Crystal structure of 3-(difluoromethyl)-1-methyl-N-(4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)-1H-pyrazole-4-carboxamide monohydrate, C23H26F2N4O3
Abstract
C23H26F2N4O3, orthorhombic, P212121 (no. 19), a = 11.6524(10) Å, b = 11.9777(10) Å, c = 15.9058(13) Å, V = 2220.0(3) Å3, Z = 4, R gt (F) = 0.0305, wR ref (F 2) = 0.0751, T = 296(2) K.
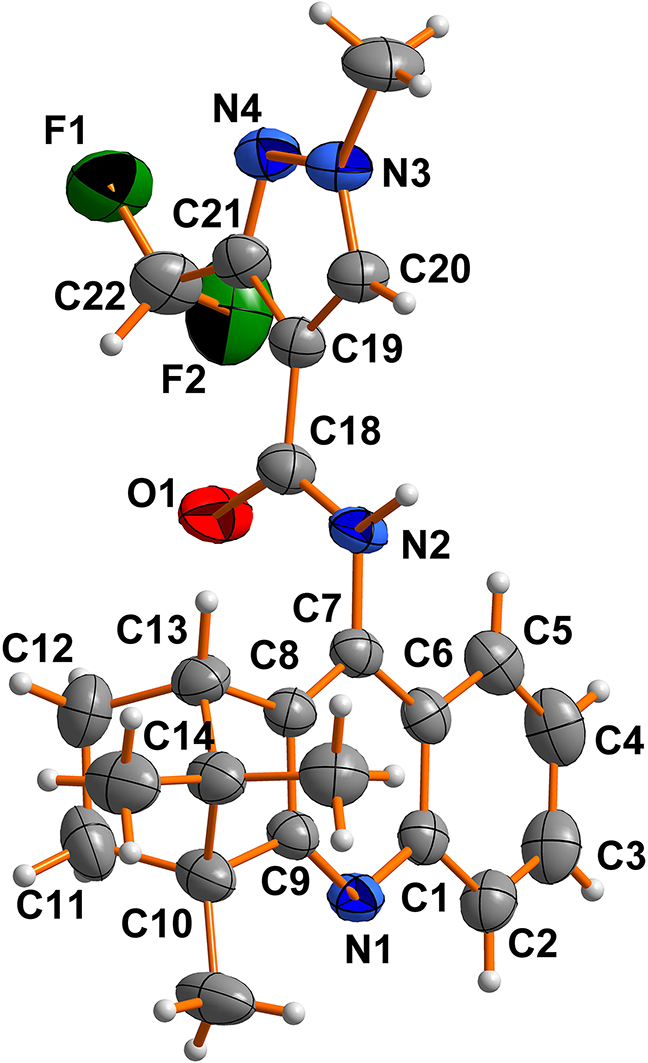
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.20 × 0.18 × 0.12 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 25.5°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 17,253, 4136, 0.022 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 3649 |
| N(param)refined: | 293 |
| Programs: | Bruker [1], SHELX [2, 3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.22419 (18) | 0.38793 (18) | 0.21920 (13) | 0.0440 (5) |
| C2 | 0.2957 (2) | 0.3521 (2) | 0.28537 (15) | 0.0552 (6) |
| H2 | 0.2967 | 0.3914 | 0.3358 | 0.066* |
| C3 | 0.3634 (2) | 0.2604 (2) | 0.27604 (18) | 0.0644 (7) |
| H3 | 0.4085 | 0.2365 | 0.3208 | 0.077* |
| C4 | 0.3661 (2) | 0.2018 (2) | 0.20050 (18) | 0.0655 (7) |
| H4 | 0.4131 | 0.1395 | 0.1950 | 0.079* |
| C5 | 0.2996 (2) | 0.23566 (19) | 0.13449 (17) | 0.0560 (6) |
| H5 | 0.3030 | 0.1969 | 0.0839 | 0.067* |
| C6 | 0.22602 (18) | 0.32866 (17) | 0.14203 (14) | 0.0439 (5) |
| C7 | 0.15056 (19) | 0.36415 (18) | 0.07673 (12) | 0.0431 (5) |
| C8 | 0.07984 (19) | 0.45060 (18) | 0.09209 (13) | 0.0430 (5) |
| C9 | 0.08387 (18) | 0.50458 (18) | 0.17189 (13) | 0.0426 (5) |
| C10 | −0.0086 (2) | 0.5927 (2) | 0.17166 (14) | 0.0546 (6) |
| C11 | −0.1216 (2) | 0.5219 (3) | 0.17370 (18) | 0.0747 (8) |
| H11A | −0.1188 | 0.4669 | 0.2184 | 0.090* |
| H11B | −0.1880 | 0.5695 | 0.1822 | 0.090* |
| C12 | −0.1265 (2) | 0.4648 (3) | 0.08708 (19) | 0.0722 (8) |
| H12A | −0.1941 | 0.4877 | 0.0559 | 0.087* |
| H12B | −0.1265 | 0.3841 | 0.0926 | 0.087* |
| C13 | −0.0156 (2) | 0.5065 (2) | 0.04409 (14) | 0.0533 (6) |
| H13 | −0.0128 | 0.4978 | −0.0171 | 0.064* |
| C14 | −0.0054 (2) | 0.6278 (2) | 0.07687 (14) | 0.0535 (6) |
| C15 | −0.1052 (3) | 0.7040 (3) | 0.05150 (17) | 0.0758 (8) |
| H15A | −0.1004 | 0.7729 | 0.0822 | 0.114* |
| H15B | −0.1766 | 0.6677 | 0.0641 | 0.114* |
| H15C | −0.1012 | 0.7191 | −0.0077 | 0.114* |
| C16 | 0.1072 (2) | 0.6833 (2) | 0.05134 (17) | 0.0660 (7) |
| H16A | 0.1098 | 0.6909 | −0.0087 | 0.099* |
| H16B | 0.1704 | 0.6381 | 0.0698 | 0.099* |
| H16C | 0.1122 | 0.7557 | 0.0769 | 0.099* |
| C17 | −0.0003 (3) | 0.6835 (3) | 0.23707 (16) | 0.0783 (9) |
| H17A | −0.0156 | 0.6525 | 0.2916 | 0.117* |
| H17B | −0.0555 | 0.7407 | 0.2249 | 0.117* |
| H17C | 0.0755 | 0.7149 | 0.2363 | 0.117* |
| C18 | 0.0837 (2) | 0.22076 (18) | −0.01835 (13) | 0.0482 (5) |
| C19 | 0.09286 (19) | 0.17012 (17) | −0.10278 (14) | 0.0456 (5) |
| C20 | 0.1474 (2) | 0.20916 (19) | −0.17316 (13) | 0.0510 (5) |
| H20 | 0.1894 | 0.2750 | −0.1773 | 0.061* |
| C21 | 0.04214 (19) | 0.06952 (18) | −0.12960 (15) | 0.0498 (6) |
| C22 | −0.0257 (3) | −0.0110 (2) | −0.07928 (18) | 0.0683 (7) |
| H22 | −0.0809 | 0.0287 | −0.0437 | 0.082* |
| C23 | 0.1679 (3) | 0.1410 (3) | −0.32149 (15) | 0.0814 (9) |
| H23A | 0.1999 | 0.2133 | −0.3325 | 0.122* |
| H23B | 0.1040 | 0.1282 | −0.3583 | 0.122* |
| H23C | 0.2253 | 0.0848 | −0.3310 | 0.122* |
| N1 | 0.15201 (16) | 0.47758 (15) | 0.23376 (10) | 0.0457 (4) |
| N2 | 0.15123 (17) | 0.30889 (15) | −0.00290 (11) | 0.0485 (5) |
| H2A | 0.1961 | 0.3328 | −0.0419 | 0.058* |
| N3 | 0.12931 (18) | 0.13556 (17) | −0.23450 (11) | 0.0554 (5) |
| N4 | 0.06475 (19) | 0.04849 (16) | −0.20956 (13) | 0.0575 (5) |
| O1 | 0.01822 (19) | 0.18373 (17) | 0.03480 (11) | 0.0791 (6) |
| O2 | 0.82110 (17) | 0.08597 (18) | 0.10387 (12) | 0.0675 (5) |
| F1 | −0.08090 (19) | −0.08452 (15) | −0.12961 (14) | 0.1092 (7) |
| F2 | 0.0441 (2) | −0.07281 (19) | −0.03227 (17) | 0.1337 (9) |
| H1W | 0.831 (2) | 0.053 (2) | 0.1491 (11) | 0.078 (9)* |
| H2W | 0.8819 (19) | 0.117 (3) | 0.0891 (18) | 0.107 (13)* |
Source of material
An amount of 10 mmol of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid and 30 mmol of thionyl chloride were mixed and one drop of N,N-dimethylformamide was added. The mixture was condensed and refluxed at 95 °C for 4 h, and 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carbonyl chloride was obtained [5]. About 10 mmol of 4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-amine was dissolved in 20 mL of dichloromethane. The prepared 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carbonyl chloride was added to the single-necked flask and dissolved in 30 mL of dichloromethane solution, Then cool to 0 °C. A mixed solution of 10 mmol of 4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methacridin-9-amine and 20 mmol of triethylamine was slowly added dropwise with stirring. After the addition was completed, the reactor was placed at room temperature and stirred for 6 h, and the reaction was monitored by thin layer chromatography (TLC) until completion [6]. Silica gel was added to the reaction solution and mixed well, eluted by column chromatography, and the title compound was obtained.
Experimental details
All H atoms were included in calculated positions and refined as riding atoms, with C–H = 0.90–0.97 Å with U iso(H) = 1.5 U eq(C) for methyl H atoms and 1.2 U eq(C) for all other H atoms.
Comment
Nitrogen-containing heterocyclic compounds are an important class of organic compounds. They are the basic structural framework of many drug molecules [7, 8]. Among them, pyrazole amide, as an important branch in the nitrogen-containing heterocyclic compound system, exhibits a variety of excellent pharmacological effects, such as insecticide [9, 10], bactericidal [11], anti-plant virus [12], and anti-tumor [13], etc. occupy an extremely important position in the fields of pesticides and medicine [14]. In addition, pyrazole amides exist in several pesticides with high recognition in the market, such as Tolfenpyrad, Chlorantraniliprole, and Sedaxane. Amide compounds formed from a carboxylic acid with a basic pyrazole skeleton as a parent usually exhibit different pharmacological effects depending on the substituent groups. Therefore, in recent years, the synthesis of pyrazole amide compounds and the determination of their biological activity have gradually attracted the attention of the majority of scientific researchers.
The asymmetric unit of the title compound (see the Figure) consists of one water molecule and one 3-(difluoromethyl)-1-methyl-N-(4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin9-yl)-1H-pyrazole-4-carboxamide molecule. Bond lengths and angles within the title structure are very similar to those given in the literature for (1R, 4S)-7,8-dichloro-1,2,3,4-tetra-hydro-1,11,11-trimethyl-1,4-methano-phenazine [15]. The atoms of pyrazole ring and quinoline ring are approximately co planar, and the dihedral angle between the pyrazole ring and the quinoline ring is 76.76(7)°. The dihedral angle between the pyrazole ring and the plane carboxamide moiety(C18–O1–N2) is 9.76(13)°. The C8–C15 part adopts a bridge ring structure. The torsion angles of C7–C8–C13–C12, C7–C8–C13–C14, N1–C9–C10–C14, N1–C9–C10–C11, and N1–C9–C10–C17 are 104.2(3)°, -149.6(3)°, 149.5(2)°, 107.0(3)°, and 20.7(3)°, respectively.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 31960295
Award Identifier / Grant number: 32160660
Funding source: Natural Science Foundation of Jiangxi Province
Award Identifier / Grant number: 20181BAB203015
Acknowledgments
X-ray data were collected at Instrumental Analysis Center Nanchang Hangkong University, Nanchang, 330063, People’s Republic of China.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported the National Natural Science Foundation of China (31960295, 32160660) and the Natural Science Foundation of Jiangxi Province (Grant No. 20181BAB203015).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System (ver. 4.0); Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
5. Bian, H., Feng, J., Xu, W. Synthesis and biological evaluation of novel AM80 derivatives as antileukemic agents. Med. Chem. Res. 2013, 22, 175–185; https://doi.org/10.1007/s00044-012-0019-9.Search in Google Scholar
6. Tian, H., Xu, Y., Liu, S., Jin, D., Zhang, J., Duan, L., Tan, W. Synthesis of gibberellic acid derivatives and their effects on plant growth. Molecules 2017, 22, 694; https://doi.org/10.3390/molecules22050694.Search in Google Scholar
7. Mermer, A., Keles, T., Sirin, Y. Recent studies of nitrogen containing heterocyclic compounds as novel antiviral agents: a review. Bioorg. Chem. 2021, 114, 105076; https://doi.org/10.1016/j.bioorg.2021.105076.Search in Google Scholar
8. Liang, K., Zhou, Q., Xun, X., Ni, Y., Li, J., Shi, Y., Zhou, H., Wu, X., Shi, J., Gao, L., Dai, H. Synthesis and insecticidal activities of novel pyrazole amide derivatives containing a thiazole unit. Chin. J. Org. Chem. 2020, 40, 1665–1672; https://doi.org/10.6023/cjoc202001029.Search in Google Scholar
9. Deng, X. L., Zhang, L., Hu, X. P., Yin, B., Liang, P., Yang, X. L. Target-based design, synthesis and biological activity of new pyrazole amide derivatives. Chin. Chem. Lett. 2016, 27, 251–255; https://doi.org/10.1016/j.cclet.2015.10.006.Search in Google Scholar
10. Jiang, B., Jin, X., Dong, Y., Guo, B., Cui, L., Deng, X., Zhang, L., Yang, Q., Li, Y., Yang, X., Smagghe, G. Design, synthesis, and biological activity of novel heptacyclic pyrazolamide derivatives: a new candidate of dual-target insect growth regulators. J. Agric. Food Chem. 2020, 68, 6347–6354; https://doi.org/10.1021/acs.jafc.0c00522.Search in Google Scholar
11. Ahmad, G., Rasool, N., Qamar, M. U., Alam, M. M., Kosar, N., Mahmood, T., Imran, M. Facile synthesis of 4-aryl-N-(5-methyl-1H-pyrazol-3-yl) benzamides via Suzuki Miyaura reaction: antibacterial activity against clinically isolated NDM-1-positive bacteria and their Docking Studies. Arab. J. Chem. 2021, 14, 103270; https://doi.org/10.1016/j.arabjc.2021.103270.Search in Google Scholar
12. Xiao, J. J., Liao, M., Chu, M. J., Ren, Z. L., Zhang, X., Lv, X. H., Gao, H. Q. Design, synthesis and anti-tobacco mosaic virus(TMV) activity of 5-chloro-N-(4-cyano-1-aryl-1H-pyrazol-5-yl)-1-aryl-3-methyl-1H-pyrazole-4-carboxamide derivatives. Molecules 2015, 20, 807–821; https://doi.org/10.3390/molecules20010807.Search in Google Scholar
13. Kim, M. H., Kim, M., Yu, H., Kim, H., Yoo, K. H., Sim, T., Hah, J. M. Structure based design and syntheses of amino-1H-pyrazole amide derivatives as selective Raf kinase inhibitors in melanoma cells. Bioorg. Med. Chem. 2011, 19, 1915–1923; https://doi.org/10.1016/j.bmc.2011.01.067.Search in Google Scholar
14. Sharp, S. Y., Prodromou, C., Boxall, K., Powers, M. V., Holmes, J. L., Box, G., Matthews, T. P., Cheung, K. J., Kalusa, A., James, K., Hayes, A., Hardcastle, A., Dymock, B., Brough, P. A., Barril, X., Cansfield, J. E., Wright, L., Surgenor, A., Foloppe, N., Hubbard, R. E., Aherne, W., Pearl, L., Jones, K., McDonald, E., Raynaud, F., Eccles, S., Drysdale, M., Workman, P. Inhibition of the heat shock protein 90 molecular chaperone in vitro and in vivo by novel, synthetic, potent resorcinylic pyrazole/isoxazole amide analogues. Mol. Cancer Therapeut. 2007, 6, 1198–1211; https://doi.org/10.1158/1535-7163.mct-07-0149.Search in Google Scholar
15. Crundwell, G., Glagovich, N. (1R,4S)-7,8-dichloro-1,2,3,4-tetra-hydro-1,11,11-trimethyl-1,4-methano-phenazine. Acta Crystallogr. 2010, E66, o3042; https://doi.org/10.1107/s1600536810044016.Search in Google Scholar
© 2022 Liang Zhong et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-(4-imidazol-1-yl-phenyl)-(2-methoxy-benzylidene)-amine monohydrate, C17H17N3O2
- Crystal structure of 6-methyl-3-(pyrrolidine-1-carbonyl)-2H-chromen-2-one, C15H15N1O3
- Crystal structure of 4-methyl-4-nitropentanoic acid, C6H11NO4
- The crystal structure of (E)-3-(furan-2-yl)acrylonitrile, C7H5NO
- Crystal structure of 3-(difluoromethyl)-1-methyl-N-(4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)-1H-pyrazole-4-carboxamide monohydrate, C23H26F2N4O3
- Crystal structure of 2-(4-bromobenzyloxy)-6-chloropyridine, C12H9BrClNO
- Crystal structure of N-(4-bromo-2,6-dichloro-phenyl)pyrazin-2-amine, C10H6BrCl2N3
- Crystal structure of (E)-1-(2–nitrophenyl)-3-phenylprop-2-en-1-one, C15H11NO3
- The crystal structure of (E)-3-chloro-2-(2-(4-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of (E)-amino(2-(thiazol-2-ylmethylene)hydrazineyl)methaniminium nitrate, C10H16N12O6S2
- Crystal structure of 9-methoxy-2,3,4,4a,5,6-hexahydro-1H-pyrido [1′,2′:1,6]pyrazino[2,3-b]quinoxaline, C15H18N4O
- The crystal structure bis(dimethylsulfoxide-κ1O)-dipyridine-κ1 N-bis(m2-(Z)-3-methyl-2-oxido-N-((Z)-oxido(phenyl)methylene)benzohydrazonato-κ5)trinickel(II) - dimethylsulfoxide (1/2), C48H56N6Ni3O10S4
- Crystal structure of bis(bis(triphenylphosphine)iminium) tetradecacarbonyltetratelluridopentaferrate(2-), (PPN)2[Fe5Te4(CO)14]
- Crystal structure of 4-Hydroxy-3-(naphthalen-2-ylthio)pent-3-en-2-one, C15H14O2S
- The crystal structure of [(1,10-phenanthroline-κ2 N,N)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)nickel(II)] monohydrate, C36H26N4O5Ni
- Crystal structure of 3,3′-(pyridine-2,6-diylbis(methylene))bis(1-propyl-1H-imidazol-3-ium) ditetrafluoroborate, C19H27B2F8N5
- The crystal structure of (E)-1-(4-aminophenyl)-3-(p-tolyl)prop-2-en-1-one, C16H15NO
- The crystal structure of poly[(μ2-terephthalato-κ4O,O′: O″,O‴)-(μ4-terephthalato-κ4O:O′:O″:O‴)-{μ4-(1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ4O:O′:O″,O‴)}dicadmium(II)] – water – acetronitrile (1/2/2), C38H36N14O10Cd2
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)cobalt(II)–water–N,N-dimethylformamide(1/2/1), C27H31N3O9Co
- The co-crystal structure of 4-hydroxy-3-methoxybenzoic acid – 4,4′-bipyridine, C8H8O4·C10H8N2
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)cobalt(II)], C32H22Br2CoN4O4
- Crystal structure of (E)-5-propyl-4-((pyridin-2-ylmethylene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione – methanol (1/1), C11H13N5S
- The crystal structure of (Z)-4-bromo-6-(((1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)amino)methylene)cyclohexa-2,4-dien-1-one monohydrate, C11H16BrNO5
- Crystal structure of bis(tetrapropylammonium) nonaselenidotetrastannate(IV), (Pr4N)2[Sn4Se9]
- Crystal structure of 2,6-di-tert-butyl-4-(4-chlorobenzylidene)cyclohexa-2,5-dien-1-one, C21H25ClO
- Crystal structure of (2,2′-((naphthalen-1-ylmethyl)azanediyl)diacetato-κ3 N,O,O′)-(1,10-phenanthroline-κ2 N,N′)-copper(II) trihydrate, CuC27H27N3O7
- The crystal structure of tetrakis(6-phenylpyridine-2-carboxylato-κ2N,O)-bis(1H-pyrazol-3-ylamine-κ2 N:N)dicobalt(II) dihydrate, C27H23N5O5Co
- The crystal structure of bis((E)-2-((tert-butylimino)methyl)-4-chlorophenolato-κ2N,O)zinc(II), C22H26Cl2N2O2Zn
- The crystal structure of poly[diaqua-(μ3-5-nitrobenzene-1,2,3-tricarboxylato-κ3O:O′:O′)-(μ2-4,4′-dipyridylamine-κ2N:N′)copper(II)], C38H30Cu3N8O20
- The crystal structure of (E)-1-ferrocenyl-3-(naphthalen-1-yl)prop-2-en-1-one, C23H18FeO
- The crystal structure of (E)-1-ferrocenyl-3-(4-isopropylphenyl)prop-2-en-1-one, C22H22FeO
- Crystal structure of 6-hydroxy-2,2-dimethyl-4Hbenzo[d][1,3]dioxin-4-one, C10H10O4
- The crystal structure of (2E,4E)-1-ferrocenyl-5-phenylpenta-2,4-dien-1-one, C21H18FeO
- Crystal structure of alaninato-κ2N,O-bis(hydroxylamido-κ2N,O)-oxido-vanadium(V), C3H10N3O5V
- Crystal structure of catena-poly[aqua-bis[μ2-6-(1H-imidazol-1-yl)nicotinato-κ2 N,O]copper(II)], C18H14N6O5Cu
- Crystal structure of diethyl 4,6-diphenyl-1,9-di-p-tolylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,5(2H)-dicarboxylate, C42H42N2O4
- The crystal structure of cobalt cadmium bis(hydrogenphosphate) bis(phosphate(V)) tetrahydrate, H10O20P4Co3.14Cd1.86
- Crystal structure of dimethyl 1,4,6,9-tetraphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,7(2H)-dicarboxylate, C38H34N2O4
- Crystal structure of (Z)-4-(furan-2-yl((4-iodophenyl)amino)methylene)-5-methyl-2(p-tolyl)-2,4-dihydro-3H-pyrazol-3-one, C21H16I N3O2
- Crystal structure of (E)-1-(4-(3,5-dimethoxystyryl)phenyl)-7-ethylheptanedioate, C25H30O6
- Crystal structure of 6-bromo-2-(4-chlorophenyl)chroman-4-one (6-bromo-4′-chloroflavanone), C15H10BrClO2
- The crystal structure of 2-(benzhydryloxy)-3-nitropyridine, C18H14N2O3
- The crystal structure of 1,3(4,1)-dipyridin-1-iuma-2(1,8)-diethynylanthracena-5(1,3)-benzenacyclohexaphane-11,31-diium bis(hexafluoridophosphate), C36H24F12N2P2
- Crystal structure of 3,6-di-tert-butyl-1-iodo-9-methyl-8-(pyren-1-ylethynyl)-9H-carbazole, C39H34IN
- The cocrystal 2-(dimethylammonio)-5-nitrobenzoate – 2-(dimethylamino)-5-nitrobenzoic acid, C9H10N2O4
- Crystal structure of 5-nitroquinazolin-4(3H)-one, C8H5N3O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-(4-imidazol-1-yl-phenyl)-(2-methoxy-benzylidene)-amine monohydrate, C17H17N3O2
- Crystal structure of 6-methyl-3-(pyrrolidine-1-carbonyl)-2H-chromen-2-one, C15H15N1O3
- Crystal structure of 4-methyl-4-nitropentanoic acid, C6H11NO4
- The crystal structure of (E)-3-(furan-2-yl)acrylonitrile, C7H5NO
- Crystal structure of 3-(difluoromethyl)-1-methyl-N-(4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)-1H-pyrazole-4-carboxamide monohydrate, C23H26F2N4O3
- Crystal structure of 2-(4-bromobenzyloxy)-6-chloropyridine, C12H9BrClNO
- Crystal structure of N-(4-bromo-2,6-dichloro-phenyl)pyrazin-2-amine, C10H6BrCl2N3
- Crystal structure of (E)-1-(2–nitrophenyl)-3-phenylprop-2-en-1-one, C15H11NO3
- The crystal structure of (E)-3-chloro-2-(2-(4-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of (E)-amino(2-(thiazol-2-ylmethylene)hydrazineyl)methaniminium nitrate, C10H16N12O6S2
- Crystal structure of 9-methoxy-2,3,4,4a,5,6-hexahydro-1H-pyrido [1′,2′:1,6]pyrazino[2,3-b]quinoxaline, C15H18N4O
- The crystal structure bis(dimethylsulfoxide-κ1O)-dipyridine-κ1 N-bis(m2-(Z)-3-methyl-2-oxido-N-((Z)-oxido(phenyl)methylene)benzohydrazonato-κ5)trinickel(II) - dimethylsulfoxide (1/2), C48H56N6Ni3O10S4
- Crystal structure of bis(bis(triphenylphosphine)iminium) tetradecacarbonyltetratelluridopentaferrate(2-), (PPN)2[Fe5Te4(CO)14]
- Crystal structure of 4-Hydroxy-3-(naphthalen-2-ylthio)pent-3-en-2-one, C15H14O2S
- The crystal structure of [(1,10-phenanthroline-κ2 N,N)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)nickel(II)] monohydrate, C36H26N4O5Ni
- Crystal structure of 3,3′-(pyridine-2,6-diylbis(methylene))bis(1-propyl-1H-imidazol-3-ium) ditetrafluoroborate, C19H27B2F8N5
- The crystal structure of (E)-1-(4-aminophenyl)-3-(p-tolyl)prop-2-en-1-one, C16H15NO
- The crystal structure of poly[(μ2-terephthalato-κ4O,O′: O″,O‴)-(μ4-terephthalato-κ4O:O′:O″:O‴)-{μ4-(1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ4O:O′:O″,O‴)}dicadmium(II)] – water – acetronitrile (1/2/2), C38H36N14O10Cd2
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)cobalt(II)–water–N,N-dimethylformamide(1/2/1), C27H31N3O9Co
- The co-crystal structure of 4-hydroxy-3-methoxybenzoic acid – 4,4′-bipyridine, C8H8O4·C10H8N2
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)cobalt(II)], C32H22Br2CoN4O4
- Crystal structure of (E)-5-propyl-4-((pyridin-2-ylmethylene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione – methanol (1/1), C11H13N5S
- The crystal structure of (Z)-4-bromo-6-(((1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)amino)methylene)cyclohexa-2,4-dien-1-one monohydrate, C11H16BrNO5
- Crystal structure of bis(tetrapropylammonium) nonaselenidotetrastannate(IV), (Pr4N)2[Sn4Se9]
- Crystal structure of 2,6-di-tert-butyl-4-(4-chlorobenzylidene)cyclohexa-2,5-dien-1-one, C21H25ClO
- Crystal structure of (2,2′-((naphthalen-1-ylmethyl)azanediyl)diacetato-κ3 N,O,O′)-(1,10-phenanthroline-κ2 N,N′)-copper(II) trihydrate, CuC27H27N3O7
- The crystal structure of tetrakis(6-phenylpyridine-2-carboxylato-κ2N,O)-bis(1H-pyrazol-3-ylamine-κ2 N:N)dicobalt(II) dihydrate, C27H23N5O5Co
- The crystal structure of bis((E)-2-((tert-butylimino)methyl)-4-chlorophenolato-κ2N,O)zinc(II), C22H26Cl2N2O2Zn
- The crystal structure of poly[diaqua-(μ3-5-nitrobenzene-1,2,3-tricarboxylato-κ3O:O′:O′)-(μ2-4,4′-dipyridylamine-κ2N:N′)copper(II)], C38H30Cu3N8O20
- The crystal structure of (E)-1-ferrocenyl-3-(naphthalen-1-yl)prop-2-en-1-one, C23H18FeO
- The crystal structure of (E)-1-ferrocenyl-3-(4-isopropylphenyl)prop-2-en-1-one, C22H22FeO
- Crystal structure of 6-hydroxy-2,2-dimethyl-4Hbenzo[d][1,3]dioxin-4-one, C10H10O4
- The crystal structure of (2E,4E)-1-ferrocenyl-5-phenylpenta-2,4-dien-1-one, C21H18FeO
- Crystal structure of alaninato-κ2N,O-bis(hydroxylamido-κ2N,O)-oxido-vanadium(V), C3H10N3O5V
- Crystal structure of catena-poly[aqua-bis[μ2-6-(1H-imidazol-1-yl)nicotinato-κ2 N,O]copper(II)], C18H14N6O5Cu
- Crystal structure of diethyl 4,6-diphenyl-1,9-di-p-tolylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,5(2H)-dicarboxylate, C42H42N2O4
- The crystal structure of cobalt cadmium bis(hydrogenphosphate) bis(phosphate(V)) tetrahydrate, H10O20P4Co3.14Cd1.86
- Crystal structure of dimethyl 1,4,6,9-tetraphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,7(2H)-dicarboxylate, C38H34N2O4
- Crystal structure of (Z)-4-(furan-2-yl((4-iodophenyl)amino)methylene)-5-methyl-2(p-tolyl)-2,4-dihydro-3H-pyrazol-3-one, C21H16I N3O2
- Crystal structure of (E)-1-(4-(3,5-dimethoxystyryl)phenyl)-7-ethylheptanedioate, C25H30O6
- Crystal structure of 6-bromo-2-(4-chlorophenyl)chroman-4-one (6-bromo-4′-chloroflavanone), C15H10BrClO2
- The crystal structure of 2-(benzhydryloxy)-3-nitropyridine, C18H14N2O3
- The crystal structure of 1,3(4,1)-dipyridin-1-iuma-2(1,8)-diethynylanthracena-5(1,3)-benzenacyclohexaphane-11,31-diium bis(hexafluoridophosphate), C36H24F12N2P2
- Crystal structure of 3,6-di-tert-butyl-1-iodo-9-methyl-8-(pyren-1-ylethynyl)-9H-carbazole, C39H34IN
- The cocrystal 2-(dimethylammonio)-5-nitrobenzoate – 2-(dimethylamino)-5-nitrobenzoic acid, C9H10N2O4
- Crystal structure of 5-nitroquinazolin-4(3H)-one, C8H5N3O3

