Abstract
C10H8FN3OS2, monoclinic, P21/c (no. 14), a = 6.2909(2) Å, b = 7.4103(2) Å, c = 24.7079(6) Å, β = 97.396(2)°, V = 1142.24(6) Å3, Z = 4, Rgt(F) = 0.0375, wRref(F2) = 0.1014, T = 293 K.
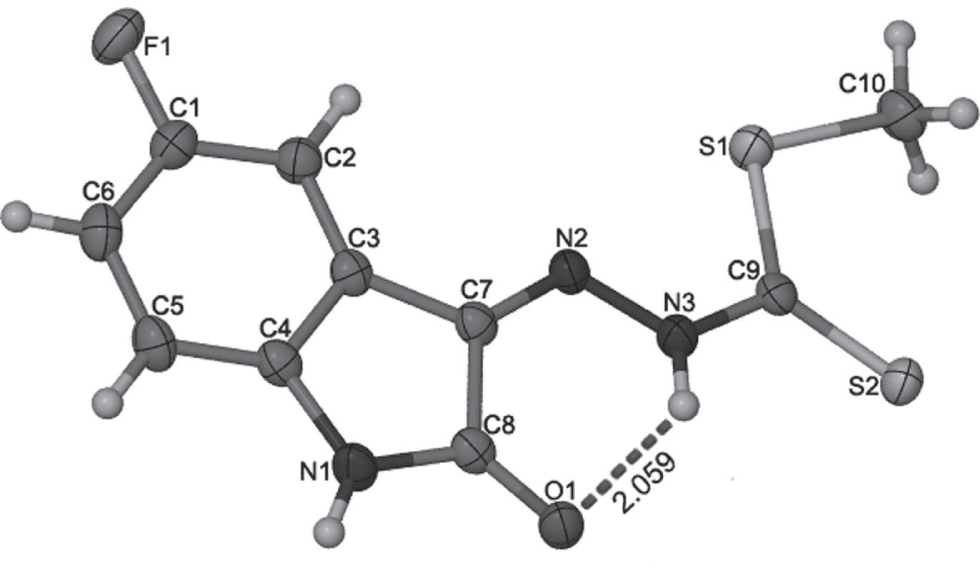
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Block, orange |
| Size: | 0.30 × 0.20 × 0.15 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.47 mm−1 |
| Diffractometer, scan mode: | Bruker FRAMBO, φ and ω-scans |
| θmax, completeness: | 26.7°, >95% |
| N(hkl)measured, N(hkl)unique, Rint: | 4237, 2316, 0.026 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2055 |
| N(param)refined: | 154 |
| Programs: | Bruker programs [1], OLEX2 [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| F1 | 0.9211(2) | 0.9622(2) | 0.41251(5) | 0.0447(4) |
| S1 | 0.87398(7) | 0.74803(7) | 0.69049(2) | 0.02957(16) |
| S2 | 0.51079(8) | 0.55752(8) | 0.73725(2) | 0.03156(16) |
| O1 | 0.1503(2) | 0.5651(2) | 0.56565(6) | 0.0299(3) |
| N1 | 0.2120(2) | 0.6427(2) | 0.47797(6) | 0.0281(4) |
| H1A | 0.0938 | 0.6056 | 0.4601 | 0.034* |
| N2 | 0.5934(2) | 0.7257(2) | 0.59387(6) | 0.0240(3) |
| N3 | 0.5034(2) | 0.6614(2) | 0.63661(6) | 0.0266(4) |
| H3A | 0.3726 | 0.6249 | 0.6319 | 0.032* |
| C1 | 0.7417(3) | 0.8824(3) | 0.42727(8) | 0.0298(4) |
| C2 | 0.7327(3) | 0.8517(3) | 0.48182(8) | 0.0276(4) |
| H2B | 0.8443 | 0.8843 | 0.5084 | 0.033* |
| C3 | 0.5478(3) | 0.7692(2) | 0.49493(8) | 0.0236(4) |
| C4 | 0.3805(3) | 0.7228(3) | 0.45399(8) | 0.0256(4) |
| C5 | 0.3935(3) | 0.7574(3) | 0.39960(8) | 0.0308(4) |
| H5A | 0.2813 | 0.7278 | 0.3728 | 0.037* |
| C6 | 0.5788(3) | 0.8378(3) | 0.38616(8) | 0.0328(4) |
| H6A | 0.5937 | 0.8616 | 0.3499 | 0.039* |
| C7 | 0.4801(3) | 0.7138(3) | 0.54628(8) | 0.0238(4) |
| C8 | 0.2607(3) | 0.6316(3) | 0.53294(7) | 0.0249(4) |
| C9 | 0.6183(3) | 0.6539(3) | 0.68739(7) | 0.0239(4) |
| C10 | 0.9652(3) | 0.7363(3) | 0.76235(8) | 0.0332(5) |
| H10A | 1.1074 | 0.7850 | 0.7694 | 0.050* |
| H10B | 0.9663 | 0.6128 | 0.7741 | 0.050* |
| H10C | 0.8705 | 0.8049 | 0.7820 | 0.050* |
Source of material
The title compound was obtained via condensation of 5-fluoroindoline-2,3-dione (0.3 g, 2 mmol) with methyl hydrazinecarbodithioate (0.2 g, 2 mmol) in 8 mL methanol at room temperature for 12 h. The mixture was isolated by column chromatography on silica gel with dichloromethane/methanol = 98:2 (v/v) as eluent to give the title compound methyl (Z)-2-(5-fluoro-2-oxoindolin-3-ylidene)hydrazine-1-carbodithioate (yield 65%; m.p. 476−478 K). Orange crystals of the title compound were obtained by slow evaporation of the mixed solvent of dichloromethane/methanol = 99:1 (v/v) in air.
Experimental details
All the H atoms were discernible in the difference electron density maps. Nevertheless, the hydrogen atoms were placed into idealized positions and allowed to ride on the carrier atoms, with C—H = 0.93 and 0.96 Å for aryl and methylene hydrogens, respectively, and N—H = 0.86 Å for −N—H group. Uiso(H) = 1.5Ueq(C)methyl. Uiso(H) = 1.2Ueq(C)aryl/methylene.
Discussion
Indolin-2-one is one of important heterocyclic scaffolds. Some derivatives have attracted much attentions because they are regarded as new anticancer agents [4], [5], [6], [7]. Recently, our group synthesized two platinum(II) complexes with methyl hydrazinecarbodithioate derivatives of indolin-2-one, which can non-covalently bind to DNA with high affinity and exhibit cytotoxicity against cancer cells by inducing apoptosis [8]. In order to investigate the structure-activity relationship of methyl hydrazinecarbodithioate derivatives of indolin-2-ones and to search for more effective ligands, we synthesized the new title compound, an isotypic structure of S-methyl 2-(5-chloro-2-oxoindolin-3-ylidene)hydrazinecarbodithioate reported [9], and characterized it via single-crystal X-ray crystallographical analysis.
As shown in the Figure, this molecule takes an almost planar configuration with the torsion angle N3—N2—C7—C8 of 1.7(3)°. Notably, intramolecular N3—H⋯O1 bonding interaction with D⋯A of 2.741(2) Å and D—H⋯A angle of 135.7° is presented, which can well account for the planar configuration of the title molecule. The double bond length between C7 and N2 (1.297(2) Å) and the N2–C7–C8 (127.39(18)°) angle are in agreement with those of the analogous compound (Z)-methyl N′-(5,7-dibromo-1-(2-morpholinoethyl)-2-oxoindolin-3-ylidene)hydrazinecarbodithioate (1.288(4) Å, 128.1(3)°) [10]. The C9 = S2 bond length is 1.6433(19) Å, and the torsion angle S1—C9—N3—N2 of 4.3(2)° also display the planar configuration.
Acknowledgements
The authors are grateful to the 973 project (Grant No. 2013CB911002) for financial support.
References
Bruker: APEX2, SAINT and SADABS. Brucker AXS Inc., Madison, WI, USA (2009).Search in Google Scholar
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–341.10.1107/S0021889808042726Search in Google Scholar
Sheldrick, G. M.: SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
Dweedar, H. E.; Mahrous, H.; Ibrahim, H. S.; Abdel-Aziz, H. A.: Analogue-based design, synthesis and biological evaluation of 3-substituted-(methylenehydrazono)indolin-2-ones as anticancer agents. Eur. J. Med. Chem. 78 (2014) 275–280.10.1016/j.ejmech.2014.03.058Search in Google Scholar PubMed
Ibrahim, H. S.; Abou-Seri, S. M.; Abdel-Aziz, H. A.: 3-Hydrazinoindolin-2-one derivatives: Chemical classification and investigation of their targets as anticancer agents. Eur. J. Med. Chem. 122 (2016) 366–381.10.1016/j.ejmech.2016.06.034Search in Google Scholar PubMed
Li, P. K.; Xiao, Z.; Hu, Z.; Pandit, B.; Sun, Y.; Sackett, D. L.; Werbovetz, K.; Lewis, A.; Johnsamuel, J.: Conformationally restricted analogs of combretastatin A-4 derived from SU5416. Bioorg. Med. Chem. Lett. 15 (2005) 5382–5385.10.1016/j.bmcl.2005.09.001Search in Google Scholar PubMed
Liu, Z.; Hou, Y.; Zhang, G.; Xu, N.; Mi, B.; Gong, P.; Zhao, Y.: Design, synthesis and antitumor activity of novel indolin-2-one derivatives containing 4-thiazolidinone moiety. Chem. Res. Chinese U. 31 (2015) 235–243.10.1007/s40242-015-4335-8Search in Google Scholar
Li, Y. S.; Peng, B.; Ma, L.; Cao, S. L.; Bai, L. L.; Yang, C. R.; Wan, C. Q.; Yan, H. J.; Ding, P. P.; Li, Z. F.; Liao, J.; Meng, Y. Y.; Wang, H. L.; Li, J.; Xu, X.: Synthesis, crystal structures and antitumor activity of two platinum(II) complexes with methyl hydrazinecarbodithioate derivatives of indolin-2-one. Eur. J. Med. Chem. 127 (2017) 137–146.10.1016/j.ejmech.2016.12.050Search in Google Scholar PubMed
Manan, M. A. F. A.; Tahir, M. I. M.; Crouse, K. A.; Watkin, D. J.: A crystallographic study of S-methyl 2-(5-chloro-2-oxoindolin-3-ylidene)hydrazinecarbodithioate. J. Chem. Crystallogr. 41 (2011) 230–235.10.1007/s10870-010-9869-5Search in Google Scholar
Ma, L.; Li, A. M.; Wan, C. Q.; Cao, S. L.: Crystal structure of (Z)-methyl N′-(5,7-dibromo-1-(2-morpholinoethyl)-2-oxoindolin-3-ylidene) hydrazinecarbodithioate. Z. Kristallogr. NCS 229 (2014) 25–26.10.1515/ncrs-2014-0020Search in Google Scholar
©2018 Xin-Hong Li et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Editorial 2018
- Crystal structure of dimethanol-bis{3-(((2-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N:O′}dizinc(II), C42H30Zn2N2O10
- Crystal structure of aqua-bis{[2,6-dimethyl-N-(pyridin-2-ylmethylene)aniline-κ2N,N′]}zinc(II) triflate monohydrate [ZnC29H31N4O]CF3SO3⋅H2O
- Crystal structure of (E)-1-(4-{[(E)-4-Diethylamino-2-hydroxybenzene methylene]amino}phenyl)ethanone methoxy oxime, C20H27ClN3O3
- Crystal structure of (E)-1-(4-(((E)-4-(diethylamino)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one oxime, C19H23N3O2
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4,4′-sulfonyldibenzoato-κ2O:O′)zinc(II)], C40H34N4O6SZn
- Crystal structure of catena-poly[diaqua(μ3-pyrazine-2,3-dicarboxylato-κ4O,N:O′:O′′)zinc(II)] 1.25 hydrate, C6H8.5N2O7.25Zn
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri-m-tolyl phosphane-κP)rhenium(I), C29H28O5PRe
- Crystal structure of bis(μ2-methanolato-κ2O:O)-bis(methanol-κ1O)-bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,O′,N}dichromium(III), C38H36Cr2N2O14
- Crystal structure of poly[aqua-(μ3-pyridine-3,5-dicarboxylato-κ5O,O′:O′′,O′′′,N)zinc(II)], C7H7NO6Zn
- Crystal structure of bis((1-(((4-(((benzyloxy)imino)methyl)phenyl)imino)methyl)naphthalen-2-yl)oxy-κ2O,N)copper(II), C52H42CuN4O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}cobalt(II), C40H38CoN4O6
- Crystal structure of diaqua-bis(N,N-dimethylformamide-κ1O)-bis{3-((5-chloro-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ4N,O,O′:O′}dinickel(II), C38H34Ni2Cl2N4O12
- Crystal structure of tetrakis(methanol-κO)bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}bicobalt(II), C38H38Co2N2O14
- Crystal structure of (S)-tert-butyl-(1-hydroxypropan-2-yl)carbamate, C8H17NO3
- Crystal structure of 4-(4′-(pyridin-4-yl)-[1,1′-biphenyl]-4-yl)pyridin-1-ium catena-poly[{5-carboxy-4′-methyl-[1,1′-biphenyl]-3-carboxylato-κ2O,O′}-(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-κ4O,O′:O′′,O′′′)lead(II)], C52H40N2O9Pb
- Crystal structure of catena-poly[diaqua-(μ2-5-methylisophthalato-κ2O:O′)(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)], NiC21H22O6N6
- Crystal structure of the salt tris(guanidinium) tris(tetrapropylammonium) bis(pyridine-2,4,6-tricarboxylate) – water (1/10), C55H126N14O22
- Crystal structure of 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7,8-trimethoxy-4H-chromen-4-one, C19H18O8
- Crystal structure of poly{[μ2-1,1′-(sulfonylbis(4,1-phenylene))bis(2-methyl-1H-imidazole)-κ2N:N′][μ2-4,4′-oxydibenzoato-κ2O:O′]cobalt(II)} hemihydrate, C34H27N4O7.5SCo
- The crystal structure of 25,27-(2,2′-[(2-thioxo-1,3-dithiole-4,5-diyl)disulfanediyl]diethanolate)-26,28-dihydroxycalix[4]arene — dichloromethane (1/1), C36H32Cl2O4S5
- The crystal structure of 1,2-bis(3-(pyridin-3-yl)-1,2,4-oxadiazol-5-yl)ethane, C16H12N6O2
- Crystal structure of 1-benzyl-3-((4-bromophenyl)amino)-4-(4-methoxyphenyl)-1H-pyrrole-2,5-dione, C24H19BrN2O3
- Crystal structure of bis(2-((allylcarbamothioyl)imino)-4-methylthiazol-3-ido-κ2N,S)palladium(II), C16H20N6PdS4
- Crystal structure of pyrimidine-2,5-dicarboxylic acid 1.5 hydrate, C12H14N4O11
- Crystal structure of trans-diaqua-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II), C8H10N4O6Mn
- Crystal structure of catena-(μ3-5-bromoisophthatato-κO,O′: O′′,O′′′′)-(1,2-bis(imidazol-1-yl)ethane-κN:N′)cobalt(II), C16H13CoN4O4Br
- Investigation of the compound La5Zn2−xPb1 + x (x = 0.20–0.32)
- Crystal structure of (OC-6-13)-diaqua-bis(3,5-di(pyridin-3-yl)-4H-1,2,4-triazol-4-amine-κ1N)-bis(dicyanamido-κ1N)zinc(II) tetrahydrate, ZnC28H32N18O6
- Crystal structure of Ga0.62(3)Sb0.38(3)Pd3
- Crystal structure of Ga0.47(1)Sb0.53(1)Pd2
- A derivative of the Corey lactone – crystal structure of (3aR,4S,5R,6aS)-4-(((tert-butyldimethylsilyl)oxy)methyl)-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl benzoate, C21H30O5Si
- A Corey lactone: crystal structure of (3aR,4R,5R,6aS)-5-benzoyloxy-4(hydroxymethyl)hexahydro-2H-cyclopenta[b]furan-2-one, C15H16O5
- Hydrothermal synthesis and crystal structure of poly[aqua-(μ2-1,3-bis(4-pyridyl)propane-κ2N:N′)-(μ2-1,4,5,6,7,7-hexachlorobicyclo[2.2.1]hept-5-ene-2,3-dicarboxylato-κ2O:O′)manganese(II) hydrate, C22H20Cl6N2O6Mn
- Crystal structure of 2-acetylpyrrole S-methylthiosemicarbazonium hydroiodide, C8H13IN4S
- Crystal structure of [N,N-bis((pyrrol-2-yl)ethylidene)butane-1,4-diamine-κ4N,N′,N′′,N′′′]-nickel(II), C16H20N4Ni
- Crystal structure of poly[aqua-(μ5-2,5-dicarboxybenzoato-κ5O:O:O′:O′′:O′′′)sodium(I)], C9H7NaO7
- Crystal structure of bis(N′-((1H-pyrrol-2-yl)methylene)-1-methylthio-methanethiohydrazido-κ2S,N)nickel(II), C14H16N6NiS4
- Crystal structure of 1-(4-((benzo[d][1,3]dioxol-5-yloxy)methyl)phenethyl)-4-(3-chlorophenyl) piperazin-1-ium chloride, C26H28Cl2N2O3
- Crystal structure of 2-(4-(2-(4-(2-fluorophenyl)piperazin-1-yl)ethyl)benzyl)benzo[d]isothiazol-3(2H)-one 1,1-dioxide, C26H26FN3O3S – a saccharin dervative
- Crystal structure of 3-(2-dimethylaminoethyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C12H15N3OS
- Crystal structure of 3-(3-dimethylaminopropyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C13H17N3OS
- The crystal structure of trans-tetraaqua-bis(p-tolylsulfinato-κO)calcium(II)), C14H22O8S2Ca
- The crystal structure of (E)-N′-(pyridin-2-ylmethylene)pyrazine-2-carbohydrazide, C11H9N5O
- Crystal structure of (E)-3-(pyren-1-yl)-1-(pyridin-4-yl)prop-2-en-1-one, C24H15NO
- Crystal structure of catena-poly[diaqua-(μ2-tartrato-κ4O,O′:O′′,O′′′)cobalt(II)], C4H8CoO8
- Crystal structure of 4-chloro-2-methyl-6-(4-(trifluoromethoxy)phenyl)pyrimidine, C12H8ClF3N2O
- Crystal structure of 1-(4-fluorophenyl)-N-(5-((triphenylstannyl)thio)thiophen-2-yl)methanimine, C27H20FN3S2Sn
- Crystal structure of methyl (Z)-2-(5-fluoro-2-oxoindolin-3-ylidene)hydrazine-1-carbodithioate, C10H8FN3OS2
- Crystal structure of tert-butyl (Z)-4-(2-(5-methoxy-3-(2-((methylthio)carbonothioyl)hydrazono)-2-oxoindolin-1-yl)ethyl)piperazine-1-carboxylate, C22H31N5O4S2
- The crystal structure of (E)-2-((2-(o-tolylcarbamothioyl)hydrazono)methyl)benzoic acid, C16H15N3O2S
- Crystal structure of 2-chloro-1,3-di-tert-pentyl-4,4-diphenyl-1,3,2λ3,4-diazaphosphasiletidine, C22H32ClN2PSi
- Crystal structure of tetramethyl 5,5′-(buta-1,3-diyne-1,4-diyl)diisophthalate, C24H18O8
- Crystal structural of 2-amino-4-(4-methoxyphenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C19H20N2O3
- Crystal structure of 1,3,5-tris((trimethylsilyl)methyl)-1,3,5-triazinane-2,4,6-trione, C15H33N3O3Si3
- The crystal structure of bis(2-benzoyl-5-hydroxylphenolato-κ2O,O′)copper(II), C26H18CuO6
- Crystal structure of 2,6-bis(3-(pyrazin-2-yl)-1H-1,2,4-triazol-5-yl)pyridine – 1-ethyl-3-methyl-1H-imidazol-3-ium bromide (1/1), C23H22N13Br
- The crystal structure of (E)-N-benzyl-N′-benzylidene-4-methylbenzenesulfonohydrazide, C21H20N2O2S
- Crystal structure of ethyl (E)-5-((2-(3-hydroxybenzoyl)hydrazono)methyl)-3,4-dimethyl-1H-pyrrole-2-carboxylate – water – ethanol (1/1/1), C19H27N3O6
- The crystal structure of (E)-4-(3-ethoxy-2-hydroxybenzylideneamino)benzoic acid, C16H15NO4
- Crystal structure of (μ2-N,N′-bis((pyridin-4-yl)methyl)ethanediamide-κ2N:N′)-tetrakis(diethylcarbamodithioato-κ2S,S′)dizinc(II), C34H54N8O2S8Zn2
Articles in the same Issue
- Cover and Frontmatter
- Editorial 2018
- Crystal structure of dimethanol-bis{3-(((2-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N:O′}dizinc(II), C42H30Zn2N2O10
- Crystal structure of aqua-bis{[2,6-dimethyl-N-(pyridin-2-ylmethylene)aniline-κ2N,N′]}zinc(II) triflate monohydrate [ZnC29H31N4O]CF3SO3⋅H2O
- Crystal structure of (E)-1-(4-{[(E)-4-Diethylamino-2-hydroxybenzene methylene]amino}phenyl)ethanone methoxy oxime, C20H27ClN3O3
- Crystal structure of (E)-1-(4-(((E)-4-(diethylamino)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one oxime, C19H23N3O2
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4,4′-sulfonyldibenzoato-κ2O:O′)zinc(II)], C40H34N4O6SZn
- Crystal structure of catena-poly[diaqua(μ3-pyrazine-2,3-dicarboxylato-κ4O,N:O′:O′′)zinc(II)] 1.25 hydrate, C6H8.5N2O7.25Zn
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri-m-tolyl phosphane-κP)rhenium(I), C29H28O5PRe
- Crystal structure of bis(μ2-methanolato-κ2O:O)-bis(methanol-κ1O)-bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,O′,N}dichromium(III), C38H36Cr2N2O14
- Crystal structure of poly[aqua-(μ3-pyridine-3,5-dicarboxylato-κ5O,O′:O′′,O′′′,N)zinc(II)], C7H7NO6Zn
- Crystal structure of bis((1-(((4-(((benzyloxy)imino)methyl)phenyl)imino)methyl)naphthalen-2-yl)oxy-κ2O,N)copper(II), C52H42CuN4O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}cobalt(II), C40H38CoN4O6
- Crystal structure of diaqua-bis(N,N-dimethylformamide-κ1O)-bis{3-((5-chloro-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ4N,O,O′:O′}dinickel(II), C38H34Ni2Cl2N4O12
- Crystal structure of tetrakis(methanol-κO)bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}bicobalt(II), C38H38Co2N2O14
- Crystal structure of (S)-tert-butyl-(1-hydroxypropan-2-yl)carbamate, C8H17NO3
- Crystal structure of 4-(4′-(pyridin-4-yl)-[1,1′-biphenyl]-4-yl)pyridin-1-ium catena-poly[{5-carboxy-4′-methyl-[1,1′-biphenyl]-3-carboxylato-κ2O,O′}-(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-κ4O,O′:O′′,O′′′)lead(II)], C52H40N2O9Pb
- Crystal structure of catena-poly[diaqua-(μ2-5-methylisophthalato-κ2O:O′)(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)], NiC21H22O6N6
- Crystal structure of the salt tris(guanidinium) tris(tetrapropylammonium) bis(pyridine-2,4,6-tricarboxylate) – water (1/10), C55H126N14O22
- Crystal structure of 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7,8-trimethoxy-4H-chromen-4-one, C19H18O8
- Crystal structure of poly{[μ2-1,1′-(sulfonylbis(4,1-phenylene))bis(2-methyl-1H-imidazole)-κ2N:N′][μ2-4,4′-oxydibenzoato-κ2O:O′]cobalt(II)} hemihydrate, C34H27N4O7.5SCo
- The crystal structure of 25,27-(2,2′-[(2-thioxo-1,3-dithiole-4,5-diyl)disulfanediyl]diethanolate)-26,28-dihydroxycalix[4]arene — dichloromethane (1/1), C36H32Cl2O4S5
- The crystal structure of 1,2-bis(3-(pyridin-3-yl)-1,2,4-oxadiazol-5-yl)ethane, C16H12N6O2
- Crystal structure of 1-benzyl-3-((4-bromophenyl)amino)-4-(4-methoxyphenyl)-1H-pyrrole-2,5-dione, C24H19BrN2O3
- Crystal structure of bis(2-((allylcarbamothioyl)imino)-4-methylthiazol-3-ido-κ2N,S)palladium(II), C16H20N6PdS4
- Crystal structure of pyrimidine-2,5-dicarboxylic acid 1.5 hydrate, C12H14N4O11
- Crystal structure of trans-diaqua-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II), C8H10N4O6Mn
- Crystal structure of catena-(μ3-5-bromoisophthatato-κO,O′: O′′,O′′′′)-(1,2-bis(imidazol-1-yl)ethane-κN:N′)cobalt(II), C16H13CoN4O4Br
- Investigation of the compound La5Zn2−xPb1 + x (x = 0.20–0.32)
- Crystal structure of (OC-6-13)-diaqua-bis(3,5-di(pyridin-3-yl)-4H-1,2,4-triazol-4-amine-κ1N)-bis(dicyanamido-κ1N)zinc(II) tetrahydrate, ZnC28H32N18O6
- Crystal structure of Ga0.62(3)Sb0.38(3)Pd3
- Crystal structure of Ga0.47(1)Sb0.53(1)Pd2
- A derivative of the Corey lactone – crystal structure of (3aR,4S,5R,6aS)-4-(((tert-butyldimethylsilyl)oxy)methyl)-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl benzoate, C21H30O5Si
- A Corey lactone: crystal structure of (3aR,4R,5R,6aS)-5-benzoyloxy-4(hydroxymethyl)hexahydro-2H-cyclopenta[b]furan-2-one, C15H16O5
- Hydrothermal synthesis and crystal structure of poly[aqua-(μ2-1,3-bis(4-pyridyl)propane-κ2N:N′)-(μ2-1,4,5,6,7,7-hexachlorobicyclo[2.2.1]hept-5-ene-2,3-dicarboxylato-κ2O:O′)manganese(II) hydrate, C22H20Cl6N2O6Mn
- Crystal structure of 2-acetylpyrrole S-methylthiosemicarbazonium hydroiodide, C8H13IN4S
- Crystal structure of [N,N-bis((pyrrol-2-yl)ethylidene)butane-1,4-diamine-κ4N,N′,N′′,N′′′]-nickel(II), C16H20N4Ni
- Crystal structure of poly[aqua-(μ5-2,5-dicarboxybenzoato-κ5O:O:O′:O′′:O′′′)sodium(I)], C9H7NaO7
- Crystal structure of bis(N′-((1H-pyrrol-2-yl)methylene)-1-methylthio-methanethiohydrazido-κ2S,N)nickel(II), C14H16N6NiS4
- Crystal structure of 1-(4-((benzo[d][1,3]dioxol-5-yloxy)methyl)phenethyl)-4-(3-chlorophenyl) piperazin-1-ium chloride, C26H28Cl2N2O3
- Crystal structure of 2-(4-(2-(4-(2-fluorophenyl)piperazin-1-yl)ethyl)benzyl)benzo[d]isothiazol-3(2H)-one 1,1-dioxide, C26H26FN3O3S – a saccharin dervative
- Crystal structure of 3-(2-dimethylaminoethyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C12H15N3OS
- Crystal structure of 3-(3-dimethylaminopropyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C13H17N3OS
- The crystal structure of trans-tetraaqua-bis(p-tolylsulfinato-κO)calcium(II)), C14H22O8S2Ca
- The crystal structure of (E)-N′-(pyridin-2-ylmethylene)pyrazine-2-carbohydrazide, C11H9N5O
- Crystal structure of (E)-3-(pyren-1-yl)-1-(pyridin-4-yl)prop-2-en-1-one, C24H15NO
- Crystal structure of catena-poly[diaqua-(μ2-tartrato-κ4O,O′:O′′,O′′′)cobalt(II)], C4H8CoO8
- Crystal structure of 4-chloro-2-methyl-6-(4-(trifluoromethoxy)phenyl)pyrimidine, C12H8ClF3N2O
- Crystal structure of 1-(4-fluorophenyl)-N-(5-((triphenylstannyl)thio)thiophen-2-yl)methanimine, C27H20FN3S2Sn
- Crystal structure of methyl (Z)-2-(5-fluoro-2-oxoindolin-3-ylidene)hydrazine-1-carbodithioate, C10H8FN3OS2
- Crystal structure of tert-butyl (Z)-4-(2-(5-methoxy-3-(2-((methylthio)carbonothioyl)hydrazono)-2-oxoindolin-1-yl)ethyl)piperazine-1-carboxylate, C22H31N5O4S2
- The crystal structure of (E)-2-((2-(o-tolylcarbamothioyl)hydrazono)methyl)benzoic acid, C16H15N3O2S
- Crystal structure of 2-chloro-1,3-di-tert-pentyl-4,4-diphenyl-1,3,2λ3,4-diazaphosphasiletidine, C22H32ClN2PSi
- Crystal structure of tetramethyl 5,5′-(buta-1,3-diyne-1,4-diyl)diisophthalate, C24H18O8
- Crystal structural of 2-amino-4-(4-methoxyphenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C19H20N2O3
- Crystal structure of 1,3,5-tris((trimethylsilyl)methyl)-1,3,5-triazinane-2,4,6-trione, C15H33N3O3Si3
- The crystal structure of bis(2-benzoyl-5-hydroxylphenolato-κ2O,O′)copper(II), C26H18CuO6
- Crystal structure of 2,6-bis(3-(pyrazin-2-yl)-1H-1,2,4-triazol-5-yl)pyridine – 1-ethyl-3-methyl-1H-imidazol-3-ium bromide (1/1), C23H22N13Br
- The crystal structure of (E)-N-benzyl-N′-benzylidene-4-methylbenzenesulfonohydrazide, C21H20N2O2S
- Crystal structure of ethyl (E)-5-((2-(3-hydroxybenzoyl)hydrazono)methyl)-3,4-dimethyl-1H-pyrrole-2-carboxylate – water – ethanol (1/1/1), C19H27N3O6
- The crystal structure of (E)-4-(3-ethoxy-2-hydroxybenzylideneamino)benzoic acid, C16H15NO4
- Crystal structure of (μ2-N,N′-bis((pyridin-4-yl)methyl)ethanediamide-κ2N:N′)-tetrakis(diethylcarbamodithioato-κ2S,S′)dizinc(II), C34H54N8O2S8Zn2

