Abstract
C12H10O3Se2, triclinic, P
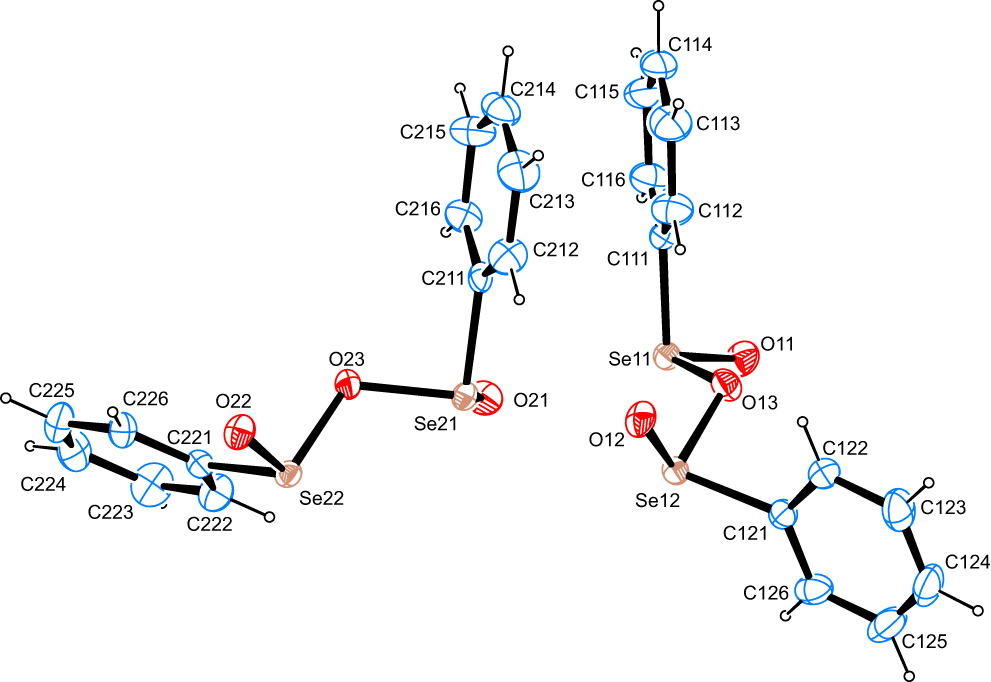
The molecular structure is shown in the Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless rod |
| Size: | 0.52 × 0.11 × 0.07 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 6.13 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 28.4°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 20,955, 6011, 0.028 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4555 |
| N(param)refined: | 307 |
| Programs: | Bruker [1], [2], SHELX [3], WinGX/ORTEP [4], Mercury [5], PLATON [6] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Se11 | 0.64277(3) | 0.65469(3) | 0.47094(3) | 0.01928(8) |
| Se12 | 0.33592(3) | 0.62604(3) | 0.64134(3) | 0.01874(8) |
| Se21 | 0.17898(3) | 0.62597(3) | 0.40703(2) | 0.01887(8) |
| Se22 | 0.02400(3) | 0.46463(3) | 0.31419(2) | 0.01857(8) |
| O11 | 0.8000(3) | 0.6490(2) | 0.51098(19) | 0.0297(5) |
| O12 | 0.1820(2) | 0.7164(2) | 0.60964(19) | 0.0271(5) |
| O13 | 0.4814(3) | 0.7146(2) | 0.59715(19) | 0.0258(5) |
| O21 | 0.3711(2) | 0.5719(2) | 0.3776(2) | 0.0284(5) |
| O22 | −0.1338(2) | 0.5356(2) | 0.27528(18) | 0.0253(5) |
| O23 | 0.1410(3) | 0.5736(2) | 0.28425(18) | 0.0266(5) |
| C111 | 0.6136(4) | 0.8058(3) | 0.3909(3) | 0.0219(6) |
| C112 | 0.4707(4) | 0.8936(3) | 0.4206(3) | 0.0338(8) |
| H112 | 0.383085 | 0.880011 | 0.480027 | 0.041* |
| C113 | 0.4578(5) | 1.0009(3) | 0.3623(4) | 0.0399(9) |
| H113 | 0.360885 | 1.062079 | 0.382593 | 0.048* |
| C114 | 0.5834(5) | 1.0204(3) | 0.2753(3) | 0.0397(9) |
| H114 | 0.573920 | 1.095019 | 0.236392 | 0.048* |
| C115 | 0.7235(5) | 0.9309(4) | 0.2446(3) | 0.0425(9) |
| H115 | 0.809076 | 0.943558 | 0.182830 | 0.051* |
| C116 | 0.7408(4) | 0.8236(3) | 0.3023(3) | 0.0327(8) |
| H116 | 0.838063 | 0.762897 | 0.281860 | 0.039* |
| C121 | 0.2900(4) | 0.6619(3) | 0.7986(3) | 0.0221(6) |
| C122 | 0.1924(4) | 0.7719(3) | 0.8402(3) | 0.0276(7) |
| H122 | 0.155233 | 0.832237 | 0.790999 | 0.033* |
| C123 | 0.1490(5) | 0.7931(4) | 0.9564(3) | 0.0391(9) |
| H123 | 0.082718 | 0.868795 | 0.987131 | 0.047* |
| C124 | 0.2023(5) | 0.7043(4) | 1.0261(3) | 0.0447(10) |
| H124 | 0.169892 | 0.718316 | 1.105437 | 0.054* |
| C125 | 0.3017(5) | 0.5954(4) | 0.9833(3) | 0.0429(10) |
| H125 | 0.338852 | 0.535506 | 1.032798 | 0.051* |
| C126 | 0.3478(4) | 0.5728(3) | 0.8685(3) | 0.0316(8) |
| H126 | 0.417362 | 0.498026 | 0.837968 | 0.038* |
| C211 | 0.1595(4) | 0.7863(3) | 0.3461(3) | 0.0210(6) |
| C212 | 0.0258(4) | 0.8756(3) | 0.3963(3) | 0.0329(8) |
| H212 | −0.054430 | 0.858349 | 0.459853 | 0.039* |
| C213 | 0.0110(5) | 0.9907(3) | 0.3524(4) | 0.0426(10) |
| H213 | −0.079507 | 1.053575 | 0.386491 | 0.051* |
| C214 | 0.1268(5) | 1.0140(3) | 0.2598(4) | 0.0387(9) |
| H214 | 0.115641 | 1.093092 | 0.229716 | 0.046* |
| C215 | 0.2589(5) | 0.9239(3) | 0.2101(4) | 0.0411(9) |
| H215 | 0.337637 | 0.940598 | 0.145198 | 0.049* |
| C216 | 0.2770(4) | 0.8090(3) | 0.2544(3) | 0.0292(7) |
| H216 | 0.369361 | 0.746866 | 0.221938 | 0.035* |
| C221 | 0.1526(3) | 0.3731(3) | 0.1791(3) | 0.0205(6) |
| C222 | 0.3017(4) | 0.2991(3) | 0.1762(3) | 0.0304(8) |
| H222 | 0.340371 | 0.293075 | 0.239802 | 0.037* |
| C223 | 0.3915(4) | 0.2350(4) | 0.0788(3) | 0.0381(9) |
| H223 | 0.494058 | 0.184812 | 0.074650 | 0.046* |
| C224 | 0.3340(4) | 0.2429(3) | −0.0131(3) | 0.0346(8) |
| H224 | 0.396669 | 0.197398 | −0.079576 | 0.042* |
| C225 | 0.1860(4) | 0.3167(3) | −0.0089(3) | 0.0317(8) |
| H225 | 0.147487 | 0.322856 | −0.072623 | 0.038* |
| C226 | 0.0937(4) | 0.3817(3) | 0.0885(3) | 0.0255(7) |
| H226 | −0.009063 | 0.431551 | 0.092705 | 0.031* |
Source of material
The compound was obtained commercially (Sigma Aldrich). Crystals suitable for the diffraction study were taken directly from the provided product.
Experimental details
Carbon-bound H atoms were placed in calculated positions (C–H 0.95 Å) and were included in the refinement in the riding model approximation, with U(H) set to 1.2Ueq(C).
Comment
The Baeyer–Villiger reaction is a useful method to convert ketones into esters. A number of substrates in this context benefit significantly from the catalytic action of seleninic acids [7], [8], [9]. In continuation of our interest in structure-property relationships of esters [10], [11], [12], [13], [14] a preparative study aimed at the synthesis of novel esters was initiated. To prevent accidental isolation of the anhydride of benzeneseleninic acid (that was used as a precursor to generate the catalytically-active benzeneseleninic acid in situ) it was subjected to a diffraction study. The molecular and crystal structures of methylseleninic acid [15] and benzeneseleninic acid [16] have been reported in the literature. Only one report about the structure of a seleninic acid anhydride has appeared in print so far [17].
The structure solution shows the presence of two complete molecules of the title compound in the asymmetric unit. Se–O bond lengths for the formal Se=O double bonds cover a narrow range of only 1.635(2)–1.648(2) Å as well as an equally small range of 1.818(2)–1.843(2) Å for the Se–O single bonds. C–Se bonds are found in between 1.920(3)–1.932(3) Å. All mentioned atomic distances are in good agreement with corresponding values reported for comparable compounds as found in the Cambridge Structural Database [18]. The least-squares planes as defined by the non-hydrogen atoms of the aromatic moieties within the two individual molecules enclose angles of 48.1(2)° and 68.76(19)°, respectively.
In the crystal, C–H⋯O contacts whose range falls by more than 0.1 Å below the sum of van-der-Waals radii of the atoms participating in them are observed. These are established between one of the hydrogen atoms on each of the two molecules present in the asymmetric unit as donors as well as – invariably – one of the formally double-bonded oxygen atoms on each of the two molecules present in the asymmetric unit as acceptors. In one case, the hydrogen atom is found in the ortho, in the other case in the meta position to the selenium atom the phenyl group is bonded to. The donor-acceptor interaction exclusively appears in between the two symmetry-independend molecules. In terms of graph-set analysis [19], [20], the descriptor for these contacts is DD level and C22(15) at the binary level. In total, the two molecules are connected to infinite strands along the crystallographic a axis. Furthermore, dispersive Se⋯O contacts whose range falls by up to more than 0.6 Å below the sum of van-der–Waals radii of the two chalcogen atoms are apparent. A number of potential C–H⋯π interactions actually exhibit D–H⋯A angles that deviate significantly from a linear arrangement. π -Stacking is not a prominent feature as the shortest distance between two centers of gravity was measured at 4.346(3) Å.
Funding source: National Research Foundation
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: The corresponding author thanks the National Research Foundation for financial support.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2; Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.Search in Google Scholar
2. Bruker. SADABS; AXS Inc.: Madison, Wisconsin, USA, 2008.Search in Google Scholar
3. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
4. Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
5. Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J., Wood, P. A. Mercury CSD 2.0 – new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470; https://doi.org/10.1107/s0021889807067908.Search in Google Scholar
6. Spek, A. L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155; https://doi.org/10.1107/s090744490804362x.Search in Google Scholar
7. ten Brink, G. J., Fernandes, B. C. M., van Vliet, M. C. A., Arends, I. W. C. E., Sheldon, R. A. Selenium catalyzed oxidations with aqueous hydrogen peroxide. Part I. Epoxidation reactions in homogeneous solution. J. Chem. Soc. 2001, 224–228; https://doi.org/10.1039/b008198l.Search in Google Scholar
8. ten Brink, G. J., Vis, J. M., Arends, I. W. C. E., Sheldon, R. A. Selenium-catalyzed oxidations with aqueous hydrogen peroxide. 2. Baeyer–Villiger reactions in homogeneous solution. J. Org. Chem. 2001, 66, 2429–2433; https://doi.org/10.1021/jo0057710.Search in Google Scholar
9. Mercier, E. A., Smith, C. D., Parvez, M., Back, T. G. Cyclic seleninate esters as catalysts for the oxidation of sulfides to sulfoxides, epoxidation of alkenes, and conversion of enamines to α-hydroxyketones. J. Org. Chem. 2012, 77, 3508–3517; https://doi.org/10.1021/jo300313v.Search in Google Scholar
10. Betz, R., Herdlicka, S., Klüfers, P. Ethyl dicyclohexylglycolate. Acta Crystallogr. 2007, E63, o3916; https://doi.org/10.1107/s1600536807041554.Search in Google Scholar
11. Garudachari, B., Isloor, A. M., Satyanarayan, M. N., Gerber, T., Hosten, E., Betz, R. Diethyl 2-{[2-(trifluoromethyl)anilino]methylidene}propanedioate. Acta Crystallogr. 2012, E68, o514–o515; https://doi.org/10.1107/s1600536812002590.Search in Google Scholar
12. Isloor, A. M., Rai, U. S., Shetty, P., Gerber, T., Hosten, E., Betz, R. Methyl 2-[(2-methylphenoxy)methyl]benzoate. Acta Crystallogr. 2012, E68, o728; https://doi.org/10.1107/s1600536812005995.Search in Google Scholar
13. Odame, F., Hosten, E. C., Tshentu, Z. R., Betz, R. Crystal structure of 3,5-diazamethyl-2-methyl-6-oxo-6-phenyl-4-thioxohexanoate, at 200 K, C12H14N2O3S. Z. Kristallogr. NCS 2015, 230, 9–10; https://doi.org/10.1515/ncrs-2014-0217.Search in Google Scholar
14. Betz, R., Gerber, T., Schalekamp, H., Hosten, E. Refinement of the crystal structure of L-valinemethylester hydrochloride, C6H13NO2⋅HCl, at 200 K. Z. Kristallogr. NCS 2011, 226, 637–638; https://doi.org/10.1524/ncrs.2011.0286.Search in Google Scholar
15. Nakahima, Y., Shimizu, T., Hirabayashi, K., Kamigata, N., Yasui, M., Nakazato, M., Iwasaki, F. Isolation, absolute configuration, and chiral crystallization of optically active seleninic acid. Tetrahedron Lett. 2004, 45, 2301–2303.10.1016/j.tetlet.2004.01.107Search in Google Scholar
16. Bryden, J. H., McCullough, J. D. The crystal structure of benzeneseleninic acid. Acta Crystallogr. 1954, 7, 833–838; https://doi.org/10.1107/s0365110x54002551.Search in Google Scholar
17. Gould, E. S., Post, B. The crystal structure of trans- ethanediseleninic anhydride. J. Am. Chem. Soc. 1956, 78, 5161–5164; https://doi.org/10.1021/ja01601a007.Search in Google Scholar
18. Allen, F. H. The Cambridge Structural Database: a quarter of a million crystal structures and rising. Acta Crystallogr. 2002, B58, 380–388; https://doi.org/10.1107/s0108768102003890.Search in Google Scholar
19. Bernstein, J., Davis, R. E., Shimoni, L., Chang, N.-L. Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573; https://doi.org/10.1002/anie.199515551.Search in Google Scholar
20. Etter, M. C., MacDonald, J. C., Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. 1990, B46, 256–262; https://doi.org/10.1107/s0108768189012929.Search in Google Scholar
© 2020 Eric C. Hosten and Richard Betz, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 4-hydroxybenzene-1,3-diaminium dichloride, C6H10Cl2N2O
- The crystal structure of 3-chloropropylammonium chloride, C3H9Cl2N
- The crystal structure of 1-chloro-2-(dimethylamino)ethane hydrochloride, C4H11Cl2N
- Crystal structure of N-(2-(trifluoromethyl)phenyl)hexanamide, C13H16F3NO
- Redetermination of the crystal structure of para-toluidine, C7H9N
- The crystal structure of bis(1,3-dihydroxy-2-methylpropan-2-aminium) carbonate, C9H24N2O7
- The crystal structure of 4-chloro-1-methylpiperidin-1-ium chloride, C6H13Cl2N
- Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO
- The crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H21F2NO4
- Crystal structure of 6,6'‐((1E,1'E)‐(propane‐1,3‐diylbis(azaneylylidene))bis(methaneylylidene))bis(3‐bromophenol), C34H32Br4N4O4
- The crystal structure of (E)-2-(2-((2-picolinoylhydrazono)methyl)phenoxy)acetic acid dihydrate, C15H17N3O6
- Crystal structure of (E)-4-bromo-N′-(3-chloro-2-hydroxybenzylidene)benzohydrazide, C14H10BrClN2O2
- Crystal structure of N,N′-bis(4-bromosalicylidene) ethylene-1,2-diaminopropan, C34H32Br4N4O4
- Crystal structure of 4-bromo-N′-[(3-bromo-2-hydroxyphenyl)methylidene]benzohydrazide methanol solvate, C15H14Br2N2O3
- The crystal structure of 1,2-bis(1H-benzo[d]imidazol-2-yl)ethane-1,2-diol — N-(2-aminophenyl)-3-(1H-benzo[d]imidazol-2-yl)-2,3-dihydroxypropanamide (1/1), C32H30N8O5
- The crystal structure of para-trifluoromethyl-aniline hemihydrate, C14H14F6N2O
- Redetermination of the crystal structure of 2-amino-2-methyl-propane-1,3-diole, C4H11NO2
- The crystal structure of methacholine chloride, C8H18ClNO2
- Crystal structure of 5,7,7-trimethyl-4,6,7,8-tetrahydrocyclopenta[g]isochromen-1(3H)-one, C15H18O2
- Crystal structure of poly[diammine-bis(μ4-4-hydroxypyridine-3-sulfonato-κ5N:O, O′:O′′:O′′)(μ2-pyrazinyl-κ2N:N′)tetrasilver(I)], C7H8Ag2N3O4S
- Crystal structure of ethyl (E)-5-(((3′,6′-bis(ethylamino)-3-oxospiro[isoindoline-1,9′-xanthen]-2-yl)imino)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxylate — ethanol (1/1), C38H45N5O5
- Crystal structure of 4-bromo-N′-[(3-chloro-2-hydroxyphenyl)methylidene]benzohydrazide, C14H7Br2N2O2
- Redetermination of the crystal structure of 3,3,3-triphenylpropanoic acid, C21H18O2 – Deposition of hydrogen atomic coordinates
- Structure redetermination of dextromethorphan hydrobromide monohydrate, C18H28BrNO2 – localization of hydrogen atoms
- Crystal structure of tris(azido-κ1N)-(N-(2-aminoethyl)-N-methyl-1,3-propanediamine-κ3N,N′,N′′)cobalt(III), C7H19CoN12
- Crystal structure of tetraaqua-bis(1H-indazole-6-carboxylate-κN)cadmium (II), C16H18CdN4O8
- Crystal structure of dichloride-bis(1-propylimidazole-κ1N)zinc(II), C12H20Cl2N4Zn
- Crystal structure of (E)-resveratrol 3-O-β-D-xylopyranoside, C19H22O8
- Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-vinyl- 1H-imidazol-3-ium) bis(hexafluoro phosphate)(V), C18H20F12N4P2
- Crystal structure of diaqua[bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″]-phthalato-κ1O-nickel(II)-methanol (1/2), C26H31N5NiO8
- Crystal structure of 6,7-difluoro-1-methyl-3-(trifluoromethyl)quinoxalin-2(1H)-one, C10H5F5N2O
- Crystal structure of dichlorido-bis(1-hexyl-1H-benzotriazole-k1N)zinc(II), C24H34N6Cl2Zn
- The crystal structre of 2-(4-bromophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C16H12BBrN2
- Crystal structure of diethyl 3,9-bis(4-fluorophenyl)-6,12-diphenyl-3,9-diazapentacyclo[6.4.0.02,7.04,11]dodecane-1,5-dicarboxylate, C40H36F2N2O4
- Crystal structure of (E)-7-methoxy-2-((5-methoxypyridin-3-yl)methylene)-3,4- dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-chloro-6-(((1,3-dihydroxy-2-(oxidomethyl)propan-2-yl)imino)methyl)phenolate-κ3N,O,O’)manganese(IV), C22H24Cl2MnN2O8
- The crystal structure of α-(meta-methoxyphenoxy)-ortho-tolylic acid, C15H14O4
- The crystal structure of N-(2-chloroethyl)-N,N-diethylammonium chloride, C6H15Cl2N
- The crystal structure of tris(2,3,4,6,7,8,9,10-octahydro-1H-pyrimido[1,2-a]azepin-5-ium) trihydrodecavanadate(V), C27H54N6O28V10
- Crystal structure of 1,3-bis(octyl)benzimidazolium perchlorate C23H39ClN2O4
- Crystal structure of tetrakis[(Z)-(2-(1-(furan-2-yl)-2-methylpropylidene)-1-phenylhydrazin-1-ido-κ2N,N′)] zirconium(IV), C56H60N8O4Zr
- The crystal structure of 2-(naphthalen-2-yloxy)-4-phenyl-6-(prop-2-yn-1-yloxy)-1,3,5-triazine, C22H15N3O2
- The crystal structure of trimethylsulfonium tris(trifluoromethylsulfonyl)methanide, C7H9F9O6S4
- Crystal structure of 4-bromo-N′-[3,5-dichloro-2-hydroxyphenyl)methylidene]benzohydrazide methanol solvate, C15H13BrCl2N2O3
- The crystal structure of 4-(4-bromophenyl)-2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)thiazole, C24H16Br2FN3S
- The crystal structure of N-(adamantan-1-yl)-piperidine-1-carbothioamide, C16H26N2S
- The crystal structure of 1-phenyl-N-(4,5,6,7-tetrabromo-1,3-dioxoisoindolin-2-yl)-5-(thiophen-2-yl)-1H-pyrazole-3-carboxamide-dimethylformamide (1/1) C22H10Br4N4O3S
- The crystal structure of benzeneseleninic acid anhydride, C12H10O3Se2
- The crystal structure of diphenyalmine hydrochloride antimony trichloride co-crystallizate, C12H12Cl4NSb – Localization of hydrogen atoms
- The crystal structure of para-nitrobenzylbromide, C7H6BrNO2 – A second polymorph and correction of 3D coordinates
- Crystal structure of catena-poly[(5H-pyrrolo[3,2-b:4,5-b′]dipyridine-κ2N,N′)-(μ4-hexaoxidodivanadato)dizinc(II)],C10H9N3O6V2Zn
- Crystal structure of N,N′-(2-hydroxypropane-1,3-diyl)bis(pyridine-2-aldimine)-κ5N,N′,N′′,N′′′,O]-tris(nitrato-κ2O,O′) cerium(III), C15H16CeN7O10
- Synthesis and crystal structure of oktakis(dimethylsulphoxide-κ1O)gadolinium(III) [tetrabromido-μ2-bromido-μ2-sulfido-di-μ3-sulfido-μ4-sulfido-tetracopper(I)-tungsten(VI)], C16H48O8S12Br5Cu4GdW
- Crystal structure of {tris((1H-benzo[d]imidazol-2- yl)methyl)amine-κ4N,N′,N′′,N′′′}-(succinato-κ2O,O′)nickel(II) – methanol (1/4), C32H41N7NiO8
- Crystal structure of catena-poly[trans-tetraaqua(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-k2N:N′)cobalt(II)] dinitrate – 1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol) – water (1/3/2), C72H68CoN18O12
- Crystal structure of bis(μ2-2-oxido-2-phenylacetate-κ3O:O,O′)-bis(1-isopropoxy-2-oxo-2-phenylethan-1-olato-κ2O,O′)-bis(propan-2-olato-κ1O)dititanium(IV), C44H52O14Ti2
- The crystal structure of 5-carboxy-2-(hydroxymethyl)-1H-imidazol-3-ium-4-carboxylate, C6H8N2O6
- The crystal structure of 2,6-dibromo-4-fluoroaniline, C6H4Br2FN
- The crystal structure of 4-chloro-N-(2-phenoxyphenyl)benzamide, C19H14ClNO2
- The crystal structure of 2-methyl-β-naphthothiazole, C12H9NS
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 4-hydroxybenzene-1,3-diaminium dichloride, C6H10Cl2N2O
- The crystal structure of 3-chloropropylammonium chloride, C3H9Cl2N
- The crystal structure of 1-chloro-2-(dimethylamino)ethane hydrochloride, C4H11Cl2N
- Crystal structure of N-(2-(trifluoromethyl)phenyl)hexanamide, C13H16F3NO
- Redetermination of the crystal structure of para-toluidine, C7H9N
- The crystal structure of bis(1,3-dihydroxy-2-methylpropan-2-aminium) carbonate, C9H24N2O7
- The crystal structure of 4-chloro-1-methylpiperidin-1-ium chloride, C6H13Cl2N
- Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO
- The crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H21F2NO4
- Crystal structure of 6,6'‐((1E,1'E)‐(propane‐1,3‐diylbis(azaneylylidene))bis(methaneylylidene))bis(3‐bromophenol), C34H32Br4N4O4
- The crystal structure of (E)-2-(2-((2-picolinoylhydrazono)methyl)phenoxy)acetic acid dihydrate, C15H17N3O6
- Crystal structure of (E)-4-bromo-N′-(3-chloro-2-hydroxybenzylidene)benzohydrazide, C14H10BrClN2O2
- Crystal structure of N,N′-bis(4-bromosalicylidene) ethylene-1,2-diaminopropan, C34H32Br4N4O4
- Crystal structure of 4-bromo-N′-[(3-bromo-2-hydroxyphenyl)methylidene]benzohydrazide methanol solvate, C15H14Br2N2O3
- The crystal structure of 1,2-bis(1H-benzo[d]imidazol-2-yl)ethane-1,2-diol — N-(2-aminophenyl)-3-(1H-benzo[d]imidazol-2-yl)-2,3-dihydroxypropanamide (1/1), C32H30N8O5
- The crystal structure of para-trifluoromethyl-aniline hemihydrate, C14H14F6N2O
- Redetermination of the crystal structure of 2-amino-2-methyl-propane-1,3-diole, C4H11NO2
- The crystal structure of methacholine chloride, C8H18ClNO2
- Crystal structure of 5,7,7-trimethyl-4,6,7,8-tetrahydrocyclopenta[g]isochromen-1(3H)-one, C15H18O2
- Crystal structure of poly[diammine-bis(μ4-4-hydroxypyridine-3-sulfonato-κ5N:O, O′:O′′:O′′)(μ2-pyrazinyl-κ2N:N′)tetrasilver(I)], C7H8Ag2N3O4S
- Crystal structure of ethyl (E)-5-(((3′,6′-bis(ethylamino)-3-oxospiro[isoindoline-1,9′-xanthen]-2-yl)imino)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxylate — ethanol (1/1), C38H45N5O5
- Crystal structure of 4-bromo-N′-[(3-chloro-2-hydroxyphenyl)methylidene]benzohydrazide, C14H7Br2N2O2
- Redetermination of the crystal structure of 3,3,3-triphenylpropanoic acid, C21H18O2 – Deposition of hydrogen atomic coordinates
- Structure redetermination of dextromethorphan hydrobromide monohydrate, C18H28BrNO2 – localization of hydrogen atoms
- Crystal structure of tris(azido-κ1N)-(N-(2-aminoethyl)-N-methyl-1,3-propanediamine-κ3N,N′,N′′)cobalt(III), C7H19CoN12
- Crystal structure of tetraaqua-bis(1H-indazole-6-carboxylate-κN)cadmium (II), C16H18CdN4O8
- Crystal structure of dichloride-bis(1-propylimidazole-κ1N)zinc(II), C12H20Cl2N4Zn
- Crystal structure of (E)-resveratrol 3-O-β-D-xylopyranoside, C19H22O8
- Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-vinyl- 1H-imidazol-3-ium) bis(hexafluoro phosphate)(V), C18H20F12N4P2
- Crystal structure of diaqua[bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″]-phthalato-κ1O-nickel(II)-methanol (1/2), C26H31N5NiO8
- Crystal structure of 6,7-difluoro-1-methyl-3-(trifluoromethyl)quinoxalin-2(1H)-one, C10H5F5N2O
- Crystal structure of dichlorido-bis(1-hexyl-1H-benzotriazole-k1N)zinc(II), C24H34N6Cl2Zn
- The crystal structre of 2-(4-bromophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C16H12BBrN2
- Crystal structure of diethyl 3,9-bis(4-fluorophenyl)-6,12-diphenyl-3,9-diazapentacyclo[6.4.0.02,7.04,11]dodecane-1,5-dicarboxylate, C40H36F2N2O4
- Crystal structure of (E)-7-methoxy-2-((5-methoxypyridin-3-yl)methylene)-3,4- dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-chloro-6-(((1,3-dihydroxy-2-(oxidomethyl)propan-2-yl)imino)methyl)phenolate-κ3N,O,O’)manganese(IV), C22H24Cl2MnN2O8
- The crystal structure of α-(meta-methoxyphenoxy)-ortho-tolylic acid, C15H14O4
- The crystal structure of N-(2-chloroethyl)-N,N-diethylammonium chloride, C6H15Cl2N
- The crystal structure of tris(2,3,4,6,7,8,9,10-octahydro-1H-pyrimido[1,2-a]azepin-5-ium) trihydrodecavanadate(V), C27H54N6O28V10
- Crystal structure of 1,3-bis(octyl)benzimidazolium perchlorate C23H39ClN2O4
- Crystal structure of tetrakis[(Z)-(2-(1-(furan-2-yl)-2-methylpropylidene)-1-phenylhydrazin-1-ido-κ2N,N′)] zirconium(IV), C56H60N8O4Zr
- The crystal structure of 2-(naphthalen-2-yloxy)-4-phenyl-6-(prop-2-yn-1-yloxy)-1,3,5-triazine, C22H15N3O2
- The crystal structure of trimethylsulfonium tris(trifluoromethylsulfonyl)methanide, C7H9F9O6S4
- Crystal structure of 4-bromo-N′-[3,5-dichloro-2-hydroxyphenyl)methylidene]benzohydrazide methanol solvate, C15H13BrCl2N2O3
- The crystal structure of 4-(4-bromophenyl)-2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)thiazole, C24H16Br2FN3S
- The crystal structure of N-(adamantan-1-yl)-piperidine-1-carbothioamide, C16H26N2S
- The crystal structure of 1-phenyl-N-(4,5,6,7-tetrabromo-1,3-dioxoisoindolin-2-yl)-5-(thiophen-2-yl)-1H-pyrazole-3-carboxamide-dimethylformamide (1/1) C22H10Br4N4O3S
- The crystal structure of benzeneseleninic acid anhydride, C12H10O3Se2
- The crystal structure of diphenyalmine hydrochloride antimony trichloride co-crystallizate, C12H12Cl4NSb – Localization of hydrogen atoms
- The crystal structure of para-nitrobenzylbromide, C7H6BrNO2 – A second polymorph and correction of 3D coordinates
- Crystal structure of catena-poly[(5H-pyrrolo[3,2-b:4,5-b′]dipyridine-κ2N,N′)-(μ4-hexaoxidodivanadato)dizinc(II)],C10H9N3O6V2Zn
- Crystal structure of N,N′-(2-hydroxypropane-1,3-diyl)bis(pyridine-2-aldimine)-κ5N,N′,N′′,N′′′,O]-tris(nitrato-κ2O,O′) cerium(III), C15H16CeN7O10
- Synthesis and crystal structure of oktakis(dimethylsulphoxide-κ1O)gadolinium(III) [tetrabromido-μ2-bromido-μ2-sulfido-di-μ3-sulfido-μ4-sulfido-tetracopper(I)-tungsten(VI)], C16H48O8S12Br5Cu4GdW
- Crystal structure of {tris((1H-benzo[d]imidazol-2- yl)methyl)amine-κ4N,N′,N′′,N′′′}-(succinato-κ2O,O′)nickel(II) – methanol (1/4), C32H41N7NiO8
- Crystal structure of catena-poly[trans-tetraaqua(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-k2N:N′)cobalt(II)] dinitrate – 1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol) – water (1/3/2), C72H68CoN18O12
- Crystal structure of bis(μ2-2-oxido-2-phenylacetate-κ3O:O,O′)-bis(1-isopropoxy-2-oxo-2-phenylethan-1-olato-κ2O,O′)-bis(propan-2-olato-κ1O)dititanium(IV), C44H52O14Ti2
- The crystal structure of 5-carboxy-2-(hydroxymethyl)-1H-imidazol-3-ium-4-carboxylate, C6H8N2O6
- The crystal structure of 2,6-dibromo-4-fluoroaniline, C6H4Br2FN
- The crystal structure of 4-chloro-N-(2-phenoxyphenyl)benzamide, C19H14ClNO2
- The crystal structure of 2-methyl-β-naphthothiazole, C12H9NS

