Abstract
C15H14O2S, monoclinic, P21/c (no. 14), a = 13.5881(11) Å, b = 7.3129(9) Å, c = 13.3284(11) Å, β = 96.125(7)°, V = 1316.9(2) Å3, Z = 4, R gt (F) = 0.0570, wRref(F2) = 0.1402, T = 293 K.
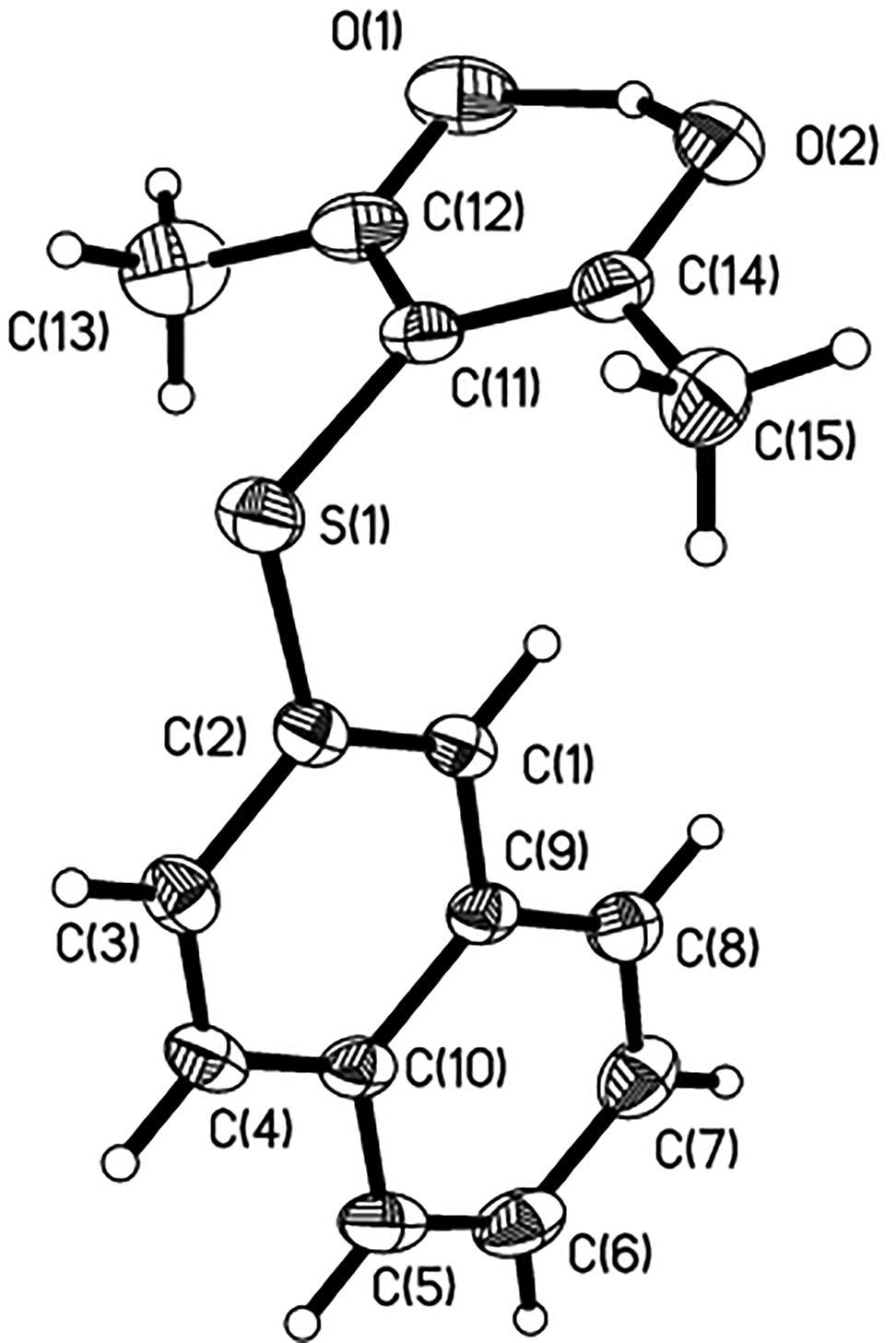
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Brown block |
| Size: | 0.20 × 0.16 × 0.15 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.24 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 28.3°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 5547, 2725, 0.022 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2014 |
| N(param)refined: | 169 |
| Programs: | CrysAlisPRO [1], Olex2 [2], SHELX [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| S1 | 0.14502 (5) | 0.74734 (11) | 0.61439 (5) | 0.0502 (2) |
| O1 | 0.12386 (15) | 0.2882 (3) | 0.45978 (18) | 0.0690 (6) |
| H1 | 0.102 (3) | 0.400 (8) | 0.376 (4) | 0.17 (2)* |
| O2 | 0.07901 (15) | 0.5401 (4) | 0.34290 (13) | 0.0642 (6) |
| C1 | 0.33959 (17) | 0.7432 (4) | 0.56455 (16) | 0.0389 (6) |
| H1A | 0.3176 | 0.6754 | 0.5075 | 0.047* |
| C2 | 0.27362 (17) | 0.7959 (4) | 0.62994 (16) | 0.0389 (6) |
| C3 | 0.30661 (18) | 0.8987 (4) | 0.71634 (16) | 0.0448 (7) |
| H3 | 0.2616 | 0.9345 | 0.7605 | 0.054* |
| C4 | 0.40301 (19) | 0.9461 (4) | 0.73587 (17) | 0.0474 (7) |
| H4 | 0.4232 | 1.0146 | 0.7931 | 0.057* |
| C5 | 0.57510 (19) | 0.9388 (4) | 0.6891 (2) | 0.0548 (8) |
| H5 | 0.5976 | 1.0050 | 0.7466 | 0.066* |
| C6 | 0.6399 (2) | 0.8874 (5) | 0.6241 (2) | 0.0631 (9) |
| H6 | 0.7065 | 0.9176 | 0.6376 | 0.076* |
| C7 | 0.6073 (2) | 0.7887 (5) | 0.5366 (2) | 0.0622 (9) |
| H7 | 0.6524 | 0.7553 | 0.4920 | 0.075* |
| C8 | 0.51042 (19) | 0.7414 (4) | 0.51634 (19) | 0.0491 (7) |
| H8 | 0.4897 | 0.6760 | 0.4581 | 0.059* |
| C9 | 0.44101 (17) | 0.7910 (4) | 0.58321 (16) | 0.0387 (6) |
| C10 | 0.47349 (17) | 0.8931 (4) | 0.67077 (16) | 0.0410 (6) |
| C11 | 0.12970 (16) | 0.5916 (4) | 0.51345 (17) | 0.0415 (6) |
| C12 | 0.14117 (17) | 0.4027 (5) | 0.5316 (2) | 0.0524 (7) |
| C13 | 0.1742 (2) | 0.3276 (6) | 0.6336 (3) | 0.0793 (10) |
| H13A | 0.1764 | 0.1965 | 0.6302 | 0.119* |
| H13B | 0.1284 | 0.3640 | 0.6800 | 0.119* |
| H13C | 0.2389 | 0.3738 | 0.6562 | 0.119* |
| C14 | 0.09843 (17) | 0.6553 (4) | 0.41561 (18) | 0.0478 (7) |
| C15 | 0.0846 (2) | 0.8509 (5) | 0.3894 (2) | 0.0712 (9) |
| H15A | 0.0690 | 0.8630 | 0.3178 | 0.107* |
| H15B | 0.1444 | 0.9166 | 0.4104 | 0.107* |
| H15C | 0.0314 | 0.8998 | 0.4231 | 0.107* |
Source of material
To a solution of acetylacetone (0.200 g, 2 mmol) and 2-naphthalenethiol (0.160 g, 1 mmol) in DMSO (1 mL) was added Cs2CO3 (0.326 mg, 1 mmol). The mixture was stirred at 45 °C under oxygen atmosphere for 10 h. The mixture was then added to water (5 mL). The resulting mixture was extracted with ethylether (10 mL) for three times. The combined organic layers were washed with water and brine, dried over anhydrous Na2SO4 and concentrated under reduced pressure. After removal of the solvent, the residue was then purified by flash column chromatography on silica gel with petroleum ether/ethyl acetate (45:1) to give the desired (245 mg, 95%) as a white solid. Single crystals suitable for X-ray diffraction were obtained by crystallization of the title compound from ethyl acetate. Melting point: 88–89 °C. 1 H NMR (400 MHz, CDCl3, 298 K) δ 17.47 (s, 1H), 7.84–7.78 (m, 2H), 7.76(d, J = 8.6 Hz, 1H), 7.53–7.43 (m, 3H), 7.32 (d, J = 8.6 Hz, 1H), 2.43 (s, 6H). 13 C NMR (101 MHz, CDCl3) δ 198.4, 135.3, 133.9, 131.4, 128.9, 127.8, 126.8, 125.4, 123.6, 121.8, 101.4, 24.4.
Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms.
Comment
β-dicarbonyl thioether belong to an important class of compounds, which are of great demand in organic synthesis as well as in the pharmaceutical industry [4, 5]. From a synthetic perspective, these compounds have frequently used as valuable starting materials or reactive intermediates in a variety of organic transformations [6], [7], [8]. For example, they have been used as precursors for a large number of target molecules [9]. Besides, β-dicarbonyl thioether have applications in the pharmaceutical and agrochemical industries. Examples of β-dicarbonyl thioethers as pharmaceutical building blocks or intermediates include anti-inflammatory [10], anti-HSV-1 [11], and antimicrobial properties [12]. In this paper we report the synthesis and crystal structure of a β-dicarbonyl thioether.
The assymmetric unit contains one molecule of the title compound, which is constructed by the acetylacetone and the 2-naphthalenethiol (see the figure). The acetylacetone fragment together with the sulfur almost in a strict plane, whereby the largest deviation for the S1 atom from the acetylacetone plane is 0.137 Å. The dihedral angle between the acetylacetone group and 2-naphthalenethiol group is found to be 88.26°. The C(2)–S(1)–C(11)–C(14) and C(2)–S(1)–C(11)–C(12) torsion angles are −97.72(19)° and 87.20(2)°. The C(2)–S(1)–C(11) bond angle is 104.71(11)°. The thioether bond distances are 1.758(3) Å for C(11)–S(1) and 1.773(2) Å for C(2)–S(1), respectively, which are typical Caryl–S bond distances. The lengths of C(2)–C(1), C(2)–C(3) bond in benzene ring are 1.371(3) and 1.408(3) Å respectively. Within the acetylacetone unit, the dimensions and planarity are consistent with their adoption of a delocalized enol form. The bond lengths of C(11)–C(14), C(11)–C(12) are 1.407(3) Å and 1.409(4) Å respectively. In the crystal structure, the two oxygen atoms are linked by an intramolecular O(1)–H(1)⃛O(2) hydrogen bond. The distance of O(1)⃛H(1) is 1.400(5) Å and the distance of O(2)⃛H(1) is 1.140(6) Å. The structure of the molecule is similar to the stereo-configuration of the compound reported in the references [13], [14], [15]. The bond lengths and angles are all in the expected ranges. The complete set of X-ray diffraction data for the title compound was deposited to the Cambridge Crystallographic Data Centre (CCDC entry no. 2123340).
Funding source: Programs for Science and Technology Development of Henan Province http://dx.doi.org/10.13039/501100011447
Award Identifier / Grant number: 212102210650
Funding source: Key Research Project for Colleges and Universities of Henan Province
Award Identifier / Grant number: 21B530002
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the Programs for Science and Technology Development of Henan Province, China (No. 212102210650) and the Key Research Project for Colleges and Universities of Henan Province, China (No. 21B530002).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Oxford Diffraction. CrysAlisPRO (version 1.171.33.42); Oxford Diffraction Ltd.: Oxford, UK, 2009.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
4. Margalef, J., Samec, J. S. M. Assessing methodologies to synthesize alpha-sulfenylated carbonyl compounds by green chemistry metrics. ChemSusChem 2021, 14, 808–823; https://doi.org/10.1002/cssc.202002409.Search in Google Scholar
5. Chen, Q., Wang, X. F., Wen, C. X., Huang, Y. L., Yan, X. X., Zeng, J. K. Cs2CO3-promoted cross-dehydrogenative coupling of thiophenols with active methylene compounds. RSC Adv. 2017, 7, 39758–39761; https://doi.org/10.1039/c7ra06904a.Search in Google Scholar
6. Parvatkar, P. T., Manetsch, R., Banik, B. K. Metal-free cross-dehydrogenative coupling (CDC): molecular iodine as a versatile catalyst/reagent for CDC reactions. Chem. Asian J. 2019, 14, 6–30; https://doi.org/10.1002/asia.201801237.Search in Google Scholar
7. Varun, B. V., Gadde, K., Prabhu, K. R. Sulfenylation of beta-diketones using C–H functionalization strategy. Org. Lett. 2015, 17, 2944–2947; https://doi.org/10.1021/acs.orglett.5b01221.Search in Google Scholar
8. Trost, B. M. α-Sulfenylated carbonyl compounds in organic synthesis. Chem. Rev. 1978, 78, 363–382; https://doi.org/10.1021/cr60314a002.Search in Google Scholar
9. Cao, H., Yuan, J. W., Liu, C., Hu, X. Q., Lei, A. W. Iodine-catalyzed C–H/S–H oxidative coupling: from 1,3-diketones and thiophenols to β-dicarbonyl thioethers. RSC Adv. 2015, 5, 41493–41496; https://doi.org/10.1039/c5ra04906g.Search in Google Scholar
10. El-Gazzar, A. B. A., Youssef, M. M., Youssef, A. M. S., Abu-Hashem, A. A., Badria, F. A. Design and synthesis of azolopyrimidoquinolines, pyrimidoquinazolines as anti-oxidant, anti-inflammatory and analgesic activities. Eur. J. Med. Chem. 2009, 44, 609–624; https://doi.org/10.1016/j.ejmech.2008.03.022.Search in Google Scholar
11. Mohamed, S. F., Flefel, E. M., Amr, A. E. G. E., Abd El-Shafy, D. N. Anti-HSV-1 activity and mechanism of action of some new synthesized substituted pyrimidine, thiopyrimidine and thiazolopyrimidine derivatives. Eur. J. Med. Chem. 2010, 45, 1494–1501; https://doi.org/10.1016/j.ejmech.2009.12.057.Search in Google Scholar
12. Abu-Hashem, A. A. Synthesis of new furothiazolo pyrimido quinazolinones from visnagenone or khellinone and antimicrobial activity. Molecules 2018, 23, 2793–2813; https://doi.org/10.3390/molecules23112793.Search in Google Scholar
13. Rashid, M. A., Rasool, N., Adeel, M., Reinke, H., Fischer, C., Langer, P. Synthesis of functionalized diaryl sulfides based on regioselective one-pot cyclizations of 1,3-bis(trimethylsilyloxy)-1,3-butadienes. Tetrahedron 2008, 64, 3782–3793; https://doi.org/10.1016/j.tet.2008.02.010.Search in Google Scholar
14. Olivier, J. H., Haefele, A., Retailleau, P., Ziessel, R. Borondipyrromethene dyes with pentane-2,4-dione anchors. Org. Lett. 2010, 12, 408–411; https://doi.org/10.1021/ol902386u.Search in Google Scholar
15. Dong, L., Guo, Y.-F., Ma, J.-Y., Wang, J.-L., Feng, S.-X., Huo, H.-K. Crystal structure of (E)-3-((4-(tert-butyl)phenyl)thio)-4-hydroxypent-3-en-2-one, C15H20O2S. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 37–39; https://doi.org/10.1515/ncrs-2021-0365.Search in Google Scholar
© 2022 Ya-Xin Wen et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-(4-imidazol-1-yl-phenyl)-(2-methoxy-benzylidene)-amine monohydrate, C17H17N3O2
- Crystal structure of 6-methyl-3-(pyrrolidine-1-carbonyl)-2H-chromen-2-one, C15H15N1O3
- Crystal structure of 4-methyl-4-nitropentanoic acid, C6H11NO4
- The crystal structure of (E)-3-(furan-2-yl)acrylonitrile, C7H5NO
- Crystal structure of 3-(difluoromethyl)-1-methyl-N-(4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)-1H-pyrazole-4-carboxamide monohydrate, C23H26F2N4O3
- Crystal structure of 2-(4-bromobenzyloxy)-6-chloropyridine, C12H9BrClNO
- Crystal structure of N-(4-bromo-2,6-dichloro-phenyl)pyrazin-2-amine, C10H6BrCl2N3
- Crystal structure of (E)-1-(2–nitrophenyl)-3-phenylprop-2-en-1-one, C15H11NO3
- The crystal structure of (E)-3-chloro-2-(2-(4-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of (E)-amino(2-(thiazol-2-ylmethylene)hydrazineyl)methaniminium nitrate, C10H16N12O6S2
- Crystal structure of 9-methoxy-2,3,4,4a,5,6-hexahydro-1H-pyrido [1′,2′:1,6]pyrazino[2,3-b]quinoxaline, C15H18N4O
- The crystal structure bis(dimethylsulfoxide-κ1O)-dipyridine-κ1 N-bis(m2-(Z)-3-methyl-2-oxido-N-((Z)-oxido(phenyl)methylene)benzohydrazonato-κ5)trinickel(II) - dimethylsulfoxide (1/2), C48H56N6Ni3O10S4
- Crystal structure of bis(bis(triphenylphosphine)iminium) tetradecacarbonyltetratelluridopentaferrate(2-), (PPN)2[Fe5Te4(CO)14]
- Crystal structure of 4-Hydroxy-3-(naphthalen-2-ylthio)pent-3-en-2-one, C15H14O2S
- The crystal structure of [(1,10-phenanthroline-κ2 N,N)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)nickel(II)] monohydrate, C36H26N4O5Ni
- Crystal structure of 3,3′-(pyridine-2,6-diylbis(methylene))bis(1-propyl-1H-imidazol-3-ium) ditetrafluoroborate, C19H27B2F8N5
- The crystal structure of (E)-1-(4-aminophenyl)-3-(p-tolyl)prop-2-en-1-one, C16H15NO
- The crystal structure of poly[(μ2-terephthalato-κ4O,O′: O″,O‴)-(μ4-terephthalato-κ4O:O′:O″:O‴)-{μ4-(1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ4O:O′:O″,O‴)}dicadmium(II)] – water – acetronitrile (1/2/2), C38H36N14O10Cd2
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)cobalt(II)–water–N,N-dimethylformamide(1/2/1), C27H31N3O9Co
- The co-crystal structure of 4-hydroxy-3-methoxybenzoic acid – 4,4′-bipyridine, C8H8O4·C10H8N2
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)cobalt(II)], C32H22Br2CoN4O4
- Crystal structure of (E)-5-propyl-4-((pyridin-2-ylmethylene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione – methanol (1/1), C11H13N5S
- The crystal structure of (Z)-4-bromo-6-(((1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)amino)methylene)cyclohexa-2,4-dien-1-one monohydrate, C11H16BrNO5
- Crystal structure of bis(tetrapropylammonium) nonaselenidotetrastannate(IV), (Pr4N)2[Sn4Se9]
- Crystal structure of 2,6-di-tert-butyl-4-(4-chlorobenzylidene)cyclohexa-2,5-dien-1-one, C21H25ClO
- Crystal structure of (2,2′-((naphthalen-1-ylmethyl)azanediyl)diacetato-κ3 N,O,O′)-(1,10-phenanthroline-κ2 N,N′)-copper(II) trihydrate, CuC27H27N3O7
- The crystal structure of tetrakis(6-phenylpyridine-2-carboxylato-κ2N,O)-bis(1H-pyrazol-3-ylamine-κ2 N:N)dicobalt(II) dihydrate, C27H23N5O5Co
- The crystal structure of bis((E)-2-((tert-butylimino)methyl)-4-chlorophenolato-κ2N,O)zinc(II), C22H26Cl2N2O2Zn
- The crystal structure of poly[diaqua-(μ3-5-nitrobenzene-1,2,3-tricarboxylato-κ3O:O′:O′)-(μ2-4,4′-dipyridylamine-κ2N:N′)copper(II)], C38H30Cu3N8O20
- The crystal structure of (E)-1-ferrocenyl-3-(naphthalen-1-yl)prop-2-en-1-one, C23H18FeO
- The crystal structure of (E)-1-ferrocenyl-3-(4-isopropylphenyl)prop-2-en-1-one, C22H22FeO
- Crystal structure of 6-hydroxy-2,2-dimethyl-4Hbenzo[d][1,3]dioxin-4-one, C10H10O4
- The crystal structure of (2E,4E)-1-ferrocenyl-5-phenylpenta-2,4-dien-1-one, C21H18FeO
- Crystal structure of alaninato-κ2N,O-bis(hydroxylamido-κ2N,O)-oxido-vanadium(V), C3H10N3O5V
- Crystal structure of catena-poly[aqua-bis[μ2-6-(1H-imidazol-1-yl)nicotinato-κ2 N,O]copper(II)], C18H14N6O5Cu
- Crystal structure of diethyl 4,6-diphenyl-1,9-di-p-tolylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,5(2H)-dicarboxylate, C42H42N2O4
- The crystal structure of cobalt cadmium bis(hydrogenphosphate) bis(phosphate(V)) tetrahydrate, H10O20P4Co3.14Cd1.86
- Crystal structure of dimethyl 1,4,6,9-tetraphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,7(2H)-dicarboxylate, C38H34N2O4
- Crystal structure of (Z)-4-(furan-2-yl((4-iodophenyl)amino)methylene)-5-methyl-2(p-tolyl)-2,4-dihydro-3H-pyrazol-3-one, C21H16I N3O2
- Crystal structure of (E)-1-(4-(3,5-dimethoxystyryl)phenyl)-7-ethylheptanedioate, C25H30O6
- Crystal structure of 6-bromo-2-(4-chlorophenyl)chroman-4-one (6-bromo-4′-chloroflavanone), C15H10BrClO2
- The crystal structure of 2-(benzhydryloxy)-3-nitropyridine, C18H14N2O3
- The crystal structure of 1,3(4,1)-dipyridin-1-iuma-2(1,8)-diethynylanthracena-5(1,3)-benzenacyclohexaphane-11,31-diium bis(hexafluoridophosphate), C36H24F12N2P2
- Crystal structure of 3,6-di-tert-butyl-1-iodo-9-methyl-8-(pyren-1-ylethynyl)-9H-carbazole, C39H34IN
- The cocrystal 2-(dimethylammonio)-5-nitrobenzoate – 2-(dimethylamino)-5-nitrobenzoic acid, C9H10N2O4
- Crystal structure of 5-nitroquinazolin-4(3H)-one, C8H5N3O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-(4-imidazol-1-yl-phenyl)-(2-methoxy-benzylidene)-amine monohydrate, C17H17N3O2
- Crystal structure of 6-methyl-3-(pyrrolidine-1-carbonyl)-2H-chromen-2-one, C15H15N1O3
- Crystal structure of 4-methyl-4-nitropentanoic acid, C6H11NO4
- The crystal structure of (E)-3-(furan-2-yl)acrylonitrile, C7H5NO
- Crystal structure of 3-(difluoromethyl)-1-methyl-N-(4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)-1H-pyrazole-4-carboxamide monohydrate, C23H26F2N4O3
- Crystal structure of 2-(4-bromobenzyloxy)-6-chloropyridine, C12H9BrClNO
- Crystal structure of N-(4-bromo-2,6-dichloro-phenyl)pyrazin-2-amine, C10H6BrCl2N3
- Crystal structure of (E)-1-(2–nitrophenyl)-3-phenylprop-2-en-1-one, C15H11NO3
- The crystal structure of (E)-3-chloro-2-(2-(4-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of (E)-amino(2-(thiazol-2-ylmethylene)hydrazineyl)methaniminium nitrate, C10H16N12O6S2
- Crystal structure of 9-methoxy-2,3,4,4a,5,6-hexahydro-1H-pyrido [1′,2′:1,6]pyrazino[2,3-b]quinoxaline, C15H18N4O
- The crystal structure bis(dimethylsulfoxide-κ1O)-dipyridine-κ1 N-bis(m2-(Z)-3-methyl-2-oxido-N-((Z)-oxido(phenyl)methylene)benzohydrazonato-κ5)trinickel(II) - dimethylsulfoxide (1/2), C48H56N6Ni3O10S4
- Crystal structure of bis(bis(triphenylphosphine)iminium) tetradecacarbonyltetratelluridopentaferrate(2-), (PPN)2[Fe5Te4(CO)14]
- Crystal structure of 4-Hydroxy-3-(naphthalen-2-ylthio)pent-3-en-2-one, C15H14O2S
- The crystal structure of [(1,10-phenanthroline-κ2 N,N)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)nickel(II)] monohydrate, C36H26N4O5Ni
- Crystal structure of 3,3′-(pyridine-2,6-diylbis(methylene))bis(1-propyl-1H-imidazol-3-ium) ditetrafluoroborate, C19H27B2F8N5
- The crystal structure of (E)-1-(4-aminophenyl)-3-(p-tolyl)prop-2-en-1-one, C16H15NO
- The crystal structure of poly[(μ2-terephthalato-κ4O,O′: O″,O‴)-(μ4-terephthalato-κ4O:O′:O″:O‴)-{μ4-(1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ4O:O′:O″,O‴)}dicadmium(II)] – water – acetronitrile (1/2/2), C38H36N14O10Cd2
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)cobalt(II)–water–N,N-dimethylformamide(1/2/1), C27H31N3O9Co
- The co-crystal structure of 4-hydroxy-3-methoxybenzoic acid – 4,4′-bipyridine, C8H8O4·C10H8N2
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)cobalt(II)], C32H22Br2CoN4O4
- Crystal structure of (E)-5-propyl-4-((pyridin-2-ylmethylene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione – methanol (1/1), C11H13N5S
- The crystal structure of (Z)-4-bromo-6-(((1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)amino)methylene)cyclohexa-2,4-dien-1-one monohydrate, C11H16BrNO5
- Crystal structure of bis(tetrapropylammonium) nonaselenidotetrastannate(IV), (Pr4N)2[Sn4Se9]
- Crystal structure of 2,6-di-tert-butyl-4-(4-chlorobenzylidene)cyclohexa-2,5-dien-1-one, C21H25ClO
- Crystal structure of (2,2′-((naphthalen-1-ylmethyl)azanediyl)diacetato-κ3 N,O,O′)-(1,10-phenanthroline-κ2 N,N′)-copper(II) trihydrate, CuC27H27N3O7
- The crystal structure of tetrakis(6-phenylpyridine-2-carboxylato-κ2N,O)-bis(1H-pyrazol-3-ylamine-κ2 N:N)dicobalt(II) dihydrate, C27H23N5O5Co
- The crystal structure of bis((E)-2-((tert-butylimino)methyl)-4-chlorophenolato-κ2N,O)zinc(II), C22H26Cl2N2O2Zn
- The crystal structure of poly[diaqua-(μ3-5-nitrobenzene-1,2,3-tricarboxylato-κ3O:O′:O′)-(μ2-4,4′-dipyridylamine-κ2N:N′)copper(II)], C38H30Cu3N8O20
- The crystal structure of (E)-1-ferrocenyl-3-(naphthalen-1-yl)prop-2-en-1-one, C23H18FeO
- The crystal structure of (E)-1-ferrocenyl-3-(4-isopropylphenyl)prop-2-en-1-one, C22H22FeO
- Crystal structure of 6-hydroxy-2,2-dimethyl-4Hbenzo[d][1,3]dioxin-4-one, C10H10O4
- The crystal structure of (2E,4E)-1-ferrocenyl-5-phenylpenta-2,4-dien-1-one, C21H18FeO
- Crystal structure of alaninato-κ2N,O-bis(hydroxylamido-κ2N,O)-oxido-vanadium(V), C3H10N3O5V
- Crystal structure of catena-poly[aqua-bis[μ2-6-(1H-imidazol-1-yl)nicotinato-κ2 N,O]copper(II)], C18H14N6O5Cu
- Crystal structure of diethyl 4,6-diphenyl-1,9-di-p-tolylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,5(2H)-dicarboxylate, C42H42N2O4
- The crystal structure of cobalt cadmium bis(hydrogenphosphate) bis(phosphate(V)) tetrahydrate, H10O20P4Co3.14Cd1.86
- Crystal structure of dimethyl 1,4,6,9-tetraphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,7(2H)-dicarboxylate, C38H34N2O4
- Crystal structure of (Z)-4-(furan-2-yl((4-iodophenyl)amino)methylene)-5-methyl-2(p-tolyl)-2,4-dihydro-3H-pyrazol-3-one, C21H16I N3O2
- Crystal structure of (E)-1-(4-(3,5-dimethoxystyryl)phenyl)-7-ethylheptanedioate, C25H30O6
- Crystal structure of 6-bromo-2-(4-chlorophenyl)chroman-4-one (6-bromo-4′-chloroflavanone), C15H10BrClO2
- The crystal structure of 2-(benzhydryloxy)-3-nitropyridine, C18H14N2O3
- The crystal structure of 1,3(4,1)-dipyridin-1-iuma-2(1,8)-diethynylanthracena-5(1,3)-benzenacyclohexaphane-11,31-diium bis(hexafluoridophosphate), C36H24F12N2P2
- Crystal structure of 3,6-di-tert-butyl-1-iodo-9-methyl-8-(pyren-1-ylethynyl)-9H-carbazole, C39H34IN
- The cocrystal 2-(dimethylammonio)-5-nitrobenzoate – 2-(dimethylamino)-5-nitrobenzoic acid, C9H10N2O4
- Crystal structure of 5-nitroquinazolin-4(3H)-one, C8H5N3O3

