Abstract
C16H20N6PdS4, monoclinic, C2/c (no. 15), a = 13.2801(8) Å, b = 18.5771(8) Å, c = 10.2577(5) Å, β = 121.906(2)°, V = 2148.29(19) Å3, Z = 4, Rgt(F) = 0.0300, WRref(F2) = 0.0721, T = 293 K.
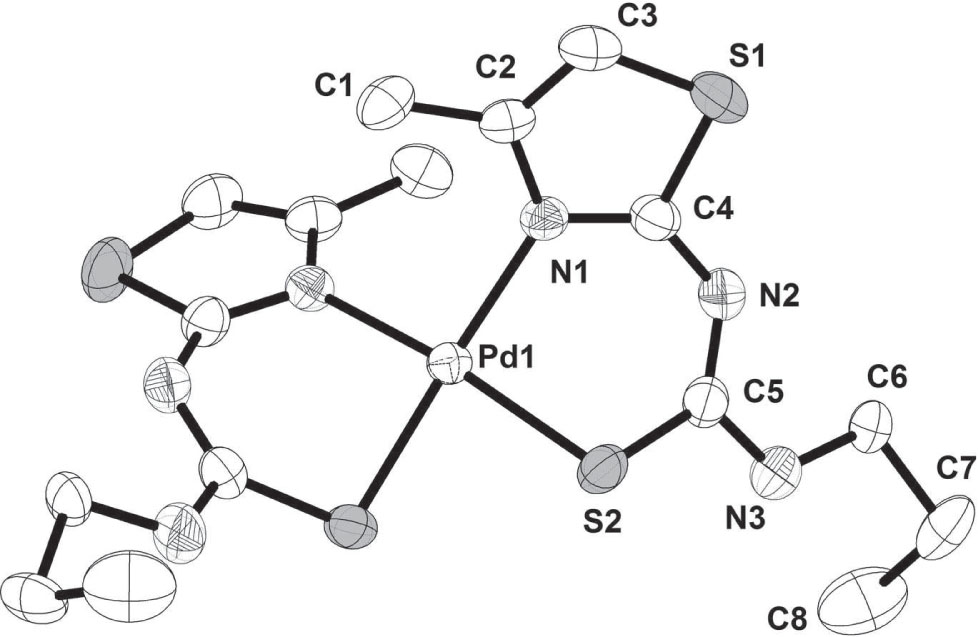
The crystal structure is shown in the figure. The disorder in the ally moiety is omitted for clarity. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Block, colorless |
| Size: | 0.34 × 0.27 × 0.11 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.27 mm−1 |
| Diffractometer, scan mode: | Bruker SMART, φ and ω-scans |
| 2θmax, completeness: | 33.2°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 49183, 4099, 0.033 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3838 |
| N(param)refined: | 159 |
| Programs: | Bruker programs [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Pd1 | 0.5000 | 0.31839(2) | 0.7500 | 0.03215(5) |
| S1 | 0.38995(6) | 0.19783(3) | 0.30890(7) | 0.05980(14) |
| S2 | 0.48422(4) | 0.40860(2) | 0.58973(6) | 0.04436(10) |
| N1 | 0.44970(12) | 0.24457(7) | 0.57476(16) | 0.0367(3) |
| N2 | 0.54461(16) | 0.30251(9) | 0.45503(19) | 0.0464(3) |
| N3 | 0.6317(2) | 0.41207(11) | 0.4937(2) | 0.0566(4) |
| C1 | 0.33325(18) | 0.16658(12) | 0.6500(3) | 0.0508(4) |
| H1A | 0.2670 | 0.1342 | 0.5999 | 0.076* |
| H1B | 0.3113 | 0.2083 | 0.6845 | 0.076* |
| H1C | 0.3987 | 0.1429 | 0.7365 | 0.076* |
| C2 | 0.36847(16) | 0.18883(9) | 0.5399(2) | 0.0413(3) |
| C3 | 0.3282(2) | 0.15846(11) | 0.4019(3) | 0.0537(5) |
| H3A | 0.2738 | 0.1209 | 0.3619 | 0.064* |
| C4 | 0.47004(16) | 0.25548(10) | 0.4628(2) | 0.0413(3) |
| C5 | 0.55713(16) | 0.36801(10) | 0.5067(2) | 0.0418(3) |
| C6a | 0.6960(14) | 0.3851(9) | 0.4193(18) | 0.0460(18) |
| H6Aa | 0.6418 | 0.3612 | 0.3229 | 0.055* |
| H6Ba | 0.7582 | 0.3516 | 0.4861 | 0.055* |
| C7a | 0.7481(9) | 0.4521(4) | 0.3926(13) | 0.060(2) |
| H7Aa | 0.695(8) | 0.484(5) | 0.325(10) | 0.072* |
| C8a | 0.8575(9) | 0.4727(6) | 0.5772(16) | 0.098(4) |
| H8Aa | 0.8688 | 0.4439 | 0.6581 | 0.118* |
| H8Ba | 0.9056 | 0.5127 | 0.5966 | 0.118* |
| C8Xb | 0.8542(8) | 0.4600(4) | 0.4428(15) | 0.146(4) |
| H8XAb | 0.8296 | 0.4772 | 0.3452 | 0.175* |
| H8XBb | 0.9308 | 0.4694 | 0.5244 | 0.175* |
| C7Xb | 0.7982(7) | 0.4313(5) | 0.4601(10) | 0.099(2) |
| H7XAb | 0.8412 | 0.4200 | 0.5642 | 0.118* |
| C6Xb | 0.6822(11) | 0.3987(7) | 0.4007(14) | 0.083(3) |
| H6XAb | 0.6282 | 0.4170 | 0.2981 | 0.099* |
| H6XBb | 0.6890 | 0.3471 | 0.3928 | 0.099* |
| H1N3 | 0.627(3) | 0.4521(16) | 0.515(3) | 0.061(8)* |
Occupancies: a0.345(9), b0.655(9).
Source of material
A mixture of K2[PtCl4] (0.001 mol in distilled water), 1-allyl-3-(4-methylthiazol-2(3H)-ylidene)thiourea (0.002 mol in ethanol), was heated and stirred for 3 h. Then the solution was cooled to room temperature, filtered and washed. The resulting compound crystallized and was dried in a final step.
Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms.
Discussion
In recent years, thioureas and thiourea derivatives have gained unprecedented fame in medical and non-medical applications. Their characteristics as versatile ligands allow them to form stable complexes [3, 4] . Many reports have documented the ability of thioureas to coordinate transition metals such as Ni(II), Cu(II), Co(II), Fe(III) [5, 6] , Zn(II) [7], Pd(II) [8], and Ru(II) [9]. Their diversified use has received attention by various scientific communities including medical [10], analytical chemistry and metallurgy fields [11]. To name a few, they have been used in antiviral [12, 13] , anti-HIV [14], antimicrobial, antitumor, anti-inflammatory [15], and insecticidal substances [16].
In the title Pd(II) complex two ligands act as bidentate N, S-donor chelates [17]. Pd(II) is four-coordinated (PtN2S2) in a square planar geometry. The Pd1—N1 bond distance is 2.0710(14) Å, the bond angles of N1—Pd1—N1A, N1—Pd1—S2, N1A—Pd1—S2A are 97.06(8), 89.79(4), 89.79(4), respectively. The Pd1—S2 bond distance is 2.2783(4), the bond angles of S2—Pd1—S2A, S2—Pd1—S2A are 85.38(7)°, 85.30(3)°, respectively. Geometric parameters of the organic ligand is within the expectations [18].
References
Bruker: APEX2, SAINT and SADABS. Brucker AXS Inc., Madison, WI, USA (2009).Search in Google Scholar
Sheldrick, G. M.: SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
Khairul, W.; Daud, A.; Hanifaah, N.; Arshad, S.; Abdul Razak, I.; Zuki, H.; Erben, F.: Structural study of a novel acetylide-thiourea derivative and its evaluation as a detector of benzene. J. Mol. Struct. 1139 (2017) 353–361.10.1016/j.molstruc.2017.03.065Search in Google Scholar
Saeed, A.; Khurshid, A.; Jasinski, J. P.; Pozzi, C. G.; Fantoni, A. C.; Erben, M. F.: Competing intramolecular N—H/O—C hydrogen bonds and extended intermolecular network in 1-(4-chlorobenzoyl)-3-(2-methyl-4-oxopentan-2-yl) thiourea analyzed by experimental and theoretical methods. Chem. Phys. 431 (2014) 39–46.10.1016/j.chemphys.2014.01.009Search in Google Scholar
Gil, D. M.; Lestard, M. D.; Estévez-Hernández, O.; Duque, J.; Reguera, E.: Quantum chemical studies on molecular structure, spectroscopic (IR, Raman, UV–Vis), NBO and Homo–Lumo analysis of 1-benzyl-3-(2-furoyl) thiourea. J. Spectrochim. Acta. A 145 (2015) 553–562.10.1016/j.saa.2015.02.071Search in Google Scholar PubMed
Khairul, W. M.; Daud, A. I.; Hanifaah, N. A. M.; Arshad, S.; Razak, I. A.; Zuki, H. M.; Erben, M. F.: Structural study of a novel acetylide-thiourea derivative and its evaluation as a detector of benzene. J. Mol. Sruct. 1139 (2017) 353–361.10.1016/j.molstruc.2017.03.065Search in Google Scholar
Bilal, S.; Bibi, S.; Ahmad, S. M.: Counterpoise-corrected energies, NBO, HOMO–LUMO and interaction energies of poly(o-aminophenol) for ammonia sensing by DFT methods. Synt. Met. 209 (2015) 143–149.10.1016/j.synthmet.2015.06.027Search in Google Scholar
Daud, A. I.; Khairul, W. M.; Zuki, H. M.; Kubulat, K.: Aerobic synthetic approach and characterisation of some acetylide–thiourea derivatives for the detection of carbon monoxide (CO) gas. J. Mol. Struct. 1093 (2015) 172–178.10.1016/j.molstruc.2015.03.065Search in Google Scholar
Liu, P.; Shu, C.; Liu, L.; Huang, Q.; Peng, Y.: Design and synthesis of thiourea derivatives with sulfur-containing heterocyclic scaffolds as potential tyrosinase inhibitors. Bioorgan. Med. Chem. 24 (2016) 1866–1871.10.1016/j.bmc.2016.03.013Search in Google Scholar PubMed
Qiao, L.; Zhang, Y.; Hu, W.; Guo, J.; Cao, W.; Ding, Z.; Guo, Z.; Fan, A.; Song, J.: Synthesis, structural characterization and quantum chemical calculations on 1-(isomeric methylbenzoyl)-3-(4-trifluoromethylphenyl) thioureas. J. Mol. Struct. 1141 (2017) 309–321.10.1016/j.molstruc.2017.03.113Search in Google Scholar
Saeed, A.; Qamar, R.; Fattah, T. A.; Flörke, U.; Erben, M. F.: Recent developments in chemistry, coordination, structure and biological aspects of 1-(acyl/aroyl)-3-thioureas. Res. Chem. Intermed. 42 (2016) 1–14.10.1007/s11164-016-2811-5Search in Google Scholar
Lal, B.; Badshah, A.; Altaf, A. A.; Khan, N.; Ullah, S.: Miscellaneous applications of ferrocene-based peptides/amides. Appl. Organomet. Chem. 25 (2011) 843–855.10.1002/aoc.1843Search in Google Scholar
Di Grandi, M. J.; Curan, K. J.; Feigelson, G.; Prashad, A.; Ross, A. A.; Visalli, R.; Fairhurst, J.; Feld, B.: Thiourea inhibitors of herpesviruses. Part 3: Inhibitors of varicella zoster virus. J.D. Bloom, Bioorg. Med. Chem. Lett. 14 (2004) 4157–4160.10.1016/j.bmcl.2004.06.025Search in Google Scholar
Venkatachalam, T. K.; Mao, C.; Ucku, F. M.: Effect of stereochemistry on the anti-HIV activity of chiral thiourea compounds. Bioorg. Med. Chem. 12 (2004) 4275–4284.10.1016/j.bmc.2004.04.050Search in Google Scholar PubMed
Kumar, H.; Javed, S. A.; Khan, S. A.; Amir, M.: 1, 3, 4-Oxadiazole/thiadiazole and 1, 2, 4-triazole derivatives of biphenyl-4-yloxy acetic acid: synthesis and preliminary evaluation of biological properties. Eur. J. Med. Chem. 43 (2008) 2688–2698.10.1016/j.ejmech.2008.01.039Search in Google Scholar PubMed
Wang, B.; Ma, Y.; Xiong, L.; Li, Z.: Synthesis and insecticidal activity of novelN-pyridylpyrazole carbonyl thioureas. Chin. J. Chem. 30 (2012) 815–821.10.1002/cjoc.201100386Search in Google Scholar
Schoultz, X.; Gerber, T.; Hosten, E.: Rhenium(I) complexes with benzothiazole-thiourea derivatives. Polyhedron 113 (2016) 55–60.10.1016/j.poly.2016.04.016Search in Google Scholar
Jambi, S. M. S.; Al-Obaid, A. M.; Hosten, E. C.; Betz, R.; Bari, A.: Crystal structure of 1-(4-methylthiazol-2-yl)-3-propylthiourea, C8H13N3S2. Z. Kristallogr. NCS 231 (2016) 901–902.10.1515/ncrs-2016-0001Search in Google Scholar
©2018 Suhair M.S. Jambi, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Editorial 2018
- Crystal structure of dimethanol-bis{3-(((2-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N:O′}dizinc(II), C42H30Zn2N2O10
- Crystal structure of aqua-bis{[2,6-dimethyl-N-(pyridin-2-ylmethylene)aniline-κ2N,N′]}zinc(II) triflate monohydrate [ZnC29H31N4O]CF3SO3⋅H2O
- Crystal structure of (E)-1-(4-{[(E)-4-Diethylamino-2-hydroxybenzene methylene]amino}phenyl)ethanone methoxy oxime, C20H27ClN3O3
- Crystal structure of (E)-1-(4-(((E)-4-(diethylamino)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one oxime, C19H23N3O2
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4,4′-sulfonyldibenzoato-κ2O:O′)zinc(II)], C40H34N4O6SZn
- Crystal structure of catena-poly[diaqua(μ3-pyrazine-2,3-dicarboxylato-κ4O,N:O′:O′′)zinc(II)] 1.25 hydrate, C6H8.5N2O7.25Zn
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri-m-tolyl phosphane-κP)rhenium(I), C29H28O5PRe
- Crystal structure of bis(μ2-methanolato-κ2O:O)-bis(methanol-κ1O)-bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,O′,N}dichromium(III), C38H36Cr2N2O14
- Crystal structure of poly[aqua-(μ3-pyridine-3,5-dicarboxylato-κ5O,O′:O′′,O′′′,N)zinc(II)], C7H7NO6Zn
- Crystal structure of bis((1-(((4-(((benzyloxy)imino)methyl)phenyl)imino)methyl)naphthalen-2-yl)oxy-κ2O,N)copper(II), C52H42CuN4O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}cobalt(II), C40H38CoN4O6
- Crystal structure of diaqua-bis(N,N-dimethylformamide-κ1O)-bis{3-((5-chloro-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ4N,O,O′:O′}dinickel(II), C38H34Ni2Cl2N4O12
- Crystal structure of tetrakis(methanol-κO)bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}bicobalt(II), C38H38Co2N2O14
- Crystal structure of (S)-tert-butyl-(1-hydroxypropan-2-yl)carbamate, C8H17NO3
- Crystal structure of 4-(4′-(pyridin-4-yl)-[1,1′-biphenyl]-4-yl)pyridin-1-ium catena-poly[{5-carboxy-4′-methyl-[1,1′-biphenyl]-3-carboxylato-κ2O,O′}-(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-κ4O,O′:O′′,O′′′)lead(II)], C52H40N2O9Pb
- Crystal structure of catena-poly[diaqua-(μ2-5-methylisophthalato-κ2O:O′)(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)], NiC21H22O6N6
- Crystal structure of the salt tris(guanidinium) tris(tetrapropylammonium) bis(pyridine-2,4,6-tricarboxylate) – water (1/10), C55H126N14O22
- Crystal structure of 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7,8-trimethoxy-4H-chromen-4-one, C19H18O8
- Crystal structure of poly{[μ2-1,1′-(sulfonylbis(4,1-phenylene))bis(2-methyl-1H-imidazole)-κ2N:N′][μ2-4,4′-oxydibenzoato-κ2O:O′]cobalt(II)} hemihydrate, C34H27N4O7.5SCo
- The crystal structure of 25,27-(2,2′-[(2-thioxo-1,3-dithiole-4,5-diyl)disulfanediyl]diethanolate)-26,28-dihydroxycalix[4]arene — dichloromethane (1/1), C36H32Cl2O4S5
- The crystal structure of 1,2-bis(3-(pyridin-3-yl)-1,2,4-oxadiazol-5-yl)ethane, C16H12N6O2
- Crystal structure of 1-benzyl-3-((4-bromophenyl)amino)-4-(4-methoxyphenyl)-1H-pyrrole-2,5-dione, C24H19BrN2O3
- Crystal structure of bis(2-((allylcarbamothioyl)imino)-4-methylthiazol-3-ido-κ2N,S)palladium(II), C16H20N6PdS4
- Crystal structure of pyrimidine-2,5-dicarboxylic acid 1.5 hydrate, C12H14N4O11
- Crystal structure of trans-diaqua-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II), C8H10N4O6Mn
- Crystal structure of catena-(μ3-5-bromoisophthatato-κO,O′: O′′,O′′′′)-(1,2-bis(imidazol-1-yl)ethane-κN:N′)cobalt(II), C16H13CoN4O4Br
- Investigation of the compound La5Zn2−xPb1 + x (x = 0.20–0.32)
- Crystal structure of (OC-6-13)-diaqua-bis(3,5-di(pyridin-3-yl)-4H-1,2,4-triazol-4-amine-κ1N)-bis(dicyanamido-κ1N)zinc(II) tetrahydrate, ZnC28H32N18O6
- Crystal structure of Ga0.62(3)Sb0.38(3)Pd3
- Crystal structure of Ga0.47(1)Sb0.53(1)Pd2
- A derivative of the Corey lactone – crystal structure of (3aR,4S,5R,6aS)-4-(((tert-butyldimethylsilyl)oxy)methyl)-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl benzoate, C21H30O5Si
- A Corey lactone: crystal structure of (3aR,4R,5R,6aS)-5-benzoyloxy-4(hydroxymethyl)hexahydro-2H-cyclopenta[b]furan-2-one, C15H16O5
- Hydrothermal synthesis and crystal structure of poly[aqua-(μ2-1,3-bis(4-pyridyl)propane-κ2N:N′)-(μ2-1,4,5,6,7,7-hexachlorobicyclo[2.2.1]hept-5-ene-2,3-dicarboxylato-κ2O:O′)manganese(II) hydrate, C22H20Cl6N2O6Mn
- Crystal structure of 2-acetylpyrrole S-methylthiosemicarbazonium hydroiodide, C8H13IN4S
- Crystal structure of [N,N-bis((pyrrol-2-yl)ethylidene)butane-1,4-diamine-κ4N,N′,N′′,N′′′]-nickel(II), C16H20N4Ni
- Crystal structure of poly[aqua-(μ5-2,5-dicarboxybenzoato-κ5O:O:O′:O′′:O′′′)sodium(I)], C9H7NaO7
- Crystal structure of bis(N′-((1H-pyrrol-2-yl)methylene)-1-methylthio-methanethiohydrazido-κ2S,N)nickel(II), C14H16N6NiS4
- Crystal structure of 1-(4-((benzo[d][1,3]dioxol-5-yloxy)methyl)phenethyl)-4-(3-chlorophenyl) piperazin-1-ium chloride, C26H28Cl2N2O3
- Crystal structure of 2-(4-(2-(4-(2-fluorophenyl)piperazin-1-yl)ethyl)benzyl)benzo[d]isothiazol-3(2H)-one 1,1-dioxide, C26H26FN3O3S – a saccharin dervative
- Crystal structure of 3-(2-dimethylaminoethyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C12H15N3OS
- Crystal structure of 3-(3-dimethylaminopropyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C13H17N3OS
- The crystal structure of trans-tetraaqua-bis(p-tolylsulfinato-κO)calcium(II)), C14H22O8S2Ca
- The crystal structure of (E)-N′-(pyridin-2-ylmethylene)pyrazine-2-carbohydrazide, C11H9N5O
- Crystal structure of (E)-3-(pyren-1-yl)-1-(pyridin-4-yl)prop-2-en-1-one, C24H15NO
- Crystal structure of catena-poly[diaqua-(μ2-tartrato-κ4O,O′:O′′,O′′′)cobalt(II)], C4H8CoO8
- Crystal structure of 4-chloro-2-methyl-6-(4-(trifluoromethoxy)phenyl)pyrimidine, C12H8ClF3N2O
- Crystal structure of 1-(4-fluorophenyl)-N-(5-((triphenylstannyl)thio)thiophen-2-yl)methanimine, C27H20FN3S2Sn
- Crystal structure of methyl (Z)-2-(5-fluoro-2-oxoindolin-3-ylidene)hydrazine-1-carbodithioate, C10H8FN3OS2
- Crystal structure of tert-butyl (Z)-4-(2-(5-methoxy-3-(2-((methylthio)carbonothioyl)hydrazono)-2-oxoindolin-1-yl)ethyl)piperazine-1-carboxylate, C22H31N5O4S2
- The crystal structure of (E)-2-((2-(o-tolylcarbamothioyl)hydrazono)methyl)benzoic acid, C16H15N3O2S
- Crystal structure of 2-chloro-1,3-di-tert-pentyl-4,4-diphenyl-1,3,2λ3,4-diazaphosphasiletidine, C22H32ClN2PSi
- Crystal structure of tetramethyl 5,5′-(buta-1,3-diyne-1,4-diyl)diisophthalate, C24H18O8
- Crystal structural of 2-amino-4-(4-methoxyphenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C19H20N2O3
- Crystal structure of 1,3,5-tris((trimethylsilyl)methyl)-1,3,5-triazinane-2,4,6-trione, C15H33N3O3Si3
- The crystal structure of bis(2-benzoyl-5-hydroxylphenolato-κ2O,O′)copper(II), C26H18CuO6
- Crystal structure of 2,6-bis(3-(pyrazin-2-yl)-1H-1,2,4-triazol-5-yl)pyridine – 1-ethyl-3-methyl-1H-imidazol-3-ium bromide (1/1), C23H22N13Br
- The crystal structure of (E)-N-benzyl-N′-benzylidene-4-methylbenzenesulfonohydrazide, C21H20N2O2S
- Crystal structure of ethyl (E)-5-((2-(3-hydroxybenzoyl)hydrazono)methyl)-3,4-dimethyl-1H-pyrrole-2-carboxylate – water – ethanol (1/1/1), C19H27N3O6
- The crystal structure of (E)-4-(3-ethoxy-2-hydroxybenzylideneamino)benzoic acid, C16H15NO4
- Crystal structure of (μ2-N,N′-bis((pyridin-4-yl)methyl)ethanediamide-κ2N:N′)-tetrakis(diethylcarbamodithioato-κ2S,S′)dizinc(II), C34H54N8O2S8Zn2
Articles in the same Issue
- Cover and Frontmatter
- Editorial 2018
- Crystal structure of dimethanol-bis{3-(((2-oxidonaphthalen-1-yl)methylene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N:O′}dizinc(II), C42H30Zn2N2O10
- Crystal structure of aqua-bis{[2,6-dimethyl-N-(pyridin-2-ylmethylene)aniline-κ2N,N′]}zinc(II) triflate monohydrate [ZnC29H31N4O]CF3SO3⋅H2O
- Crystal structure of (E)-1-(4-{[(E)-4-Diethylamino-2-hydroxybenzene methylene]amino}phenyl)ethanone methoxy oxime, C20H27ClN3O3
- Crystal structure of (E)-1-(4-(((E)-4-(diethylamino)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one oxime, C19H23N3O2
- Crystal structure of poly[(μ2-1,4-bis((2-ethyl-1H-benzo[d]imidazol-1-yl)methyl)benzene-κ2N:N′)-(μ2-4,4′-sulfonyldibenzoato-κ2O:O′)zinc(II)], C40H34N4O6SZn
- Crystal structure of catena-poly[diaqua(μ3-pyrazine-2,3-dicarboxylato-κ4O,N:O′:O′′)zinc(II)] 1.25 hydrate, C6H8.5N2O7.25Zn
- Crystal structure of fac-(acetylacetonato-κ2O,O′)tricarbonyl(tri-m-tolyl phosphane-κP)rhenium(I), C29H28O5PRe
- Crystal structure of bis(μ2-methanolato-κ2O:O)-bis(methanol-κ1O)-bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,O′,N}dichromium(III), C38H36Cr2N2O14
- Crystal structure of poly[aqua-(μ3-pyridine-3,5-dicarboxylato-κ5O,O′:O′′,O′′′,N)zinc(II)], C7H7NO6Zn
- Crystal structure of bis((1-(((4-(((benzyloxy)imino)methyl)phenyl)imino)methyl)naphthalen-2-yl)oxy-κ2O,N)copper(II), C52H42CuN4O4
- Crystal structure of bis{5-(diethylamino)-2-(((2-oxo-2H-chromen-6-yl)imino)methyl)phenolato-κ2O,N}cobalt(II), C40H38CoN4O6
- Crystal structure of diaqua-bis(N,N-dimethylformamide-κ1O)-bis{3-((5-chloro-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ4N,O,O′:O′}dinickel(II), C38H34Ni2Cl2N4O12
- Crystal structure of tetrakis(methanol-κO)bis{3-((4-methoxy-2-oxidobenzylidene)amino)-2-oxo-2H-chromen-4-olato-κ3O,N,O′}bicobalt(II), C38H38Co2N2O14
- Crystal structure of (S)-tert-butyl-(1-hydroxypropan-2-yl)carbamate, C8H17NO3
- Crystal structure of 4-(4′-(pyridin-4-yl)-[1,1′-biphenyl]-4-yl)pyridin-1-ium catena-poly[{5-carboxy-4′-methyl-[1,1′-biphenyl]-3-carboxylato-κ2O,O′}-(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-κ4O,O′:O′′,O′′′)lead(II)], C52H40N2O9Pb
- Crystal structure of catena-poly[diaqua-(μ2-5-methylisophthalato-κ2O:O′)(μ2-1,4-bis((1H-1,2,4-triazol-1-yl)methyl)benzene-κ2N:N′)], NiC21H22O6N6
- Crystal structure of the salt tris(guanidinium) tris(tetrapropylammonium) bis(pyridine-2,4,6-tricarboxylate) – water (1/10), C55H126N14O22
- Crystal structure of 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7,8-trimethoxy-4H-chromen-4-one, C19H18O8
- Crystal structure of poly{[μ2-1,1′-(sulfonylbis(4,1-phenylene))bis(2-methyl-1H-imidazole)-κ2N:N′][μ2-4,4′-oxydibenzoato-κ2O:O′]cobalt(II)} hemihydrate, C34H27N4O7.5SCo
- The crystal structure of 25,27-(2,2′-[(2-thioxo-1,3-dithiole-4,5-diyl)disulfanediyl]diethanolate)-26,28-dihydroxycalix[4]arene — dichloromethane (1/1), C36H32Cl2O4S5
- The crystal structure of 1,2-bis(3-(pyridin-3-yl)-1,2,4-oxadiazol-5-yl)ethane, C16H12N6O2
- Crystal structure of 1-benzyl-3-((4-bromophenyl)amino)-4-(4-methoxyphenyl)-1H-pyrrole-2,5-dione, C24H19BrN2O3
- Crystal structure of bis(2-((allylcarbamothioyl)imino)-4-methylthiazol-3-ido-κ2N,S)palladium(II), C16H20N6PdS4
- Crystal structure of pyrimidine-2,5-dicarboxylic acid 1.5 hydrate, C12H14N4O11
- Crystal structure of trans-diaqua-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II), C8H10N4O6Mn
- Crystal structure of catena-(μ3-5-bromoisophthatato-κO,O′: O′′,O′′′′)-(1,2-bis(imidazol-1-yl)ethane-κN:N′)cobalt(II), C16H13CoN4O4Br
- Investigation of the compound La5Zn2−xPb1 + x (x = 0.20–0.32)
- Crystal structure of (OC-6-13)-diaqua-bis(3,5-di(pyridin-3-yl)-4H-1,2,4-triazol-4-amine-κ1N)-bis(dicyanamido-κ1N)zinc(II) tetrahydrate, ZnC28H32N18O6
- Crystal structure of Ga0.62(3)Sb0.38(3)Pd3
- Crystal structure of Ga0.47(1)Sb0.53(1)Pd2
- A derivative of the Corey lactone – crystal structure of (3aR,4S,5R,6aS)-4-(((tert-butyldimethylsilyl)oxy)methyl)-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl benzoate, C21H30O5Si
- A Corey lactone: crystal structure of (3aR,4R,5R,6aS)-5-benzoyloxy-4(hydroxymethyl)hexahydro-2H-cyclopenta[b]furan-2-one, C15H16O5
- Hydrothermal synthesis and crystal structure of poly[aqua-(μ2-1,3-bis(4-pyridyl)propane-κ2N:N′)-(μ2-1,4,5,6,7,7-hexachlorobicyclo[2.2.1]hept-5-ene-2,3-dicarboxylato-κ2O:O′)manganese(II) hydrate, C22H20Cl6N2O6Mn
- Crystal structure of 2-acetylpyrrole S-methylthiosemicarbazonium hydroiodide, C8H13IN4S
- Crystal structure of [N,N-bis((pyrrol-2-yl)ethylidene)butane-1,4-diamine-κ4N,N′,N′′,N′′′]-nickel(II), C16H20N4Ni
- Crystal structure of poly[aqua-(μ5-2,5-dicarboxybenzoato-κ5O:O:O′:O′′:O′′′)sodium(I)], C9H7NaO7
- Crystal structure of bis(N′-((1H-pyrrol-2-yl)methylene)-1-methylthio-methanethiohydrazido-κ2S,N)nickel(II), C14H16N6NiS4
- Crystal structure of 1-(4-((benzo[d][1,3]dioxol-5-yloxy)methyl)phenethyl)-4-(3-chlorophenyl) piperazin-1-ium chloride, C26H28Cl2N2O3
- Crystal structure of 2-(4-(2-(4-(2-fluorophenyl)piperazin-1-yl)ethyl)benzyl)benzo[d]isothiazol-3(2H)-one 1,1-dioxide, C26H26FN3O3S – a saccharin dervative
- Crystal structure of 3-(2-dimethylaminoethyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C12H15N3OS
- Crystal structure of 3-(3-dimethylaminopropyl)-2,3-dihydro-2-thioxoquinazolin-4(1H)-one, C13H17N3OS
- The crystal structure of trans-tetraaqua-bis(p-tolylsulfinato-κO)calcium(II)), C14H22O8S2Ca
- The crystal structure of (E)-N′-(pyridin-2-ylmethylene)pyrazine-2-carbohydrazide, C11H9N5O
- Crystal structure of (E)-3-(pyren-1-yl)-1-(pyridin-4-yl)prop-2-en-1-one, C24H15NO
- Crystal structure of catena-poly[diaqua-(μ2-tartrato-κ4O,O′:O′′,O′′′)cobalt(II)], C4H8CoO8
- Crystal structure of 4-chloro-2-methyl-6-(4-(trifluoromethoxy)phenyl)pyrimidine, C12H8ClF3N2O
- Crystal structure of 1-(4-fluorophenyl)-N-(5-((triphenylstannyl)thio)thiophen-2-yl)methanimine, C27H20FN3S2Sn
- Crystal structure of methyl (Z)-2-(5-fluoro-2-oxoindolin-3-ylidene)hydrazine-1-carbodithioate, C10H8FN3OS2
- Crystal structure of tert-butyl (Z)-4-(2-(5-methoxy-3-(2-((methylthio)carbonothioyl)hydrazono)-2-oxoindolin-1-yl)ethyl)piperazine-1-carboxylate, C22H31N5O4S2
- The crystal structure of (E)-2-((2-(o-tolylcarbamothioyl)hydrazono)methyl)benzoic acid, C16H15N3O2S
- Crystal structure of 2-chloro-1,3-di-tert-pentyl-4,4-diphenyl-1,3,2λ3,4-diazaphosphasiletidine, C22H32ClN2PSi
- Crystal structure of tetramethyl 5,5′-(buta-1,3-diyne-1,4-diyl)diisophthalate, C24H18O8
- Crystal structural of 2-amino-4-(4-methoxyphenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran, C19H20N2O3
- Crystal structure of 1,3,5-tris((trimethylsilyl)methyl)-1,3,5-triazinane-2,4,6-trione, C15H33N3O3Si3
- The crystal structure of bis(2-benzoyl-5-hydroxylphenolato-κ2O,O′)copper(II), C26H18CuO6
- Crystal structure of 2,6-bis(3-(pyrazin-2-yl)-1H-1,2,4-triazol-5-yl)pyridine – 1-ethyl-3-methyl-1H-imidazol-3-ium bromide (1/1), C23H22N13Br
- The crystal structure of (E)-N-benzyl-N′-benzylidene-4-methylbenzenesulfonohydrazide, C21H20N2O2S
- Crystal structure of ethyl (E)-5-((2-(3-hydroxybenzoyl)hydrazono)methyl)-3,4-dimethyl-1H-pyrrole-2-carboxylate – water – ethanol (1/1/1), C19H27N3O6
- The crystal structure of (E)-4-(3-ethoxy-2-hydroxybenzylideneamino)benzoic acid, C16H15NO4
- Crystal structure of (μ2-N,N′-bis((pyridin-4-yl)methyl)ethanediamide-κ2N:N′)-tetrakis(diethylcarbamodithioato-κ2S,S′)dizinc(II), C34H54N8O2S8Zn2

