Abstract
C27H20FNO, monoclinic, P21/n (no. 14), a = 13.1620 Å, b = 13.8779 Å, c = 11.1618 Å, β = 99.710(1)°, V = 2009.62(6) Å3, Z = 4, Rgt(F) = 0.0436, wRref(F2) = 0.1310, T = 173(2) K.
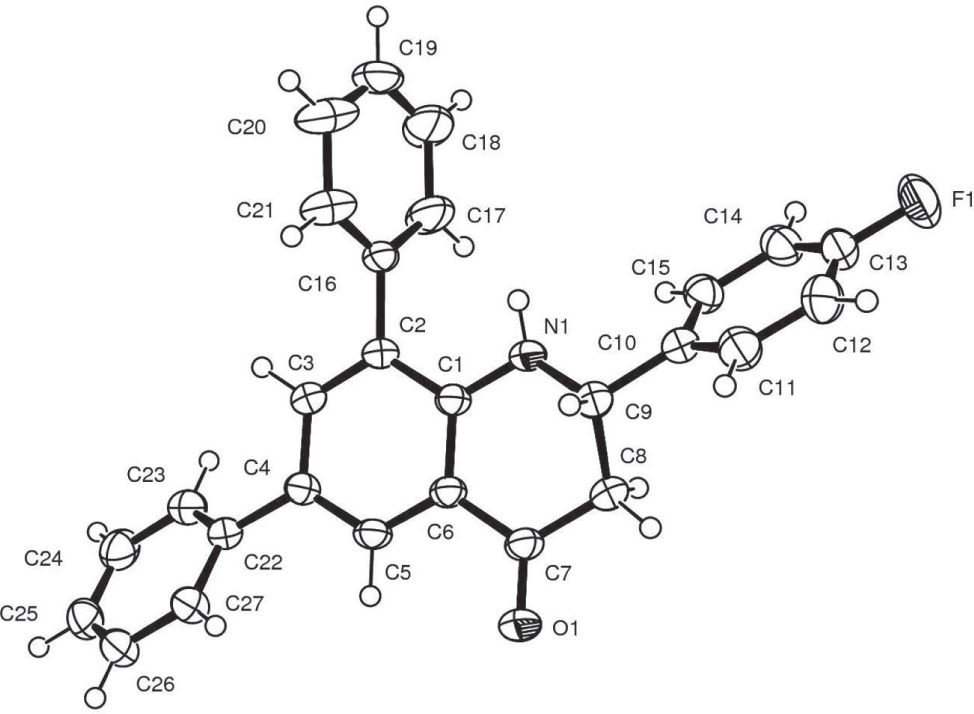
The asymmetric unit of the title crystal structure is shown in the figure. Tables 1 and 2 contain details of the measurement method and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.46 × 0.43 × 0.11 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.9 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω scans |
| 2θmax, completeness: | 56°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 25349, 4862, 0.071 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3566 |
| N(param)refined: | 275 |
| Programs: | Bruker programs [1], SHELX [2], PLATON [3–4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 1.08261(9) | 0.87850(9) | 0.41526(10) | 0.0289(3) |
| C2 | 1.17171(10) | 0.90592(9) | 0.36769(10) | 0.0311(3) |
| C3 | 1.25833(10) | 0.93585(9) | 0.44637(10) | 0.0328(3) |
| H3 | 1.3176 | 0.9540 | 0.4135 | 0.039* |
| C4 | 1.26273(10) | 0.94058(9) | 0.57288(10) | 0.0314(3) |
| C5 | 1.17516(10) | 0.91407(9) | 0.61790(10) | 0.0313(3) |
| H5 | 1.1760 | 0.9164 | 0.7031 | 0.038* |
| C6 | 1.08569(9) | 0.88402(9) | 0.54193(10) | 0.0292(3) |
| C7 | 0.99421(10) | 0.85745(9) | 0.59438(11) | 0.0327(3) |
| C8 | 0.89689(10) | 0.84094(10) | 0.50465(11) | 0.0351(3) |
| H8A | 0.8624 | 0.9036 | 0.4838 | 0.042* |
| H8B | 0.8495 | 0.8001 | 0.5428 | 0.042* |
| C9 | 0.91765(10) | 0.79226(10) | 0.38812(11) | 0.0342(3) |
| H9 | 0.9453 | 0.7261 | 0.4085 | 0.041* |
| C10 | 0.81990(10) | 0.78413(10) | 0.29418(11) | 0.0347(3) |
| C11 | 0.75634(11) | 0.70484(12) | 0.29512(13) | 0.0461(4) |
| H11 | 0.7748 | 0.6557 | 0.3539 | 0.055* |
| C12 | 0.66576(12) | 0.69599(13) | 0.21132(15) | 0.0520(4) |
| H12 | 0.6221 | 0.6416 | 0.2123 | 0.062* |
| C13 | 0.64132(11) | 0.76773(12) | 0.12755(13) | 0.0438(4) |
| C14 | 0.70189(11) | 0.84705(11) | 0.12228(13) | 0.0425(3) |
| H14 | 0.6831 | 0.8953 | 0.0623 | 0.051* |
| C15 | 0.79166(11) | 0.85511(10) | 0.20713(12) | 0.0377(3) |
| H15 | 0.8345 | 0.9101 | 0.2056 | 0.045* |
| C16 | 1.17435(10) | 0.89780(10) | 0.23490(11) | 0.0350(3) |
| C17 | 1.12024(15) | 0.96057(18) | 0.15243(15) | 0.0726(6) |
| H17 | 1.0799 | 1.0103 | 0.1793 | 0.087* |
| C18 | 1.12499(16) | 0.9508(2) | 0.02920(16) | 0.0955(9) |
| H18 | 1.0871 | 0.9939 | −0.0276 | 0.115* |
| C19 | 1.18271(15) | 0.88077(17) | −0.01086(14) | 0.0701(6) |
| H19 | 1.1852 | 0.8753 | −0.0951 | 0.084* |
| C20 | 1.23712(16) | 0.81826(13) | 0.06951(14) | 0.0607(5) |
| H20 | 1.2778 | 0.7692 | 0.0417 | 0.073* |
| C21 | 1.23267(13) | 0.82689(11) | 0.19263(13) | 0.0482(4) |
| H21 | 1.2705 | 0.7831 | 0.2487 | 0.058* |
| C22 | 1.35742(10) | 0.97269(10) | 0.65462(11) | 0.0335(3) |
| C23 | 1.41426(11) | 1.05112(11) | 0.62505(12) | 0.0410(3) |
| H23 | 1.3934 | 1.0838 | 0.5501 | 0.049* |
| C24 | 1.50092(11) | 1.08207(12) | 0.70378(14) | 0.0476(4) |
| H24 | 1.5385 | 1.1362 | 0.6829 | 0.057* |
| C25 | 1.53311(11) | 1.03461(13) | 0.81255(14) | 0.0491(4) |
| H25 | 1.5928 | 1.0558 | 0.8660 | 0.059* |
| C26 | 1.47814(11) | 0.95644(12) | 0.84296(13) | 0.0453(4) |
| H26 | 1.5000 | 0.9237 | 0.9176 | 0.054* |
| C27 | 1.39090(10) | 0.92552(11) | 0.76470(11) | 0.0389(3) |
| H27 | 1.3535 | 0.8715 | 0.7863 | 0.047* |
| N1 | 0.99530(8) | 0.84800(8) | 0.33891(10) | 0.0342(3) |
| O1 | 0.99674(8) | 0.85217(8) | 0.70421(8) | 0.0477(3) |
| F1 | 0.55246(7) | 0.75914(8) | 0.04504(9) | 0.0631(3) |
| H1 | 1.0078(13) | 0.8267(12) | 0.2691(16) | 0.054(5)* |
Source of material
A mixture of 6,8-dibromo-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(1H)-one (1.0 mmol), phenylboronic acid (2.2 mmol), dichlorido-bis(triphenylphosphine)palladium(II) (0.05 mmol), tricyclohexylphosphine (0.1 mmol) and potassium carbonate (2.0 mmol) in a dioxane-water mixture (3:1, v/v) was purged with argon for 0.5 h. A balloon filled with argon gas was then connected to the top of the condenser and the mixture was heated with stirring at 80–90 °C under argon atmosphere for 3 h. The mixture was quenched with ice cold water and the product extracted with chloroform. The combined organic extracts were sequentially washed with brine, dried over anhyd. MgSO4, filtered and then evaporated under reduced pressure. The crude product was purified by column chromatography on silica gel to afford the title compound [5]. Single crystals were obtained by slow evaporation of ethanol at room temperature.
Experimental details
The collection method involved ω-scans of width 0.3°. Data reduction was carried out using the program SAINT+[1] and absorption corrections were made using the program SADABS [1]. The crystal structure was solved by direct methods using SHELXTL [2]. Hydrogen atoms were positioned geometrically and allowed to ride on their respective parent atoms. Aromatic C—H distances were set to 0.93 Å and their Uiso set to 1.2 times the Ueq for the atoms to which they are bonded. Hydrogen atoms involved in hydrogen bonding were refined freely.
Comment
In recent years, there has been growing interest in the design and synthesis of 2-aryl-2,3-dihydroquinolin-4(1H)-ones due to their wide range of biological properties [6]. 2-Aryl-2,3-dihydroquinolin-4(1H)-one derivatives serve as antitumor agents [7] and others exhibit microRNA inhibitory properties [8]. The synthesis of the title compound follows the transition metal catalysed Suzuki-Miyaura cross coupling reaction as a tool for carbon-carbon bond formation. The crystal structure of the analogous 2,3-diphenylquinoline showed that the quinolone ring was essentially planar [9]. The heterocyclic ring of the title compound exists in half-chair conformation. The 2-aryl ring is twisted from co-planarity expressed by a torsion angle of 91.70° for [C(8)—C(9)—C(10)—C(15)]. The 6- and 8-aryl rings are also turned out of the plane of the heterocyclic moiety with average torsion angels of −110° and −139°, respectively. The crystal structure shows an intermolecular hydrogen bond between N—H of one molecule and the carbonyl oxygen of another one with a D—H⋅⋅⋅A angle of 124° and H⋅⋅⋅A distance of 2.58 Å.
Acknowledgement
The authors are grateful to the University of South Africa and national research foundation (NRF) for financial assistance and M. A. Fernandes at Witwatersrand University for X-ray data.
References
1 Bruker. APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, WI, USA (2013).Suche in Google Scholar
2 Sheldrick, G. M.: A Short History of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar
3 Farrugia, L. J.: WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 32 (1999) 837–838.10.1107/S0021889899006020Suche in Google Scholar
4 Spek, A. L.: Structure validation in chemical crystallography. Acta Crystallogr. D65 (2009) 148–155.10.1107/S090744490804362XSuche in Google Scholar
5 Mphahlele, M. J.; Oyeyiola F. A.: Suzuki-Miyaura cross-coupling of 2-aryl-6,8-dibromo-1,2,3,4-tetrahydroquinolin-4-ones and subsequent dehydrogenation and oxidative aromatization of the resulting 2,6,8-triaryl-1,2,3,4-tetrahydroquinolin-4-ones. Tetrahedron 67 (2011) 6819–6825.10.1016/j.tet.2011.06.085Suche in Google Scholar
6 Zhang, S.-X.; Feng, J.; Kuo, S.-C.; Brossi, A.; Hamel, E.; Tropsha, A.; Lee, K.-H.: Antitumor agents. 199. Three-dimensional quantitative structure-activity relationship study of the colchicine binding site ligands using comparative molecular field analysis. J. Med. Chem. 43 (2000) 167–176.10.1021/jm990333aSuche in Google Scholar
7 Xia, Y.; Yang, Z.-Y.; Xia, P.; Batow, K. F.; Tachibana, Y.; Kuo, S.-C.; Hamel, E.; Hackl, GT.; Lee, K.-H.: Synthesis and biological evaluation of 6,7,2′,3′,4′-substituted-1,2,3,4-tetrahydro-2-phenyl-4-quinolones as a new class of antimitotic antitumor agents. J. Med. Chem. 41 (1988) 1155–1162.10.1021/jm9707479Suche in Google Scholar
8 Chandrasekhar, S.; Pushpavalli, S. N. C. V. L.; Chatla, S.; Mukhopadhyay, D.; Changanna, B.; Vijeender, K.; Srihari, P.; Reddy, C. R.; Ramaiah, M. J.; Bhadra, U.: Aza-flavanones as potent cross-species microRNA inhibitors that arrest cell cycle. Bioorg. Med. Chem. Lett. 22 (2012) 645–648.10.1016/j.bmcl.2011.10.061Suche in Google Scholar
9 Carloni, P.; Damiani, E.; Greci, L.; Stipa , P.; Rizzoli, C.; Sgarabotto, P.; Ugozzoli, F.: Oxidative dimerization of quinolinic nitroxides in the presence of trichloro- and trifluoro- acetic acid. Crystal structures of 6,6υ008242-bis-(1-oxide-1,2,6,8a-tetrahydroquinoline)ylidene and of 2,3-diphenylquinoline. Tetrahedron 49 (1993) 5099–5108.10.1016/S0040-4020(01)81875-5Suche in Google Scholar
©2017 Marole M. Maluleka et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16


