Abstract
C26H28Cl2N2O3, monoclinic, P21/c (no. 14), a = 13.422(3) Å, b = 7.0011(14) Å, c = 26.249(5) Å, β = 101.06(3)°, V = 2420.8(9) Å3, Z = 4, Rgt(F) = 0.0516, wRref(F2) = 0.1370, T = 296 K.
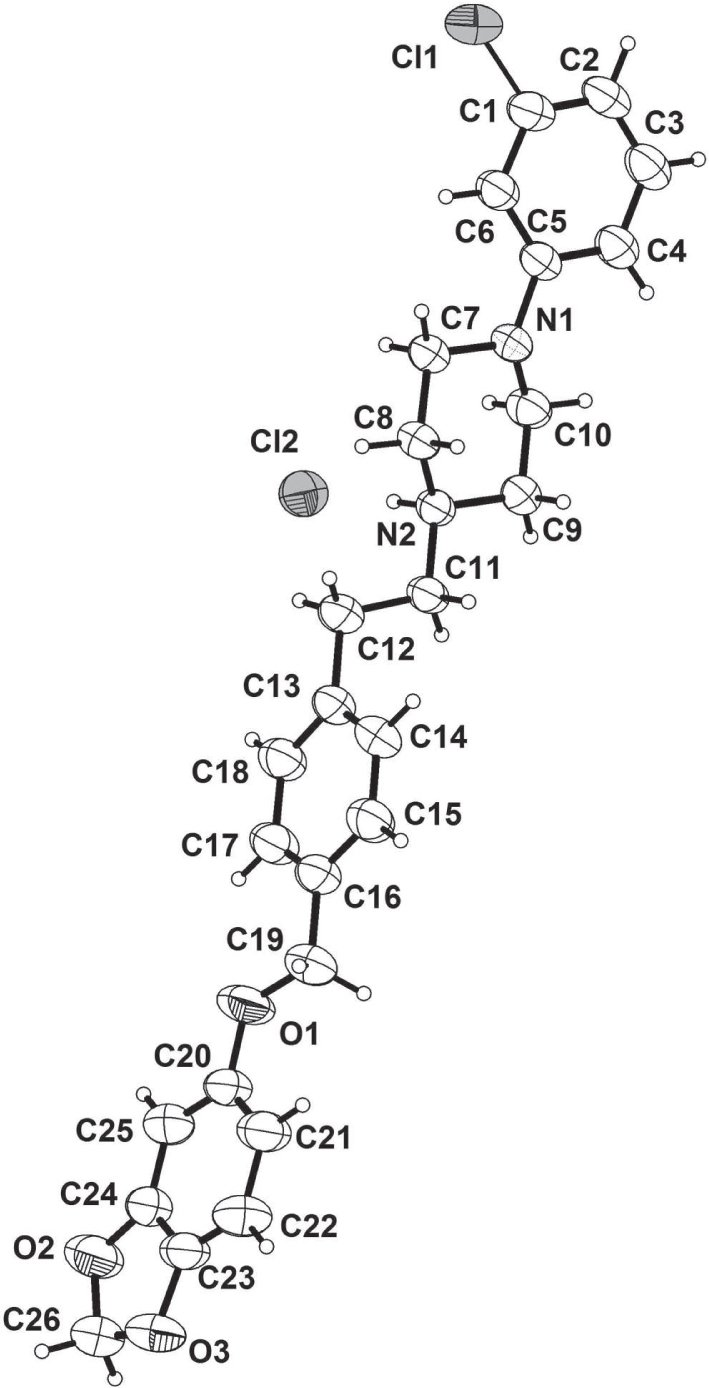
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Block, colorless |
| Size: | 0.30 × 0.10 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 9.5 mm−1 |
| Diffractometer, scan mode: | Bruker P4, ω-scans |
| 2θmax, completeness: | 68.2°, >98% |
| N(hkl)measured, N(hkl)unique, Rint: | 39188, 4439, 0.089 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3123 |
| N(param)refined: | 299 |
| Programs: | Bruker programs [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Cl1 | 0.74454(6) | 0.60009(15) | 0.80257(3) | 0.0877(3) |
| Cl2 | 0.36791(6) | 0.23715(10) | 0.52937(3) | 0.0672(2) |
| O1 | 0.10602(19) | 0.8289(4) | 0.22125(8) | 0.0908(8) |
| O2 | −0.0435(2) | 0.6095(4) | 0.05155(8) | 0.0959(8) |
| O3 | −0.09168(18) | 0.9128(4) | 0.02059(8) | 0.0864(7) |
| N1 | 0.42623(15) | 0.6717(3) | 0.65995(7) | 0.0493(5) |
| N2 | 0.34226(14) | 0.6588(3) | 0.55024(7) | 0.0452(5) |
| H2 | 0.3504 | 0.5216 | 0.5449 | 0.054* |
| C1 | 0.6136(2) | 0.6255(4) | 0.78260(10) | 0.0577(7) |
| C2 | 0.5547(2) | 0.6326(4) | 0.81984(10) | 0.0603(7) |
| H2A | 0.5833 | 0.6298 | 0.8550 | 0.072* |
| C3 | 0.4514(2) | 0.6440(4) | 0.80275(10) | 0.0638(8) |
| H3 | 0.4092 | 0.6457 | 0.8270 | 0.077* |
| C4 | 0.4087(2) | 0.6530(4) | 0.75071(10) | 0.0574(7) |
| H4 | 0.3386 | 0.6613 | 0.7405 | 0.069* |
| C5 | 0.4695(2) | 0.6498(4) | 0.71292(9) | 0.0473(6) |
| C6 | 0.5737(2) | 0.6333(4) | 0.73020(9) | 0.0551(7) |
| H6 | 0.6166 | 0.6274 | 0.7063 | 0.066* |
| C7 | 0.49202(19) | 0.6530(4) | 0.62203(9) | 0.0525(6) |
| H7A | 0.5053 | 0.5187 | 0.6171 | 0.063* |
| H7B | 0.5564 | 0.7151 | 0.6353 | 0.063* |
| C8 | 0.44482(18) | 0.7409(4) | 0.57028(9) | 0.0493(6) |
| H8A | 0.4391 | 0.8779 | 0.5743 | 0.059* |
| H8B | 0.4884 | 0.7175 | 0.5454 | 0.059* |
| C9 | 0.27692(19) | 0.6832(4) | 0.58989(9) | 0.0535(6) |
| H9A | 0.2113 | 0.6245 | 0.5775 | 0.064* |
| H9B | 0.2662 | 0.8182 | 0.5952 | 0.064* |
| C10 | 0.3261(2) | 0.5928(4) | 0.64064(9) | 0.0564(7) |
| H10A | 0.2835 | 0.6134 | 0.6661 | 0.068* |
| H10B | 0.3318 | 0.4561 | 0.6358 | 0.068* |
| C11 | 0.2909(2) | 0.7439(4) | 0.49953(9) | 0.0502(6) |
| H11A | 0.2824 | 0.8801 | 0.5041 | 0.060* |
| H11B | 0.2239 | 0.6880 | 0.4894 | 0.060* |
| C12 | 0.3498(2) | 0.7121(5) | 0.45654(10) | 0.0683(8) |
| H12A | 0.4135 | 0.7814 | 0.4643 | 0.082* |
| H12B | 0.3651 | 0.5773 | 0.4544 | 0.082* |
| C13 | 0.2891(2) | 0.7794(5) | 0.40509(10) | 0.0593(7) |
| C14 | 0.2689(2) | 0.9716(5) | 0.39575(10) | 0.0680(8) |
| H14 | 0.2923 | 1.0606 | 0.4216 | 0.082* |
| C15 | 0.2143(2) | 1.0324(5) | 0.34838(11) | 0.0691(8) |
| H15 | 0.2012 | 1.1620 | 0.3428 | 0.083* |
| C16 | 0.1789(2) | 0.9027(5) | 0.30907(10) | 0.0623(7) |
| C17 | 0.1981(2) | 0.7126(5) | 0.31815(11) | 0.0678(8) |
| H17 | 0.1748 | 0.6240 | 0.2922 | 0.081* |
| C18 | 0.2524(2) | 0.6507(5) | 0.36587(10) | 0.0650(8) |
| H18 | 0.2642 | 0.5208 | 0.3715 | 0.078* |
| C19 | 0.1216(3) | 0.9784(5) | 0.25782(11) | 0.0778(9) |
| H19A | 0.0567 | 1.0300 | 0.2623 | 0.093* |
| H19B | 0.1601 | 1.0802 | 0.2456 | 0.093* |
| C20 | 0.0572(2) | 0.8698(5) | 0.17149(10) | 0.0678(8) |
| C21 | 0.0305(2) | 1.0506(5) | 0.15351(11) | 0.0749(9) |
| H21 | 0.0462 | 1.1551 | 0.1755 | 0.090* |
| C22 | −0.0204(3) | 1.0779(5) | 0.10207(12) | 0.0793(10) |
| H22 | −0.0396 | 1.1992 | 0.0895 | 0.095* |
| C23 | −0.0407(2) | 0.9218(5) | 0.07151(11) | 0.0660(8) |
| C24 | −0.0132(2) | 0.7428(5) | 0.08970(11) | 0.0675(8) |
| C25 | 0.0361(2) | 0.7112(5) | 0.13948(11) | 0.0761(9) |
| H25 | 0.0548 | 0.5888 | 0.1514 | 0.091* |
| C26 | −0.0928(3) | 0.7162(6) | 0.00771(13) | 0.0915(12) |
| H26A | −0.0582 | 0.6967 | −0.0211 | 0.110* |
| H26B | −0.1623 | 0.6728 | −0.0028 | 0.110* |
Source of material
The title compound was synthesized from 2-(4-((benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl 4-methylbenzenesulfonate (65% yield) in form of colorless crystals. To a solution of 2-(4-((benzo[d][1,3]dioxol-5-yl-oxy)methyl)phenyl)ethyl 4-methylbenzenesulfonate (100 mg, 0.23 mmol) in acetonitrile (CH3CN, 10 mL) was added 1-(3-chlorophenyl)piperazine (1.2 equiv) and potassium carbonate (6.0 equiv). The reaction mixture was stirred at reflux for 16 h. After cooling to ambient temperature, the reaction mixture was filtered through a Büchner funnel. After filtration the filtrate was concentrated in vacuo and the residue was purified by silica gel column chromatography using ethyl acetate/petroleum ether (1/5, v/v) as eluent to afford the product, and the title compound was recrystallized from trichloromethane and n-hexane [3].
Experimental details
The hydrogen atoms were assigned with isotropic displacement factors Uiso(H) = 1.2Ueq (N and imidazol C), or Uiso(H) = 1.5Ueq (methyl C) and included in the final refinements by using geometrical restraints, with C—H = 0.93 Å (imidazol) or C–H = 0.96 Å (methyl), and N—H = 0.86 Å.
Discussion
Compounds with arylpiperazine moieties have a wide range of activities including antiarrhythmic [4], diuretic [5], antiallergic [6], antidepressant [7], anxiolytic [8], antipsychotic [9], antimalarial [10], antiplasmodial [11] and anti-proliferative [12], [13], [14] properties. In addition, these compounds also display receptor-blocking properties [15], [16], [17], [18], [19]. Moreover, arylpiperazine derivatives have been reported as anticancer drugs for site-directed chemotherapy of prostate cancer in our previous work [20, 21] , and some derivatives showed significant cytotoxic activity against the tested prostate cancer cell lines.
The molecule of the title compound shows an aryl, a piperazine and the benzo[d][1,3]dioxole dihydrosulfide moieties. The 2-chloro-phenyl moiety is connected to the 1,4-piperazine moiety via a C—N bond, then the above fragment further is connected by a C6H4 unit via an ethylene group. The termial fragment of molecule is the benzo[d][1,3]dioxole dihydrosulfide unit. The central aryl ring and benzo[d][1,3]dioxole dihydrosulfide ring are almost into the same plane. In the molecule, the N(1)—C(5), N(1)—C(7), N(1)—C(10), N(2)—C(11), N(2)—C(9), N(2)—N(8) bond lengths are found to be 1.452(3) Å, 1.456(3) Å, 458(3) Å, 1.500(3) Å, 1.494(3) Å and 1.491(3) Å, respectively, which are nearly equal to other typical single bonds. The bond lengths of C(24)—O(2) and C(26)—O(2) are found to be 1.372(4) and 1.424(4) Å, respectively. The bond angles C5—N1—C10, C5—N1—C7, C10—N1—C7, C9—N2—C11, C8—N2—C11 and C8—N2—C9 are 118.8(2)°, 118.4(2)°, 111.7(19)°, 109.97(18)°, 113.34(19)° and 109.64(18)°, respectively. N2—C11—C12 and C11—C12—C13 are 112.7(2)°, 110.5(3)°. Bond lengths and all other geometic parameters of the title molecule are in the expected ranges [22].
Acknowledgements
This work was supported by the Natural Science Foundation of China (no. 81401462), Henan Province Science and Technology Attack Plan Foundation (no. 162102310477), the Key Scientific Research Project of Higher Education of Henan Province (nos. 16A350008, 17A150039 and 17B350001) and National Scientific Research Foundation of Luoyang Normal University (no. 2015-PYJJ-005).
References
Bruker: APEX2, SAINT and SADABS. Brucker AXS Inc., Madison, WI, USA (2009).Search in Google Scholar
Sheldrick, G. M.: SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053273314026370Search in Google Scholar
Chen, H.; Liang, X.; Xu, F.; Xu, B.-B.; He, X.-L.; Huang, B.-Y.; Yuan, M.: Synthesis and cytotoxic activity evaluation of novel arylpiperazine derivatives on human prostate cancer cell lines. Molecules 9 (2014) 12048–12064.10.3390/molecules190812048Search in Google Scholar
Szkaradek, N.; Rapacz, A.; Pytka, K.; Filipek, B.; Siwek, A.; Cegla, M.; Marona, H.: Synthesis and preliminary evaluation of pharmacological properties of some piperazine derivatives of xanthone. Bioorg. Med. Chem. 9 (2013) 514–522.10.1016/j.bmc.2012.11.014Search in Google Scholar
Cecchetti, V.; Fravolini, A.; Schiaffella, F.; Tabarrini, O.; Bruni, G.; Segret, G.: o-Chlorobenzenesulfonamidic derivatives of (aryloxy)propanolamines as beta-blocking/diuretic agents. J. Med. Chem. 9 (1993) 157–161.10.1021/jm00053a020Search in Google Scholar
Walsh, D. A.; Chen, Y. H.; Green, J. B.; Nolan, J. C.; Yannit, J. M.: The synthesis and antiallergy activity of 1-(aryloxy)-4-(4-arylpiperazinyl)-2-butanol derivatives. J. Med. Chem. 9 (1990) 1823–1827.10.1021/jm00168a044Search in Google Scholar
Seo, H. J.; Park, E. J.; Kim, M. J.; Kang, S. Y.; Lee, S. H.; Kim, H. J.; Lee, K. N.; Jung, M. E.; Lee, M.; Kim, M. S.; Son, E. J.; Park, W. K.; Kim, J.; Lee, J.: Design and synthesis of novel arylpiperazine derivatives containing the imidazole core targeting 5-HT(2A) receptor and 5-HT transporter. J. Med. Chem. 9 (2011) 6305–6318.10.1021/jm200682bSearch in Google Scholar
Kikumoto, R.; Tobe, A.; Fukami, H.; Egawa, M.: Synthesis and antianxiety activity of (omega-piperazinylalkoxy)indan derivatives. J. Med. Chem. 9 (1983) 246–250.10.1021/jm00356a024Search in Google Scholar
Jaen, J. C.; Wise, L. D.; Heffner, T. G.; Pugsley, T. A.; Meltzed, L. T.: Dopamine autoreceptor agonists as potential antipsychotics 1 (Aminoalkoxy)anilines. J. Med. Chem. 9 (1988) 1621–1625.10.1021/jm00403a022Search in Google Scholar
Cross, R. M.; Namelikonda, N. K.; Mutka, T. S.; Luong, L.; Kyle, D. E.; Manetsch, R.: Synthesis, antimalarial activity, and structure-activity relationship of 7-(2-phenoxyethoxy)-4(1H)-quinolones. J. Med. Chem. 9 (2011) 8321–8327.10.1021/jm200718mSearch in Google Scholar
Clarkson, C.; Musonda, C. C.; Chibale, K.; Campbella, W. E.; Smitha, P.: Synthesis of totarol amino alcohol derivatives and their antiplasmodial activity and cytotoxicity. Bioorg. Med. Chem. 9 (2003) 4417–4422.10.1016/S0968-0896(03)00491-7Search in Google Scholar
Berardi, F.; Abate, C.; Ferorelli, S.; De Robertis, A. F.; Leopoldo, M.; Colabufo, N. A.; Niso, M.; Perrone, R.: Novel 4-(4-aryl)cyclohexyl-1-(2-pyridyl)piperazines as Delta(8)-Delta(7) sterol isomerase (emopamil binding protein) selective ligands with antiproliferative activity. J. Med. Chem. 9 (2008) 7523–7531.10.1021/jm800965bSearch in Google Scholar PubMed
Abate, C.; Niso, M.; Contino, M.; Colabufo, N. A.; Ferorelli, S.; Perrone, R.; Berardi, F.: 1-Cyclohexyl-4-(4-arylcyclohexyl)piperazines: Mixed and human (8)–(7) sterol isomerase ligands with antiproliferative and P-glycoprotein inhibitory activity. ChemMedChem 9 (2011) 73–80.10.1002/cmdc.201000371Search in Google Scholar PubMed
Liu, W.-H.; Chang, J.-X.; Liu, Y.; Luo, J.-W.; Zhang, J.-W.: Design, synthesis and activities of novel benzothiazole derivatives containing arylpiperazine. Acta Pharm. Sin. B9 (2013) 1259–1265.Search in Google Scholar
Leopoldo, M.; Lacivita, E.; Passafiume, E.; Contino, M.; Colabufo, N. A.; Berardi, F.; Perrone, R.: 4-[omega-[4-arylpiperazin-1-yl]alkoxy]phenyl)imidazo[1,2-a]pyridine derivatives: fluorescent high-affinity dopamine D3 receptor ligands as potential probes for receptor visualization. J. Med. Chem. 9 (2007) 5043–5047.10.1021/jm070721+Search in Google Scholar PubMed
Chen, X.; Sassano, M. F.; Zheng, L. Y.; Setola, V.; Chen, M.; Bai, X.; Frye, S. V.; Wetsel, W. C.; Roth, B. L.; Jin, J.: Structure-functional selectivity relationship studies of α-arrestin-biased dopamine D2 receptor agonists. J. Med. Chem. 9 (2012) 7141–7153.10.1021/jm300603ySearch in Google Scholar PubMed PubMed Central
Romeiro, L. A.; Da Silva Ferreira, M.; Da Silva, L. L.; Castro, H. C.; Miranda, A. L.; Silva, C. L.; Noël, F.; Nascimento, J. B.; Araújo, C. V.; Tibiri cá, E.; Barreiro, E. J.; Fraga, C. A.: Discovery of LASSBio-772, a 1,3-benzodioxole N-phenylpiperazine derivative with potent alpha 1A/D-adrenergic receptor blocking properties. Eur. J. Med. Chem. 9 (2011) 3000–3012.10.1016/j.ejmech.2011.04.032Search in Google Scholar PubMed
Baran, M.; Kepczynska, E.; Zylewski, M.; Siwek, A.; Bednarski, M.; Cegla, M. T.: Studies on novel pyridine and 2-pyridone derivatives of N-arylpiperazine as α-adrenoceptor ligands. Med. Chem. 9 (2014) 144–153.10.2174/0929867320999131122114922Search in Google Scholar PubMed
Ananthan, S.; Saini, S. K.; Zhou, G.; Hobrath, J. V.; Padmalayam, I.; Zhai, L.; Bostwick, J. R.; Antonio, T.; Reith, M. E.; McDowell, S.; Cho, E.; McAleer, L.; Taylor, M.; Luedtke, R. R.: Design, synthesis, and structure-activity relationship studies of a series of [4-(4-carboxamidobutyl)]-1-arylpiperazines: insights into structural features contributing to dopamine D3 versus D2 receptor subtype selectivity. J. Med. Chem. 9 (2014) 7042–7060.10.1021/jm500801rSearch in Google Scholar PubMed PubMed Central
Chen, H.; Xu, F.; Liang, X.; Xu, B.-B.; Yang, Z.-L.; He, X.-L.; Huang, B.-Y.; Yuan, M.: Design, synthesis and biological evaluation of novel arylpiperazine derivatives on human prostate cancer cell lines. Bioorg. Med. Chem. Lett. 9 (2015) 285–287.10.1016/j.bmcl.2014.11.049Search in Google Scholar PubMed
Chen, H.; Xu, F.; Xu, B.-B.; Xu, J.-Y.; Shao, B.-H.; Huang, B.-Y.; Yuan, M.: Design, synthesis and biological evaluation of novel arylpiperazine derivatives on human prostate cancer cell lines. Chinese Chem. Lett. 9 (2016) 277–282.10.1016/j.cclet.2015.09.016Search in Google Scholar
Al-Wahaibi, L. H.; Hassan, H. M.; Abo-Kamar, A. M.; Ghabbourand, H. A.; El-Emam, A. A.: Crystal structure of 4-bromobenzyl (Z)-N′-(adamantan-1-yl)-4-phenylpiperazine-1-carbothioimidate, C28H34BrN3S. Z. Kristallogr. NCS 232 (2017) 189–191.10.1515/ncrs-2016-0184Search in Google Scholar
©2018 Hong Chen et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Editorial 2018
- Crystal structure of dimethanol-bis{3-(((2-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N:O′}dizinc(II), C42H30Zn2N2O10
- Crystal structure of aqua-bis{[2,6-dimethyl-N-(pyridin-2-ylmethylene)aniline-κ2N,N′]}zinc(II) triflate monohydrate [ZnC29H31N4O]CF3SO3⋅H2O
- Crystal structure of (E)-1-(4-{[(E)-4-Diethylamino-2-hydroxybenzene methylene]amino}phenyl)ethanone methoxy oxime, C20H27ClN3O3
- Crystal structure of (E)-1-(4-(((E)-4-(diethylamino)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one oxime, C19H23N3O2
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4,4′-sulfonyldibenzoato-κ2O:O′)zinc(II)], C40H34N4O6SZn
- Crystal structure of catena-poly[diaqua(μ3-pyrazine-2,3-dicarboxylato-κ4O,N:O′:O′′)zinc(II)] 1.25 hydrate, C6H8.5N2O7.25Zn
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri-m-tolyl phosphane-κP)rhenium(I), C29H28O5PRe
- Crystal structure of bis(μ2-methanolato-κ2O:O)-bis(methanol-κ1O)-bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,O′,N}dichromium(III), C38H36Cr2N2O14
- Crystal structure of poly[aqua-(μ3-pyridine-3,5-dicarboxylato-κ5O,O′:O′′,O′′′,N)zinc(II)], C7H7NO6Zn
- Crystal structure of bis((1-(((4-(((benzyloxy)imino)methyl)phenyl)imino)methyl)naphthalen-2-yl)oxy-κ2O,N)copper(II), C52H42CuN4O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}cobalt(II), C40H38CoN4O6
- Crystal structure of diaqua-bis(N,N-dimethylformamide-κ1O)-bis{3-((5-chloro-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ4N,O,O′:O′}dinickel(II), C38H34Ni2Cl2N4O12
- Crystal structure of tetrakis(methanol-κO)bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}bicobalt(II), C38H38Co2N2O14
- Crystal structure of (S)-tert-butyl-(1-hydroxypropan-2-yl)carbamate, C8H17NO3
- Crystal structure of 4-(4′-(pyridin-4-yl)-[1,1′-biphenyl]-4-yl)pyridin-1-ium catena-poly[{5-carboxy-4′-methyl-[1,1′-biphenyl]-3-carboxylato-κ2O,O′}-(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-κ4O,O′:O′′,O′′′)lead(II)], C52H40N2O9Pb
- Crystal structure of catena-poly[diaqua-(μ2-5-methylisophthalato-κ2O:O′)(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)], NiC21H22O6N6
- Crystal structure of the salt tris(guanidinium) tris(tetrapropylammonium) bis(pyridine-2,4,6-tricarboxylate) – water (1/10), C55H126N14O22
- Crystal structure of 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7,8-trimethoxy-4H-chromen-4-one, C19H18O8
- Crystal structure of poly{[μ2-1,1′-(sulfonylbis(4,1-phenylene))bis(2-methyl-1H-imidazole)-κ2N:N′][μ2-4,4′-oxydibenzoato-κ2O:O′]cobalt(II)} hemihydrate, C34H27N4O7.5SCo
- The crystal structure of 25,27-(2,2′-[(2-thioxo-1,3-dithiole-4,5-diyl)disulfanediyl]diethanolate)-26,28-dihydroxycalix[4]arene — dichloromethane (1/1), C36H32Cl2O4S5
- The crystal structure of 1,2-bis(3-(pyridin-3-yl)-1,2,4-oxadiazol-5-yl)ethane, C16H12N6O2
- Crystal structure of 1-benzyl-3-((4-bromophenyl)amino)-4-(4-methoxyphenyl)-1H-pyrrole-2,5-dione, C24H19BrN2O3
- Crystal structure of bis(2-((allylcarbamothioyl)imino)-4-methylthiazol-3-ido-κ2N,S)palladium(II), C16H20N6PdS4
- Crystal structure of pyrimidine-2,5-dicarboxylic acid 1.5 hydrate, C12H14N4O11
- Crystal structure of trans-diaqua-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II), C8H10N4O6Mn
- Crystal structure of catena-(μ3-5-bromoisophthatato-κO,O′: O′′,O′′′′)-(1,2-bis(imidazol-1-yl)ethane-κN:N′)cobalt(II), C16H13CoN4O4Br
- Investigation of the compound La5Zn2−xPb1 + x (x = 0.20–0.32)
- Crystal structure of (OC-6-13)-diaqua-bis(3,5-di(pyridin-3-yl)-4H-1,2,4-triazol-4-amine-κ1N)-bis(dicyanamido-κ1N)zinc(II) tetrahydrate, ZnC28H32N18O6
- Crystal structure of Ga0.62(3)Sb0.38(3)Pd3
- Crystal structure of Ga0.47(1)Sb0.53(1)Pd2
- A derivative of the Corey lactone – crystal structure of (3aR,4S,5R,6aS)-4-(((tert-butyldimethylsilyl)oxy)methyl)-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl benzoate, C21H30O5Si
- A Corey lactone: crystal structure of (3aR,4R,5R,6aS)-5-benzoyloxy-4(hydroxymethyl)hexahydro-2H-cyclopenta[b]furan-2-one, C15H16O5
- Hydrothermal synthesis and crystal structure of poly[aqua-(μ2-1,3-bis(4-pyridyl)propane-κ2N:N′)-(μ2-1,4,5,6,7,7-hexachlorobicyclo[2.2.1]hept-5-ene-2,3-dicarboxylato-κ2O:O′)manganese(II) hydrate, C22H20Cl6N2O6Mn
- Crystal structure of 2-acetylpyrrole S-methylthiosemicarbazonium hydroiodide, C8H13IN4S
- Crystal structure of [N,N-bis((pyrrol-2-yl)ethylidene)butane-1,4-diamine-κ4N,N′,N′′,N′′′]-nickel(II), C16H20N4Ni
- Crystal structure of poly[aqua-(μ5-2,5-dicarboxybenzoato-κ5O:O:O′:O′′:O′′′)sodium(I)], C9H7NaO7
- Crystal structure of bis(N′-((1H-pyrrol-2-yl)methylene)-1-methylthio-methanethiohydrazido-κ2S,N)nickel(II), C14H16N6NiS4
- Crystal structure of 1-(4-((benzo[d][1,3]dioxol-5-yloxy)methyl)phenethyl)-4-(3-chlorophenyl) piperazin-1-ium chloride, C26H28Cl2N2O3
- Crystal structure of 2-(4-(2-(4-(2-fluorophenyl)piperazin-1-yl)ethyl)benzyl)benzo[d]isothiazol-3(2H)-one 1,1-dioxide, C26H26FN3O3S – a saccharin dervative
- Crystal structure of 3-(2-dimethylaminoethyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C12H15N3OS
- Crystal structure of 3-(3-dimethylaminopropyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C13H17N3OS
- The crystal structure of trans-tetraaqua-bis(p-tolylsulfinato-κO)calcium(II)), C14H22O8S2Ca
- The crystal structure of (E)-N′-(pyridin-2-ylmethylene)pyrazine-2-carbohydrazide, C11H9N5O
- Crystal structure of (E)-3-(pyren-1-yl)-1-(pyridin-4-yl)prop-2-en-1-one, C24H15NO
- Crystal structure of catena-poly[diaqua-(μ2-tartrato-κ4O,O′:O′′,O′′′)cobalt(II)], C4H8CoO8
- Crystal structure of 4-chloro-2-methyl-6-(4-(trifluoromethoxy)phenyl)pyrimidine, C12H8ClF3N2O
- Crystal structure of 1-(4-fluorophenyl)-N-(5-((triphenylstannyl)thio)thiophen-2-yl)methanimine, C27H20FN3S2Sn
- Crystal structure of methyl (Z)-2-(5-fluoro-2-oxoindolin-3-ylidene)hydrazine-1-carbodithioate, C10H8FN3OS2
- Crystal structure of tert-butyl (Z)-4-(2-(5-methoxy-3-(2-((methylthio)carbonothioyl)hydrazono)-2-oxoindolin-1-yl)ethyl)piperazine-1-carboxylate, C22H31N5O4S2
- The crystal structure of (E)-2-((2-(o-tolylcarbamothioyl)hydrazono)methyl)benzoic acid, C16H15N3O2S
- Crystal structure of 2-chloro-1,3-di-tert-pentyl-4,4-diphenyl-1,3,2λ3,4-diazaphosphasiletidine, C22H32ClN2PSi
- Crystal structure of tetramethyl 5,5′-(buta-1,3-diyne-1,4-diyl)diisophthalate, C24H18O8
- Crystal structural of 2-amino-4-(4-methoxyphenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C19H20N2O3
- Crystal structure of 1,3,5-tris((trimethylsilyl)methyl)-1,3,5-triazinane-2,4,6-trione, C15H33N3O3Si3
- The crystal structure of bis(2-benzoyl-5-hydroxylphenolato-κ2O,O′)copper(II), C26H18CuO6
- Crystal structure of 2,6-bis(3-(pyrazin-2-yl)-1H-1,2,4-triazol-5-yl)pyridine – 1-ethyl-3-methyl-1H-imidazol-3-ium bromide (1/1), C23H22N13Br
- The crystal structure of (E)-N-benzyl-N′-benzylidene-4-methylbenzenesulfonohydrazide, C21H20N2O2S
- Crystal structure of ethyl (E)-5-((2-(3-hydroxybenzoyl)hydrazono)methyl)-3,4-dimethyl-1H-pyrrole-2-carboxylate – water – ethanol (1/1/1), C19H27N3O6
- The crystal structure of (E)-4-(3-ethoxy-2-hydroxybenzylideneamino)benzoic acid, C16H15NO4
- Crystal structure of (μ2-N,N′-bis((pyridin-4-yl)methyl)ethanediamide-κ2N:N′)-tetrakis(diethylcarbamodithioato-κ2S,S′)dizinc(II), C34H54N8O2S8Zn2
Articles in the same Issue
- Cover and Frontmatter
- Editorial 2018
- Crystal structure of dimethanol-bis{3-(((2-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N:O′}dizinc(II), C42H30Zn2N2O10
- Crystal structure of aqua-bis{[2,6-dimethyl-N-(pyridin-2-ylmethylene)aniline-κ2N,N′]}zinc(II) triflate monohydrate [ZnC29H31N4O]CF3SO3⋅H2O
- Crystal structure of (E)-1-(4-{[(E)-4-Diethylamino-2-hydroxybenzene methylene]amino}phenyl)ethanone methoxy oxime, C20H27ClN3O3
- Crystal structure of (E)-1-(4-(((E)-4-(diethylamino)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one oxime, C19H23N3O2
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4,4′-sulfonyldibenzoato-κ2O:O′)zinc(II)], C40H34N4O6SZn
- Crystal structure of catena-poly[diaqua(μ3-pyrazine-2,3-dicarboxylato-κ4O,N:O′:O′′)zinc(II)] 1.25 hydrate, C6H8.5N2O7.25Zn
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri-m-tolyl phosphane-κP)rhenium(I), C29H28O5PRe
- Crystal structure of bis(μ2-methanolato-κ2O:O)-bis(methanol-κ1O)-bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,O′,N}dichromium(III), C38H36Cr2N2O14
- Crystal structure of poly[aqua-(μ3-pyridine-3,5-dicarboxylato-κ5O,O′:O′′,O′′′,N)zinc(II)], C7H7NO6Zn
- Crystal structure of bis((1-(((4-(((benzyloxy)imino)methyl)phenyl)imino)methyl)naphthalen-2-yl)oxy-κ2O,N)copper(II), C52H42CuN4O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}cobalt(II), C40H38CoN4O6
- Crystal structure of diaqua-bis(N,N-dimethylformamide-κ1O)-bis{3-((5-chloro-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ4N,O,O′:O′}dinickel(II), C38H34Ni2Cl2N4O12
- Crystal structure of tetrakis(methanol-κO)bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}bicobalt(II), C38H38Co2N2O14
- Crystal structure of (S)-tert-butyl-(1-hydroxypropan-2-yl)carbamate, C8H17NO3
- Crystal structure of 4-(4′-(pyridin-4-yl)-[1,1′-biphenyl]-4-yl)pyridin-1-ium catena-poly[{5-carboxy-4′-methyl-[1,1′-biphenyl]-3-carboxylato-κ2O,O′}-(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-κ4O,O′:O′′,O′′′)lead(II)], C52H40N2O9Pb
- Crystal structure of catena-poly[diaqua-(μ2-5-methylisophthalato-κ2O:O′)(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)], NiC21H22O6N6
- Crystal structure of the salt tris(guanidinium) tris(tetrapropylammonium) bis(pyridine-2,4,6-tricarboxylate) – water (1/10), C55H126N14O22
- Crystal structure of 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7,8-trimethoxy-4H-chromen-4-one, C19H18O8
- Crystal structure of poly{[μ2-1,1′-(sulfonylbis(4,1-phenylene))bis(2-methyl-1H-imidazole)-κ2N:N′][μ2-4,4′-oxydibenzoato-κ2O:O′]cobalt(II)} hemihydrate, C34H27N4O7.5SCo
- The crystal structure of 25,27-(2,2′-[(2-thioxo-1,3-dithiole-4,5-diyl)disulfanediyl]diethanolate)-26,28-dihydroxycalix[4]arene — dichloromethane (1/1), C36H32Cl2O4S5
- The crystal structure of 1,2-bis(3-(pyridin-3-yl)-1,2,4-oxadiazol-5-yl)ethane, C16H12N6O2
- Crystal structure of 1-benzyl-3-((4-bromophenyl)amino)-4-(4-methoxyphenyl)-1H-pyrrole-2,5-dione, C24H19BrN2O3
- Crystal structure of bis(2-((allylcarbamothioyl)imino)-4-methylthiazol-3-ido-κ2N,S)palladium(II), C16H20N6PdS4
- Crystal structure of pyrimidine-2,5-dicarboxylic acid 1.5 hydrate, C12H14N4O11
- Crystal structure of trans-diaqua-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II), C8H10N4O6Mn
- Crystal structure of catena-(μ3-5-bromoisophthatato-κO,O′: O′′,O′′′′)-(1,2-bis(imidazol-1-yl)ethane-κN:N′)cobalt(II), C16H13CoN4O4Br
- Investigation of the compound La5Zn2−xPb1 + x (x = 0.20–0.32)
- Crystal structure of (OC-6-13)-diaqua-bis(3,5-di(pyridin-3-yl)-4H-1,2,4-triazol-4-amine-κ1N)-bis(dicyanamido-κ1N)zinc(II) tetrahydrate, ZnC28H32N18O6
- Crystal structure of Ga0.62(3)Sb0.38(3)Pd3
- Crystal structure of Ga0.47(1)Sb0.53(1)Pd2
- A derivative of the Corey lactone – crystal structure of (3aR,4S,5R,6aS)-4-(((tert-butyldimethylsilyl)oxy)methyl)-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl benzoate, C21H30O5Si
- A Corey lactone: crystal structure of (3aR,4R,5R,6aS)-5-benzoyloxy-4(hydroxymethyl)hexahydro-2H-cyclopenta[b]furan-2-one, C15H16O5
- Hydrothermal synthesis and crystal structure of poly[aqua-(μ2-1,3-bis(4-pyridyl)propane-κ2N:N′)-(μ2-1,4,5,6,7,7-hexachlorobicyclo[2.2.1]hept-5-ene-2,3-dicarboxylato-κ2O:O′)manganese(II) hydrate, C22H20Cl6N2O6Mn
- Crystal structure of 2-acetylpyrrole S-methylthiosemicarbazonium hydroiodide, C8H13IN4S
- Crystal structure of [N,N-bis((pyrrol-2-yl)ethylidene)butane-1,4-diamine-κ4N,N′,N′′,N′′′]-nickel(II), C16H20N4Ni
- Crystal structure of poly[aqua-(μ5-2,5-dicarboxybenzoato-κ5O:O:O′:O′′:O′′′)sodium(I)], C9H7NaO7
- Crystal structure of bis(N′-((1H-pyrrol-2-yl)methylene)-1-methylthio-methanethiohydrazido-κ2S,N)nickel(II), C14H16N6NiS4
- Crystal structure of 1-(4-((benzo[d][1,3]dioxol-5-yloxy)methyl)phenethyl)-4-(3-chlorophenyl) piperazin-1-ium chloride, C26H28Cl2N2O3
- Crystal structure of 2-(4-(2-(4-(2-fluorophenyl)piperazin-1-yl)ethyl)benzyl)benzo[d]isothiazol-3(2H)-one 1,1-dioxide, C26H26FN3O3S – a saccharin dervative
- Crystal structure of 3-(2-dimethylaminoethyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C12H15N3OS
- Crystal structure of 3-(3-dimethylaminopropyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C13H17N3OS
- The crystal structure of trans-tetraaqua-bis(p-tolylsulfinato-κO)calcium(II)), C14H22O8S2Ca
- The crystal structure of (E)-N′-(pyridin-2-ylmethylene)pyrazine-2-carbohydrazide, C11H9N5O
- Crystal structure of (E)-3-(pyren-1-yl)-1-(pyridin-4-yl)prop-2-en-1-one, C24H15NO
- Crystal structure of catena-poly[diaqua-(μ2-tartrato-κ4O,O′:O′′,O′′′)cobalt(II)], C4H8CoO8
- Crystal structure of 4-chloro-2-methyl-6-(4-(trifluoromethoxy)phenyl)pyrimidine, C12H8ClF3N2O
- Crystal structure of 1-(4-fluorophenyl)-N-(5-((triphenylstannyl)thio)thiophen-2-yl)methanimine, C27H20FN3S2Sn
- Crystal structure of methyl (Z)-2-(5-fluoro-2-oxoindolin-3-ylidene)hydrazine-1-carbodithioate, C10H8FN3OS2
- Crystal structure of tert-butyl (Z)-4-(2-(5-methoxy-3-(2-((methylthio)carbonothioyl)hydrazono)-2-oxoindolin-1-yl)ethyl)piperazine-1-carboxylate, C22H31N5O4S2
- The crystal structure of (E)-2-((2-(o-tolylcarbamothioyl)hydrazono)methyl)benzoic acid, C16H15N3O2S
- Crystal structure of 2-chloro-1,3-di-tert-pentyl-4,4-diphenyl-1,3,2λ3,4-diazaphosphasiletidine, C22H32ClN2PSi
- Crystal structure of tetramethyl 5,5′-(buta-1,3-diyne-1,4-diyl)diisophthalate, C24H18O8
- Crystal structural of 2-amino-4-(4-methoxyphenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C19H20N2O3
- Crystal structure of 1,3,5-tris((trimethylsilyl)methyl)-1,3,5-triazinane-2,4,6-trione, C15H33N3O3Si3
- The crystal structure of bis(2-benzoyl-5-hydroxylphenolato-κ2O,O′)copper(II), C26H18CuO6
- Crystal structure of 2,6-bis(3-(pyrazin-2-yl)-1H-1,2,4-triazol-5-yl)pyridine – 1-ethyl-3-methyl-1H-imidazol-3-ium bromide (1/1), C23H22N13Br
- The crystal structure of (E)-N-benzyl-N′-benzylidene-4-methylbenzenesulfonohydrazide, C21H20N2O2S
- Crystal structure of ethyl (E)-5-((2-(3-hydroxybenzoyl)hydrazono)methyl)-3,4-dimethyl-1H-pyrrole-2-carboxylate – water – ethanol (1/1/1), C19H27N3O6
- The crystal structure of (E)-4-(3-ethoxy-2-hydroxybenzylideneamino)benzoic acid, C16H15NO4
- Crystal structure of (μ2-N,N′-bis((pyridin-4-yl)methyl)ethanediamide-κ2N:N′)-tetrakis(diethylcarbamodithioato-κ2S,S′)dizinc(II), C34H54N8O2S8Zn2

