Abstract
The stiffness of arterial wall in response to cardiovascular diseases has been associated with the changes in extracellular matrix (ECM) proteins, i.e., collagen and elastin. Vascular smooth muscle cells (VSMCs) helped to regulate the ECM reorganizations and thus contributed to arterial stiffness. This article reviewed experimental and computational studies for quantifying the roles of ECM proteins and VSMCs in mechanical properties of arteries, including nanostructure and mechanical properties of VSMCs and ECMs, cell-ECM interaction, and biomimetic gels/scaffolds induced contractile properties and phenotype changing of VSMCs. This work will facilitate our understanding of how the microenvironments and mechanotransduction impact and regulate the arterial adaptation.
1 Load bearing filaments in VSMC cytoskeleton
The cytoskeletal of vascular smooth muscles encompasses filaments and organelles. The density and number of these components can vary with respect to different internal and external signals [1, 2]. The filaments inside cytoskeleton can be classified as actin stress fibers (SFs), microtubules (MTs), and intermediate filaments (IFs), as shown in Figure 1.
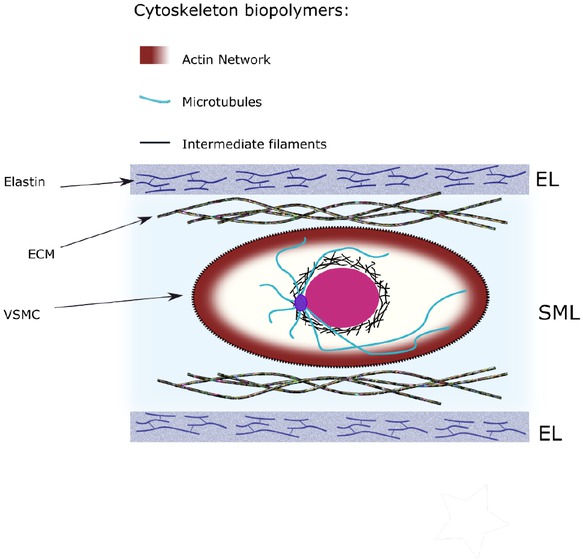
Cytoskeleton structure of the cell and the load carrying fibers
These filaments play a principal role in the mechanical properties of vascular smooth muscle cells including proliferation 3, differentiation [4, 5], cell migration 6, and apoptosis [7, 8]. Therefore, mechanical properties of these fibers are critical for the deformation and stability of vascular smooth muscle cells.
1.1 Stress fibers (SFs)
It has been reported that stress fibers (SFs), which mainly alighted in major axis of the cell, are the principal contributor to contractile forces through actomyosin activation 9. Deguchi et al. 10 performed tensile tests of SFs by isolating these fibers from cultured bovine VSMCs. Each SF is composed of a bundle of actin filaments (AFs). These bundles are held together by the actin-crosslinking protein α-actin. The elastic modulus of SFs was approximately 1.45 MPa which was three orders of magnitude lower than that of single AF (1.8-2.6 GPa) 11. On the other hand, the breaking force of single AF was determined to be 600 pN, whereas the breaking force of a single SF is approximately 380 nN, i.e., 600 times higher. In addition, the stress-strain relation was linear for the single AF, although SFs exhibited a highly non-linear strain-induced hardening behavior 12. Cell contraction is based on two vital structures, SFs and focal adhesion sites. Rho GTPase promotes the formation of SFs and cell adhesion sites, resulted in higher contractility 13. It is reported that the tension applied to focal adhesions increased from 10 nN to 100 nN upon contraction of the VSMCs 14. Moreover, disruption of SFs during the tensile tests decreased the cell’s stiffness by 50% 15.
1.2 Microtubules (MTs)
Microtubules (MTs) have a cylindrical shape with inner and outer diameters of 14 and 25 nm 16. MTs are rigid filaments with bending stiffness of 100 times higher than that of AFs and with elastic modulus of 1.2 GPa 17. MTs have a remarkable contribution in stabilization of cells elongation through attaching to the cell membrane via certain capping proteins 18. The contribution of MTs on cell locomotion and migration by regulating of actin polymerization has been reported 19. Kato et al. 20 showed that tracheal fusion cells form polarized microtubule bundles oriented towards the leading edge of migrating cells. The function of these microtubules is twofold: to concentrate E-cadherin to the newly contacted cell interface and to initiate the formation of new adherent’s junctions. Microtubule depolymerization enhances isometric contraction of vascular smooth muscle cell, which is not receptor dependent 21. Besides the principal contribution of SFs in contractility and MTs in migration, MTs are acknowledged to indirectly affect the contractility of VSMCs. Specifically, MTs growth favors dissolution of focal adhesions, whereas disruption of MTs leads to enhanced cell contractility by formation of SFs and focal adhesions 22. In addition, disruption of the MTs decreased the tensile stiffness of VSMCs by 30% at large strain levels. Insignificant contribution of MTs was observed under small tensile strain which stem from wavy morphology of these fibers 15.
1.3 Intermediate filaments (IFs)
The intermediate filament (IF) network is one of three cytoskeletal systems. IFs are widely distributed from the plasma membrane to nucleus, providing mechanical and structural integrity for the cell 23. In conjunction with associated proteins, IFs generate networks that serve to generate and support cell shapes. Spatial reorganization of IFs along with the development of SFs make VSMCs able to adjust their contraction/relaxation states. Moreover, the dynamic IFs play a crucial role in regulating various cellular functions including signal transduction; tension development; cell division and migration 24. The IFs, with the diameter of approximate 10 nm, have been grouped into five types, or sequence homology classes (SHC), on the basis of amino-acid-sequence identity 25. The most prominent IFs in VSMC cytoskeleton is vimentin, which forms a dynamic network and varies during contraction 26. The elastic modulus of IFs has been reported in the range of 300-900 MPa 27. The contribution of IFs in tensile properties of SMCs is remained to be determined even though IFs play important roles in tensile properties of the cells during large deformation 28. Green et al. 29 speculated that it is impossible to disrupt IFs themselves due to the interaction between IFs and AF structure.
Although the characteristics of each filament in the VSMCs cytoskeleton has been studied separately, the intracellular force balance, contraction, and cell stiffness are strongly influenced by the interaction of cytoskeleton with extracellular matrix (ECM) and signaling pathways as described below.
2 Interaction of VSMCs within the extracellular matrix (ECM)
Structural constituents of ECM, that regulate its passive mechanical behavior, are elastin fibers, collagens, and glycosaminoglycans (GAGs) 30. Interaction of these structural constituents and VSMCs can trigger significant variations of stiffness of both ECM and VSMCs. The adhesive glycoproteins fibronectin and laminin form connections between ECM and VSMCs via specific integrin receptors. Fibronectin is a multifunctional adhesive protein present in the plasma and also synthesized by vascular cells 31. VSMCs express both β-1 and β-3 integrins and 32 demonstrated greater functional significance in adhesive processes of β-3 integrin essential for SMC migration. On way to study the interaction between VSMC (and other cells in general) and ECM is to culture the cell on substrate and study the deformations under different circumstances 33. Adhesion rate, spread area, cytoskeletal assembly, and focal adhesion signaling was evaluated by culturing VSMCs on substrates with different stiffness and coated with fibroactin- or laminin- 34. When VSMCs were cultured on fibroactin substrates with varied mechanical gradient, it was found out that cells preferentially migrate toward stiffer regions [35, 36]. On the other side, Hartman et al. 37 observed the migration of VSMCs toward the stiffer region of gradient substrate coated with fibroactin, whereas the migration on laminin-coated gradient substrate appeared to be random. This observation indicated that the deformation and migration of VSMCs are not only dependent on the stiffness of ECM but also the type of interacting proteins and the engaged integrins 34.
The ECM stiffness can also affect the phenotype of VSMCs 38. A stiffer ECM led to synthetic phenotype in the VSMC. Specifically, the VSMC decreases the number of cytoskeletal filaments and exhibits lower stiffness than that of contractile phenotype. Fibronectin drives cells away from the contractile phenotype in vitro, whereas laminin has been shown to conserve it 39. Cell culture in 2D has been widely used to study the mechanotransduction of VSMCs due to ease of handling, maintenance, and application of mechanical loads [40, 41, 42]. However, culturing cells on a 2D substrate affects the cellular deformation, adhesion force and stiffness. To address this issue, engineering 3D gels 43 or scaffolds [44, 45] as the cell culture environment has been suggested.
Artery and its cellular components are continuously exposed to hemodynamic stimuli including cyclic strain, flow shear stress, and blood pressure [46, 47]. These mechanical loadings correlated with VSMC behaviors, ECM remodeling, and vasoregulation 48. Cyclic mechanical stimulation possesses dual effect on proliferation of VSMC 49, enhance the collagen production 50, and increases the capability of transformation from synthetic SMC phenotype into contractile phenotype 51. A cyclic tensile strain of 5% reduced SMC proliferation 52. Conflicting variation of VSMCs phenotype with respect to the level of cyclic loading has been reported 53, whereas over-expression of contractile phenotype proteins has been observed [54, 55, 56, 57, 58]. Solan et al. 59 showed that cyclic strain had a direct impact on increasing collagen content and organization in ECMs. Bono et al. 60 studied the effects of cyclic strain (7%) on the VSMCs behavior which were cultured on 2D substrates and in 3D matrix composed of type I collagen. It was demonstrated that in the 3D culture environment there are more VSMCs aligned in the direction of strain (nearly 60%).Additionally, the level of SM α-actin in VSMCs cultured in the 3D collagen matrix was higher than that cultured on the monolayer 2D substrate. This research indicated that in 3D culture environment and under cyclic loading the density of contractile proteins inside VSMC’s cytoskeleton increases remarkably. It is worth mentioning that in the cardiac cycle VSMCs are cyclically stretched by ~ 10% with a 25-50% mean strain, and their mechanical properties should be evaluated over a large range of deformations 61.
It was noted that the ECM mechanical properties including its heterogeneity are the key factors to impact the 3D VSMC contractility 62. Novel hydrogels have been developed to resemble the composition of ECM and thus in vivo mechanical environment [63, 64]. Ding et al. 65 developed a biomimetic fibrous hydrogel with tunable structure and stiffness. The developed ECM array consisted of collagen I, III, IV, fibroactin, and laminin. The effect of ECM deposition and stiffening during vascular disease progression on VSMCs contraction/relaxation was investigated. Although, the developed hydrogel encompassed the composition of ECM components, the challenges lie in the control of the architecture and alignments of collagen fibers. It has been illustrated that fibers orientation affect their load sharing contribution to the media tunica 66. Phillippi et al. 67 reported that a remarkable variation of collagen fiber orientation distribution exists in the diseased aortic media. Considering the limitation in reproducing a complex in vivo ECM environment, the load sharing of VSMCs with respect to these structural components of ECM remained to be explored.
3 Arterial constituents
The artery wall exhibits three major layers: Intima, media and adventitia. The intima layer is predominantly populated with endothelial cells (ECs), which synthesize proteins, such as collagen IV and laminin, to create basal lamina. Its main function is to transmit signals that control vascular tone. It has a minimal contribution to the artery’s mechanical properties. The adventitia mainly consists of fibroblast and a collagen-rich ECM. Adventitial fibroblasts respond to a variety of chemical and mechanical cues. For example, hypertensive environments result in increased fibroblast proliferation and collagens I and III synthesis. Adventitia bears over half of the load at abnormal pressure due to collagen’s role as structural reinforcement 68. The media is the thickest layer, between the intima and adventitia layers. It serves as the primary load bearing components. The media are composed by multiple lamellar units (LU), which consists elastic lamellae encompassing smooth muscle cells (SMC), interposed with collagen fiber network, as shown in Figure 1.
The LU was comprised of approximately 29% elastin, 24% SMCs, and 47% collagen and ground substance 69. The volume of a single medial SMC was 1630±640 μm3. The healthy aortic media SMC was in the shape of ellipse. The average length of minor and major axis is 3.1±0.8 μm and 19.0 ±3.3 μm, respectively. The average aspect ratio, i.e., major/minor axis is 6.2±1.4. At the relaxed state, the elastic modulus of the rat aortic VSMC in the major and minor direction is 14.8 KPa and 2.8 KPa, respectively 70. Upon contraction, the elastic modulus in major and minor direction is 88.1 KPa and 59 KPa, respectively. The average density of SMCs within the media is 3.7±0.6 ×105 cells/mm369. Between lamellae, the major axis of each nucleus aligned in the circumferential direction with a 19±3∘ radial tilt, resulting in cytoplasmic ends directed toward top and bottom of the lamellae. Collagen type I is the most abundant within blood vessels and had been proposed as the primary determinant of tensile properties 71. Collagen was organized as bundles of fibers (numbering 24 ±15 fibers per bundle), thinner bundles or individual fibers. Collagen fibers aligned preferentially circumferential in the media but showed random orientation in the adventitia. The LU thickness ranges 13-15 μm 66 with an elastic lamellar thickness of 1.0-2.2 μm. The number of LUs of the media layer is established during arterial development and is directly related to the tension in the wall. It was noted that the tension per lamellar unit is constant across mammalian species and throughout the arterial tree 72.
Elastic modulus of elastin and collagen fibers was reported as approximately 0.6 MPa and 1 GPa, respectively 73. Collagen fibers have a wavy nature and low contributions to mechanical behaviors at low pressure load. This is due to the waviness of collagen fibers 74, which was gradually straightened under pressure. Only 6-7% of collagen fibers are engaged at physiological pressure 69. Microscopy studies on male adult rats revealed that collagen fibers aligned in the longitudinal-circumferential plane of the media layer of aorta. On the other hand, elastin fibers tended to align in the circumferential direction in SML, but often formed a longitudinally network structure in Els 69. Collagen fibers were observed more in ELs than in SMLs, and ELs comprise elastin and collagen fibers. Collagen fibers have a diameter of 3 μm and average segment length of 13-17 μm. The diameter of elastin fibers is measured around 0.1 μm which placed in ELs with an interconnecting, fenestrated network.
4 Arterial stiffness
The stiffness of artery is directly related to the function of each component in the LU. Due to their higher elastic modulus, elastin and collagen fibers were classically considered as the main load bearing elements in LU. At physiological pressure, arterial stiffness was predominantly determined by elastin fibers, while wavy collagen fibers, without being straightened yet, did not bear much load. Then, the abnormally large mechanical load could straighten the collagen fibers, which were able to carry more load than elastin fibers. These sequential participation of elastin and collagen fibers in arterial stiffness led to non-linear stress-strain response of the arterial wall, while it was suggested that VSMCs have no contribution in the mechanical response of the artery 75, as illustrated in Figure 2.
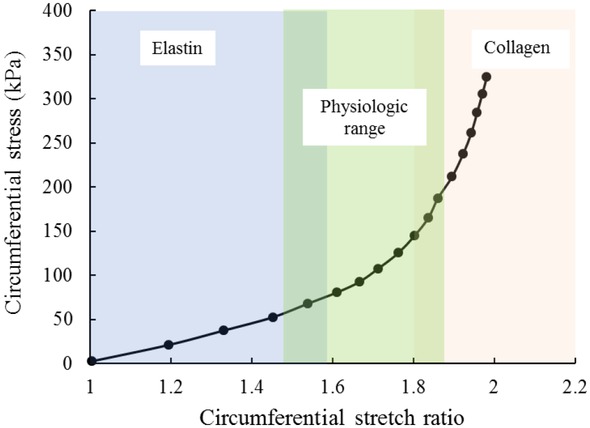
Representative circumferential stress–stretch relationship for the mouse ascending aorta.
Increased arterial stiffness is correlated with a larger collagen/elastin ratio in LU. Aging is associated with the defragmentation and discontinuity of elastin fibers. The damaged elastic fibers are generally not replaced, because elastin expression is turned off in adult species. This damage alone will weaken the artery. Then the arterial remodeling lead to more collagen fibers production, and usually increase the arterial stiffness [76, 77]. It has been reported that blood pressure and arterial stiffness are inversely related to elastin’s amount in the media layer [72, 78, 79, 80, 81]. Many cardiovascular disease, specifically hypertension, are related to high stiffness of artery induced by elastin reduction and collagen fiber production 72. Advanced glycation end-products (AGEs), which accumulate slowly with normal aging or in diabetes at a faster rate, has been considered as a major index factor for arterial wall stiffening [82, 83]. This was attributed to the increased protein–protein crosslinks on the collagen molecule [84, 85] and implied that collagen/elastin components alone are not the only inclusive parts to determine the arterial stiffness in certain situations. Using hypertensive rat models, several groups observed minimal changes in collagen content of artery [86, 87, 88, 89]. Instead, reduced collagen content were reported in some cases [90, 91]. Hu et al. 92 monitored over 8 weeks of ECM content in a coarctated mini-pig aorta. They observed that relative collagen content was increased at 2 weeks of hypertension, stayed at this high level for 4 weeks, and then declined to the baseline level at 6 weeks. The relative elastin content decreased at 2 weeks and remained at a similar level thereafter. The incongruous observations in the literature might be due to the variations in experimental protocols, including measurement methods of arterial collagen content, the hypertension degree, and the location of harvested artery 88.
Apart from the variation of collagen/elastin fibers content and ECM in general, VSMCs might have a contribution to arterial wall stiffness. Sehgel et al. 93 suggested to look into the contribution of VSMC to arterial stiffness since variation in elastin density was not enough to alter a major change in aortic stiffness. Animal studies (spontaneously hypertensive monkeys [94, 95] and rats 96) showed that VSMC in the aortic media layer is stiffer due to hypertension or aging. These observations indicated that VSMC alone might contribute to arterial stiffness but has not been measured yet.
5 VSMC
Stiffness measurement of vascular smooth muscle cells are challenging due to its sensitivity to phenotypic switching in response of the environment. It has been reported that cultured VSMC on substrate might change their phenotype to synthetic 97. VSMCs are aligned circumferentially in the media layer and undergo large deformations in physiological conditions. When the artery enlarges due to the hemodynamic pressures, VSMCs stretch along their major axis. However, AFM technique is only able to measure the elastic properties of local regions of cells under small deformations and cannot not provide enough information associated with the tensile properties of whole VSMC in physiological strain range (median strain of 25-50%). Due to the aforementioned reasons, it is vital to obtain tensile properties of the cells freshly isolated from the artery wall. In this regard, different methods for gripping the VSMC and performing tensile test have been suggested. Knotting 98, aspiration 99, adhesion on pipette 100, plate 101 and micropillar array substrate 14 are among the popular cell gripping methods for the tensile testing of VSMCs.
6 Mechanical contribution of arterial constituents
6.1 Experimental studies
There is a range of techniques to quantify the mechanical behaviors of cells, such as Atomic Force Microscopy (AFM) [88, 93, 95, 96]. The contraction response of VSMC can be measured directly by AFM tests, or in an indirect way by comparing the expression of primary SMC-specific contractile markers such as SM α-actin. It is well known that by phenotype changing of VSMCs to synthetic type, the number of stress fibers decreases, and the number of organelles increases which prepare the cell to proliferate and generate ECM proteins. These changes in the cytoskeleton decrease contractility and stiffness of VSMCs (by one-third or one-fourth). Thus, the initiation of cell proliferation can be counted as an indicator of relaxed VSMCs. Hu et al. 92 reported that cell proliferation occurred at 2, 4, and 6 weeks, but not at 8 weeks of hypertension. The highest proliferation rate was captured at 2 weeks of hypertension. Xu et al. 102 found that proliferation of medial VSMCs was induced rapidly within 3 days after acute coarctation of the rat aorta and continued for 2 weeks. In addition, In addition, fluctuations in VSMCs stiffness was detected over 8 weeks of high tension loading of rat aorta 103. Tosun and McFetridge 104 used cardiac output to define frequency profile of cyclic stretch of human VSMCs which was against with the previous in vitro models which were stimulated with constant pulse frequencies. It was revealed that the phenotypic outcome may be more dependent on the variation in the stimuli, rather than specific amplitude of change.
These studies indicate that VSMC’s stiffness could decrease sharply at the early stages of hypertension because of their dedifferentiation. However, it is reported that medial VSMCs expressing contractile proteins could also proliferate and actively synthesize ECM proteins 92. On the other hand, the dedifferentiated cells express low levels of contractile markers and high levels of signaling molecules associated with cell growth, migration, fibrosis, and inflammation 105. Matsumoto et al. 103 investigated the effects of hypertension on morphological, contractile and mechanical properties of rat aortic VSMCs. They found that the density of SFs and the stiffness of each SF may dependent on the intensity and duration of hypertension. The contraction and stiffness of VSMCs increased to its maximum at 8 weeks of hypertension and decreased thereafter. However, these observations were not correlated with the previous studies. The potential explanation could be the level of hypertension, measurement techniques, level of VSMCs tension and VSMCs alignment.
The mechanical properties of elastin fiber network in the media layer were evaluated under uniaxial or biaxial tension [106, 107, 108, 109, 110].Weisbecker et al. 111 compared the mechanical behavior of elastase and collagenase treated media from human thoracic aorta to untreated control specimens. VSMCs were still visible after elastase treatment and it was noted that their passive response might slightly affect the anisotropy of the tissue. One limitation of this work was neglecting the dependency of the mechanical properties on age or on the location of the artery. Martinez and Han 112 showed that collagenase treatment (collagen content decreased by 15%) caused an enhancement in the axial deformation but not in the circumferential deformation. This was explained by the dominating circumferential alignment of collagen in the vessel wall. While collagenase treatment may equally break the collagen fibers aligned in both the axial and circumferential directions, the ratio of change in the circumferential direction would be much smaller due to the large amount of collagen at the baseline 112. However, Dorbin et al. 113 observed a considerable reduction in the arterial wall stiffness in the circumferential direction of collagenase treated dog arteries. The difference might be associated with the type of species, the density of collagenase used, or the implemented testing conditions 114. Moreover, compared to elastase treatment, collagenase treatment seemed had less effect on the physiological pressures as that collagen is not fully engaged in the bearing arterial wall stresses. Reportedly, a decrease VSMC content by 11±7% in porcine carotid arteries was associated with enlargement of arterial wall at pressures up to 120 mm Hg and mechanical stiffening of the arterial wall at higher pressures 115. Despite the valuable results, the conducted researches had limitations such as being performed under static loading conditions, and the collagen fibers or VSMCs were partially removed in the treated specimens.
Although there have been many experiments to quantify the contribution of medial fibrous matrix in mechanical properties of the artery, the load sharing capacity of VSMCs has been underestimated. Previous studies about determining the stiffness and contraction of VSMCs in hypertension provided valuable information but sometimes are inconsistent which makes it difficult to evaluate the mechanical contribution of VSMCs in normotension and hypertension arteries. In addition, the load sharing capacity of VSMCs in LU is still not clear. Heterogeneity of LU and different mechanical properties of each component are the problematic issues to determine the portion of load taken by each constituent when the artery is exposed to hemodynamic pressures.
6.2 Computational methods
Numerical simulations have been implemented for many years to study the mechanical behavior of arteries. In the previous developed models, the arterial wall has been modeled as a single layer 116, two or three layers [117, 118]. The applied constitutive relations to the arterial wall have been formulated by hyperelastic material with orthotropic, transverse isotropic, and isotropic behavior [119, 120, 121, 122, 123]. The main concern about these models was to predict the macroscopic mechanical properties of the artery and evaluate its deformation [124, 125, 126, 127]. Considering the highly heterogeneous microstructure of the arterial layers has been challenging in these studies.
Furthermore, micromechanical modeling approach has been employed to include clearly distinguishable constituents inherited different material properties. The goal was to predict the anisotropic response of the heterogeneous material on the basis of the geometries and properties of the individual constituents, a task known as homogenization 128. Application of micromechanical modeling in arterial mechanics is vast. Capturing the responses of hyperelastic tissues with multiple families of collagen fibers 129, elucidating the interaction between collagen and non-fibrillar matrix 130, strain hardening of collagen-I gel and realignment of the network 131 can be counted as the micromechanical modeling applications associated with the behavior of fiber matrix. Thunes et al. 66 developed a micromechanical model to detect the stress field of the fiber matrix after collagen recruitment. The VSMCs were simplified and replaced by a homogenous medium as the non-fibrous part.
In order to study the VSMC contraction effects in the media tunica and stress distribution through the thickness of artery, Lukes and Rohan 132 proposed a 3D micromechanical model, which consisted of a hyperelastic matrix (ECM), an incompressible inclusion (VSMC), and contractile bars (SFs). The micro-scale model was then coupled with a 2D macro-scale model of the arterial wall consisted of two layers of tunica media and tunica adventitia.
Nakamachi et al. 51 constructed a multi-scale FE model for stress and strain evaluation of VSMC of the human artery. Their micro-scale model was based on a Representative Volume Element (RVE) model and consisted of a VSMC embedded in a homogenous matrix, Figure 3. Despite of the novelty of the developed model, the simplified ECM structure and neglecting the distribution of collagen/elastin fibers could be influential on the obtained results. Moreover, there were lack of discussion about mechanical contribution of the constituents in the arterial wall.
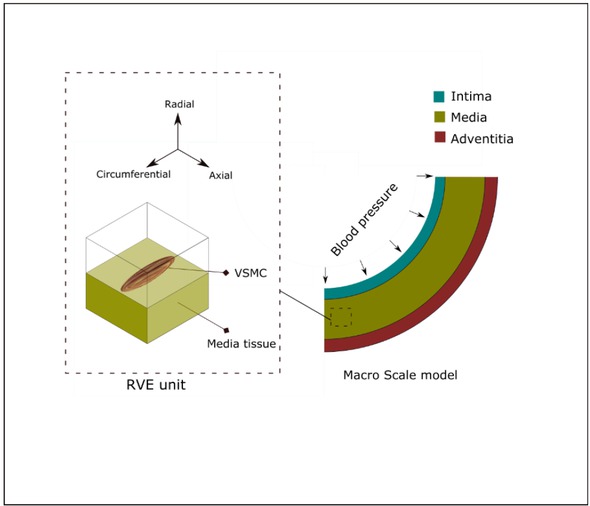
Macro scale model of the arterial wall with three layers (right); arterial VSMC and RVE model (left)
It has been found that the microstructure of ECM can vary by some diseases. Collagen disposition and cross-link disruption has been observed in the arteries with Marfan syndrome 133.Moreover, Marfan aortic samples are histologically characterized by the fragmentation of elastic laminae (almost 50% lower [134, 135, 136]), which leads to the formation of aneurysms. Therefore, considering the heterogeneous structure of ECM will allow to detect the ongoing mechanisms behind the arterial disease which change the properties of ECM and VSMC state.
7 Sumamry
This review summarized the mechanical contribution of VSMCs to the arterial stiffness with focus on the load sharing of collagen/elastin fibers and contracted/relaxed VSMCs in the media layer of artery. In view of VSMCs cytoskeleton, it was noted that stress fibers have the major contribution in VSMCs contraction, however, microtubules and intermediate filaments can indirectly affect contractility of the cells. In addition, the cytoskeleton responses are strongly related to the interaction of integrin receptors and extracellular matrix.
VSMCs alter their proliferation and contractility or change their phenotype with respect to the mechanical environment, such as 2D or 3D ECM, and level of cyclic strains. Specifically, the cultured VSMCs change their phenotype compared with in vivo conditions. The responses of VSMCs subjected to cyclic loading is dependent on the time period of the applied load.
The mechanics of VSMCs could be better delineated using numerical simulation. The interaction between collagen and non-fibrillar matrix, alignment and recruitment of collagen fibers and induced stresses in VSMCs during extension have been elucidated. However, the load sharing capacity of VSMCs in Lamellar unit as well as the influence of phenotype changing on the VSMCs contribution in arterial stiffness remained to be determined.
The focus of this review paper was on the tunica media. However, the contribution of tunica adventitia and intima should not be neglected. Adventitia prevents the arterial wall from overexpansion. The most abundant cell type in adventitia is the fibroblast with a stiffness ranging 1-27 kPa, which synthesize the extracellular matrix and collagen fibers. Endothelial cells in intima have a relative lower stiffness of 1-2kPa, but plays an important role in contraction and relaxation of VSMCs and arterial stiffness.
Acknowledgement
This work was supported by the National Science Foundation CAREER award (CBET-1254095).
References
[1] Tang, D.D. and B.D. Gerlach, The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respiratory Research, 2017. 18(1): p. 54.10.1186/s12931-017-0544-7Search in Google Scholar PubMed PubMed Central
[2] Huber, F., et al., Emergent complexity of the cytoskeleton: from single filaments to tissue. Advances in physics, 2013. 62(1): p. 1-112.10.1080/00018732.2013.771509Search in Google Scholar PubMed PubMed Central
[3] Ingber, D.E., Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proceedings of the National Academy of Sciences of the United States of America, 1990. 87(9): p. 3579-3583.10.1073/pnas.87.9.3579Search in Google Scholar PubMed PubMed Central
[4] Collinsworth, A.M., et al., Apparent elastic modulus and hysteresis of skeletal muscle cells throughout differentiation. American Journal of Physiology-Cell Physiology, 2002. 283(4): p. C1219-C1227.10.1152/ajpcell.00502.2001Search in Google Scholar PubMed
[5] Jacob, J.A., J.M.M. Salmani, and B. Chen, Magnetic nanoparticles: mechanistic studies on the cancer cell interaction. Nanotechnology Reviews, 2016. 5(5): p. 481-488.10.1515/ntrev-2016-0022Search in Google Scholar
[6] Pelling, A.E. and M.A. Horton, An historical perspective on cell mechanics. Pflügers Archiv - European Journal of Physiology, 2008. 456(1): p. 3-12.10.1007/s00424-007-0405-1Search in Google Scholar PubMed
[7] Chen, C.S., et al., Geometric Control of Cell Life and Death. Science, 1997. 276(5317): p. 1425.10.1126/science.276.5317.1425Search in Google Scholar PubMed
[8] Mohammad, F., et al., Targeted hyperthermia-induced cancer cell death by superparamagnetic iron oxide nanoparticles conjugated to luteinizing hormone-releasing hormone. Nanotechnology Reviews, 2014. 3(4): p. 389-400.10.1515/ntrev-2013-0041Search in Google Scholar
[9] Katoh, K., et al., Isolation and Contraction of the Stress Fiber. Molecular Biology of the Cell, 1998. 9(7): p. 1919-1938.10.1091/mbc.9.7.1919Search in Google Scholar PubMed PubMed Central
[10] Deguchi, S., T. Ohashi, and M. Sato, Tensile properties of single stress fibers isolated from cultured vascular smooth muscle cells. Journal of Biomechanics, 2006. 39(14): p. 2603-2610.10.1016/j.jbiomech.2005.08.026Search in Google Scholar PubMed
[11] Liu, X. and G.H. Pollack, Mechanics of F-actin characterized with microfabricated cantilevers. Biophysical Journal, 2002. 83(5): p. 2705-2715.10.1016/S0006-3495(02)75280-6Search in Google Scholar
[12] Tsuda, Y., et al., Torsional rigidity of single actin filaments and actin–actin bond breaking force under torsion measured directly by in vitro micromanipulation. Proceedings of the National Academy of Sciences of the United States of America, 1996. 93(23): p. 12937-12942.10.1073/pnas.93.23.12937Search in Google Scholar PubMed PubMed Central
[13] Etienne-Manneville, S., Actin and Microtubules in Cell Motility: Which One is in Control? Traffic, 2004. 5(7): p. 470-477.10.1111/j.1600-0854.2004.00196.xSearch in Google Scholar PubMed
[14] Nagayama, K. and T. Matsumoto, Dynamic Change in Morphology and Traction Forces at Focal Adhesions in Cultured Vascular Smooth Muscle Cells During Contraction. Cellular and Molecular Bioengineering, 2011. 4(3): p. 348-357.10.1007/s12195-011-0166-ySearch in Google Scholar
[15] Nagayama, K. and T. Matsumoto, Contribution of actin filaments and microtubules to quasi-in situ tensile properties and internal force balance of cultured smooth muscle cells on a substrate. American Journal of Physiology-Cell Physiology, 2008. 295(6): p. C1569-C1578.10.1152/ajpcell.00098.2008Search in Google Scholar PubMed
[16] Nogales, E., Structural Insights into Microtubule Function. Annual Review of Biochemistry, 2000. 69(1): p. 277-302.10.1146/annurev.biochem.69.1.277Search in Google Scholar PubMed
[17] Gittes, F., et al., Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. The Journal of Cell Biology, 1993. 120(4): p. 923.10.1083/jcb.120.4.923Search in Google Scholar PubMed PubMed Central
[18] Reilein, A. and W.J. Nelson, APC is a component of an organizing template for cortical microtubule networks. Nature Cell Biology, 2005. 7(5): p. 463-473.10.1038/ncb1248Search in Google Scholar PubMed PubMed Central
[19] Goldman, R.D., THE ROLE OF THREE CYTOPLASMIC FIBERS IN BHK-21 CELL MOTILITY : I. Microtubules and the Effects of Colchicine. The Journal of Cell Biology, 1971. 51(3): p. 752-762.10.1083/jcb.51.3.752Search in Google Scholar PubMed PubMed Central
[20] Kato, K., et al., Microtubule-dependent balanced cell contraction and luminal-matrix modification accelerate epithelial tube fusion. Nature Communications, 2016. 7: p. 11141.10.1038/ncomms11141Search in Google Scholar PubMed PubMed Central
[21] Zhang, D., et al., Influence of microtubules on vascular smooth muscle contraction. Journal of Muscle Research & Cell Motility, 2000. 21(3): p. 293-300.10.1023/A:1005600118157Search in Google Scholar
[22] Liu, B.P., M. Chrzanowska-Wodnicka, and K. Burridge, Microtubule depolymerization induces stress fibers, focal adhesions, and DNA synthesis via the GTP-binding protein Rho. Cell adhesion and communication, 1998. 5(4): p. 249-255.10.3109/15419069809040295Search in Google Scholar PubMed
[23] Chang, L. and R.D. Goldman, Intermediate filaments mediate cytoskeletal crosstalk. Nature Reviews Molecular Cell Biology, 2004. 5: p. 601.10.1038/nrm1438Search in Google Scholar PubMed
[24] Li, Q.-F., et al., Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase (PAK) in vimentin cytoskeleton signaling. The Journal of biological chemistry, 2006. 281(45): p. 34716-34724.10.1074/jbc.M607715200Search in Google Scholar PubMed PubMed Central
[25] Fuchs, E. and K. Weber, Intermediate Filaments: Structure, Dynamics, Function and Disease. Annual Review of Biochemistry, 1994. 63(1): p. 345-382.10.1146/annurev.bi.63.070194.002021Search in Google Scholar PubMed
[26] Wede, O.K., et al., Mechanical function of intermediate filaments in arteries of different size examined using desmin deficient mice. The Journal of Physiology, 2002. 540(Pt 3): p. 941-949.10.1113/jphysiol.2001.014910Search in Google Scholar PubMed PubMed Central
[27] Guzmán, C., et al., Exploring the Mechanical Properties of Single Vimentin Intermediate Filaments by Atomic Force Microscopy. Journal of Molecular Biology, 2006. 360(3): p. 623-630.10.1016/j.jmb.2006.05.030Search in Google Scholar PubMed
[28] Wang, N. and D. Stamenovic, Contribution of intermediate filaments to cell stiffness, stiffening, and growth. American Journal of Physiology-Cell Physiology, 2000. 279(1): p. C188-C194.10.1152/ajpcell.2000.279.1.C188Search in Google Scholar PubMed
[29] Green, K.J., et al., The relationship between intermediate filaments and microfilaments before and during the formation of desmosomes and adherens-type junctions in mouse epidermal keratinocytes. The Journal of cell biology, 1987. 104(5): p. 1389-1402.10.1083/jcb.104.5.1389Search in Google Scholar PubMed PubMed Central
[30] Humphrey, J.D., E.R. Dufresne, and M.A. Schwartz, Mechanotransduction and extracellular matrix homeostasis. Nature reviews Molecular cell biology, 2014. 15(12): p. 802.10.1038/nrm3896Search in Google Scholar PubMed PubMed Central
[31] Raines, E.W., The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. International Journal of Experimental Pathology, 2000. 81(3): p. 173-182.10.1046/j.1365-2613.2000.00155.xSearch in Google Scholar PubMed PubMed Central
[32] Bendeck, M.P., et al., Smooth muscle cell matrix metalloproteinase production is stimulated via αvβ3 integrin. Arteriosclerosis, thrombosis, and vascular biology, 2000. 20(6): p. 1467-1472.10.1161/01.ATV.20.6.1467Search in Google Scholar
[33] Hedin, U. and J. Thyberg, Plasma fibronectin promotes modulation of arterial smooth-muscle cells from contractile to synthetic phenotype. Differentiation, 1987. 33(3): p. 239-246.10.1111/j.1432-0436.1987.tb01563.xSearch in Google Scholar PubMed
[34] Sazonova, O.V., et al., Extracellular matrix presentation modulates vascular smooth muscle cell mechanotransduction. Matrix Biology, 2015. 41: p. 36-43.10.1016/j.matbio.2014.11.001Search in Google Scholar PubMed
[35] Isenberg, B.C., et al., Vascular Smooth Muscle Cell Durotaxis Depends on Substrate Stiffness Gradient Strength. Biophysical Journal, 2009. 97(5): p. 1313-1322.10.1016/j.bpj.2009.06.021Search in Google Scholar PubMed PubMed Central
[36] Wong, J.Y., et al., Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir, 2003. 19(5): p. 1908-1913.10.1021/la026403pSearch in Google Scholar
[37] Hartman, C.D., et al., Vascular smooth muscle cell durotaxis depends on extracellular matrix composition. Proceedings of the National Academy of Sciences, 2016. 113(40): p. 11190.10.1073/pnas.1611324113Search in Google Scholar PubMed PubMed Central
[38] Timraz, S.B.H., et al., Stiffness of Extracellular Matrix Components Modulates the Phenotype of Human Smooth Muscle Cells in Vitro and Allows for the Control of Properties of Engineered Tissues. Procedia Engineering, 2015. 110: p. 29-36.10.1016/j.proeng.2015.07.006Search in Google Scholar
[39] Qin, H., et al., Effects of Extracellular Matrix on Phenotype Modulation and MAPK Transduction of Rat Aortic Smooth Muscle Cells in Vitro. Experimental and Molecular Pathology, 2000. 69(2): p. 79-90.10.1006/exmp.2000.2321Search in Google Scholar PubMed
[40] Morrow, D., et al., Cyclic strain inhibits Notch receptor signaling in vascular smooth muscle cells in vitro. Circulation research, 2005. 96(5): p. 567-575.10.1161/01.RES.0000159182.98874.43Search in Google Scholar PubMed
[41] Ritchie, A.C., et al., Dependence of alignment direction on magnitude of strain in esophageal smooth muscle cells. Biotechnology and Bioengineering, 2008. 102(6): p. 1703-1711.10.1002/bit.22190Search in Google Scholar PubMed
[42] Lin, S., et al., Eigenstrain as a mechanical set-point of cells. Biomechanics and Modeling in Mechanobiology, 2018. 17(4): p. 951-959.10.1007/s10237-018-1004-0Search in Google Scholar PubMed
[43] Floren, M. and W. Tan, Three-dimensional, soft neotissue arrays as high throughput platforms for the interrogation of engineered tissue environments. Biomaterials, 2015. 59: p. 39-52.10.1016/j.biomaterials.2015.04.036Search in Google Scholar PubMed PubMed Central
[44] Svenja, H., et al., In vitro elastogenesis: instructing human vascular smooth muscle cells to generate an elastic fiber-containing extracellular matrix scaffold. Biomedical Materials, 2015. 10(3): p. 034102.10.1088/1748-6041/10/3/034102Search in Google Scholar PubMed
[45] Katja, H., et al., Bioink properties before, during and after 3D bioprinting. Biofabrication, 2016. 8(3): p. 032002.10.1088/1758-5090/8/3/032002Search in Google Scholar PubMed
[46] Chen, L.J., S.Y. Wei, and J.J. Chiu, Mechanical regulation of epigenetics in vascular biology and pathobiology. Journal of cellular and molecular medicine, 2013. 17(4): p. 437-448.10.1111/jcmm.12031Search in Google Scholar PubMed PubMed Central
[47] Lin, S., et al., Fluid-Structure Interaction in Abdominal Aortic Aneurysm: Effect of Modeling Techniques. BioMed Research International, 2017. 2017: p. 10.10.1155/2017/7023078Search in Google Scholar PubMed PubMed Central
[48] Schad, J.F., et al., Cyclic strain upregulates VEGF and attenuates proliferation of vascular smooth muscle cells. Vascular Cell, 2011(1): p. 21%V 3.10.1186/2045-824X-3-21Search in Google Scholar PubMed PubMed Central
[49] Birukov, K.G., et al., Stretch affects phenotype and proliferation of vascular smooth muscle cells. Molecular and Cellular Biochemistry, 1995. 144(2): p. 131-139.10.1007/BF00944392Search in Google Scholar PubMed
[50] Leung, D.Y., S. Glagov, and M.B. Mathews, Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science, 1976. 191(4226): p. 475.10.1126/science.128820Search in Google Scholar PubMed
[51] Nakamachi, E., et al., Multi-scale finite element analyses for stress and strain evaluations of braid fibril artificial blood vessel and smooth muscle cell. International Journal for Numerical Methods in Biomedical Engineering, 2014. 30(8): p. 796-813.10.1002/cnm.2630Search in Google Scholar
[52] Colombo, A., et al., Cyclic strain amplitude dictates the growth response of vascular smooth muscle cells in vitro: role in in-stent restenosis and inhibition with a sirolimus drug-eluting stent. Biomechanics and Modeling in Mechanobiology, 2013. 12(4): p. 671-683.10.1007/s10237-012-0433-4Search in Google Scholar
[53] Reusch, P., et al., Mechanical strain increases smoothmuscle and decreases nonmuscle myosin expression in rat vascular smooth muscle cells. Circulation research, 1996. 79(5): p. 1046-1053.10.1161/01.RES.79.5.1046Search in Google Scholar
[54] Qu, M.J., et al., Frequency-Dependent Phenotype Modulation of Vascular Smooth Muscle Cells under Cyclic Mechanical Strain. Journal of Vascular Research, 2007. 44(5): p. 345-353.10.1159/000102278Search in Google Scholar
[55] Sharifpoor, S., et al., Functional characterization of human coronary artery smooth muscle cells under cyclic mechanical strain in a degradable polyurethane scaffold. Biomaterials, 2011. 32(21): p. 4816-4829.10.1016/j.biomaterials.2011.03.034Search in Google Scholar
[56] Sharifpoor, S., et al., A study of vascular smooth muscle cell function under cyclic mechanical loading in a polyurethane scaffold with optimized porosity. Acta Biomaterialia, 2010. 6(11): p. 4218-4228.10.1016/j.actbio.2010.06.018Search in Google Scholar
[57] Stegemann, J.P. and R.M. Nerem, Phenotype Modulation in Vascular Tissue Engineering Using Biochemical and Mechanical Stimulation. Annals of Biomedical Engineering, 2003. 31(4): p. 391-402.10.1114/1.1558031Search in Google Scholar
[58] Tock, J., et al., Induction of SM-α-actin expression by mechanical strain in adult vascular smooth muscle cells is mediated through activation of JNK and p38 MAP kinase. Biochemical and Biophysical Research Communications, 2003. 301(4): p. 1116-1121.10.1016/S0006-291X(03)00087-1Search in Google Scholar
[59] Solan, A., S.L.M. Dahl, and L.E. Niklason, Effects of Mechanical Stretch on Collagen and Cross-Linking in Engineered Blood Vessels. Cell Transplantation, 2009. 18(8): p. 915-921.10.3727/096368909X471161Search in Google Scholar PubMed PubMed Central
[60] Bono, N., et al., Unraveling the role of mechanical stimulation on smooth muscle cells: A comparative study between 2D and 3D models. Biotechnology and Bioengineering, 2016. 113(10): p. 2254-2263.10.1002/bit.25979Search in Google Scholar PubMed
[61] Matsumoto, T. and K. Nagayama, Tensile properties of vascular smooth muscle cells: Bridging vascular and cellular biomechanics. Journal of Biomechanics, 2012. 45(5): p. 745-755.10.1016/j.jbiomech.2011.11.014Search in Google Scholar PubMed
[62] Lin, S., et al., Active stiffening of F-actin network dominated by structural transition of actin filaments into bundles. Composites Part B: Engineering, 2017. 116: p. 377-381.10.1016/j.compositesb.2016.10.079Search in Google Scholar
[63] Barreto-Ortiz, S.F., et al., Fabrication of 3-dimensional multicellular microvascular structures. The FASEB Journal, 2015. 29(8): p. 3302-3314.10.1096/fj.14-263343Search in Google Scholar PubMed PubMed Central
[64] Baker, B.M., et al., Cell-mediated fibre recruitment drives extra-cellular matrix mechanosensing in engineered fibrillar microenvironments. Nature materials, 2015. 14(12): p. 1262.10.1038/nmat4444Search in Google Scholar PubMed PubMed Central
[65] Ding, Y., et al., Biomimetic soft fibrous hydrogels for contractile and pharmacologically responsive smooth muscle. Acta Biomaterialia, 2018. 74: p. 121-130.10.1016/j.actbio.2018.05.015Search in Google Scholar PubMed PubMed Central
[66] Thunes, J.R., et al., A structural finite element model for lamellar unit of aortic media indicates heterogeneous stress field after collagen recruitment. Journal of Biomechanics, 2016. 49(9): p. 1562-1569.10.1016/j.jbiomech.2016.03.034Search in Google Scholar PubMed PubMed Central
[67] Phillippi, J.A., et al., Mechanism of aortic medial matrix remodeling is distinct in patients with bicuspid aortic valve. The Journal of Thoracic and Cardiovascular Surgery, 2014. 147(3): p. 1056-1064.10.1016/j.jtcvs.2013.04.028Search in Google Scholar PubMed PubMed Central
[68] Beenakker, J.-W.M., et al., Mechanical properties of the extracellular matrix of the aorta studied by enzymatic treatments. Biophysical journal, 2012. 102(8): p. 1731-1737.10.1016/j.bpj.2012.03.041Search in Google Scholar PubMed PubMed Central
[69] O’Connell, M.K., et al., The Three-Dimensional Micro- and Nanostructure of the Aortic Medial Lamellar Unit Measured Using 3D Confocal & Electron Microscopy Imaging. Matrix biology : journal of the International Society for Matrix Biology, 2008. 27(3): p. 171-181.10.1016/j.matbio.2007.10.008Search in Google Scholar PubMed PubMed Central
[70] Nagayama, K. and T. Matsumoto, Mechanical Anisotropy of Rat Aortic Smooth Muscle Cells Decreases with Their Contraction (Possible Effect of Actin Filament Orientation). JSME International Journal Series C Mechanical Systems, Machine Elements and Manufacturing, 2004. 47(4): p. 985-991.10.1299/jsmec.47.985Search in Google Scholar
[71] Roeder, B.A., et al., Tensile Mechanical Properties of Three-Dimensional Type I Collagen Extracellular Matrices With Varied Microstructure. Journal of Biomechanical Engineering, 2002. 124(2): p. 214-222.10.1115/1.1449904Search in Google Scholar PubMed
[72] Wagenseil, J.E. and R.P. Mecham, Elastin in Large Artery Stiffness and Hypertension. Journal of Cardiovascular Translational Research, 2012. 5(3): p. 264-273.10.1007/s12265-012-9349-8Search in Google Scholar PubMed PubMed Central
[73] Sugita, S. and T. Matsumoto, Multiphoton microscopy observations of 3D elastin and collagen fiber microstructure changes during pressurization in aortic media. Biomechanics and Modeling in Mechanobiology, 2017. 16(3): p. 763-773.10.1007/s10237-016-0851-9Search in Google Scholar PubMed
[74] Lin, S. and L. Gu, Contribution of Fiber Undulation to Mechanics of Three-Dimensional Collagen-I Gel. Macromolecular Symposia, 2016. 365(1): p. 112-117.10.1002/masy.201650020Search in Google Scholar
[75] Carta, L., et al., Discrete contributions of elastic fiber components to arterial development and mechanical compliance. Arteriosclerosis, thrombosis, and vascular biology, 2009. 29(12): p. 2083.10.1161/ATVBAHA.109.193227Search in Google Scholar PubMed PubMed Central
[76] Szabo, Z., et al., Aortic aneurysmal disease and cutis laxa caused by defects in the elastin gene. Journal of Medical Genetics, 2006. 43(3): p. 255-258.10.1136/jmg.2005.034157Search in Google Scholar PubMed PubMed Central
[77] Zhao, S. and L. Gu, Implementation and Validation of Aortic Remodeling in Hypertensive Rats. Journal of Biomechanical Engineering, 2014. 136(9): p. 091007-091007-8.10.1115/1.4027939Search in Google Scholar PubMed
[78] Faury, G., et al., Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. The Journal of clinical investigation, 2003. 112(9): p. 1419-1428.10.1172/JCI19028Search in Google Scholar PubMed PubMed Central
[79] Hirano, E., et al., Functional rescue of elastin insufficiency in mice by the human elastin gene: implications for mouse models of human disease. Circulation research, 2007. 101(5): p. 523-531.10.1161/CIRCRESAHA.107.153510Search in Google Scholar PubMed
[80] Wagenseil, J.E., et al., Elastin-insufficient mice show normal cardiovascular remodeling in 2K1C hypertension despite higher baseline pressure and unique cardiovascular architecture. American Journal of Physiology-Heart and Circulatory Physiology, 2007. 293(1): p. H574-H582.10.1152/ajpheart.00205.2007Search in Google Scholar PubMed
[81] Wagenseil, J.E., et al., Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. American Journal of Physiology-Heart and Circulatory Physiology, 2005. 289(3): p. H1209-H1217.10.1152/ajpheart.00046.2005Search in Google Scholar
[82] Aronson, D., Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. Journal of Hypertension, 2003. 21(1): p. 3-12.10.1097/00004872-200301000-00002Search in Google Scholar
[83] Konova, E., et al., Age-related changes in the glycation of human aortic elastin. Experimental Gerontology, 2004. 39(2): p. 249-254.10.1016/j.exger.2003.10.003Search in Google Scholar
[84] Lin, S., et al., Towards Tuning the Mechanical Properties of Three-Dimensional Collagen Scaffolds Using a Coupled Fiber-Matrix Model. Materials (Basel, Switzerland), 2015. 8(8): p. 5376-5384.10.3390/ma8085254Search in Google Scholar
[85] Lin, S. and L. Gu, Influence of Crosslink Density and Stiffness on Mechanical Properties of Type I Collagen Gel. Materials (Basel, Switzerland), 2015. 8(2): p. 551-560.10.3390/ma8020551Search in Google Scholar
[86] Bezie, Y., et al., Connection of smooth muscle cells to elastic lamellae in aorta of spontaneously hypertensive rats. Hypertension, 1998. 32(1): p. 166-169.10.1161/01.HYP.32.1.166Search in Google Scholar
[87] Koffi, I., et al., Prevention of arterial structural alterations with verapamil and trandolapril and consequences for mechanical properties in spontaneously hypertensive rats. European journal of pharmacology, 1998. 361(1): p. 51-60.10.1016/S0014-2999(98)00691-8Search in Google Scholar
[88] Sehgel, N.L., et al., Augmented vascular smooth muscle cell stiffness and adhesion when hypertension is superimposed on aging. Hypertension, 2015. 65(2): p. 370.10.1161/HYPERTENSIONAHA.114.04456Search in Google Scholar PubMed PubMed Central
[89] van Gorp, A.W., et al., In spontaneously hypertensive rats alterations in aortic wall properties precede development of hypertension. American Journal of Physiology-Heart and Circulatory Physiology, 2000. 278(4): p. H1241-H1247.10.1152/ajpheart.2000.278.4.H1241Search in Google Scholar PubMed
[90] Cox, R.H., Basis for the altered arterial wall mechanics in the spontaneously hypertensive rat. Hypertension, 1981. 3(4): p. 485-495.10.1161/01.HYP.3.4.485Search in Google Scholar
[91] Mizutani, K., et al., Biomechanical properties and chemical composition of the aorta in genetic hypertensive rats. Journal of hypertension, 1999. 17(4): p. 481-487.10.1097/00004872-199917040-00005Search in Google Scholar PubMed
[92] Hu, J.-J., et al., Time Courses of Growth and Remodeling of Porcine Aortic Media During Hypertension: A Quantitative Immunohistochemical Examination. Journal of Histochemistry and Cytochemistry, 2008. 56(4): p. 359-370.10.1369/jhc.7A7324.2007Search in Google Scholar PubMed PubMed Central
[93] Sehgel, N.L., S.F. Vatner, and G.A. Meininger, “Smooth Muscle Cell Stiffness Syndrome”—Revisiting the Structural Basis of Arterial Stiffness. Frontiers in Physiology, 2015. 6(335).10.3389/fphys.2015.00335Search in Google Scholar PubMed PubMed Central
[94] Qiu, H., et al., Vascular Smooth Muscle Cell Stiffness as a Mechanism for Increased Aortic Stiffness with Aging. Circulation research, 2010. 107(5): p. 615-619.10.1161/CIRCRESAHA.110.221846Search in Google Scholar PubMed PubMed Central
[95] Sehgel, N.L., et al., Increased vascular smooth muscle cell stiffness: a novel mechanism for aortic stiffness in hypertension. American Journal of Physiology-Heart and Circulatory Physiology, 2013. 305(9): p. H1281-H1287.10.1152/ajpheart.00232.2013Search in Google Scholar PubMed PubMed Central
[96] Zhu, Y., et al., Temporal analysis of vascular smooth muscle cell elasticity and adhesion reveals oscillation waveforms that differ with aging. Aging cell, 2012. 11(5): p. 741-750.10.1111/j.1474-9726.2012.00840.xSearch in Google Scholar PubMed PubMed Central
[97] Campbell, G.R. and J.H. Campbell, - Development of the Vessel Wall: Overview in The Vascular Smooth Muscle Cell S.M. Schwartz and R.P. Mecham, Editors. 1995, Academic Press: San Diego. p. 1-15.10.1016/B978-012632310-8/50002-4Search in Google Scholar
[98] Warshaw, D.M., et al., Pharmacology and force development of single freshly isolated bovine carotid artery smooth muscle cells. Circ Res, 1986. 58(3): p. 399-406.10.1161/01.RES.58.3.399Search in Google Scholar PubMed
[99] Matsumoto, T., et al., Smooth muscle cells freshly isolated from rat thoracic aortas are much stiffer than cultured bovine cells: possible effect of phenotype. JSME International Journal Series C Mechanical Systems, Machine Elements and Manufacturing, 2000. 43(4): p. 867-874.10.1299/jsmec.43.867Search in Google Scholar
[100] Smith, P.G., et al., Selected contribution: mechanical strain increases force production and calcium sensitivity in cultured airway smooth muscle cells. Journal of applied physiology, 2000. 89(5): p. 2092-2098.10.1152/jappl.2000.89.5.2092Search in Google Scholar PubMed
[101] Thoumine, O. and A. Ott, Time scale dependent viscoelastic and contractile regimes in fibroblasts probed by microplate manipulation. Journal of cell science, 1997. 110(17): p. 2109-2116.10.1242/jcs.110.17.2109Search in Google Scholar PubMed
[102] Xu, C., et al., Molecular mechanisms of aortic wall remodeling in response to hypertension. Journal of Vascular Surgery, 2001. 33(3): p. 570-578.10.1067/mva.2001.112231Search in Google Scholar PubMed
[103] Matsumoto, T., et al., Effects of hypertension on morphological, contractile and mechanical properties of rat aortic smooth muscle cells. Cellular and Molecular Bioengineering, 2011. 4(3): p. 340-347.10.1007/s12195-011-0182-ySearch in Google Scholar
[104] Tosun, Z. and P.S. McFetridge, Variation in Cardiac Pulse Frequencies Modulates vSMC Phenotype Switching During Vascular Remodeling. Cardiovascular Engineering and Technology, 2015. 6(1): p. 59-70.10.1007/s13239-014-0204-8Search in Google Scholar PubMed
[105] Owens, G.K., M.S. Kumar, and B.R. Wamhoff, Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological reviews, 2004. 84(3): p. 767-801.10.1152/physrev.00041.2003Search in Google Scholar PubMed
[106] Gundiah, N., M. B Ratcliffe, and L. A Pruitt, Determination of strain energy function for arterial elastin: Experiments using histology and mechanical tests. Journal of Biomechanics, 2007. 40(3): p. 586-594.10.1016/j.jbiomech.2006.02.004Search in Google Scholar PubMed
[107] Gundiah, N., M.B. Ratcliffe, and L.A. Pruitt, The biomechanics of arterial elastin. Journal of the Mechanical Behavior of Biomedical Materials, 2009. 2(3): p. 288-296.10.1016/j.jmbbm.2008.10.007Search in Google Scholar PubMed
[108] Lillie, M.A., R.E. Shadwick, and J.M. Gosline, Mechanical anisotropy of inflated elastic tissue from the pig aorta. Journal of Biomechanics, 2010. 43(11): p. 2070-2078.10.1016/j.jbiomech.2010.04.014Search in Google Scholar PubMed
[109] Zou, Y. and Y. Zhang, An Experimental and Theoretical Study on the Anisotropy of Elastin Network. Annals of Biomedical Engineering, 2009. 37(8): p. 1572-1583.10.1007/s10439-009-9724-zSearch in Google Scholar PubMed
[110] Zou, Y. and Y. Zhang, The orthotropic viscoelastic behavior of aortic elastin. Biomechanics and Modeling in Mechanobiology, 2011. 10(5): p. 613-625.10.1007/s10237-010-0260-4Search in Google Scholar PubMed
[111] Weisbecker, H., et al., The role of elastin and collagen in the softening behavior of the human thoracic aortic media. Journal of Biomechanics, 2013. 46(11): p. 1859-1865.10.1016/j.jbiomech.2013.04.025Search in Google Scholar
[112] Martinez, R. and H.-C. Han, THE EFFECT OF COLLAGENASE ON THE CRITICAL BUCKLING PRESSURE OF ARTERIES. Molecular & cellular biomechanics : MCB, 2012. 9(1): p. 55-75.Search in Google Scholar
[113] Dobrin, P.B. and T.R. Canfield, Elastase, collagenase, and the biaxial elastic properties of dog carotid artery. American Journal of Physiology-Heart and Circulatory Physiology, 1984. 247(1): p. H124-H131.10.1152/ajpheart.1984.247.1.H124Search in Google Scholar
[114] Dobrin, P.B., T. Schwarcz, and W. Baker, Mechanisms of arterial and aneurysmal tortuosity. Surgery, 1988. 104(3): p. 568-571.Search in Google Scholar
[115] Kochová, P., et al., The contribution of vascular smooth muscle, elastin and collagen on the passive mechanics of porcine carotid arteries. Physiological Measurement, 2012. 33(8): p. 1335.10.1088/0967-3334/33/8/1335Search in Google Scholar
[116] Kiousis, D.E., et al., A Methodology to Analyze Changes in Lipid Core and Calcification Onto Fibrous Cap Vulnerability: The Human Atherosclerotic Carotid Bifurcation as an Illustratory Example. Journal of Biomechanical Engineering, 2009. 131(12): p. 121002-121002-9.10.1115/1.4000078Search in Google Scholar
[117] Kock, S.A., et al., Mechanical stresses in carotid plaques using MRI-based fluid–structure interaction models. Journal of biomechanics, 2008. 41(8): p. 1651-1658.10.1016/j.jbiomech.2008.03.019Search in Google Scholar
[118] Holzapfel, G.A. and R.W. Ogden, Modelling the layer-specific three-dimensional residual stresses in arteries, with an application to the human aorta. Journal of The Royal Society Interface, 2009.10.1098/rsif.2009.0357Search in Google Scholar
[119] Delfino, A., et al., Residual strain effects on the stress field in a thick wall finite element model of the human carotid bifurcation. Journal of Biomechanics, 1997. 30(8): p. 777-786.10.1016/S0021-9290(97)00025-0Search in Google Scholar
[120] Holzapfel, G.A., T.C. Gasser, and R.W. Ogden, A new constitutive framework for arterial wall mechanics and a comparative study of material models. Journal of elasticity and the physical science of solids, 2000. 61(1-3): p. 1-48.10.1007/0-306-48389-0_1Search in Google Scholar
[121] Kural, M.H., et al., Planar biaxial characterization of diseased human coronary and carotid arteries for computational modeling. Journal of biomechanics, 2012. 45(5): p. 790-798.10.1016/j.jbiomech.2011.11.019Search in Google Scholar PubMed PubMed Central
[122] Taber, L.A. and J.D. Humphrey, Stress-modulated growth, residual stress, and vascular heterogeneity. Journal of biomechanical engineering, 2001. 123(6): p. 528-535.10.1115/1.1412451Search in Google Scholar PubMed
[123] Yamada, H., et al., Age-related distensibility and histology of the ascending aorta in elderly patients with acute aortic dissection. Journal of biomechanics, 2015. 48(12): p. 3267-3273.10.1016/j.jbiomech.2015.06.025Search in Google Scholar
[124] Masson, I., et al., Carotid artery mechanical properties and stresses quantified using in vivo data from normotensive and hypertensive humans. Biomechanics and modeling in mechanobiology, 2011. 10(6): p. 867-882.10.1007/s10237-010-0279-6Search in Google Scholar
[125] Peterson, S. and R. Okamoto, Effect of residual stress and heterogeneity on circumferential stress in the arterial wall. Journal of biomechanical engineering, 2000. 122(4): p. 454-456.10.1115/1.1288210Search in Google Scholar
[126] Sommer, G. and G.A. Holzapfel, 3D constitutive modeling of the biaxial mechanical response of intact and layer-dissected human carotid arteries. Journal of the mechanical behavior of biomedical materials, 2012. 5(1): p. 116-128.10.1016/j.jmbbm.2011.08.013Search in Google Scholar
[127] Von Maltzahn, W.-W., D. Besdo, and W. Wiemer, Elastic properties of arteries: a nonlinear two-layer cylindrical model. Journal of Biomechanics, 1981. 14(6): p. 389-397.10.1016/0021-9290(81)90056-7Search in Google Scholar
[128] Hudson, J., Overall properties of heterogeneous material. Geophysical journal international, 1991. 107(3): p. 505-511.10.1111/j.1365-246X.1991.tb01411.xSearch in Google Scholar
[129] Oren, T., Analytical and numerical analyses of the micromechanics of soft fibrous connective tissues. Biomechanics and modeling in mechanobiology, 2013. 12(1): p. 151-166.10.1007/s10237-012-0388-5Search in Google Scholar PubMed
[130] Lake, S.P., et al., Mechanics of a Fiber Network Within a Non-Fibrillar Matrix: Model and Comparison with Collagen-Agarose Co-gels. Annals of Biomedical Engineering, 2012. 40(10): p. 2111-2121.10.1007/s10439-012-0584-6Search in Google Scholar PubMed PubMed Central
[131] Stein, A.M., et al., The micromechanics of three-dimensional collagen-I gels. Complexity, 2010. 16(4): p. 22-28.10.1002/cplx.20332Search in Google Scholar
[132] Lukeš, V. and E. Rohan, Microstructure based two-scale modelling of soft tissues. Mathematics and Computers in Simulation, 2010. 80(6): p. 1289-1301.10.1016/j.matcom.2009.02.016Search in Google Scholar
[133] Lindeman, J.H.N., et al., Distinct defects in collagen microarchitecture underlie vessel-wall failure in advanced abdominal aneurysms and aneurysms in Marfan syndrome. Proceedings of the National Academy of Sciences of the United States of America, 2010. 107(2): p. 862-865.10.1073/pnas.0910312107Search in Google Scholar PubMed PubMed Central
[134] López-Guimet, J., et al., High-Resolution Morphological Approach to Analyse Elastic Laminae Injuries of the Ascending Aorta in a Murine Model of Marfan Syndrome. Scientific Reports, 2017. 7(1): p. 1505.10.1038/s41598-017-01620-8Search in Google Scholar PubMed PubMed Central
[135] Abraham, P.A., et al., Marfan syndrome. Demonstration of abnormal elastin in aorta. The Journal of Clinical Investigation, 1982. 70(6): p. 1245-1252.10.1172/JCI110723Search in Google Scholar
[136] Tsamis, A., J.T. Krawiec, and D.A. Vorp, Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. Journal of The Royal Society Interface, 2013. 10(83).10.1098/rsif.2012.1004Search in Google Scholar PubMed PubMed Central
© 2019 H. Mozafari et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Research Articles
- Investigation of rare earth upconversion fluorescent nanoparticles in biomedical field
- Carbon Nanotubes Coated Paper as Current Collectors for Secondary Li-ion Batteries
- Insight into the working wavelength of hotspot effects generated by popular nanostructures
- Novel Lead-free biocompatible piezoelectric Hydroxyapatite (HA) – BCZT (Ba0.85Ca0.15Zr0.1Ti0.9O3) nanocrystal composites for bone regeneration
- Effect of defects on the motion of carbon nanotube thermal actuator
- Dynamic mechanical behavior of nano-ZnO reinforced dental composite
- Fabrication of Ag Np-coated wetlace nonwoven fabric based on amino-terminated hyperbranched polymer
- Fractal analysis of pore structures in graphene oxide-carbon nanotube based cementitious pastes under different ultrasonication
- Effect of PVA fiber on durability of cementitious composite containing nano-SiO2
- Cr effects on the electrical contact properties of the Al2O3-Cu/15W composites
- Experimental evaluation of self-expandable metallic tracheobronchial stents
- Experimental study on the existence of nano-scale pores and the evolution of organic matter in organic-rich shale
- Mechanical characterizations of braided composite stents made of helical polyethylene terephthalate strips and NiTi wires
- Mechanical properties of boron nitride sheet with randomly distributed vacancy defects
- Fabrication, mechanical properties and failure mechanism of random and aligned nanofiber membrane with different parameters
- Micro- structure and rheological properties of graphene oxide rubber asphalt
- First-principles calculations of mechanical and thermodynamic properties of tungsten-based alloy
- Adsorption performance of hydrophobic/hydrophilic silica aerogel for low concentration organic pollutant in aqueous solution
- Preparation of spherical aminopropyl-functionalized MCM-41 and its application in removal of Pb(II) ion from aqueous solution
- Electrical conductivity anisotropy of copper matrix composites reinforced with SiC whiskers
- Miniature on-fiber extrinsic Fabry-Perot interferometric vibration sensors based on micro-cantilever beam
- Electric-field assisted growth and mechanical bactericidal performance of ZnO nanoarrays with gradient morphologies
- Flexural behavior and mechanical model of aluminum alloy mortise-and-tenon T-joints for electric vehicle
- Synthesis of nano zirconium oxide and its application in dentistry
- Surface modification of nano-sized carbon black for reinforcement of rubber
- Temperature-dependent negative Poisson’s ratio of monolayer graphene: Prediction from molecular dynamics simulations
- Finite element nonlinear transient modelling of carbon nanotubes reinforced fiber/polymer composite spherical shells with a cutout
- Preparation of low-permittivity K2O–B2O3–SiO2–Al2O3 composites without the addition of glass
- Large amplitude vibration of doubly curved FG-GRC laminated panels in thermal environments
- Enhanced flexural properties of aramid fiber/epoxy composites by graphene oxide
- Correlation between electrochemical performance degradation and catalyst structural parameters on polymer electrolyte membrane fuel cell
- Materials characterization of advanced fillers for composites engineering applications
- Humic acid assisted stabilization of dispersed single-walled carbon nanotubes in cementitious composites
- Test on axial compression performance of nano-silica concrete-filled angle steel reinforced GFRP tubular column
- Multi-scale modeling of the lamellar unit of arterial media
- The multiscale enhancement of mechanical properties of 3D MWK composites via poly(oxypropylene) diamines and GO nanoparticles
- Mechanical properties of circular nano-silica concrete filled stainless steel tube stub columns after being exposed to freezing and thawing
- Arc erosion behavior of TiB2/Cu composites with single-scale and dual-scale TiB2 particles
- Yb3+-containing chitosan hydrogels induce B-16 melanoma cell anoikis via a Fak-dependent pathway
- Template-free synthesis of Se-nanorods-rGO nanocomposite for application in supercapacitors
- Effect of graphene oxide on chloride penetration resistance of recycled concrete
- Bending resistance of PVA fiber reinforced cementitious composites containing nano-SiO2
- Review Articles
- Recent development of Supercapacitor Electrode Based on Carbon Materials
- Mechanical contribution of vascular smooth muscle cells in the tunica media of artery
- Applications of polymer-based nanoparticles in vaccine field
- Toxicity of metallic nanoparticles in the central nervous system
- Parameter control and concentration analysis of graphene colloids prepared by electric spark discharge method
- A critique on multi-jet electrospinning: State of the art and future outlook
- Electrospun cellulose acetate nanofibers and Au@AgNPs for antimicrobial activity - A mini review
- Recent progress in supercapacitors based on the advanced carbon electrodes
- Recent progress in shape memory polymer composites: methods, properties, applications and prospects
- In situ capabilities of Small Angle X-ray Scattering
- Review of nano-phase effects in high strength and conductivity copper alloys
- Progress and challenges in p-type oxide-based thin film transistors
- Advanced materials for flexible solar cell applications
- Phenylboronic acid-decorated polymeric nanomaterials for advanced bio-application
- The effect of nano-SiO2 on concrete properties: a review
- A brief review for fluorinated carbon: synthesis, properties and applications
- A review on the mechanical properties for thin film and block structure characterised by using nanoscratch test
- Cotton fibres functionalized with plasmonic nanoparticles to promote the destruction of harmful molecules: an overview
Articles in the same Issue
- Research Articles
- Investigation of rare earth upconversion fluorescent nanoparticles in biomedical field
- Carbon Nanotubes Coated Paper as Current Collectors for Secondary Li-ion Batteries
- Insight into the working wavelength of hotspot effects generated by popular nanostructures
- Novel Lead-free biocompatible piezoelectric Hydroxyapatite (HA) – BCZT (Ba0.85Ca0.15Zr0.1Ti0.9O3) nanocrystal composites for bone regeneration
- Effect of defects on the motion of carbon nanotube thermal actuator
- Dynamic mechanical behavior of nano-ZnO reinforced dental composite
- Fabrication of Ag Np-coated wetlace nonwoven fabric based on amino-terminated hyperbranched polymer
- Fractal analysis of pore structures in graphene oxide-carbon nanotube based cementitious pastes under different ultrasonication
- Effect of PVA fiber on durability of cementitious composite containing nano-SiO2
- Cr effects on the electrical contact properties of the Al2O3-Cu/15W composites
- Experimental evaluation of self-expandable metallic tracheobronchial stents
- Experimental study on the existence of nano-scale pores and the evolution of organic matter in organic-rich shale
- Mechanical characterizations of braided composite stents made of helical polyethylene terephthalate strips and NiTi wires
- Mechanical properties of boron nitride sheet with randomly distributed vacancy defects
- Fabrication, mechanical properties and failure mechanism of random and aligned nanofiber membrane with different parameters
- Micro- structure and rheological properties of graphene oxide rubber asphalt
- First-principles calculations of mechanical and thermodynamic properties of tungsten-based alloy
- Adsorption performance of hydrophobic/hydrophilic silica aerogel for low concentration organic pollutant in aqueous solution
- Preparation of spherical aminopropyl-functionalized MCM-41 and its application in removal of Pb(II) ion from aqueous solution
- Electrical conductivity anisotropy of copper matrix composites reinforced with SiC whiskers
- Miniature on-fiber extrinsic Fabry-Perot interferometric vibration sensors based on micro-cantilever beam
- Electric-field assisted growth and mechanical bactericidal performance of ZnO nanoarrays with gradient morphologies
- Flexural behavior and mechanical model of aluminum alloy mortise-and-tenon T-joints for electric vehicle
- Synthesis of nano zirconium oxide and its application in dentistry
- Surface modification of nano-sized carbon black for reinforcement of rubber
- Temperature-dependent negative Poisson’s ratio of monolayer graphene: Prediction from molecular dynamics simulations
- Finite element nonlinear transient modelling of carbon nanotubes reinforced fiber/polymer composite spherical shells with a cutout
- Preparation of low-permittivity K2O–B2O3–SiO2–Al2O3 composites without the addition of glass
- Large amplitude vibration of doubly curved FG-GRC laminated panels in thermal environments
- Enhanced flexural properties of aramid fiber/epoxy composites by graphene oxide
- Correlation between electrochemical performance degradation and catalyst structural parameters on polymer electrolyte membrane fuel cell
- Materials characterization of advanced fillers for composites engineering applications
- Humic acid assisted stabilization of dispersed single-walled carbon nanotubes in cementitious composites
- Test on axial compression performance of nano-silica concrete-filled angle steel reinforced GFRP tubular column
- Multi-scale modeling of the lamellar unit of arterial media
- The multiscale enhancement of mechanical properties of 3D MWK composites via poly(oxypropylene) diamines and GO nanoparticles
- Mechanical properties of circular nano-silica concrete filled stainless steel tube stub columns after being exposed to freezing and thawing
- Arc erosion behavior of TiB2/Cu composites with single-scale and dual-scale TiB2 particles
- Yb3+-containing chitosan hydrogels induce B-16 melanoma cell anoikis via a Fak-dependent pathway
- Template-free synthesis of Se-nanorods-rGO nanocomposite for application in supercapacitors
- Effect of graphene oxide on chloride penetration resistance of recycled concrete
- Bending resistance of PVA fiber reinforced cementitious composites containing nano-SiO2
- Review Articles
- Recent development of Supercapacitor Electrode Based on Carbon Materials
- Mechanical contribution of vascular smooth muscle cells in the tunica media of artery
- Applications of polymer-based nanoparticles in vaccine field
- Toxicity of metallic nanoparticles in the central nervous system
- Parameter control and concentration analysis of graphene colloids prepared by electric spark discharge method
- A critique on multi-jet electrospinning: State of the art and future outlook
- Electrospun cellulose acetate nanofibers and Au@AgNPs for antimicrobial activity - A mini review
- Recent progress in supercapacitors based on the advanced carbon electrodes
- Recent progress in shape memory polymer composites: methods, properties, applications and prospects
- In situ capabilities of Small Angle X-ray Scattering
- Review of nano-phase effects in high strength and conductivity copper alloys
- Progress and challenges in p-type oxide-based thin film transistors
- Advanced materials for flexible solar cell applications
- Phenylboronic acid-decorated polymeric nanomaterials for advanced bio-application
- The effect of nano-SiO2 on concrete properties: a review
- A brief review for fluorinated carbon: synthesis, properties and applications
- A review on the mechanical properties for thin film and block structure characterised by using nanoscratch test
- Cotton fibres functionalized with plasmonic nanoparticles to promote the destruction of harmful molecules: an overview

