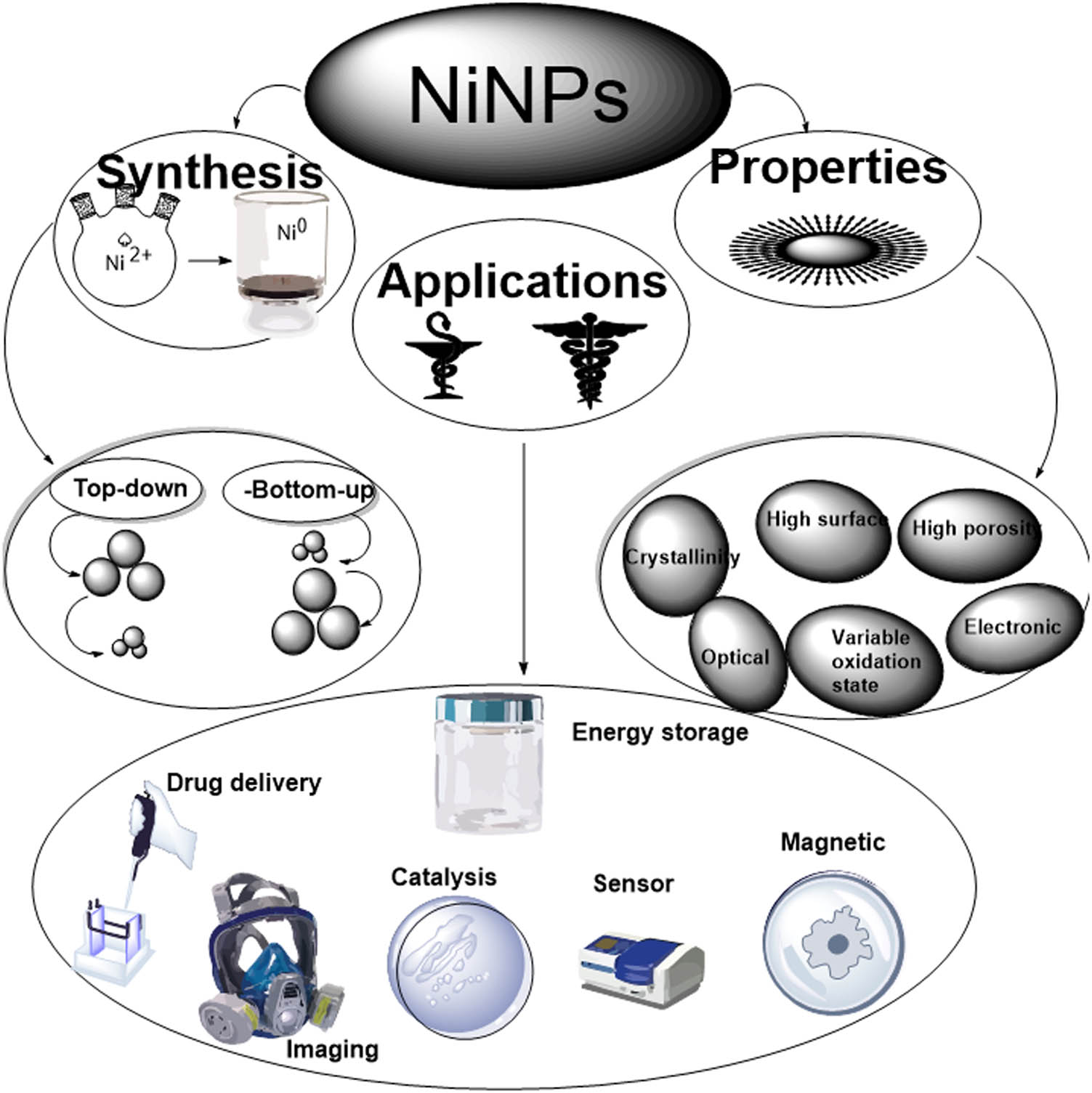
Terpenoids play a significant role in the biosynthesis of silver ions to metallic nanoparticles when Geranium leaves extracts are used in the reactions; the major terpenoid of Cinnamomum zeylanisum is eugenol, which was found to be involved in the bio-reduction of HAuCl4 as well as AgNO3 to nanomaterial [91]. Using FT-IR info, proton dissociation in the eugenol OH group was proposed to create hybrid structures that can be further oxidized. The successful reduction of metal ions contributes to the synthesis of the nanoparticle. The proposed mechanism is shown in Figure 4. In this mechanism, the metal ions interact with plant reducing agents (O–H) leading to the formation of a reaction intermediate (M═O), which subsequently breaks down to metal atoms (M) followed by growth and stabilization of the metal atoms.
Scheme of reaction mechanism of Ni nanoparticle formation.
4.2 Mechanisms of Ni nanoparticle formation involving flavonoids
Another class of compounds contained in the plant extracts are the flavonoids, which are a group of polyphenolic functional groups that are made up of various classes, i.e. isoflavonoids, anthocyanins, chalcones, flavonols, flavonones, and flavones. These compounds can effectively chelate and reduce metals to nanoparticles [92]. Flavonoids have different functional groups that can facilitate the development of nanoparticles [93]. The hybrid conversion from enol to keto will generate reactive hydrogen to convert metal ions into metallic nanoparticles as has been reported. It has been proposed [90] that for Ocimum basilicum extracts, the forming of silver nanoparticles from Ag ion is due to the transition of the flavonoids luteolin and rosmarinic acid from enol to keto form.
Likewise, a pathway to convert ketones into carboxylic acids in flavonoids is likely to reduce Au3+ ion. Metal ions may be chelated with their carbonyl groups or π-electrons from some flavonoids. For example, the flavonoid quercetin is very active in chelating, as it can be used in three positions in C3, C5, and C3 and C4 in the carbonyl and the hydroxylic groups and C3 and C4 in the catechol, as seen in a quercetin molecule in Figure 5. These compounds can chelate transition metal ions like Pb2+, Co2+, Fe2+, Al3+, Zn2+, Ni2+, Cr3+, and Fe3+. Chelation reactions can clarify the potential adsorption of flavonoids on the surface of the metal nanoparticles. Kasthuri et al. [94] reported that probably it constitutes the initiation stage of the nanoparticle formation and subsequent growth, in addition to the bio-reduction stages. Likewise, the formation of metal nanoparticles is generated by isolated flavonoids and glucosides.
The numbering system in quercetin molecule, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one showing carbon 1–8.
Isolated reducing sugars from plants initiate reduction reactions leading to nanoparticle formation. Monosaccharides like glucose can initiate reduction reactions. Monosaccharides contain a keto group like fructose, which can act as an antioxidant after undergoing several tautomeric transformations from ketone to an aldehyde [95]. Disaccharides and polysaccharides have their reduced tendency to follow a conformation within an oligomer based upon the specific monosaccharide structure, thereby making the metal ion available to an aldehyde molecule [96]. The disaccharide lactose and maltose have reduction potential, for example, because one of their monomers can shift in an open-chain shape. Sucrose cannot reduce metal ions as the monomers glucose and fructose are joined in a manner that does not promote the reduction of the open-chain configuration. Glucose can induce metal nanoparticle synthesis with different morphologies, while fructose initiates a synthesis with monodispersed silver and gold nanosized materials.
4.3 Mechanisms of Ni nanoparticle formation involving amino acids
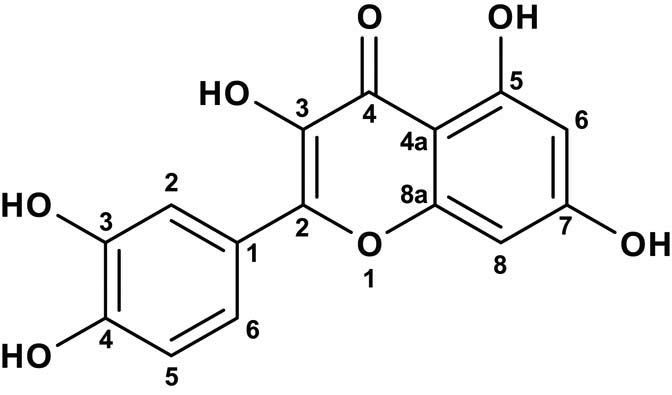
It has been suggested that nanomaterial synthesized with plant extracts or in plants showed the as-synthesized nanomaterials are often found in association with proteins [97]. The ability of amino acids to bind and reduce metal ions can differ if kept on the peptide chain. The formation of peptide linkage, for example, modifies the functionality of the amines R-carbon of certain amino acids and carboxylic acids; hence, they are unavailable to react with metal ions. Din and Rani [19] formulated a mechanism of plant extracts-assisted synthesis of metal oxides and metals as represented in Figure 6. In this mechanism, nickel ions (Ni2+), in the presence of plant reducing agents (O–H), undergo a reaction to form (Ni═O) intermediate. This is followed by the reduction of the intermediate to form nickel atoms (Ni0). The Ni nanoparticles formed undergo growth and stabilization as they are capped by the phytochemicals.
Proposed reaction scheme for the formation of NiO.
4.4 Reduction, growth, and stabilization stages in Ni nanoparticles formation
Plant-mediated synthesis of Ni nanoparticles consists of three major steps, namely, activation stage, growth stage, and termination stage. According to Kim et al. [98], the comprehensive reaction process for plant and plant extract synthesis of Ni nanoparticles involves three main phases; initiation step at which the Ni ions are reduced and subsequent nucleation of reduced Ni atoms occurs. The second step is the growth point, in which the neighbouring nanoparticles are naturally aggregated into bigger particles and accompanied by an enhancement in nanoparticles’ stability and, finally, the stage which determines nanoparticles’ size and crystallinity. pH variation influences the activity of the phytochemicals contained in an extract, which determines their binding capacity and the subsequent reduction of metal cations and anion at the time of nanoparticle formation, leading to the acquisition of various shapes, sizes, and yield of nanoparticles [99].
Nanoparticle nucleation has been formulated as the operation by which nuclei act as a template for the growth of crystals. Primary nucleation is when nucleation occurs in the absence of other crystalline matter [100]. Homogeneous nucleation is said to occur when there is a uniform formation of nuclei all over the phase, while heterogeneous nucleation occurs at fundamental inhomogeneity, dislocations, and grain boundaries [100]. The total energy possessed by a nanoparticle is given by the sum of the bulk-free energy and the surface-free energy [71]. Mittal et al. [101] suggested a scheme of nanoparticle formation through the stages of reduction of Ni ions to Ni atoms, growth of Ni atoms, and stabilization of the atoms during synthesis. The detailed mechanism of metal nanoparticle formation can be summarized as shown in Figure 7, in which the metal ions (Ni+) are reduced to metal atoms (Ni0). The nickel atoms then aggregate and grow. Finally, the metal atoms are capped with stabilizing agents to form stable NiNPs.
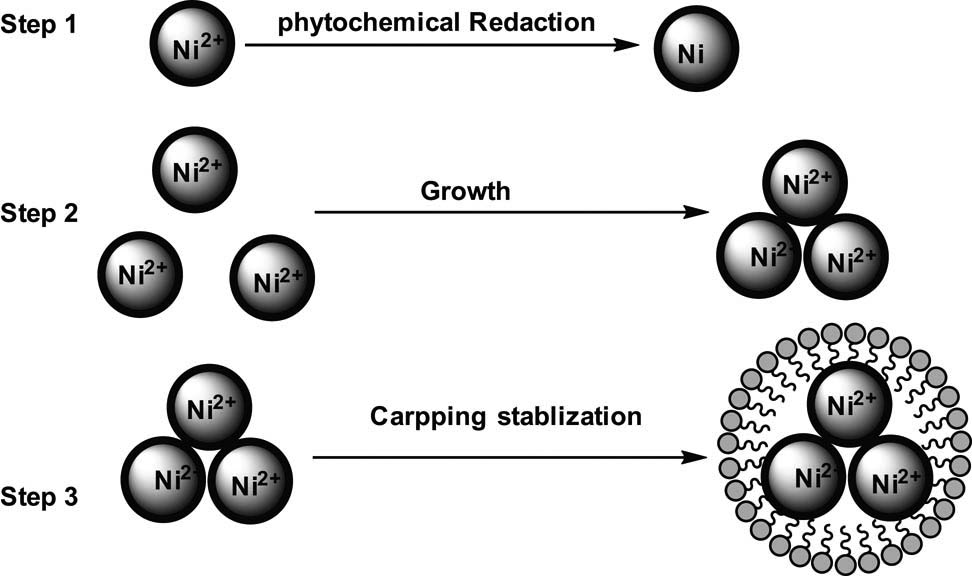
Mechanism of nanoparticle formation.
Pandian et al. specifically formulated the mechanism of reaction in Ni nanoparticles formation employing Ocimum sanctum leaf extract [74]. The mechanism involves oxidative damage, surface bond, and the release of Ni ions, and finally, the reduction of Ni ions to Ni, as represented in Figure 8. A summary of the reported reaction mechanisms contains the three main phases of reduction, growth, and stabilization.
![Figure 8
The mechanism of reaction for NiNP formation [74].](/document/doi/10.1515/ntrev-2020-0109/asset/graphic/j_ntrev-2020-0109_fig_008.jpg)
The mechanism of reaction for NiNP formation [74].
5 Solvothermal synthesis of Ni nanoparticles
Solvothermal synthesis of Ni nanoparticles, in which solvent at high temperature and pressure acts as reducing agent, has been reported. Several reports describing hydrothermal fabrication of Ni nanoparticles are obtainable in literature [21,102,103,104]. Libor and Zhang [103] described the procedure for the synthesis of Ni nanoparticles in which an aqueous solution of Ni acetate was heated and then hydrazine was added to the solution with continuous stirring. They concluded that the crystal shape can be controlled by controlling various Ni-ions reduction parameters to adjust the solution pH of core and shell particles. They also reported the successful coating of Ni nanoparticles onto SiO2 particles. Liu et al. [102] reported the synthesis of Ni nanosized belts using a hydrothermal reduction technique involving a complex surface-active reagent. Figure 9 shows a schematic diagram of the solvothermal synthesis of NiNPS [105].
Chen and Wu synthesized nanoparticles of Ni using a microemulsion of water-in-oil as a reducing agent and Ni chloride as a precursor. They concluded that the main factor in the synthesis of smaller Ni nanoparticles was the higher ratio of the solvent system [21]. The dependence of particle sizes on the concentration of hydrazine and Ni chloride could be explained by the reduction, nucleation, and growth process. Figure 9 shows a schematic diagram of the microemulsion solvothermal synthesis of NiNPs [106].
Kumar et al. described the fabrication of size-controlled Ni and NiO nanoparticles by employing microemulsions of water-in-oil. The size of the Ni nanoparticles can easily be managed by adjusting the water-to-surfactant molar ratio or adjusting the reducing agent concentration [107]. Hou et al. reported the fabrication of size-controlled Ni nanoparticles using a standard airless procedure in the synthesis. It was found that the introduction of hexadecylamine and trioctylphosphineoxide (TOPO) is an effective approach to obtain smaller monodispersed Ni nanoparticles. They believed this method provided a good approach for the synthesis of other metal nanoparticles [22].
Roselina and Azizan fabricated Ni nanoparticles by applying adjusted polyol by employing ethylene glycol and N2H4·6H2O. It was found that the molar ratio of N2H4/Ni was significant in controlling the rate of reaction. They reported that the reaction rate increases with the increase in N2H4/Ni molar ratio. However, an increase in agglomeration was observed as a rather unfavourable condition in the synthesis of Ni nanoparticles [108]. These results can be generalized to other metal nanoparticles, notably magnetic nanoparticles such as Ni nanoparticles.
The above reports show that solvothermal techniques for the synthesis of Ni nanoparticles are well-documented. In solvothermal synthesis, the crystal size and shape of the nanoparticles can be controlled by reaction parameters such as concentration of reactants, reaction temperature, reaction time, pH of reactants, and finally concentration of stabilizing and capping agents. Specific size can be produced by controlling the surface charge on the nanoparticles and the pH of the precursor, while the crystal shape can be controlled by adjusting the crystal nucleation and growth process.
In solvothermal synthesis by water in oil procedure, the main factor to produce smaller Ni nanoparticles was the ratio of water to cetyltrimethylammonium bromide to n-hexanol system. Higher ratio of cetyltrimethylammonium bromide to n-hexanol favoured the production of smaller nanoparticles. The average diameter of Ni nanoparticles was determined by the number of nuclei formed at the beginning of the reduction process. The magnetization of Ni nanoparticles was found to increase with decreasing temperature.
In surfactant-assisted solvothermal synthesis, a slow reaction rate is favourable for isolating the growth step from the nucleation step. In the solution phase synthesis, the main factor determining the size and shape of the final products was the kinetic control of the nucleation and growth step. The shape of the nanoparticles can be effectively modulated by adding chemical capping agents to the synthesis system. Thus, the selective interaction of the capping agents on a facet of the first-formed Ni nanoparticles is the key to its growth.
The polyol assisted solvothermal synthesis using Ni salt and hydrazine hydrate as precursors, sodium hydroxide as stabilizing agent, and ethylene glycol as capping agent; the molar ratio of N2H4/Ni was found to play an important role in controlling the reaction rate. However, the increase in reaction rate led to agglomeration of the Ni nanoparticles which is a drawback in the fabrication of the nanoparticles.
In the standard airless solvothermal synthesis of Ni nanoparticles using trioctylphosphine oxide and pure hexadecylamine as the stabilizing agents, the shape of the nanocrystal is significantly affected by reaction parameters such as reaction temperature, time, solvent, and the chemical potential between the reacting species. The surfactant trioctylphosphine oxide was effective in controlling the particle size. Hence, in solvothermal synthesis various factors play important role in determining the final size and shape of the Ni nanoparticles.
6 Hybrid approach of top-down and bottom-up methods for the synthesis of Ni nanoparticles
Several physical techniques abound in literature for the fabrication of Ni nanoparticles, among which are radiolysis techniques such as ultrasonic sonication, microwave, and laser methods, and mechanical milling. Laser ablation protocols for the fabrication of nanosized metal alloys and oxides in liquid medium employing solid target are available in the literature. This technique is cost-effective with no need for high vacuum pumps and expensive chambers and is considered environmentally friendly.
6.1 Electrochemical pulse-current deposition technique for the fabrication of Ni nanoparticles
Tu et al. reported an electrochemical deposition technique for the synthesis of metallic nanoparticles. They adopted electrochemical deposition with pulse-current to synthesize Ni nanoparticles for application as catalysts in the development of aligned nanotubes of carbon. The density of the nanoparticle nucleation site was monitored through the alteration of the pulse-current period and magnitude [109]. The plasma-enhanced hot filament CVD generated aligned carbon nanotubes of Ni nanoparticles. The goal was to produce many particles on a large area with low density. To attain this goal, they employed the technique of electrochemical deposition by pulse current. A two-electrode system was adopted for the experiment, and eventually, Ni nanoparticles were obtained. Bao et al. reported the successful preparation of a highly ordered Ni nanotube array by electrodeposition into the holes of an alumina membrane altered with an organo-amine as a pore-wall altering agent. The resulting magnetic property was analysed [110].
It is fundamental to the Ni nanotube assembly procedure that the pore wall of the alumina template is altered with methyl-γ-diethyltriaminopropyldimethoxysilane. When Ni is electrochemically deposited in the pores, the presence of the amino group preferentially deposits the Ni on the walls of the unmodified alumina membrane to form magnetic Ni nanotubes. At higher current density, nickel hydroxide was produced on the alumina membrane at the time of electrodeposition. These metal nanotubes with open ends could be used for the creation of materials with special magnetic, optical, and electrical properties [110].
Pirota et al. synthesized novel magnetic materials by electrochemical deposition techniques. NiNPs were deposited inside alumina nanopores by applying a steady current pulse in a sequence [111]. Lee et al. developed a unique process to synthesize nanotubes of metallic elements by electrodeposition of preferential metal on the surface of membrane walls of the nanoparticles. In this nano synthesis process, many metals such as Fe, Au, Pl, Ag, Pt, Ni, and Co could be incorporated into the nanotube structure, thereby enabling the synthesis of barcode-type nanomaterial [112]. Clusters of staked Ni nanotubes and multisegmented nanotubes can be analysed by a quantum interference magnetometer to compare their magnetic properties. At lower fields of saturation, the clusters of disconnected nanotubes were effectively magnetized as compared to the array of continuous Ni nanotubes [112] as shown in Figure 10.
![Figure 10
The images of multisegmented nickel nanotubes using SEM Lee et al. [112].](/document/doi/10.1515/ntrev-2020-0109/asset/graphic/j_ntrev-2020-0109_fig_010.jpg)
The images of multisegmented nickel nanotubes using SEM Lee et al. [112].
6.2 Synthesis of Ni nanoparticles employing pulsed laser ablation in liquids technique
Gondal et al. synthesized nanoparticles of NiO by applying pulsed laser ablation (PLA) in liquid. They reported a homemade PLA setup consisting of a laser and a cell with a pure Ni metal plate dipped in liquid. Before laser irradiation, a pure Ni metal target was held on a magnetic rotator at the basement of a glass container. A colloidal mixture of NiO was produced, and Ni nanoparticles were isolated from water by centrifugation [113].
6.3 Fabrication of Ni nanoparticles by arc discharge method
The fabrication of Ni nanoparticles with unique structural and magnetic properties was reported by El-Khatib et al. They employed an arc and discharge technique with an ultrasonic nebulizer in an argon atmosphere to generate NiNPs. They suggested that the technique is economical and environmentally friendly. Similarly, the technique leads to the production of the small size distribution of NiNPs with high purity [114].
6.4 Production of Ni nanoparticles by microwave combustion technique
The production of nanometal by microwave combustion has the advantage of being highly exothermic, which leads to the uniform distribution of temperature within the bulk material and the surface, and hence, the fast production of nanoparticles. Ragupathi et al. reported a technique of microwave combustion in the fabrication of nanoparticles of Ni aluminate. Aluminium nitrate and Ni nitrate were used as precursors. The microwave oven was programmed such that it led to the formation of Ni nanoparticles as the product [115]. The microwave combustion leads to the formation of fine particles with uniform morphology. The microwave combustion technique is a rapid and economic technique for the fabrication of Ni nanocomposites within terms of time, simplicity, and energy. The NiAl2O4 nanoparticles generated by the microwave combustion technique have been used in the catalysis of benzyl alcohol in the liquid phase. It resulted in excellent reactivity, good recyclability, and high stability and was environmentally favourable.
Similarly, LaGrow and coworkers fabricated highly monodispersed NiNPs. They stated that face-cantered cubic crystals were obtained by employing trioctylphosphine surfactants under a reducing hydrogen atmosphere to favour thermodynamic growth stabilization of the nanoparticles [116]. They reported that changing the nickel precursor concentration to trioctylphosphine ratio was found to alter the face shape and size from spherical at 5 nm to cubic at 12 nm.
7 Applications of Ni nanoparticles
Generally, nanostructures such as nanoparticles, nanotubes, nanowires, nano-springs, nanobelts, and nano-rings are utilized in the development of flat-panel display, nonlinear optical devices, light-emitting diodes, transistors, and logic gates. Ni-based materials were also applied in turbines, automobile molds, and aerospace [117]. The application of Ni nanoparticles includes medical applications, as a catalyst, applications in sensor development, enhancement of materials, and adsorption of dyes. These are discussed in the following sections.
7.1 Biomedical applications of Ni nanoparticles
The use of Ni nanoparticles in biomedical applications and as an antibacterial agent has been reported in the literature. These include drug and gene delivery, magnetic resonance imaging, cell separation, biomedical detection, and diagnostics [118]. Guo et al. reported that functionally charged NiNPs could increase cell membrane permeability and promote cellular absorption into cancer cells of the outer target molecules [119]. These results show that NiNPs may have a possible mechanism for targeting the cytotoxicity of leukaemia cancer cells and that NiNPs may be implemented in related biomedical and clinical fields.
Ivanov et al. noted the synergistic effect of Ni nanoparticles with reduced graphene oxide to enable the designing of high-performance applications in bioinspired microelectronics for medical therapy [120]. Angajala et al. showed that Ni nanoparticles possess high larvicidal efficacy against Culex quinquefasciatus and excellent anti-inflammatory activity comparable to that of a standard essay. They suggested that the Ni nanoparticles can also be used as a good drug carrier as well as for the control of C. quinquefasciatus, a vector in the transmission of lymphatic filariasis, and dengue fever [68]. Figure 11 shows an illustration of biomedical applications of magnetic functionalized metal nanoparticles [121].
![Figure 11
Biomedical applications of magnetic functionalized metal nanoparticles [121].](/document/doi/10.1515/ntrev-2020-0109/asset/graphic/j_ntrev-2020-0109_fig_011.jpg)
Biomedical applications of magnetic functionalized metal nanoparticles [121].
Similarly, Helan et al. suggested the potential application of Ni nanoparticles in the suppression of microbial pathogens such as Staphylococcus aureus and Escherichia coli [122]. Roselina and coworkers noted the excellent catalytic and magnetic properties of Ni nanoparticles, given its tremendous potential in many applications including biomedical and medical fields [108]. Gong et al. reported that algae can accelerate the aggregation of nanoparticles as well as reduce NiO to Ni. They suggested that green algae may be promising for bioremediation of nano-pollution [89]. Sudhasree et al. reported that green Ni nanoparticles exhibit colloidal stability with antioxidant property, thereby making them good antimicrobial agents [76]. Table 5 presents the biomedical applications of Ni nanoparticles.
Biomedical applications of Ni nanoparticles
| Type of medical application | Structure of Ni nanoparticles | Size of Ni nanoparticles (nm) | Reference |
|---|---|---|---|
| Cancer treatment | Colloidal | 38.2 | Gorgizadeh et al. [123] |
| Integrated biomaterial | hcp | 0.5–24 | Ivanov et al. [120] |
| Antibacterial activity | hcp | 12 | Helan et al. [122] |
| Anti-inflammatory | fcc | 80–100 | Angajala et al. [68] |
| Absorbent of safranin-O | Amorphous | 320 | Ghaedi et al. [124] |
| Hydrodeoxygenation of microalgae | Colloidal | 3 | Song et al. [88] |
| Biomedical | fcc | 2–600 | Roselina et al. [108] |
| Thermo-therapeutic | Crystalline | 28 | Hoque et al. [125] |
| Electrochemical sensor | hcp | 8.9 | Neiva et al. [125] |
| Glucose sensor | fcc | 15 | Liu et al. [126] |
| High bioimaging resolution | Ni2+ ions doped NaYF4:Yb3+/Er3+ | 100 nm | Jia et al. [127] |
7.2 Applications of Ni nanoparticles as catalyst
The application of Ni nanoparticles as a catalyst has appeared in several reports. Simonsen et al. reported that Ni nanoparticles can be used as catalysts in the separation of emulsions, separation, cleaning of the oil spills, purification of water, and the separation of impurities from samples. The important utility of Ni nanoparticles is due to their strong magnetic response as well as interfacial properties which play important role in the efficient adsorption and rapid separation [128]. Woodard et al. reported that Ni nanoparticles are currently being investigated as a promising non-precious metal catalyst in magnetic nanomaterials and biomedicine and as a catalyst in optoelectronics devices [129]. Nie et al. fabricated a nonenzymatic, NiNP/straight multi-walled nanotubes-based amperometric sensor which was investigated for glucose oxidation [130]. NPS/straight multi-walled nanotubes were found to have an incredibly large active electrochemical surface area and elevated electrocatalytic activity in basic conditions for the electro-oxide of glucose. Figure 12 shows catalytic activity of NiNPs [131].
![Figure 12
Typical catalytic activity of NiNPs [131]. (a) reduction of CO2 bubbles, (b) aggregation of NiNPs and (c) adsorption of polymers.](/document/doi/10.1515/ntrev-2020-0109/asset/graphic/j_ntrev-2020-0109_fig_012.jpg)
Typical catalytic activity of NiNPs [131]. (a) reduction of CO2 bubbles, (b) aggregation of NiNPs and (c) adsorption of polymers.
Similarly, Song et al. noted that the catalytic Ni nanoparticle maintained a high level of activity even after four cycles of reaction because of the presence of more uniformly spaced, sintering, resilient Ni nanoparticles [88]. Gong et al. reported that Ni nanoparticle has great potential in catalysis of hydrogen evolution reactions under alkaline condition. it is comparable in activity to the most active hydrogen evolution reaction catalysed in the acid medium [132]. It was also found to be close to that of platinum-based catalyst. Furthermore, Feng et al. reported that Ni nanoparticles act both as protective and as a catalyst for hydrogen evolution [133]. Ragupathi et al. reported that Ni nanoparticles displayed a highly efficient optoelectronic property route for the selective oxidation of benzyl alcohol to benzaldehyde [115]. Dander and Garg reported the use of Ni nanoparticles as catalysts to break the C–N bond in amides; this resulted in a variety of improvements such as esterification and transamidation as well as Suzuki-Miyaura coupling and Negishi couplings. They suggested that the breaking of the C–N bond provides a new route to obtain C–C bonds and C-heteroatom using amide [134]. The applications of Ni nanoparticles in catalysis are summarized in Table 6.
The employment of Ni nanoparticles in catalysis
| Type of application | Structure of Ni nanoparticles | Size of Ni nanoparticle (nm) | Reference |
|---|---|---|---|
| Reductive amination | fcc | 15.35 | |
| Nonprecious catalyst | Crystalline | 3–40 | Woodard et al. [129] |
| Adsorption of dyes | Crystalline | 30 | Jin et al. [135] |
| Recoverable catalyst | Crystalline | 3.2 | Zhao et al. [136] |
| Dye-sensitized solar cells | fcc | 12–56 | Krishnapriya et al. [137] |
| Hydrogen storage | hcp/fcc | 5–10 | Wegner et al. [138] |
| Dehydrogenation of ammonia | Crystalline | 12 | Manna et al. [139] |
| Hydrogen evolution | hcp | 375 | Gong et al. [140] |
| Water splitting | Film | 5 | Feng et al. [133] |
| Absorbent in sewage treatment | fcc | 10–30 | Zhang et al. [145] |
| Photocatalysis | Amorphous | 2–3 | Agegnehu et al. [142] |
| Energy storage | Graphene carbon nanotubes/Ni foams | Ni vapour | Li et al. [143] |
| Resin graphitization | Crystalline | 50–100 | Pan et al. [144] |
7.3 Applications of Ni nanoparticles in the adsorption of dyes from industrial waste
Magnetic metallic Ni nanoparticles are good candidates for the adsorption of dyes. The removal of dyes from wastewater is crucial because organic dyes are major pollutants in wastewater. Organic dyes reduce the quality of water, thereby posing a significant impact on human health. Most organic dyes are toxic, mutagenic, and carcinogenic. Jin et al. confirmed the use of composites of Ni nanoparticles for the isolation of dyes from aqueous solution. They found that Ni nanocomposite with a large pore volume and high surface area can be easily separated from the aqueous solution by an external magnet [135]. Ghaedi et al. reported the use of Ni sulphide nanoparticles loaded on activated carbon as a novel adsorbent for the individual and simultaneous adsorption of methylene blue and safranin-O [124].
Furthermore, Sudhasree et al. reported the fabrication of Ni nanoparticles free of surfactants which were subsequently used in the removal of Congo red, an azo dye from industrial wastewater [76]. Zhang and coworkers confirmed the fabrication of Ni nanoparticles having excellent magnetic properties and crystallite size of 10–30 nm. These synthesized Ni nanoparticles were subsequently used as adsorbents of Congo red from industrial wastewater [141].
7.4 Application of Ni nanoparticles in dye-sensitized solar cells and sensors
Krishnapriya et al. described the fabrication of dye-sensitized solar cells with various nanostructured TiO2. It was incorporated with Ni nanocomposites with morphologies like interconnected bead-like, spindle shape-like, square platelets-like, and porous sphere-like to yield dye-sensitized solar cells. The nanostructures were synthesized in different solvents, namely ethanol, a mixture of ethanol and water, as well as HF in conjunction with shape- and size-tuned Ni nanocomposites of mixed triangular and hexagonal morphological crystals. These had sizes ranging from 15 to 62 nm. They reported that the incorporation of Ni nanocomposites effectively traps incident light and successively improves the rate of electron-hole pair formation and short circuit current. The fabricated dye-sensitized solar cells were found to exhibit excellent stability in conventional electrolytes over a duration of a month [137].
Furthermore, Liu et al. developed a high-performance nonenzymatic sensor on Ni nanoparticle-chitosan nanocomposite. They suggested that nonenzymatic sensors can be used for monitoring blood glucose due to their low cost, simple preparation, and excellent detection of glucose [25].
7.5 Application of Ni nanoparticles in superconductors and enhancement of materials
Zhang et al. reported the fabrication of electrodes employing Ni nanocomposite materials. The electrodes showed low charge-transfer resistance, outstanding cycle stability, high specific capacitance, and good rate performance. The high capacitation of the electrode nanocomposite was due to enhanced conductivity, regularly scattered nanoparticles of Ni(OH)2, low interfacial resistance, and synergetic effects of each portion. They suggested the composite as a promising material for high energy supercapacitor application with the improved electrochemical performance [145].
Similarly, Li et al. developed a high-performance electrochemical supercapacitor from amorphous Ni (OH)2 nanospheres. The developed electrode was found to exhibit high capacitance, high energy density, and long life. They suggested that the electrode can be used in advanced electrochemical pseudocapacitor material [146]. Agegnehu et al. reported the use of Ni and NiO nanoparticles for the enhancement of photocatalytic hydrogen evolution from aqueous methanol. They suggested that the Ni/graphene oxide exhibits high activity attributed to ease of trapping photogenerated electrons by Ni and NiO nanoparticles [142].
7.6 Tabulation of other applications of NiNPs
The other applications of Ni nanoparticles in various fields are hereby reported in Table 7. It shows the field of application of the Ni nanoparticles, the structure of the nanoparticles, the size of the nanoparticles, and the references. The nanoparticle crystallinity influences nanomaterials applications [69].
Other applications of Ni nanoparticles
| Application of Ni nanoparticles | Structures | Sizes in (nm) | Reference |
|---|---|---|---|
| Ultra-high compressive strength | Crystalline | 100–1,000 | Sharma et al. [147] |
| Catalyst for p-nitrophenol reduction | Crystalline | 34.8–143.2 | Jiang et al. [148] |
| Magnetic separation | Crystalline | 5–20 | Simonsen et al. [128] |
| Voltammetric determination of Rifampicin | fcc | 3 | Oliveira et al. [149] |
| Added to polyester fabric | Crystalline | 40 | Afshari et al. [50] |
| Microwave absorption | fcc | 200–800 | Guo et al. [105] |
| Catalyst for hydrodecylchlorination | hcp | 6 | Duraisamy et al. [151] |
| Catalyst for the oxidation of benzyl alcohol | fcc | 19.8 | Ragupathi et al. [115] |
| Free radical scavenging activity | fcc | 2,453–2,695 | Sudhasree et al. [76] |
| Catalyst for hydrodesulfurization | Crystalline | 5–20 | Layan Savithra et al. [152] |
| Electrochemical pseudocapacitor material | Amorphous | 100 | Li et al. [146] |
| Catalyst for hydrogen evolution | Crystalline | 10–20 | Karami et al. [153] |
| Electroless Ni plating | Amorphous | 10–60 | Wu et al. [154] |
| Optoelectronic applications | fcc | 80–400 | Libor et al. [103] |
| Magnetic data storage application | fcc | 30–50 | Yu et al. [81] |
| Conducting material | fcc/hcp | 12.9 | Chen et al. [155] |
| Photocatalyst | Crystalline | 22 | Asaithambi et al. [156] |
8 The advantages of using Ni as catalyst
The advantages of using Ni nanoparticles as catalysts are hereby reported with specific reactions and conversion techniques. The physical and chemical characteristics of Ni nanoparticles determine their potential applications in research as well as in the industries. Various measurement techniques [157] are employed in the characterization of Ni nanoparticles as illustrated in Table 8.
Characterization techniques for NiNPs
| Technique | Acronym | Utility |
|---|---|---|
| UV-Visible spectroscopy | UV-Vis | Chemical analysis |
| Electron probe microanalysis | EPMA | Particle size/chemical analysis |
| Transmission electron microscopy | TEM | Imaging particle/size/shape |
| Scanning electron microscopy | SEM | Imaging/topology/size |
| Energy dispersive spectroscopy | EDS | Elemental composition |
| High resolution transmission electron microscopy | HRTEM | Imaging structure/chemical analysis |
| X-ray diffraction | XRD | Crystallinity/size/shape |
| Brunauer–Emmett–Teller analysis | BET | Porosity/surface area |
| Fourier transform infrared spectroscopy | FT-IR | Organic functional groups |
| Atomic force microscopy | AFM | Topology/imaging/surface structure |
| Thermal gravimetric analysis | TGA | Mass loss vs temperature |
| Differential scanning calorimetry | DSC | Reaction heat/phase changes |
| Auger electron spectroscopy | AES | Chemical structure analysis |
8.1 The advantages of Ni nanoparticles as a cheap catalyst
Nickel in comparison to commonly used metal catalysts such as some transition metals and noble metals is relatively cheap. Dander and Garg reported the potential benefits of Ni catalysis due to the abundance of Ni, resulting in low cost, acceptable levels of toxicological requirement for orally administered medication, opportunity in green chemistry, and the ability to draw on alternative reactivity profiles to create novel chemical transitions [134]. According to Tobias and Chatani, arene preparation can be improved by the application of Ni catalyst in cross-coupling reactions. The aryl halogens can be coupled with organic nucleophiles and organometallic. Ni-catalysed reductive cleavage of aryl ethers in the absence of an external reducing agent provides strong support for this oxidative addition process [158]. Martindale et al. documented the complex nickel bis-diphosphine as a catalyst to produce solar hydrogen [159]. That is because nickel has the advantages of being an active non-noble metal catalyst to produce hydrogen in acidic, organic, and aqueous solutions. Nickel catalyst has a low overpotential for the reduction of protons in water. Furthermore, with solar light irradiation, Ni catalyses ruthenium dye inhomogeneous schemes as well as hybrid systems with nanoparticulate light absorbers [159].
8.2 Advantages of Ni nanoparticles as a catalyst in the conversion of biomass to fuels; Ni catalyst is being employed in various reactions involving the conversion of biomass to fuels
Lignin is a polymer of monomeric aromatic compounds and has the potential to be a source for liquid fuels and valuable chemicals. Luo et al. found Ni to be an efficient catalyst for catalytic depolymerization of lignin, leading to its conversion into four phenolic products. The conversion of carbon dioxide to methane is a famous reaction called the Sabatier reaction [160]. Fukuhara et al. found that the conversion of carbon dioxide to methane using Ni catalyst showed steady catalytic performance. It maintained a high activity and high selectivity during a durability test. It was found that Ni catalyst has the potential for producing energy from carbon dioxide [161]. Sweeney et al. described the use of Ni catalyst for the allylation reactions using readily available, inexpensive, and air-insensitive catalysts and reagents [162]. The use of allyl alcohols as substrates and an inexpensive Ni salt as a precursor of the catalyst offers significant advantages.
8.3 Advantages of using Ni catalyst in Suzuki-Miyaura reactions
Guard et al. described the advantages of using Ni catalyst in Suzuki-Miyaura reactions. Suzuki-Miyaura reactions are generally employed in the formation of new carbon-carbon bonds [163]. Currently, the best catalyst in use is based on precious metals such as Palladium. The use of cheap Ni-based catalyst results in affordable systems. Nickel has a smaller size compared to palladium, leading to weaker binding and increased nucleophilicity in heterocyclic coordinating atoms. The chemical advantages of using Ni over palladium are that the former can be used in Suzuki-Miyaura coupling in acyliminium, carbonate, carbamate, sulfamate, and sp3-generated substrates. The catalytic application of NiNPs in the Suzuki-Miyaura cross-coupling reaction [164] is illustrated in Figure 13.
![Figure 13
Illustration of the application of NiNPs in Suzuki-Miyaura cross-coupling reaction [164].](/document/doi/10.1515/ntrev-2020-0109/asset/graphic/j_ntrev-2020-0109_fig_013.jpg)
Illustration of the application of NiNPs in Suzuki-Miyaura cross-coupling reaction [164].
8.4 Advantages of using Ni nanoparticle due to its various oxidation states
Tasker and Jamison reported that, among the many advantages of using Ni-catalyst, one is the availability of multiple oxidation states, including Ni0, Ni1+, Ni2+, and Ni3+, and recently reported a well-defined Ni4+ species. Nickel-catalysed reactions either proceed via a Ni0/Ni2+ pathway or via a Ni+/Ni3+ pathway. The formation of products is likely enabled by a mechanistically distinct direct oxidation of Ni intermediates [165]. The features described above help in the wide use of Ni as a catalyst and in its commercialization in the fields of organic synthesis, inorganic synthesis, environmental cleaning, and medicinal drug administration. The electrochemical oxidation of urea based on Ni(ii/iii) sites [166] is shown in Figure 14.
![Figure 14
(a) Voltammograms recorded for Ni/C in KOH 1 mol L−1 and urea 0.1 mol L−1, at 10 mV s−1. (b) Voltammograms of Ni100−xRhx/C in KOH 1 mol L−1 and urea 0.1 mol L−1 (10 mV s−1 [166]).](/document/doi/10.1515/ntrev-2020-0109/asset/graphic/j_ntrev-2020-0109_fig_014.jpg)
(a) Voltammograms recorded for Ni/C in KOH 1 mol L−1 and urea 0.1 mol L−1, at 10 mV s−1. (b) Voltammograms of Ni100−xRhx/C in KOH 1 mol L−1 and urea 0.1 mol L−1 (10 mV s−1 [166]).
Mirzaei et al. suggested that the Voltammograms obtained with nickel-rich compounds indicate the presence of a new peak at a lower positive potential (−50 mV) and a comparable strength than the urea oxidation peak normally found with nickel [166]. Further studies are required to explain its nature.
9 Conclusion
In this review, several NiNPs synthetic techniques have been reported, ranging from the use of plant extracts, plant secretions, fungi, algae, and microorganisms, as well as some physical and chemical methods of synthesis. Plant extracts are economically viable, and it can be upgraded and environmentally harmless. Biogenically synthesized nickel nanomaterials are free of toxic chemicals and are especially suitable for applications in biomedicine. Various physical and chemical methods for the fabrication of nickel nanomaterials, such as mechanical milling, microwave, sonochemical, and electrochemical techniques, have been described. These methods yield NiNPs with high quality magnetic properties, controlled size, and monodispersed population. Proposed reaction mechanisms of NiNPs synthesis based on plant extracts and physical methods as reported in literature have been outlined with the specific functional groups involved in the reduction of nickel to NiNPs. The applications of nickel nanosized materials in various fields such as catalysis, biomedical applications, adsorption of dyes from industrial waste, fabrication of dye-sensitized solar cells and sensors, and in the development of supercapacitors have also been discussed. The specific advantages of using Ni nanoparticles as a cheap catalyst in the conversion of biomass to fuels, in Suzuki-Miyaura reactions, as well as the advantages of Ni nanoparticles due to its multiple oxidation states have been reported.
Extensive research has been published on the synthesis, characterization, and applications of Ni nanoparticles. However, there are no universal conclusions [167] made on the biomedical effects of Ni nanoparticles on human, animal, and plant lives. The reported reaction mechanisms in literature are inconclusive; hence, the need to ascertain the mechanisms leading to the formation of Ni nanoparticles more especially in plant-mediated synthesis. While the advantages of using Ni nanoparticles as catalyst have been stressed, the disadvantages have not been enumerated.
Acknowledgments
The authors wish to acknowledge Universiti Sains Malaysia for sponsoring this project under USM-STG-6315076 and RUI-1001/P Kimia/8011086, School of Chemical Sciences, Universiti Sains Malaysia for technical support. N. J. wish to thank Federal College of Education Technical Gombe, Nigeria, for TETFUND Postgraduate Scholarship.
-
Author contributions: Muhammad Bisyrul Hafi Othman, Hooi Ling Lee, Mohd Hazwan Hussin: Conceptualization; Nuru-Deen Jaji: Writing-original reviewer draft preparation, visualization, investigation; Muhammad Bisyrul Hafi Othman, Hazizan Md Akil, Muhammad Razlan Zakaria: Reviewing and Editing; Muhammad Bisyrul Hafi Othman, Hooi Ling Lee: Supervision, funding acquisition. This article has been read and approved by all listed authors.
-
Conflict of interest: The authors declare no conflict of interest regarding the publication of this paper.
References
[1] Paramo LA, Feregrino-Perez AA, Guevara R, Mendoza S, Esquivel K. Nanoparticles in agroindustry: applications, toxicity, challenges, and trends. Nanomaterials. 2020;10(9):1654–86.10.3390/nano10091654Search in Google Scholar PubMed PubMed Central
[2] Magaye R, Zhao J. Recent progress in studies of metallic nickel and nickel-based nanoparticles’ genotoxicity and carcinogenicity. Environ Toxicol Pharmacol. 2012;34(3):644–50.10.1016/j.etap.2012.08.012Search in Google Scholar PubMed
[3] Bibi I, Kamal S, Ahmed A, Iqbal M, Nouren S, Jilani K, et al. Nickel nanoparticle synthesis using camellia sinensis as reducing and capping agent: growth mechanism and photo-catalytic activity evaluation. Int J Biol Macromol. 2017;103:783–90.10.1016/j.ijbiomac.2017.05.023Search in Google Scholar PubMed
[4] Cheng Y, Guo M, Zhai M, Yu Y, Hu J. Nickel nanoparticles anchored onto Ni foam for supercapacitors with high specific capacitance. J Nanosci Nanotechnol. 2020;20(4):2402–7.10.1166/jnn.2020.17377Search in Google Scholar PubMed
[5] Abdel Fattah AR, Majdi T, Abdalla AM, Ghosh S, Puri IK. Nickel nanoparticles entangled in carbon nanotubes: novel ink for nanotube printing. ACS Appl Mater Interfaces. 2016;8(3):1589–93.10.1021/acsami.5b11700Search in Google Scholar PubMed
[6] Jiao M, Yao Y, Pastel G, Li T, Liang Z, Xie H, et al. Fly-through synthesis of nanoparticles on textile and paper substrates. Nanoscale. 2019;11(13):6174–81.10.1039/C8NR10137JSearch in Google Scholar
[7] Ni H, Zhu J, Wang Z, Lv H, Su Y, Zhang X. A brief overview on grain growth of bulk electrodeposited nanocrystalline nickel and nickel-iron alloys. Rev Adv Mater Sci. 2019;58(1):98–106.10.1515/rams-2019-0011Search in Google Scholar
[8] Reena Mary AP, Suchand Sandeep CS, Narayanan TN, Philip R, Moloney P, Ajayan PM, et al. Nonlinear and magneto-optical transmission studies on magnetic nanofluids of non-interacting metallic nickel nanoparticles. Materials. 2011;22(37):375702–8.10.1088/0957-4484/22/37/375702Search in Google Scholar PubMed
[9] Barsan MM, Enache TA, Preda N, Stan G, Apostol NG, Matei E, et al. Direct immobilization of biomolecules through magnetic forces on Ni electrodes via Ni nanoparticles: applications in electrochemical biosensors. ACS Appl Mater Interfaces. 2019;11(22):19867–77.10.1021/acsami.9b04990Search in Google Scholar PubMed
[10] Kiran S, Rafique MA, Iqbal S, Nosheen S, Naz S, Rasheed A. Synthesis of nickel nanoparticles using citrullus colocynthis stem extract for remediation of reactive yellow 160 dye. Environ Sci Pollut Res. 2020;27(26):32998–33007.10.1007/s11356-020-09510-9Search in Google Scholar PubMed
[11] Hill D, Barron AR, Alexander S. Comparison of hydrophobicity and durability of functionalized aluminium oxide nanoparticle coatings with magnetite nanoparticles-links between morphology and wettability. J Colloid Interface Sci. 2019;555:323–30.10.1016/j.jcis.2019.07.080Search in Google Scholar PubMed
[12] Bian Z, Das S, Wai MH, Hongmanorom P, Kawi S. A review on bimetallic nickel-based catalysts for CO2 reforming of methane. Chemphyschem. 2017;18(22):3117–34.10.1002/cphc.201700529Search in Google Scholar PubMed
[13] Sagasti A, Palomares V, Porro JM, Orue I, Sanchez-Ilarduya MB, Lopes AC, et al. Magnetic, magnetoelastic and corrosion resistant properties of (Fe-Ni)-based metallic glasses for structural health monitoring applications. Materials. 2019;13(1):57–70.10.3390/ma13010057Search in Google Scholar PubMed PubMed Central
[14] Wang D, Jia Y, He Y, Wang L, Fan J, Xie H, et al. Enhanced photothermal conversion properties of magnetic nanofluids through rotating magnetic field for direct absorption solar collector. J Colloid Interface Sci. 2019;557:266–75.10.1016/j.jcis.2019.09.022Search in Google Scholar PubMed
[15] Thellaputta GR, Chandra PS, Rao C. Machinability of nickel-based superalloys: a review. Mater Today Proc. 2017;4(2):3712–21.10.1016/j.matpr.2017.02.266Search in Google Scholar
[16] Kate RS, Khalate SA, Deokate RJ. Overview of nanostructured metal oxides and pure nickel oxide (NiO) electrodes for supercapacitors: a review. J Alloy Compd. 2018;734:89–111.10.1016/j.jallcom.2017.10.262Search in Google Scholar
[17] Zhang L, Shi D, Liu T, Jaroniec M, Yu J. Nickel-based materials for supercapacitors. Mater Today. 2019;25:35–65.10.1016/j.mattod.2018.11.002Search in Google Scholar
[18] Sequeira CA, Cardoso DS, Amaral L, Šljukić B, Santos DM. On the performance of commercially available corrosion-resistant nickel alloys: a review. Corros Rev. 2016;34(4):187–200.10.1515/corrrev-2016-0014Search in Google Scholar
[19] Imran Din M, Rani A. Recent advances in the synthesis and stabilization of nickel and nickel oxide nanoparticles: a green adeptness. Int J Anal Chem. 2016;2016:1–4.10.1155/2016/3512145Search in Google Scholar PubMed PubMed Central
[20] Hossain MD, Mayanovic RA, Dey S, Sakidja R, Benamara M. Room-temperature ferromagnetism in Ni(ii)-chromia based core–shell nanoparticles: experiment and first principles calculations. Phys Chem Chem Phys. 2018;20(15):10396–406.10.1039/C7CP08597DSearch in Google Scholar
[21] Chen DH, Wu SH. Synthesis of nickel nanoparticles in water-in-oil microemulsions. Chem Mater. 2000;12(5):1354–60.10.1021/cm991167ySearch in Google Scholar
[22] Hou Y, Kondoh H, Ohta T, Gao S. Size-controlled synthesis of nickel nanoparticles. Appl Surf Sci. 2005;241(1–2):218–22.10.1016/j.apsusc.2004.09.045Search in Google Scholar
[23] Ely TO, Amiens C, Chaudret B, Snoeck E, Verelst M, Respaud M, et al. Synthesis of nickel nanoparticles. Influence of aggregation induced by modification of poly (vinylpyrrolidone) chain length on their magnetic properties. Chem Mater. 1999;11(3):526–9.10.1021/cm980675pSearch in Google Scholar
[24] Chen DH, Hsieh CH. Synthesis of nickel nanoparticles in aqueous cationic surfactant solutions. J Mater Chem. 2002;12(8):2412–5.10.1039/b200603kSearch in Google Scholar
[25] Liu Z, Guo Y, Dong C. A high performance nonenzymatic electrochemical glucose sensor based on polyvinylpyrrolidone–graphene nanosheets–nickel nanoparticles–chitosan nanocomposite. Talanta. 2015;137:87–93.10.1016/j.talanta.2015.01.037Search in Google Scholar PubMed
[26] You W, Che R. Excellent NiO-Ni nanoplate microwave absorber via pinning effect of antiferromagnetic-ferromagnetic interface. ACS Appl Mater Interfaces. 2018;10(17):15104–11.10.1021/acsami.8b03610Search in Google Scholar PubMed
[27] Karam L, Reboul J, El Hassan N, Nelayah J, Massiani P. Nanostructured nickel aluminate as a key intermediate for the production of highly dispersed and stable nickel nanoparticles supported within mesoporous alumina for dry reforming of methane. Molecules. 2019;24(22):4107–19.10.3390/molecules24224107Search in Google Scholar PubMed PubMed Central
[28] Lyu F, Wang Q, Choi SM, Yin Y. Noble-metal-free electrocatalysts for oxygen evolution. Small. 2019;15(1):1804201–17.10.1002/smll.201804201Search in Google Scholar PubMed
[29] Ealias AM, Saravanakumar M. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf Ser Mater Sci Eng. 2017;263:32019–32.10.1088/1757-899X/263/3/032019Search in Google Scholar
[30] Basu JK, Sengupta S. Catalytic reduction of nitrobenzene using silver nanoparticles embedded calcium alginate film. J Nanosci Nanotechnol. 2019;19(11):7487–92.10.1166/jnn.2019.16669Search in Google Scholar PubMed
[31] Adam AA, Szabados M, Varga G, Papp A, Musza K, Konya Z, et al. Ultrasound-assisted hydrazine reduction method for the preparation of nickel nanoparticles, physicochemical characterization and catalytic application in Suzuki-Miyaura cross-coupling reaction. Nanomaterials. 2020;10(4):632–49.10.3390/nano10040632Search in Google Scholar PubMed PubMed Central
[32] Marzun G, Levish A, Mackert V, Kallio T, Barcikowski S, Wagener P. Laser synthesis, structure and chemical properties of colloidal nickel-molybdenum nanoparticles for the substitution of noble metals in heterogeneous catalysis. J Colloid Interface Sci. 2017;489:57–67.10.1016/j.jcis.2016.09.014Search in Google Scholar PubMed
[33] Menezes JC, Ferreira NS, Abracado LG, Macedo MA. Synthesis and characterization of nickel nanoparticles prepared using the aquolif approach. J Nanosci Nanotechnol. 2014;14(8):5903–10.10.1166/jnn.2014.8727Search in Google Scholar PubMed
[34] Molnarova O, Malek P, Vesely J, Minarik P, Lukac F, Chraska T, et al. The influence of milling and spark plasma sintering on the microstructure and properties of the Al7075 alloy. Materials. 2018;11(4):547–64.10.3390/ma11040547Search in Google Scholar PubMed PubMed Central
[35] Oujja M, Izquierdo JG, Banares L, de Nalda R, Castillejo M. Observation of middle-sized metal clusters in femtosecond laser ablation plasmas through nonlinear optics. Phys Chem Chem Phys. 2018;20(25):16956–65.10.1039/C8CP02825GSearch in Google Scholar PubMed
[36] Yao Z, Wang G, Shi Y, Zhao Y, Jiang J, Zhang Y, et al. One-step synthesis of nickel and cobalt phosphide nanomaterials via decomposition of hexamethylenetetramine-containing precursors. Dalton Trans. 2015;44(31):14122–9.10.1039/C5DT02319JSearch in Google Scholar
[37] Kobayashi J, Kobayashi N, Itaya Y, Hasatani M. Synthesis of Ni base nanoparticles by W/O emulsion combustion. J Nanostruct Chem. 2019;9(3):189–95.10.1007/s40097-019-0309-6Search in Google Scholar
[38] Reena Mary AP, Suchand Sandeep CS, Narayanan TN, Philip R, Moloney P, Ajayan PM, et al. Nonlinear and magneto-optical transmission studies on magnetic nanofluids of non-interacting metallic nickel nanoparticles. Nanotechnology. 2011;22(37):375702375702–8.10.1088/0957-4484/22/37/375702Search in Google Scholar PubMed
[39] Kumar DS, Kumar BJ, Mahesh H. Quantum nanostructures (QDs): an overview in synthesis of inorganic. Nanomaterials. 2018;15:59–88.Search in Google Scholar
[40] Rodrigues MS, Borges J, Proença M, Pedrosa P, Martin N, Romanyuk K, et al. Nanoplasmonic response of porous Au-TiO2 thin films prepared by oblique angle deposition. Nanotechnology. 2019;30(22):1–29.10.1088/1361-6528/ab068eSearch in Google Scholar PubMed
[41] Park J, Kang E, Son SU, Park HM, Lee MK, Kim J, et al. Monodisperse nanoparticles of Ni and NiO: synthesis, characterization, self-assembled superlattices, and catalytic applications in the Suzuki coupling reaction. Adv Mater. 2005;17(4):429–34.10.1002/adma.200400611Search in Google Scholar
[42] Pandey RK, Chen L, Teraji S, Nakanishi H, Soh S. Eco-friendly, direct deposition of metal nanoparticles on graphite for electrochemical energy conversion and storage. ACS Appl Mater Interfaces. 2019;11(40):36525–34.10.1021/acsami.9b09273Search in Google Scholar PubMed
[43] Gonzalez-Martinez IG, Bachmatiuk A, Gemming T, Cuniberti G, Trzebicka B, Rummeli MH. Room temperature single-step synthesis of metal decorated boron-rich nanowires via laser ablation. Nano Convergence. 2019;6(1):8–21.10.1186/s40580-019-0185-2Search in Google Scholar PubMed PubMed Central
[44] Kylian O, Shelemin A, Solar P, Pleskunov P, Nikitin D, Kuzminova A, et al. Magnetron sputtering of polymeric targets: from thin films to heterogeneous metal/plasma polymer nanoparticles. Materials. 2019;12(15):2366–82.10.3390/ma12152366Search in Google Scholar PubMed PubMed Central
[45] Fu X, Cai J, Zhang X, Li WD, Ge H, Hu Y. Twn fabrication of shape-controlled, monodisperse nanoparticles for biomedical applications. Adv Drug Deliv Rev. 2018;132:169–87.10.1016/j.addr.2018.07.006Search in Google Scholar PubMed
[46] Heilmann M, Kulla H, Prinz C, Bienert R, Reinholz U, Guilherme Buzanich A, et al. Advances in nickel nanoparticle synthesis via oleylamine route. Nanomaterials. 2020;10(4):713.10.3390/nano10040713Search in Google Scholar PubMed PubMed Central
[47] Liu Y, Xu X, Guo C, Kong H. Analysis on machining performance of nickel-base superalloy by electrochemical micro-milling with high-speed spiral electrode. Micromachines. 2019;10(7):476–90.10.3390/mi10070476Search in Google Scholar PubMed PubMed Central
[48] Ochirkhuyag A, Sapi A, Szamosvolgyi A, Kozma G, Kukovecz A, Konya Z. One-pot mechanochemical ball milling synthesis of the MnOx nanostructures as efficient catalysts for CO2 hydrogenation reactions. Phys Chem Chem Phys. 2020;22(25):13999–4012.10.1039/D0CP01855DSearch in Google Scholar PubMed
[49] Bussamara R, Eberhardt D, Feil AF, Migowski P, Wender H, de Moraes DP, et al. Sputtering deposition of magnetic Ni nanoparticles directly onto an enzyme surface: a novel method to obtain a magnetic biocatalyst. Chem Commun. 2013;49(13):1273–5.10.1039/c2cc38737aSearch in Google Scholar PubMed
[50] Li B, Zeng HC. Formation combined with intercalation of Ni and its alloy nanoparticles within mesoporous silica for robust catalytic reactions. ACS Appl Mater Interfaces. 2018;10(35):29435–47.10.1021/acsami.8b07896Search in Google Scholar PubMed
[51] Kim SJ, Kim BH, Chung MC, Ahn HG, Kim SC, Kim HG, et al. The synthesis of nickel nanoparticles by liquid phase plasma processing. J Nanosci Nanotechnol. 2013;13(3):1997–2000.10.1166/jnn.2013.6971Search in Google Scholar PubMed
[52] Li FL, Zhang HJ. Synthesis of hollow sphere and 1D structural materials by sol–gel process. Materials. 2017;10(9):995–1011.10.3390/ma10090995Search in Google Scholar PubMed PubMed Central
[53] Parashar M, Shukla VK, Singh R. Metal oxides nanoparticles via sol–gel method: a review on synthesis, characterization and applications. J Mater Sci. 2020;31(2):1–21.10.1007/s10854-020-02994-8Search in Google Scholar
[54] Manzano Martínez AN, van Eeten KM, Schouten JC, van der Schaaf J. Micromixing in a rotor–stator spinning disc reactor. Ind Eng Chem Res. 2017;56(45):13454–60.10.1021/acs.iecr.7b01324Search in Google Scholar PubMed PubMed Central
[55] Khodashenas B, Zadghaffari R, Jafari SD. Process intensification approach for the synthesis of metal nanoparticles: a mini review. Orient J Chem. 2015;31(1):249–57.10.13005/ojc/31.Special-Issue1.30Search in Google Scholar
[56] Riikonen S, Krasheninnikov A, Halonen L, Nieminen R. The role of stable and mobile carbon adspecies in copper-promoted graphene growth. J Phys Chem C. 2012;116(9):5802–9.10.1021/jp211818sSearch in Google Scholar
[57] Clemente A, Lobera MP, Balas F, Santamaria J. A versatile generator of nanoparticle aerosols. A novel tool in environmental and occupational exposure assessment. Sci Total Env. 2018;625:978–86.10.1016/j.scitotenv.2017.12.125Search in Google Scholar PubMed
[58] Ali M, Ahmed T, Wu W, Hossain A, Hafeez R, Islam Masum M, et al. Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials. 2020;10(6):1146–79.10.3390/nano10061146Search in Google Scholar PubMed PubMed Central
[59] Alsammarraie FK, Wang W, Zhou P, Mustapha A, Lin M. Green synthesis of silver nanoparticles using turmeric extracts and investigation of their antibacterial activities. Colloids Surf B. 2018;171:398–405.10.1016/j.colsurfb.2018.07.059Search in Google Scholar PubMed
[60] Chaudhary J, Tailor G, Yadav B, Michael O. Synthesis and biological function of nickel and copper nanoparticles. Heliyon. 2019;5(6):e01878.10.1016/j.heliyon.2019.e01878Search in Google Scholar PubMed PubMed Central
[61] Li PY, Cao ZH, Meng XK. Facile synthesis of superparamagnetic Ni-Fe ultrafine nanoalloy nanoparticles with equilibrium ordered phase structure via a sol–gel process. Dalton Trans. 2012;41(39):12101–5.10.1039/c2dt31484cSearch in Google Scholar PubMed
[62] McKeown JT, Roberts NA, Fowlkes JD, Wu Y, LaGrange T, Reed BW, et al. Real-time observation of nanosecond liquid-phase assembly of nickel nanoparticles via pulsed-laser heating. Langmuir. 2012;28(49):17168–75.10.1021/la303657eSearch in Google Scholar PubMed
[63] Li H, Ci S, Zhang M, Chen J, Lai K, Wen Z. Facile spray-pyrolysis synthesis of yolk-shell earth-abundant elemental nickel-iron-based nanohybrid electrocatalysts for full water splitting. ChemSusChem. 2017;10(23):4756–63.10.1002/cssc.201701521Search in Google Scholar PubMed
[64] Fadil NA, Saravanan G, Ramesh GV, Matsumoto F, Yoshikawa H, Ueda S, et al. Synthesis and electrocatalytic performance of atomically ordered nickel carbide (Ni3C) nanoparticles. Chem Commun. 2014;50(49):6451–3.10.1039/C4CC01336KSearch in Google Scholar
[65] Bhattacharjee D, Sheet SK, Khatua S, Biswas K, Joshi S, Myrboh B. A reusable magnetic nickel nanoparticle-based catalyst for the aqueous synthesis of diverse heterocycles and their evaluation as potential anti-bacterial agent. Bioorg Med Chem. 2018;26(18):5018–28.10.1016/j.bmc.2018.08.033Search in Google Scholar PubMed
[66] Khalid M, Hassan A, Honorato AMB, Crespilho FN, Varela H. Nano-flocks of a bimetallic organic framework for efficient hydrogen evolution electrocatalysis. Chem Commun. 2018;54(78):11048–51.10.1039/C8CC06918BSearch in Google Scholar
[67] Machado IM, Atsumi S. Cyanobacterial biofuel production. J Biotechnol. 2012;162(1):50–6.10.1016/j.jbiotec.2012.03.005Search in Google Scholar PubMed
[68] Angajala G, Ramya R, Subashini R. In-vitro anti-inflammatory and mosquito larvicidal efficacy of nickel nanoparticles phytofabricated from aqueous leaf extracts of Aegle marmelos Correa. Acta Trop. 2014;135:19–26.10.1016/j.actatropica.2014.03.012Search in Google Scholar PubMed
[69] Khatami M, Sarani M, Mosazadeh F, Rajabalipour M, Izadi A, Abdollahpour-Alitappeh M, et al. Nickel-doped cerium oxide nanoparticles: green synthesis using stevia and protective effect against harmful ultraviolet rays. Molecules. 2019;24(24):4424.10.3390/molecules24244424Search in Google Scholar PubMed PubMed Central
[70] Nguyen DH, Lee JS, Park KD, Ching YC, Nguyen XT, Phan VHG, et al. Green silver nanoparticles formed by phyllanthus urinaria, pouzolzia zeylanica, and scoparia dulcis leaf extracts and the antifungal activity. Nanomaterials. 2020;10(3):542–54.10.3390/nano10030542Search in Google Scholar PubMed PubMed Central
[71] Thanh NT, Maclean N, Mahiddine S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem Rev. 2014;114(15):7610–30.10.1021/cr400544sSearch in Google Scholar PubMed
[72] Cohen JM, Beltran-Huarac J, Pyrgiotakis G, Demokritou P. Effective delivery of sonication energy to fast settling and agglomerating nanomaterial suspensions for cellular studies: implications for stability, particle kinetics, dosimetry and toxicity. NanoImpact. 2018;10:81–6.10.1016/j.impact.2017.12.002Search in Google Scholar PubMed PubMed Central
[73] Huang J, Lin L, Sun D, Chen H, Yang D, Li Q. Bio-inspired synthesis of metal nanomaterials and applications. Chem Soc Rev. 2015;44(17):6330–74.10.1039/C5CS00133ASearch in Google Scholar
[74] Jeyaraj Pandian C, Palanivel R, Dhanasekaran S. Screening antimicrobial activity of nickel nanoparticles synthesized using Ocimum sanctum leaf extract. J Nanopart. 2016;2016:1–13.10.1155/2016/4694367Search in Google Scholar
[75] Nouneh K, Oyama M, Diaz R, Abd-Lefdil M, Kityk I, Bousmina M. Nanoscale synthesis and optical features of metallic nickel nanoparticles by wet chemical approaches. J Alloy Compd. 2011;509(19):5882–6.10.1016/j.jallcom.2011.02.164Search in Google Scholar
[76] Sudhasree S, Shakila Banu A, Brindha P, Kurian GA. Synthesis of nickel nanoparticles by chemical and green route and their comparison in respect to biological effect and toxicity. Toxicol Env Chem. 2014;96(5):743–54.10.1080/02772248.2014.923148Search in Google Scholar
[77] Mariam AA, Kashif M, Arokiyaraj S, Bououdina M, Sankaracharyulu M, Jayachandran M, et al. Bio-synthesis of NiO and Ni nanoparticles and their characterization. Dig J Nanomater Biostruct. 2014;9(3):1007–19.Search in Google Scholar
[78] Chen H, Wang J, Huang D, Chen X, Zhu J, Sun D, et al. Plant-mediated synthesis of size-controllable Ni nanoparticles with alfalfa extract. Mater Lett. 2014;122:166–9.10.1016/j.matlet.2014.02.028Search in Google Scholar
[79] Mamuru SA, Bello AS, Hamman SB. Annona squamosa leaf extract as an efficient bioreducing agent in the synthesis of chromium and nickel nanoparticles. IJASBT. 2015;3(2):167–9.10.3126/ijasbt.v3i2.11651Search in Google Scholar
[80] Fardood ST, Ramazani A, Moradi S. A novel green synthesis of nickel oxide nanoparticles using Arabic Gum. Chem J Mold. 2017;12(1):115–8.10.19261/cjm.2017.383Search in Google Scholar
[81] Yu C, Qiu J. Preparation and magnetic behavior of carbon-encapsulated cobalt and nickel nanoparticles from starch. Chem Eng Res Des. 2008;86(8):904–8.10.1016/j.cherd.2008.02.006Search in Google Scholar
[82] Dias M, Lacerda I, Pimentel P, De Castro H, Rosa C. Removal of heavy metals by an Aspergillus terreus strain immobilized in a polyurethane matrix. Lett Appl Microbiol. 2002;34(1):46–50.10.1046/j.1472-765x.2002.01040.xSearch in Google Scholar PubMed
[83] Hulkoti NI, Taranath T. Biosynthesis of nanoparticles using microbes—a review. Colloids Surf B. 2014;121:474–83.10.1016/j.colsurfb.2014.05.027Search in Google Scholar PubMed
[84] Konishi Y, Tsukiyama T, Tachimi T, Saitoh N, Nomura T, Nagamine S. Microbial deposition of gold nanoparticles by the metal-reducing bacterium Shewanella algae. Electrochim Acta. 2007;53(1):186–92.10.1016/j.electacta.2007.02.073Search in Google Scholar
[85] Narayanan KB, Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci. 2010;156(1–2):1–13.10.1016/j.cis.2010.02.001Search in Google Scholar PubMed
[86] Gahlawat G, Choudhury AR. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019;9(23):12944–67.10.1039/C8RA10483BSearch in Google Scholar
[87] Garcia-Muelas R, Li Q, Lopez N. Initial stages in the formation of nickel phosphides. J Phys Chem B. 2018;122(2):672–8.10.1021/acs.jpcb.7b06020Search in Google Scholar PubMed
[88] Song W, Zhao C, Lercher JA. Importance of size and distribution of Ni nanoparticles for the hydrodeoxygenation of microalgae oil. Chem Eur J. 2013;19(30):9833–42.10.1002/chem.201301005Search in Google Scholar PubMed
[89] Gong N, Shao K, Feng W, Lin Z, Liang C, Sun Y. Biotoxicity of nickel oxide nanoparticles and bio-remediation by microalgae Chlorella vulgaris. Chemosphere. 2011;83(4):510–6.10.1016/j.chemosphere.2010.12.059Search in Google Scholar PubMed
[90] Makarov V, Love A, Sinitsyna O, Makarova S, Yaminsky I, Taliansky M, et al. Green nanotechnologies: synthesis of metal nanoparticles using plants. Acta Nat. 2014;6(1):20.10.32607/20758251-2014-6-1-35-44Search in Google Scholar
[91] Shankar SS, Ahmad A, Pasricha R, Sastry M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem. 2003;13(7):1822–6.10.1039/b303808bSearch in Google Scholar
[92] Fardoun MM, Maaliki D, Halabi N, Iratni R, Bitto A, Baydoun E, et al. Flavonoids in adipose tissue inflammation and atherosclerosis: one arrow, two targets. Clin Sci. 2020;134(12):1403–32.10.1042/CS20200356Search in Google Scholar PubMed
[93] Jo S, Kim H, Kim S, Shin DH, Kim MS. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem Biol Drug Des. 2019;94(6):2023–30.10.1111/cbdd.13604Search in Google Scholar PubMed PubMed Central
[94] Kasthuri J, Veerapandian S, Rajendiran N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf B. 2009;68(1):55–60.10.1016/j.colsurfb.2008.09.021Search in Google Scholar PubMed
[95] Cao H, Ma S, Guo H, Cui X, Wang S, Zhong X, et al. Comparative study on the monosaccharide compositions, antioxidant and hypoglycemic activities in vitro of intracellular and extracellular polysaccharides of liquid fermented Coprinus comatus. Int J Biol Macromol. 2019;139:543–9.10.1016/j.ijbiomac.2019.08.017Search in Google Scholar PubMed
[96] Frihart CR, Pizzi A, Xi X, Lorenz LF. Reactions of soy flour and soy protein by non-volatile aldehydes generation by specific oxidation. Polymers. 2019;11(9):1478–95.10.3390/polym11091478Search in Google Scholar PubMed PubMed Central
[97] Zayed MF, Eisa WH, Shabaka A. Malva parviflora extract assisted green synthesis of silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc. 2012;98:423–8.10.1016/j.saa.2012.08.072Search in Google Scholar PubMed
[98] Kim J, Rheem Y, Yoo B, Chong Y, Bozhilov KN, Kim D, et al. Peptide-mediated shape-and size-tunable synthesis of gold nanostructures. Acta Biomater. 2010;6(7):2681–9.10.1016/j.actbio.2010.01.019Search in Google Scholar PubMed
[99] Sathishkumar M, Sneha K, Yun YS. Immobilization of silver nanoparticles synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Bioresour Technol. 2010;101(20):7958–65.10.1016/j.biortech.2010.05.051Search in Google Scholar PubMed
[100] Johari GP. Effects of microstructure and sample’s surface to volume ratio on pressure-induced nucleation and transformation to crystalline and apparently amorphous solids. J Phys Chem B. 2019;123(46):9992–9.10.1021/acs.jpcb.9b09218Search in Google Scholar PubMed
[101] Mittal AK, Chisti Y, Banerjee UC. Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31(2):346–56.10.1016/j.biotechadv.2013.01.003Search in Google Scholar PubMed
[102] Liu ZP, Li S, Yang Y, Peng S, Hu ZK, Qian Y, et al. Complex‐surfactant‐assisted hydrothermal route to ferromagnetic nickel nanobelts. Adv Mater. 2003;15(22):1946–8.10.1002/adma.200305663Search in Google Scholar
[103] Libor Z, Zhang Q. The synthesis of nickel nanoparticles with controlled morphology and SiO2/Ni core–shell structures. Mater Chem Phys. 2009;114(2–3):902–7.10.1016/j.matchemphys.2008.10.068Search in Google Scholar
[104] Krishnamoorthy K, Veerasubramani GK, Radhakrishnan S, Kim SJ. One pot hydrothermal growth of hierarchical nanostructured Ni3S2 on Ni foam for supercapacitor application. Chem Eng J. 2014;251:116–22.10.1016/j.cej.2014.04.006Search in Google Scholar
[105] Guo H, Pu B, Chen H, Yang J, Zhou Y, Yang J, et al. Surfactant-assisted solvothermal synthesis of pure nickel submicron spheres with microwave-absorbing properties. Nanoscale Res Lett. 2016;11(1):352–67.10.1186/s11671-016-1562-ySearch in Google Scholar
[106] Dhand C, Dwivedi N, Loh XJ, Ying ANJ, Verma NK, Beuerman RW, et al. Methods and strategies for the synthesis of diverse nanoparticles and their applications: a comprehensive overview. RSC Adv. 2015;5(127):105003–37.10.1039/C5RA19388ESearch in Google Scholar
[107] Kumar A, Saxena A, De A, Shankar R, Mozumdar S. Controlled synthesis of size-tunable nickel and nickel oxide nanoparticles using water-in-oil microemulsions. Adv Nat Sci Nanosci Nanotechnol. 2013;4(2):25009–17.10.1088/2043-6262/4/2/025009Search in Google Scholar
[108] Roselina NN, Azizan A. Ni nanoparticles: study of particles formation and agglomeration. Procedia Eng. 2012;41:1620–6.10.1016/j.proeng.2012.07.359Search in Google Scholar
[109] Tu Y, Huang Z, Wang D, Wen J, Ren Z. Growth of aligned carbon nanotubes with controlled site density. Appl Phys Lett. 2002;80(21):4018–20.10.1063/1.1482790Search in Google Scholar
[110] Bao J, Tie C, Xu Z, Zhou Q, Shen D, Ma Q. Template synthesis of an array of nickel nanotubules and its magnetic behavior. Adv Mater. 2001;13(21):1631–3.10.1002/1521-4095(200111)13:21<1631::AID-ADMA1631>3.0.CO;2-RSearch in Google Scholar
[111] Pirota K, Navas D, Hernández-Vélez M, Nielsch K, Vázquez M. Novel magnetic materials prepared by electrodeposition techniques: arrays of nanowires and multi-layered microwires. J Alloy Compd. 2004;369(1–2):18–26.10.1016/j.jallcom.2003.09.040Search in Google Scholar
[112] Lee W, Scholz R, Nielsch K, Gösele U. A template‐based electrochemical method for the synthesis of multisegmented metallic nanotubes. Angew Chem Int Ed. 2005;44(37):6050–4.10.1002/anie.200501341Search in Google Scholar
[113] Gondal M, Saleh TA, Drmosh Q. Synthesis of nickel oxide nanoparticles using pulsed laser ablation in liquids and their optical characterization. Appl Surf Sci. 2012;258(18):6982–6.10.1016/j.apsusc.2012.03.147Search in Google Scholar
[114] Yu Y, Yang W, Sun X, Zhu W, Li XZ, Sellmyer DJ, et al. Monodisperse MPt (M═Fe, Co, Ni, Cu, Zn) nanoparticles prepared from a facile oleylamine reduction of metal salts. Nano Lett. 2014;14(5):2778–82.10.1021/nl500776eSearch in Google Scholar PubMed
[115] Ragupathi C, Vijaya JJ, Kennedy LJ. Synthesis, characterization of nickel aluminate nanoparticles by microwave combustion method and their catalytic properties. Mater Sci Eng. 2014;184:18–25.10.1016/j.mseb.2014.01.010Search in Google Scholar
[116] LaGrow AP, Ingham B, Cheong S, Williams GV, Dotzler C, Toney MF, et al. Synthesis, alignment, and magnetic properties of monodisperse nickel nanocubes. J Am Chem Soc. 2012;134(2):855–8.10.1021/ja210209rSearch in Google Scholar PubMed
[117] Yinghua L, Xuelong P, Jiacai K, Yingjun D. Improving the microstructure and mechanical properties of laser cladded Ni-based alloy coatings by changing their composition: a review. Rev Adv Mater Sci. 2020;59(1):340–51.10.1515/rams-2020-0027Search in Google Scholar
[118] Ban I, Stergar J, Maver U. NiCu magnetic nanoparticles: review of synthesis methods, surface functionalization approaches, and biomedical applications. Nanotechnol Rev. 2018;7(2):187–207.10.1515/ntrev-2017-0193Search in Google Scholar
[119] Guo D, Wu C, Li X, Jiang H, Wang X, Chen B. In vitro cellular uptake and cytotoxic effect of functionalized nickel nanoparticles on leukemia cancer cells. J Nanosci Nanotechnol. 2008;8(5):2301–7.10.1166/jnn.2008.18272Search in Google Scholar
[120] Ivanov M, Khomchenko V, Salimian M, Nikitin T, Kopyl S, Buryakov A, et al. Self-assembled diphenylalanine peptide microtubes covered by reduced graphene oxide/spiky nickel nanocomposite: an integrated nanobiomaterial for multifunctional applications. Mater Des. 2018;142:149–57.10.1016/j.matdes.2018.01.018Search in Google Scholar
[121] Anderson SD, Gwenin VV, Gwenin CD. Magnetic functionalized nanoparticles for biomedical, drug delivery and imaging applications. Nanoscale Res Lett. 2019;14(1):1–16.10.1186/s11671-019-3019-6Search in Google Scholar PubMed PubMed Central
[122] Helan V, Prince JJ, Al-Dhabi NA, Arasu MV, Ayeshamariam A, Madhumitha G, et al. Neem leaves mediated preparation of NiO nanoparticles and its magnetization, coercivity and antibacterial analysis. Results Phys. 2016;6:712–8.10.1016/j.rinp.2016.10.005Search in Google Scholar
[123] Gorgizadeh M, Azarpira N, Lotfi M, Daneshvar F, Salehi F, Sattarahmady N. Sonodynamic cancer therapy by a nickel ferrite/carbon nanocomposite on melanoma tumor: in vitro and in vivo studies. Photodiagn Photodyn Ther. 2019;27:27–33.10.1016/j.pdpdt.2019.05.023Search in Google Scholar PubMed
[124] Ghaedi M, Pakniat M, Mahmoudi Z, Hajati S, Sahraei R, Daneshfar A. Synthesis of nickel sulfide nanoparticles loaded on activated carbon as a novel adsorbent for the competitive removal of methylene blue and safranin-O. Spectrochim Acta A. 2014;123:402–9.10.1016/j.saa.2013.12.083Search in Google Scholar PubMed
[125] Hoque SM, Tariq M, Liba SI, Salehin F, Mahmood ZH, Khan MN, et al. Thermo-therapeutic applications of chitosan- and PEG-coated NiFe2O4 nanoparticles. Nanotechnology. 2016;27(28):285702–11.10.1088/0957-4484/27/28/285702Search in Google Scholar PubMed
[126] Neiva EG, Bergamini MF, Oliveira MM, Marcolino Jr, LH, Zarbin AJ. PVP-capped nickel nanoparticles: synthesis, characterization and utilization as a glycerol electrosensor. Sens Actuators B Chem. 2014;196:574–81.10.1016/j.snb.2014.02.041Search in Google Scholar
[127] Jia F, Li G, Yang B, Yu B, Shen Y, Cong H. Investigation of rare earth upconversion fluorescent nanoparticles in biomedical field. Nanotechnol Rev. 2019;8(1):1–17.10.1515/ntrev-2019-0001Search in Google Scholar
[128] Simonsen G, Strand M, Øye G. Potential applications of magnetic nanoparticles within separation in the petroleum industry. J Pet Sci Eng. 2018;165:488–95.10.1016/j.petrol.2018.02.048Search in Google Scholar
[129] Woodard A, Xu L, Barragan AA, Nava G, Wong BM, Mangolini L. On the non-thermal plasma synthesis of nickel nanoparticles. Plasma Process Polym. 2018;15(1):1700104–8.10.1002/ppap.201700104Search in Google Scholar
[130] Nie H, Yao Z, Zhou X, Yang Z, Huang S. Nonenzymatic electrochemical detection of glucose using well-distributed nickel nanoparticles on straight multi-walled carbon nanotubes. Biosens Bioelectron. 2011;30(1):28–34.10.1016/j.bios.2011.08.022Search in Google Scholar PubMed
[131] Seo S, Perez GA, Tewari K, Comas X, Kim M. Catalytic activity of nickel nanoparticles stabilized by adsorbing polymers for enhanced carbon sequestration. Sci Rep. 2018;8(1):1–11.10.1038/s41598-018-29605-1Search in Google Scholar PubMed PubMed Central
[132] Gong M, Wang DY, Chen CC, Hwang BJ, Dai H. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction. Nano Res. 2016;9(1):28–46.10.1007/s12274-015-0965-xSearch in Google Scholar
[133] Feng J, Gong M, Kenney MJ, Wu JZ, Zhang B, Li Y, et al. Nickel-coated silicon photocathode for water splitting in alkaline electrolytes. Nano Res. 2015;8(5):1577–83.10.1007/s12274-014-0643-4Search in Google Scholar
[134] Dander JE, Garg NK. Breaking amides using nickel catalysis. ACS Catal. 2017;7(2):1413–23.10.1021/acscatal.6b03277Search in Google Scholar PubMed PubMed Central
[135] Jin L, Zhao X, Qian X, Dong M. Nickel nanoparticles encapsulated in porous carbon and carbon nanotube hybrids from bimetallic metal-organic-frameworks for highly efficient adsorption of dyes. J Colloid Interface Sci. 2018;509:245–53.10.1016/j.jcis.2017.09.002Search in Google Scholar PubMed
[136] Zhao X, Chen H, Wang S, Wu Q, Xia N, Kong F. Electroless decoration of cellulose paper with nickel nanoparticles: a hybrid carbon fiber for supercapacitors. Mater Chem Phys. 2018;215:157–62.10.1016/j.matchemphys.2018.05.024Search in Google Scholar
[137] Krishnapriya R, Praneetha S, Murugan AV. Microwave-solvothermal synthesis of various TiO2 nano-morphologies with enhanced efficiency by incorporating Ni nanoparticles in an electrolyte for dye-sensitized solar cells. Inorg Chem Front. 2017;4(10):1665–78.10.1039/C7QI00329CSearch in Google Scholar
[138] Wegner S, Rutz C, Schütte K, Barthel J, Bushmelev A, Schmidt A, et al. Soft, wet‐chemical synthesis of metastable superparamagnetic hexagonal close‐packed nickel nanoparticles in different ionic liquids. Chem Eur J. 2017;23(26):6330–40.10.1002/chem.201605251Search in Google Scholar PubMed
[139] Manna J, Akbayrak S, Ozkar S. Nickel(0) nanoparticles supported on bare or coated cobalt ferrite as highly active, magnetically isolable and reusable catalyst for hydrolytic dehydrogenation of ammonia borane. J Colloid Interface Sci. 2017;508:359–68.10.1016/j.jcis.2017.08.045Search in Google Scholar PubMed
[140] Gong M, Zhou W, Tsai MC, Zhou J, Guan M, Lin MC, et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat Commun. 2014;5:4695–700.10.1038/ncomms5695Search in Google Scholar PubMed
[141] Zhang G, Li J, Zhang G, Zhao L. Room-temperature synthesis of Ni nanoparticles as the absorbent used for sewage treatment. Adv Mater Sci Eng. 2015;2015:1–4.10.1155/2015/973648Search in Google Scholar
[142] Agegnehu AK, Pan CJ, Rick J, Lee JF, Su WN, Hwang BJ. Enhanced hydrogen generation by cocatalytic Ni and NiO nanoparticles loaded on graphene oxide sheets. J Mater Chem. 2012;22(27):13849–54.10.1039/c2jm30474kSearch in Google Scholar
[143] Li Z, Xu K, Pan Y. Recent development of supercapacitor electrode based on carbon materials. Nanotechnol Rev. 2019;8(1):35–49.10.1515/ntrev-2019-0004Search in Google Scholar
[144] Pan Y, Xu K, Wu C. Recent progress in supercapacitors based on the advanced carbon electrodes. Nanotechnol Rev. 2019;8(1):299–314.10.1515/ntrev-2019-0029Search in Google Scholar
[145] Zhang J, Liu Y, Guan H, Zhao Y, Zhang B. Decoration of nickel hydroxide nanoparticles onto polypyrrole nanotubes with enhanced electrochemical performance for supercapacitors. J Alloy Compd. 2017;721:731–40.10.1016/j.jallcom.2017.06.061Search in Google Scholar
[146] Li H, Yu M, Wang F, Liu P, Liang Y, Xiao J, et al. Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials. Nat Commun. 2013;4:1894–900.10.1038/ncomms2932Search in Google Scholar PubMed PubMed Central
[147] Sharma A, Hickman J, Gazit N, Rabkin E, Mishin Y. Nickel nanoparticles set a new record of strength. Nat Commun. 2018;9(1):4102–9.10.1038/s41467-018-06575-6Search in Google Scholar PubMed PubMed Central
[148] Jiang Z, Xie J, Jiang D, Wei X, Chen M. Modifiers-assisted formation of nickel nanoparticles and their catalytic application to p-nitrophenol reduction. CrystEngComm. 2013;15(3):560–9.10.1039/C2CE26398JSearch in Google Scholar
[149] Oliveira PR, Schibelbain AF, Neiva EG, Zarbin AJ, Marcolino-Junior LH, Bergamini MF. Nickel hexacyanoferrate supported at nickel nanoparticles for voltammetric determination of Rifampicin. Sens Actuators B Chem. 2018;260:816–23.10.1016/j.snb.2017.12.198Search in Google Scholar
[150] Afshari S, Montazer M. In-Situ sonosynthesis of Hedgehog-like nickel nanoparticles on polyester fabric producing magnetic properties. Ultrason Sonochem. 2018;42:679–88.10.1016/j.ultsonch.2017.12.009Search in Google Scholar PubMed
[151] Duraisamy N, Numan A, Fatin SO, Ramesh K, Ramesh S. Facile sonochemical synthesis of nanostructured NiO with different particle sizes and its electrochemical properties for supercapacitor application. J Colloid Interface Sci. 2016;471:136–44.10.1016/j.jcis.2016.03.013Search in Google Scholar PubMed
[152] Layan Savithra GH, Muthuswamy E, Bowker RH, Carrillo BA, Bussell ME, Brock SL. Rational design of nickel phosphide hydrodesulfurization catalysts: controlling particle size and preventing sintering. Chem Mater. 2013;25(6):825–33.10.1021/cm302680jSearch in Google Scholar
[153] Karami H, Mohammadi S. Pulsed current electrochemical synthesis of nickel nanoclusters and application as catalyst for hydrogen and oxygen revolution. J Clust Sci. 2010;21(4):739–52.10.1007/s10876-010-0341-7Search in Google Scholar
[154] Wu Z, Ge S, Zhang M, Li W, Tao K. Synthesis of nickel nanoparticles supported on metal oxides using electroless plating: controlling the dispersion and size of nickel nanoparticles. J Colloid Interface Sci. 2009;330(2):359–66.10.1016/j.jcis.2008.10.083Search in Google Scholar PubMed
[155] Chen Y, Peng DL, Lin D, Luo X. Preparation and magnetic properties of nickel nanoparticles via the thermal decomposition of nickel organometallic precursor in alkylamines. Nanotechnology. 2007;18(50):505703–8.10.1088/0957-4484/18/50/505703Search in Google Scholar
[156] Asaithambi S, Murugan R, Sakthivel P, Karuppaiah M, Rajendran S, Ravi G. Influence of Ni doping in SnO(2) nanoparticles with enhanced visible light photocatalytic activity for degradation of methylene blue dye. J Nanosci Nanotechnol. 2019;19(8):4438–46.10.1166/jnn.2019.16493Search in Google Scholar PubMed
[157] Baranwal A, Mahato K, Srivastava A, Maurya PK, Chandra P. Phytofabricated metallic nanoparticles and their clinical applications. RSC Adv. 2016;6(107):105996–6010.10.1039/C6RA23411ASearch in Google Scholar
[158] Tobisu M, Chatani N. Cross-couplings using aryl ethers via C–O bond activation enabled by nickel catalysts. Acc Chem Res. 2015;48(6):1717–26.10.1021/acs.accounts.5b00051Search in Google Scholar PubMed
[159] Martindale BC, Hutton GA, Caputo CA, Reisner E. Solar hydrogen production using carbon quantum dots and a molecular nickel catalyst. J Am Chem Soc. 2015;137(18):6018–25.10.1021/jacs.5b01650Search in Google Scholar PubMed
[160] Luo H, Klein IM, Jiang Y, Zhu H, Liu B, Kenttämaa HI, et al. Total utilization of miscanthus biomass, lignin and carbohydrates, using earth abundant nickel catalyst. ACS Sustain Chem Eng. 2016;4(4):2316–22.10.1021/acssuschemeng.5b01776Search in Google Scholar
[161] Fukuhara C, Hayakawa K, Suzuki Y, Kawasaki W, Watanabe R. A novel nickel-based structured catalyst for CO2 methanation: a honeycomb-type Ni/CeO2 catalyst to transform greenhouse gas into useful resources. Appl Catal A-Gen. 2017;532:12–8.10.1016/j.apcata.2016.11.036Search in Google Scholar
[162] Sweeney JB, Ball AK, Lawrence PA, Sinclair MC, Smith LJ. A simple, broad-scope nickel (0) precatalyst system for the direct amination of allyl alcohols. Angew Chem. 2018;130(32):10359–63.10.1002/ange.201805611Search in Google Scholar
[163] Guard LM, Mohadjer Beromi M, Brudvig GW, Hazari N, Vinyard DJ. Comparison of dppf-supported nickel precatalysts for the Suzuki–Miyaura reaction: the observation and activity of nickel (I). Angew Chem. 2015;127(45):13550–4.10.1002/ange.201505699Search in Google Scholar
[164] Ádám AA, Szabados M, Varga G, Papp Á, Musza K, Kónya Z, et al. Ultrasound-assisted hydrazine reduction method for the preparation of nickel nanoparticles, physicochemical characterization and catalytic application in Suzuki-Miyaura cross-coupling reaction. Nanomaterials. 2020;10(4):632–49.10.3390/nano10040632Search in Google Scholar
[165] Tasker SZ, Jamison TF. Highly regioselective indoline synthesis under nickel/photoredox dual catalysis. J Am Chem Soc. 2015;137(30):9531–4.10.1021/jacs.5b05597Search in Google Scholar
[166] Mirzaei P, Bastide S, Dassy A, Bensimon R, Bourgon J, Aghajani A, et al. Electrochemical oxidation of urea on nickel-rhodium nanoparticles/carbon composites. Electrochim Acta. 2019;297:715–24.10.1016/j.electacta.2018.11.205Search in Google Scholar
[167] Kin-Tak L, David H. The revolutionary creation of new advanced material-carbon nanotube composite. Compos B Eng. 2002;33:263–77.10.1016/S1359-8368(02)00012-4Search in Google Scholar
© 2020 Nuru-Deen Jaji et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Generalized locally-exact homogenization theory for evaluation of electric conductivity and resistance of multiphase materials
- Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide
- Characterization of mechanical properties of epoxy/nanohybrid composites by nanoindentation
- Graphene and CNT impact on heat transfer response of nanocomposite cylinders
- A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites
- Ultrasmall Fe3O4 nanoparticles induce S-phase arrest and inhibit cancer cells proliferation
- Effect of aging on properties and nanoscale precipitates of Cu-Ag-Cr alloy
- Effect of nano-strengthening on the properties and microstructure of recycled concrete
- Stabilizing effect of methylcellulose on the dispersion of multi-walled carbon nanotubes in cementitious composites
- Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites
- Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation
- Preparation and properties of 3D interconnected CNTs/Cu composites
- On factors affecting surface free energy of carbon black for reinforcing rubber
- Nano-silica modified phenolic resin film: manufacturing and properties
- Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete
- Halloysite nanotubes in polymer science: purification, characterization, modification and applications
- Cellulose hydrogel skeleton by extrusion 3D printing of solution
- Crack closure and flexural tensile capacity with SMA fibers randomly embedded on tensile side of mortar beams
- An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites
- Utilization of red mud for producing a high strength binder by composition optimization and nano strengthening
- One-pot synthesis of nano titanium dioxide in supercritical water
- Printability of photo-sensitive nanocomposites using two-photon polymerization
- In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries
- Effect of nano and micro conductive materials on conductive properties of carbon fiber reinforced concrete
- Tribological performance of nano-diamond composites-dispersed lubricants on commercial cylinder liner mating with CrN piston ring
- Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance
- Genetic mechanisms of deep-water massive sandstones in continental lake basins and their significance in micro–nano reservoir storage systems: A case study of the Yanchang formation in the Ordos Basin
- Effects of nanoparticles on engineering performance of cementitious composites reinforced with PVA fibers
- Band gap manipulation of viscoelastic functionally graded phononic crystal
- Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution
- Manipulating conductive network formation via 3D T-ZnO: A facile approach for a CNT-reinforced nanocomposite
- Microstructure and mechanical properties of WC–Ni multiphase ceramic materials with NiCl2·6H2O as a binder
- Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite
- Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function
- Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing
- Corrosion behaviour of multilayer CrN coatings deposited by hybrid HIPIMS after oxidation treatment
- Surface hydrophobicity and oleophilicity of hierarchical metal structures fabricated using ink-based selective laser melting of micro/nanoparticles
- Research on bond–slip performance between pultruded glass fiber-reinforced polymer tube and nano-CaCO3 concrete
- Antibacterial polymer nanofiber-coated and high elastin protein-expressing BMSCs incorporated polypropylene mesh for accelerating healing of female pelvic floor dysfunction
- Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films
- A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination
- Arrangement structure of carbon nanofiber with excellent spectral radiation characteristics
- Effect of different particle sizes of nano-SiO2 on the properties and microstructure of cement paste
- Superior Fe x N electrocatalyst derived from 1,1′-diacetylferrocene for oxygen reduction reaction in alkaline and acidic media
- Facile growth of aluminum oxide thin film by chemical liquid deposition and its application in devices
- Liquid crystallinity and thermal properties of polyhedral oligomeric silsesquioxane/side-chain azobenzene hybrid copolymer
- Laboratory experiment on the nano-TiO2 photocatalytic degradation effect of road surface oil pollution
- Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage
- Conversion of sub-µm calcium carbonate (calcite) particles to hollow hydroxyapatite agglomerates in K2HPO4 solutions
- Exact solutions of bending deflection for single-walled BNNTs based on the classical Euler–Bernoulli beam theory
- Effects of substrate properties and sputtering methods on self-formation of Ag particles on the Ag–Mo(Zr) alloy films
- Enhancing carbonation and chloride resistance of autoclaved concrete by incorporating nano-CaCO3
- Effect of SiO2 aerogels loading on photocatalytic degradation of nitrobenzene using composites with tetrapod-like ZnO
- Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles
- Hydration activity, crystal structural, and electronic properties studies of Ba-doped dicalcium silicate
- Microstructure and mechanical properties of brazing joint of silver-based composite filler metal
- Polymer nanocomposite sunlight spectrum down-converters made by open-air PLD
- Cryogenic milling and formation of nanostructured machined surface of AISI 4340
- Braided composite stent for peripheral vascular applications
- Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre–reinforced starch biopolymer composites
- Study on influencing factors of photocatalytic performance of CdS/TiO2 nanocomposite concrete
- Improving flexural and dielectric properties of carbon fiber epoxy composite laminates reinforced with carbon nanotubes interlayer using electrospray deposition
- Scalable fabrication of carbon materials based silicon rubber for highly stretchable e-textile sensor
- Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery
- Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries
- Synthesis of functionalized carbon nanotubes for fluorescent biosensors
- Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete
- Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage
- Microstructural evolution and properties of Cu–20 wt% Ag alloy wire by multi-pass continuous drawing
- Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene
- Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications
- Preparation and piezoresistivity of carbon nanotube-coated sand reinforced cement mortar
- Vibrational analysis of an irregular single-walled carbon nanotube incorporating initial stress effects
- Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials
- Single pulse laser removal of indium tin oxide film on glass and polyethylene terephthalate by nanosecond and femtosecond laser
- Mechanical reinforcement with enhanced electrical and heat conduction of epoxy resin by polyaniline and graphene nanoplatelets
- High-efficiency method for recycling lithium from spent LiFePO4 cathode
- Degradable tough chitosan dressing for skin wound recovery
- Static and dynamic analyses of auxetic hybrid FRC/CNTRC laminated plates
- Review articles
- Carbon nanomaterials enhanced cement-based composites: advances and challenges
- Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide
- Review on modeling and application of chemical mechanical polishing
- Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites
- Advances in modelling and analysis of nano structures: a review
- Mechanical properties of nanomaterials: A review
- New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics
- A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials
- Recent development and applications of nanomaterials for cancer immunotherapy
- Advances in biomaterials for adipose tissue reconstruction in plastic surgery
- Advances of graphene- and graphene oxide-modified cementitious materials
- Theories for triboelectric nanogenerators: A comprehensive review
- Nanotechnology of diamondoids for the fabrication of nanostructured systems
- Material advancement in technological development for the 5G wireless communications
- Nanoengineering in biomedicine: Current development and future perspectives
- Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview
- Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review
- Carbon nanotube–reinforced polymer composite for electromagnetic interference application: A review
- Functionalized layered double hydroxide applied to heavy metal ions absorption: A review
- A new classification method of nanotechnology for design integration in biomaterials
- Finite element analysis of natural fibers composites: A review
- Phase change materials for building construction: An overview of nano-/micro-encapsulation
- Recent advance in surface modification for regulating cell adhesion and behaviors
- Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions
- Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review
- Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications
- A review of passive methods in microchannel heat sink application through advanced geometric structure and nanofluids: Current advancements and challenges
- Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices
- Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging
- Synthesis of graphene: Potential carbon precursors and approaches
- A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE)
- Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water
- Analysis of functionally graded carbon nanotube-reinforced composite structures: A review
- Application of nanomaterials in ultra-high performance concrete: A review
- Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer
- Advanced nickel nanoparticles technology: From synthesis to applications
- Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable?
- Progress in construction of bio-inspired physico-antimicrobial surfaces
- From materials to devices using fused deposition modeling: A state-of-art review
- A review for modified Li composite anode: Principle, preparation and challenge
- Naturally or artificially constructed nanocellulose architectures for epoxy composites: A review
Articles in the same Issue
- Research Articles
- Generalized locally-exact homogenization theory for evaluation of electric conductivity and resistance of multiphase materials
- Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide
- Characterization of mechanical properties of epoxy/nanohybrid composites by nanoindentation
- Graphene and CNT impact on heat transfer response of nanocomposite cylinders
- A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites
- Ultrasmall Fe3O4 nanoparticles induce S-phase arrest and inhibit cancer cells proliferation
- Effect of aging on properties and nanoscale precipitates of Cu-Ag-Cr alloy
- Effect of nano-strengthening on the properties and microstructure of recycled concrete
- Stabilizing effect of methylcellulose on the dispersion of multi-walled carbon nanotubes in cementitious composites
- Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites
- Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation
- Preparation and properties of 3D interconnected CNTs/Cu composites
- On factors affecting surface free energy of carbon black for reinforcing rubber
- Nano-silica modified phenolic resin film: manufacturing and properties
- Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete
- Halloysite nanotubes in polymer science: purification, characterization, modification and applications
- Cellulose hydrogel skeleton by extrusion 3D printing of solution
- Crack closure and flexural tensile capacity with SMA fibers randomly embedded on tensile side of mortar beams
- An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites
- Utilization of red mud for producing a high strength binder by composition optimization and nano strengthening
- One-pot synthesis of nano titanium dioxide in supercritical water
- Printability of photo-sensitive nanocomposites using two-photon polymerization
- In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries
- Effect of nano and micro conductive materials on conductive properties of carbon fiber reinforced concrete
- Tribological performance of nano-diamond composites-dispersed lubricants on commercial cylinder liner mating with CrN piston ring
- Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance
- Genetic mechanisms of deep-water massive sandstones in continental lake basins and their significance in micro–nano reservoir storage systems: A case study of the Yanchang formation in the Ordos Basin
- Effects of nanoparticles on engineering performance of cementitious composites reinforced with PVA fibers
- Band gap manipulation of viscoelastic functionally graded phononic crystal
- Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution
- Manipulating conductive network formation via 3D T-ZnO: A facile approach for a CNT-reinforced nanocomposite
- Microstructure and mechanical properties of WC–Ni multiphase ceramic materials with NiCl2·6H2O as a binder
- Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite
- Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function
- Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing
- Corrosion behaviour of multilayer CrN coatings deposited by hybrid HIPIMS after oxidation treatment
- Surface hydrophobicity and oleophilicity of hierarchical metal structures fabricated using ink-based selective laser melting of micro/nanoparticles
- Research on bond–slip performance between pultruded glass fiber-reinforced polymer tube and nano-CaCO3 concrete
- Antibacterial polymer nanofiber-coated and high elastin protein-expressing BMSCs incorporated polypropylene mesh for accelerating healing of female pelvic floor dysfunction
- Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films
- A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination
- Arrangement structure of carbon nanofiber with excellent spectral radiation characteristics
- Effect of different particle sizes of nano-SiO2 on the properties and microstructure of cement paste
- Superior Fe x N electrocatalyst derived from 1,1′-diacetylferrocene for oxygen reduction reaction in alkaline and acidic media
- Facile growth of aluminum oxide thin film by chemical liquid deposition and its application in devices
- Liquid crystallinity and thermal properties of polyhedral oligomeric silsesquioxane/side-chain azobenzene hybrid copolymer
- Laboratory experiment on the nano-TiO2 photocatalytic degradation effect of road surface oil pollution
- Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage
- Conversion of sub-µm calcium carbonate (calcite) particles to hollow hydroxyapatite agglomerates in K2HPO4 solutions
- Exact solutions of bending deflection for single-walled BNNTs based on the classical Euler–Bernoulli beam theory
- Effects of substrate properties and sputtering methods on self-formation of Ag particles on the Ag–Mo(Zr) alloy films
- Enhancing carbonation and chloride resistance of autoclaved concrete by incorporating nano-CaCO3
- Effect of SiO2 aerogels loading on photocatalytic degradation of nitrobenzene using composites with tetrapod-like ZnO
- Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles
- Hydration activity, crystal structural, and electronic properties studies of Ba-doped dicalcium silicate
- Microstructure and mechanical properties of brazing joint of silver-based composite filler metal
- Polymer nanocomposite sunlight spectrum down-converters made by open-air PLD
- Cryogenic milling and formation of nanostructured machined surface of AISI 4340
- Braided composite stent for peripheral vascular applications
- Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre–reinforced starch biopolymer composites
- Study on influencing factors of photocatalytic performance of CdS/TiO2 nanocomposite concrete
- Improving flexural and dielectric properties of carbon fiber epoxy composite laminates reinforced with carbon nanotubes interlayer using electrospray deposition
- Scalable fabrication of carbon materials based silicon rubber for highly stretchable e-textile sensor
- Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery
- Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries
- Synthesis of functionalized carbon nanotubes for fluorescent biosensors
- Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete
- Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage
- Microstructural evolution and properties of Cu–20 wt% Ag alloy wire by multi-pass continuous drawing
- Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene
- Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications
- Preparation and piezoresistivity of carbon nanotube-coated sand reinforced cement mortar
- Vibrational analysis of an irregular single-walled carbon nanotube incorporating initial stress effects
- Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials
- Single pulse laser removal of indium tin oxide film on glass and polyethylene terephthalate by nanosecond and femtosecond laser
- Mechanical reinforcement with enhanced electrical and heat conduction of epoxy resin by polyaniline and graphene nanoplatelets
- High-efficiency method for recycling lithium from spent LiFePO4 cathode
- Degradable tough chitosan dressing for skin wound recovery
- Static and dynamic analyses of auxetic hybrid FRC/CNTRC laminated plates
- Review articles
- Carbon nanomaterials enhanced cement-based composites: advances and challenges
- Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide
- Review on modeling and application of chemical mechanical polishing
- Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites
- Advances in modelling and analysis of nano structures: a review
- Mechanical properties of nanomaterials: A review
- New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics
- A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials
- Recent development and applications of nanomaterials for cancer immunotherapy
- Advances in biomaterials for adipose tissue reconstruction in plastic surgery
- Advances of graphene- and graphene oxide-modified cementitious materials
- Theories for triboelectric nanogenerators: A comprehensive review
- Nanotechnology of diamondoids for the fabrication of nanostructured systems
- Material advancement in technological development for the 5G wireless communications
- Nanoengineering in biomedicine: Current development and future perspectives
- Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview
- Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review
- Carbon nanotube–reinforced polymer composite for electromagnetic interference application: A review
- Functionalized layered double hydroxide applied to heavy metal ions absorption: A review
- A new classification method of nanotechnology for design integration in biomaterials
- Finite element analysis of natural fibers composites: A review
- Phase change materials for building construction: An overview of nano-/micro-encapsulation
- Recent advance in surface modification for regulating cell adhesion and behaviors
- Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions
- Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review
- Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications
- A review of passive methods in microchannel heat sink application through advanced geometric structure and nanofluids: Current advancements and challenges
- Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices
- Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging
- Synthesis of graphene: Potential carbon precursors and approaches
- A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE)
- Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water
- Analysis of functionally graded carbon nanotube-reinforced composite structures: A review
- Application of nanomaterials in ultra-high performance concrete: A review
- Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer
- Advanced nickel nanoparticles technology: From synthesis to applications
- Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable?
- Progress in construction of bio-inspired physico-antimicrobial surfaces
- From materials to devices using fused deposition modeling: A state-of-art review
- A review for modified Li composite anode: Principle, preparation and challenge
- Naturally or artificially constructed nanocellulose architectures for epoxy composites: A review

![Figure 1
Illustration of (a) thermal decomposition [37] and (b) nanolithography technique [39] of NiNPs production.](/document/doi/10.1515/ntrev-2020-0109/asset/graphic/j_ntrev-2020-0109_fig_001.jpg)
![Figure 2
Illustration of (a) sol–gel procedure [13] and (b) biosynthesis techniques [14] for NiNPs production.](/document/doi/10.1515/ntrev-2020-0109/asset/graphic/j_ntrev-2020-0109_fig_002.jpg)
![Figure 9
Schematic diagram of (a) polyol [105], and (b) microemulsion solvothermal [106] synthesis of NiNPs.](/document/doi/10.1515/ntrev-2020-0109/asset/graphic/j_ntrev-2020-0109_fig_009.jpg)
