Abstract
C15H15N1O3, triclinic,
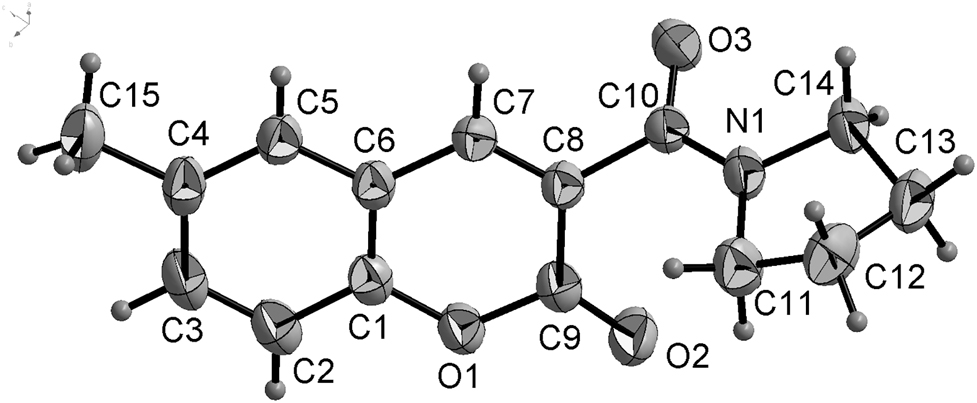
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.16 × 0.16 × 0.12 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 25.5°, 99% |
| N(hkl)measured, N(hkl)unique, R int: | 4943, 2357, 0.019 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 2003 |
| N(param)refined: | 174 |
| Programs: | Bruker [1], SHELX [2, 3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.25591 (18) | 0.49996 (16) | 0.42669 (13) | 0.0406 (3) |
| C2 | 0.2102 (2) | 0.64990 (18) | 0.47322 (15) | 0.0505 (4) |
| H2 | 0.1932 | 0.7405 | 0.4209 | 0.061* |
| C3 | 0.1905 (2) | 0.66205 (19) | 0.59861 (15) | 0.0537 (4) |
| H3 | 0.1613 | 0.7628 | 0.6303 | 0.064* |
| C4 | 0.21277 (19) | 0.5279 (2) | 0.68005 (14) | 0.0496 (4) |
| C5 | 0.26202 (19) | 0.38007 (19) | 0.63054 (13) | 0.0451 (3) |
| H5 | 0.2803 | 0.2897 | 0.6828 | 0.054* |
| C6 | 0.28481 (18) | 0.36396 (16) | 0.50350 (12) | 0.0393 (3) |
| C7 | 0.34343 (19) | 0.21508 (16) | 0.44652 (12) | 0.0407 (3) |
| H7 | 0.3682 | 0.1232 | 0.4956 | 0.049* |
| C8 | 0.36317 (18) | 0.20602 (16) | 0.32403 (12) | 0.0389 (3) |
| C9 | 0.32487 (19) | 0.34800 (16) | 0.24441 (13) | 0.0428 (3) |
| C10 | 0.44890 (19) | 0.05567 (16) | 0.26349 (12) | 0.0396 (3) |
| C11 | 0.1470 (2) | 0.0181 (2) | 0.18220 (17) | 0.0617 (5) |
| H11A | 0.1375 | 0.1069 | 0.1211 | 0.074* |
| H11B | 0.0694 | 0.0450 | 0.2601 | 0.074* |
| C12 | 0.0876 (3) | −0.1380 (2) | 0.14008 (16) | 0.0636 (5) |
| H12A | 0.0443 | −0.2129 | 0.2099 | 0.076* |
| H12B | −0.0138 | −0.1183 | 0.0916 | 0.076* |
| C13 | 0.2681 (3) | −0.2022 (2) | 0.06258 (15) | 0.0593 (4) |
| H13A | 0.2867 | −0.1495 | −0.0198 | 0.071* |
| H13B | 0.2642 | −0.3179 | 0.0565 | 0.071* |
| C14 | 0.4249 (2) | −0.16274 (17) | 0.13075 (13) | 0.0482 (4) |
| H14A | 0.4505 | −0.2514 | 0.1886 | 0.058* |
| H14B | 0.5420 | −0.1393 | 0.0738 | 0.058* |
| C15 | 0.1845 (3) | 0.5447 (3) | 0.81726 (16) | 0.0681 (5) |
| H15A | 0.0526 | 0.5299 | 0.8525 | 0.102* |
| H15B | 0.2196 | 0.6504 | 0.8327 | 0.102* |
| H15C | 0.2631 | 0.4644 | 0.8539 | 0.102* |
| N1 | 0.34679 (16) | −0.01884 (14) | 0.19575 (11) | 0.0456 (3) |
| O1 | 0.27427 (14) | 0.49030 (11) | 0.30093 (9) | 0.0456 (3) |
| O2 | 0.33608 (18) | 0.35333 (13) | 0.13411 (9) | 0.0594 (3) |
| O3 | 0.60951 (15) | 0.00625 (13) | 0.27901 (10) | 0.0578 (3) |
Source of material
To a stirred solution of 6-methyl-2-oxo-2H-chromene-3-carboxylic acid (0.612 g, 3 mmol), N,N-Dimethylformamide (DMF) (0.007 g, 0.1 mmol) was added dropwise SOCl2 (2.975 g, 25 mmol) at 0 ∘C for 30 min, then enhanced to 95 ∘C for 4 h. The reaction was monitored by thin-layer chromatography (TLC). Next, unreacted SOCl2 was removed. Then 15 mL of anhydrous CH2Cl2 was added. Pyrrolidine (0.22 g, 3.1 mmol) and TEA (0.607 g, 6 mmol) were dissolved in 10 mL of anhydrous CH2Cl2. This solution was added dropwise to the reaction mixture at room temperature. The reaction was monitored by TLC. After, CH2Cl2 was removed. At last, the reaction mixture was allowed to recrystallize from methanol to obtain 6-methyl-3-(pyrrolidine-1-carbonyl)-2H-chromen-2-one.
Experimental details
All H atoms were included in calculated positions and refined as riding atoms, with C–H = 0.90–0.97 Å with U iso(H) = 1.5 U eq(C) for methyl H atoms and 1.2 U eq(C) for all other H atoms.
Comment
Coumarin and its derivatives are heterocyclic compounds with a benzopyrone structure [5, 6], which may have excellent antiviral [7], antioxidation [8] antibacterial [9, 10], anticancer [11] and many other physiological, pharmacological activities and optical properties [12]. Among them, coumarin-3-carboxylic acids are vital precursors for the synthesis of active compounds such as dicoumarol, fluorocoumarin carboxylate and coumarin amide [13, 14]. Therefore, it is a research hotspot to explore the efficient synthesis of coumarin-3-carboxylic acids [15], [16], [17].
In the molecule of the title compound (fig.), bond lengths and angles are very similar to those given in the literature for coumarin derivatives [15–18]. In the molecules of the title structure, the coumarin moiety is approximately planar. The dihedral angle between the coumarin moiety and the plane of amides part (C10–N1–O3) is 62.8°. The pyrrolidine part adopts an envelope conformation. The torsion angles of C8–C10–N1–C11, C10–N1–C11–C12, N1–C11–C12–C13, C11–C12–C13–C14 and C12–C13–C14–N1 are −7.0(2)°, −158.5(1)°, −33.7(2)°, 38.6(2)° and −27.7(2)°, respectively.
Funding source: National Key Research Program of China
Award Identifier / Grant number: 2017YFD0301604
Funding source: National Natural Science Foundation of China http://dx.doi.org/10.13039/501100001809
Award Identifier / Grant number: 32160660
Award Identifier / Grant number: 21562022
Funding source: Natural Science Foundation of Jiangxi Province
Award Identifier / Grant number: 20181BAB203015
Acknowledgments
X-ray data were collected at Instrumental Analysis Center Nanchang Hangkong University, Nanchang, 330063, People’s Republic of China.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the “13th Five-Year” National Key Research Program of China (2017YFD0301604), the National Natural Science Foundation of China (32160660, 21562022), the Natural Science Foundation of Jiangxi Province (Grant No. 20181BAB203015).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. Apex2, Saint and Sadabs; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Suche in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Brandenburg, K. Diamond. Visual Crystal Structure Information System (ver. 4.0); Crystal Impact: Bonn, Germany, 2015.Suche in Google Scholar
5. Li, M. Y., Li, F. H., Lan, M. X., Li, Q., Ren, M. R., Wu, G. X. A study on the antioxidant active components of Trigonostemon howii. Acta Agric. Univ. Jiangxiensis 2021, 43, 305–312; https://doi.org/10.13836/j.jjau.2021035.Suche in Google Scholar
6. Tang, W. W., Liu, X. L., Zeng, D. Q. Natural products with bioactivity against Plutella xylostella L. isolated from Solanum torvum swartz. Acta Agric. Univ. Jiangxiensis 2012, 34, 483–486; https://doi.org/10.13836/j.jjau.2012090.Suche in Google Scholar
7. Liu, L., Song, D. W., Liu, G. L., Shan, L. P., Qiu, T. X., Chen, J. Hydroxycoumarin efficiently inhibits spring viraemia of carp virus infection in vitro and in vivo. Zool. Res. 2020, 41, 395–409; https://doi.org/10.24272/j.issn.2095-8137.2020.037.Suche in Google Scholar
8. Nicolaides, D. N., Gautam, D. R., Litinas, K. E., Hadjipavlou-Litina, D. J., Fylaktakidou, K. C. Synthesis and evaluation of the antioxidant and antiinflammatory activities of some benzo[l]khellactone derivatives and analogues. Eur. J. Med. Chem. 2004, 39, 323–332; https://doi.org/10.1016/j.ejmech.2004.01.003.Suche in Google Scholar
9. Wang, Y. H., Jiang, S. C., Chen, Y., Guo, T., Xia, R. J., Tang, X., He, M., Xue, W. Synthesis and antibacterial activity of novel chalcone derivatives bearing a coumarin moiety. Chem. Pap. 2019, 73, 2493–2500; https://doi.org/10.1007/s11696-019-00802-0.Suche in Google Scholar
10. Jardosh, H. H., Patel, M. P. Microwave-assisted CAN-catalyzed solvent-free synthesis of N-allyl quinolone-based pyrano [4, 3-b] chromene and benzopyrano [3, 2-c] chromene derivatives and their antimicrobial activity. Med. Chem. Res. 2013, 22, 905–915; https://doi.org/10.1007/s00044-012-0085-z.Suche in Google Scholar
11. Goud, N. S., Pooladanda, V., Mahammad, G. S., Jakkula, P., Gatreddi, S., Qureshi, I. A., Chandraiah Godugu, R. A., Alvala, M. Synthesis and biological evaluation of morpholines linked coumarin-triazole hybrids as anticancer agents. Chem. Biol. Drug. Des. 2019, 94, 1919–1929; https://doi.org/10.1111/cbdd.13578.Suche in Google Scholar
12. Ren, X., Kondakova, M. E., Giesen, D. J., Rajeswaran, M., Madaras, M., Lenhart, W. C. Coumarin-based, electron-trapping iridium complexes as highly efficient and stable phosphorescent emitters for organic light-emitting diodes. Inorg. Chem. 2010, 49, 1301–1303; https://doi.org/10.1021/ic9022097.Suche in Google Scholar
13. Bakare, S. B. Synthesis and anticancer evaluation of some coumarin and azacoumarin derivatives. Pol. J. Chem. Technol. 2021, 23, 27–34; https://doi.org/10.2478/pjct-2021-0013.Suche in Google Scholar
14. Vega-Granados, K., Medina-O’Donnell, M., Rivas, F., Reyes-Zurita, F. J., Martinez, A., Alvarez de Cienfuegos, L., Lupianez, J. A., Parra, A. Synthesis and biological activity of triterpene coumarin conjugates. J. Nat. Prod. 2021, 84, 1587–1597; https://doi.org/10.1021/acs.jnatprod.1c00128.Suche in Google Scholar
15. Mzozoyana, V., Zamisa, S. J. Crystal structure of 4-(3-fluorophenyl)-7-hydroxy-2H-chromen-2-one, C15H9FO3. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 631–633.10.1515/ncrs-2019-0869Suche in Google Scholar
16. Yu, X., Chen, Y. F., Huang, G. J., Zhang, Y. F., Yang, W. D. Synthesis and crystal structure of 4-(3-acetyl-5-(thiophen-2-yl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)-7-(diethylamino)-2H-chromen-2-one, C21H21N3O4S. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 561–562.10.1515/ncrs-2019-0807Suche in Google Scholar
17. Mzozoyana, V., Zamisa, S. J. Crystal structure of 7-hydroxy-4-phenyl-2H-chromen-2-one, C15H10O3. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 595–597.10.1515/ncrs-2019-0839Suche in Google Scholar
18. Zeng, F. X., Nie, X. L., Shang-guan, X. C., Yin, Z. P., Peng, D. Y. Crystal structure of 3-methyl-2H-chromen-2-one, C10H8O2. Z. Kristallogr. N. Cryst. Struct. 2016, 231, 975–976.10.1515/ncrs-2016-0028Suche in Google Scholar
© 2022 Jie Wang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-(4-imidazol-1-yl-phenyl)-(2-methoxy-benzylidene)-amine monohydrate, C17H17N3O2
- Crystal structure of 6-methyl-3-(pyrrolidine-1-carbonyl)-2H-chromen-2-one, C15H15N1O3
- Crystal structure of 4-methyl-4-nitropentanoic acid, C6H11NO4
- The crystal structure of (E)-3-(furan-2-yl)acrylonitrile, C7H5NO
- Crystal structure of 3-(difluoromethyl)-1-methyl-N-(4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)-1H-pyrazole-4-carboxamide monohydrate, C23H26F2N4O3
- Crystal structure of 2-(4-bromobenzyloxy)-6-chloropyridine, C12H9BrClNO
- Crystal structure of N-(4-bromo-2,6-dichloro-phenyl)pyrazin-2-amine, C10H6BrCl2N3
- Crystal structure of (E)-1-(2–nitrophenyl)-3-phenylprop-2-en-1-one, C15H11NO3
- The crystal structure of (E)-3-chloro-2-(2-(4-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of (E)-amino(2-(thiazol-2-ylmethylene)hydrazineyl)methaniminium nitrate, C10H16N12O6S2
- Crystal structure of 9-methoxy-2,3,4,4a,5,6-hexahydro-1H-pyrido [1′,2′:1,6]pyrazino[2,3-b]quinoxaline, C15H18N4O
- The crystal structure bis(dimethylsulfoxide-κ1O)-dipyridine-κ1 N-bis(m2-(Z)-3-methyl-2-oxido-N-((Z)-oxido(phenyl)methylene)benzohydrazonato-κ5)trinickel(II) - dimethylsulfoxide (1/2), C48H56N6Ni3O10S4
- Crystal structure of bis(bis(triphenylphosphine)iminium) tetradecacarbonyltetratelluridopentaferrate(2-), (PPN)2[Fe5Te4(CO)14]
- Crystal structure of 4-Hydroxy-3-(naphthalen-2-ylthio)pent-3-en-2-one, C15H14O2S
- The crystal structure of [(1,10-phenanthroline-κ2 N,N)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)nickel(II)] monohydrate, C36H26N4O5Ni
- Crystal structure of 3,3′-(pyridine-2,6-diylbis(methylene))bis(1-propyl-1H-imidazol-3-ium) ditetrafluoroborate, C19H27B2F8N5
- The crystal structure of (E)-1-(4-aminophenyl)-3-(p-tolyl)prop-2-en-1-one, C16H15NO
- The crystal structure of poly[(μ2-terephthalato-κ4O,O′: O″,O‴)-(μ4-terephthalato-κ4O:O′:O″:O‴)-{μ4-(1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ4O:O′:O″,O‴)}dicadmium(II)] – water – acetronitrile (1/2/2), C38H36N14O10Cd2
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)cobalt(II)–water–N,N-dimethylformamide(1/2/1), C27H31N3O9Co
- The co-crystal structure of 4-hydroxy-3-methoxybenzoic acid – 4,4′-bipyridine, C8H8O4·C10H8N2
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)cobalt(II)], C32H22Br2CoN4O4
- Crystal structure of (E)-5-propyl-4-((pyridin-2-ylmethylene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione – methanol (1/1), C11H13N5S
- The crystal structure of (Z)-4-bromo-6-(((1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)amino)methylene)cyclohexa-2,4-dien-1-one monohydrate, C11H16BrNO5
- Crystal structure of bis(tetrapropylammonium) nonaselenidotetrastannate(IV), (Pr4N)2[Sn4Se9]
- Crystal structure of 2,6-di-tert-butyl-4-(4-chlorobenzylidene)cyclohexa-2,5-dien-1-one, C21H25ClO
- Crystal structure of (2,2′-((naphthalen-1-ylmethyl)azanediyl)diacetato-κ3 N,O,O′)-(1,10-phenanthroline-κ2 N,N′)-copper(II) trihydrate, CuC27H27N3O7
- The crystal structure of tetrakis(6-phenylpyridine-2-carboxylato-κ2N,O)-bis(1H-pyrazol-3-ylamine-κ2 N:N)dicobalt(II) dihydrate, C27H23N5O5Co
- The crystal structure of bis((E)-2-((tert-butylimino)methyl)-4-chlorophenolato-κ2N,O)zinc(II), C22H26Cl2N2O2Zn
- The crystal structure of poly[diaqua-(μ3-5-nitrobenzene-1,2,3-tricarboxylato-κ3O:O′:O′)-(μ2-4,4′-dipyridylamine-κ2N:N′)copper(II)], C38H30Cu3N8O20
- The crystal structure of (E)-1-ferrocenyl-3-(naphthalen-1-yl)prop-2-en-1-one, C23H18FeO
- The crystal structure of (E)-1-ferrocenyl-3-(4-isopropylphenyl)prop-2-en-1-one, C22H22FeO
- Crystal structure of 6-hydroxy-2,2-dimethyl-4Hbenzo[d][1,3]dioxin-4-one, C10H10O4
- The crystal structure of (2E,4E)-1-ferrocenyl-5-phenylpenta-2,4-dien-1-one, C21H18FeO
- Crystal structure of alaninato-κ2N,O-bis(hydroxylamido-κ2N,O)-oxido-vanadium(V), C3H10N3O5V
- Crystal structure of catena-poly[aqua-bis[μ2-6-(1H-imidazol-1-yl)nicotinato-κ2 N,O]copper(II)], C18H14N6O5Cu
- Crystal structure of diethyl 4,6-diphenyl-1,9-di-p-tolylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,5(2H)-dicarboxylate, C42H42N2O4
- The crystal structure of cobalt cadmium bis(hydrogenphosphate) bis(phosphate(V)) tetrahydrate, H10O20P4Co3.14Cd1.86
- Crystal structure of dimethyl 1,4,6,9-tetraphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,7(2H)-dicarboxylate, C38H34N2O4
- Crystal structure of (Z)-4-(furan-2-yl((4-iodophenyl)amino)methylene)-5-methyl-2(p-tolyl)-2,4-dihydro-3H-pyrazol-3-one, C21H16I N3O2
- Crystal structure of (E)-1-(4-(3,5-dimethoxystyryl)phenyl)-7-ethylheptanedioate, C25H30O6
- Crystal structure of 6-bromo-2-(4-chlorophenyl)chroman-4-one (6-bromo-4′-chloroflavanone), C15H10BrClO2
- The crystal structure of 2-(benzhydryloxy)-3-nitropyridine, C18H14N2O3
- The crystal structure of 1,3(4,1)-dipyridin-1-iuma-2(1,8)-diethynylanthracena-5(1,3)-benzenacyclohexaphane-11,31-diium bis(hexafluoridophosphate), C36H24F12N2P2
- Crystal structure of 3,6-di-tert-butyl-1-iodo-9-methyl-8-(pyren-1-ylethynyl)-9H-carbazole, C39H34IN
- The cocrystal 2-(dimethylammonio)-5-nitrobenzoate – 2-(dimethylamino)-5-nitrobenzoic acid, C9H10N2O4
- Crystal structure of 5-nitroquinazolin-4(3H)-one, C8H5N3O3
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-(4-imidazol-1-yl-phenyl)-(2-methoxy-benzylidene)-amine monohydrate, C17H17N3O2
- Crystal structure of 6-methyl-3-(pyrrolidine-1-carbonyl)-2H-chromen-2-one, C15H15N1O3
- Crystal structure of 4-methyl-4-nitropentanoic acid, C6H11NO4
- The crystal structure of (E)-3-(furan-2-yl)acrylonitrile, C7H5NO
- Crystal structure of 3-(difluoromethyl)-1-methyl-N-(4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)-1H-pyrazole-4-carboxamide monohydrate, C23H26F2N4O3
- Crystal structure of 2-(4-bromobenzyloxy)-6-chloropyridine, C12H9BrClNO
- Crystal structure of N-(4-bromo-2,6-dichloro-phenyl)pyrazin-2-amine, C10H6BrCl2N3
- Crystal structure of (E)-1-(2–nitrophenyl)-3-phenylprop-2-en-1-one, C15H11NO3
- The crystal structure of (E)-3-chloro-2-(2-(4-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of (E)-amino(2-(thiazol-2-ylmethylene)hydrazineyl)methaniminium nitrate, C10H16N12O6S2
- Crystal structure of 9-methoxy-2,3,4,4a,5,6-hexahydro-1H-pyrido [1′,2′:1,6]pyrazino[2,3-b]quinoxaline, C15H18N4O
- The crystal structure bis(dimethylsulfoxide-κ1O)-dipyridine-κ1 N-bis(m2-(Z)-3-methyl-2-oxido-N-((Z)-oxido(phenyl)methylene)benzohydrazonato-κ5)trinickel(II) - dimethylsulfoxide (1/2), C48H56N6Ni3O10S4
- Crystal structure of bis(bis(triphenylphosphine)iminium) tetradecacarbonyltetratelluridopentaferrate(2-), (PPN)2[Fe5Te4(CO)14]
- Crystal structure of 4-Hydroxy-3-(naphthalen-2-ylthio)pent-3-en-2-one, C15H14O2S
- The crystal structure of [(1,10-phenanthroline-κ2 N,N)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)nickel(II)] monohydrate, C36H26N4O5Ni
- Crystal structure of 3,3′-(pyridine-2,6-diylbis(methylene))bis(1-propyl-1H-imidazol-3-ium) ditetrafluoroborate, C19H27B2F8N5
- The crystal structure of (E)-1-(4-aminophenyl)-3-(p-tolyl)prop-2-en-1-one, C16H15NO
- The crystal structure of poly[(μ2-terephthalato-κ4O,O′: O″,O‴)-(μ4-terephthalato-κ4O:O′:O″:O‴)-{μ4-(1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ4O:O′:O″,O‴)}dicadmium(II)] – water – acetronitrile (1/2/2), C38H36N14O10Cd2
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)cobalt(II)–water–N,N-dimethylformamide(1/2/1), C27H31N3O9Co
- The co-crystal structure of 4-hydroxy-3-methoxybenzoic acid – 4,4′-bipyridine, C8H8O4·C10H8N2
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)cobalt(II)], C32H22Br2CoN4O4
- Crystal structure of (E)-5-propyl-4-((pyridin-2-ylmethylene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione – methanol (1/1), C11H13N5S
- The crystal structure of (Z)-4-bromo-6-(((1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)amino)methylene)cyclohexa-2,4-dien-1-one monohydrate, C11H16BrNO5
- Crystal structure of bis(tetrapropylammonium) nonaselenidotetrastannate(IV), (Pr4N)2[Sn4Se9]
- Crystal structure of 2,6-di-tert-butyl-4-(4-chlorobenzylidene)cyclohexa-2,5-dien-1-one, C21H25ClO
- Crystal structure of (2,2′-((naphthalen-1-ylmethyl)azanediyl)diacetato-κ3 N,O,O′)-(1,10-phenanthroline-κ2 N,N′)-copper(II) trihydrate, CuC27H27N3O7
- The crystal structure of tetrakis(6-phenylpyridine-2-carboxylato-κ2N,O)-bis(1H-pyrazol-3-ylamine-κ2 N:N)dicobalt(II) dihydrate, C27H23N5O5Co
- The crystal structure of bis((E)-2-((tert-butylimino)methyl)-4-chlorophenolato-κ2N,O)zinc(II), C22H26Cl2N2O2Zn
- The crystal structure of poly[diaqua-(μ3-5-nitrobenzene-1,2,3-tricarboxylato-κ3O:O′:O′)-(μ2-4,4′-dipyridylamine-κ2N:N′)copper(II)], C38H30Cu3N8O20
- The crystal structure of (E)-1-ferrocenyl-3-(naphthalen-1-yl)prop-2-en-1-one, C23H18FeO
- The crystal structure of (E)-1-ferrocenyl-3-(4-isopropylphenyl)prop-2-en-1-one, C22H22FeO
- Crystal structure of 6-hydroxy-2,2-dimethyl-4Hbenzo[d][1,3]dioxin-4-one, C10H10O4
- The crystal structure of (2E,4E)-1-ferrocenyl-5-phenylpenta-2,4-dien-1-one, C21H18FeO
- Crystal structure of alaninato-κ2N,O-bis(hydroxylamido-κ2N,O)-oxido-vanadium(V), C3H10N3O5V
- Crystal structure of catena-poly[aqua-bis[μ2-6-(1H-imidazol-1-yl)nicotinato-κ2 N,O]copper(II)], C18H14N6O5Cu
- Crystal structure of diethyl 4,6-diphenyl-1,9-di-p-tolylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,5(2H)-dicarboxylate, C42H42N2O4
- The crystal structure of cobalt cadmium bis(hydrogenphosphate) bis(phosphate(V)) tetrahydrate, H10O20P4Co3.14Cd1.86
- Crystal structure of dimethyl 1,4,6,9-tetraphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,7(2H)-dicarboxylate, C38H34N2O4
- Crystal structure of (Z)-4-(furan-2-yl((4-iodophenyl)amino)methylene)-5-methyl-2(p-tolyl)-2,4-dihydro-3H-pyrazol-3-one, C21H16I N3O2
- Crystal structure of (E)-1-(4-(3,5-dimethoxystyryl)phenyl)-7-ethylheptanedioate, C25H30O6
- Crystal structure of 6-bromo-2-(4-chlorophenyl)chroman-4-one (6-bromo-4′-chloroflavanone), C15H10BrClO2
- The crystal structure of 2-(benzhydryloxy)-3-nitropyridine, C18H14N2O3
- The crystal structure of 1,3(4,1)-dipyridin-1-iuma-2(1,8)-diethynylanthracena-5(1,3)-benzenacyclohexaphane-11,31-diium bis(hexafluoridophosphate), C36H24F12N2P2
- Crystal structure of 3,6-di-tert-butyl-1-iodo-9-methyl-8-(pyren-1-ylethynyl)-9H-carbazole, C39H34IN
- The cocrystal 2-(dimethylammonio)-5-nitrobenzoate – 2-(dimethylamino)-5-nitrobenzoic acid, C9H10N2O4
- Crystal structure of 5-nitroquinazolin-4(3H)-one, C8H5N3O3

