Abstract
Uniform and continuous Al2O3 thin films were prepared by the chemical liquid deposition (CLD) method. The breakdown field strength of the amorphous CLD-Al2O3 film is 1.74 MV/cm, making it could be used as a candidate dielectric film for electronic devices. It was further proposed to use the CLD-Al2O3 film as an electron blocking layer in a triboelectric nanogenerator (TENG) for output performances enhancement. Output voltages and currents of about 200 V and 9 µA were obtained, respectively, which were 2.6 times and 3 times, respectively, higher than TENG device without an Al2O3. A colloidal condensation-based procedure controlled by adjusting the pH value of the solution was proposed to be the mechanism of CLD, which was confirmed by the Tyndall effect observed in the growth liquid. The results indicated that the CLD could serve as a low-cost, room temperature, nontoxic and facile new method for the growth of functional thin films for semiconductor device applications.
1 Introduction
Al2O3 is a functional material with good electrical insulation, large forbidden band width (E g ≈ 8.8 eV), high transparency, high refractive index, large mechanical strength, and good thermal conductivity [1,2,3,4,5,6,7,8]. It has stable chemical properties at high temperature. Al2O3 has a dielectric constant of about 8–10, which is more than twice as high as SiO2. Al2O3 is also superior to SiO2 and silicon nitride in its ability to resist radiation and to block impurities such as sodium ions [9]. It is more resistant to various acids and base corrosion compared to SiO2. Therefore, Al2O3 could be used as an excellent dielectric and passivation layer in semiconductor electronic devices.
Currently, standard Al2O3 film preparation processes primarily comprise electron beam evaporation [10], magnetron sputtering [11], atomic layer deposition (ALD) [12], and plasma-enhanced chemical vapor deposition (PECVD) [13]. Even though Al2O3 films prepared by these methods are typically of high quality, these methods are complicated in process and generally require growth of the film under high temperature and vacuum conditions relying on very expensive equipment.
In this article, we introduce a simple and low-cost method that uses nontoxic liquid precursors to grow the Al2O3 film. It is named as chemical liquid deposition (CLD), in parallel with the commonly known chemical vapor deposition method. The thin film growth setup was simple, inexpensive, and easy to operate. Al2O3 thin films were prepared on the surface of Si substrates and indium tin oxide (ITO)-coated glasses (i.e., ITO glass) under ambient pressure and room temperature conditions. The deposition had the characteristics of uniform film growth on the substrate. The surface morphology, structural composition, electrical properties, and passivation properties of Al2O3 films prepared by the CLD method (CLD-Al2O3) were characterized by comprehensive material analysis. Triboelectric nanogenerators (TENGs) are a new type of simple and low-cost energy harvesting devices that could effectively convert various daily wasted mechanical energy into electric energy by utilizing triboelectric charging and electrostatic induction effects [14,15,16,17]. As an example of the device application, we used CLD-Al2O3 as an electron-blocking layer in TENG, which effectively prevented charge leakage during operation and enhanced the output performance of TENG. Experiments showed that the output performance of the TENG with the Al2O3 blocking layer was significantly improved compared to the conventional TENG structure. In addition, the electron blocking layer located at the bottom of the friction layer provided a long-term performance enhancement for TENG. Finally, through the observation of the Tyndall effect, we had revealed that the growth mechanism of the CLD method was essentially a colloidal condensation process caused by adjusting the pH value. Note that this mechanism should not be confused with the traditional sol–gel method. Our results are important for the theoretical interpretation of CLD and could be used as the guideline for the design of CLD of other functional materials.
2 Materials and methods
2.1 Preparation of Al2O3 thin films by CLD
The chemical reagents used in this experiment were purchased from Sinopharm Chemical Reagent Co., Ltd. Figure 1a shows a schematic process for the Al2O3-CLD, and the specific preparation procedures are as follows:
Al2(SO4)3·18H2O (50 g) was dissolved in 50 mL of deionized (DI) water at room temperature. The solution was filtered (maximum pore size 15 µm) to remove few undissolved large particles and to obtain 73.25 mL colorless viscous liquid, whose nominal pH is about −0.26.
The prepared Al2(SO4)3·18H2O solution was poured into a beaker and stirred using a magnetic stirrer, and 16.115 g of NaHCO3 powder was slowly added into the solution. Each time when the NaHCO3 was added, it was necessary to wait for the CO2 foam to substantially disappear before continuing to add more. A small amount of residual NaHCO3 pellets were treated with an ultrasonic device to dissolve them thoroughly to obtain 74.72 mL of a colorless transparent viscous liquid, whose pH was about 2.89. This liquid was prone to produce aluminum–sodium vanadium, so it should not be placed for a long time.
The as-prepared liquid was immediately diluted with water by a volume ratio of 1:1, and the suspended residual matter in the liquid was filtered out, thereby obtaining 149.43 mL of colorless transparent liquid. Now the pH value is about 3.35.
This liquid was further diluted with water with a volume ratio of 1:5 to obtain 896.58 mL of the growth liquid, whose pH was about 3.74. The Si substrates or ITO glasses were ultrasonically washed in acetone, absolute ethanol, and DI water for 10 min each and then dried. After the cleaning was completed, the surface was treated with oxygen plasma for 15 min to obtain some degree of hydrophilicity, so that a large amount of surface hydroxyl groups (OH groups) were decorated on the surface, which were advantageous for enhancing the chemical adsorption of the film material to the substrates. The samples were then placed on the surface of a Teflon stent in a Teflon beaker and immersed in the growth liquid, as shown in Figure 1b. The liquid was agitated with a magnetic stirrer at 200 rpm. After few hours at room temperature, the growth liquid eventually turned little turbid. The samples were taken out, cleaned with DI water, and dried in an oven at 60°C.
Finally, the as-prepared samples were thermally annealed optional at 400°C for 1 h using a muffle furnace (heating rate 1.5°C/min) and were taken out after natural cooling.
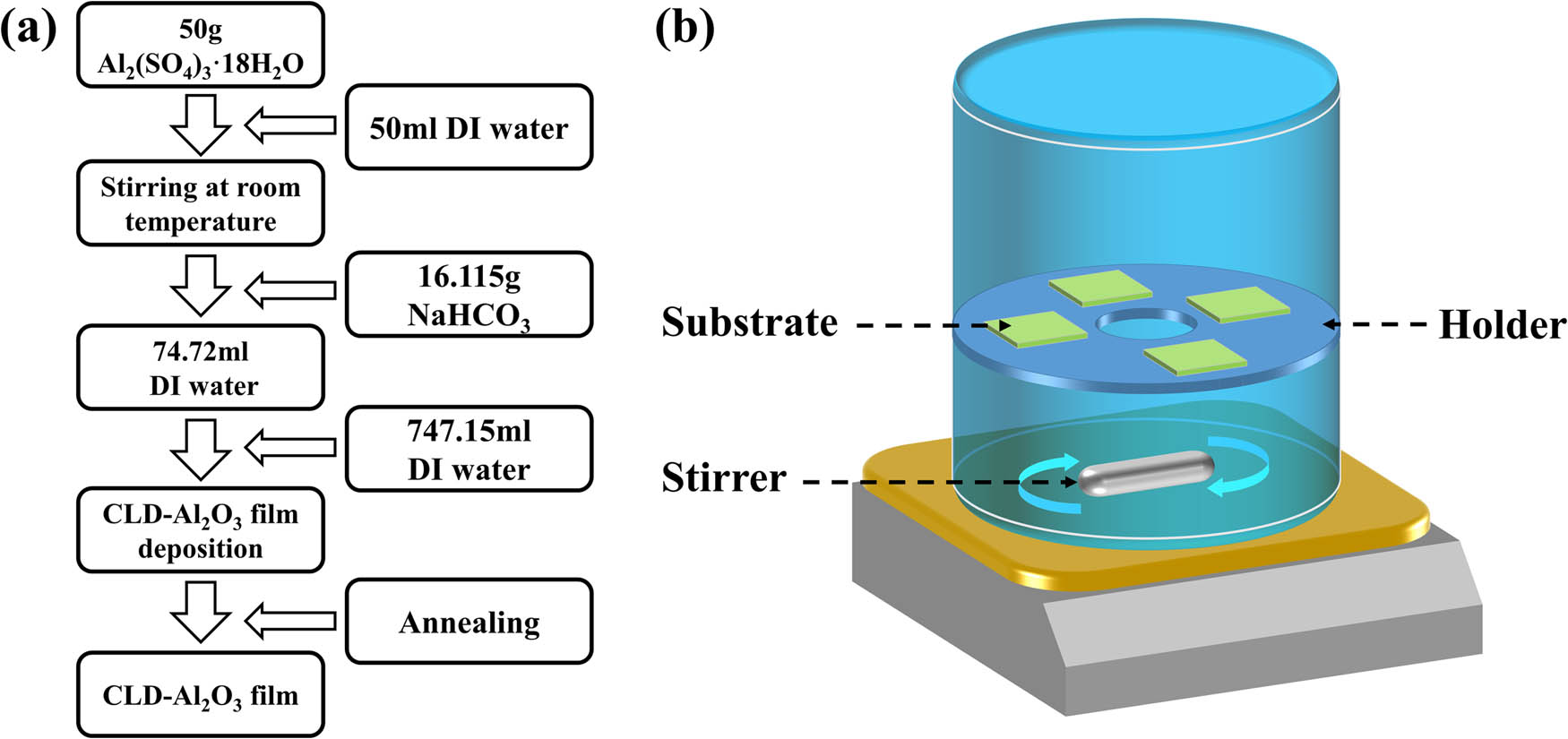
Illustration of the preparation of CLD-Al2O3 films. (a) Process flow chart for preparing Al2O3 thin films by the CLD method. (b) Schematic diagram of the Al2O3 growth setup.
2.2 Fabrication process of the TENG device with Al2O3 electron blocking layer
The TENG device consisted of a polydimethylsiloxane (PDMS)/Al2O3/ITO complex film and an Al metal layer operating in a vertical contact-separation mode [18]. PDMS (Sylgard 184, Dow Corning) base and a curing agent were mixed at a mass ratio of 10:1 and were spin coated at 3,000 rpm for 30 s onto the Al2O3-coated ITO glass. The PDMS was then cured at 150°C for 10 min. PDMS and Al act as negative and positive friction layers, respectively. The surfaces (contact area: 2 × 2 cm2) of the PDMS and Al film were periodically contacted and separated to generate a potential difference.
2.3 Measurement and characterization
The chemical compositions of the samples were analyzed by energy-dispersive spectroscopy (EDS, AMETEK) and X-ray photo-electron spectroscopy (XPS, Thermo Scientific, ESCALAB 250). The morphology of the Al2O3 films was characterized by ultra-high resolution field emission scanning electron microscopy (SEM, Thermo Scientific, Verios G4 UC) and atomic force microscopy (AFM, Bruker, Multimode 8). The crystal structure analysis of the Al2O3 films was performed using X-ray diffraction (XRD, Empyrean, DY1602). The thickness and the refractive index of the Al2O3 films were measured using ellipsometry (J.A. Woollam, M2000). The breakdown voltage of the film was measured by the semiconductor characterization system (Keithley, 4200). A signal generator (RIGOL, DG4162), a power amplifier (SINOCERA, YE5872A), and a vibration exciter (SINOCERA, JKZ-10) were combined to form a controllable tapping system to drive the TENG to work with the same external force and frequency. An oscilloscope (RIGOL, DS1102E) was used to record the voltage signal of the TENG output. The output current and the transferred charge were measured by using an electrometer (Keithley, 6514).
3 Results and discussion
The double hydrolysis reaction of Al2(SO4)3 and NaHCO3 in the experiment is given as follows:
where Al(OH)3 is actually xAl2O3·yH2O and ΔH is the chemical reaction enthalpy (ΔH > 0). Al(OH)3 is a typical amphoteric hydroxide, which is soluble not only in strong acids but also in strong bases to form hydroxyl complexes. The solubility of Al(OH)3 has a certain relationship with the pH value. Al(OH)3 can be dissolved in the acidic environment with pH < 3.4 or an alkaline environment with pH > 12.9, and it does not substantially dissolve in the pH rang of 4–11.
In this experiment, a very high concentration of Al2(SO4)3 solution was first prepared, and the solution exhibited strong acidity due to the hydrolysis of Al2(SO4)3. After slowly adding an appropriate amount of NaHCO3 powder, the Al(OH)3 nominally formed by the double hydrolysis reaction will then all “dissolve” in the still strongly acidic liquid. However, Na2SO4, one of the reaction products, could form a double-salt aluminum–sodium vanadium (NaAl(SO4)2·12H2O) with Al2(SO4)3. As aluminum–sodium vanadium could easily crystallize, it was necessary to dilute the liquid with water rapidly after the reaction of Al2(SO4)3 with NaHCO3 was completed. Then, the added water (volume ratio 1:1) had adjusted the pH to 3.35, very close to the critical value. This liquid was chemically relatively stable and could be stored if necessary (not more than a few weeks). Just before the film growth, DI water with five times the volume of the liquid was poured into the liquid to adjust the pH to 3.74, which was greater than the critical value of 3.4. At this point, the Al(OH)3 in the liquid gradually precipitated onto the sample to initiate the nucleation and the subsequent film formation. The surface was therefore deposited with a layer of Al(OH)3 film, which was then annealed to eliminate the possibly existing H element, although this step was usually optional, since the amount of H is negligible.
3.1 Characterization of Al2O3 thin films grown by CLD
Figure 2a and b are AFM images of the CLD-Al2O3 film on Si substrate with a scan range of 4 × 4 µm2, and the AFM images of the corresponding Si substrate are shown in Figure 2c and d. The root-mean-square (RMS) surface roughness of the CLD-Al2O3 film and bare Si substrate was measured to be 0.068 and 0.232 nm, respectively. It shows that the surface of Al2O3 film is smooth and uniform, without obvious protrusions and holes. Figure 3a and the inset are SEM images of the Al2O3 film observed at magnifications of 1,00,000 and 2,00,000, respectively. This figure also shows that the surface morphology of the Al2O3 film is continuous, relatively flat, and uniform, with a few bumps occasionally. Generally, an insulating layer with the smooth surface is favorable for forming good interface contact with electron devices, which can improve the electrical performance and the stability of the devices [19].
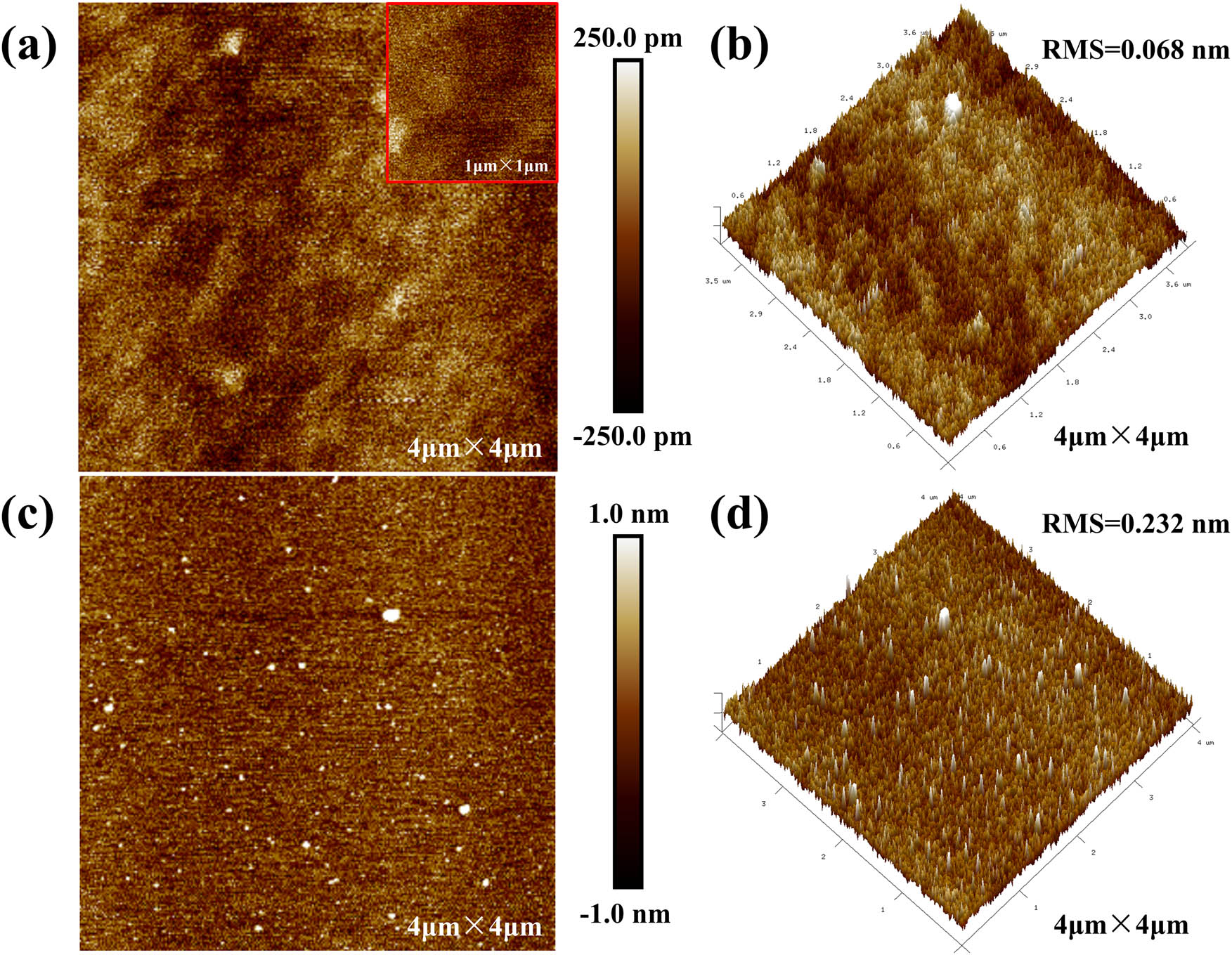
AFM micrographs of the CLD-Al2O3 and the Si substrate. (a) 2D and (b) 3D AFM images of the CLD-Al2O3 thin film on Si substrate. The inset is an AFM image with much higher magnification. (c) 2D and (d) 3D AFM images of the bare Si substrate.
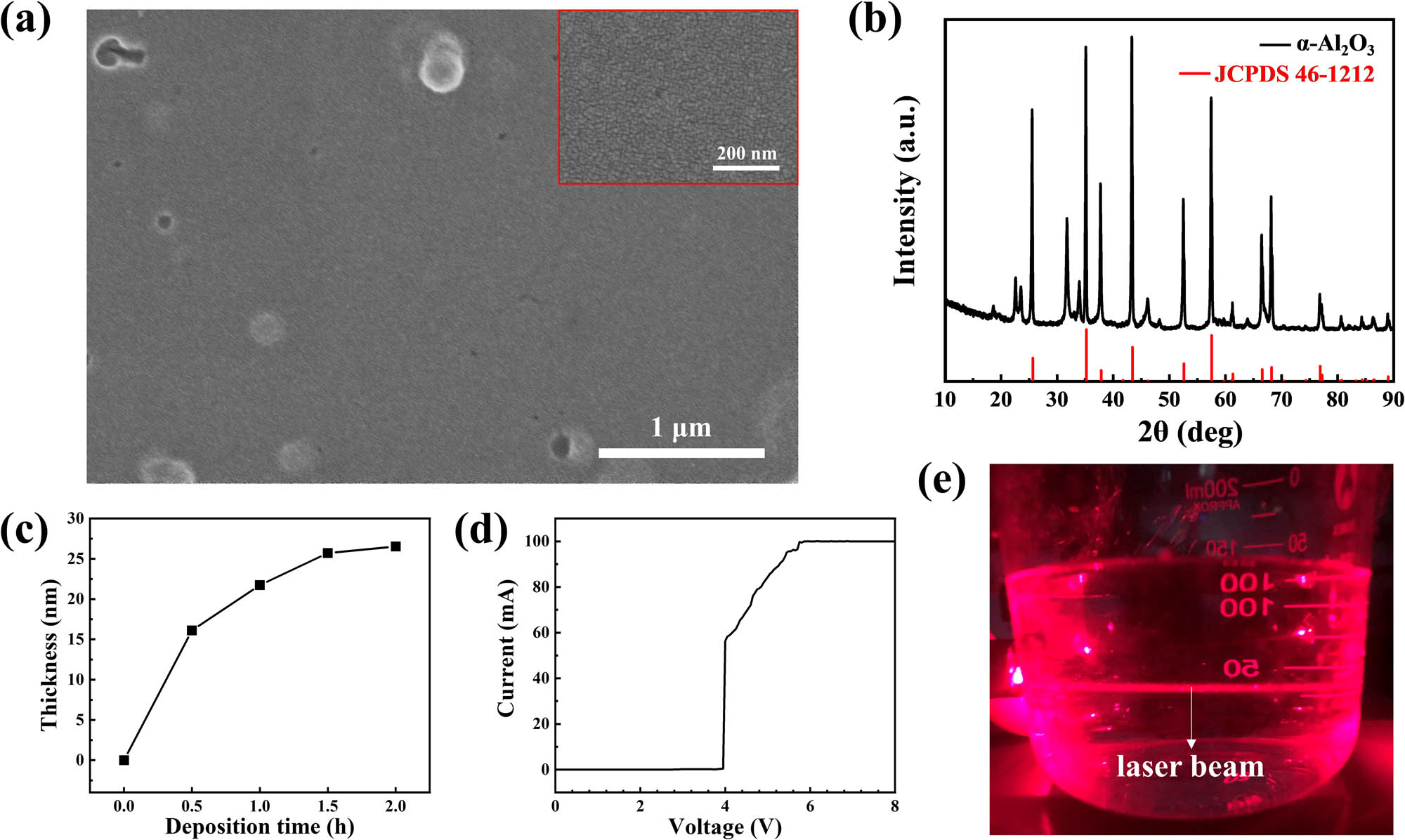
Material characterizations of the CLD-Al2O3. (a) SEM image of the CLD-Al2O3 thin film on ITO glass. The inset is an SEM image with much higher magnification where no features other than a continuous and flat film have been detected. (b) XRD curve of the CLD-Al2O3 (1,000°C annealing). (c) Deposition thickness of the CLD-Al2O3 film as a function of the growth time. (d) I–V characteristics of the 23 nm CLD-Al2O3 thin film sandwiched between two electrodes. The electrical breakdown occurs at ∼4 V. (e) The Tyndall effect observed in the 1:1 diluted liquid. The horizontal laser beam is clearly visible.
Because the Al2O3 film was very thin, we did not detect any signal in the small incident angle XRD measurement [20]. Therefore, we performed XRD tests on the precipitate collected in the growth liquid instead, because the precipitate had exactly the same composition and structure as the film. The precipitate was filtered out from the growth liquid that had grown for 12 h, washed, dried, and annealed in a muffle furnace at 1,000°C for 1 h. The XRD curve was compared with the JCPDS card (46-1212 [21]) and confirmed as α-Al2O3, as shown in Figure 3b. We also characterized the powder annealed at 500°C but did not obtain conclusive results indicating a crystallization. Therefore, increasing the annealing temperature can indeed crystallize this material. However, for most applications in electronic devices, this crystallization is unnecessary. In general, films with an amorphous structure are isotropic, and their insulation and other properties are usually more uniform than the polycrystalline counterpart. In addition, the amorphous structure usually makes the surface of the dielectric layer flatter. If the film has a polycrystalline structure, the grain boundaries will become channels for impurity scattering and leakage current. The anisotropy will reduce the dielectric stability of the film. Furthermore, the grain boundary will increase the surface roughness of the film, increase the density of defect states at the interface with which the semiconductor layer is in contact, and directly affect the application of the dielectric film.
Several ITO glass substrates were simultaneously immersed in the growth liquid, and one sample was taken out every half an hour. After annealing at high temperature, the thickness and the refractive index of the grown Al2O3 films were measured by ellipsometry. The relation between the thickness and the growth time is shown in Figure 3c. It can be seen from the figure that the deposition rate is faster in the range of 0–0.5 h, which is about 32 nm/h. As the growth time increases, the growth rate of the film decreases, and it remains nearly constant at about 10 nm/h at 0.5–1.5 h. When the growth time exceeds 1.5 h, the film thickness remains almost unchanged, indicating that the liquid has already substantially lost its growth ability.
Figures 4a and b show the EDS spectrum of the Al2O3 film grown on the ITO surface and Si substrate (with a scan range of 0 to 5 keV, 400°C annealing), respectively. This figure also shows that there are clear O and Al signals at 0.53 and 1.49 keV, respectively. Apart from the In peak, there are no additional peaks detected, which indicates that the chemical composition of the CLD-Al2O3 film method is very pure. According to the peak values in Figure 4a, it can be quasi quantitatively inferred that the atomic ratio of the O element to the Al element is about 7:1, which deviates from the expected 3:2 ratio. The main reason is that the thickness of the Al2O3 film is small. A large amount of O element contained in the thick ITO film on the base surface is inevitably detected, so that the content of the O element appears significantly larger. The chemical composition of the CLD-Al2O3 film on the Si substrate (400°C annealing) was investigated by XPS measurements as well. In Figure 4c, the XPS spectrum shows a singlet Al 2p peak at 74.4 eV, which is consistent with the peak position of the oxidation state Al in the literature [22]. The XPS O 1s peak of the CLD-Al2O3 film is shown in Figure 4d. The black solid line shows the measured data, and the green dotted line represents the Gaussian peak fitting result. The O 1s peak in spectrum is deconvoluted into two sub-peaks corresponding to O2− bonded with Al3+ ions in the Al2O3 film and oxygen associated with the OH− in aluminum hydroxide, whose centers are located at around 531.05 and 532.35 eV, respectively [23]. It is worth noting that the associated OH groups are mainly due to water absorbed from the environment in the Al2O3 film [22].
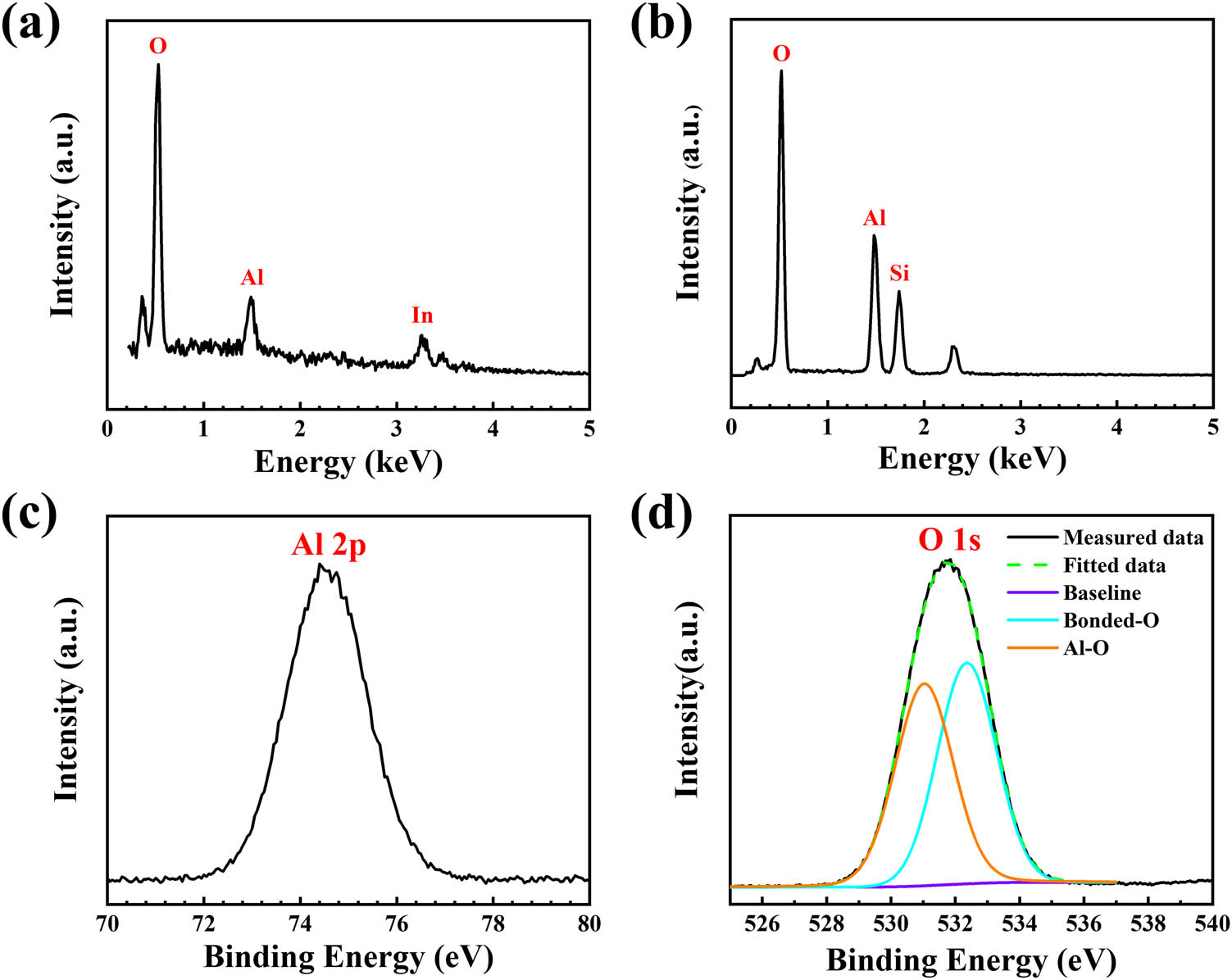
Elemental analysis of the CLD-Al2O3. EDS spectra of the CLD-Al2O3 on (a) ITO and (b) silicon after annealing at 400°C. XPS (c) Al 2p and (d) O 1s spectra of CLD-Al2O3 after annealing at 400°C.
3.2 Output performance of the TENG with CLD-Al2O3 layer
We applied the CLD-Al2O3 thin film in TENG as an electron blocking layer to enhance the output performance. At the same time, we also fabricated TENG without Al2O3 as a reference sample. The fabrication process is described in detail in the experimental section, and Figure 5a schematically shows the TENG with an Al2O3 film between the PDMS friction layer and the bottom electrodes. The cross-sectional SEM image of an Al2O3/ITO glass sample is shown in Figure 5b, where the Al2O3 film is about 260 nm thick. The TENG devices operate in a vertical contact-separation mode. The open circuit voltage, short circuit current, and amount of transferred charge amount of these devices are measured under the same applied force and frequency, as shown in Figures 5c–e. In Figure 5c, the open circuit voltage of the Al2O3-TENG is about 200 V, which is nearly 2.6 times that of the Al2O3-free TENG’s open circuit voltage. The short circuit current also increases from about 3 to 9 µA, which is three times that of the TENG without Al2O3, as illustrated in Figure 5d. Meanwhile, the amount of transferred charge in the TENG with an Al2O3 layer is about 2.4 times higher than that of the TENG without Al2O3 (see Figure 5e).
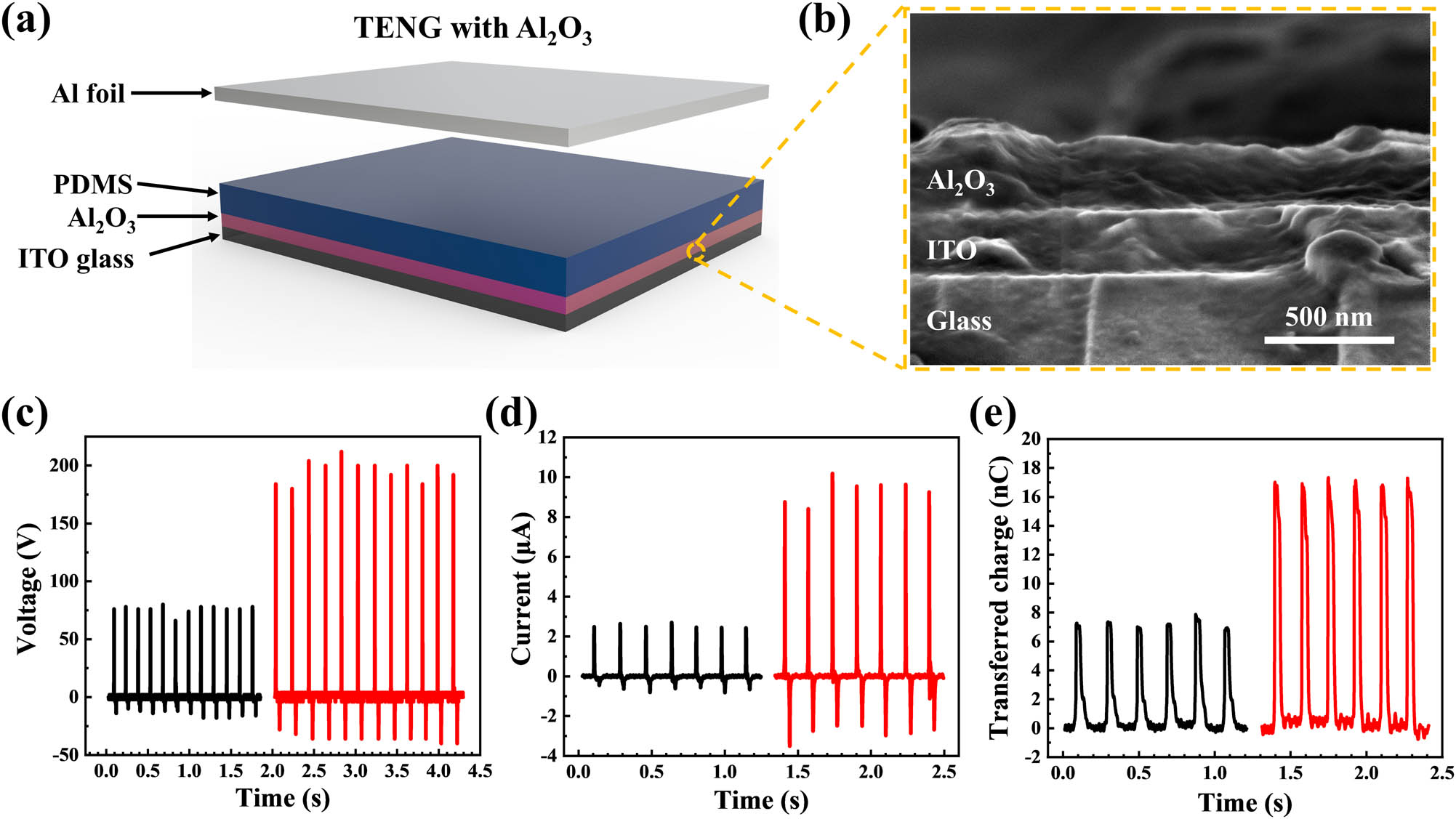
Device structure and performance of the TENG with CLD-Al2O3 layer. (a) Schematic diagram of the TENG device with the Al2O3 layer. (b) A cross-sectional view of SEM image of the Al2O3/ITO glass sample showing the sharp interfaces between different materials. (c) Open-circuit voltage of the TENG without (black)/with (red) the Al2O3 layer. (d) Short-circuit current of the TENG without (black)/with (red) the Al2O3 layer. (e) The amount of transferred charge of TENG without (black)/with (red) Al2O3.
Figures 6a–d schematically illustrates the principle of the Al2O3 electron blocking layer enhancing the TENG output performance. After several contact and separation cycles, the friction generated electrons are accumulated on the surface of the PDMS friction layer. At the same time, the bottom electrode will be positively charged due to electrostatic induction, so an electric field will be generated between the contact surface and the bottom electrode (the direction of the field is upward), as shown in Figure 6a. At this moment, there are two electron transfer procedures: a drift process caused by the electric field and a diffusion process caused by the concentration gradient of electrons [24]. Since the electric field intensity generated by the electrostatic induction is large, the electron diffusion transfer is comparably negligible. Therefore, in Figure 6b, only the drift motion is considered. Although the number of actually drifted electrons are not large due to the fact that the PDMS is an insulator, some electrons do have high enough energy to climb over the lowest unoccupied molecular orbital (LUMO) level of the PDMS (equivalent to the conduction band bottom of an insulator). These hot electrons eventually reach the bottom of the PDMS and escape from the bottom electrode, leading to the attenuation of the triboelectric charge stored in the friction layer. This reduces the output performance of TENG. However, the band gap of Al2O3 is wider than the highest occupied molecular orbital (HOMO)–LUMO gap of PDMS [25,26,27]. Therefore, after the introduction of Al2O3, it blocks the hot electrons, suppressing the charge transport. That is, the Al2O3 can effectively prevent the electrons from escaping at the bottom of PDMS, thereby improving the triboelectric charge storage capacity (see Figures 6c and d). We have measured the breakdown field of the CLD-Al2O3 thin film to be about 1.74 MV/cm, as shown in Figure 3d. In this measurement, a 23 nm Al2O3 film was sandwiched between two parallel electrodes. This breakdown field value is in the same order as that of the standard Al2O3 [28]. Today, in TENG community, to enhance the device output performance, most research has focused on the surface optimization of the surface friction layer [29,30,31,32]. However, these approaches yield little contributions toward the performance gain in long-term operation when surface modification/engineering is inevitably physically worn out by intimate contact and friction between the triboelectric materials [33]. Different from that strategy, the Al2O3 electron blocking layer here is located far away from the friction layer surface. Therefore, the resulted enhancement would not diminish even after the friction layer surface was worn during long-term operation. In addition, instead of competing with other methods, our technique could actually complement them. It is generic and could be applied in combination with other methods.
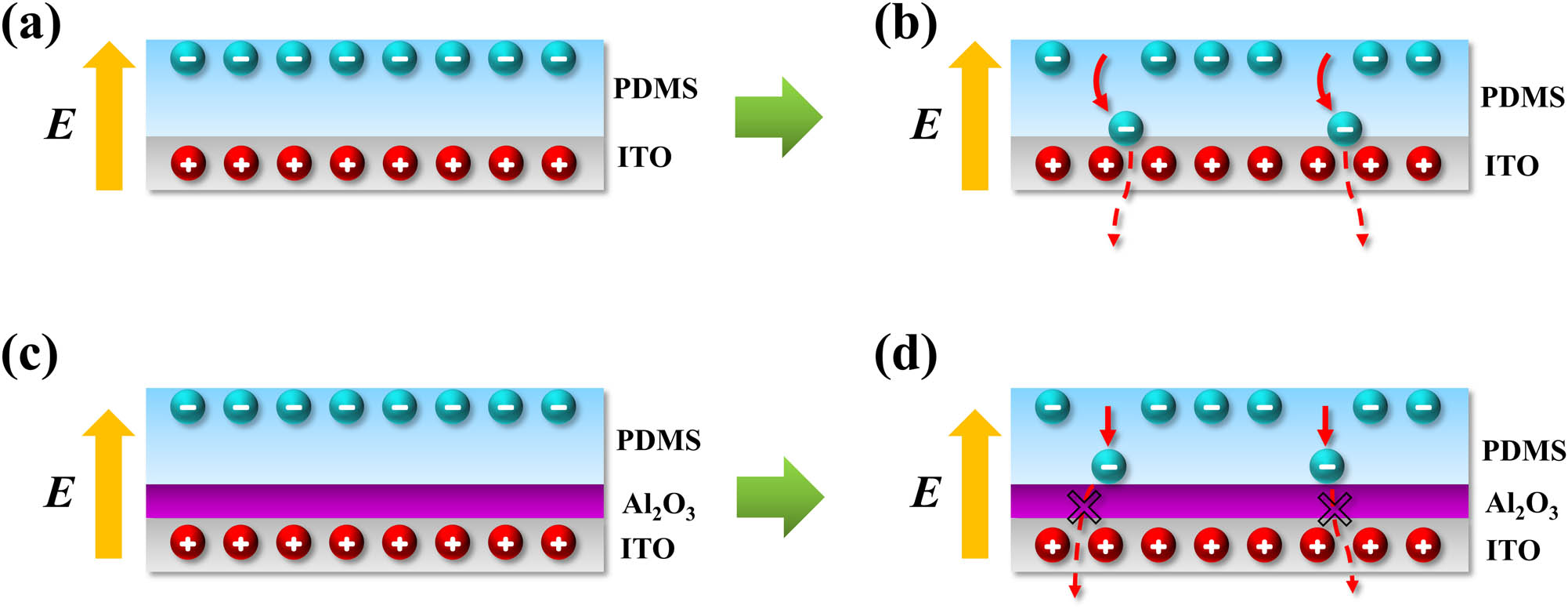
Working principle of the Al2O3 electron blocking layer. Schematic diagram of (a) the charge distribution and (b) electron drift process in the TENG without Al2O3. Schematic diagram of (c) the charge distribution and (d) the electron drift motion blocked by the Al2O3 layer in the TENG with an inserted Al2O3 layer.
3.3 Growth mechanism of the CLD-Al2O3
Comparing with other more conventional Al2O3 deposition methods, the CLD technique may achieve the similar material quality while using cheaper and more convenient setup that does not rely on vacuum systems. Especially, the deposition temperature of CLD is much lower than other methods (see Table 1). Therefore, unless the substrate is sensitive to the aqueous environment, CLD could be used as a facile method to grow aluminum oxide thin films on electron devices. Nevertheless, an interesting question remains to be solved. That is, the deposition mechanism is yet to be unveiled. We propose the CLD-Al2O3 thin film growth mechanism as follows. First, owing to the abundance of OH groups in the growth liquid, OH are decorated onto the surface of substrate. Then, the OH groups and the Al(OH)3 clusters undergo a continuous dehydration and polymerization, which result in the CLD-Al2O3 particle and subsequently thin film deposition. However, what is the status of the precursor? Are the Al(OH)3 in ionic form, i.e., Al3+ and OH−, like in a solution, or are they already forming colloids? To clarify this, we have let a laser beam pass through a 1:1 diluted liquid (see step 3 in Section 2.1 and Figure 1a). In a dark room, observing from the side, a bright “light path” of the incident light travelling in the liquid can be vividly seen, i.e., the Tyndall effect, as shown in Figure 3e. Therefore, some Al(OH)3 colloids are already formed in the liquid in step 3 (see Section 2.1) before the deposition. Nevertheless, we note that our CLD method is fundamentally different from the existing sol–gel method and should not be mixed up. CLD has the advantage that a solid film can be directly grown on the surface of the sample in the growth liquid, and postannealing is not mandatory in the process flow. If needed, however, postannealing could be used to increase the crystallinity of the film. On the contrary, the sol–gel method usually requires a plurality of heat treatments in the process of preparing the sol. After immersing the substrate in the sol, no film is formed in situ. Taking the sample out of the sol, a solid film is produced only after the high-temperature annealing, which is not an optional procedure. Therefore, the CLD method is intrinsically a room temperature process that accomplishes thin film deposition already in the growth liquid, which is not possible using the sol–gel method. The growth is in essence a pH-controlled condensation process of colloidal nanoparticles. No matter we put in a substrate or not, the condensation will anyway take place and that is why the liquid gradually becomes turbid. When a substrate is immersed in the growth liquid, the nanoparticles will nucleate on its surface and growth into a thin film. The particles are so fine that the film is rather flat and pin hole free. Of course, it is suitable for conformal coating of ultrathin films. Eventually, when the nanoparticles are exhausted, the liquid losses its ability to grow films. Actually, the growth already essentially stops when the colloidal particles become too large to be adsorbed. Note that our experiments do not exclude the growth from single ions. Although the liquid is proved to contain Al(OH)3 sol, since the chemical reaction (see equation (1)) is dynamically taking place, it does not exclude the possibility that single Al(OH)3 particles coming from the hydrolysis of single Al3+ ions can be directly incorporated into the thin film (without forming nanoparticles). Therefore, the CLD of Al2O3 is a complicated procedure involving Al(OH)3 formation at both nanometer and single-ion level. With the growth mechanism revealed, one could specifically select chemical reactions that generate colloids, which can be condensed in a controlled fashion to grow the needed thin films in a facile manner. It opens the gateway to the synthesis of a bunch of functional films.
Comparison of different traditional Al2O3 deposition methods with CLD
| No. | Method | Deposition temperature [°C] | Deposition rate [nm/min] | RMS [nm] | Breakdown field [MV/cm] | Ref. |
|---|---|---|---|---|---|---|
| 1 | CLD | 25 | 0.17–0.25 | ∼0.07 | 1.74 | This work |
| 2 | ALD | 180 | ∼0.25 nm/cycle | 0.1–1 | ∼1 | [12] |
| 3 | PECVD | 800 | ∼200 | 2.4–8 | — | [13] |
| 4 | Sputtering | 500 | ∼5 | — | — | [11] |
| 5 | Evaporation | 65 | 24 | — | 1 | [10] |
4 Conclusions
In summary, Al2O3 thin films were deposited on substrates by using the CLD method, which is a facile and low-cost method. The growth conditions are carefully optimized. The CLD method can grow large area thin films at room temperature with a reasonably good material quality suitable for electronic applications. The results show that the surface of the Al2O3 films is very flat. The breakdown field strength can reach 1.74 MV/cm. We have applied the as-grown Al2O3 films as the electron blocking layer of TENG, which provides a simple, low-cost, and effective method for improving the output performance of TENG. The open-circuit voltage and short-circuit current of the Al2O3-TENG is about 2.6 and 3 times, respectively, that of the Al2O3-free TENG. Finally, the growth mechanism is confirmed to include a pH-controlled colloidal condensation procedure, which could be used as the guideline to design the CLD of other thin films.
Acknowledgments
The authors gratefully acknowledge the help in measurement from Fujian Zhaoyuan Photoelectric Co., Ltd. and the financial support from National Natural Science Foundation of China (11674016).
-
Conflict of interest: The authors declare no conflict of interest regarding the publication of this paper.
References
[1] Abbott RA, Kamins TI. Sodium migration through electron-gun evaporated Al2O3 and double layer Al2O3 SiO2 structures. Solid State Electron. 1970;13:565–76.10.1016/0038-1101(70)90137-1Search in Google Scholar
[2] Robertson J. High dielectric constant oxides. Eur Phys J Appl Phys. 2004;28:265–91.10.1051/epjap:2004206Search in Google Scholar
[3] Shang Y, Zhong C, Jia R, Xiong H, Li H, Li X, et al. Preparation of low-permittivity K2O–B2O3–SiO2–Al2O3 composites without the addition of glass. Nanotechnol Rev. 2019;8:459–66.10.1515/ntrev-2019-0041Search in Google Scholar
[4] Zaininger KH, Waxman AS. Radiation resistance of Al2O3 MOS devices. IEEE Trans Electron Dev. 1969;16:333–8.10.1109/T-ED.1969.16753Search in Google Scholar
[5] Jo YJ, Jin HS, Ha MW, Park TJ. Sulfur incorporation at interface between atomic-layer-deposited Al2O3 thin film and AlGaN/GaN heterostructure. Electron Mater Lett. 2019;15:179–85.10.1007/s13391-018-00110-xSearch in Google Scholar
[6] Zhang X, Zhang Y, Tian B, Jia Y, Liu Y, Song K, et al. Cr effects on the electrical contact properties of the Al2O3-Cu/15W composites. Nanotechnol Rev. 2019;8:128–35.10.1515/ntrev-2019-0012Search in Google Scholar
[7] Lee J, Kim JH, Im S. Pentacene thin-film transistors with Al2O3+x gate dielectric films deposited on indium-tin-oxide glass. Appl Phys Lett. 2003;83:2689–91.10.1063/1.1613997Search in Google Scholar
[8] Nam Y, Lindvall N, Sun J, Park YW, Yurgens A. Graphene p–n–p junctions controlled by local gates made of naturally oxidized thin aluminium films. Carbon. 2006;50:1987–92.10.1016/j.carbon.2011.12.056Search in Google Scholar
[9] Saraie J, Ngan SF. Photo-CVD of Al2O3 thin films. Jan J Appl Phys. 1990;29:L1877.10.1143/JJAP.29.L1877Search in Google Scholar
[10] Shamala KS, Murthy LCS, Rao KN. Studies on optical and dielectric properties of Al2O3 thin films prepared by electron beam evaporation and spray pyrolysis method. Mater Sci Eng B. 2004;106:269–74.10.1016/j.mseb.2003.09.036Search in Google Scholar
[11] Musil J, Blažek J, Zeman P, Prokšová Š, Šašek M, Čerstvý R. Thermal stability of alumina thin films containing γ-Al2O3 phase prepared by reactive magnetron sputtering. Appl Surf Sci. 2010;257:1058–62.10.1016/j.apsusc.2010.07.107Search in Google Scholar
[12] Ghiraldelli E, Pelosi C, Gombia E, Chiavarotti G, Vanzetti L. ALD growth, thermal treatments and characterisation of Al2O3 layers. Thin Solid Films. 2008;517:434–6.10.1016/j.tsf.2008.08.052Search in Google Scholar
[13] Cibert C, Hidalgo H, Champeaux C, Tristant P, Tixier C, Desmaison J, et al. Properties of aluminum oxide thin films deposited by pulsed laser deposition and plasma enhanced chemical vapor deposition. Thin Solid Films. 2008;516:1290–6.10.1016/j.tsf.2007.05.064Search in Google Scholar
[14] Fan FR, Tian ZQ, Wang ZL. Flexible triboelectric generator. Nano Energy. 2012;1:328–34.10.1016/j.nanoen.2012.01.004Search in Google Scholar
[15] Wu C, Kim TW, Park JH, An H, Shao J, Chen X, et al. Enhanced triboelectric nanogenerators based on MoS2 monolayer nanocomposites acting as electron-acceptor layers. ACS Nano. 2017;11:8356–63.10.1021/acsnano.7b03657Search in Google Scholar PubMed
[16] Xu L, Wu H, Yao G, Chen L, Yang X, Chen B, et al. Giant voltage enhancement via triboelectric charge supplement channel for self-powered electroadhesion. ACS Nano. 2018;12:10262–71.10.1021/acsnano.8b05359Search in Google Scholar PubMed
[17] Mallineni SSK, Behlow H, Podila R, Rao AM. A low-cost approach for measuring electrical load currents in triboelectric nanogenerators. Nanotechnol Rev. 2018;7:149–56.10.1515/ntrev-2017-0178Search in Google Scholar
[18] Wu C, Wang AC, Ding W, Guo H, Wang ZL. Triboelectric nanogenerator: a foundation of the energy for the new era. Adv Energy Mater. 2019;9:1802906.10.1002/aenm.201802906Search in Google Scholar
[19] Shang ZW, Hsu HH, Zheng ZW, Cheng CH. Progress and challenges in p-type oxide-based thin film transistors. Nanotechnol Rev. 2019;8:422–43.10.1515/ntrev-2019-0038Search in Google Scholar
[20] Feng J, Kriechbaum M, Liu LE. In situ capabilities of small angle x-ray scattering. Nanotechnol Rev. 2019;8:352–69.10.1515/ntrev-2019-0032Search in Google Scholar
[21] JCPDS File NO. 46-1212. JCPDS-International Center for Diffraction Data. ICDD; 2001.Search in Google Scholar
[22] Nayak PK, Hedhili MN, Cha D, Alshareef HN. High performance In2O3 thin film transistors using chemically derived aluminum oxide dielectric. Appl Phys Lett. 2013;103:033518.10.1063/1.4816060Search in Google Scholar
[23] Brand JVD, Sloof WG, Terryn H, De Wit JHW. Correlation between hydroxyl fraction and O/Al atomic ratio as determined from XPS spectra of aluminium oxide layers. Surf Interface Anal. 2004;36:81–88.10.1002/sia.1653Search in Google Scholar
[24] Cui N, Gu L, Lei Y, Liu J, Qin Y, Ma X, et al. Dynamic behavior of the triboelectric charges and structural optimization of the friction layer for a triboelectric nanogenerator. ACS Nano. 2016;10:6131–8.10.1021/acsnano.6b02076Search in Google Scholar PubMed
[25] Wang B, Huang W, Chi L, Al-Hashimi M, Marks TJ, Facchetti A. High-k gate dielectrics for emerging flexible and stretchable electronics. Chem Rev. 2018;118:5690–754.10.1021/acs.chemrev.8b00045Search in Google Scholar PubMed
[26] Zhao D, Liu Y, Zhang Q, Zhang Y, Zhang W, Duan Q, et al. Surface stress-based biosensor with stable conductive AuNPs network for biomolecules detection. Appl Surf Sci. 2019;491:443–50.10.1016/j.apsusc.2019.06.178Search in Google Scholar
[27] Guo QZ, Yang LC, Wang RC, Liu CP. Tunable work function of MgxZn1–xO as a viable friction material for a triboelectric nanogenerator. ACS Appl Mater Interfaces. 2018;11:1420–5.10.1021/acsami.8b17416Search in Google Scholar PubMed
[28] Kolodzey J, Chowdhury EA, Adam TN, Qui G, Rau I, Olowolafe JO, et al. Electrical conduction and dielectric breakdown in aluminum oxide insulators on silicon. IEEE Trans Electron Dev. 2000;47:121–8.10.1109/16.817577Search in Google Scholar
[29] Ding P, Chen J, Farooq U, Zhao P, Soin N, Yu L, et al. Realizing the potential of polyethylene oxide as new positive tribo-material: over 40 W/m2 high power flat surface triboelectric nanogenerators. Nano Energy. 2018;46:63–72.10.1016/j.nanoen.2018.01.034Search in Google Scholar
[30] Chen SN, Chen CH, Lin ZH, Tsao YH, Liu CP. On enhancing capability of tribocharge transfer of ZnO nanorod arrays by Sb doping for anomalous output performance improvement of triboelectric nanogenerators. Nano Energy. 2018;45:311–8.10.1016/j.nanoen.2018.01.013Search in Google Scholar
[31] Wang S, Zi Y, Zhou YS, Li S, Fan F, Lin L, et al. Molecular surface functionalization to enhance the power output of triboelectric nanogenerators. J Mater Chem A. 2016;4:3728–34.10.1039/C5TA10239ASearch in Google Scholar
[32] Shin SH, Kwon YH, Kim YH. Triboelectric charging sequence induced by surface functionalization as a method to fabricate high performance triboelectric generators. ACS Nano. 2015;9:4621–7.10.1021/acsnano.5b01340Search in Google Scholar PubMed
[33] Yu Y, Li Z, Wang Y, Gong S, Wang X. Sequential infiltration synthesis of doped polymer films with tunable electrical properties for efficient triboelectric nanogenerator development. Adv Mater. 2015;27:4938–44.10.1002/adma.201502546Search in Google Scholar PubMed
© 2020 Dianlun Li et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Generalized locally-exact homogenization theory for evaluation of electric conductivity and resistance of multiphase materials
- Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide
- Characterization of mechanical properties of epoxy/nanohybrid composites by nanoindentation
- Graphene and CNT impact on heat transfer response of nanocomposite cylinders
- A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites
- Ultrasmall Fe3O4 nanoparticles induce S-phase arrest and inhibit cancer cells proliferation
- Effect of aging on properties and nanoscale precipitates of Cu-Ag-Cr alloy
- Effect of nano-strengthening on the properties and microstructure of recycled concrete
- Stabilizing effect of methylcellulose on the dispersion of multi-walled carbon nanotubes in cementitious composites
- Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites
- Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation
- Preparation and properties of 3D interconnected CNTs/Cu composites
- On factors affecting surface free energy of carbon black for reinforcing rubber
- Nano-silica modified phenolic resin film: manufacturing and properties
- Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete
- Halloysite nanotubes in polymer science: purification, characterization, modification and applications
- Cellulose hydrogel skeleton by extrusion 3D printing of solution
- Crack closure and flexural tensile capacity with SMA fibers randomly embedded on tensile side of mortar beams
- An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites
- Utilization of red mud for producing a high strength binder by composition optimization and nano strengthening
- One-pot synthesis of nano titanium dioxide in supercritical water
- Printability of photo-sensitive nanocomposites using two-photon polymerization
- In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries
- Effect of nano and micro conductive materials on conductive properties of carbon fiber reinforced concrete
- Tribological performance of nano-diamond composites-dispersed lubricants on commercial cylinder liner mating with CrN piston ring
- Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance
- Genetic mechanisms of deep-water massive sandstones in continental lake basins and their significance in micro–nano reservoir storage systems: A case study of the Yanchang formation in the Ordos Basin
- Effects of nanoparticles on engineering performance of cementitious composites reinforced with PVA fibers
- Band gap manipulation of viscoelastic functionally graded phononic crystal
- Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution
- Manipulating conductive network formation via 3D T-ZnO: A facile approach for a CNT-reinforced nanocomposite
- Microstructure and mechanical properties of WC–Ni multiphase ceramic materials with NiCl2·6H2O as a binder
- Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite
- Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function
- Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing
- Corrosion behaviour of multilayer CrN coatings deposited by hybrid HIPIMS after oxidation treatment
- Surface hydrophobicity and oleophilicity of hierarchical metal structures fabricated using ink-based selective laser melting of micro/nanoparticles
- Research on bond–slip performance between pultruded glass fiber-reinforced polymer tube and nano-CaCO3 concrete
- Antibacterial polymer nanofiber-coated and high elastin protein-expressing BMSCs incorporated polypropylene mesh for accelerating healing of female pelvic floor dysfunction
- Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films
- A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination
- Arrangement structure of carbon nanofiber with excellent spectral radiation characteristics
- Effect of different particle sizes of nano-SiO2 on the properties and microstructure of cement paste
- Superior Fe x N electrocatalyst derived from 1,1′-diacetylferrocene for oxygen reduction reaction in alkaline and acidic media
- Facile growth of aluminum oxide thin film by chemical liquid deposition and its application in devices
- Liquid crystallinity and thermal properties of polyhedral oligomeric silsesquioxane/side-chain azobenzene hybrid copolymer
- Laboratory experiment on the nano-TiO2 photocatalytic degradation effect of road surface oil pollution
- Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage
- Conversion of sub-µm calcium carbonate (calcite) particles to hollow hydroxyapatite agglomerates in K2HPO4 solutions
- Exact solutions of bending deflection for single-walled BNNTs based on the classical Euler–Bernoulli beam theory
- Effects of substrate properties and sputtering methods on self-formation of Ag particles on the Ag–Mo(Zr) alloy films
- Enhancing carbonation and chloride resistance of autoclaved concrete by incorporating nano-CaCO3
- Effect of SiO2 aerogels loading on photocatalytic degradation of nitrobenzene using composites with tetrapod-like ZnO
- Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles
- Hydration activity, crystal structural, and electronic properties studies of Ba-doped dicalcium silicate
- Microstructure and mechanical properties of brazing joint of silver-based composite filler metal
- Polymer nanocomposite sunlight spectrum down-converters made by open-air PLD
- Cryogenic milling and formation of nanostructured machined surface of AISI 4340
- Braided composite stent for peripheral vascular applications
- Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre–reinforced starch biopolymer composites
- Study on influencing factors of photocatalytic performance of CdS/TiO2 nanocomposite concrete
- Improving flexural and dielectric properties of carbon fiber epoxy composite laminates reinforced with carbon nanotubes interlayer using electrospray deposition
- Scalable fabrication of carbon materials based silicon rubber for highly stretchable e-textile sensor
- Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery
- Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries
- Synthesis of functionalized carbon nanotubes for fluorescent biosensors
- Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete
- Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage
- Microstructural evolution and properties of Cu–20 wt% Ag alloy wire by multi-pass continuous drawing
- Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene
- Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications
- Preparation and piezoresistivity of carbon nanotube-coated sand reinforced cement mortar
- Vibrational analysis of an irregular single-walled carbon nanotube incorporating initial stress effects
- Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials
- Single pulse laser removal of indium tin oxide film on glass and polyethylene terephthalate by nanosecond and femtosecond laser
- Mechanical reinforcement with enhanced electrical and heat conduction of epoxy resin by polyaniline and graphene nanoplatelets
- High-efficiency method for recycling lithium from spent LiFePO4 cathode
- Degradable tough chitosan dressing for skin wound recovery
- Static and dynamic analyses of auxetic hybrid FRC/CNTRC laminated plates
- Review articles
- Carbon nanomaterials enhanced cement-based composites: advances and challenges
- Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide
- Review on modeling and application of chemical mechanical polishing
- Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites
- Advances in modelling and analysis of nano structures: a review
- Mechanical properties of nanomaterials: A review
- New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics
- A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials
- Recent development and applications of nanomaterials for cancer immunotherapy
- Advances in biomaterials for adipose tissue reconstruction in plastic surgery
- Advances of graphene- and graphene oxide-modified cementitious materials
- Theories for triboelectric nanogenerators: A comprehensive review
- Nanotechnology of diamondoids for the fabrication of nanostructured systems
- Material advancement in technological development for the 5G wireless communications
- Nanoengineering in biomedicine: Current development and future perspectives
- Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview
- Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review
- Carbon nanotube–reinforced polymer composite for electromagnetic interference application: A review
- Functionalized layered double hydroxide applied to heavy metal ions absorption: A review
- A new classification method of nanotechnology for design integration in biomaterials
- Finite element analysis of natural fibers composites: A review
- Phase change materials for building construction: An overview of nano-/micro-encapsulation
- Recent advance in surface modification for regulating cell adhesion and behaviors
- Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions
- Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review
- Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications
- A review of passive methods in microchannel heat sink application through advanced geometric structure and nanofluids: Current advancements and challenges
- Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices
- Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging
- Synthesis of graphene: Potential carbon precursors and approaches
- A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE)
- Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water
- Analysis of functionally graded carbon nanotube-reinforced composite structures: A review
- Application of nanomaterials in ultra-high performance concrete: A review
- Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer
- Advanced nickel nanoparticles technology: From synthesis to applications
- Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable?
- Progress in construction of bio-inspired physico-antimicrobial surfaces
- From materials to devices using fused deposition modeling: A state-of-art review
- A review for modified Li composite anode: Principle, preparation and challenge
- Naturally or artificially constructed nanocellulose architectures for epoxy composites: A review
Articles in the same Issue
- Research Articles
- Generalized locally-exact homogenization theory for evaluation of electric conductivity and resistance of multiphase materials
- Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide
- Characterization of mechanical properties of epoxy/nanohybrid composites by nanoindentation
- Graphene and CNT impact on heat transfer response of nanocomposite cylinders
- A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites
- Ultrasmall Fe3O4 nanoparticles induce S-phase arrest and inhibit cancer cells proliferation
- Effect of aging on properties and nanoscale precipitates of Cu-Ag-Cr alloy
- Effect of nano-strengthening on the properties and microstructure of recycled concrete
- Stabilizing effect of methylcellulose on the dispersion of multi-walled carbon nanotubes in cementitious composites
- Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites
- Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation
- Preparation and properties of 3D interconnected CNTs/Cu composites
- On factors affecting surface free energy of carbon black for reinforcing rubber
- Nano-silica modified phenolic resin film: manufacturing and properties
- Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete
- Halloysite nanotubes in polymer science: purification, characterization, modification and applications
- Cellulose hydrogel skeleton by extrusion 3D printing of solution
- Crack closure and flexural tensile capacity with SMA fibers randomly embedded on tensile side of mortar beams
- An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites
- Utilization of red mud for producing a high strength binder by composition optimization and nano strengthening
- One-pot synthesis of nano titanium dioxide in supercritical water
- Printability of photo-sensitive nanocomposites using two-photon polymerization
- In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries
- Effect of nano and micro conductive materials on conductive properties of carbon fiber reinforced concrete
- Tribological performance of nano-diamond composites-dispersed lubricants on commercial cylinder liner mating with CrN piston ring
- Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance
- Genetic mechanisms of deep-water massive sandstones in continental lake basins and their significance in micro–nano reservoir storage systems: A case study of the Yanchang formation in the Ordos Basin
- Effects of nanoparticles on engineering performance of cementitious composites reinforced with PVA fibers
- Band gap manipulation of viscoelastic functionally graded phononic crystal
- Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution
- Manipulating conductive network formation via 3D T-ZnO: A facile approach for a CNT-reinforced nanocomposite
- Microstructure and mechanical properties of WC–Ni multiphase ceramic materials with NiCl2·6H2O as a binder
- Effect of ball milling process on the photocatalytic performance of CdS/TiO2 composite
- Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function
- Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing
- Corrosion behaviour of multilayer CrN coatings deposited by hybrid HIPIMS after oxidation treatment
- Surface hydrophobicity and oleophilicity of hierarchical metal structures fabricated using ink-based selective laser melting of micro/nanoparticles
- Research on bond–slip performance between pultruded glass fiber-reinforced polymer tube and nano-CaCO3 concrete
- Antibacterial polymer nanofiber-coated and high elastin protein-expressing BMSCs incorporated polypropylene mesh for accelerating healing of female pelvic floor dysfunction
- Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films
- A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination
- Arrangement structure of carbon nanofiber with excellent spectral radiation characteristics
- Effect of different particle sizes of nano-SiO2 on the properties and microstructure of cement paste
- Superior Fe x N electrocatalyst derived from 1,1′-diacetylferrocene for oxygen reduction reaction in alkaline and acidic media
- Facile growth of aluminum oxide thin film by chemical liquid deposition and its application in devices
- Liquid crystallinity and thermal properties of polyhedral oligomeric silsesquioxane/side-chain azobenzene hybrid copolymer
- Laboratory experiment on the nano-TiO2 photocatalytic degradation effect of road surface oil pollution
- Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage
- Conversion of sub-µm calcium carbonate (calcite) particles to hollow hydroxyapatite agglomerates in K2HPO4 solutions
- Exact solutions of bending deflection for single-walled BNNTs based on the classical Euler–Bernoulli beam theory
- Effects of substrate properties and sputtering methods on self-formation of Ag particles on the Ag–Mo(Zr) alloy films
- Enhancing carbonation and chloride resistance of autoclaved concrete by incorporating nano-CaCO3
- Effect of SiO2 aerogels loading on photocatalytic degradation of nitrobenzene using composites with tetrapod-like ZnO
- Radiation-modified wool for adsorption of redox metals and potentially for nanoparticles
- Hydration activity, crystal structural, and electronic properties studies of Ba-doped dicalcium silicate
- Microstructure and mechanical properties of brazing joint of silver-based composite filler metal
- Polymer nanocomposite sunlight spectrum down-converters made by open-air PLD
- Cryogenic milling and formation of nanostructured machined surface of AISI 4340
- Braided composite stent for peripheral vascular applications
- Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre–reinforced starch biopolymer composites
- Study on influencing factors of photocatalytic performance of CdS/TiO2 nanocomposite concrete
- Improving flexural and dielectric properties of carbon fiber epoxy composite laminates reinforced with carbon nanotubes interlayer using electrospray deposition
- Scalable fabrication of carbon materials based silicon rubber for highly stretchable e-textile sensor
- Degradation modeling of poly-l-lactide acid (PLLA) bioresorbable vascular scaffold within a coronary artery
- Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries
- Synthesis of functionalized carbon nanotubes for fluorescent biosensors
- Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete
- Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage
- Microstructural evolution and properties of Cu–20 wt% Ag alloy wire by multi-pass continuous drawing
- Transparent ultraviolet-shielding composite films made from dispersing pristine zinc oxide nanoparticles in low-density polyethylene
- Microfluidic-assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications
- Preparation and piezoresistivity of carbon nanotube-coated sand reinforced cement mortar
- Vibrational analysis of an irregular single-walled carbon nanotube incorporating initial stress effects
- Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials
- Single pulse laser removal of indium tin oxide film on glass and polyethylene terephthalate by nanosecond and femtosecond laser
- Mechanical reinforcement with enhanced electrical and heat conduction of epoxy resin by polyaniline and graphene nanoplatelets
- High-efficiency method for recycling lithium from spent LiFePO4 cathode
- Degradable tough chitosan dressing for skin wound recovery
- Static and dynamic analyses of auxetic hybrid FRC/CNTRC laminated plates
- Review articles
- Carbon nanomaterials enhanced cement-based composites: advances and challenges
- Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide
- Review on modeling and application of chemical mechanical polishing
- Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites
- Advances in modelling and analysis of nano structures: a review
- Mechanical properties of nanomaterials: A review
- New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics
- A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials
- Recent development and applications of nanomaterials for cancer immunotherapy
- Advances in biomaterials for adipose tissue reconstruction in plastic surgery
- Advances of graphene- and graphene oxide-modified cementitious materials
- Theories for triboelectric nanogenerators: A comprehensive review
- Nanotechnology of diamondoids for the fabrication of nanostructured systems
- Material advancement in technological development for the 5G wireless communications
- Nanoengineering in biomedicine: Current development and future perspectives
- Recent advances in ocean wave energy harvesting by triboelectric nanogenerator: An overview
- Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review
- Carbon nanotube–reinforced polymer composite for electromagnetic interference application: A review
- Functionalized layered double hydroxide applied to heavy metal ions absorption: A review
- A new classification method of nanotechnology for design integration in biomaterials
- Finite element analysis of natural fibers composites: A review
- Phase change materials for building construction: An overview of nano-/micro-encapsulation
- Recent advance in surface modification for regulating cell adhesion and behaviors
- Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions
- Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review
- Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications
- A review of passive methods in microchannel heat sink application through advanced geometric structure and nanofluids: Current advancements and challenges
- Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices
- Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging
- Synthesis of graphene: Potential carbon precursors and approaches
- A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE)
- Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water
- Analysis of functionally graded carbon nanotube-reinforced composite structures: A review
- Application of nanomaterials in ultra-high performance concrete: A review
- Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer
- Advanced nickel nanoparticles technology: From synthesis to applications
- Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable?
- Progress in construction of bio-inspired physico-antimicrobial surfaces
- From materials to devices using fused deposition modeling: A state-of-art review
- A review for modified Li composite anode: Principle, preparation and challenge
- Naturally or artificially constructed nanocellulose architectures for epoxy composites: A review

