Abstract
C16H15NO4, triclinic, P1̅ (no. 2), a = 4.9960(4) Å, b = 6.9139(5) Å, c = 20.7650(15) Å, α = 83.718(6)°, β = 84.805(6)°, γ = 78.648(6)°, V = 697.25(9) Å3, Z = 2, Rgt(F) = 0.0480, wRref(F2) = 0.0988, T = 296 K.
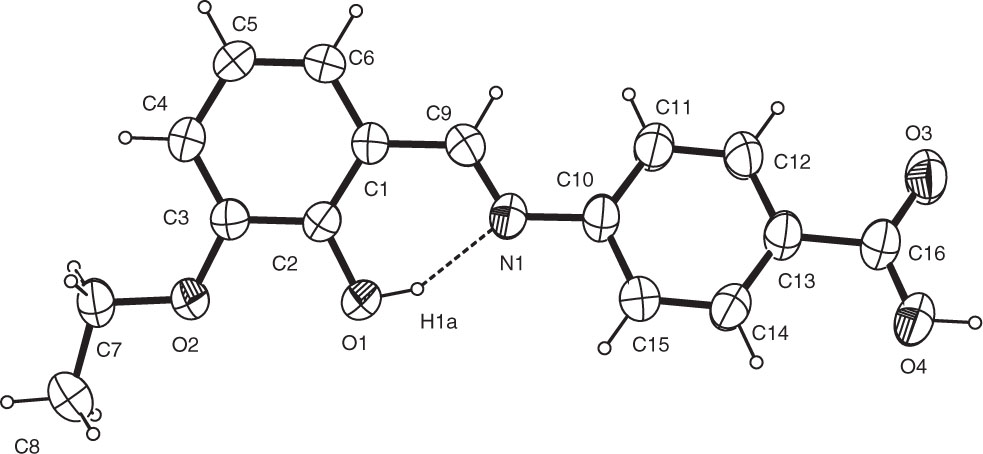
The asymmetric unit of the title crystal structure is shown in the figure. Tables 1 and 2 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Brown needle |
| Size: | 0.76 × 0.30 × 0.03 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.0 cm−1 |
| Diffractometer, scan mode: | STOE IPDS 2, ω scans |
| 2θmax, completeness: | 52°, 98.8% |
| N(hkl)measured, N(hkl)unique, Rint: | 7346, 2710, 0.141 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1509 |
| N(param)refined: | 198 |
| Programs: | Stoe programs [1], SHELX [2], ORTEP-3 [3], WinGX [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | −0.0486(4) | 0.5847(3) | 0.28544(10) | 0.0414(5) |
| C2 | −0.1998(4) | 0.4428(3) | 0.31452(10) | 0.0416(5) |
| C3 | −0.4125(4) | 0.4952(3) | 0.36158(10) | 0.0437(5) |
| C4 | −0.4763(5) | 0.6873(3) | 0.37826(11) | 0.0488(6) |
| H4 | −0.6198 | 0.7229 | 0.4090 | 0.059* |
| C5 | −0.3276(5) | 0.8288(3) | 0.34940(11) | 0.0528(6) |
| H5 | −0.3722 | 0.9581 | 0.3609 | 0.063* |
| C6 | −0.1167(5) | 0.7773(3) | 0.30435(10) | 0.0500(6) |
| H6 | −0.0164 | 0.8719 | 0.2859 | 0.060* |
| C7 | −0.7522(5) | 0.3840(3) | 0.43814(11) | 0.0516(6) |
| H7A | −0.8995 | 0.4881 | 0.4231 | 0.062* |
| H7B | −0.6788 | 0.4261 | 0.4748 | 0.062* |
| C8 | −0.8560(5) | 0.1955(4) | 0.45762(12) | 0.0664(7) |
| H8A | −0.7084 | 0.0936 | 0.4723 | 0.080* |
| H8B | −0.9290 | 0.1558 | 0.4210 | 0.080* |
| H8C | −0.9972 | 0.2162 | 0.4921 | 0.080* |
| C9 | 0.1799(5) | 0.5306(3) | 0.23893(10) | 0.0466(6) |
| H9 | 0.2836 | 0.6252 | 0.2225 | 0.056* |
| C10 | 0.4725(4) | 0.3035(3) | 0.17547(10) | 0.0458(6) |
| C11 | 0.5881(5) | 0.4351(4) | 0.13141(11) | 0.0609(7) |
| H11 | 0.5124 | 0.5695 | 0.1289 | 0.073* |
| C12 | 0.8139(5) | 0.3671(3) | 0.09157(11) | 0.0599(7) |
| H12 | 0.8913 | 0.4564 | 0.0625 | 0.072* |
| C13 | 0.9279(5) | 0.1666(3) | 0.09416(10) | 0.0488(6) |
| C14 | 0.8084(5) | 0.0359(3) | 0.13666(11) | 0.0537(6) |
| H14 | 0.8811 | −0.0990 | 0.1382 | 0.064* |
| C15 | 0.5821(5) | 0.1029(3) | 0.17687(11) | 0.0515(6) |
| H15 | 0.5024 | 0.0129 | 0.2051 | 0.062* |
| C16 | 1.1746(5) | 0.0938(4) | 0.05226(10) | 0.0500(6) |
| N1 | 0.2443(4) | 0.3570(3) | 0.21973(8) | 0.0471(5) |
| O1 | −0.1432(4) | 0.2540(2) | 0.29939(8) | 0.0572(5) |
| O2 | −0.5430(3) | 0.3452(2) | 0.38719(7) | 0.0551(5) |
| O3 | 1.2797(4) | 0.2190(3) | 0.01362(8) | 0.0686(5) |
| O4 | 1.2701(4) | −0.0897(3) | 0.05632(9) | 0.0663(5) |
| H1A | −0.013(5) | 0.249(4) | 0.2681(11) | 0.089(10)* |
| H4A | 1.423(6) | −0.129(6) | 0.0302(18) | 0.19(2)* |
Source of materials
The title compound was prepared by refluxing a mixture of a solution containing 3-ethoxy-2-hydroxybenzaldehyde (0.5 g; 3.01 mmol) in 20 mL ethanol and a solution containing 4-aminobenzoic acid (0.41 g; 3.01 mmol) in 20 mL ethanol. The reaction mixture was stirred for 1 h under reflux. The crystals for the crystal structure determination were obtained from acetone by slow evaporation (yield 72%, m.p. 488–490 K).
Experimental details
All H atoms except for H1A and H4A were refined using riding models, with C—H distances of 0.96 Å for CH3 group, 0.97 Å for CH2 group and 0.93 Å for aromatic groups. The displacement parameters of these H atoms were fixed at 1.2Ueq of their parent carbon atom for aromatic groups and methylene, 1.5Ueq of their parent atoms for methyl group.
Comment
The reaction of an aldehyde and a primary amine forms the Schiff base compounds having a −C=N− double bond. These compounds have a wide range of application in the fields of coordination chemistry, biochemistry, pharmacy, nanotechnology, optical devices and textile industries [5], [6], [7]. Particularly the o-hydroxy Schiff base class is more attractive for physicists and chemists because of its interesting photo-, solvato- and thermochromic features which are caused by an intramolecular proton transfer from the hydroxyl O atom to the imine N atom with a change in the π-electronic system in the solid state. o-Hydroxy Schiff base compounds adopt OH (enol-imine/benzenoid) [8] or NH (keto-amine/quinoid) [9] tautomeric forms with reference to the location of the transferred proton.
The title compound adopts enol-imine tautomeric form and has E configuration around the C=N double bond. The bond lengths and angles are in the expected ranges and comparable with those of similar compounds in literature [10, 11] . The compound displays a strong intramolecular hydrogen bond including the atoms O1 and N1 (O⋯N = 2.588(2) Å) as a common feature of o-hydroxysalicylidene systems. As can be seen from the figure, this strong hydrogen bond constitutes a S(6) ring. In three dimensional network, the crystal structure is stabilized by an intermolecular O—H⋯O hydrogen bond and weak van der Waals interactions. The donor-acceptor distance of this intermolecular O4—H4A⋯O3′ hydrogen bond is 2.621(2) Å [symmetry code (’) = 3−x, −y, −z].
References
Stoe & Cie: X-AREA and X-RED. Stoe & Cie, Darmstadt, Germany (2002).Search in Google Scholar
Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar
Farrugia, L. J.: ORTEP-3 for Windows–a version of ORTEP-III with a graphical user interface (GUI). J. Appl. Cryst. 30 (1997) 565.10.1107/S0021889897003117Search in Google Scholar
Farrugia, L. J.: WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 32 (1999) 837–838.10.1107/S0021889899006020Search in Google Scholar
Krygowski, T. M.; Zachara-Horegland, J. E.; Palusiak, M.; Pelloni, S.; Lazzaretti, P.: Relation between π-electron localization/delocalization and H-bond strength in derivatives of o-Hydroxy-Schiff bases. J. Org. Chem. 73 (2008) 2138–2145.10.1021/jo7023174Search in Google Scholar
Kukawska-Tarnawska, B.; Les, A.; Dziembowska, T.; Rozwadowski, Z. J.: Tautomeric forms of N-(5-nitrosalicylidene)-2-butylamine: Experimental and theoretical DFT study. J. Mol. Struct. 928 (2009) 25–31.10.1016/j.molstruc.2009.03.007Search in Google Scholar
Ren, S.; Tokes, Z. A.; Osipke, C.; Zhou, B.; Yen, Y.; Lien, E. J.: Inhibition of tumor cell growth by Schiff bases of hydroxysemicarbazide. Anticancer Res. 21 (2001) 3445–3451.Search in Google Scholar
Casasnovas, R.; Salva, A.; Frau, J.; Donoso, J.; Munoz, F.: Theoretical study on the distribution of atomic charges in the Schiff bases of 3-hydroxypyridine-4-aldehyde and alanine. The effect of the protonation state of the pyridine and imine nitrogen atoms. Chem. Phys. 355 (2009) 149–156.10.1016/j.chemphys.2008.12.006Search in Google Scholar
Dziembowska, T.; Szafran, M.; Katrusrak, A.; Raqzwadowski, Z.: Crystal structure of and solvent effect on tautomeric equilibrium in Schiff base derived from 2-hydroxy-1-naphthaldehyde and methylamine studied by X-ray diffraction, DFT, NMR and IR methods. J. Mol. Struct. 929 (2009) 32–42.10.1016/j.molstruc.2009.04.001Search in Google Scholar
Albayrak, C.; Kaştaş, G.; Odaba soğlu, M.; Frank, R.: The prototropic tautomerism and substituent effect through strong electron-withdrawing group in (E)-5-(diethylamino)-2-[(3-nitrophenylimino)methyl]phenol. Spectrochim. Acta A114 (2013) 205–213.10.1016/j.saa.2013.05.044Search in Google Scholar
Dey, D. K.; Dey, S. P.; Elmali, A.; Elerman, Y.: Molecular structure and conformation of N-2-[3′-(methoxysalicylideneimino)benzyl]-3′-methoxysalicylideneimine. J. Mol. Struct. 562 (2001) 177–184.10.1016/S0022-2860(00)00970-4Search in Google Scholar
©2018 Başak Koşar Kirca, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Editorial 2018
- Crystal structure of dimethanol-bis{3-(((2-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N:O′}dizinc(II), C42H30Zn2N2O10
- Crystal structure of aqua-bis{[2,6-dimethyl-N-(pyridin-2-ylmethylene)aniline-κ2N,N′]}zinc(II) triflate monohydrate [ZnC29H31N4O]CF3SO3⋅H2O
- Crystal structure of (E)-1-(4-{[(E)-4-Diethylamino-2-hydroxybenzene methylene]amino}phenyl)ethanone methoxy oxime, C20H27ClN3O3
- Crystal structure of (E)-1-(4-(((E)-4-(diethylamino)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one oxime, C19H23N3O2
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4,4′-sulfonyldibenzoato-κ2O:O′)zinc(II)], C40H34N4O6SZn
- Crystal structure of catena-poly[diaqua(μ3-pyrazine-2,3-dicarboxylato-κ4O,N:O′:O′′)zinc(II)] 1.25 hydrate, C6H8.5N2O7.25Zn
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri-m-tolyl phosphane-κP)rhenium(I), C29H28O5PRe
- Crystal structure of bis(μ2-methanolato-κ2O:O)-bis(methanol-κ1O)-bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,O′,N}dichromium(III), C38H36Cr2N2O14
- Crystal structure of poly[aqua-(μ3-pyridine-3,5-dicarboxylato-κ5O,O′:O′′,O′′′,N)zinc(II)], C7H7NO6Zn
- Crystal structure of bis((1-(((4-(((benzyloxy)imino)methyl)phenyl)imino)methyl)naphthalen-2-yl)oxy-κ2O,N)copper(II), C52H42CuN4O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}cobalt(II), C40H38CoN4O6
- Crystal structure of diaqua-bis(N,N-dimethylformamide-κ1O)-bis{3-((5-chloro-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ4N,O,O′:O′}dinickel(II), C38H34Ni2Cl2N4O12
- Crystal structure of tetrakis(methanol-κO)bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}bicobalt(II), C38H38Co2N2O14
- Crystal structure of (S)-tert-butyl-(1-hydroxypropan-2-yl)carbamate, C8H17NO3
- Crystal structure of 4-(4′-(pyridin-4-yl)-[1,1′-biphenyl]-4-yl)pyridin-1-ium catena-poly[{5-carboxy-4′-methyl-[1,1′-biphenyl]-3-carboxylato-κ2O,O′}-(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-κ4O,O′:O′′,O′′′)lead(II)], C52H40N2O9Pb
- Crystal structure of catena-poly[diaqua-(μ2-5-methylisophthalato-κ2O:O′)(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)], NiC21H22O6N6
- Crystal structure of the salt tris(guanidinium) tris(tetrapropylammonium) bis(pyridine-2,4,6-tricarboxylate) – water (1/10), C55H126N14O22
- Crystal structure of 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7,8-trimethoxy-4H-chromen-4-one, C19H18O8
- Crystal structure of poly{[μ2-1,1′-(sulfonylbis(4,1-phenylene))bis(2-methyl-1H-imidazole)-κ2N:N′][μ2-4,4′-oxydibenzoato-κ2O:O′]cobalt(II)} hemihydrate, C34H27N4O7.5SCo
- The crystal structure of 25,27-(2,2′-[(2-thioxo-1,3-dithiole-4,5-diyl)disulfanediyl]diethanolate)-26,28-dihydroxycalix[4]arene — dichloromethane (1/1), C36H32Cl2O4S5
- The crystal structure of 1,2-bis(3-(pyridin-3-yl)-1,2,4-oxadiazol-5-yl)ethane, C16H12N6O2
- Crystal structure of 1-benzyl-3-((4-bromophenyl)amino)-4-(4-methoxyphenyl)-1H-pyrrole-2,5-dione, C24H19BrN2O3
- Crystal structure of bis(2-((allylcarbamothioyl)imino)-4-methylthiazol-3-ido-κ2N,S)palladium(II), C16H20N6PdS4
- Crystal structure of pyrimidine-2,5-dicarboxylic acid 1.5 hydrate, C12H14N4O11
- Crystal structure of trans-diaqua-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II), C8H10N4O6Mn
- Crystal structure of catena-(μ3-5-bromoisophthatato-κO,O′: O′′,O′′′′)-(1,2-bis(imidazol-1-yl)ethane-κN:N′)cobalt(II), C16H13CoN4O4Br
- Investigation of the compound La5Zn2−xPb1 + x (x = 0.20–0.32)
- Crystal structure of (OC-6-13)-diaqua-bis(3,5-di(pyridin-3-yl)-4H-1,2,4-triazol-4-amine-κ1N)-bis(dicyanamido-κ1N)zinc(II) tetrahydrate, ZnC28H32N18O6
- Crystal structure of Ga0.62(3)Sb0.38(3)Pd3
- Crystal structure of Ga0.47(1)Sb0.53(1)Pd2
- A derivative of the Corey lactone – crystal structure of (3aR,4S,5R,6aS)-4-(((tert-butyldimethylsilyl)oxy)methyl)-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl benzoate, C21H30O5Si
- A Corey lactone: crystal structure of (3aR,4R,5R,6aS)-5-benzoyloxy-4(hydroxymethyl)hexahydro-2H-cyclopenta[b]furan-2-one, C15H16O5
- Hydrothermal synthesis and crystal structure of poly[aqua-(μ2-1,3-bis(4-pyridyl)propane-κ2N:N′)-(μ2-1,4,5,6,7,7-hexachlorobicyclo[2.2.1]hept-5-ene-2,3-dicarboxylato-κ2O:O′)manganese(II) hydrate, C22H20Cl6N2O6Mn
- Crystal structure of 2-acetylpyrrole S-methylthiosemicarbazonium hydroiodide, C8H13IN4S
- Crystal structure of [N,N-bis((pyrrol-2-yl)ethylidene)butane-1,4-diamine-κ4N,N′,N′′,N′′′]-nickel(II), C16H20N4Ni
- Crystal structure of poly[aqua-(μ5-2,5-dicarboxybenzoato-κ5O:O:O′:O′′:O′′′)sodium(I)], C9H7NaO7
- Crystal structure of bis(N′-((1H-pyrrol-2-yl)methylene)-1-methylthio-methanethiohydrazido-κ2S,N)nickel(II), C14H16N6NiS4
- Crystal structure of 1-(4-((benzo[d][1,3]dioxol-5-yloxy)methyl)phenethyl)-4-(3-chlorophenyl) piperazin-1-ium chloride, C26H28Cl2N2O3
- Crystal structure of 2-(4-(2-(4-(2-fluorophenyl)piperazin-1-yl)ethyl)benzyl)benzo[d]isothiazol-3(2H)-one 1,1-dioxide, C26H26FN3O3S – a saccharin dervative
- Crystal structure of 3-(2-dimethylaminoethyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C12H15N3OS
- Crystal structure of 3-(3-dimethylaminopropyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C13H17N3OS
- The crystal structure of trans-tetraaqua-bis(p-tolylsulfinato-κO)calcium(II)), C14H22O8S2Ca
- The crystal structure of (E)-N′-(pyridin-2-ylmethylene)pyrazine-2-carbohydrazide, C11H9N5O
- Crystal structure of (E)-3-(pyren-1-yl)-1-(pyridin-4-yl)prop-2-en-1-one, C24H15NO
- Crystal structure of catena-poly[diaqua-(μ2-tartrato-κ4O,O′:O′′,O′′′)cobalt(II)], C4H8CoO8
- Crystal structure of 4-chloro-2-methyl-6-(4-(trifluoromethoxy)phenyl)pyrimidine, C12H8ClF3N2O
- Crystal structure of 1-(4-fluorophenyl)-N-(5-((triphenylstannyl)thio)thiophen-2-yl)methanimine, C27H20FN3S2Sn
- Crystal structure of methyl (Z)-2-(5-fluoro-2-oxoindolin-3-ylidene)hydrazine-1-carbodithioate, C10H8FN3OS2
- Crystal structure of tert-butyl (Z)-4-(2-(5-methoxy-3-(2-((methylthio)carbonothioyl)hydrazono)-2-oxoindolin-1-yl)ethyl)piperazine-1-carboxylate, C22H31N5O4S2
- The crystal structure of (E)-2-((2-(o-tolylcarbamothioyl)hydrazono)methyl)benzoic acid, C16H15N3O2S
- Crystal structure of 2-chloro-1,3-di-tert-pentyl-4,4-diphenyl-1,3,2λ3,4-diazaphosphasiletidine, C22H32ClN2PSi
- Crystal structure of tetramethyl 5,5′-(buta-1,3-diyne-1,4-diyl)diisophthalate, C24H18O8
- Crystal structural of 2-amino-4-(4-methoxyphenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C19H20N2O3
- Crystal structure of 1,3,5-tris((trimethylsilyl)methyl)-1,3,5-triazinane-2,4,6-trione, C15H33N3O3Si3
- The crystal structure of bis(2-benzoyl-5-hydroxylphenolato-κ2O,O′)copper(II), C26H18CuO6
- Crystal structure of 2,6-bis(3-(pyrazin-2-yl)-1H-1,2,4-triazol-5-yl)pyridine – 1-ethyl-3-methyl-1H-imidazol-3-ium bromide (1/1), C23H22N13Br
- The crystal structure of (E)-N-benzyl-N′-benzylidene-4-methylbenzenesulfonohydrazide, C21H20N2O2S
- Crystal structure of ethyl (E)-5-((2-(3-hydroxybenzoyl)hydrazono)methyl)-3,4-dimethyl-1H-pyrrole-2-carboxylate – water – ethanol (1/1/1), C19H27N3O6
- The crystal structure of (E)-4-(3-ethoxy-2-hydroxybenzylideneamino)benzoic acid, C16H15NO4
- Crystal structure of (μ2-N,N′-bis((pyridin-4-yl)methyl)ethanediamide-κ2N:N′)-tetrakis(diethylcarbamodithioato-κ2S,S′)dizinc(II), C34H54N8O2S8Zn2
Articles in the same Issue
- Cover and Frontmatter
- Editorial 2018
- Crystal structure of dimethanol-bis{3-(((2-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N:O′}dizinc(II), C42H30Zn2N2O10
- Crystal structure of aqua-bis{[2,6-dimethyl-N-(pyridin-2-ylmethylene)aniline-κ2N,N′]}zinc(II) triflate monohydrate [ZnC29H31N4O]CF3SO3⋅H2O
- Crystal structure of (E)-1-(4-{[(E)-4-Diethylamino-2-hydroxybenzene methylene]amino}phenyl)ethanone methoxy oxime, C20H27ClN3O3
- Crystal structure of (E)-1-(4-(((E)-4-(diethylamino)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one oxime, C19H23N3O2
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4,4′-sulfonyldibenzoato-κ2O:O′)zinc(II)], C40H34N4O6SZn
- Crystal structure of catena-poly[diaqua(μ3-pyrazine-2,3-dicarboxylato-κ4O,N:O′:O′′)zinc(II)] 1.25 hydrate, C6H8.5N2O7.25Zn
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri-m-tolyl phosphane-κP)rhenium(I), C29H28O5PRe
- Crystal structure of bis(μ2-methanolato-κ2O:O)-bis(methanol-κ1O)-bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,O′,N}dichromium(III), C38H36Cr2N2O14
- Crystal structure of poly[aqua-(μ3-pyridine-3,5-dicarboxylato-κ5O,O′:O′′,O′′′,N)zinc(II)], C7H7NO6Zn
- Crystal structure of bis((1-(((4-(((benzyloxy)imino)methyl)phenyl)imino)methyl)naphthalen-2-yl)oxy-κ2O,N)copper(II), C52H42CuN4O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}cobalt(II), C40H38CoN4O6
- Crystal structure of diaqua-bis(N,N-dimethylformamide-κ1O)-bis{3-((5-chloro-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ4N,O,O′:O′}dinickel(II), C38H34Ni2Cl2N4O12
- Crystal structure of tetrakis(methanol-κO)bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}bicobalt(II), C38H38Co2N2O14
- Crystal structure of (S)-tert-butyl-(1-hydroxypropan-2-yl)carbamate, C8H17NO3
- Crystal structure of 4-(4′-(pyridin-4-yl)-[1,1′-biphenyl]-4-yl)pyridin-1-ium catena-poly[{5-carboxy-4′-methyl-[1,1′-biphenyl]-3-carboxylato-κ2O,O′}-(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-κ4O,O′:O′′,O′′′)lead(II)], C52H40N2O9Pb
- Crystal structure of catena-poly[diaqua-(μ2-5-methylisophthalato-κ2O:O′)(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)], NiC21H22O6N6
- Crystal structure of the salt tris(guanidinium) tris(tetrapropylammonium) bis(pyridine-2,4,6-tricarboxylate) – water (1/10), C55H126N14O22
- Crystal structure of 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7,8-trimethoxy-4H-chromen-4-one, C19H18O8
- Crystal structure of poly{[μ2-1,1′-(sulfonylbis(4,1-phenylene))bis(2-methyl-1H-imidazole)-κ2N:N′][μ2-4,4′-oxydibenzoato-κ2O:O′]cobalt(II)} hemihydrate, C34H27N4O7.5SCo
- The crystal structure of 25,27-(2,2′-[(2-thioxo-1,3-dithiole-4,5-diyl)disulfanediyl]diethanolate)-26,28-dihydroxycalix[4]arene — dichloromethane (1/1), C36H32Cl2O4S5
- The crystal structure of 1,2-bis(3-(pyridin-3-yl)-1,2,4-oxadiazol-5-yl)ethane, C16H12N6O2
- Crystal structure of 1-benzyl-3-((4-bromophenyl)amino)-4-(4-methoxyphenyl)-1H-pyrrole-2,5-dione, C24H19BrN2O3
- Crystal structure of bis(2-((allylcarbamothioyl)imino)-4-methylthiazol-3-ido-κ2N,S)palladium(II), C16H20N6PdS4
- Crystal structure of pyrimidine-2,5-dicarboxylic acid 1.5 hydrate, C12H14N4O11
- Crystal structure of trans-diaqua-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II), C8H10N4O6Mn
- Crystal structure of catena-(μ3-5-bromoisophthatato-κO,O′: O′′,O′′′′)-(1,2-bis(imidazol-1-yl)ethane-κN:N′)cobalt(II), C16H13CoN4O4Br
- Investigation of the compound La5Zn2−xPb1 + x (x = 0.20–0.32)
- Crystal structure of (OC-6-13)-diaqua-bis(3,5-di(pyridin-3-yl)-4H-1,2,4-triazol-4-amine-κ1N)-bis(dicyanamido-κ1N)zinc(II) tetrahydrate, ZnC28H32N18O6
- Crystal structure of Ga0.62(3)Sb0.38(3)Pd3
- Crystal structure of Ga0.47(1)Sb0.53(1)Pd2
- A derivative of the Corey lactone – crystal structure of (3aR,4S,5R,6aS)-4-(((tert-butyldimethylsilyl)oxy)methyl)-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl benzoate, C21H30O5Si
- A Corey lactone: crystal structure of (3aR,4R,5R,6aS)-5-benzoyloxy-4(hydroxymethyl)hexahydro-2H-cyclopenta[b]furan-2-one, C15H16O5
- Hydrothermal synthesis and crystal structure of poly[aqua-(μ2-1,3-bis(4-pyridyl)propane-κ2N:N′)-(μ2-1,4,5,6,7,7-hexachlorobicyclo[2.2.1]hept-5-ene-2,3-dicarboxylato-κ2O:O′)manganese(II) hydrate, C22H20Cl6N2O6Mn
- Crystal structure of 2-acetylpyrrole S-methylthiosemicarbazonium hydroiodide, C8H13IN4S
- Crystal structure of [N,N-bis((pyrrol-2-yl)ethylidene)butane-1,4-diamine-κ4N,N′,N′′,N′′′]-nickel(II), C16H20N4Ni
- Crystal structure of poly[aqua-(μ5-2,5-dicarboxybenzoato-κ5O:O:O′:O′′:O′′′)sodium(I)], C9H7NaO7
- Crystal structure of bis(N′-((1H-pyrrol-2-yl)methylene)-1-methylthio-methanethiohydrazido-κ2S,N)nickel(II), C14H16N6NiS4
- Crystal structure of 1-(4-((benzo[d][1,3]dioxol-5-yloxy)methyl)phenethyl)-4-(3-chlorophenyl) piperazin-1-ium chloride, C26H28Cl2N2O3
- Crystal structure of 2-(4-(2-(4-(2-fluorophenyl)piperazin-1-yl)ethyl)benzyl)benzo[d]isothiazol-3(2H)-one 1,1-dioxide, C26H26FN3O3S – a saccharin dervative
- Crystal structure of 3-(2-dimethylaminoethyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C12H15N3OS
- Crystal structure of 3-(3-dimethylaminopropyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C13H17N3OS
- The crystal structure of trans-tetraaqua-bis(p-tolylsulfinato-κO)calcium(II)), C14H22O8S2Ca
- The crystal structure of (E)-N′-(pyridin-2-ylmethylene)pyrazine-2-carbohydrazide, C11H9N5O
- Crystal structure of (E)-3-(pyren-1-yl)-1-(pyridin-4-yl)prop-2-en-1-one, C24H15NO
- Crystal structure of catena-poly[diaqua-(μ2-tartrato-κ4O,O′:O′′,O′′′)cobalt(II)], C4H8CoO8
- Crystal structure of 4-chloro-2-methyl-6-(4-(trifluoromethoxy)phenyl)pyrimidine, C12H8ClF3N2O
- Crystal structure of 1-(4-fluorophenyl)-N-(5-((triphenylstannyl)thio)thiophen-2-yl)methanimine, C27H20FN3S2Sn
- Crystal structure of methyl (Z)-2-(5-fluoro-2-oxoindolin-3-ylidene)hydrazine-1-carbodithioate, C10H8FN3OS2
- Crystal structure of tert-butyl (Z)-4-(2-(5-methoxy-3-(2-((methylthio)carbonothioyl)hydrazono)-2-oxoindolin-1-yl)ethyl)piperazine-1-carboxylate, C22H31N5O4S2
- The crystal structure of (E)-2-((2-(o-tolylcarbamothioyl)hydrazono)methyl)benzoic acid, C16H15N3O2S
- Crystal structure of 2-chloro-1,3-di-tert-pentyl-4,4-diphenyl-1,3,2λ3,4-diazaphosphasiletidine, C22H32ClN2PSi
- Crystal structure of tetramethyl 5,5′-(buta-1,3-diyne-1,4-diyl)diisophthalate, C24H18O8
- Crystal structural of 2-amino-4-(4-methoxyphenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C19H20N2O3
- Crystal structure of 1,3,5-tris((trimethylsilyl)methyl)-1,3,5-triazinane-2,4,6-trione, C15H33N3O3Si3
- The crystal structure of bis(2-benzoyl-5-hydroxylphenolato-κ2O,O′)copper(II), C26H18CuO6
- Crystal structure of 2,6-bis(3-(pyrazin-2-yl)-1H-1,2,4-triazol-5-yl)pyridine – 1-ethyl-3-methyl-1H-imidazol-3-ium bromide (1/1), C23H22N13Br
- The crystal structure of (E)-N-benzyl-N′-benzylidene-4-methylbenzenesulfonohydrazide, C21H20N2O2S
- Crystal structure of ethyl (E)-5-((2-(3-hydroxybenzoyl)hydrazono)methyl)-3,4-dimethyl-1H-pyrrole-2-carboxylate – water – ethanol (1/1/1), C19H27N3O6
- The crystal structure of (E)-4-(3-ethoxy-2-hydroxybenzylideneamino)benzoic acid, C16H15NO4
- Crystal structure of (μ2-N,N′-bis((pyridin-4-yl)methyl)ethanediamide-κ2N:N′)-tetrakis(diethylcarbamodithioato-κ2S,S′)dizinc(II), C34H54N8O2S8Zn2

