Abstract
C19H18O8, monoclinic, P21/c (no. 14), a = 12.689(2) Å, b = 20.321(4) Å, c = 7.0820(13) Å, β = 105.368(13)°, V = 1760.8(6) Å3, Z = 4, Rgt = 0.0662, wRref(F2) = 0.1788, T = 295 K.
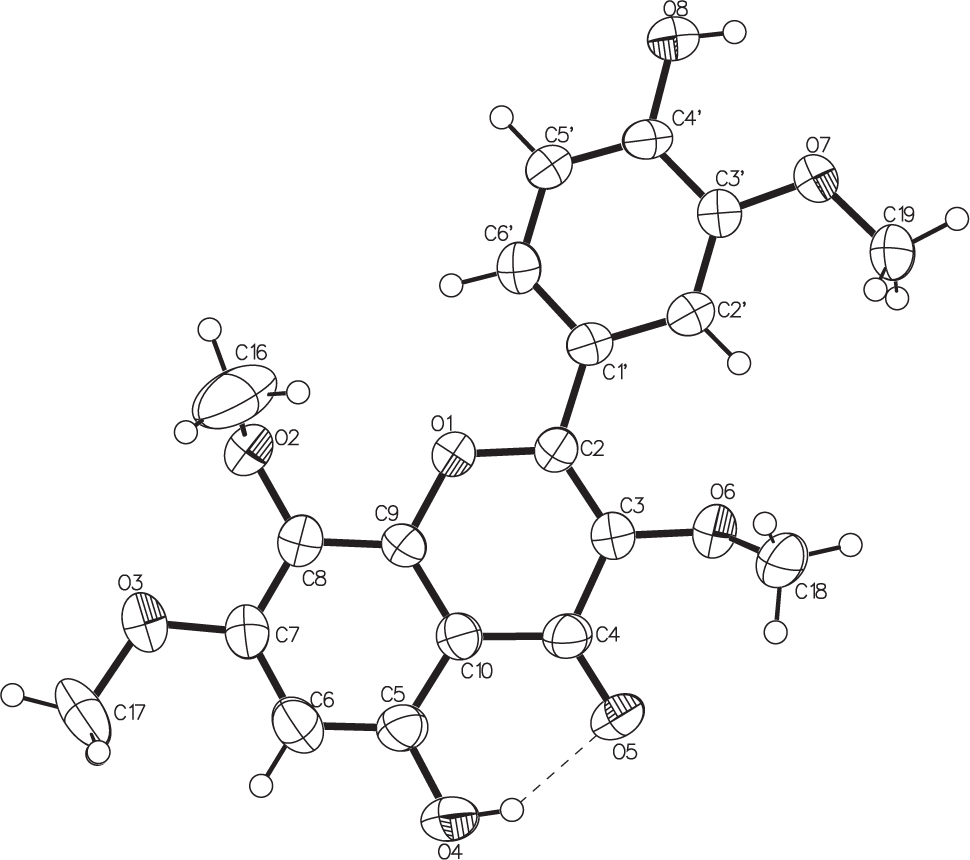
The title crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.22 × 0.20 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 0.94 mm−1 |
| Diffractometer, scan mode: | Bruker D8-Venture, φ and ω-scans |
| θmax, completeness: | 59.6°, >98% |
| N(hkl)measured, N(hkl)unique, Rint: | 27492, 2531, 0.317 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1251 |
| N(param)refined: | 251 |
| Programs: | Bruker programs [27], SHELX [28], OLEX2 [29] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.6930(2) | 0.39194(13) | 0.6743(4) | 0.0469(9) |
| O2 | 0.4768(2) | 0.39502(15) | 0.6405(5) | 0.0586(10) |
| O3 | 0.3695(2) | 0.28331(15) | 0.6637(5) | 0.0666(11) |
| O4 | 0.6911(2) | 0.15607(13) | 0.7049(5) | 0.0654(10) |
| H4 | 0.7569 | 0.1618 | 0.7230 | 0.098* |
| O5 | 0.8656(2) | 0.22207(14) | 0.6981(5) | 0.0586(10) |
| O6 | 0.9641(2) | 0.34225(13) | 0.6641(4) | 0.0538(9) |
| O7 | 1.0806(2) | 0.56956(13) | 0.7812(5) | 0.0570(10) |
| O8 | 0.9222(2) | 0.66056(13) | 0.6624(5) | 0.0620(10) |
| H8 | 0.9888 | 0.6646 | 0.7001 | 0.093* |
| C1′ | 0.8346(3) | 0.46317(19) | 0.6687(7) | 0.0406(12) |
| C2′ | 0.9446(3) | 0.4822(2) | 0.7249(7) | 0.0453(13) |
| H2′ | 0.9983 | 0.4502 | 0.7637 | 0.054* |
| C3′ | 0.9753(3) | 0.5473(2) | 0.7243(6) | 0.0423(12) |
| C4′ | 0.8951(4) | 0.5955(2) | 0.6645(7) | 0.0458(12) |
| C5′ | 0.7876(3) | 0.5781(2) | 0.6054(7) | 0.0486(13) |
| H5′ | 0.7347 | 0.6103 | 0.5619 | 0.058* |
| C6′ | 0.7563(3) | 0.5127(2) | 0.6097(7) | 0.0479(13) |
| H6′ | 0.6825 | 0.5018 | 0.5729 | 0.057* |
| C2 | 0.8006(3) | 0.3949(2) | 0.6779(6) | 0.0389(12) |
| C3 | 0.8600(3) | 0.3391(2) | 0.6846(6) | 0.0424(12) |
| C4 | 0.8125(3) | 0.2743(2) | 0.6939(6) | 0.0446(12) |
| C5 | 0.6404(4) | 0.2148(2) | 0.6947(7) | 0.0473(13) |
| C6 | 0.5310(4) | 0.2166(2) | 0.6854(7) | 0.0538(14) |
| H6 | 0.4926 | 0.1776 | 0.6859 | 0.065* |
| C7 | 0.4774(3) | 0.2771(2) | 0.6750(7) | 0.0489(13) |
| C8 | 0.5325(3) | 0.3358(2) | 0.6675(7) | 0.0464(12) |
| C9 | 0.6415(3) | 0.3330(2) | 0.6786(6) | 0.0399(12) |
| C10 | 0.6998(3) | 0.2739(2) | 0.6918(6) | 0.0419(12) |
| C16 | 0.4761(5) | 0.4283(3) | 0.8144(10) | 0.109(2) |
| H16A | 0.4480 | 0.4720 | 0.7836 | 0.163* |
| H16B | 0.5492 | 0.4307 | 0.8977 | 0.163* |
| H16C | 0.4306 | 0.4049 | 0.8805 | 0.163* |
| C17 | 0.3041(4) | 0.2245(2) | 0.6496(8) | 0.0830(19) |
| H17C | 0.3098 | 0.1992 | 0.5383 | 0.125* |
| H17A | 0.2291 | 0.2365 | 0.6347 | 0.125* |
| H17B | 0.3298 | 0.1988 | 0.7664 | 0.125* |
| C18 | 1.0481(4) | 0.3224(2) | 0.8311(8) | 0.0777(19) |
| H18A | 1.1181 | 0.3326 | 0.8109 | 0.117* |
| H18B | 1.0430 | 0.2759 | 0.8505 | 0.117* |
| H18C | 1.0396 | 0.3454 | 0.9446 | 0.117* |
| C19 | 1.1645(3) | 0.5215(2) | 0.8348(7) | 0.0569(14) |
| H19A | 1.1604 | 0.4923 | 0.7265 | 0.085* |
| H19B | 1.1554 | 0.4968 | 0.9450 | 0.085* |
| H19C | 1.2345 | 0.5429 | 0.8691 | 0.085* |
Source of material
The title compound is a highly biologically active poly-methoxylated flavonoid, which is anti-inflammatory and antiproliferative [1, 2] , can inhibit leukocyte chemotaxis and oxygen free radical formation [3, 4] and prevents cyclophosphamide and ifosfamide-induced hemorrhagic cystitis in rats [5]. The title compound was isolated using medium pressure column chromatography (MPCC) from Parastrephia quadrangularis, a medicinal plant used by the Aymara Amerindians [6, 7] , a known producer of several flavonoids and benzoic acid derivatives with antioxidant activity [8], [9], [10], [11]. Following our program to isolate interesting metabolites from the Atacama Desert Flora, Northern Chile [12], [13], [14], [15], [16], [17], [18], dried aerial parts of P. quadrangularis (1622 g) collected in april 2015 in “El Tatio”, Andean mountain range of the of Atacama Desert, II Region, Northern Chile, were defatted with hexane (3 liters, 3 times in the dark, 24 hours each time) and 54.82 g were obtained after evaporation of the solvent. Then the plant material was extracted with ethyl acetate (3 liters, 3 times in the dark, 24 hours each time. After evaporation of the solvent under vacuo at 40 oC, 485 g of a dark gummy extract was obtained. A portion of the extract (10.0 g) was filtered and submitted to a medium pressure column chromatography system composed of an 2.5 cm × 48 cm medium pressure column packed with silicagel using an isocratic solvent system of n-hexane-ethyl acetate (8.0:2.0 v:v) pumped with a medium pressure pump with a flow rate of 10 mL-minute. The collected fractions (120) were combined according to TLC analysis (Kieselgel F254 plates, developed with hexane: EtOAc 7:3 v/v, and spots visualized by spraying with vanillin: sulfuric acid 2% in ethanol and heating) and twenty combined fractions were obtained. Fraction 15, enriched in flavonoids (120 mg) was re-chromatographed using Sephadex LH 20 (200 g, solvent methanol) and 60 fractions (S1–S60) were obtained. From fractions S25–33, the known compound: 5,7,4′-trihydroxy-3,8,3′trymethoxyflavone (25 mg) was isolated and from fractions S45–57 the title compound (15 mg) was isolated. The NMR data for both compounds are consistent with literature [19], [20], [21], [22], [23]. Recrystallization from ethyl acetate at room temperature yielded pure yellow crystals of the title compound (8 mg). Yellow crystals, m.p. 215–217 °C. The molecular weight was determined by HESI-MS/MS with a mass spectrometer (Q-exactive Focus, Bremen, Germany) [M—H]−: 373.09290, calcd. for C19H17O8−: 373.09289. 1H NMR (Bruker Avance 300 MHz, CDCl3) δ ppm: 7.80 (1H, s, H-2′), 7.79 (1H, d, J = 9.0 Hz, H-6′), 7.07 (1H, d, J = 9.0 Hz, H-5′), 6.42 (1H, s, H-6), 6.09 (1H, s, 4′-OH), 12.5 (1H, s, 5-OH), 3.99 (3H, s, OCH3), 3.95 (3H, s, OCH3), 3.92 (3H, s, OCH3), 3.88 (3H, s, OCH3). 13C NMR (13C NMR Bruker Avance 75 MHz, DMSO-d6) δ ppm: 158.4 (C-2), 138.6 (C-3), 179.01 (C-4), 157.3 (C-5), 95.4 (C-6), 155.8 (C-7), 128.7 (C-8), 148.5 (C-9), 105.3 (C-10), 122.9 (C-1′), 110.8 (C-2′), 148.4 (C-3′), 146.4 (C-4′), 114.8 (C-5′), 122.6 (C-6′), 61.6 (O-CH3), 60.2 (O-CH3), 56.4 (O-CH3), 56.0 (O-CH3). These data, together with ESI-MS/MS and correlations observed in the HSQC and HMBC spectra, are consistent with the structure of the title compound (cf. the figure) confirmed by comparison of spectroscopic data with those reported in the literature for this structure [19, 20] and other similar methoxilated flavones [21], [22], [23].
Experimental details
H atoms were refined with fixed individual displacement parameters, using a riding model with C—H distances of 0.93 Å (for aromatic rings), 0.96 Å ( for CH3 group) and O—H of 0.86 Å with U(H) values of 1.2Ueq(C,O) (for CH in aromatic moiety and OH group), and 1.5Ueq(C) (for CH3). Problems during data collection and the successive reduction yielded many reflections with a negative intensity, which produced a non-optimal Rint factor. Nevertheless the topology of this structure is in any case correct.
Discussion
The title compound (1) is a positional isomer of 5-hydroxy-2-(3′-hydroxy-4-methoxyphenyl)-3,6,7′-trimethoxychromen-4-one (casticin) (2) [24] and 5,12-Dihydroxy-2,6,7,13-tetramethoxyflavone (3) [25]. In the compounds (2) and (3) the fused chromene ring system and the benzene ring bonded to it are close to coplanar, with a dihedral angle between their respective mean planes of: 8.30(12)° and 2.7(1)° for (2) and (3) respectively, however for the compound (1) the dihedral angle value is 17.17(18)°, which is the main difference with the isomers (2) and (3). The two hydroxy H atoms of the title compound are involved in intramolecular and intermolecular O—H⋯O hydrogen bonding in the solid state. Intramolecular O4—H4⋯O5 and intermolecular O8—H8⋯O5 (2 − x, 1/2 + y, 1/2 − z) hydrogen bonds and π-π interactions help to stabilize the crystal structure. In the crystal packing the molecules are associated by one strong intermolecular hydrogen bond forming zig-zag chain with graph-set motif C(10) [26] along [010] direction. All distances and angles are normal and comparable with the compounds (2) and (3).
Acknowledgements
IB thanks to Fondequip (EQM13-0021). JB and MS thanks to FONDECYT (Chile) (Grant 1140178) and DID-PEF 2017 Universidad Austral de Chile for financial support.
References
Pessoa, C.; Silveira, E. R.; Lemos, T. L. G.; Wetmore, L. A.; Moraes, M. O.; Leyva, A.: Antiproliferative effects of compounds derived from plants of Northeast Brazil. Phytother. Res. 14 (2000) 187–119.10.1002/(SICI)1099-1573(200005)14:3<187::AID-PTR572>3.0.CO;2-ISearch in Google Scholar
Souza, M. F.; Rao, V. S. N.; Silveira, E. R.: Antianaphylactic and antiinflammatory effects of ternatin, a flavonoid isolated from egletes-viscosa less. Braz. J. Med. Biol. Res. 25 (1992) 1029–1032.Search in Google Scholar
Souza, M. F.; Cunha, G. M. A.; Fontenele, J. B.; Viana, G. S. B.; Rao, V. S. N.; Silveira, E. R.: Antithrombotic activity of ternatin, a tetramethoxy flavone from Egletes viscosa less. Phytother. Res. 8 (1994) 478–481.10.1002/ptr.2650080808Search in Google Scholar
Rao, V. S. N.; Figueiredo, E. G.; Melo, C. L.; Viana, G. S. B.; Menezes, D. B.; Matos, M. S. F.; Silveira, E. R.: Protective effect of ternatin, a flavonoid isolated from Egletes viscosa less., in experimental liver injury. Pharmacology 48 (1994) 392–397.10.1159/000139206Search in Google Scholar
Vieira, M. M.; Macedo, F. Y. B.; Belarmino, J. N.; Costa, A.; Cunha, A. N.; Silveira, E. R.; Brito, G. A. C.; Ribeiro, R. A.: Ternatin, a flavonoid, prevents cyclophosphamide and ifosfamide-induced hemorrhagic cystitis in rats. Phytother. Res. 18 (2004) 135–141.10.1002/ptr.1379Search in Google Scholar
Cortes, A.; Rau, J. R.; Miranda, E.; Jimenez, J. E.: Hábitos alimenticios de Lagidium viscacia y Abrocoma cinerea: roedores sintópicos en ambientes altoandinos del norte de Chile. Rev. Chil. Hist . Nat. 75 (2002) 583–593.10.4067/S0716-078X2002000300009Search in Google Scholar
Moreira-Munoz, A.; Munoz-Schick, M.; Marticorena, A.; Morales, V.: Catálogo de Asteraceae (Compositae) de la Región de Arica y Parinacota, Chile. Gayana Bot. 73 (2016) 226–267.10.4067/S0717-66432016000200226Search in Google Scholar
Loyola, L. A.; Naranjo, J.; Morales, G.: 5,7-Dihydroxy-3,8,3′,4′-tetramethoxyflavone from Parastrephia Quadrangularis. Phytochemistry 24 (1985) 1871–1872.10.1016/S0031-9422(00)82580-7Search in Google Scholar
Bohlmann, F.; Fritz, U.; King, R. M.: Tremeton-derivate aus parastrephia lepidophylla. Phytochemistry 18 (1979) 1403–1405.10.1016/0031-9422(79)83037-XSearch in Google Scholar
D’Almeida, R. E.; Alberto, M. R.; Quispe, C.; Schmeda-Hirschmann, G.; Isla, M. I.: Antimicrobial phenylpropanoids from the argentinean highland plant Parastrephia lucida (Meyen). J. Ethnopharmacol. 142 (2012) 407–414.10.1016/j.jep.2012.05.010Search in Google Scholar
Echiburu-Chau, C.; Pastén, L.; Parra, C.; Bórquez, J.; Mocan, A.; Simirgiotis, M. J.: High resolution UHPLC-MS characterization and isolation of main compounds from the antioxidant medicinal plant Parastrephia lucida (Meyen). Saudi Pharm. J. 25 (2017) 1032–1039.10.1016/j.jsps.2017.03.001Search in Google Scholar
Bórquez, J.; Ardiles, A.; Loyola, L. A.; Peña-Rodriguez, L. M.; Molina-Salinas, G. M.; Vallejos, J.; Collado, I. G.; Simirgiotis, M. J.: Further mulinane and azorellane diterpenoids isolated from mulinum crassifolium and azorella compacta. Molecules 19 (2014) 3898–3908.10.3390/molecules19043898Search in Google Scholar PubMed PubMed Central
Bórquez, J.; Bartolucci, N. L.; Echiburú-Chau, C.; Winterhalter, P.; Vallejos, J.; Jerz, G.; Simirgiotis, M. J.: Isolation of cytotoxic diterpenoids from the Chilean medicinal plant Azorella compacta Phil from the Atacama Desert by high-speed counter-current chromatography. J. Sci. Food Agric. 96 (2016) 2832–2838.10.1002/jsfa.7451Search in Google Scholar PubMed
Brito, I.; Bórquez, J.; Simirgiotis, M.; Neves-Vieira, M.; Jerz, G.; Winterhalter, P.; Bolte, M.; Cárdenas, A.: Crystal structure of nor-1,2-secolycoserone, C24H32O4. Z. Kristallogr. NCS 229 (2014) 399–400.10.1515/ncrs-2014-0212Search in Google Scholar
Brito, I.; Borquez, J.; Simirgiotis, M.; Cardenas, A.; Lopez-Rodriguez, M.: 5-Dihydroxy-7-methyloxyflavanone dihydrate 4′. Acta Crystallogr. E68 (2012) o32–o33.10.1107/S1600536811051221Search in Google Scholar PubMed PubMed Central
Brito, I.; Borquez, J.; Simirgiotis, M.; Cardenas, A.; Molina-Salinas, G. M.; Jerz, G.; Pena-Rodriguez, L. M.; Winterhalter, P.: Crystal structure of methyl 8-hydroxy-3-isopropyl-5a,8-dimethyl-2,3,4,5,5a,6,7,8,10a, 10bdecahydrocyclohepta[e]indene-3a(1H)-carboxylate, C21H34O3. Z. Kristallogr. NCS 231 (2016) 579–582.10.1515/ncrs-2015-0197Search in Google Scholar
Brito, I.; Simirgiotis, M.; Jerz, G.; Werner, M. R.; Borques, J.; Winterhalter, P.; Cardenas, A.: Crystal structure of 3′,4′,5-trihydroxy-3,7dimethoxyflavone, C17H14O7. Z. Kristallogr. NCS 231 (2016) 113–115.10.1515/ncrs-2015-0054Search in Google Scholar
Brito, I.; Simirgiotis, M.; Muñoz, R.; Benites, J.; Pasten, L.; Bórquez, J.; Cárdenas, A.: Crystal structure of 11-(p-coumaroyloxy)-tremetone, C22H20O5. Z. Kristallogr. NCS 232 (2017) 13–14.10.1515/ncrs-2016-0105Search in Google Scholar
Wollenweber, E.; Fischer, R.; Dörr, M.; Irvine, K.; Pereira, C.; Stevens Jan, F.: Chemodiversity of exudate flavonoids in cassinia and ozothamnus(asteraceae, naphalieae). Z. Naturforsch., C: Biosci. 63 (2008) 731–739.10.1515/znc-2008-9-1019Search in Google Scholar PubMed
Gedara Sahar, R.; Abdel-Halim Osama, B.; El-Sharkawy Saleh, H.; Salama Osama, M.; Shier Thomas, W.; Halim Ahmed, F.: New erythroxane-type diterpenoids from fagonia boveana (hadidi) hadidi & graf. Z. Naturforsch. C 58 (2003) 23–32.10.1515/znc-2003-1-204Search in Google Scholar PubMed
Yoo, H.; Kim, S. H.; Lee, J.; Kim, H. J.; Seo, S. H.; Chung, B. Y.; Jin, C.; Lee, Y. S.: Synthesis and antioxidant activity of 3-methoxyflavones. Bull. Korean Chem. Soc. 26 (2005) 2057–2060.10.5012/bkcs.2005.26.12.2057Search in Google Scholar
Agrawal, P. K.: Carbon-13 NMR of Flavonoids. Elsevier, Michigan (1989).10.1016/B978-0-444-87449-8.50011-0Search in Google Scholar
Modak, B.; Rojas, M.; Torres, R.: Chemical analysis of the resinous exudate isolated from Heliotropium taltalense and evaluation of the antioxidant activity of the phenolics components and the resin in homogeneous and heterogeneous systems. Molecules 14 (2009) 1980–1989.10.3390/molecules14061980Search in Google Scholar PubMed PubMed Central
Asker, E.; Akin, S.; Hökelek, T.: 5,3′-Dihydroxy-3,6,7,4′-tetramethoxyflavone. Acta Crystallogr. E62 (2006) o4159–o416110.1107/S1600536806033630Search in Google Scholar
Meng, Z.-L.; Qi, Y.-Y.; Liu, R.-M.; Sun, A.-L.; Wang, D.-Q.: 5,12-Dihydroxy-2,6,7,13-tetramethoxyflavone. Acta Crystallogr. E62 (2006) o3831–o3832.10.1107/S1600536806031175Search in Google Scholar
Bernstein, J.; Davis, R. E.; Shimoni, L.; Chang, N.-L.: Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. Engl. 34 (1995) 1555–1573.10.1002/anie.199515551Search in Google Scholar
Bruker: APEX3, SAINT Bruker AXS Inc., Madison, WI, USA (2016).Search in Google Scholar
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–341.10.1107/S0021889808042726Search in Google Scholar
©2018 Iván Brito et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Editorial 2018
- Crystal structure of dimethanol-bis{3-(((2-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N:O′}dizinc(II), C42H30Zn2N2O10
- Crystal structure of aqua-bis{[2,6-dimethyl-N-(pyridin-2-ylmethylene)aniline-κ2N,N′]}zinc(II) triflate monohydrate [ZnC29H31N4O]CF3SO3⋅H2O
- Crystal structure of (E)-1-(4-{[(E)-4-Diethylamino-2-hydroxybenzene methylene]amino}phenyl)ethanone methoxy oxime, C20H27ClN3O3
- Crystal structure of (E)-1-(4-(((E)-4-(diethylamino)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one oxime, C19H23N3O2
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4,4′-sulfonyldibenzoato-κ2O:O′)zinc(II)], C40H34N4O6SZn
- Crystal structure of catena-poly[diaqua(μ3-pyrazine-2,3-dicarboxylato-κ4O,N:O′:O′′)zinc(II)] 1.25 hydrate, C6H8.5N2O7.25Zn
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri-m-tolyl phosphane-κP)rhenium(I), C29H28O5PRe
- Crystal structure of bis(μ2-methanolato-κ2O:O)-bis(methanol-κ1O)-bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,O′,N}dichromium(III), C38H36Cr2N2O14
- Crystal structure of poly[aqua-(μ3-pyridine-3,5-dicarboxylato-κ5O,O′:O′′,O′′′,N)zinc(II)], C7H7NO6Zn
- Crystal structure of bis((1-(((4-(((benzyloxy)imino)methyl)phenyl)imino)methyl)naphthalen-2-yl)oxy-κ2O,N)copper(II), C52H42CuN4O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}cobalt(II), C40H38CoN4O6
- Crystal structure of diaqua-bis(N,N-dimethylformamide-κ1O)-bis{3-((5-chloro-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ4N,O,O′:O′}dinickel(II), C38H34Ni2Cl2N4O12
- Crystal structure of tetrakis(methanol-κO)bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}bicobalt(II), C38H38Co2N2O14
- Crystal structure of (S)-tert-butyl-(1-hydroxypropan-2-yl)carbamate, C8H17NO3
- Crystal structure of 4-(4′-(pyridin-4-yl)-[1,1′-biphenyl]-4-yl)pyridin-1-ium catena-poly[{5-carboxy-4′-methyl-[1,1′-biphenyl]-3-carboxylato-κ2O,O′}-(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-κ4O,O′:O′′,O′′′)lead(II)], C52H40N2O9Pb
- Crystal structure of catena-poly[diaqua-(μ2-5-methylisophthalato-κ2O:O′)(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)], NiC21H22O6N6
- Crystal structure of the salt tris(guanidinium) tris(tetrapropylammonium) bis(pyridine-2,4,6-tricarboxylate) – water (1/10), C55H126N14O22
- Crystal structure of 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7,8-trimethoxy-4H-chromen-4-one, C19H18O8
- Crystal structure of poly{[μ2-1,1′-(sulfonylbis(4,1-phenylene))bis(2-methyl-1H-imidazole)-κ2N:N′][μ2-4,4′-oxydibenzoato-κ2O:O′]cobalt(II)} hemihydrate, C34H27N4O7.5SCo
- The crystal structure of 25,27-(2,2′-[(2-thioxo-1,3-dithiole-4,5-diyl)disulfanediyl]diethanolate)-26,28-dihydroxycalix[4]arene — dichloromethane (1/1), C36H32Cl2O4S5
- The crystal structure of 1,2-bis(3-(pyridin-3-yl)-1,2,4-oxadiazol-5-yl)ethane, C16H12N6O2
- Crystal structure of 1-benzyl-3-((4-bromophenyl)amino)-4-(4-methoxyphenyl)-1H-pyrrole-2,5-dione, C24H19BrN2O3
- Crystal structure of bis(2-((allylcarbamothioyl)imino)-4-methylthiazol-3-ido-κ2N,S)palladium(II), C16H20N6PdS4
- Crystal structure of pyrimidine-2,5-dicarboxylic acid 1.5 hydrate, C12H14N4O11
- Crystal structure of trans-diaqua-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II), C8H10N4O6Mn
- Crystal structure of catena-(μ3-5-bromoisophthatato-κO,O′: O′′,O′′′′)-(1,2-bis(imidazol-1-yl)ethane-κN:N′)cobalt(II), C16H13CoN4O4Br
- Investigation of the compound La5Zn2−xPb1 + x (x = 0.20–0.32)
- Crystal structure of (OC-6-13)-diaqua-bis(3,5-di(pyridin-3-yl)-4H-1,2,4-triazol-4-amine-κ1N)-bis(dicyanamido-κ1N)zinc(II) tetrahydrate, ZnC28H32N18O6
- Crystal structure of Ga0.62(3)Sb0.38(3)Pd3
- Crystal structure of Ga0.47(1)Sb0.53(1)Pd2
- A derivative of the Corey lactone – crystal structure of (3aR,4S,5R,6aS)-4-(((tert-butyldimethylsilyl)oxy)methyl)-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl benzoate, C21H30O5Si
- A Corey lactone: crystal structure of (3aR,4R,5R,6aS)-5-benzoyloxy-4(hydroxymethyl)hexahydro-2H-cyclopenta[b]furan-2-one, C15H16O5
- Hydrothermal synthesis and crystal structure of poly[aqua-(μ2-1,3-bis(4-pyridyl)propane-κ2N:N′)-(μ2-1,4,5,6,7,7-hexachlorobicyclo[2.2.1]hept-5-ene-2,3-dicarboxylato-κ2O:O′)manganese(II) hydrate, C22H20Cl6N2O6Mn
- Crystal structure of 2-acetylpyrrole S-methylthiosemicarbazonium hydroiodide, C8H13IN4S
- Crystal structure of [N,N-bis((pyrrol-2-yl)ethylidene)butane-1,4-diamine-κ4N,N′,N′′,N′′′]-nickel(II), C16H20N4Ni
- Crystal structure of poly[aqua-(μ5-2,5-dicarboxybenzoato-κ5O:O:O′:O′′:O′′′)sodium(I)], C9H7NaO7
- Crystal structure of bis(N′-((1H-pyrrol-2-yl)methylene)-1-methylthio-methanethiohydrazido-κ2S,N)nickel(II), C14H16N6NiS4
- Crystal structure of 1-(4-((benzo[d][1,3]dioxol-5-yloxy)methyl)phenethyl)-4-(3-chlorophenyl) piperazin-1-ium chloride, C26H28Cl2N2O3
- Crystal structure of 2-(4-(2-(4-(2-fluorophenyl)piperazin-1-yl)ethyl)benzyl)benzo[d]isothiazol-3(2H)-one 1,1-dioxide, C26H26FN3O3S – a saccharin dervative
- Crystal structure of 3-(2-dimethylaminoethyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C12H15N3OS
- Crystal structure of 3-(3-dimethylaminopropyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C13H17N3OS
- The crystal structure of trans-tetraaqua-bis(p-tolylsulfinato-κO)calcium(II)), C14H22O8S2Ca
- The crystal structure of (E)-N′-(pyridin-2-ylmethylene)pyrazine-2-carbohydrazide, C11H9N5O
- Crystal structure of (E)-3-(pyren-1-yl)-1-(pyridin-4-yl)prop-2-en-1-one, C24H15NO
- Crystal structure of catena-poly[diaqua-(μ2-tartrato-κ4O,O′:O′′,O′′′)cobalt(II)], C4H8CoO8
- Crystal structure of 4-chloro-2-methyl-6-(4-(trifluoromethoxy)phenyl)pyrimidine, C12H8ClF3N2O
- Crystal structure of 1-(4-fluorophenyl)-N-(5-((triphenylstannyl)thio)thiophen-2-yl)methanimine, C27H20FN3S2Sn
- Crystal structure of methyl (Z)-2-(5-fluoro-2-oxoindolin-3-ylidene)hydrazine-1-carbodithioate, C10H8FN3OS2
- Crystal structure of tert-butyl (Z)-4-(2-(5-methoxy-3-(2-((methylthio)carbonothioyl)hydrazono)-2-oxoindolin-1-yl)ethyl)piperazine-1-carboxylate, C22H31N5O4S2
- The crystal structure of (E)-2-((2-(o-tolylcarbamothioyl)hydrazono)methyl)benzoic acid, C16H15N3O2S
- Crystal structure of 2-chloro-1,3-di-tert-pentyl-4,4-diphenyl-1,3,2λ3,4-diazaphosphasiletidine, C22H32ClN2PSi
- Crystal structure of tetramethyl 5,5′-(buta-1,3-diyne-1,4-diyl)diisophthalate, C24H18O8
- Crystal structural of 2-amino-4-(4-methoxyphenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C19H20N2O3
- Crystal structure of 1,3,5-tris((trimethylsilyl)methyl)-1,3,5-triazinane-2,4,6-trione, C15H33N3O3Si3
- The crystal structure of bis(2-benzoyl-5-hydroxylphenolato-κ2O,O′)copper(II), C26H18CuO6
- Crystal structure of 2,6-bis(3-(pyrazin-2-yl)-1H-1,2,4-triazol-5-yl)pyridine – 1-ethyl-3-methyl-1H-imidazol-3-ium bromide (1/1), C23H22N13Br
- The crystal structure of (E)-N-benzyl-N′-benzylidene-4-methylbenzenesulfonohydrazide, C21H20N2O2S
- Crystal structure of ethyl (E)-5-((2-(3-hydroxybenzoyl)hydrazono)methyl)-3,4-dimethyl-1H-pyrrole-2-carboxylate – water – ethanol (1/1/1), C19H27N3O6
- The crystal structure of (E)-4-(3-ethoxy-2-hydroxybenzylideneamino)benzoic acid, C16H15NO4
- Crystal structure of (μ2-N,N′-bis((pyridin-4-yl)methyl)ethanediamide-κ2N:N′)-tetrakis(diethylcarbamodithioato-κ2S,S′)dizinc(II), C34H54N8O2S8Zn2
Articles in the same Issue
- Cover and Frontmatter
- Editorial 2018
- Crystal structure of dimethanol-bis{3-(((2-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N:O′}dizinc(II), C42H30Zn2N2O10
- Crystal structure of aqua-bis{[2,6-dimethyl-N-(pyridin-2-ylmethylene)aniline-κ2N,N′]}zinc(II) triflate monohydrate [ZnC29H31N4O]CF3SO3⋅H2O
- Crystal structure of (E)-1-(4-{[(E)-4-Diethylamino-2-hydroxybenzene methylene]amino}phenyl)ethanone methoxy oxime, C20H27ClN3O3
- Crystal structure of (E)-1-(4-(((E)-4-(diethylamino)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one oxime, C19H23N3O2
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4,4′-sulfonyldibenzoato-κ2O:O′)zinc(II)], C40H34N4O6SZn
- Crystal structure of catena-poly[diaqua(μ3-pyrazine-2,3-dicarboxylato-κ4O,N:O′:O′′)zinc(II)] 1.25 hydrate, C6H8.5N2O7.25Zn
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri-m-tolyl phosphane-κP)rhenium(I), C29H28O5PRe
- Crystal structure of bis(μ2-methanolato-κ2O:O)-bis(methanol-κ1O)-bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,O′,N}dichromium(III), C38H36Cr2N2O14
- Crystal structure of poly[aqua-(μ3-pyridine-3,5-dicarboxylato-κ5O,O′:O′′,O′′′,N)zinc(II)], C7H7NO6Zn
- Crystal structure of bis((1-(((4-(((benzyloxy)imino)methyl)phenyl)imino)methyl)naphthalen-2-yl)oxy-κ2O,N)copper(II), C52H42CuN4O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}cobalt(II), C40H38CoN4O6
- Crystal structure of diaqua-bis(N,N-dimethylformamide-κ1O)-bis{3-((5-chloro-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ4N,O,O′:O′}dinickel(II), C38H34Ni2Cl2N4O12
- Crystal structure of tetrakis(methanol-κO)bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}bicobalt(II), C38H38Co2N2O14
- Crystal structure of (S)-tert-butyl-(1-hydroxypropan-2-yl)carbamate, C8H17NO3
- Crystal structure of 4-(4′-(pyridin-4-yl)-[1,1′-biphenyl]-4-yl)pyridin-1-ium catena-poly[{5-carboxy-4′-methyl-[1,1′-biphenyl]-3-carboxylato-κ2O,O′}-(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-κ4O,O′:O′′,O′′′)lead(II)], C52H40N2O9Pb
- Crystal structure of catena-poly[diaqua-(μ2-5-methylisophthalato-κ2O:O′)(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)], NiC21H22O6N6
- Crystal structure of the salt tris(guanidinium) tris(tetrapropylammonium) bis(pyridine-2,4,6-tricarboxylate) – water (1/10), C55H126N14O22
- Crystal structure of 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7,8-trimethoxy-4H-chromen-4-one, C19H18O8
- Crystal structure of poly{[μ2-1,1′-(sulfonylbis(4,1-phenylene))bis(2-methyl-1H-imidazole)-κ2N:N′][μ2-4,4′-oxydibenzoato-κ2O:O′]cobalt(II)} hemihydrate, C34H27N4O7.5SCo
- The crystal structure of 25,27-(2,2′-[(2-thioxo-1,3-dithiole-4,5-diyl)disulfanediyl]diethanolate)-26,28-dihydroxycalix[4]arene — dichloromethane (1/1), C36H32Cl2O4S5
- The crystal structure of 1,2-bis(3-(pyridin-3-yl)-1,2,4-oxadiazol-5-yl)ethane, C16H12N6O2
- Crystal structure of 1-benzyl-3-((4-bromophenyl)amino)-4-(4-methoxyphenyl)-1H-pyrrole-2,5-dione, C24H19BrN2O3
- Crystal structure of bis(2-((allylcarbamothioyl)imino)-4-methylthiazol-3-ido-κ2N,S)palladium(II), C16H20N6PdS4
- Crystal structure of pyrimidine-2,5-dicarboxylic acid 1.5 hydrate, C12H14N4O11
- Crystal structure of trans-diaqua-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II), C8H10N4O6Mn
- Crystal structure of catena-(μ3-5-bromoisophthatato-κO,O′: O′′,O′′′′)-(1,2-bis(imidazol-1-yl)ethane-κN:N′)cobalt(II), C16H13CoN4O4Br
- Investigation of the compound La5Zn2−xPb1 + x (x = 0.20–0.32)
- Crystal structure of (OC-6-13)-diaqua-bis(3,5-di(pyridin-3-yl)-4H-1,2,4-triazol-4-amine-κ1N)-bis(dicyanamido-κ1N)zinc(II) tetrahydrate, ZnC28H32N18O6
- Crystal structure of Ga0.62(3)Sb0.38(3)Pd3
- Crystal structure of Ga0.47(1)Sb0.53(1)Pd2
- A derivative of the Corey lactone – crystal structure of (3aR,4S,5R,6aS)-4-(((tert-butyldimethylsilyl)oxy)methyl)-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl benzoate, C21H30O5Si
- A Corey lactone: crystal structure of (3aR,4R,5R,6aS)-5-benzoyloxy-4(hydroxymethyl)hexahydro-2H-cyclopenta[b]furan-2-one, C15H16O5
- Hydrothermal synthesis and crystal structure of poly[aqua-(μ2-1,3-bis(4-pyridyl)propane-κ2N:N′)-(μ2-1,4,5,6,7,7-hexachlorobicyclo[2.2.1]hept-5-ene-2,3-dicarboxylato-κ2O:O′)manganese(II) hydrate, C22H20Cl6N2O6Mn
- Crystal structure of 2-acetylpyrrole S-methylthiosemicarbazonium hydroiodide, C8H13IN4S
- Crystal structure of [N,N-bis((pyrrol-2-yl)ethylidene)butane-1,4-diamine-κ4N,N′,N′′,N′′′]-nickel(II), C16H20N4Ni
- Crystal structure of poly[aqua-(μ5-2,5-dicarboxybenzoato-κ5O:O:O′:O′′:O′′′)sodium(I)], C9H7NaO7
- Crystal structure of bis(N′-((1H-pyrrol-2-yl)methylene)-1-methylthio-methanethiohydrazido-κ2S,N)nickel(II), C14H16N6NiS4
- Crystal structure of 1-(4-((benzo[d][1,3]dioxol-5-yloxy)methyl)phenethyl)-4-(3-chlorophenyl) piperazin-1-ium chloride, C26H28Cl2N2O3
- Crystal structure of 2-(4-(2-(4-(2-fluorophenyl)piperazin-1-yl)ethyl)benzyl)benzo[d]isothiazol-3(2H)-one 1,1-dioxide, C26H26FN3O3S – a saccharin dervative
- Crystal structure of 3-(2-dimethylaminoethyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C12H15N3OS
- Crystal structure of 3-(3-dimethylaminopropyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C13H17N3OS
- The crystal structure of trans-tetraaqua-bis(p-tolylsulfinato-κO)calcium(II)), C14H22O8S2Ca
- The crystal structure of (E)-N′-(pyridin-2-ylmethylene)pyrazine-2-carbohydrazide, C11H9N5O
- Crystal structure of (E)-3-(pyren-1-yl)-1-(pyridin-4-yl)prop-2-en-1-one, C24H15NO
- Crystal structure of catena-poly[diaqua-(μ2-tartrato-κ4O,O′:O′′,O′′′)cobalt(II)], C4H8CoO8
- Crystal structure of 4-chloro-2-methyl-6-(4-(trifluoromethoxy)phenyl)pyrimidine, C12H8ClF3N2O
- Crystal structure of 1-(4-fluorophenyl)-N-(5-((triphenylstannyl)thio)thiophen-2-yl)methanimine, C27H20FN3S2Sn
- Crystal structure of methyl (Z)-2-(5-fluoro-2-oxoindolin-3-ylidene)hydrazine-1-carbodithioate, C10H8FN3OS2
- Crystal structure of tert-butyl (Z)-4-(2-(5-methoxy-3-(2-((methylthio)carbonothioyl)hydrazono)-2-oxoindolin-1-yl)ethyl)piperazine-1-carboxylate, C22H31N5O4S2
- The crystal structure of (E)-2-((2-(o-tolylcarbamothioyl)hydrazono)methyl)benzoic acid, C16H15N3O2S
- Crystal structure of 2-chloro-1,3-di-tert-pentyl-4,4-diphenyl-1,3,2λ3,4-diazaphosphasiletidine, C22H32ClN2PSi
- Crystal structure of tetramethyl 5,5′-(buta-1,3-diyne-1,4-diyl)diisophthalate, C24H18O8
- Crystal structural of 2-amino-4-(4-methoxyphenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C19H20N2O3
- Crystal structure of 1,3,5-tris((trimethylsilyl)methyl)-1,3,5-triazinane-2,4,6-trione, C15H33N3O3Si3
- The crystal structure of bis(2-benzoyl-5-hydroxylphenolato-κ2O,O′)copper(II), C26H18CuO6
- Crystal structure of 2,6-bis(3-(pyrazin-2-yl)-1H-1,2,4-triazol-5-yl)pyridine – 1-ethyl-3-methyl-1H-imidazol-3-ium bromide (1/1), C23H22N13Br
- The crystal structure of (E)-N-benzyl-N′-benzylidene-4-methylbenzenesulfonohydrazide, C21H20N2O2S
- Crystal structure of ethyl (E)-5-((2-(3-hydroxybenzoyl)hydrazono)methyl)-3,4-dimethyl-1H-pyrrole-2-carboxylate – water – ethanol (1/1/1), C19H27N3O6
- The crystal structure of (E)-4-(3-ethoxy-2-hydroxybenzylideneamino)benzoic acid, C16H15NO4
- Crystal structure of (μ2-N,N′-bis((pyridin-4-yl)methyl)ethanediamide-κ2N:N′)-tetrakis(diethylcarbamodithioato-κ2S,S′)dizinc(II), C34H54N8O2S8Zn2

