Abstract
C38H30Cu3N8O20, triclinic,
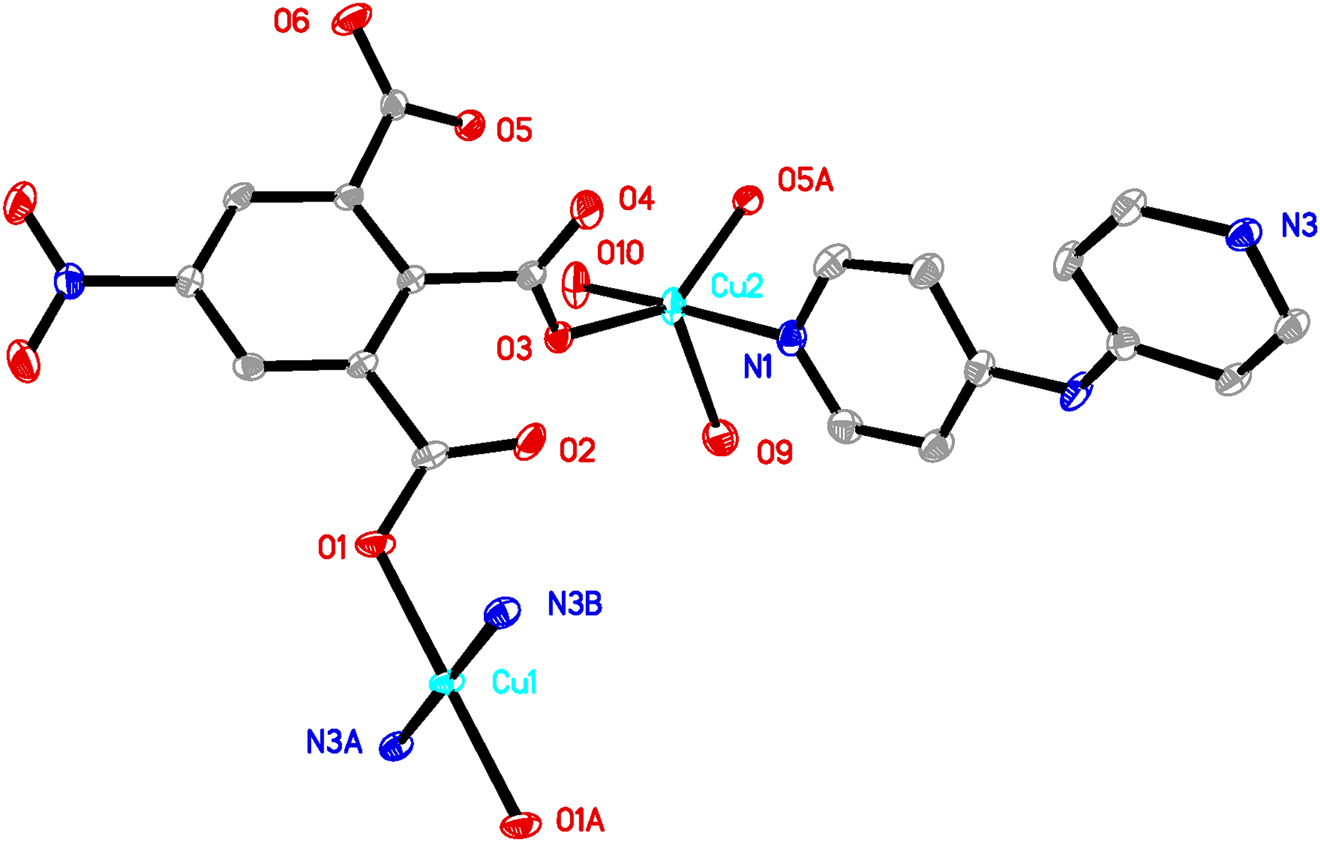
A part of the polymeric title crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Violet block |
| Size: | 0.32 × 0.27 × 0.23 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.69 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 25.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 12,847, 3694, 0.027 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3279 |
| N(param)refined: | 315 |
| Programs: | CrysAlisPRO [1], SHELX [2, 3], Olex2 [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Cu1 | 0.98971 (5) | 0.46012 (3) | 0.67007 (3) | 0.02904 (12) |
| Cu2 | 0.5000 | 0.0000 | 1.0000 | 0.02065 (13) |
| O1 | 0.8797 (3) | 0.32333 (16) | 0.67205 (15) | 0.0266 (4) |
| O2 | 0.6156 (4) | 0.42361 (19) | 0.60852 (19) | 0.0476 (6) |
| O3 | 0.5864 (3) | 0.02127 (17) | 0.83849 (14) | 0.0299 (5) |
| O4 | 0.5375 (3) | 0.22050 (18) | 0.81274 (15) | 0.0314 (5) |
| O5 | 0.9523 (3) | 0.38562 (16) | 0.41512 (15) | 0.0284 (4) |
| O6 | 0.8209 (4) | 0.3406 (2) | 0.29458 (17) | 0.0493 (7) |
| O7 | 1.2280 (3) | 0.38774 (19) | 0.56804 (19) | 0.0437 (6) |
| H7A | 1.2557 | 0.4372 | 0.5075 | 0.066* |
| H7B | 1.2058 | 0.3289 | 0.5459 | 0.066* |
| O8 | 1.2015 (3) | 0.4228 (2) | 0.79244 (18) | 0.0424 (5) |
| H8A | 1.2484 | 0.4838 | 0.7880 | 0.064* |
| H8B | 1.3028 | 0.3707 | 0.7722 | 0.064* |
| O9 | 0.7941 (4) | −0.0814 (2) | 0.35523 (17) | 0.0445 (6) |
| O10 | 0.7030 (4) | −0.17260 (19) | 0.52149 (19) | 0.0462 (6) |
| N1 | 0.7795 (3) | 0.5413 (2) | 0.78330 (19) | 0.0282 (5) |
| N2 | −0.2345 (3) | 0.9066 (2) | 1.00702 (17) | 0.0231 (5) |
| N3 | 0.3448 (3) | 0.7372 (2) | 1.0063 (2) | 0.0343 (6) |
| H3 | 0.3812 | 0.7448 | 1.0630 | 0.041* |
| N4 | 0.7493 (3) | −0.0857 (2) | 0.4555 (2) | 0.0281 (5) |
| C1 | 0.7399 (4) | 0.3342 (2) | 0.6261 (2) | 0.0253 (6) |
| C2 | 0.7378 (4) | 0.2217 (2) | 0.5839 (2) | 0.0194 (5) |
| C3 | 0.6750 (4) | 0.1219 (2) | 0.6555 (2) | 0.0197 (5) |
| C4 | 0.6822 (4) | 0.0202 (2) | 0.6141 (2) | 0.0216 (5) |
| H4 | 0.6445 | −0.0468 | 0.6618 | 0.026* |
| C5 | 0.7465 (4) | 0.0202 (2) | 0.5004 (2) | 0.0215 (5) |
| C6 | 0.8026 (4) | 0.1179 (2) | 0.4281 (2) | 0.0228 (6) |
| H6 | 0.8415 | 0.1166 | 0.3520 | 0.027* |
| C7 | 0.8007 (4) | 0.2188 (2) | 0.4695 (2) | 0.0210 (5) |
| C8 | 0.5927 (4) | 0.1239 (3) | 0.7786 (2) | 0.0228 (6) |
| C9 | 0.8624 (4) | 0.3236 (2) | 0.3851 (2) | 0.0247 (6) |
| C10 | 0.7851 (4) | 0.5321 (3) | 0.8903 (2) | 0.0326 (7) |
| H10 | 0.8881 | 0.4793 | 0.9154 | 0.039* |
| C11 | 0.6457 (4) | 0.5972 (3) | 0.9636 (2) | 0.0331 (7) |
| H11 | 0.6560 | 0.5884 | 1.0366 | 0.040* |
| C12 | 0.4887 (4) | 0.6763 (3) | 0.9289 (2) | 0.0274 (6) |
| C13 | 0.4908 (4) | 0.6914 (3) | 0.8165 (2) | 0.0340 (7) |
| H13 | 0.3960 | 0.7485 | 0.7875 | 0.041* |
| C14 | 0.6340 (4) | 0.6215 (3) | 0.7492 (2) | 0.0351 (7) |
| H14 | 0.6294 | 0.6305 | 0.6752 | 0.042* |
| C15 | 0.1519 (4) | 0.7872 (2) | 1.0055 (2) | 0.0260 (6) |
| C16 | 0.0599 (4) | 0.7793 (3) | 0.9251 (2) | 0.0296 (6) |
| H16 | 0.1253 | 0.7332 | 0.8697 | 0.036* |
| C17 | −0.1286 (4) | 0.8405 (3) | 0.9287 (2) | 0.0275 (6) |
| H17 | −0.1863 | 0.8357 | 0.8733 | 0.033* |
| C18 | −0.1501 (4) | 0.9055 (2) | 1.0895 (2) | 0.0251 (6) |
| H18 | −0.2234 | 0.9456 | 1.1480 | 0.030* |
| C19 | 0.0376 (4) | 0.8485 (3) | 1.0919 (2) | 0.0267 (6) |
| H19 | 0.0888 | 0.8505 | 1.1508 | 0.032* |
Source of material
All chemicals were used without further purification. The title compound was prepared under the hydrothermal conditions by the following procedure: a mixture of 5-nitro-1,2,3-benzenetricarboxylic acid (0.1 mmol, 0.026 g), Cu(OAc)2 ·H2O (0.1 mmol, 0.020 g), 4,4′-dipyridylamine (0.1 mmol, 0.017 g), and deionized water (6 mL) was sealed in a 25 mL Teflon-lined stainless steel vessel and heated at 413 K for three days. After cooling to room temperature at a rate of 5 Kh−1, purple block crystals were collected by filtration and washed with distilled water in 37% yield (based on Cu).
Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms.
Comment
The design and construction of coordination polymers is of current interest in the fields of crystal engineering and supramolecular chemistry, not only for their structural diversities and intriguing topologies, but also for their applications as functional materials [5], [6], [7], [8]. In the processes of synthesizing desired coordination polymers, the choice of organic ligands, central metal ions, the temperature, the ratio of solvent, and counterions are important factors in construction of target coordination polymers. It is worth mentioned that aromatic multicarboxylates such as 1,3-benzenedicarboxylate, 1,4-benzenedicarboxylate, 1,3,5-benzenetricarboxylic acid, and 5-nitro-1,2,3-benzenetricarboxylate (nbta) as organic ligands have been widely used to construct various coordination polymers [9], [10], [11], [12]. On the other hand, 4,4′-dipyridylamine (dpa), as a flexible dipyridyl coligand, has attracted significant attention during the construction of coordination polymers [13], [14], [15]. Here, we present a new Cu(II) coordination polymer based on nbta and dpa.
The title compound was prepared under mild hydrothermal conditions. Single crystal X-ray structural analysis shows that the compound is a layered structure and crystallizes in the triclinic space group
Funding source: Luoyang Normal University
Award Identifier / Grant number: (DT2100009147)
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the grants from Luoyang Normal University (DT2100009147).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Agilent Technologies. CrysAlisPRO Software system (version 1.171.39.46); Agilent Technologies UK Ltd: Oxford, UK, 2018.Suche in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
5. Mohamedally, K. Magnetic metal-organic frameworks. Chem. Soc. Rev. 2009, 8, 1353–1379.10.1039/b804757jSuche in Google Scholar
6. Silva, P., Vilela, S. M., Tomé, J. P., Paz, F. A. A multifunctional metal–organic frameworks: from academia to industrial applications. Chem. Soc. Rev. 2015, 44, 6774–6803; https://doi.org/10.1039/c5cs00307e.Suche in Google Scholar
7. Wang, Z., Hu, B., Qi, X., Shen, N., Huang, X. Microwave-assisted ionothermal synthesis of a water-stable Eu-coordination polymer: Ba2+ ion detector and fluorescence thermometer. Dalton Trans. 2016, 45, 8745–8752; https://doi.org/10.1039/c6dt00641h.Suche in Google Scholar
8. Wu, Z., Tan, B., Wang, J., Du, C., Deng, Z., Huang, X. Tunable photoluminescence and direct white-light emission in Mg-based coordination networks. Chem. Commun. 2015, 51, 157–160; https://doi.org/10.1039/c4cc07634f.Suche in Google Scholar
9. Zhu, X., Wang, N., Luo, Y., Pang, Y., Tian, D., Zhang, H. Three novel polymeric CoII/CuII complexes assembled from 5-nitro-1,2,3-benzenetricarboxylate and 4,4′-bipyridine: syntheses, crystal structures, and magnetic properties. Aust. J. Chem. 2011, 64, 1346–1354; https://doi.org/10.1071/ch10431.Suche in Google Scholar
10. Shi, C., Wang, Z., Chen, Y., Zhang, X., Zhao, Y., Tao, Y., Wu, H. Structural diversity of four coordination polymers based on 5-nitro-1,2,3-benzenetricarboxylic acid (H3nbta): solvothermal syntheses, structural characterizations and properties. J. Solid State Chem. 2017, 253, 35–42; https://doi.org/10.1016/j.jssc.2017.05.010.Suche in Google Scholar
11. Wang, X., Li, Z., Yu, B., Van Hecke, K., Cui, G. Synthesis and characterizations of a bis(triazole)-based 3D crystalline copper(II) MOF with high adsorption capacity for congo red dye. Inorg. Chem. Commun. 2015, 54, 9–11; https://doi.org/10.1016/j.inoche.2015.01.030.Suche in Google Scholar
12. He, J., Zhang, Y., Pan, Q., Yu, J., Ding, H., Xu, R. Three metal-organic frameworks prepared from mixed solvents of DMF and HAc. Microporous Mesoporous Mater. 2006, 90, 145–152; https://doi.org/10.1016/j.micromeso.2005.11.049.Suche in Google Scholar
13. Cordes, D. B., Hanton, L. R., Spicer, M. D. Helices versus zigzag chains: one-dimensional coordination polymers of AgI and bis(4-pyridyl)amine. Inorg. Chem. 2006, 45, 7651–7664; https://doi.org/10.1021/ic060487y.Suche in Google Scholar
14. Krishnan, S. M., Montney, M. R., LaDuca, R. L. Two-dimensional divalent metal/pimelate coordination polymers incorporating dipodal organodiimines: crystal structures, thermal properties, and magnetic studies. Polyhedron 2008, 27, 821–834; https://doi.org/10.1016/j.poly.2007.11.006.Suche in Google Scholar
15. Lucas, J. S., Bell, L. D., Gandolfo, C. M., LaDuca, R. L. Substituent dependent dimensionalities in cobalt isophthalate supramolecular complexes and coordination polymers containing dipyridylamine ligands. Inorg. Chim. Acta 2011, 378, 269–279; https://doi.org/10.1016/j.ica.2011.09.016.Suche in Google Scholar
© 2022 Dong-Feng Hong et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-(4-imidazol-1-yl-phenyl)-(2-methoxy-benzylidene)-amine monohydrate, C17H17N3O2
- Crystal structure of 6-methyl-3-(pyrrolidine-1-carbonyl)-2H-chromen-2-one, C15H15N1O3
- Crystal structure of 4-methyl-4-nitropentanoic acid, C6H11NO4
- The crystal structure of (E)-3-(furan-2-yl)acrylonitrile, C7H5NO
- Crystal structure of 3-(difluoromethyl)-1-methyl-N-(4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)-1H-pyrazole-4-carboxamide monohydrate, C23H26F2N4O3
- Crystal structure of 2-(4-bromobenzyloxy)-6-chloropyridine, C12H9BrClNO
- Crystal structure of N-(4-bromo-2,6-dichloro-phenyl)pyrazin-2-amine, C10H6BrCl2N3
- Crystal structure of (E)-1-(2–nitrophenyl)-3-phenylprop-2-en-1-one, C15H11NO3
- The crystal structure of (E)-3-chloro-2-(2-(4-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of (E)-amino(2-(thiazol-2-ylmethylene)hydrazineyl)methaniminium nitrate, C10H16N12O6S2
- Crystal structure of 9-methoxy-2,3,4,4a,5,6-hexahydro-1H-pyrido [1′,2′:1,6]pyrazino[2,3-b]quinoxaline, C15H18N4O
- The crystal structure bis(dimethylsulfoxide-κ1O)-dipyridine-κ1 N-bis(m2-(Z)-3-methyl-2-oxido-N-((Z)-oxido(phenyl)methylene)benzohydrazonato-κ5)trinickel(II) - dimethylsulfoxide (1/2), C48H56N6Ni3O10S4
- Crystal structure of bis(bis(triphenylphosphine)iminium) tetradecacarbonyltetratelluridopentaferrate(2-), (PPN)2[Fe5Te4(CO)14]
- Crystal structure of 4-Hydroxy-3-(naphthalen-2-ylthio)pent-3-en-2-one, C15H14O2S
- The crystal structure of [(1,10-phenanthroline-κ2 N,N)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)nickel(II)] monohydrate, C36H26N4O5Ni
- Crystal structure of 3,3′-(pyridine-2,6-diylbis(methylene))bis(1-propyl-1H-imidazol-3-ium) ditetrafluoroborate, C19H27B2F8N5
- The crystal structure of (E)-1-(4-aminophenyl)-3-(p-tolyl)prop-2-en-1-one, C16H15NO
- The crystal structure of poly[(μ2-terephthalato-κ4O,O′: O″,O‴)-(μ4-terephthalato-κ4O:O′:O″:O‴)-{μ4-(1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ4O:O′:O″,O‴)}dicadmium(II)] – water – acetronitrile (1/2/2), C38H36N14O10Cd2
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)cobalt(II)–water–N,N-dimethylformamide(1/2/1), C27H31N3O9Co
- The co-crystal structure of 4-hydroxy-3-methoxybenzoic acid – 4,4′-bipyridine, C8H8O4·C10H8N2
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)cobalt(II)], C32H22Br2CoN4O4
- Crystal structure of (E)-5-propyl-4-((pyridin-2-ylmethylene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione – methanol (1/1), C11H13N5S
- The crystal structure of (Z)-4-bromo-6-(((1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)amino)methylene)cyclohexa-2,4-dien-1-one monohydrate, C11H16BrNO5
- Crystal structure of bis(tetrapropylammonium) nonaselenidotetrastannate(IV), (Pr4N)2[Sn4Se9]
- Crystal structure of 2,6-di-tert-butyl-4-(4-chlorobenzylidene)cyclohexa-2,5-dien-1-one, C21H25ClO
- Crystal structure of (2,2′-((naphthalen-1-ylmethyl)azanediyl)diacetato-κ3 N,O,O′)-(1,10-phenanthroline-κ2 N,N′)-copper(II) trihydrate, CuC27H27N3O7
- The crystal structure of tetrakis(6-phenylpyridine-2-carboxylato-κ2N,O)-bis(1H-pyrazol-3-ylamine-κ2 N:N)dicobalt(II) dihydrate, C27H23N5O5Co
- The crystal structure of bis((E)-2-((tert-butylimino)methyl)-4-chlorophenolato-κ2N,O)zinc(II), C22H26Cl2N2O2Zn
- The crystal structure of poly[diaqua-(μ3-5-nitrobenzene-1,2,3-tricarboxylato-κ3O:O′:O′)-(μ2-4,4′-dipyridylamine-κ2N:N′)copper(II)], C38H30Cu3N8O20
- The crystal structure of (E)-1-ferrocenyl-3-(naphthalen-1-yl)prop-2-en-1-one, C23H18FeO
- The crystal structure of (E)-1-ferrocenyl-3-(4-isopropylphenyl)prop-2-en-1-one, C22H22FeO
- Crystal structure of 6-hydroxy-2,2-dimethyl-4Hbenzo[d][1,3]dioxin-4-one, C10H10O4
- The crystal structure of (2E,4E)-1-ferrocenyl-5-phenylpenta-2,4-dien-1-one, C21H18FeO
- Crystal structure of alaninato-κ2N,O-bis(hydroxylamido-κ2N,O)-oxido-vanadium(V), C3H10N3O5V
- Crystal structure of catena-poly[aqua-bis[μ2-6-(1H-imidazol-1-yl)nicotinato-κ2 N,O]copper(II)], C18H14N6O5Cu
- Crystal structure of diethyl 4,6-diphenyl-1,9-di-p-tolylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,5(2H)-dicarboxylate, C42H42N2O4
- The crystal structure of cobalt cadmium bis(hydrogenphosphate) bis(phosphate(V)) tetrahydrate, H10O20P4Co3.14Cd1.86
- Crystal structure of dimethyl 1,4,6,9-tetraphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,7(2H)-dicarboxylate, C38H34N2O4
- Crystal structure of (Z)-4-(furan-2-yl((4-iodophenyl)amino)methylene)-5-methyl-2(p-tolyl)-2,4-dihydro-3H-pyrazol-3-one, C21H16I N3O2
- Crystal structure of (E)-1-(4-(3,5-dimethoxystyryl)phenyl)-7-ethylheptanedioate, C25H30O6
- Crystal structure of 6-bromo-2-(4-chlorophenyl)chroman-4-one (6-bromo-4′-chloroflavanone), C15H10BrClO2
- The crystal structure of 2-(benzhydryloxy)-3-nitropyridine, C18H14N2O3
- The crystal structure of 1,3(4,1)-dipyridin-1-iuma-2(1,8)-diethynylanthracena-5(1,3)-benzenacyclohexaphane-11,31-diium bis(hexafluoridophosphate), C36H24F12N2P2
- Crystal structure of 3,6-di-tert-butyl-1-iodo-9-methyl-8-(pyren-1-ylethynyl)-9H-carbazole, C39H34IN
- The cocrystal 2-(dimethylammonio)-5-nitrobenzoate – 2-(dimethylamino)-5-nitrobenzoic acid, C9H10N2O4
- Crystal structure of 5-nitroquinazolin-4(3H)-one, C8H5N3O3
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-(4-imidazol-1-yl-phenyl)-(2-methoxy-benzylidene)-amine monohydrate, C17H17N3O2
- Crystal structure of 6-methyl-3-(pyrrolidine-1-carbonyl)-2H-chromen-2-one, C15H15N1O3
- Crystal structure of 4-methyl-4-nitropentanoic acid, C6H11NO4
- The crystal structure of (E)-3-(furan-2-yl)acrylonitrile, C7H5NO
- Crystal structure of 3-(difluoromethyl)-1-methyl-N-(4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)-1H-pyrazole-4-carboxamide monohydrate, C23H26F2N4O3
- Crystal structure of 2-(4-bromobenzyloxy)-6-chloropyridine, C12H9BrClNO
- Crystal structure of N-(4-bromo-2,6-dichloro-phenyl)pyrazin-2-amine, C10H6BrCl2N3
- Crystal structure of (E)-1-(2–nitrophenyl)-3-phenylprop-2-en-1-one, C15H11NO3
- The crystal structure of (E)-3-chloro-2-(2-(4-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of (E)-amino(2-(thiazol-2-ylmethylene)hydrazineyl)methaniminium nitrate, C10H16N12O6S2
- Crystal structure of 9-methoxy-2,3,4,4a,5,6-hexahydro-1H-pyrido [1′,2′:1,6]pyrazino[2,3-b]quinoxaline, C15H18N4O
- The crystal structure bis(dimethylsulfoxide-κ1O)-dipyridine-κ1 N-bis(m2-(Z)-3-methyl-2-oxido-N-((Z)-oxido(phenyl)methylene)benzohydrazonato-κ5)trinickel(II) - dimethylsulfoxide (1/2), C48H56N6Ni3O10S4
- Crystal structure of bis(bis(triphenylphosphine)iminium) tetradecacarbonyltetratelluridopentaferrate(2-), (PPN)2[Fe5Te4(CO)14]
- Crystal structure of 4-Hydroxy-3-(naphthalen-2-ylthio)pent-3-en-2-one, C15H14O2S
- The crystal structure of [(1,10-phenanthroline-κ2 N,N)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)nickel(II)] monohydrate, C36H26N4O5Ni
- Crystal structure of 3,3′-(pyridine-2,6-diylbis(methylene))bis(1-propyl-1H-imidazol-3-ium) ditetrafluoroborate, C19H27B2F8N5
- The crystal structure of (E)-1-(4-aminophenyl)-3-(p-tolyl)prop-2-en-1-one, C16H15NO
- The crystal structure of poly[(μ2-terephthalato-κ4O,O′: O″,O‴)-(μ4-terephthalato-κ4O:O′:O″:O‴)-{μ4-(1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ4O:O′:O″,O‴)}dicadmium(II)] – water – acetronitrile (1/2/2), C38H36N14O10Cd2
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)cobalt(II)–water–N,N-dimethylformamide(1/2/1), C27H31N3O9Co
- The co-crystal structure of 4-hydroxy-3-methoxybenzoic acid – 4,4′-bipyridine, C8H8O4·C10H8N2
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)cobalt(II)], C32H22Br2CoN4O4
- Crystal structure of (E)-5-propyl-4-((pyridin-2-ylmethylene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione – methanol (1/1), C11H13N5S
- The crystal structure of (Z)-4-bromo-6-(((1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)amino)methylene)cyclohexa-2,4-dien-1-one monohydrate, C11H16BrNO5
- Crystal structure of bis(tetrapropylammonium) nonaselenidotetrastannate(IV), (Pr4N)2[Sn4Se9]
- Crystal structure of 2,6-di-tert-butyl-4-(4-chlorobenzylidene)cyclohexa-2,5-dien-1-one, C21H25ClO
- Crystal structure of (2,2′-((naphthalen-1-ylmethyl)azanediyl)diacetato-κ3 N,O,O′)-(1,10-phenanthroline-κ2 N,N′)-copper(II) trihydrate, CuC27H27N3O7
- The crystal structure of tetrakis(6-phenylpyridine-2-carboxylato-κ2N,O)-bis(1H-pyrazol-3-ylamine-κ2 N:N)dicobalt(II) dihydrate, C27H23N5O5Co
- The crystal structure of bis((E)-2-((tert-butylimino)methyl)-4-chlorophenolato-κ2N,O)zinc(II), C22H26Cl2N2O2Zn
- The crystal structure of poly[diaqua-(μ3-5-nitrobenzene-1,2,3-tricarboxylato-κ3O:O′:O′)-(μ2-4,4′-dipyridylamine-κ2N:N′)copper(II)], C38H30Cu3N8O20
- The crystal structure of (E)-1-ferrocenyl-3-(naphthalen-1-yl)prop-2-en-1-one, C23H18FeO
- The crystal structure of (E)-1-ferrocenyl-3-(4-isopropylphenyl)prop-2-en-1-one, C22H22FeO
- Crystal structure of 6-hydroxy-2,2-dimethyl-4Hbenzo[d][1,3]dioxin-4-one, C10H10O4
- The crystal structure of (2E,4E)-1-ferrocenyl-5-phenylpenta-2,4-dien-1-one, C21H18FeO
- Crystal structure of alaninato-κ2N,O-bis(hydroxylamido-κ2N,O)-oxido-vanadium(V), C3H10N3O5V
- Crystal structure of catena-poly[aqua-bis[μ2-6-(1H-imidazol-1-yl)nicotinato-κ2 N,O]copper(II)], C18H14N6O5Cu
- Crystal structure of diethyl 4,6-diphenyl-1,9-di-p-tolylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,5(2H)-dicarboxylate, C42H42N2O4
- The crystal structure of cobalt cadmium bis(hydrogenphosphate) bis(phosphate(V)) tetrahydrate, H10O20P4Co3.14Cd1.86
- Crystal structure of dimethyl 1,4,6,9-tetraphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,7(2H)-dicarboxylate, C38H34N2O4
- Crystal structure of (Z)-4-(furan-2-yl((4-iodophenyl)amino)methylene)-5-methyl-2(p-tolyl)-2,4-dihydro-3H-pyrazol-3-one, C21H16I N3O2
- Crystal structure of (E)-1-(4-(3,5-dimethoxystyryl)phenyl)-7-ethylheptanedioate, C25H30O6
- Crystal structure of 6-bromo-2-(4-chlorophenyl)chroman-4-one (6-bromo-4′-chloroflavanone), C15H10BrClO2
- The crystal structure of 2-(benzhydryloxy)-3-nitropyridine, C18H14N2O3
- The crystal structure of 1,3(4,1)-dipyridin-1-iuma-2(1,8)-diethynylanthracena-5(1,3)-benzenacyclohexaphane-11,31-diium bis(hexafluoridophosphate), C36H24F12N2P2
- Crystal structure of 3,6-di-tert-butyl-1-iodo-9-methyl-8-(pyren-1-ylethynyl)-9H-carbazole, C39H34IN
- The cocrystal 2-(dimethylammonio)-5-nitrobenzoate – 2-(dimethylamino)-5-nitrobenzoic acid, C9H10N2O4
- Crystal structure of 5-nitroquinazolin-4(3H)-one, C8H5N3O3


