Abstract
C38H45N5O5, triclinic, P
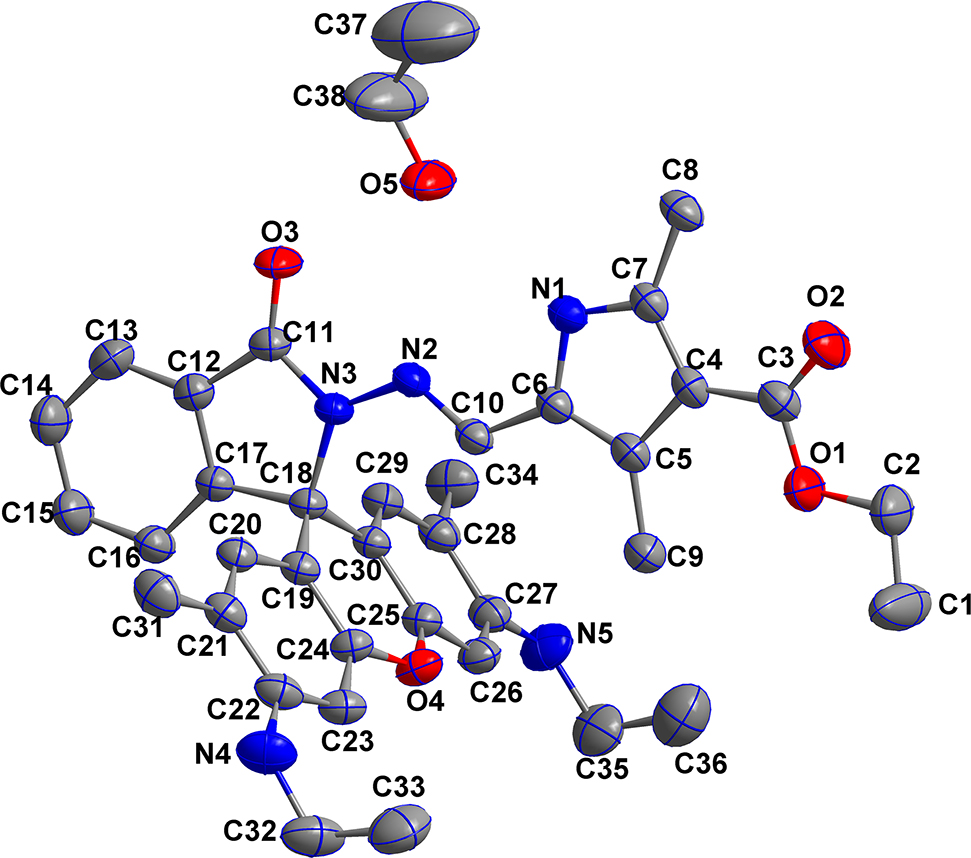
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.25 × 0.24 × 0.22 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 25.0°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 9253, 6305, 0.026 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3251 |
| N(param)refined: | 462 |
| Programs: | Bruker [1], SHELX [2], [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| N1 | −0.0797 (2) | 0.0662 (2) | 0.38943 (18) | 0.0585 (7) |
| H1 | −0.0771 | 0.1423 | 0.3653 | 0.070* |
| N2 | 0.1181 (2) | 0.1667 (2) | 0.22061 (18) | 0.0557 (6) |
| N3 | 0.2141 (2) | 0.2195 (2) | 0.13828 (18) | 0.0524 (6) |
| N4 | 0.1356 (3) | −0.1142 (3) | −0.1069 (3) | 0.0922 (10) |
| H4 | 0.0951 | −0.0789 | −0.1518 | 0.111* |
| N5 | 0.6077 (3) | −0.1031 (3) | 0.3494 (2) | 0.0888 (9) |
| H5 | 0.6450 | −0.0663 | 0.3761 | 0.107* |
| O1 | −0.1681 (2) | −0.3258 (2) | 0.56339 (19) | 0.0960 (8) |
| O2 | −0.3029 (2) | −0.2224 (2) | 0.6266 (2) | 0.1036 (9) |
| O3 | 0.1584 (2) | 0.40561 (19) | 0.12996 (18) | 0.0867 (8) |
| O4 | 0.3342 (2) | −0.09311 (17) | 0.14233 (17) | 0.0679 (6) |
| O5 | −0.0600 (3) | 0.3229 (2) | 0.2949 (2) | 0.1240 (11) |
| H5Aa | 0.0072 | 0.3271 | 0.2567 | 0.186* |
| H5Bb | 0.0019 | 0.3427 | 0.2481 | 0.186* |
| C1 | −0.1859 (6) | −0.5303 (4) | 0.6149 (5) | 0.211 (4) |
| H1A | −0.1773 | −0.5112 | 0.5426 | 0.317* |
| H1B | −0.2404 | −0.5998 | 0.6537 | 0.317* |
| H1C | −0.1081 | −0.5451 | 0.6307 | 0.317* |
| C2 | −0.2336 (4) | −0.4333 (3) | 0.6413 (3) | 0.1097 (15) |
| H2A | −0.2229 | −0.4420 | 0.7085 | 0.132* |
| H2B | −0.3196 | −0.4308 | 0.6434 | 0.132* |
| C3 | −0.2128 (3) | −0.2240 (3) | 0.5655 (3) | 0.0704 (9) |
| C4 | −0.1380 (3) | −0.1211 (3) | 0.4849 (2) | 0.0597 (8) |
| C5 | −0.0307 (3) | −0.1178 (3) | 0.4081 (2) | 0.0568 (8) |
| C6 | 0.0027 (3) | −0.0007 (3) | 0.3501 (2) | 0.0536 (8) |
| C7 | −0.1642 (3) | −0.0053 (3) | 0.4708 (2) | 0.0590 (8) |
| C8 | −0.2611 (3) | 0.0442 (3) | 0.5301 (2) | 0.0728 (10) |
| H8A | −0.2506 | 0.0230 | 0.5987 | 0.109* |
| H8B | −0.3392 | 0.0125 | 0.5331 | 0.109* |
| H8C | −0.2557 | 0.1289 | 0.4968 | 0.109* |
| C9 | 0.0355 (3) | −0.2200 (3) | 0.3932 (2) | 0.0726 (9) |
| H9A | 0.0598 | −0.2665 | 0.4549 | 0.109* |
| H9B | 0.1062 | −0.1906 | 0.3371 | 0.109* |
| H9C | −0.0169 | −0.2685 | 0.3780 | 0.109* |
| C10 | 0.1035 (3) | 0.0537 (3) | 0.2638 (2) | 0.0556 (8) |
| H10 | 0.1581 | 0.0071 | 0.2393 | 0.067* |
| C11 | 0.2259 (3) | 0.3413 (3) | 0.0970 (2) | 0.0613 (8) |
| C12 | 0.3318 (3) | 0.3756 (3) | 0.0089 (2) | 0.0586 (8) |
| C13 | 0.3817 (4) | 0.4868 (3) | −0.0599 (3) | 0.0795 (10) |
| H13 | 0.3481 | 0.5551 | −0.0544 | 0.095* |
| C14 | 0.4827 (4) | 0.4933 (3) | −0.1369 (3) | 0.0920 (12) |
| H14 | 0.5170 | 0.5669 | −0.1851 | 0.110* |
| C15 | 0.5334 (3) | 0.3911 (3) | −0.1429 (3) | 0.0862 (11) |
| H15 | 0.6024 | 0.3972 | −0.1945 | 0.103* |
| C16 | 0.4836 (3) | 0.2806 (3) | −0.0738 (2) | 0.0667 (9) |
| H16 | 0.5179 | 0.2124 | −0.0785 | 0.080* |
| C17 | 0.3823 (3) | 0.2738 (2) | 0.0019 (2) | 0.0517 (7) |
| C18 | 0.3113 (2) | 0.1623 (2) | 0.0850 (2) | 0.0482 (7) |
| C19 | 0.2605 (2) | 0.0870 (2) | 0.0376 (2) | 0.0513 (7) |
| C20 | 0.1984 (2) | 0.1355 (3) | −0.0403 (2) | 0.0546 (8) |
| H20 | 0.1862 | 0.2159 | −0.0613 | 0.066* |
| C21 | 0.1544 (3) | 0.0715 (3) | −0.0877 (2) | 0.0605 (8) |
| C22 | 0.1755 (3) | −0.0493 (3) | −0.0583 (3) | 0.0641 (9) |
| C23 | 0.2368 (3) | −0.0999 (3) | 0.0187 (3) | 0.0638 (8) |
| H23 | 0.2505 | −0.1800 | 0.0394 | 0.077* |
| C24 | 0.2774 (3) | −0.0316 (3) | 0.0645 (2) | 0.0545 (7) |
| C25 | 0.3975 (3) | −0.0290 (3) | 0.1790 (2) | 0.0535 (7) |
| C26 | 0.4698 (3) | −0.0941 (3) | 0.2420 (2) | 0.0631 (8) |
| H26 | 0.4740 | −0.1747 | 0.2555 | 0.076* |
| C27 | 0.5365 (3) | −0.0399 (3) | 0.2853 (2) | 0.0621 (8) |
| C28 | 0.5296 (3) | 0.0819 (3) | 0.2635 (2) | 0.0613 (8) |
| C29 | 0.4573 (3) | 0.1431 (3) | 0.1992 (2) | 0.0564 (8) |
| H29 | 0.4540 | 0.2241 | 0.1840 | 0.068* |
| C30 | 0.3889 (2) | 0.0907 (2) | 0.1555 (2) | 0.0496 (7) |
| C31 | 0.0874 (3) | 0.1284 (3) | −0.1694 (3) | 0.0815 (10) |
| H31A | 0.0068 | 0.0908 | −0.1476 | 0.122* |
| H31B | 0.1293 | 0.1198 | −0.2320 | 0.122* |
| H31C | 0.0830 | 0.2110 | −0.1810 | 0.122* |
| C32 | 0.1562 (4) | −0.2349 (4) | −0.0887 (4) | 0.1075 (14) |
| H32A | 0.2377 | −0.2487 | −0.0790 | 0.129* |
| H32B | 0.1515 | −0.2504 | −0.1492 | 0.129* |
| C33 | 0.0700 (4) | −0.3182 (4) | 0.0009 (4) | 0.1278 (17) |
| H33A | −0.0112 | −0.2971 | −0.0026 | 0.192* |
| H33B | 0.0857 | −0.3152 | 0.0626 | 0.192* |
| H33C | 0.0791 | −0.3969 | 0.0014 | 0.192* |
| C34 | 0.5948 (3) | 0.1427 (3) | 0.3128 (3) | 0.0869 (11) |
| H34A | 0.5615 | 0.1114 | 0.3859 | 0.130* |
| H34B | 0.5852 | 0.2263 | 0.2854 | 0.130* |
| H34C | 0.6796 | 0.1294 | 0.2985 | 0.130* |
| C35 | 0.6230 (4) | −0.2283 (4) | 0.3744 (4) | 0.1064 (14) |
| H35A | 0.6985 | −0.2472 | 0.3956 | 0.128* |
| H35B | 0.6283 | −0.2464 | 0.3134 | 0.128* |
| C36 | 0.5228 (5) | −0.3012 (5) | 0.4564 (4) | 0.1394 (19) |
| H36A | 0.4475 | −0.2794 | 0.4374 | 0.209* |
| H36B | 0.5221 | −0.2892 | 0.5187 | 0.209* |
| H36C | 0.5325 | −0.3832 | 0.4673 | 0.209* |
| C37a | −0.2251 (11) | 0.4332 (15) | 0.3339 (12) | 0.178 (7) |
| H37Aa | −0.2795 | 0.4842 | 0.2986 | 0.268* |
| H37Ba | −0.2051 | 0.4624 | 0.3822 | 0.268* |
| H37Ca | −0.2636 | 0.3545 | 0.3700 | 0.268* |
| C37Ab | −0.179 (2) | 0.486 (2) | 0.2497 (18) | 0.226 (11) |
| H37Db | −0.1302 | 0.5325 | 0.1828 | 0.339* |
| H37Eb | −0.2259 | 0.5363 | 0.2792 | 0.339* |
| H37Fb | −0.2329 | 0.4301 | 0.2436 | 0.339* |
| C38a | −0.1137 (12) | 0.4308 (10) | 0.2588 (11) | 0.149 (6) |
| H38Aa | −0.1329 | 0.4412 | 0.1940 | 0.178* |
| H38Ba | −0.0568 | 0.4959 | 0.2466 | 0.178* |
| C38Ab | −0.1000 (19) | 0.420 (2) | 0.3168 (15) | 0.164 (8) |
| H38Cb | −0.0307 | 0.4726 | 0.3067 | 0.196* |
| H38Db | −0.1445 | 0.3948 | 0.3883 | 0.196* |
aOccupancy: 0.560(16), bOccupancy: 0.440(16).
Source of material
Rhodamine 6 G hydrazide was condensed with equimolar ethyl 2,4-dimethyl-5-formylpyrrole-3-carboxylate in boiling ethanol and refluxed for 6 h. After cooling to room temperature, a white powder appeared, which was filtered off, washed with ethanol to receive the title compound. Yield: ca 80%. Single crystals of the title compound were obtained by recrystallization from ethanol solution.
Experimental details
The structure was solved by direct methods and refined with the SHELX crystallographic software package [3]. The C37/C38 atoms occupied two positions, with the occupancy value of C37(C38)/C37A(C38A) being 0.56/0.44. All hydrogen atoms were placed at calculated positions and refined as riding atoms with isotropic displacement parameters.
Comment
Rhodamine derivatives have attracted much attention in the design of chemosensors or chemodosimeters for metal ions [4]. Particularly, rhodamine hydrazones bearing a pyrrole unit may act as fluorescent probes for Cu2+ or Cd2+ [5], [6]. Herein, we report the crystal structure of the title compound derived from rhodamine 6 G hydrazide and a substituted pyrrole-2-aldehyde, which is related to the anticancer drug Sunitinib.
The asymmetric unit contains a neutral hydrazone molecule in a ring-closed form and one ethanol molecule. The C11=O3 bond distance is 1.229(3) Å, indicating the keto form of the amide. The bond length of C10–N2 is 1.279(4) Å, suggesting the existence of the Schiff base C=N moiety. All bond lengths are in the expected ranges [7], [8] and comparable with those of rhodamine 6 G hydrazones containing pyrrole ring [5], [6]. The benzamide and pyrrole ring are almost coplanar with the dihedral angle of 0.83°. The torsion angle of N1–C6–C10–N2 is −0.8(4)°, which reveals that the title compound might form chelate complexes with metal ions in this ring closed structure [6]. As expected, the intermolecular N–H…O, O–H…O and O–H…N hydrogen bonds are present between the hydrazone molecules and crystal ethanol molecules.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 21671124
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: National Natural Science Foundation of China (grant no. 21671124).
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. Smart and Saint; Bruker AXS Inc.: Madison, Wisconsin, USA, 2007.Search in Google Scholar
2. Sheldrick, G. M. SHELXT-integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELX. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Chen, X. Q., Pradhan, T., Wang, F., Kim, J. S., Yoon, J. Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem. Rev. 2012, 112, 1910–1956; https://doi.org/10.1021/cr200201z.Search in Google Scholar
5. Puangploy, P., Smanmoo, S., Surareungchai, W. A new rhodamine derivative-based chemosensor for highly selective and sensitive determination of Cu2+. Sens. Actuators, B 2014, 193, 679–686; https://doi.org/10.1016/j.snb.2013.12.037.Search in Google Scholar
6. Wang, Y., Chang, H.-Q., Wu, W.-N., Peng, W.-B., Yan, Y.-F., He, C.-M., Chen, T.-T., Zhao, X.-L., Xu, Z.-Q. Rhodamine 6G hydrazone bearing pyrrole unit: ratiometric and selective fluorescent sensor for Cu2+ based on two different approaches. Sens. Actuators, B 2016, 228, 395–400; https://doi.org/10.1016/j.snb.2016.01.052.Search in Google Scholar
7. Yuan, J., Cheng, D. Crystal structure of (E)-3′,6′-bis(ethylamino)-2′,7′-dimethyl-2-((pyridin-2-ylmethylene)amino) spiro[isoindoline-1,9′-xanthen]-3-one, C32H31N5O2. Z. Kristallogr. NCS 2019, 234, 149–151.10.1515/ncrs-2018-0239Search in Google Scholar
8. Chu, Y., Li, K., Yang, H., Yuan, J. Crystal structure of (E)-3′,6′-bis(ethylamino)-2′,7′-dimethyl-2-(2-((quinolin-2-ylmethylene)amino) ethyl)spiro[isoindoline-1,9′-xanthen]-3-one, C38H37N5O2. Z. Kristallogr. NCS 2020, 235, 1175–1177; https://doi.org/10.1515/ncrs-2020-0233.Search in Google Scholar
© 2020 Xiao-Li Gao et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 4-hydroxybenzene-1,3-diaminium dichloride, C6H10Cl2N2O
- The crystal structure of 3-chloropropylammonium chloride, C3H9Cl2N
- The crystal structure of 1-chloro-2-(dimethylamino)ethane hydrochloride, C4H11Cl2N
- Crystal structure of N-(2-(trifluoromethyl)phenyl)hexanamide, C13H16F3NO
- Redetermination of the crystal structure of para-toluidine, C7H9N
- The crystal structure of bis(1,3-dihydroxy-2-methylpropan-2-aminium) carbonate, C9H24N2O7
- The crystal structure of 4-chloro-1-methylpiperidin-1-ium chloride, C6H13Cl2N
- Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO
- The crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H21F2NO4
- Crystal structure of 6,6'‐((1E,1'E)‐(propane‐1,3‐diylbis(azaneylylidene))bis(methaneylylidene))bis(3‐bromophenol), C34H32Br4N4O4
- The crystal structure of (E)-2-(2-((2-picolinoylhydrazono)methyl)phenoxy)acetic acid dihydrate, C15H17N3O6
- Crystal structure of (E)-4-bromo-N′-(3-chloro-2-hydroxybenzylidene)benzohydrazide, C14H10BrClN2O2
- Crystal structure of N,N′-bis(4-bromosalicylidene) ethylene-1,2-diaminopropan, C34H32Br4N4O4
- Crystal structure of 4-bromo-N′-[(3-bromo-2-hydroxyphenyl)methylidene]benzohydrazide methanol solvate, C15H14Br2N2O3
- The crystal structure of 1,2-bis(1H-benzo[d]imidazol-2-yl)ethane-1,2-diol — N-(2-aminophenyl)-3-(1H-benzo[d]imidazol-2-yl)-2,3-dihydroxypropanamide (1/1), C32H30N8O5
- The crystal structure of para-trifluoromethyl-aniline hemihydrate, C14H14F6N2O
- Redetermination of the crystal structure of 2-amino-2-methyl-propane-1,3-diole, C4H11NO2
- The crystal structure of methacholine chloride, C8H18ClNO2
- Crystal structure of 5,7,7-trimethyl-4,6,7,8-tetrahydrocyclopenta[g]isochromen-1(3H)-one, C15H18O2
- Crystal structure of poly[diammine-bis(μ4-4-hydroxypyridine-3-sulfonato-κ5N:O, O′:O′′:O′′)(μ2-pyrazinyl-κ2N:N′)tetrasilver(I)], C7H8Ag2N3O4S
- Crystal structure of ethyl (E)-5-(((3′,6′-bis(ethylamino)-3-oxospiro[isoindoline-1,9′-xanthen]-2-yl)imino)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxylate — ethanol (1/1), C38H45N5O5
- Crystal structure of 4-bromo-N′-[(3-chloro-2-hydroxyphenyl)methylidene]benzohydrazide, C14H7Br2N2O2
- Redetermination of the crystal structure of 3,3,3-triphenylpropanoic acid, C21H18O2 – Deposition of hydrogen atomic coordinates
- Structure redetermination of dextromethorphan hydrobromide monohydrate, C18H28BrNO2 – localization of hydrogen atoms
- Crystal structure of tris(azido-κ1N)-(N-(2-aminoethyl)-N-methyl-1,3-propanediamine-κ3N,N′,N′′)cobalt(III), C7H19CoN12
- Crystal structure of tetraaqua-bis(1H-indazole-6-carboxylate-κN)cadmium (II), C16H18CdN4O8
- Crystal structure of dichloride-bis(1-propylimidazole-κ1N)zinc(II), C12H20Cl2N4Zn
- Crystal structure of (E)-resveratrol 3-O-β-D-xylopyranoside, C19H22O8
- Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-vinyl- 1H-imidazol-3-ium) bis(hexafluoro phosphate)(V), C18H20F12N4P2
- Crystal structure of diaqua[bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″]-phthalato-κ1O-nickel(II)-methanol (1/2), C26H31N5NiO8
- Crystal structure of 6,7-difluoro-1-methyl-3-(trifluoromethyl)quinoxalin-2(1H)-one, C10H5F5N2O
- Crystal structure of dichlorido-bis(1-hexyl-1H-benzotriazole-k1N)zinc(II), C24H34N6Cl2Zn
- The crystal structre of 2-(4-bromophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C16H12BBrN2
- Crystal structure of diethyl 3,9-bis(4-fluorophenyl)-6,12-diphenyl-3,9-diazapentacyclo[6.4.0.02,7.04,11]dodecane-1,5-dicarboxylate, C40H36F2N2O4
- Crystal structure of (E)-7-methoxy-2-((5-methoxypyridin-3-yl)methylene)-3,4- dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-chloro-6-(((1,3-dihydroxy-2-(oxidomethyl)propan-2-yl)imino)methyl)phenolate-κ3N,O,O’)manganese(IV), C22H24Cl2MnN2O8
- The crystal structure of α-(meta-methoxyphenoxy)-ortho-tolylic acid, C15H14O4
- The crystal structure of N-(2-chloroethyl)-N,N-diethylammonium chloride, C6H15Cl2N
- The crystal structure of tris(2,3,4,6,7,8,9,10-octahydro-1H-pyrimido[1,2-a]azepin-5-ium) trihydrodecavanadate(V), C27H54N6O28V10
- Crystal structure of 1,3-bis(octyl)benzimidazolium perchlorate C23H39ClN2O4
- Crystal structure of tetrakis[(Z)-(2-(1-(furan-2-yl)-2-methylpropylidene)-1-phenylhydrazin-1-ido-κ2N,N′)] zirconium(IV), C56H60N8O4Zr
- The crystal structure of 2-(naphthalen-2-yloxy)-4-phenyl-6-(prop-2-yn-1-yloxy)-1,3,5-triazine, C22H15N3O2
- The crystal structure of trimethylsulfonium tris(trifluoromethylsulfonyl)methanide, C7H9F9O6S4
- Crystal structure of 4-bromo-N′-[3,5-dichloro-2-hydroxyphenyl)methylidene]benzohydrazide methanol solvate, C15H13BrCl2N2O3
- The crystal structure of 4-(4-bromophenyl)-2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)thiazole, C24H16Br2FN3S
- The crystal structure of N-(adamantan-1-yl)-piperidine-1-carbothioamide, C16H26N2S
- The crystal structure of 1-phenyl-N-(4,5,6,7-tetrabromo-1,3-dioxoisoindolin-2-yl)-5-(thiophen-2-yl)-1H-pyrazole-3-carboxamide-dimethylformamide (1/1) C22H10Br4N4O3S
- The crystal structure of benzeneseleninic acid anhydride, C12H10O3Se2
- The crystal structure of diphenyalmine hydrochloride antimony trichloride co-crystallizate, C12H12Cl4NSb – Localization of hydrogen atoms
- The crystal structure of para-nitrobenzylbromide, C7H6BrNO2 – A second polymorph and correction of 3D coordinates
- Crystal structure of catena-poly[(5H-pyrrolo[3,2-b:4,5-b′]dipyridine-κ2N,N′)-(μ4-hexaoxidodivanadato)dizinc(II)],C10H9N3O6V2Zn
- Crystal structure of N,N′-(2-hydroxypropane-1,3-diyl)bis(pyridine-2-aldimine)-κ5N,N′,N′′,N′′′,O]-tris(nitrato-κ2O,O′) cerium(III), C15H16CeN7O10
- Synthesis and crystal structure of oktakis(dimethylsulphoxide-κ1O)gadolinium(III) [tetrabromido-μ2-bromido-μ2-sulfido-di-μ3-sulfido-μ4-sulfido-tetracopper(I)-tungsten(VI)], C16H48O8S12Br5Cu4GdW
- Crystal structure of {tris((1H-benzo[d]imidazol-2- yl)methyl)amine-κ4N,N′,N′′,N′′′}-(succinato-κ2O,O′)nickel(II) – methanol (1/4), C32H41N7NiO8
- Crystal structure of catena-poly[trans-tetraaqua(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-k2N:N′)cobalt(II)] dinitrate – 1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol) – water (1/3/2), C72H68CoN18O12
- Crystal structure of bis(μ2-2-oxido-2-phenylacetate-κ3O:O,O′)-bis(1-isopropoxy-2-oxo-2-phenylethan-1-olato-κ2O,O′)-bis(propan-2-olato-κ1O)dititanium(IV), C44H52O14Ti2
- The crystal structure of 5-carboxy-2-(hydroxymethyl)-1H-imidazol-3-ium-4-carboxylate, C6H8N2O6
- The crystal structure of 2,6-dibromo-4-fluoroaniline, C6H4Br2FN
- The crystal structure of 4-chloro-N-(2-phenoxyphenyl)benzamide, C19H14ClNO2
- The crystal structure of 2-methyl-β-naphthothiazole, C12H9NS
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 4-hydroxybenzene-1,3-diaminium dichloride, C6H10Cl2N2O
- The crystal structure of 3-chloropropylammonium chloride, C3H9Cl2N
- The crystal structure of 1-chloro-2-(dimethylamino)ethane hydrochloride, C4H11Cl2N
- Crystal structure of N-(2-(trifluoromethyl)phenyl)hexanamide, C13H16F3NO
- Redetermination of the crystal structure of para-toluidine, C7H9N
- The crystal structure of bis(1,3-dihydroxy-2-methylpropan-2-aminium) carbonate, C9H24N2O7
- The crystal structure of 4-chloro-1-methylpiperidin-1-ium chloride, C6H13Cl2N
- Crystal structure of (Z)-3-(6-bromo-1H-indol-3-yl)-1,3-diphenylprop-2-en-1-one, C23H16BrNO
- The crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H21F2NO4
- Crystal structure of 6,6'‐((1E,1'E)‐(propane‐1,3‐diylbis(azaneylylidene))bis(methaneylylidene))bis(3‐bromophenol), C34H32Br4N4O4
- The crystal structure of (E)-2-(2-((2-picolinoylhydrazono)methyl)phenoxy)acetic acid dihydrate, C15H17N3O6
- Crystal structure of (E)-4-bromo-N′-(3-chloro-2-hydroxybenzylidene)benzohydrazide, C14H10BrClN2O2
- Crystal structure of N,N′-bis(4-bromosalicylidene) ethylene-1,2-diaminopropan, C34H32Br4N4O4
- Crystal structure of 4-bromo-N′-[(3-bromo-2-hydroxyphenyl)methylidene]benzohydrazide methanol solvate, C15H14Br2N2O3
- The crystal structure of 1,2-bis(1H-benzo[d]imidazol-2-yl)ethane-1,2-diol — N-(2-aminophenyl)-3-(1H-benzo[d]imidazol-2-yl)-2,3-dihydroxypropanamide (1/1), C32H30N8O5
- The crystal structure of para-trifluoromethyl-aniline hemihydrate, C14H14F6N2O
- Redetermination of the crystal structure of 2-amino-2-methyl-propane-1,3-diole, C4H11NO2
- The crystal structure of methacholine chloride, C8H18ClNO2
- Crystal structure of 5,7,7-trimethyl-4,6,7,8-tetrahydrocyclopenta[g]isochromen-1(3H)-one, C15H18O2
- Crystal structure of poly[diammine-bis(μ4-4-hydroxypyridine-3-sulfonato-κ5N:O, O′:O′′:O′′)(μ2-pyrazinyl-κ2N:N′)tetrasilver(I)], C7H8Ag2N3O4S
- Crystal structure of ethyl (E)-5-(((3′,6′-bis(ethylamino)-3-oxospiro[isoindoline-1,9′-xanthen]-2-yl)imino)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxylate — ethanol (1/1), C38H45N5O5
- Crystal structure of 4-bromo-N′-[(3-chloro-2-hydroxyphenyl)methylidene]benzohydrazide, C14H7Br2N2O2
- Redetermination of the crystal structure of 3,3,3-triphenylpropanoic acid, C21H18O2 – Deposition of hydrogen atomic coordinates
- Structure redetermination of dextromethorphan hydrobromide monohydrate, C18H28BrNO2 – localization of hydrogen atoms
- Crystal structure of tris(azido-κ1N)-(N-(2-aminoethyl)-N-methyl-1,3-propanediamine-κ3N,N′,N′′)cobalt(III), C7H19CoN12
- Crystal structure of tetraaqua-bis(1H-indazole-6-carboxylate-κN)cadmium (II), C16H18CdN4O8
- Crystal structure of dichloride-bis(1-propylimidazole-κ1N)zinc(II), C12H20Cl2N4Zn
- Crystal structure of (E)-resveratrol 3-O-β-D-xylopyranoside, C19H22O8
- Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-vinyl- 1H-imidazol-3-ium) bis(hexafluoro phosphate)(V), C18H20F12N4P2
- Crystal structure of diaqua[bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″]-phthalato-κ1O-nickel(II)-methanol (1/2), C26H31N5NiO8
- Crystal structure of 6,7-difluoro-1-methyl-3-(trifluoromethyl)quinoxalin-2(1H)-one, C10H5F5N2O
- Crystal structure of dichlorido-bis(1-hexyl-1H-benzotriazole-k1N)zinc(II), C24H34N6Cl2Zn
- The crystal structre of 2-(4-bromophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C16H12BBrN2
- Crystal structure of diethyl 3,9-bis(4-fluorophenyl)-6,12-diphenyl-3,9-diazapentacyclo[6.4.0.02,7.04,11]dodecane-1,5-dicarboxylate, C40H36F2N2O4
- Crystal structure of (E)-7-methoxy-2-((5-methoxypyridin-3-yl)methylene)-3,4- dihydronaphthalen-1(2H)-one, C18H17NO3
- Crystal structure of (E)-2-chloro-6-(((1,3-dihydroxy-2-(oxidomethyl)propan-2-yl)imino)methyl)phenolate-κ3N,O,O’)manganese(IV), C22H24Cl2MnN2O8
- The crystal structure of α-(meta-methoxyphenoxy)-ortho-tolylic acid, C15H14O4
- The crystal structure of N-(2-chloroethyl)-N,N-diethylammonium chloride, C6H15Cl2N
- The crystal structure of tris(2,3,4,6,7,8,9,10-octahydro-1H-pyrimido[1,2-a]azepin-5-ium) trihydrodecavanadate(V), C27H54N6O28V10
- Crystal structure of 1,3-bis(octyl)benzimidazolium perchlorate C23H39ClN2O4
- Crystal structure of tetrakis[(Z)-(2-(1-(furan-2-yl)-2-methylpropylidene)-1-phenylhydrazin-1-ido-κ2N,N′)] zirconium(IV), C56H60N8O4Zr
- The crystal structure of 2-(naphthalen-2-yloxy)-4-phenyl-6-(prop-2-yn-1-yloxy)-1,3,5-triazine, C22H15N3O2
- The crystal structure of trimethylsulfonium tris(trifluoromethylsulfonyl)methanide, C7H9F9O6S4
- Crystal structure of 4-bromo-N′-[3,5-dichloro-2-hydroxyphenyl)methylidene]benzohydrazide methanol solvate, C15H13BrCl2N2O3
- The crystal structure of 4-(4-bromophenyl)-2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)thiazole, C24H16Br2FN3S
- The crystal structure of N-(adamantan-1-yl)-piperidine-1-carbothioamide, C16H26N2S
- The crystal structure of 1-phenyl-N-(4,5,6,7-tetrabromo-1,3-dioxoisoindolin-2-yl)-5-(thiophen-2-yl)-1H-pyrazole-3-carboxamide-dimethylformamide (1/1) C22H10Br4N4O3S
- The crystal structure of benzeneseleninic acid anhydride, C12H10O3Se2
- The crystal structure of diphenyalmine hydrochloride antimony trichloride co-crystallizate, C12H12Cl4NSb – Localization of hydrogen atoms
- The crystal structure of para-nitrobenzylbromide, C7H6BrNO2 – A second polymorph and correction of 3D coordinates
- Crystal structure of catena-poly[(5H-pyrrolo[3,2-b:4,5-b′]dipyridine-κ2N,N′)-(μ4-hexaoxidodivanadato)dizinc(II)],C10H9N3O6V2Zn
- Crystal structure of N,N′-(2-hydroxypropane-1,3-diyl)bis(pyridine-2-aldimine)-κ5N,N′,N′′,N′′′,O]-tris(nitrato-κ2O,O′) cerium(III), C15H16CeN7O10
- Synthesis and crystal structure of oktakis(dimethylsulphoxide-κ1O)gadolinium(III) [tetrabromido-μ2-bromido-μ2-sulfido-di-μ3-sulfido-μ4-sulfido-tetracopper(I)-tungsten(VI)], C16H48O8S12Br5Cu4GdW
- Crystal structure of {tris((1H-benzo[d]imidazol-2- yl)methyl)amine-κ4N,N′,N′′,N′′′}-(succinato-κ2O,O′)nickel(II) – methanol (1/4), C32H41N7NiO8
- Crystal structure of catena-poly[trans-tetraaqua(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-k2N:N′)cobalt(II)] dinitrate – 1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol) – water (1/3/2), C72H68CoN18O12
- Crystal structure of bis(μ2-2-oxido-2-phenylacetate-κ3O:O,O′)-bis(1-isopropoxy-2-oxo-2-phenylethan-1-olato-κ2O,O′)-bis(propan-2-olato-κ1O)dititanium(IV), C44H52O14Ti2
- The crystal structure of 5-carboxy-2-(hydroxymethyl)-1H-imidazol-3-ium-4-carboxylate, C6H8N2O6
- The crystal structure of 2,6-dibromo-4-fluoroaniline, C6H4Br2FN
- The crystal structure of 4-chloro-N-(2-phenoxyphenyl)benzamide, C19H14ClNO2
- The crystal structure of 2-methyl-β-naphthothiazole, C12H9NS

