Abstract
C10H16N12O6S2, triclinic,
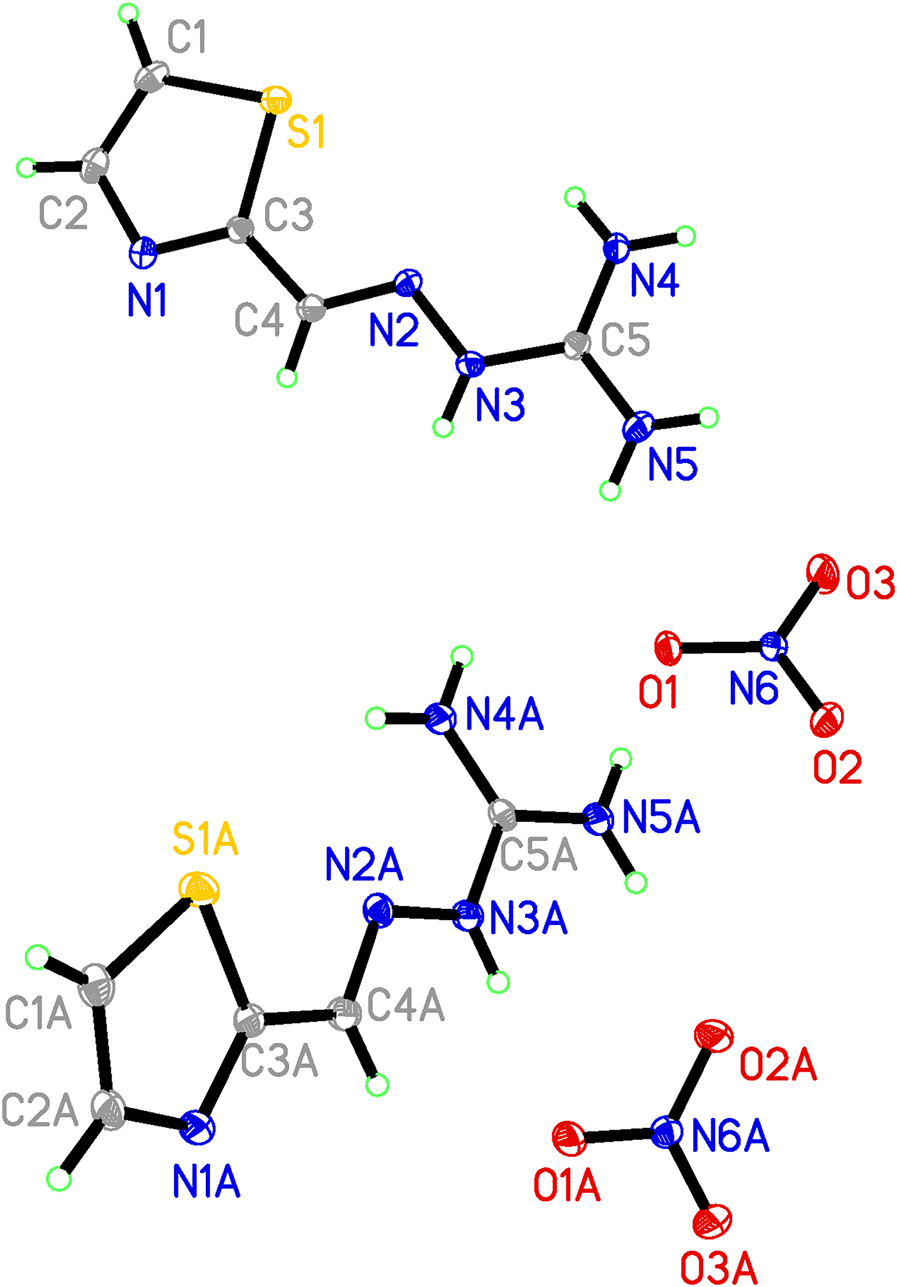
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.35 × 0.25 × 0.22 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.34 mm−1 |
| Diffractometer, scan mode: | XtaLAB AFC10 (RCD3), ω |
| θ max, completeness: | 30.9°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 14,660, 5143, 0.014 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 4688 |
| N(param)refined: | 271 |
| Programs: | CrysAlisPRO [1], SHELX [2], Olex2 [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| S1 | −0.02938 (4) | 1.14231 (2) | 0.24276 (3) | 0.02553 (8) |
| N1 | −0.08184 (12) | 0.92768 (9) | 0.41287 (8) | 0.02321 (18) |
| N2 | 0.31167 (11) | 1.00396 (8) | 0.23385 (8) | 0.02025 (17) |
| N3 | 0.46678 (11) | 0.94566 (8) | 0.22837 (8) | 0.02179 (18) |
| H3 | 0.489769 | 0.872227 | 0.281252 | 0.026* |
| N4 | 0.54896 (12) | 1.11884 (9) | 0.06023 (9) | 0.02497 (19) |
| H4A | 0.623089 | 1.156278 | 0.002427 | 0.030* |
| H4B | 0.452541 | 1.155728 | 0.066689 | 0.030* |
| N5 | 0.72923 (12) | 0.94579 (10) | 0.13173 (9) | 0.0273 (2) |
| H5A | 0.805461 | 0.981200 | 0.074764 | 0.033* |
| H5B | 0.748427 | 0.871488 | 0.184202 | 0.033* |
| C1 | −0.22320 (15) | 1.11768 (12) | 0.32364 (12) | 0.0284 (2) |
| H1 | −0.312389 | 1.176945 | 0.311035 | 0.034* |
| C2 | −0.22817 (14) | 0.99984 (11) | 0.40844 (11) | 0.0266 (2) |
| H2 | −0.324413 | 0.969667 | 0.460351 | 0.032* |
| C3 | 0.03466 (13) | 0.99220 (9) | 0.33083 (9) | 0.01957 (19) |
| C4 | 0.20394 (14) | 0.93955 (10) | 0.31734 (10) | 0.0211 (2) |
| H4 | 0.234070 | 0.858913 | 0.369479 | 0.025* |
| C5 | 0.58299 (13) | 1.00537 (10) | 0.13861 (9) | 0.01993 (19) |
| S1A | 1.22478 (4) | 0.55622 (3) | 1.00624 (3) | 0.02790 (8) |
| N1A | 1.32956 (13) | 0.32877 (9) | 1.06870 (9) | 0.0256 (2) |
| N2A | 1.19702 (12) | 0.51244 (8) | 0.76713 (8) | 0.02126 (18) |
| N3A | 1.20609 (12) | 0.50465 (8) | 0.65399 (8) | 0.02169 (18) |
| H3A | 1.260960 | 0.443648 | 0.642744 | 0.026* |
| N4A | 1.01502 (13) | 0.67214 (9) | 0.58345 (9) | 0.02411 (19) |
| H4AA | 0.962792 | 0.728763 | 0.523990 | 0.029* |
| H4AB | 0.994036 | 0.666128 | 0.657812 | 0.029* |
| N5A | 1.16450 (13) | 0.59928 (9) | 0.44882 (8) | 0.02395 (19) |
| H5AA | 1.114858 | 0.654655 | 0.387025 | 0.029* |
| H5AB | 1.238466 | 0.547044 | 0.437522 | 0.029* |
| C1A | 1.27698 (16) | 0.47576 (12) | 1.15541 (10) | 0.0286 (2) |
| H1A | 1.270341 | 0.508253 | 1.216909 | 0.034* |
| C2A | 1.32931 (16) | 0.35815 (11) | 1.17141 (10) | 0.0279 (2) |
| H2A | 1.363031 | 0.300708 | 1.247257 | 0.033* |
| C3A | 1.27680 (14) | 0.42520 (10) | 0.97454 (10) | 0.0208 (2) |
| C4A | 1.27005 (15) | 0.42237 (10) | 0.85270 (10) | 0.0234 (2) |
| H4AC | 1.318780 | 0.355105 | 0.836762 | 0.028* |
| C5A | 1.12731 (13) | 0.59449 (9) | 0.56061 (9) | 0.01926 (19) |
| O1 | 0.98220 (10) | 0.74665 (8) | 0.23715 (7) | 0.02680 (17) |
| O2 | 1.24634 (11) | 0.74315 (8) | 0.16780 (8) | 0.03120 (19) |
| O3 | 1.05183 (13) | 0.86196 (10) | 0.05015 (8) | 0.0437 (3) |
| N6 | 1.09389 (12) | 0.78448 (8) | 0.15090 (8) | 0.02186 (18) |
| O1A | 1.39236 (12) | 0.29907 (8) | 0.61837 (7) | 0.0319 (2) |
| O2A | 1.40854 (13) | 0.41839 (8) | 0.42539 (8) | 0.0350 (2) |
| O3A | 1.51643 (12) | 0.23101 (8) | 0.48687 (9) | 0.0339 (2) |
| N6A | 1.43877 (12) | 0.31603 (9) | 0.50890 (9) | 0.02292 (18) |
Source of material
2-Thiazolecarboxaldehyde (1.13 g, 0.01 mol) was placed in 15 ml ethanol at room temperature, added into aminoguanidine nitrate (1.37 g, 0.01 mol) solution containing 10 ml water and 8 ml ethanol. The mixture was heated and stirred for 8 h, cooled to room temperature. Then the precipitate was removed, and the filtrate was left standing to precipitate colorless block crystal.
Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms.
Comment
Schiff Base compounds have attracted many researchers due to their catalysis [4], simulation of biological enzymes and antibodies, molecular recognition [5] and other aspects. This is mainly due to the C=N group interaction. In recent decades, the preparation, physical and chemical properties of Schiff bases and their complexes have been extensively reported. They can be used as chelating agents [6], stabilizers [7] and bioactive agents [8]. The thiazole ring exists in many kinds of bioactive heterocycles and natural products, and is considered as the main component of many bioactive compounds. It is reported that Schiff bases containing thiazole may show a wide range of applications in medicine [9], as an antioxidant [10], or in catalysis [11]. Therefore, the synthesis and properties of thiazoles Schiff bases have become a very active topic in heterocyclic chemistry and coordination chemistry. According to previous studies [12, 13], we synthesized the title compound.
The structure of the title compound contains a pair of independent cations and anions (see the figure). Moreover, all bond lengths and bond angles of the title compound are in the normal range [14].
Funding source: Henan University of Science and Technology Distinguished Professor Open Fund http://dx.doi.org/10.13039/501100003172
Award Identifier / Grant number: 135100001
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This study was funded by the Henan University of Science and Technology Distinguished Professor Open Fund (Grant No. 135100001).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Agilent Technologies. CRYSALISPRO; Agilent Technologies: Santa Clara, CA, USA, 2017.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar
3. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
4. Gayen, F. R., Ali, A. A., Bora, D., Roy, S., Saha, S., Saikia, L., Goswamee, R. L., Saha, B. A ferrocene functionalized Schiff base containing Cu(II) complex: synthesis, characterization and parts-per- million level catalysis for azide alkyne cycloaddition. Dalton Trans. 2021, 50, 6735; https://doi.org/10.1039/d1dt90072b.Search in Google Scholar
5. Daniel, S. Colorimetric molecular receptors for the sensing of acetate, fluoride and mercury based on Schiff’s bases. Bull. Mater. Sci. 2020, 43, 157; https://doi.org/10.1007/s12034-020-02143-1.Search in Google Scholar
6. Ismael, M., Abdel-Mawgoud, A. M. M., Rabia, M. K., Abdou, A. Ni(II) mixed-ligand chelates based on 2-hydroxy-1-naphthaldehyde as antimicrobial agents: synthesis, characterization, and molecular modeling. J. Mol. Liq. 2021, 330, 115611; https://doi.org/10.1016/j.molliq.2021.115611.Search in Google Scholar
7. Elmehbad, N. Y., Mohamed, N. A. Evaluation of poly(N-benzoyl-4-(N-itaconimido)benzhydrazide) and its metal complexes as microbial inhibitors and thermal stabilizers for poly(vinyl chloride). Polym. Bull. 2021, 1–26; https://doi.org/10.1080/00914037.2021.1963724.Search in Google Scholar
8. Uddin, M. N., Ahmed, S. S., Alam, S. M. R. REVIEW: biomedical applications of Schiff base metal complexes. J. Coord. Chem. 2020, 73, 3109–3149; https://doi.org/10.1080/00958972.2020.1854745.Search in Google Scholar
9. Cordeiro, R., Kachroo, M. Synthesis and biological evaluation of anti-tubercular activity of Schiff bases of 2-amino thiazoles. Bioorg. Med. Chem. Lett 2020, 30, 127655; https://doi.org/10.1016/j.bmcl.2020.127655.Search in Google Scholar
10. Lemilemu, F., Bitew, M., Demissie, T. B., Eswaramoorthy, R., Endale, M. Synthesis, antibacterial and antioxidant activities of thiazole-based Schiff base derivatives: a combined experimental and computational study. BMC Chem. 2021, 15, 67; https://doi.org/10.1186/s13065-021-00791-w.Search in Google Scholar
11. Kumar, P., Lymperopoulou, S., Loukopoulos, E., Matsuda, W., Kourkoumelis, N., Seki, S., Kostakis, G. E. Catalytic and conductivity studies in two dimensional coordination polymers built with a thiazole based ligand. Polyhedron 2018, 150, 21–27; https://doi.org/10.1016/j.poly.2018.04.040.Search in Google Scholar
12. Jin, Z.-S., Liu, X.-J., Li, Z.-Y., Liu, E., Jian, F.-F., Liang, T. Crystal structure of (E)-(2-((1H-pyrrol-2-yl)methylene) hydrazineyl)(amino)methaniminium nitrate monohydrate, C6H12N6O4. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 143–145; https://doi.org/10.1515/ncrs-2021-0419.Search in Google Scholar
13. Wang, B., Zhang, P.-Z., Chen, X., Jia, A.-Q., Zhang, Q.-F. Syntheses and crystal structures of guanidine hydrochlorides with two Schiff base functions as efficient colorimetric and selective sensors for fluoride. Z. Naturforsch., B 2018, 150, 601–609; https://doi.org/10.1515/znb-2018-0102.Search in Google Scholar
14. Laverick, R. J., Carter, A. B., Klein, H. A., Fitzpatrick, A. J., Keene, T. D., Morgan, G. G., Kitchen, J. A. Synthesis and characterisation of Fe(III) and Co(III) complexes of thiazole- containing thiosemicarbazone ligands. Inorg. Chim. Acta 2017, 463, 126–133; https://doi.org/10.1016/j.ica.2017.04.008.Search in Google Scholar
© 2022 Ze-Sen Jin et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-(4-imidazol-1-yl-phenyl)-(2-methoxy-benzylidene)-amine monohydrate, C17H17N3O2
- Crystal structure of 6-methyl-3-(pyrrolidine-1-carbonyl)-2H-chromen-2-one, C15H15N1O3
- Crystal structure of 4-methyl-4-nitropentanoic acid, C6H11NO4
- The crystal structure of (E)-3-(furan-2-yl)acrylonitrile, C7H5NO
- Crystal structure of 3-(difluoromethyl)-1-methyl-N-(4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)-1H-pyrazole-4-carboxamide monohydrate, C23H26F2N4O3
- Crystal structure of 2-(4-bromobenzyloxy)-6-chloropyridine, C12H9BrClNO
- Crystal structure of N-(4-bromo-2,6-dichloro-phenyl)pyrazin-2-amine, C10H6BrCl2N3
- Crystal structure of (E)-1-(2–nitrophenyl)-3-phenylprop-2-en-1-one, C15H11NO3
- The crystal structure of (E)-3-chloro-2-(2-(4-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of (E)-amino(2-(thiazol-2-ylmethylene)hydrazineyl)methaniminium nitrate, C10H16N12O6S2
- Crystal structure of 9-methoxy-2,3,4,4a,5,6-hexahydro-1H-pyrido [1′,2′:1,6]pyrazino[2,3-b]quinoxaline, C15H18N4O
- The crystal structure bis(dimethylsulfoxide-κ1O)-dipyridine-κ1 N-bis(m2-(Z)-3-methyl-2-oxido-N-((Z)-oxido(phenyl)methylene)benzohydrazonato-κ5)trinickel(II) - dimethylsulfoxide (1/2), C48H56N6Ni3O10S4
- Crystal structure of bis(bis(triphenylphosphine)iminium) tetradecacarbonyltetratelluridopentaferrate(2-), (PPN)2[Fe5Te4(CO)14]
- Crystal structure of 4-Hydroxy-3-(naphthalen-2-ylthio)pent-3-en-2-one, C15H14O2S
- The crystal structure of [(1,10-phenanthroline-κ2 N,N)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)nickel(II)] monohydrate, C36H26N4O5Ni
- Crystal structure of 3,3′-(pyridine-2,6-diylbis(methylene))bis(1-propyl-1H-imidazol-3-ium) ditetrafluoroborate, C19H27B2F8N5
- The crystal structure of (E)-1-(4-aminophenyl)-3-(p-tolyl)prop-2-en-1-one, C16H15NO
- The crystal structure of poly[(μ2-terephthalato-κ4O,O′: O″,O‴)-(μ4-terephthalato-κ4O:O′:O″:O‴)-{μ4-(1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ4O:O′:O″,O‴)}dicadmium(II)] – water – acetronitrile (1/2/2), C38H36N14O10Cd2
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)cobalt(II)–water–N,N-dimethylformamide(1/2/1), C27H31N3O9Co
- The co-crystal structure of 4-hydroxy-3-methoxybenzoic acid – 4,4′-bipyridine, C8H8O4·C10H8N2
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)cobalt(II)], C32H22Br2CoN4O4
- Crystal structure of (E)-5-propyl-4-((pyridin-2-ylmethylene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione – methanol (1/1), C11H13N5S
- The crystal structure of (Z)-4-bromo-6-(((1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)amino)methylene)cyclohexa-2,4-dien-1-one monohydrate, C11H16BrNO5
- Crystal structure of bis(tetrapropylammonium) nonaselenidotetrastannate(IV), (Pr4N)2[Sn4Se9]
- Crystal structure of 2,6-di-tert-butyl-4-(4-chlorobenzylidene)cyclohexa-2,5-dien-1-one, C21H25ClO
- Crystal structure of (2,2′-((naphthalen-1-ylmethyl)azanediyl)diacetato-κ3 N,O,O′)-(1,10-phenanthroline-κ2 N,N′)-copper(II) trihydrate, CuC27H27N3O7
- The crystal structure of tetrakis(6-phenylpyridine-2-carboxylato-κ2N,O)-bis(1H-pyrazol-3-ylamine-κ2 N:N)dicobalt(II) dihydrate, C27H23N5O5Co
- The crystal structure of bis((E)-2-((tert-butylimino)methyl)-4-chlorophenolato-κ2N,O)zinc(II), C22H26Cl2N2O2Zn
- The crystal structure of poly[diaqua-(μ3-5-nitrobenzene-1,2,3-tricarboxylato-κ3O:O′:O′)-(μ2-4,4′-dipyridylamine-κ2N:N′)copper(II)], C38H30Cu3N8O20
- The crystal structure of (E)-1-ferrocenyl-3-(naphthalen-1-yl)prop-2-en-1-one, C23H18FeO
- The crystal structure of (E)-1-ferrocenyl-3-(4-isopropylphenyl)prop-2-en-1-one, C22H22FeO
- Crystal structure of 6-hydroxy-2,2-dimethyl-4Hbenzo[d][1,3]dioxin-4-one, C10H10O4
- The crystal structure of (2E,4E)-1-ferrocenyl-5-phenylpenta-2,4-dien-1-one, C21H18FeO
- Crystal structure of alaninato-κ2N,O-bis(hydroxylamido-κ2N,O)-oxido-vanadium(V), C3H10N3O5V
- Crystal structure of catena-poly[aqua-bis[μ2-6-(1H-imidazol-1-yl)nicotinato-κ2 N,O]copper(II)], C18H14N6O5Cu
- Crystal structure of diethyl 4,6-diphenyl-1,9-di-p-tolylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,5(2H)-dicarboxylate, C42H42N2O4
- The crystal structure of cobalt cadmium bis(hydrogenphosphate) bis(phosphate(V)) tetrahydrate, H10O20P4Co3.14Cd1.86
- Crystal structure of dimethyl 1,4,6,9-tetraphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,7(2H)-dicarboxylate, C38H34N2O4
- Crystal structure of (Z)-4-(furan-2-yl((4-iodophenyl)amino)methylene)-5-methyl-2(p-tolyl)-2,4-dihydro-3H-pyrazol-3-one, C21H16I N3O2
- Crystal structure of (E)-1-(4-(3,5-dimethoxystyryl)phenyl)-7-ethylheptanedioate, C25H30O6
- Crystal structure of 6-bromo-2-(4-chlorophenyl)chroman-4-one (6-bromo-4′-chloroflavanone), C15H10BrClO2
- The crystal structure of 2-(benzhydryloxy)-3-nitropyridine, C18H14N2O3
- The crystal structure of 1,3(4,1)-dipyridin-1-iuma-2(1,8)-diethynylanthracena-5(1,3)-benzenacyclohexaphane-11,31-diium bis(hexafluoridophosphate), C36H24F12N2P2
- Crystal structure of 3,6-di-tert-butyl-1-iodo-9-methyl-8-(pyren-1-ylethynyl)-9H-carbazole, C39H34IN
- The cocrystal 2-(dimethylammonio)-5-nitrobenzoate – 2-(dimethylamino)-5-nitrobenzoic acid, C9H10N2O4
- Crystal structure of 5-nitroquinazolin-4(3H)-one, C8H5N3O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-(4-imidazol-1-yl-phenyl)-(2-methoxy-benzylidene)-amine monohydrate, C17H17N3O2
- Crystal structure of 6-methyl-3-(pyrrolidine-1-carbonyl)-2H-chromen-2-one, C15H15N1O3
- Crystal structure of 4-methyl-4-nitropentanoic acid, C6H11NO4
- The crystal structure of (E)-3-(furan-2-yl)acrylonitrile, C7H5NO
- Crystal structure of 3-(difluoromethyl)-1-methyl-N-(4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)-1H-pyrazole-4-carboxamide monohydrate, C23H26F2N4O3
- Crystal structure of 2-(4-bromobenzyloxy)-6-chloropyridine, C12H9BrClNO
- Crystal structure of N-(4-bromo-2,6-dichloro-phenyl)pyrazin-2-amine, C10H6BrCl2N3
- Crystal structure of (E)-1-(2–nitrophenyl)-3-phenylprop-2-en-1-one, C15H11NO3
- The crystal structure of (E)-3-chloro-2-(2-(4-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of (E)-amino(2-(thiazol-2-ylmethylene)hydrazineyl)methaniminium nitrate, C10H16N12O6S2
- Crystal structure of 9-methoxy-2,3,4,4a,5,6-hexahydro-1H-pyrido [1′,2′:1,6]pyrazino[2,3-b]quinoxaline, C15H18N4O
- The crystal structure bis(dimethylsulfoxide-κ1O)-dipyridine-κ1 N-bis(m2-(Z)-3-methyl-2-oxido-N-((Z)-oxido(phenyl)methylene)benzohydrazonato-κ5)trinickel(II) - dimethylsulfoxide (1/2), C48H56N6Ni3O10S4
- Crystal structure of bis(bis(triphenylphosphine)iminium) tetradecacarbonyltetratelluridopentaferrate(2-), (PPN)2[Fe5Te4(CO)14]
- Crystal structure of 4-Hydroxy-3-(naphthalen-2-ylthio)pent-3-en-2-one, C15H14O2S
- The crystal structure of [(1,10-phenanthroline-κ2 N,N)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)nickel(II)] monohydrate, C36H26N4O5Ni
- Crystal structure of 3,3′-(pyridine-2,6-diylbis(methylene))bis(1-propyl-1H-imidazol-3-ium) ditetrafluoroborate, C19H27B2F8N5
- The crystal structure of (E)-1-(4-aminophenyl)-3-(p-tolyl)prop-2-en-1-one, C16H15NO
- The crystal structure of poly[(μ2-terephthalato-κ4O,O′: O″,O‴)-(μ4-terephthalato-κ4O:O′:O″:O‴)-{μ4-(1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ4O:O′:O″,O‴)}dicadmium(II)] – water – acetronitrile (1/2/2), C38H36N14O10Cd2
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)cobalt(II)–water–N,N-dimethylformamide(1/2/1), C27H31N3O9Co
- The co-crystal structure of 4-hydroxy-3-methoxybenzoic acid – 4,4′-bipyridine, C8H8O4·C10H8N2
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)cobalt(II)], C32H22Br2CoN4O4
- Crystal structure of (E)-5-propyl-4-((pyridin-2-ylmethylene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione – methanol (1/1), C11H13N5S
- The crystal structure of (Z)-4-bromo-6-(((1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)amino)methylene)cyclohexa-2,4-dien-1-one monohydrate, C11H16BrNO5
- Crystal structure of bis(tetrapropylammonium) nonaselenidotetrastannate(IV), (Pr4N)2[Sn4Se9]
- Crystal structure of 2,6-di-tert-butyl-4-(4-chlorobenzylidene)cyclohexa-2,5-dien-1-one, C21H25ClO
- Crystal structure of (2,2′-((naphthalen-1-ylmethyl)azanediyl)diacetato-κ3 N,O,O′)-(1,10-phenanthroline-κ2 N,N′)-copper(II) trihydrate, CuC27H27N3O7
- The crystal structure of tetrakis(6-phenylpyridine-2-carboxylato-κ2N,O)-bis(1H-pyrazol-3-ylamine-κ2 N:N)dicobalt(II) dihydrate, C27H23N5O5Co
- The crystal structure of bis((E)-2-((tert-butylimino)methyl)-4-chlorophenolato-κ2N,O)zinc(II), C22H26Cl2N2O2Zn
- The crystal structure of poly[diaqua-(μ3-5-nitrobenzene-1,2,3-tricarboxylato-κ3O:O′:O′)-(μ2-4,4′-dipyridylamine-κ2N:N′)copper(II)], C38H30Cu3N8O20
- The crystal structure of (E)-1-ferrocenyl-3-(naphthalen-1-yl)prop-2-en-1-one, C23H18FeO
- The crystal structure of (E)-1-ferrocenyl-3-(4-isopropylphenyl)prop-2-en-1-one, C22H22FeO
- Crystal structure of 6-hydroxy-2,2-dimethyl-4Hbenzo[d][1,3]dioxin-4-one, C10H10O4
- The crystal structure of (2E,4E)-1-ferrocenyl-5-phenylpenta-2,4-dien-1-one, C21H18FeO
- Crystal structure of alaninato-κ2N,O-bis(hydroxylamido-κ2N,O)-oxido-vanadium(V), C3H10N3O5V
- Crystal structure of catena-poly[aqua-bis[μ2-6-(1H-imidazol-1-yl)nicotinato-κ2 N,O]copper(II)], C18H14N6O5Cu
- Crystal structure of diethyl 4,6-diphenyl-1,9-di-p-tolylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,5(2H)-dicarboxylate, C42H42N2O4
- The crystal structure of cobalt cadmium bis(hydrogenphosphate) bis(phosphate(V)) tetrahydrate, H10O20P4Co3.14Cd1.86
- Crystal structure of dimethyl 1,4,6,9-tetraphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,7(2H)-dicarboxylate, C38H34N2O4
- Crystal structure of (Z)-4-(furan-2-yl((4-iodophenyl)amino)methylene)-5-methyl-2(p-tolyl)-2,4-dihydro-3H-pyrazol-3-one, C21H16I N3O2
- Crystal structure of (E)-1-(4-(3,5-dimethoxystyryl)phenyl)-7-ethylheptanedioate, C25H30O6
- Crystal structure of 6-bromo-2-(4-chlorophenyl)chroman-4-one (6-bromo-4′-chloroflavanone), C15H10BrClO2
- The crystal structure of 2-(benzhydryloxy)-3-nitropyridine, C18H14N2O3
- The crystal structure of 1,3(4,1)-dipyridin-1-iuma-2(1,8)-diethynylanthracena-5(1,3)-benzenacyclohexaphane-11,31-diium bis(hexafluoridophosphate), C36H24F12N2P2
- Crystal structure of 3,6-di-tert-butyl-1-iodo-9-methyl-8-(pyren-1-ylethynyl)-9H-carbazole, C39H34IN
- The cocrystal 2-(dimethylammonio)-5-nitrobenzoate – 2-(dimethylamino)-5-nitrobenzoic acid, C9H10N2O4
- Crystal structure of 5-nitroquinazolin-4(3H)-one, C8H5N3O3

