Crystal structure of 2-oxo-1-(pyrimidin-5-ylmethyl)-3-(3-(trifluoromethyl)phenyl)-1,2-dihydro-5l4-pyrido[1,2-a]pyrimidin-4-olate, C20H13F3N4O2
-
Shi Yun-lian
, Shi Xu-Gen
und Li Bao-Tong
Abstract
C20H13F3N4O2, triclinic, P1̄ (no. 2), a = 11.3525(10) Å, b = 12.7530(11) Å, c = 13.1926(12) Å, α = 83.894(1)°, β = 69.314(1)°, γ = 84.720(1)°, V = 1773.7(3) Å3, Z = 4, Rgt(F) = 0.0477, wRref(F2) = 0.1433, T = 296(2) K.
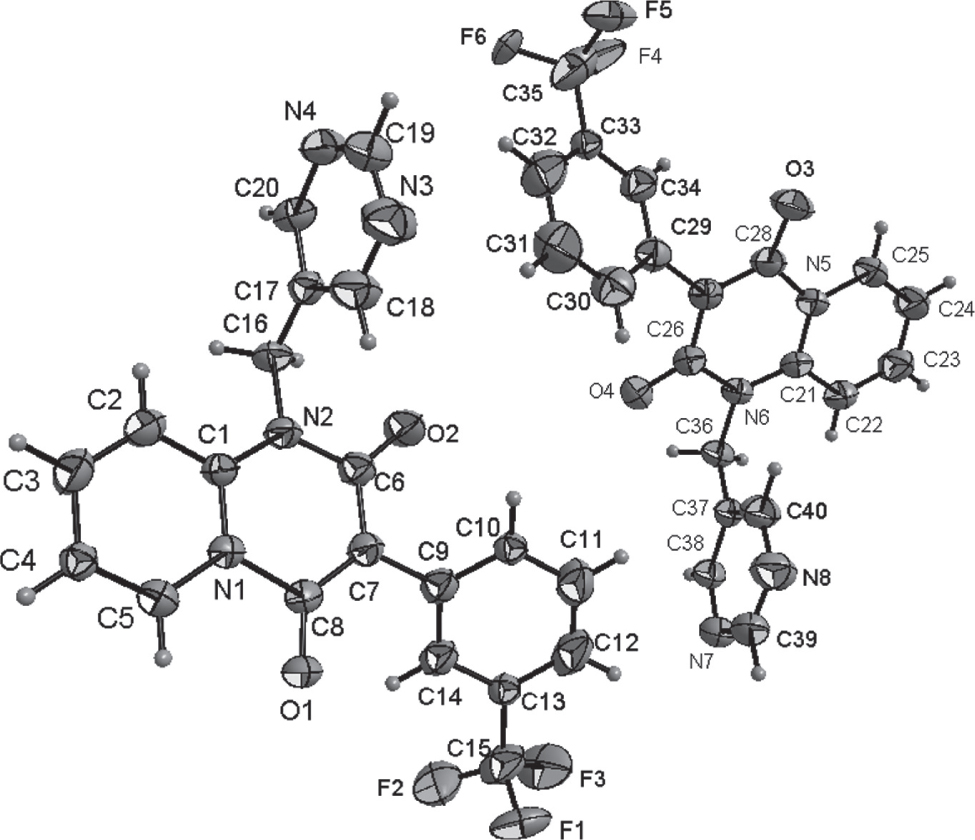
The crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.16 × 0.14 × 0.12 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.12 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω-scans |
| θmax, completeness: | 25.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 13789, 6557, 0.018 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4729 |
| N(param)refined: | 524 |
| Programs: | Bruker programs [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| N1 | 0.64548(15) | 0.61208(12) | −0.02771(13) | 0.0359(4) |
| N2 | 0.60039(16) | 0.79195(13) | −0.05909(13) | 0.0402(4) |
| N3 | 0.4451(2) | 0.9377(2) | −0.2909(2) | 0.0761(7) |
| N4 | 0.5802(2) | 1.07862(17) | −0.32960(15) | 0.0583(5) |
| N5 | 0.28673(17) | 0.71963(13) | 0.74578(14) | 0.0426(4) |
| N6 | 0.16724(16) | 0.82321(14) | 0.65745(13) | 0.0419(4) |
| N7 | 0.1696(2) | 1.15172(17) | 0.43342(18) | 0.0611(6) |
| N8 | 0.2367(2) | 1.14491(18) | 0.5847(2) | 0.0712(6) |
| O1 | 0.51386(14) | 0.51400(11) | 0.11790(12) | 0.0476(4) |
| O2 | 0.42007(16) | 0.87504(12) | 0.04180(14) | 0.0603(5) |
| O3 | 0.21238(17) | 0.68711(15) | 0.93115(13) | 0.0666(5) |
| O4 | −0.02772(15) | 0.89287(13) | 0.74796(13) | 0.0546(4) |
| F1 | 0.1578(3) | 0.46935(15) | 0.47081(18) | 0.1224(8) |
| F2 | 0.3103(2) | 0.53887(18) | 0.48375(14) | 0.1027(6) |
| F3 | 0.1282(2) | 0.60544(18) | 0.55855(14) | 0.1252(9) |
| F4 | −0.1767(4) | 0.5352(2) | 1.16110(19) | 0.1722(14) |
| F5 | −0.1498(3) | 0.6141(2) | 1.2769(2) | 0.1437(11) |
| F6 | −0.3229(2) | 0.6230(3) | 1.2607(3) | 0.1748(14) |
| C1 | 0.67559(19) | 0.70382(15) | −0.09094(15) | 0.0358(4) |
| C2 | 0.7812(2) | 0.70287(18) | −0.18643(18) | 0.0484(5) |
| H2 | 0.8005 | 0.7638 | −0.2330 | 0.058* |
| C3 | 0.8556(2) | 0.61269(19) | −0.21097(19) | 0.0543(6) |
| H3 | 0.9264 | 0.6123 | −0.2740 | 0.065* |
| C4 | 0.8263(2) | 0.52106(18) | −0.14232(19) | 0.0523(6) |
| H4 | 0.8785 | 0.4598 | −0.1576 | 0.063* |
| C5 | 0.7214(2) | 0.52231(17) | −0.05343(18) | 0.0442(5) |
| H5 | 0.7001 | 0.4606 | −0.0084 | 0.053* |
| C6 | 0.4833(2) | 0.79085(16) | 0.02807(17) | 0.0421(5) |
| C7 | 0.4508(2) | 0.69391(16) | 0.09154(16) | 0.0400(5) |
| C8 | 0.52883(19) | 0.60225(16) | 0.06985(16) | 0.0376(5) |
| C9 | 0.3300(2) | 0.69155(16) | 0.18443(17) | 0.0430(5) |
| C10 | 0.2207(2) | 0.74638(18) | 0.1770(2) | 0.0540(6) |
| H10 | 0.2233 | 0.7854 | 0.1127 | 0.065* |
| C11 | 0.1088(3) | 0.7433(2) | 0.2643(3) | 0.0686(8) |
| H11 | 0.0372 | 0.7812 | 0.2584 | 0.082* |
| C12 | 0.1017(3) | 0.6850(2) | 0.3597(2) | 0.0697(8) |
| H12 | 0.0258 | 0.6826 | 0.4178 | 0.084* |
| C13 | 0.2088(2) | 0.62991(18) | 0.36827(19) | 0.0557(6) |
| C14 | 0.3223(2) | 0.63378(17) | 0.28196(17) | 0.0474(5) |
| H14 | 0.3940 | 0.5974 | 0.2893 | 0.057* |
| C15 | 0.1999(3) | 0.5625(2) | 0.4691(2) | 0.0702(8) |
| C16 | 0.6417(2) | 0.89679(15) | −0.11010(17) | 0.0419(5) |
| H16A | 0.7329 | 0.8924 | −0.1437 | 0.050* |
| H16B | 0.6198 | 0.9456 | −0.0538 | 0.050* |
| C17 | 0.5861(2) | 0.94077(16) | −0.19411(16) | 0.0393(5) |
| C18 | 0.4933(3) | 0.8965(2) | −0.2154(2) | 0.0617(7) |
| H18 | 0.4619 | 0.8344 | −0.1753 | 0.074* |
| C19 | 0.4937(3) | 1.0261(2) | −0.3447(2) | 0.0642(7) |
| H19 | 0.4634 | 1.0545 | −0.3993 | 0.077* |
| C20 | 0.6247(2) | 1.03481(18) | −0.25337(17) | 0.0482(5) |
| H20 | 0.6852 | 1.0695 | −0.2393 | 0.058* |
| C21 | 0.2768(2) | 0.76818(16) | 0.65200(16) | 0.0403(5) |
| C22 | 0.3796(2) | 0.75795(19) | 0.55549(19) | 0.0516(6) |
| H22 | 0.3757 | 0.7919 | 0.4907 | 0.062* |
| C23 | 0.4852(2) | 0.6985(2) | 0.5560(2) | 0.0621(7) |
| H23 | 0.5532 | 0.6918 | 0.4916 | 0.074* |
| C24 | 0.4918(2) | 0.6478(2) | 0.6526(2) | 0.0642(7) |
| H24 | 0.5632 | 0.6061 | 0.6533 | 0.077* |
| C25 | 0.3932(2) | 0.65997(18) | 0.7453(2) | 0.0534(6) |
| H25 | 0.3977 | 0.6271 | 0.8103 | 0.064* |
| C26 | 0.0642(2) | 0.83797(17) | 0.75605(17) | 0.0435(5) |
| C27 | 0.0771(2) | 0.78767(17) | 0.85265(17) | 0.0438(5) |
| C28 | 0.1863(2) | 0.72968(17) | 0.85416(17) | 0.0464(5) |
| C29 | −0.0294(2) | 0.79774(19) | 0.95717(18) | 0.0483(5) |
| C30 | −0.0955(3) | 0.8940(2) | 0.9842(2) | 0.0640(7) |
| H30 | −0.0719 | 0.9541 | 0.9370 | 0.077* |
| C31 | −0.1959(3) | 0.9013(3) | 1.0808(2) | 0.0856(10) |
| H31 | −0.2396 | 0.9662 | 1.0976 | 0.103* |
| C32 | −0.2319(3) | 0.8139(3) | 1.1524(2) | 0.0827(9) |
| H32 | −0.2992 | 0.8193 | 1.2174 | 0.099* |
| C33 | −0.1672(2) | 0.7182(2) | 1.12660(19) | 0.0615(7) |
| C34 | −0.0667(2) | 0.7098(2) | 1.03022(18) | 0.0533(6) |
| H34 | −0.0236 | 0.6447 | 1.0140 | 0.064* |
| C35 | −0.2035(3) | 0.6248(3) | 1.2050(2) | 0.0790(9) |
| C36 | 0.1484(2) | 0.87329(17) | 0.55879(17) | 0.0453(5) |
| H36A | 0.0627 | 0.8640 | 0.5635 | 0.054* |
| H36B | 0.2051 | 0.8380 | 0.4967 | 0.054* |
| C37 | 0.17099(19) | 0.98893(17) | 0.54056(16) | 0.0414(5) |
| C38 | 0.1503(2) | 1.04929(19) | 0.45452(18) | 0.0495(6) |
| H38 | 0.1212 | 1.0165 | 0.4089 | 0.059* |
| C39 | 0.2129(2) | 1.1930(2) | 0.5001(2) | 0.0639(7) |
| H39 | 0.2283 | 1.2644 | 0.4855 | 0.077* |
| C40 | 0.2136(3) | 1.0427(2) | 0.6042(2) | 0.0590(6) |
| H40 | 0.2271 | 1.0059 | 0.6643 | 0.071* |
Source of materials
The synthesis of triflumezopyrim was carried out using 2-[3-(trifluoromethyl)phenyl]malonic acid and N-(pyrimidin-5-ylmethyl)pyridin-2-amine as start material, using a modified literature known reaction [3], [4], [5]. The educt 2-[3-(trifluoromethyl)phenyl]malonic acid (0.36 g, 1.45 mmol) was dissolved in dichloromethane (20 mL), oxalyl chloride (0.37 mL, 2.9 mmol) and two drops of DMF was added. The reaction was stirred for 1 h at room temperature. The mixture was concentrated under reduced pressure, 10 mL dichloromethane was added for next step use. And then triethylamine (1.1 mL, 7.94 mmol) and N-(pyrimidin-5-ylmethyl)pyridin-2-amine (0.27 g, 1.45 mmol) in 10 mL CH2Cl2 was slowly added to the above solution at 0 °C and then stirred at room temperature. The reaction was monitored by TLC. After completion of the reaction, the solvent was removed under reduced pressure, and the residue was purified by column chromatography to give the title compound (0.15 g), Yield 26%. The product was recrystallized from acetone. m.p. 182–184 °C. Elemental Anal. Calcd. (%) for C20H13F3N4O2 (398.38): C, 60.30; H, 3.29; Found. (%). C, 60.13; H, 3.56.
Experimental details
All H atoms were included in calculated positions and refined as riding atoms, with C—H = 0.93 Å with Uiso(H) = 1.5 Ueq(C) for methyl H atoms and 1.2 Ueq(C) for all other H atoms.
Comment
The title compound Triflumezopyrim is a novel mesoionic insecticide, which has a good internal absorption conduction activity and is the only inhibitory compound in insecticides acting on nicotinic acetylcholine receptors (nAChRs) [6], [7], [8]. It mainly controls rice planthoppers and cicadellidae, and has good control effect on various pests such as lepidoptera and homoptera. It can be used in crops like cotton, rice, corn and soybeans [9], [10]. It is used in non-agricultural fields and has a broad market [6]. Herein we report the crystal structure of triflumezopyrim, which makes this kind of compound better understood and paves the way for the development of more novel mesoionic compounds.
There are two crystallographic independent molecules in the asymmetric unit (shown in the figure). There are a pair of external racemate trifluorabrimidine in the figure. In the molecule of the title compound bond lengths and angles are very similar to those given in the literature for 2,4-diphenyl-4,5,6,7-tetrahydro-4,7a,12b-triazadibenzo(e,g)azulene-1,3,8-trione [11]. In the title structure, all the non-hydrogen atoms in pyrido[1,2-a]pyrimidine-2,4(3H)-dione ring are approximately coplanar. The dihedral angles of pyrido[1,2-a]pyrimidine-2,4(3H)-dione ring, pyrimidine ring and benzene ring are 80.4, 40.5 and 55.7°, respectively.
Acknowledgements
X-ray data were collected at Instrumental Analysis Center Nanchang Hangkong University, Nanchang, 330063, People’s Republic of China. This research has been supported by the China National Key R and D Program during the 13th Five-year Plan Period (Grant No. 2017YFD0301604), The National Natural Science Foundation of China (Grant Nos. 2156200, 31660527), The Natural Science Foundation of Jiangxi Province (Grant No. 20181BAB203015), The Research Foundation of Educational Department of Jiangxi Province [GJJ180204, GJJ160379].
References
1. Bruker. APEX2, SAINT and SADABS. Brucker AXS Inc., Madison, WI, USA (2009).Suche in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
3. Zhang, W.: Mesoionic Pyrido[1,2a]pyrimidinone insecticides: from discovery to tri umezopyrim and dicloromezotiaz. Acc. Chem. Res. 50 (2017) 2381–2388.10.1021/acs.accounts.7b00311Suche in Google Scholar PubMed
4. Zhang, W.; Caleb, W. H. Jr.; James, B.; Robert, M. L.; Daniel, C.; Daniel, R. V.; Kenneth, A. H.; My-Hanh, T. T.; Stephen, F.; Xu, M.; Twyla, A. B.; Thomas, F. P.; George, P. L.: Mesoionic pyrido[1,2-a]pyrimidinones: a novel class of insecticides inhibiting nicotinic acetylcholine receptors. Bioorg. Med. Chem. Lett. 26 (2016) 5444–5449.10.1016/j.bmcl.2016.10.031Suche in Google Scholar PubMed
5. Shi, Y. L.; Huang, T.; Nie, X. L.; Peng, D. Y.; Liu, J. C.; Li, B. T.: Crystal structure of bis(2,4,6-trichlorophenyl)malonate, C15H6Cl6O4. Z. Kristallogr. NCS 2 (2019) 331–332.Suche in Google Scholar
6. Gu, L. L.: Market prospects of three insecticides acting on nicotinic acetylcholine receptors. Pestic Market Inform. 24 (2018) 33.Suche in Google Scholar
7. Zhu, Y. J.; Gu, L. L.: Development and progress of new insecticides on nicotinic acetylcholine receptor. Modern Agro. 4 (2018) 1–7.Suche in Google Scholar
8. Daniel, C.; Eric, A. B.; Mark, E. S.; Caleb, W. H. Jr.; Zhang, W.; Thomas, F. P.; Robert, M. L.; Daniel, R. V.; Jason, C. H.: Mode of action of triflumezopyrim: a novel mesoionic insecticide which inhibits the nicotinic acetylcholine receptor. Insect Biochem. Mol. Biol. 74 (2016) 32–41.10.1016/j.ibmb.2016.04.008Suche in Google Scholar PubMed
9. Ying, J. W.; Lei, G. Y.; Song, Y. Q.: Synthesis and bioactivity of triflumezopyrim. Modern Agro. 2 (2017) 14–16.Suche in Google Scholar
10. Li, R. X.; Xu, X. D.; Gao, Z.: Preliminary report of control effect of triflunezopyrim on rice planthopper. Hunan Agric. Sci. 8 (2017) 61–63.Suche in Google Scholar
11. Schmidt, A.; Shilabin, A. G.; Namyslo, J. C.; Nieger, M.; Hemmen, S.: Pyrimidine-annulated pyrrolobenzodiazepines. A new ring system related to aspergillus alkaloids. Eur. J. Org. Chem. 9 (2005) 1781–1789.10.1002/chin.200535190Suche in Google Scholar
©2019 Shi Yun-lian et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Frontmatter
- Crystal structure of poly[diaqua-(μ8-1,1′:2′,1′′-terphenyl-3,3′′,4′,5′-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)dicopper(II)], C22H14Cu2O10
- Crystal structure of 2-((1H-benzo[d]imidazol-2-ylimino)methyl)-4,6-di-tert-butylphenol, C22H27N3O
- Crystal structure of (4-ethoxynaphthalen-1-yl)(furan-2-yl)methanone, C17H14O3
- Crystal structure of 1-nonylpyridazin-1-ium iodide, C13H23N2I
- Crystal structure of bis[diaqua(1,10-phenanthroline-κ2N, N′)-copper(II)]diphenylphosphopentamolybdate dihydrate, C36H38Cu2Mo5N4O27P2
- The crystal structure of tetrakis(imidazole)-copper(I) hexafluorophosphate, C12H16CuF6PN8
- The crystal structure of dimethyl ((3,5-di-tert-butyl-4-hydroxyphenyl)(phenyl)methyl)phosphonate, C23H33O4P
- Crystal structure of diaqua-bis(1,10-phenanthroline κ2N,N′)nickel(II) trifluoroacetate- trifluoroacetic acid (1/1), C30H21F9N4NiO8
- Crystal structure of 2-(naphthalen-2-yl)-1,8-naphthyridine, C18H12N2
- Synthesis and crystal structure of a new polymorph of diisopropylammonium trichloroacetate, C8H16Cl3NO2
- Crystal structure of dimethanol-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)cadmium(II) C34H34CdN12O2S2
- Crystal structure of ethyl 2,2-difluoro-2-(7-methoxy-2-oxo-2H-chromen-3-yl)acetate, C14H12F2O5
- The crystal structure of bis[μ2-(N,N-diethylcarbamodithioato-κS:κS,κS′)] bis[1′-(diphenylphosphino-κP)-1-cyanoferrocene]disilver(I), C56H56Ag2Fe2N4P2S4
- Crystal structure of bis(di-n-butylammonium) tetrachloridodiphenylstannate(IV), C28H50Cl4N2Sn
- The crystal structure of poly[(μ5-2-((5-bromo-3-formyl-2-hydroxybenzylidene)amino)benzenesulfonato-κ6O:O:O,O′:O′:O′′)sodium(I)], C13H9O4NSBrNa
- Crystal structure of catena-{poly[bis(O,O′-diethyldithiophosphato-S)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)-zinc(II)] di-acetonitrile solvate}, {C20H30N4O4P2S4Zn ⋅ 2 C2H3N}n
- Halogen and hydrogen bonding in the layered crystal structure of 2-iodoanilinium triiodide, C6H7I4N
- Crystal structure of cyclohexane-1,4-diammonium 2-[(2-carboxylatophenyl)disulfanyl]benzoate — dimethylformamide — monohydrate (1/1/1), [C6H16N2][C14H8O4S2] ⋅ C3H7NO⋅H2O
- The synthesis and crystal structure of isobutyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C16H13Cl2F6N3O3S
- Isolation and crystal structure of bufotalinin — methanol (1/1), C25H34O7
- Crystal structure of benzylbis(1,3-diphenylpropane-1,3-dionato-κ2O,O′) chloridotin(IV), C37H29ClO4Sn
- Crystal structure of Bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imidazol)}diiodidocadmium(II), [Cd(C11H11N5)2I2], C22H22N10I2Cd
- Crystal structure of 4-isobutoxybenzaldehyde oxime, C11H15NO2
- The crystal structure of bis(acetato-κ1O)-bis(N′-hydroxypyrimidine-2-carboximidamide-κ2N,N′)manganese(II) — methanol (1/2), C14H18MnN8O6, 2(CH3OH)′
- Crystal structure of poly[bis(μ2-bis(4-(1H-imidazol-1-yl)phenyl)amine-κ2N:N′)-bis(nitrato-κO)cadmium(II)], C36H30CdN12O6
- Crystal structure and optical properties of 1,6-bis(methylthio)pyrene, C18H14S2
- The crystal structure of hexaquamagnesium(II) bis(3,4-dinitropyrazol-1-ide), C6H14MgN8O14
- Halogen bonds in the crystal structure of 4,3:5,4-terpyridine – 1,4-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure and photochromic properties of a novel photochromic perfluordiarylethene containing a triazole bridged pyridine group moiety, C24H18F6N4S2
- Crystal structure of bis[(μ3-oxido)-(μ2-(N,N-diisopropylthiocarbamoylthio) acetato-κ2O,O′)-((N,N-diisopropylthiocarbamoylthio)acetato-κO)-bis(di-4-methylbenzyl-tin(IV))], C100H136N4O10S8Sn4
- Crystal structure of dibromidobis(4-bromobenzyl)tin(IV), C14H12Br4Sn
- The crystal structure of (4Z)-2-[(E)-(1-ethyl-3,3-dimethyl-1,3-dihydro-2H-indol-2-ylidene)methyl]-4-[(1-ethyl-3,3-dimethyl-3H-indolium-2-yl)methylidene]-3-oxocyclobut-1-en-1-olate, C30H32N2O2
- The crystal structure of (E)-3-(4-(dimethylamino)styryl)-5,5-dimethylcyclohex-2-en-1-one, C18H23NO
- Crystal structure of dihydrazinium 1H-pyrazole-3,5-dicarboxylate, C5H12N6O4
- Crystal structure of poly[μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4-sulfidobenzoate-κ2O:S)cobalt(II)] dihydrate, C42H44Co2N8O7S2
- Crystal structure of 8-(3,4-dimethylbenzylidene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C17H18O4
- Crystal structure of 4-(2-bromo-4-(6-morpholino-3-phenyl-3H-benzo[f]chromen-3-yl) cyclohexa-2,5-dien-1-yl)morpholine, C33H31BrN2O
- Synthesis and crystal structure of 2-((1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)methylene)-2,3-dihydro-1H-inden-1-one, C23H16N2OS
- Crystal structure of poly[(μ2-1,1′-(oxybis(4,1-phenylene)bis(1H-imidazole)-κ2N,N′)(μ2-1,3-benzenecarboxylato-κ3O,O′:O′′)zinc(II)] dihydrate, C26H22N4O7Zn
- Crystal structure of diaqua-bis(cinnamato-κ2O,O′)zinc(II), C18H18ZnO6
- Crystal structure of 2-(prop-2-yn-1-yloxy)-1-naphthaldehyde, C14H10O2
- Crystal structure and photochromic properties of 1-(2-methyl-5-phenyl-3-thienyl)-2-{2-methyl-5-[4-(9-fluorenone hydrazone)-phenyl]-3-thienyl}perfluorocyclopentene, C41H26F6N2S2
- Hydrothermal synthesis and crystal structure of cylo[tetraaqua-bis(μ2-1,4-bis(1H-benzo[d]imidazol-1-yl)but-2-ene-κ2N:N′)-bis(μ2-4-nitro-phthalate-κ2O,O′)dinickel(II)], C26H23N5O8Ni
- Crystal structure of 3-[methyl(phenyl)amino]-1-phenylthiourea, C14H15N3S
- Crystal structure of 1-(4-chlorophenyl)-3-[methyl(phenyl)amino]thiourea, C14H14ClN3S
- Crystal structure of 2-tert-butyl-1H-imidazo[4,5-b]pyridine, C10H13N3
- Crystal structure of 5-carboxy-2-(2-carboxyphenyl)-1H-imidazol-3-ium-4-carboxylate dihydrate, C12H8N2O6⋅2(H2O)
- The crystal structure of dichlorido-μ2-dichlorido-(η2-1,4-bis(4-vinylbenzyl)-1,4-diazabicyclo[2.2.2]octane-1,4-diium)dicopper(I), C24H30N2Cu2Cl4
- Crystal structure of 4-bromobenzyl (Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C22H29BrN2OS
- Crystal structure of (4S,4aS,6aR,6bR,12aS,12bR,14aS,14bR)-3,3,6a,6b,9,9,12a-heptamethyloctadecahydro-1H,3H-4,14b-ethanophenanthro[1,2-h]isochromene-1(6bH)-one, C30H48O2
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C30H33F6N3S
- The crystal structure of 3-methoxyphenanthridin-6(5H)-one, C14H11NO2
- Crystal structure of 4-(5,5-difluoro-1,3,7,9-tetramethyl-3H,5H-5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)pyridin-1-ium tetraiodidoferrate(III), C18H19BF2FeI4N3
- Crystal structure of 2-(3-methoxyphenyl)-3-((phenylsulfonyl)methyl)imidazo[1,2-a]pyridine, C21H18N2O3S
- Crystal structure of [(2-(2-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) perchlorate, C29H50Cl2N4NiO8
- Crystal structure of (Z)-6-(dimethylamino)-3,3-bis(4-(dimethylamino)phenyl)-2-(2-(quinoxalin-2-ylmethylene)hydrazinyl)-2,3-dihydroinden-1-one, C35H35N7O
- 5-Methyl-N′-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbonyl]-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbohydrazide, C22H22N8O2
- Crystal structure of 2,3-dichloro-6-methoxyquinoxaline, C9H6Cl2N2O
- Synthesis and crystal structure of 7-chloro-2-(ethylsulfinyl)-6-fluoro-3-(1H-pyrazole-1-yl)-4H-thiochromen-4-one, C13H10FN3OS2
- Crystal structure of 4-ethylpiperazine-1-carbothioic dithioperoxyanhydride, C14H26N4S4
- Crystal structure of 2-(2-(6-methylpyridin-2-yl)naphthalen-1-yl)pyrimidine, C20H15N3
- The crystal structure of N′-((1E,2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-ylidene)-3-methylbenzohydrazide, C23H22N2O4
- Crystal structure of catena-poly[(μ2-isophthalato-κ2O:O′)-(2,5-di(pyrazin-2-yl)-4,4′-bipyridine-κ3N,N′,N′′)zinc(II)] — water (2/5), C26H21N6O6.5Zn
- Crystal structure of (3E,5E)-3,5-bis(3-nitrobenzylidene)-1-((4-(trifluoromethyl)phenyl)sulfonyl)piperidin-4-one — dichloromethane (2/1), C53H38Cl2F6N6O14S2
- Crystal structure of (μ2-oxido)-bis(N,N′-o-phenylenebis(salicylideneiminato))diiron(III) — N,N′-dimethylformamide, C47H43Fe2N4O9
- Crystal structure of N1,N3-bis(2-hydroxyethyl)-N1, N1,N3,N3-tetramethylpropane-1,3-diaminium dibromide, C11H28Br2N2O2
- Crystal structure of (E)-N-(4-chlorophenyl)-1-(pyridin-2-yl)methanimine, C12H9ClN2
- Crystal structure of 8-bromo-6-oxo-2-phenyl-6H-pyrrolo[3,2,1-ij]quinoline-5-carbaldehyde, C18H11BrNO2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride trihydrate, C8H18N8Cl2 ⋅ 3 H2O
- Crystal structure of (E)-4-bromo-N-(pyridin-2-ylmethylene)aniline, C12H9BrN2
- Crystal structure of bis[(2-(3-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ-O)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Br2N4NiO8
- The crystal structure of (1E,2E)-2-methyl-4-((7-oxo-7H-furo[3,2-g]chromen-9-yl)oxy)but-2-enal O-isonicotinoyl oxime–trichloromethane (3/1), C67H49Cl3N6O18
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-methyl-1H-imidazol-3-ium hexafluoridophosphate(V), C8H13F6N2O2P
- Crystal structure of bis[(2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κO)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) hemihydrate C42H65Br2N4NiO8.5
- The crystal structure of N-(7-(4-fluorobenzylidene)-3-(4-fluorophenyl)-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbonothioyl)benzamide, C28H23F2N3OS
- The crystal structure of N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide, C18H20N4O2
- Crystal structure of (E)-2-(3,6-bis(ethylamino)-2,7-dimethyl-9H-xanthen-9-yl)-N′-((6-methylpyridin-2-yl)methylene)benzohydrazide – methanol (1/1), C34H37N5O3
- Crystal structure of 2-oxo-1-(pyrimidin-5-ylmethyl)-3-(3-(trifluoromethyl)phenyl)-1,2-dihydro-5l4-pyrido[1,2-a]pyrimidin-4-olate, C20H13F3N4O2
- Crystal structure of poly[(μ3-9H-carbazole-3,6-dicarboxylato-κ3O1: O2: O3)(μ2-4-(pyridin-4-yl)pyridine-κ2N1:N1′)zinc(II)], C19H11N2O4Zn
- Crystal structure of (E)-N′-((1,8-dihydropyren-1-yl)-methylene)picolinohydrazide, C23H15N3O
- Crystal structure of catena-poly{[μ2-1,2-bis(diphenylphosphino)ethane]dichloridocadmium(II)}, C26H24CdCl2P2
- Crystal structure of the 1:2 co-crystal between N,N′-bis(4-pyridylmethyl)oxalamide and acetic acid as a dihydrate, C14H14N4O2⋅2 C2H4O2⋅2 H2O
- Crystal structure of the co-crystal N,N′-bis(3-pyridylmethyl)oxalamide acetic acid (1/2), C14H14N4O2⋅2C2H4O2
- Crystal structure of the co-crystal N,N′-bis(4-pyridylmethyl)oxalamide and 2,3,5,6-tetrafluoro-1,4-di-iodobenzene (1/1), C14H14N4O2⋅C6F4I2
- Crystal structure of the co-crystal 4-[(4-carboxyphenyl)disulfanyl]benzoic acid–(1E,4E)-1-N,4-N-bis(pyridin-4-ylmethylidene)cyclohexane-1,4-diamine (1/1), C14H10O4S2⋅C18H20N4
- Crystal structure of hexacarbonyl-bis(μ2-di-n-propyldithiocarbamato-κ3S,S′:S;κ3S:S:S′)-di-rhenium(I), C20H28N2O6Re2S4
- Crystal structure of fac-tricarbonyl-morpholine-κN-(morpholinocarbamodithioato-κ2S,S′)rhenium(I), C12H17N2O5ReS2
Artikel in diesem Heft
- Frontmatter
- Crystal structure of poly[diaqua-(μ8-1,1′:2′,1′′-terphenyl-3,3′′,4′,5′-tetracarboxylato-κ8O1:O2:O3:O4:O5:O6:O7:O8)dicopper(II)], C22H14Cu2O10
- Crystal structure of 2-((1H-benzo[d]imidazol-2-ylimino)methyl)-4,6-di-tert-butylphenol, C22H27N3O
- Crystal structure of (4-ethoxynaphthalen-1-yl)(furan-2-yl)methanone, C17H14O3
- Crystal structure of 1-nonylpyridazin-1-ium iodide, C13H23N2I
- Crystal structure of bis[diaqua(1,10-phenanthroline-κ2N, N′)-copper(II)]diphenylphosphopentamolybdate dihydrate, C36H38Cu2Mo5N4O27P2
- The crystal structure of tetrakis(imidazole)-copper(I) hexafluorophosphate, C12H16CuF6PN8
- The crystal structure of dimethyl ((3,5-di-tert-butyl-4-hydroxyphenyl)(phenyl)methyl)phosphonate, C23H33O4P
- Crystal structure of diaqua-bis(1,10-phenanthroline κ2N,N′)nickel(II) trifluoroacetate- trifluoroacetic acid (1/1), C30H21F9N4NiO8
- Crystal structure of 2-(naphthalen-2-yl)-1,8-naphthyridine, C18H12N2
- Synthesis and crystal structure of a new polymorph of diisopropylammonium trichloroacetate, C8H16Cl3NO2
- Crystal structure of dimethanol-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)cadmium(II) C34H34CdN12O2S2
- Crystal structure of ethyl 2,2-difluoro-2-(7-methoxy-2-oxo-2H-chromen-3-yl)acetate, C14H12F2O5
- The crystal structure of bis[μ2-(N,N-diethylcarbamodithioato-κS:κS,κS′)] bis[1′-(diphenylphosphino-κP)-1-cyanoferrocene]disilver(I), C56H56Ag2Fe2N4P2S4
- Crystal structure of bis(di-n-butylammonium) tetrachloridodiphenylstannate(IV), C28H50Cl4N2Sn
- The crystal structure of poly[(μ5-2-((5-bromo-3-formyl-2-hydroxybenzylidene)amino)benzenesulfonato-κ6O:O:O,O′:O′:O′′)sodium(I)], C13H9O4NSBrNa
- Crystal structure of catena-{poly[bis(O,O′-diethyldithiophosphato-S)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)-zinc(II)] di-acetonitrile solvate}, {C20H30N4O4P2S4Zn ⋅ 2 C2H3N}n
- Halogen and hydrogen bonding in the layered crystal structure of 2-iodoanilinium triiodide, C6H7I4N
- Crystal structure of cyclohexane-1,4-diammonium 2-[(2-carboxylatophenyl)disulfanyl]benzoate — dimethylformamide — monohydrate (1/1/1), [C6H16N2][C14H8O4S2] ⋅ C3H7NO⋅H2O
- The synthesis and crystal structure of isobutyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C16H13Cl2F6N3O3S
- Isolation and crystal structure of bufotalinin — methanol (1/1), C25H34O7
- Crystal structure of benzylbis(1,3-diphenylpropane-1,3-dionato-κ2O,O′) chloridotin(IV), C37H29ClO4Sn
- Crystal structure of Bis{1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-methyl-imidazol)}diiodidocadmium(II), [Cd(C11H11N5)2I2], C22H22N10I2Cd
- Crystal structure of 4-isobutoxybenzaldehyde oxime, C11H15NO2
- The crystal structure of bis(acetato-κ1O)-bis(N′-hydroxypyrimidine-2-carboximidamide-κ2N,N′)manganese(II) — methanol (1/2), C14H18MnN8O6, 2(CH3OH)′
- Crystal structure of poly[bis(μ2-bis(4-(1H-imidazol-1-yl)phenyl)amine-κ2N:N′)-bis(nitrato-κO)cadmium(II)], C36H30CdN12O6
- Crystal structure and optical properties of 1,6-bis(methylthio)pyrene, C18H14S2
- The crystal structure of hexaquamagnesium(II) bis(3,4-dinitropyrazol-1-ide), C6H14MgN8O14
- Halogen bonds in the crystal structure of 4,3:5,4-terpyridine – 1,4-diiodotetrafluorobenzene (1/1), C21H11F4I2N3
- Crystal structure and photochromic properties of a novel photochromic perfluordiarylethene containing a triazole bridged pyridine group moiety, C24H18F6N4S2
- Crystal structure of bis[(μ3-oxido)-(μ2-(N,N-diisopropylthiocarbamoylthio) acetato-κ2O,O′)-((N,N-diisopropylthiocarbamoylthio)acetato-κO)-bis(di-4-methylbenzyl-tin(IV))], C100H136N4O10S8Sn4
- Crystal structure of dibromidobis(4-bromobenzyl)tin(IV), C14H12Br4Sn
- The crystal structure of (4Z)-2-[(E)-(1-ethyl-3,3-dimethyl-1,3-dihydro-2H-indol-2-ylidene)methyl]-4-[(1-ethyl-3,3-dimethyl-3H-indolium-2-yl)methylidene]-3-oxocyclobut-1-en-1-olate, C30H32N2O2
- The crystal structure of (E)-3-(4-(dimethylamino)styryl)-5,5-dimethylcyclohex-2-en-1-one, C18H23NO
- Crystal structure of dihydrazinium 1H-pyrazole-3,5-dicarboxylate, C5H12N6O4
- Crystal structure of poly[μ2-1,4-bis((1H-imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4-sulfidobenzoate-κ2O:S)cobalt(II)] dihydrate, C42H44Co2N8O7S2
- Crystal structure of 8-(3,4-dimethylbenzylidene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C17H18O4
- Crystal structure of 4-(2-bromo-4-(6-morpholino-3-phenyl-3H-benzo[f]chromen-3-yl) cyclohexa-2,5-dien-1-yl)morpholine, C33H31BrN2O
- Synthesis and crystal structure of 2-((1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)methylene)-2,3-dihydro-1H-inden-1-one, C23H16N2OS
- Crystal structure of poly[(μ2-1,1′-(oxybis(4,1-phenylene)bis(1H-imidazole)-κ2N,N′)(μ2-1,3-benzenecarboxylato-κ3O,O′:O′′)zinc(II)] dihydrate, C26H22N4O7Zn
- Crystal structure of diaqua-bis(cinnamato-κ2O,O′)zinc(II), C18H18ZnO6
- Crystal structure of 2-(prop-2-yn-1-yloxy)-1-naphthaldehyde, C14H10O2
- Crystal structure and photochromic properties of 1-(2-methyl-5-phenyl-3-thienyl)-2-{2-methyl-5-[4-(9-fluorenone hydrazone)-phenyl]-3-thienyl}perfluorocyclopentene, C41H26F6N2S2
- Hydrothermal synthesis and crystal structure of cylo[tetraaqua-bis(μ2-1,4-bis(1H-benzo[d]imidazol-1-yl)but-2-ene-κ2N:N′)-bis(μ2-4-nitro-phthalate-κ2O,O′)dinickel(II)], C26H23N5O8Ni
- Crystal structure of 3-[methyl(phenyl)amino]-1-phenylthiourea, C14H15N3S
- Crystal structure of 1-(4-chlorophenyl)-3-[methyl(phenyl)amino]thiourea, C14H14ClN3S
- Crystal structure of 2-tert-butyl-1H-imidazo[4,5-b]pyridine, C10H13N3
- Crystal structure of 5-carboxy-2-(2-carboxyphenyl)-1H-imidazol-3-ium-4-carboxylate dihydrate, C12H8N2O6⋅2(H2O)
- The crystal structure of dichlorido-μ2-dichlorido-(η2-1,4-bis(4-vinylbenzyl)-1,4-diazabicyclo[2.2.2]octane-1,4-diium)dicopper(I), C24H30N2Cu2Cl4
- Crystal structure of 4-bromobenzyl (Z)-N-(adamantan-1-yl)morpholine-4-carbothioimidate, C22H29BrN2OS
- Crystal structure of (4S,4aS,6aR,6bR,12aS,12bR,14aS,14bR)-3,3,6a,6b,9,9,12a-heptamethyloctadecahydro-1H,3H-4,14b-ethanophenanthro[1,2-h]isochromene-1(6bH)-one, C30H48O2
- Crystal structure of 3,5-bis(trifluoromethyl)benzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C30H33F6N3S
- The crystal structure of 3-methoxyphenanthridin-6(5H)-one, C14H11NO2
- Crystal structure of 4-(5,5-difluoro-1,3,7,9-tetramethyl-3H,5H-5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)pyridin-1-ium tetraiodidoferrate(III), C18H19BF2FeI4N3
- Crystal structure of 2-(3-methoxyphenyl)-3-((phenylsulfonyl)methyl)imidazo[1,2-a]pyridine, C21H18N2O3S
- Crystal structure of [(2-(2-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′) (5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) perchlorate, C29H50Cl2N4NiO8
- Crystal structure of (Z)-6-(dimethylamino)-3,3-bis(4-(dimethylamino)phenyl)-2-(2-(quinoxalin-2-ylmethylene)hydrazinyl)-2,3-dihydroinden-1-one, C35H35N7O
- 5-Methyl-N′-[5-methyl-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbonyl]-1-(4-methylphenyl)-1H-1,2,3-triazole-4-carbohydrazide, C22H22N8O2
- Crystal structure of 2,3-dichloro-6-methoxyquinoxaline, C9H6Cl2N2O
- Synthesis and crystal structure of 7-chloro-2-(ethylsulfinyl)-6-fluoro-3-(1H-pyrazole-1-yl)-4H-thiochromen-4-one, C13H10FN3OS2
- Crystal structure of 4-ethylpiperazine-1-carbothioic dithioperoxyanhydride, C14H26N4S4
- Crystal structure of 2-(2-(6-methylpyridin-2-yl)naphthalen-1-yl)pyrimidine, C20H15N3
- The crystal structure of N′-((1E,2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-ylidene)-3-methylbenzohydrazide, C23H22N2O4
- Crystal structure of catena-poly[(μ2-isophthalato-κ2O:O′)-(2,5-di(pyrazin-2-yl)-4,4′-bipyridine-κ3N,N′,N′′)zinc(II)] — water (2/5), C26H21N6O6.5Zn
- Crystal structure of (3E,5E)-3,5-bis(3-nitrobenzylidene)-1-((4-(trifluoromethyl)phenyl)sulfonyl)piperidin-4-one — dichloromethane (2/1), C53H38Cl2F6N6O14S2
- Crystal structure of (μ2-oxido)-bis(N,N′-o-phenylenebis(salicylideneiminato))diiron(III) — N,N′-dimethylformamide, C47H43Fe2N4O9
- Crystal structure of N1,N3-bis(2-hydroxyethyl)-N1, N1,N3,N3-tetramethylpropane-1,3-diaminium dibromide, C11H28Br2N2O2
- Crystal structure of (E)-N-(4-chlorophenyl)-1-(pyridin-2-yl)methanimine, C12H9ClN2
- Crystal structure of 8-bromo-6-oxo-2-phenyl-6H-pyrrolo[3,2,1-ij]quinoline-5-carbaldehyde, C18H11BrNO2
- Crystal structure of 1,4-bis(2-azidoethyl)piperazine-1,4-diium dichloride trihydrate, C8H18N8Cl2 ⋅ 3 H2O
- Crystal structure of (E)-4-bromo-N-(pyridin-2-ylmethylene)aniline, C12H9BrN2
- Crystal structure of bis[(2-(3-bromophenyl)-5-methyl-1,3-dioxane-5-carboxylato-κ-O)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II), C40H60Br2N4NiO8
- The crystal structure of (1E,2E)-2-methyl-4-((7-oxo-7H-furo[3,2-g]chromen-9-yl)oxy)but-2-enal O-isonicotinoyl oxime–trichloromethane (3/1), C67H49Cl3N6O18
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-methyl-1H-imidazol-3-ium hexafluoridophosphate(V), C8H13F6N2O2P
- Crystal structure of bis[(2-(2-bromophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κO)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)]nickel(II) hemihydrate C42H65Br2N4NiO8.5
- The crystal structure of N-(7-(4-fluorobenzylidene)-3-(4-fluorophenyl)-3,3a,4,5,6,7-hexahydro-2H-indazole-2-carbonothioyl)benzamide, C28H23F2N3OS
- The crystal structure of N1,N4-bis(pyridin-3-yl)cyclohexane-1,4-dicarboxamide, C18H20N4O2
- Crystal structure of (E)-2-(3,6-bis(ethylamino)-2,7-dimethyl-9H-xanthen-9-yl)-N′-((6-methylpyridin-2-yl)methylene)benzohydrazide – methanol (1/1), C34H37N5O3
- Crystal structure of 2-oxo-1-(pyrimidin-5-ylmethyl)-3-(3-(trifluoromethyl)phenyl)-1,2-dihydro-5l4-pyrido[1,2-a]pyrimidin-4-olate, C20H13F3N4O2
- Crystal structure of poly[(μ3-9H-carbazole-3,6-dicarboxylato-κ3O1: O2: O3)(μ2-4-(pyridin-4-yl)pyridine-κ2N1:N1′)zinc(II)], C19H11N2O4Zn
- Crystal structure of (E)-N′-((1,8-dihydropyren-1-yl)-methylene)picolinohydrazide, C23H15N3O
- Crystal structure of catena-poly{[μ2-1,2-bis(diphenylphosphino)ethane]dichloridocadmium(II)}, C26H24CdCl2P2
- Crystal structure of the 1:2 co-crystal between N,N′-bis(4-pyridylmethyl)oxalamide and acetic acid as a dihydrate, C14H14N4O2⋅2 C2H4O2⋅2 H2O
- Crystal structure of the co-crystal N,N′-bis(3-pyridylmethyl)oxalamide acetic acid (1/2), C14H14N4O2⋅2C2H4O2
- Crystal structure of the co-crystal N,N′-bis(4-pyridylmethyl)oxalamide and 2,3,5,6-tetrafluoro-1,4-di-iodobenzene (1/1), C14H14N4O2⋅C6F4I2
- Crystal structure of the co-crystal 4-[(4-carboxyphenyl)disulfanyl]benzoic acid–(1E,4E)-1-N,4-N-bis(pyridin-4-ylmethylidene)cyclohexane-1,4-diamine (1/1), C14H10O4S2⋅C18H20N4
- Crystal structure of hexacarbonyl-bis(μ2-di-n-propyldithiocarbamato-κ3S,S′:S;κ3S:S:S′)-di-rhenium(I), C20H28N2O6Re2S4
- Crystal structure of fac-tricarbonyl-morpholine-κN-(morpholinocarbamodithioato-κ2S,S′)rhenium(I), C12H17N2O5ReS2


