Abstract
C28H37N3O2, monoclinic, P21/c (no. 14), a = 11.0454(4) Å, b = 21.9125(8) Å, c = 11.6544(4) Å, β = 111.783(1)°, V = 2619.33(16) Å3, Z = 4, Rgt(F) = 0.0658, wRref(F2) = 0.1940, T = 293(2) K.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.42 × 0.27 × 0.11 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 80.7 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| 2θmax, completeness: | 53°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 38550, 5420, 0.065 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3079 |
| N(param)refined: | 301 |
| Programs: | Bruker programs [1], SHELX [2, 3] |
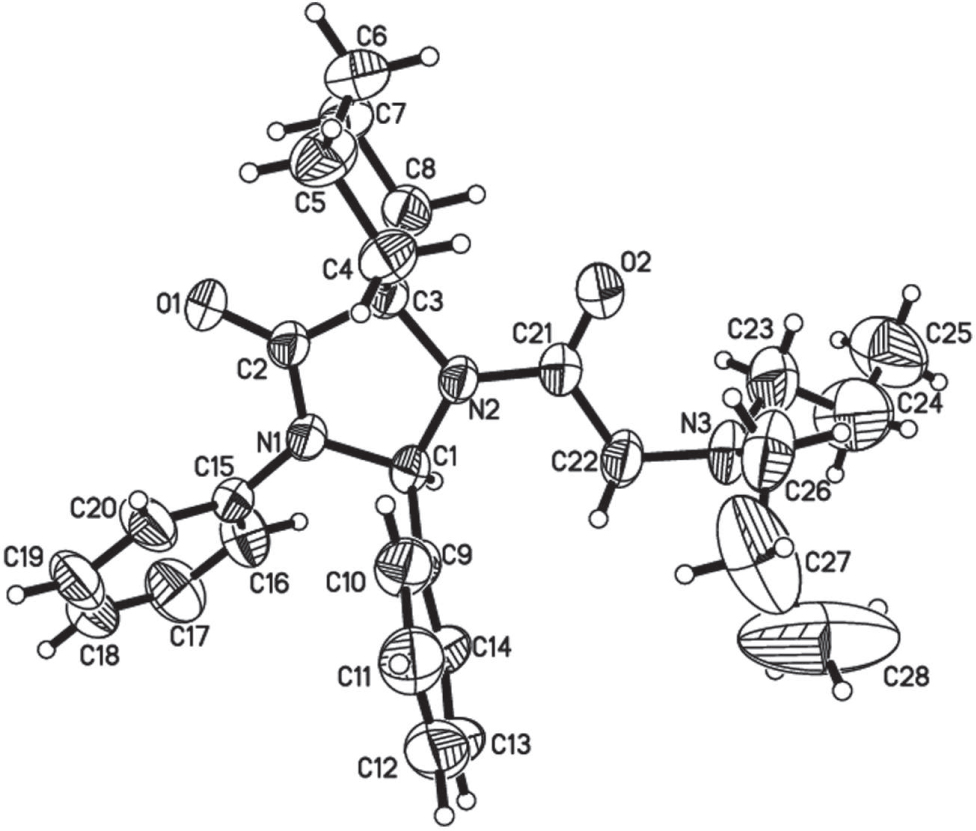
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.48261(18) | 0.25128(10) | 1.11038(18) | 0.0807(7) |
| O2 | 0.2053(2) | 0.40213(10) | 0.76116(19) | 0.0809(7) |
| N1 | 0.55635(19) | 0.28601(9) | 0.96390(17) | 0.0487(5) |
| N2 | 0.39265(19) | 0.35021(9) | 0.85567(16) | 0.0481(5) |
| N3 | 0.2873(2) | 0.45341(11) | 0.5799(2) | 0.0713(7) |
| C1 | 0.5210(2) | 0.32920(11) | 0.8608(2) | 0.0466(6) |
| H1A | 0.5116 | 0.3075 | 0.7844 | 0.056* |
| C2 | 0.4692(2) | 0.28220(12) | 1.0196(2) | 0.0536(6) |
| C3 | 0.3515(2) | 0.32153(11) | 0.9513(2) | 0.0486(6) |
| C4 | 0.3271(3) | 0.36928(13) | 1.0353(3) | 0.0674(8) |
| H4A | 0.4091 | 0.3890 | 1.0828 | 0.081* |
| H4B | 0.2690 | 0.4002 | 0.9844 | 0.081* |
| C5 | 0.2679(4) | 0.34356(17) | 1.1240(3) | 0.0877(10) |
| H5A | 0.3308 | 0.3171 | 1.1835 | 0.105* |
| H5B | 0.2476 | 0.3768 | 1.1689 | 0.105* |
| C6 | 0.1454(3) | 0.30788(19) | 1.0555(4) | 0.0949(11) |
| H6A | 0.1101 | 0.2915 | 1.1139 | 0.114* |
| H6B | 0.0805 | 0.3347 | 0.9991 | 0.114* |
| C7 | 0.1750(3) | 0.25661(16) | 0.9844(3) | 0.0809(10) |
| H7A | 0.0956 | 0.2341 | 0.9406 | 0.097* |
| H7B | 0.2364 | 0.2288 | 1.0416 | 0.097* |
| C8 | 0.2322(3) | 0.27994(13) | 0.8920(3) | 0.0652(7) |
| H8A | 0.1655 | 0.3024 | 0.8273 | 0.078* |
| H8B | 0.2574 | 0.2453 | 0.8539 | 0.078* |
| C9 | 0.6238(2) | 0.37832(11) | 0.8857(2) | 0.0529(6) |
| C10 | 0.6433(3) | 0.42103(13) | 0.9776(3) | 0.0700(8) |
| H10A | 0.5915 | 0.4200 | 1.0247 | 0.084* |
| C11 | 0.7373(4) | 0.46486(15) | 1.0009(4) | 0.0942(11) |
| H11A | 0.7492 | 0.4933 | 1.0633 | 0.113* |
| C12 | 0.8135(4) | 0.4666(2) | 0.9320(5) | 0.1121(15) |
| H12A | 0.8766 | 0.4968 | 0.9467 | 0.135* |
| C13 | 0.7978(4) | 0.4238(2) | 0.8404(5) | 0.1080(13) |
| H13A | 0.8512 | 0.4247 | 0.7947 | 0.130* |
| C14 | 0.7016(3) | 0.37936(15) | 0.8171(3) | 0.0782(9) |
| H14A | 0.6900 | 0.3505 | 0.7553 | 0.094* |
| C15 | 0.6764(2) | 0.25289(11) | 1.0025(2) | 0.0518(6) |
| C16 | 0.7058(3) | 0.21710(14) | 0.9209(3) | 0.0783(9) |
| H16A | 0.6489 | 0.2149 | 0.8389 | 0.094* |
| C17 | 0.8209(3) | 0.18395(16) | 0.9605(4) | 0.0948(11) |
| H17A | 0.8410 | 0.1596 | 0.9047 | 0.114* |
| C18 | 0.9048(3) | 0.18674(18) | 1.0805(4) | 0.0905(11) |
| H18A | 0.9818 | 0.1643 | 1.1070 | 0.109* |
| C19 | 0.8748(3) | 0.2225(2) | 1.1603(3) | 0.1077(14) |
| H19A | 0.9312 | 0.2242 | 1.2425 | 0.129* |
| C20 | 0.7620(3) | 0.25640(19) | 1.1218(3) | 0.0877(11) |
| H20A | 0.7441 | 0.2818 | 1.1774 | 0.105* |
| C21 | 0.3129(3) | 0.38735(12) | 0.7644(2) | 0.0588(7) |
| C22 | 0.3644(3) | 0.40860(13) | 0.6659(2) | 0.0676(8) |
| H22A | 0.4512 | 0.4252 | 0.7075 | 0.081* |
| H22B | 0.3727 | 0.3732 | 0.6195 | 0.081* |
| C23 | 0.1641(4) | 0.4297(2) | 0.4917(3) | 0.1065(13) |
| H23A | 0.1648 | 0.3856 | 0.4995 | 0.128* |
| H23B | 0.0938 | 0.4451 | 0.5145 | 0.128* |
| C24 | 0.1358(5) | 0.4445(3) | 0.3646(4) | 0.168(2) |
| H24A | 0.2112 | 0.4338 | 0.3452 | 0.202* |
| H24B | 0.1249 | 0.4884 | 0.3554 | 0.202* |
| C25 | 0.0205(5) | 0.4155(3) | 0.2724(4) | 0.161(2) |
| H25A | 0.0125 | 0.4282 | 0.1911 | 0.241* |
| H25B | −0.0562 | 0.4274 | 0.2871 | 0.241* |
| H25C | 0.0299 | 0.3719 | 0.2789 | 0.241* |
| C26 | 0.2777(5) | 0.51213(17) | 0.6310(4) | 0.1036(13) |
| H26A | 0.2507 | 0.5062 | 0.7005 | 0.124* |
| H26B | 0.2101 | 0.5355 | 0.5690 | 0.124* |
| C27 | 0.4016(7) | 0.5488(3) | 0.6737(6) | 0.180(3) |
| H27A | 0.3883 | 0.5857 | 0.7134 | 0.216* |
| H27B | 0.4697 | 0.5253 | 0.7349 | 0.216* |
| C28 | 0.4457(9) | 0.5656(4) | 0.5737(12) | 0.283(6) |
| H28A | 0.4915 | 0.6038 | 0.5932 | 0.424* |
| H28B | 0.3716 | 0.5696 | 0.4979 | 0.424* |
| H28C | 0.5028 | 0.5345 | 0.5647 | 0.424* |
Source of material
n-Dipropylamine (2.3 g, 0.023 mol) was added to a solution of 1-(chloroacetyl)-2,3-diphenyl-1,3-diazaspiro[4.5]decan-4-one (1.8 g, 0.0047 mol) in toluene (40 mL) and the reaction mixture was heated at 90 °C for 20 h. After cooling to room temperature the solvent was removed under reduced pressure to give a residual oil which was dissolved in dichloromethane (100 mL). The organic phase was washed with water, dried (Na2SO4) and evaporated under vacuum to yield the title compound as a viscous oil. The crude oil was purified by column chromatography using silica gel as a stationary phase and a mixture of petroleum ether (40–60 °C): ethyl acetate (7:3) as a mobile phase to give the title compound as a white solid; m.p. 411–413 K in 65% yield [4]. The title compound was re-crystallized from ethyl acetate to afford colorless single crystals.
Experimental details
Carbon-bound hydrogen atoms were placed in calculated positions and were included in the refinement using the riding model approximation, with Uiso(H) set to 1.2Ueq(C) and 1.5Ueq(Methyl).
Discussion
Epilepsy is a chronic disorder of the brain that affects approximately 50 million people, making it one of the most common neurological diseases worldwide [5]. Many new antiepileptic drugs have been introduced in the market, however the available antiepileptics fail to prevent the seizures in approximately 30% of patients [6]. Moreover, these medications are associated with severe adverse effects such as gingival hyperplasia, megaloblastic anaemia and hepatotoxicity [7, 8] . Therefore, there is a great need for the development of new, more effective and less toxic antiepileptic agents. The title compound displayed 83% protection toward subcutaneous pentylenetetrazole -induced convulsions in mice at dose level 0.035 mmol/kg [3].
In the crystal structure of the title compound, the asymmetric unit contains one independent molecule. Molecules form extended chains along the crystallographic c direction via one non-classical intermolecular hydrogen bond, C1—H1A ⋯O1i, symmetry code: (i) x, − y + 1/2, z-1/2, with a D ⋯A distance of 3.300 Å and a D—H ⋯A angle of 175°.
Acknowledgement
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RGP-196.
References
1 Brucker. APEX2, SAINT and SADABS. Brucker AXS Inc., Madison, Wisconsin, USA, (2009).Suche in Google Scholar
2 Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar
3 Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar
4 Aboul-Enein, M. N.; El-Azzouny, A. A.; Saleh, O. A.; Amin, K. M.; Maklad, Y. A.; Hassan, R. M.: Synthesis and anticonvulsant activity of aubstituted-1,3-diazaspiro[4.5]decan-4-ones. Arch. Pharm. Chem. Life Sci. 348 (2015) 575–588.10.1002/ardp.201500092Suche in Google Scholar
5 Epilepsy: epidemiology, aetiology and prognosis, in: WHO Factsheet, World Health Organization, (2015).Suche in Google Scholar
6 Plech, T.; Luszczki, J. J.; Wujec, M.; Flieger, J.; Pizoń, M.: Synthesis, characterization and preliminary anticonvulsant evaluation of some 4-alkyl-1,2,4-triazoles. Eur. J. Med. Chem. 60 (2013) 208–215.10.1016/j.ejmech.2012.11.026Suche in Google Scholar
7 Lin, Z.; Kadaba, P. K.: Molecular targets for the rational design of antiepileptic drugs and related neuroprotective agents. Med. Res. Rev. 17 (1997) 537–572.10.1002/(SICI)1098-1128(199711)17:6<537::AID-MED3>3.0.CO;2-2Suche in Google Scholar
8 Zaccara, G.; Franciotta, D.; Perucca, E.: Idiosyncratic adverse reactions to antiepileptic drugs. Epilepsia 48 (2007) 1223–1244.10.1111/j.1528-1167.2007.01041.xSuche in Google Scholar
©2017 Rasha M. Hassan et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16


