Abstract
C16H15NO, monoclinic, P21/c (no. 14), a = 5.9233(3) Å, b = 14.5788(9) Å, c = 14.7095(9) Å, β = 99.073(2)°, V = 1254.34(13) Å3, Z = 4, Rgt (F) = 0.0600, wRref (F 2) = 0.1512, T = 170 K.
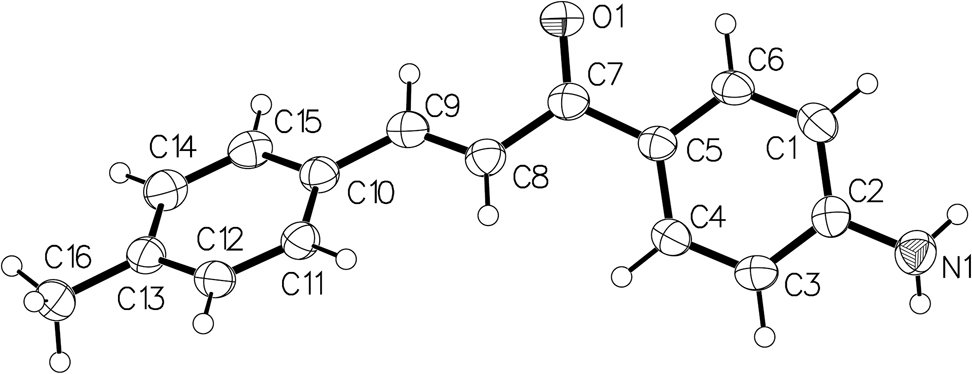
The molecular structure is shown in Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.15 × 0.08 × 0.05 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | D8 VENTURE, φ and ω |
| θ max, completeness: | 26.4°, 99% |
| N(hkl)measured, N(hkl)unique, R int: | 9288, 2525, 0.062 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 1561 |
| N(param)refined: | 165 |
| Programs: | Bruker [1], Olex2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.0665 (4) | 0.65406 (17) | 0.73260 (18) | 0.0368 (6) |
| H1 | −0.025558 | 0.686228 | 0.769335 | 0.044* |
| C2 | 0.2691 (4) | 0.69388 (17) | 0.71327 (17) | 0.0339 (6) |
| C3 | 0.4017 (4) | 0.64440 (17) | 0.65972 (18) | 0.0348 (6) |
| H3 | 0.538568 | 0.670646 | 0.645369 | 0.042* |
| C4 | 0.3368 (4) | 0.55821 (17) | 0.62747 (17) | 0.0342 (6) |
| H4 | 0.431085 | 0.525377 | 0.592139 | 0.041* |
| C5 | 0.1338 (4) | 0.51812 (16) | 0.64597 (16) | 0.0298 (6) |
| C6 | 0.0006 (4) | 0.56888 (17) | 0.69874 (18) | 0.0344 (6) |
| H6 | −0.138946 | 0.543599 | 0.711388 | 0.041* |
| C7 | 0.0561 (4) | 0.42645 (17) | 0.61382 (18) | 0.0337 (6) |
| C8 | 0.2148 (4) | 0.36410 (16) | 0.57528 (18) | 0.0350 (6) |
| H8 | 0.374422 | 0.375960 | 0.587377 | 0.042* |
| C9 | 0.1389 (4) | 0.29222 (17) | 0.52436 (18) | 0.0353 (6) |
| H9 | −0.022393 | 0.285782 | 0.509914 | 0.042* |
| C10 | 0.2762 (4) | 0.22144 (16) | 0.48778 (18) | 0.0330 (6) |
| C11 | 0.5047 (4) | 0.20595 (17) | 0.52362 (18) | 0.0344 (6) |
| H11 | 0.578029 | 0.243631 | 0.572153 | 0.041* |
| C12 | 0.6266 (4) | 0.13633 (17) | 0.48949 (18) | 0.0374 (6) |
| H12 | 0.781757 | 0.126501 | 0.515750 | 0.045* |
| C13 | 0.5266 (4) | 0.08044 (17) | 0.41751 (18) | 0.0362 (6) |
| C14 | 0.3003 (5) | 0.09620 (18) | 0.38163 (19) | 0.0407 (7) |
| H14 | 0.228638 | 0.059223 | 0.332185 | 0.049* |
| C15 | 0.1750 (4) | 0.16482 (18) | 0.41610 (18) | 0.0379 (6) |
| H15 | 0.018811 | 0.173386 | 0.390669 | 0.046* |
| C16 | 0.6631 (5) | 0.00734 (18) | 0.3791 (2) | 0.0466 (8) |
| H16A | 0.769847 | 0.035940 | 0.343057 | 0.070* |
| H16B | 0.559583 | −0.033613 | 0.339385 | 0.070* |
| H16C | 0.748961 | −0.028048 | 0.429739 | 0.070* |
| N1 | 0.3355 (4) | 0.77931 (14) | 0.74641 (16) | 0.0428 (6) |
| H1A | 0.410274 | 0.806343 | 0.706709 | 0.051* |
| H1B | 0.214020 | 0.812081 | 0.752672 | 0.051* |
| O1 | −0.1388 (3) | 0.39956 (12) | 0.62087 (14) | 0.0459 (5) |
Source of material
The substrate 4-methylbenzaldehyde (12 mmol) was initially added to 10 mL ethanol in a 100 mL three-mouth flask. Then 1-(4-aminophenyl)ethan-1-one (10 mmol) was added to the solution and stirred at room temperature until the reactants were mixed evenly. Subsequently, 10 mL potassium hydroxide solution (20%) was slowly added to the reaction mixture and continued stirring for 30 min with solid precipitates. After the disappearance of raw materials monitored by thin-layer chromatography (TLC), the reactants were poured into 50 mL water. The solids were filtered by suction and washed successively with 50 mL water and 50 mL 30% ethanol. The crystals of the title compound were obtained after further recrystallization.
Experimental details
All hydrogen atoms were included in the refinement in the riding model approximation. The U iso values of the hydrogen atoms of phenolic hydroxyl groups were set to 1.5U eq(C), and the U iso values of all other hydrogen atoms were arranged to 1.2U eq(C).
Comment
Chalcones are aromatic ketones whose configurations are α, β-unsaturated ketones substituted with diaryl groups [5], [6], [7]. They are widely distributed in nature and have various biological activities [8], [9], [10], [11]. Because of their multiple reaction centers, they can bind to a variety of receptors. They have many pharmacological properties, [12], [13], [14]. Furthermore chalcones serve as an essential intermediate in organic synthesis and novel drug discovery in medicinal chemistry. The research and development of chalcones have become a hot research field of pharmaceutical chemistry [15], [16], [17], and many relative structures were reported [18], [19], [20], [21]. chalcones can be extracted from natural products and synthesized by chemical and biological methods [5, 15, 17].
The asymmetric unit of the title structure consists of one (E)-1-(4-aminophenyl)-3-(p-tolyl)prop-2-en-1-one molecule. In the title compound, as displayed in the figure, a methyl group and an amino group replace the hydrogen atoms on the opposite positions of the two benzene rings in the chalcone structure, respectively. Among them, the bond length of C13–C16 is 1.501(4) Å, and the bond length of C2–N1 is 1.372(4) Å. The bond angles of C12–C13–C16 and C14–C13–C16 are 120.8(3)° and 121.5(3)°, respectively [22]. In addition, the angle in the C10⋯C15 ring is in the range of 117.7°–121.5°, and the bond angles at substitutions sites are the smallest, indicating that the substitution on the benzene ring reduces the bond angle. The dihedral angle between the C10⋯C15 ring plane and the ketone plane is 12.4°, and the dihedral angle between the C1⋯C6 benzene ring plane and the ketone plane is 26.0°. Weak NH⋯O hydrogen bonds connects neighboring molecules to form chains along [10].
Acknowledgements
This work was financially supported by the key scientific research projects of colleges and universities in Henan Province for financial support (22A430032).
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Key scientific research projects of colleges and universities in Henan Province for financial support (22A430032).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SMART APEX-II CCD; Bruker AXS Inc.: Madison, WI, USA, 2006.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. Olex2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar
5. Jasim, H. A., Nahar, L., Jasim, M. A., Moore, S. A., Ritchie, K. J., Sarker, S. D. Chalcones: synthetic chemistry follows where nature leads. Biomolecules 2021, 11, 1203; https://doi.org/10.3390/biom11081203.Search in Google Scholar
6. Elkhalifa, D., Al-Hashimi, I., Al Moustafa, A.-E., Khalil, A. A comprehensive review on the antiviral activities of chalcones. J. Drug Target. 2021, 29, 403–419; https://doi.org/10.1080/1061186x.2020.1853759.Search in Google Scholar
7. Adelusi, T. I., Akinbolaji, G. R., Yin, X., Ayinde, K. S., Olaoba, O. T. Neurotrophic, anti-neuroinflammatory, and redox balance mechanisms of chalcones. Eur. J. Pharmacol. 2021, 891, 173695; https://doi.org/10.1016/j.ejphar.2020.173695.Search in Google Scholar
8. Aktar, B. S. K., Sıcak, Y., Tok, T. T., Oruç-Emre, E. E., Yağlıoğlu, A. Ş., Iyidoğan, A. K., Öztürk, M., Demirtaş, I. Designing heterocyclic chalcones, benzoyl/sulfonyl hydrazones: an insight into their biological activities and molecular docking study. J. Mol. Struct. 2020, 1211, 128059; https://doi.org/10.1016/j.molstruc.2020.128059.Search in Google Scholar
9. Wang, D., Liang, J., Zhang, J., Wang, Y., Cai, X. Natural chalcones in Chinese materia medica: licorice. Evid.-Based Complement. Altern. Med. 2020, 2020, 3821248; https://doi.org/10.1155/2020/3821248.Search in Google Scholar
10. Rozmer, Z., Perjési, P. Naturally occurring chalcones and their biological activities. Phytochem. Rev. 2016, 15, 87–120; https://doi.org/10.1007/s11101-014-9387-8.Search in Google Scholar
11. Singh, P., Anand, A., Kumar, V. Recent developments in biological activities of chalcones: a mini review. Eur. J. Med. Chem. 2014, 85, 758–777; https://doi.org/10.1016/j.ejmech.2014.08.033.Search in Google Scholar
12. ur Rashid, H., Xu, Y., Ahmad, N., Muhammad, Y., Wang, L. Promising anti-inflammatory effects of chalcones via inhibition of cyclooxygenase, prostaglandin E2, inducible NO synthase and nuclear factor κb activities. Bioorg. Chem. 2019, 87, 335–365; https://doi.org/10.1016/j.bioorg.2019.03.033.Search in Google Scholar
13. Rocha, S., Ribeiro, D., Fernandes, E., Freitas, M. A systematic review on anti-diabetic properties of chalcones. Curr. Med. Chem. 2020, 27, 2257–2321; https://doi.org/10.2174/0929867325666181001112226.Search in Google Scholar
14. Sinha, S., Batovska, D. I., Medhi, B., Radotra, B., Bhalla, A., Markova, N., Sehgal, R. In vitro anti-malarial efficacy of chalcones: cytotoxicity profile, mechanism of action and their effect on erythrocytes. Malar. J. 2019, 18, 1–11; https://doi.org/10.1186/s12936-019-3060-z.Search in Google Scholar
15. Rammohan, A., Reddy, J. S., Sravya, G., Rao, C. N., Zyryanov, G. V. Chalcone synthesis, properties and medicinal applications: a review. Environ. Chem. Lett. 2020, 18, 433–458; https://doi.org/10.1007/s10311-019-00959-w.Search in Google Scholar
16. Lagu, S. B., Yejella, R. P., Bhandare, R. R., Shaik, A. B. Design, synthesis, and antibacterial and antifungal activities of novel trifluoromethyl and trifluoromethoxy substituted chalcone derivatives. Pharmaceuticals 2020, 13, 375; https://doi.org/10.3390/ph13110375.Search in Google Scholar
17. Goyal, K., Kaur, R., Goyal, A., Awasthi, R. Chalcones: a review on synthesis and pharmacological activities. J. Appl. Pharmaceut. Sci. 2021, 11, 1–14.Search in Google Scholar
18. Zaini, M. F., Arshad, S., Thanigaimani, K., Khalib, N. C., Zainuri, D. A., Abdullah, M., Razak, I. A. New halogenated chalcones: synthesis, crystal structure, spectroscopic and theoretical analyses for third-order nonlinear optical properties. J. Mol. Struct. 2019, 1195, 606–619; https://doi.org/10.1016/j.molstruc.2019.05.122.Search in Google Scholar
19. Hall, C. L., Guo, R., Potticary, J., Cremeens, M. E., Warren, S. D., Andrusenko, I., Gemmi, M., Zwijnenburg, M. A., Sparkes, H. A., Pridmore, N. E. Color differences highlight concomitant polymorphism of chalcones. Cryst. Growth Des. 2020, 20, 6346–6355; https://doi.org/10.1021/acs.cgd.0c00285.Search in Google Scholar
20. Asad, M., Arshad, M. N., Khan, S. A., Oves, M., Khalid, M., Asiri, A. M., Braga, A. A. Cyclization of chalcones into N-propionyl pyrazolines for their single crystal X-ray, computational and antibacterial studies. J. Mol. Struct. 2020, 1201, 127186; https://doi.org/10.1016/j.molstruc.2019.127186.Search in Google Scholar
21. Wang, C., Wang, L., Meng, Q.-G., Huang, Z.-X., Ma, N.-N., Wang, C.-H. Crystal structure of (E)-2-((3-fluoropyridin-4-yl)methylene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 1073–1075; https://doi.org/10.1515/ncrs-2021-0223.Search in Google Scholar
22. Fun, H.-K., Chantrapromma, S., Patil, P. S., Silva, E. D., Dharmaprakash, S. M. (E)-3-(4-Methylphenyl)-1-(4-nitrophenyl)prop-2-en-1-one. Acta Crystallogr. 2008, E64, o954–o955; https://doi.org/10.1107/s1600536808012257.Search in Google Scholar
© 2022 Jingxiao Zhang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-(4-imidazol-1-yl-phenyl)-(2-methoxy-benzylidene)-amine monohydrate, C17H17N3O2
- Crystal structure of 6-methyl-3-(pyrrolidine-1-carbonyl)-2H-chromen-2-one, C15H15N1O3
- Crystal structure of 4-methyl-4-nitropentanoic acid, C6H11NO4
- The crystal structure of (E)-3-(furan-2-yl)acrylonitrile, C7H5NO
- Crystal structure of 3-(difluoromethyl)-1-methyl-N-(4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)-1H-pyrazole-4-carboxamide monohydrate, C23H26F2N4O3
- Crystal structure of 2-(4-bromobenzyloxy)-6-chloropyridine, C12H9BrClNO
- Crystal structure of N-(4-bromo-2,6-dichloro-phenyl)pyrazin-2-amine, C10H6BrCl2N3
- Crystal structure of (E)-1-(2–nitrophenyl)-3-phenylprop-2-en-1-one, C15H11NO3
- The crystal structure of (E)-3-chloro-2-(2-(4-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of (E)-amino(2-(thiazol-2-ylmethylene)hydrazineyl)methaniminium nitrate, C10H16N12O6S2
- Crystal structure of 9-methoxy-2,3,4,4a,5,6-hexahydro-1H-pyrido [1′,2′:1,6]pyrazino[2,3-b]quinoxaline, C15H18N4O
- The crystal structure bis(dimethylsulfoxide-κ1O)-dipyridine-κ1 N-bis(m2-(Z)-3-methyl-2-oxido-N-((Z)-oxido(phenyl)methylene)benzohydrazonato-κ5)trinickel(II) - dimethylsulfoxide (1/2), C48H56N6Ni3O10S4
- Crystal structure of bis(bis(triphenylphosphine)iminium) tetradecacarbonyltetratelluridopentaferrate(2-), (PPN)2[Fe5Te4(CO)14]
- Crystal structure of 4-Hydroxy-3-(naphthalen-2-ylthio)pent-3-en-2-one, C15H14O2S
- The crystal structure of [(1,10-phenanthroline-κ2 N,N)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)nickel(II)] monohydrate, C36H26N4O5Ni
- Crystal structure of 3,3′-(pyridine-2,6-diylbis(methylene))bis(1-propyl-1H-imidazol-3-ium) ditetrafluoroborate, C19H27B2F8N5
- The crystal structure of (E)-1-(4-aminophenyl)-3-(p-tolyl)prop-2-en-1-one, C16H15NO
- The crystal structure of poly[(μ2-terephthalato-κ4O,O′: O″,O‴)-(μ4-terephthalato-κ4O:O′:O″:O‴)-{μ4-(1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ4O:O′:O″,O‴)}dicadmium(II)] – water – acetronitrile (1/2/2), C38H36N14O10Cd2
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)cobalt(II)–water–N,N-dimethylformamide(1/2/1), C27H31N3O9Co
- The co-crystal structure of 4-hydroxy-3-methoxybenzoic acid – 4,4′-bipyridine, C8H8O4·C10H8N2
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)cobalt(II)], C32H22Br2CoN4O4
- Crystal structure of (E)-5-propyl-4-((pyridin-2-ylmethylene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione – methanol (1/1), C11H13N5S
- The crystal structure of (Z)-4-bromo-6-(((1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)amino)methylene)cyclohexa-2,4-dien-1-one monohydrate, C11H16BrNO5
- Crystal structure of bis(tetrapropylammonium) nonaselenidotetrastannate(IV), (Pr4N)2[Sn4Se9]
- Crystal structure of 2,6-di-tert-butyl-4-(4-chlorobenzylidene)cyclohexa-2,5-dien-1-one, C21H25ClO
- Crystal structure of (2,2′-((naphthalen-1-ylmethyl)azanediyl)diacetato-κ3 N,O,O′)-(1,10-phenanthroline-κ2 N,N′)-copper(II) trihydrate, CuC27H27N3O7
- The crystal structure of tetrakis(6-phenylpyridine-2-carboxylato-κ2N,O)-bis(1H-pyrazol-3-ylamine-κ2 N:N)dicobalt(II) dihydrate, C27H23N5O5Co
- The crystal structure of bis((E)-2-((tert-butylimino)methyl)-4-chlorophenolato-κ2N,O)zinc(II), C22H26Cl2N2O2Zn
- The crystal structure of poly[diaqua-(μ3-5-nitrobenzene-1,2,3-tricarboxylato-κ3O:O′:O′)-(μ2-4,4′-dipyridylamine-κ2N:N′)copper(II)], C38H30Cu3N8O20
- The crystal structure of (E)-1-ferrocenyl-3-(naphthalen-1-yl)prop-2-en-1-one, C23H18FeO
- The crystal structure of (E)-1-ferrocenyl-3-(4-isopropylphenyl)prop-2-en-1-one, C22H22FeO
- Crystal structure of 6-hydroxy-2,2-dimethyl-4Hbenzo[d][1,3]dioxin-4-one, C10H10O4
- The crystal structure of (2E,4E)-1-ferrocenyl-5-phenylpenta-2,4-dien-1-one, C21H18FeO
- Crystal structure of alaninato-κ2N,O-bis(hydroxylamido-κ2N,O)-oxido-vanadium(V), C3H10N3O5V
- Crystal structure of catena-poly[aqua-bis[μ2-6-(1H-imidazol-1-yl)nicotinato-κ2 N,O]copper(II)], C18H14N6O5Cu
- Crystal structure of diethyl 4,6-diphenyl-1,9-di-p-tolylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,5(2H)-dicarboxylate, C42H42N2O4
- The crystal structure of cobalt cadmium bis(hydrogenphosphate) bis(phosphate(V)) tetrahydrate, H10O20P4Co3.14Cd1.86
- Crystal structure of dimethyl 1,4,6,9-tetraphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,7(2H)-dicarboxylate, C38H34N2O4
- Crystal structure of (Z)-4-(furan-2-yl((4-iodophenyl)amino)methylene)-5-methyl-2(p-tolyl)-2,4-dihydro-3H-pyrazol-3-one, C21H16I N3O2
- Crystal structure of (E)-1-(4-(3,5-dimethoxystyryl)phenyl)-7-ethylheptanedioate, C25H30O6
- Crystal structure of 6-bromo-2-(4-chlorophenyl)chroman-4-one (6-bromo-4′-chloroflavanone), C15H10BrClO2
- The crystal structure of 2-(benzhydryloxy)-3-nitropyridine, C18H14N2O3
- The crystal structure of 1,3(4,1)-dipyridin-1-iuma-2(1,8)-diethynylanthracena-5(1,3)-benzenacyclohexaphane-11,31-diium bis(hexafluoridophosphate), C36H24F12N2P2
- Crystal structure of 3,6-di-tert-butyl-1-iodo-9-methyl-8-(pyren-1-ylethynyl)-9H-carbazole, C39H34IN
- The cocrystal 2-(dimethylammonio)-5-nitrobenzoate – 2-(dimethylamino)-5-nitrobenzoic acid, C9H10N2O4
- Crystal structure of 5-nitroquinazolin-4(3H)-one, C8H5N3O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-(4-imidazol-1-yl-phenyl)-(2-methoxy-benzylidene)-amine monohydrate, C17H17N3O2
- Crystal structure of 6-methyl-3-(pyrrolidine-1-carbonyl)-2H-chromen-2-one, C15H15N1O3
- Crystal structure of 4-methyl-4-nitropentanoic acid, C6H11NO4
- The crystal structure of (E)-3-(furan-2-yl)acrylonitrile, C7H5NO
- Crystal structure of 3-(difluoromethyl)-1-methyl-N-(4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)-1H-pyrazole-4-carboxamide monohydrate, C23H26F2N4O3
- Crystal structure of 2-(4-bromobenzyloxy)-6-chloropyridine, C12H9BrClNO
- Crystal structure of N-(4-bromo-2,6-dichloro-phenyl)pyrazin-2-amine, C10H6BrCl2N3
- Crystal structure of (E)-1-(2–nitrophenyl)-3-phenylprop-2-en-1-one, C15H11NO3
- The crystal structure of (E)-3-chloro-2-(2-(4-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of (E)-amino(2-(thiazol-2-ylmethylene)hydrazineyl)methaniminium nitrate, C10H16N12O6S2
- Crystal structure of 9-methoxy-2,3,4,4a,5,6-hexahydro-1H-pyrido [1′,2′:1,6]pyrazino[2,3-b]quinoxaline, C15H18N4O
- The crystal structure bis(dimethylsulfoxide-κ1O)-dipyridine-κ1 N-bis(m2-(Z)-3-methyl-2-oxido-N-((Z)-oxido(phenyl)methylene)benzohydrazonato-κ5)trinickel(II) - dimethylsulfoxide (1/2), C48H56N6Ni3O10S4
- Crystal structure of bis(bis(triphenylphosphine)iminium) tetradecacarbonyltetratelluridopentaferrate(2-), (PPN)2[Fe5Te4(CO)14]
- Crystal structure of 4-Hydroxy-3-(naphthalen-2-ylthio)pent-3-en-2-one, C15H14O2S
- The crystal structure of [(1,10-phenanthroline-κ2 N,N)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)nickel(II)] monohydrate, C36H26N4O5Ni
- Crystal structure of 3,3′-(pyridine-2,6-diylbis(methylene))bis(1-propyl-1H-imidazol-3-ium) ditetrafluoroborate, C19H27B2F8N5
- The crystal structure of (E)-1-(4-aminophenyl)-3-(p-tolyl)prop-2-en-1-one, C16H15NO
- The crystal structure of poly[(μ2-terephthalato-κ4O,O′: O″,O‴)-(μ4-terephthalato-κ4O:O′:O″:O‴)-{μ4-(1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ4O:O′:O″,O‴)}dicadmium(II)] – water – acetronitrile (1/2/2), C38H36N14O10Cd2
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)cobalt(II)–water–N,N-dimethylformamide(1/2/1), C27H31N3O9Co
- The co-crystal structure of 4-hydroxy-3-methoxybenzoic acid – 4,4′-bipyridine, C8H8O4·C10H8N2
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)cobalt(II)], C32H22Br2CoN4O4
- Crystal structure of (E)-5-propyl-4-((pyridin-2-ylmethylene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione – methanol (1/1), C11H13N5S
- The crystal structure of (Z)-4-bromo-6-(((1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)amino)methylene)cyclohexa-2,4-dien-1-one monohydrate, C11H16BrNO5
- Crystal structure of bis(tetrapropylammonium) nonaselenidotetrastannate(IV), (Pr4N)2[Sn4Se9]
- Crystal structure of 2,6-di-tert-butyl-4-(4-chlorobenzylidene)cyclohexa-2,5-dien-1-one, C21H25ClO
- Crystal structure of (2,2′-((naphthalen-1-ylmethyl)azanediyl)diacetato-κ3 N,O,O′)-(1,10-phenanthroline-κ2 N,N′)-copper(II) trihydrate, CuC27H27N3O7
- The crystal structure of tetrakis(6-phenylpyridine-2-carboxylato-κ2N,O)-bis(1H-pyrazol-3-ylamine-κ2 N:N)dicobalt(II) dihydrate, C27H23N5O5Co
- The crystal structure of bis((E)-2-((tert-butylimino)methyl)-4-chlorophenolato-κ2N,O)zinc(II), C22H26Cl2N2O2Zn
- The crystal structure of poly[diaqua-(μ3-5-nitrobenzene-1,2,3-tricarboxylato-κ3O:O′:O′)-(μ2-4,4′-dipyridylamine-κ2N:N′)copper(II)], C38H30Cu3N8O20
- The crystal structure of (E)-1-ferrocenyl-3-(naphthalen-1-yl)prop-2-en-1-one, C23H18FeO
- The crystal structure of (E)-1-ferrocenyl-3-(4-isopropylphenyl)prop-2-en-1-one, C22H22FeO
- Crystal structure of 6-hydroxy-2,2-dimethyl-4Hbenzo[d][1,3]dioxin-4-one, C10H10O4
- The crystal structure of (2E,4E)-1-ferrocenyl-5-phenylpenta-2,4-dien-1-one, C21H18FeO
- Crystal structure of alaninato-κ2N,O-bis(hydroxylamido-κ2N,O)-oxido-vanadium(V), C3H10N3O5V
- Crystal structure of catena-poly[aqua-bis[μ2-6-(1H-imidazol-1-yl)nicotinato-κ2 N,O]copper(II)], C18H14N6O5Cu
- Crystal structure of diethyl 4,6-diphenyl-1,9-di-p-tolylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,5(2H)-dicarboxylate, C42H42N2O4
- The crystal structure of cobalt cadmium bis(hydrogenphosphate) bis(phosphate(V)) tetrahydrate, H10O20P4Co3.14Cd1.86
- Crystal structure of dimethyl 1,4,6,9-tetraphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,7(2H)-dicarboxylate, C38H34N2O4
- Crystal structure of (Z)-4-(furan-2-yl((4-iodophenyl)amino)methylene)-5-methyl-2(p-tolyl)-2,4-dihydro-3H-pyrazol-3-one, C21H16I N3O2
- Crystal structure of (E)-1-(4-(3,5-dimethoxystyryl)phenyl)-7-ethylheptanedioate, C25H30O6
- Crystal structure of 6-bromo-2-(4-chlorophenyl)chroman-4-one (6-bromo-4′-chloroflavanone), C15H10BrClO2
- The crystal structure of 2-(benzhydryloxy)-3-nitropyridine, C18H14N2O3
- The crystal structure of 1,3(4,1)-dipyridin-1-iuma-2(1,8)-diethynylanthracena-5(1,3)-benzenacyclohexaphane-11,31-diium bis(hexafluoridophosphate), C36H24F12N2P2
- Crystal structure of 3,6-di-tert-butyl-1-iodo-9-methyl-8-(pyren-1-ylethynyl)-9H-carbazole, C39H34IN
- The cocrystal 2-(dimethylammonio)-5-nitrobenzoate – 2-(dimethylamino)-5-nitrobenzoic acid, C9H10N2O4
- Crystal structure of 5-nitroquinazolin-4(3H)-one, C8H5N3O3

