Abstract
Due to a recent lack of clean water as a result of an increasing water demand, new wastewater solutions are required. Many researchers have looked into the removal of organic dyes from wastewater, with adsorption being an easy and effective method for removing organic and inorganic contaminants from contaminated water. Conjugated modified polymers, primarily polyaniline (Pani), have been widely used in the wastewater treatment because of their unique properties, such as easy synthesis, tunable morphology, porous structure, good electrorheological property, biodegradability, and nontoxic nature. Modified surface polymers are more reactive for the removal of dyes from wastewater and have outstanding dye removal capabilities in the wastewater treatment. This review article elaborates on wastewater treatment by utilizing silica gel-impregnated polyaniline nanocomposites as adsorbents. The use of polyaniline-modified silica gel in dye migration behavior to the most suited system for the resolution of co-existing dyes is referred to as the separation of organic dyes from their mixtures. Adsorption of important organic dyes to optimize conditions for efficient organic dye removal and comparison with another commercially available adsorbent. Chemical modification with the introduction of acidic or basic surface functionality could increase cationic and anionic chemical adsorption, as well as charged organic species such as dyes. The carrier is thus obtained with a chelating reagent on the surface of a silica gel after impregnation with polyaniline.
1 Introduction
Water plays an important role in all living organisms to perform the basic necessities. All living species require hygienic water to survive. Water contamination has happened around the world for a variety of reasons, including rapid urbanization, industrialization, and a lack of effective planning and accomplishment, which further exacerbates the water crisis [1,2]. One of the key troubles that the world faces today is the pollution of drinking water. According to the Encyclopedia Britannica, human-accessible freshwater accounts for just around 0.007% of all the water on the earth, making clean water scarcity a global issue. Filtration, micro/ultra/nanofiltration, precipitation, flotation, centrifugation, coprecipitation, adsorption, oxidation, coagulation, ion exchange, solvent extraction, evaporation, electrolysis, reverse osmosis, distillation, and other treatment processes for contaminated groundwater are common [3,4,5,6]. The adsorption technique, which is used to remove numerous organic, inorganic, and biological contaminants from unclean water, is the most cost-effective method of the treatment [7,8,9,10,11,12]. Dyes are organic chemicals that provide color, and as a result, they are widely employed in the paint, plastic, textile, leather, paper, cosmetics, and photographic industries [13,14,15]. Large amounts of water are used for dyeing applications in dye industries.
The majority of dyes can hold on for a long time in the environment and cannot be degraded by natural processes. When dyes present in water bodies alter the phenomenon of photosynthesis, the aquatic plant and animal environments are affected in the ecosystem. Silica aerogels have attracted attention in recent years as outstanding nanoporous materials with low-density solids, large specific surface areas, and high porosity [16,17]. Silica aerogels have emerged as a promising class of hybrid porous materials with a wide variety of adsorption applications [18]. Hydrophilic silica aerogels had good adsorption results, which shows the performance of the cationic dye [19,20,21,22,23]. The adsorbent’s adsorption property is mostly determined by the surface functional groups, the surface area, and surface charge [24]. The rate of elimination of organic dyes is affected by a variety of factors, including dye concentration, pH, and contact duration [25].
Organic dyes’ adsorption capability on Pani-modified silica aerogel is determined by the characteristics of both the adsorbent and the adsorbates. Organic dyes are chemically stable, have low toxicity, and have high water solubility. In this review article, a novel solid-phase polyaniline silica gel nanocomposite adsorbent material for dye adsorption was employed. Polymeric-modified adsorbent Pani@/SG has been considered for the adsorption of organic dyes. Pani is a well-known in-situ polymerization technique that may be used to easily produce conductive polymer [26]. Pani is an important conducting polymer due to its relatively easy processability, superior electrical conductivity, and high environmental durability. Its composites with inorganic materials such as silica gel have better adsorption capabilities. Chemical and physical adsorption phenomenon is shown in Figure 1.
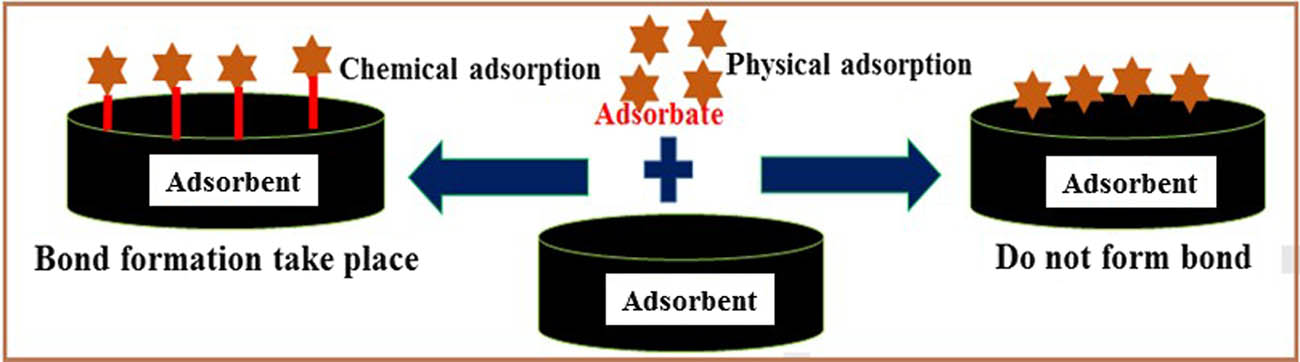
Chemical and physical adsorption phenomenon.
2 Organic compounds
The organic compound molecule contains carbon. It is possible to categorize it in a variety of ways. The contrast between natural and manufactured chemicals is significant. In ancient times, human beings used coloring materials. The term “attractive natural substances” refers to chemicals generated from plants and animals. As a result, natural dyes are generally safe and nontoxic to the environment. Natural colors are obtained from plants, fruits, animals, insects, minerals, and other natural resources, and many of them are still collected from natural sources since artificial production would be prohibitively expensive. Mordants used for the application are toxic substances of mordents, such as copper, aluminum, iron, and chromium, which were employed to help the natural dye attach to clothes [27,28,29]. Natural dyes are rare and expensive since they require a large amount of land to produce. Their colors may wash out over time, posing a sustainability challenge. Synthetic compounds are produced by the reaction of other compounds [30,31]. Many polymers, including all plastics, are synthetic compounds. Synthetic dyes are used in a variety of applications, including food, wood, clothing, and pharmaceuticals since they are less expensive to produce and for application to surfaces. Direct, acid, basic, reactive, metal complex, mordant, dispersion dye, sulfur, and other dyes fall under this category [32].
2.1 Organic dyes
Organic dyes are capable of coloring fabrics. Rubbing or washing will not remove them. People have utilized natural colors derived from animal and vegetable sources from ancient times, such as Turkey red or alizarin derived from the roots of the madder plant and indigo dye derived from the leaves of the indigoid plant (Table 1). The majority of the synthetic organic colors, from clothing to household products, are obtained from synthetic organic dyes. William Henry Perkin discovered the first synthetic organic dye by accident in 1856 [33]. He attempted to manufacture quinine, a medication used to treat malaria. He ended up with purple dye as a result of his efforts.
Structure of organic dyes [34]
| Organic dyes | Structure |
|---|---|
| Tartrazine |
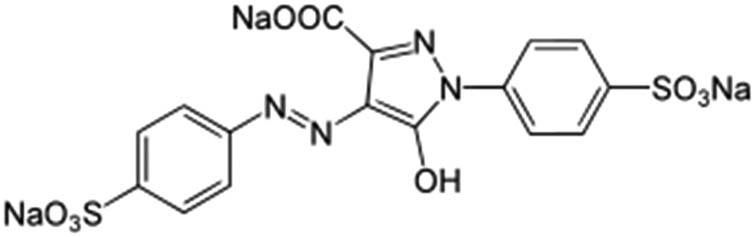
|
| Brilliant blue |
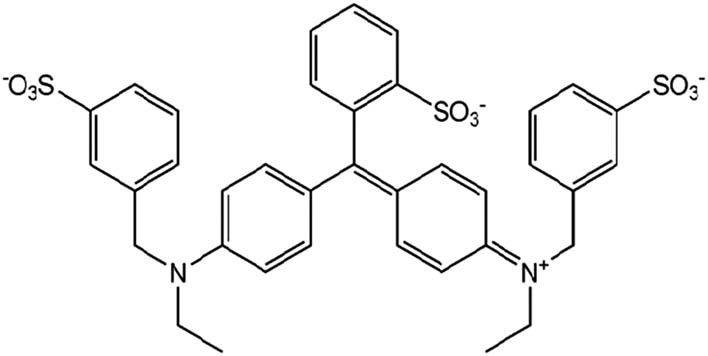
|
| Carmoisine |
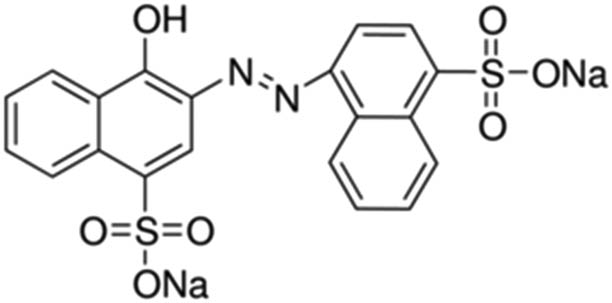
|
| Bromocresol green |
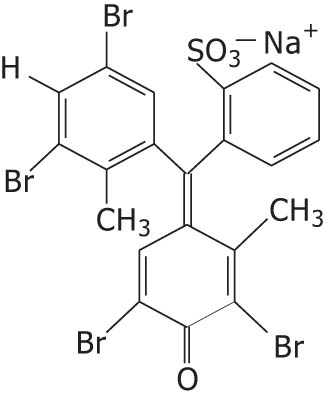
|
| Amidoblack 10B |
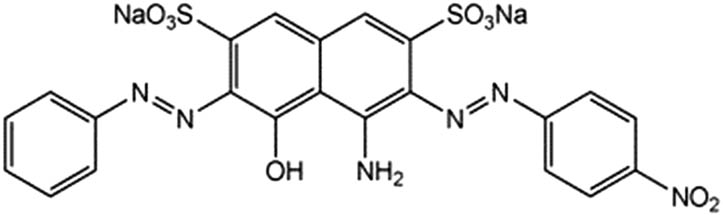
|
| 4-Nitrobenzene dizonium tetrafluoroborate |
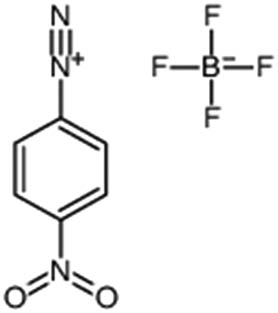
|
| Xylenol orange |
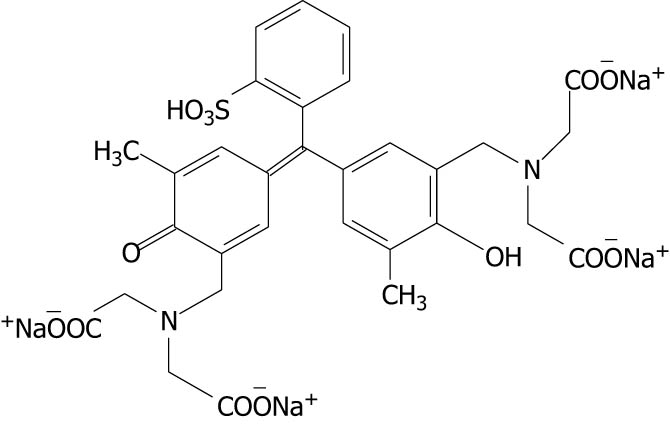
|
| Rose Bengal |
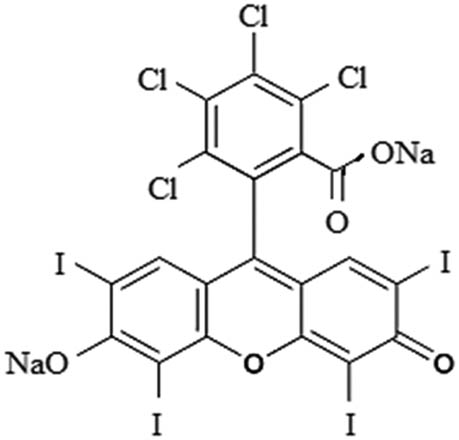
|
| Aluminone |
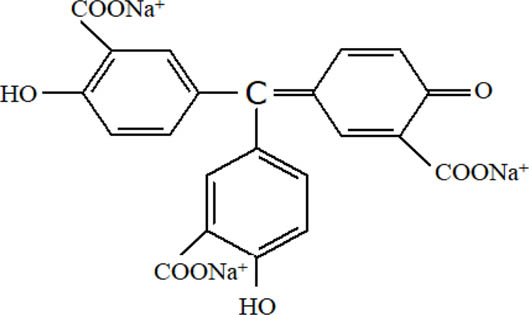
|
| Alizarin red S |
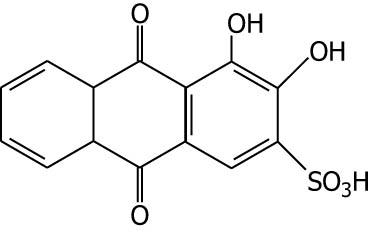
|
| 1-(2-Pyridylazo)-2-napthol (PAN) |
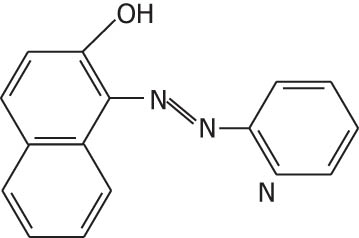
|
| Pyrocatechol violet |
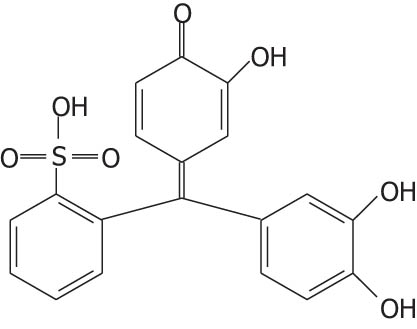
|
| Bromopyrogallol red |
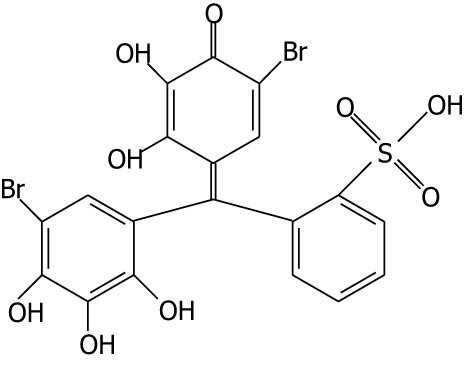
|
| Ammonium purpurate |
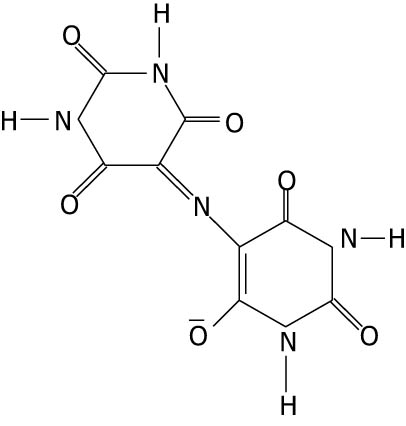
|
| Thorin indicator |
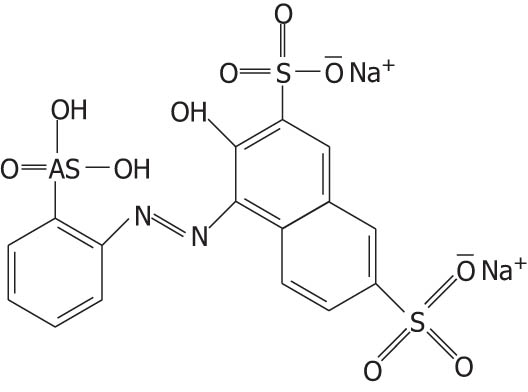
|
| Bromocresol purple |
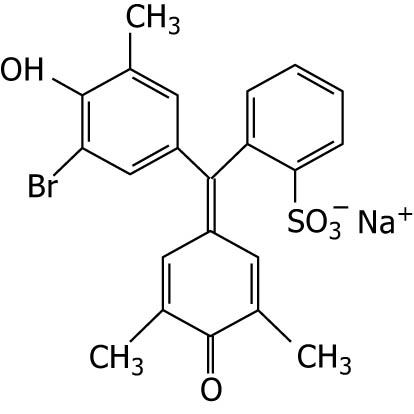
|
| Methyl red |
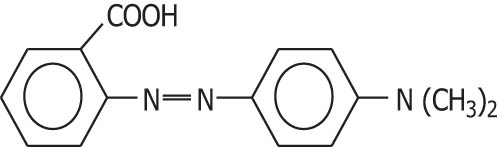
|
| Congo red |
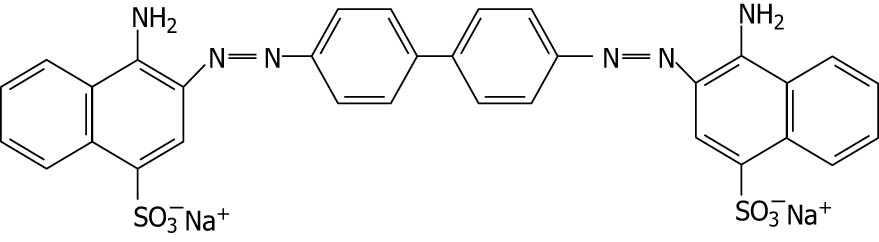
|
| Malachite green |
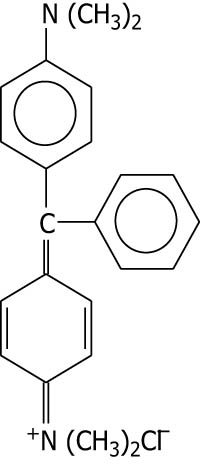
|
| Brilliant green |
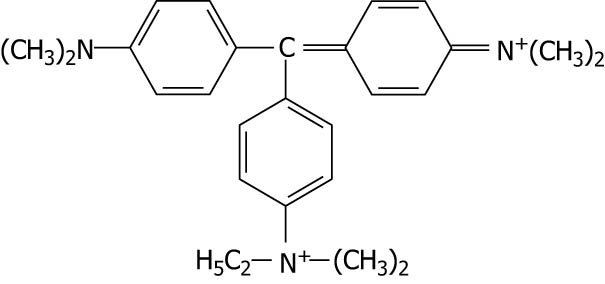
|
| Patent blue V |
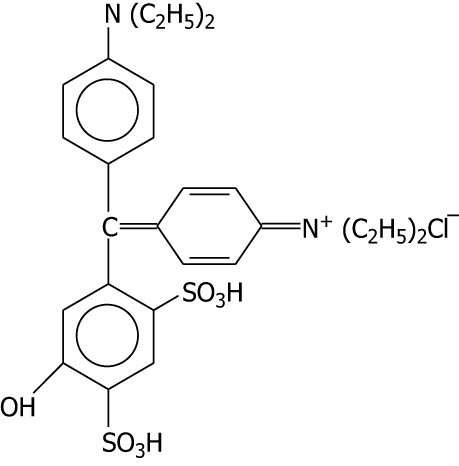
|
| Saffranine |
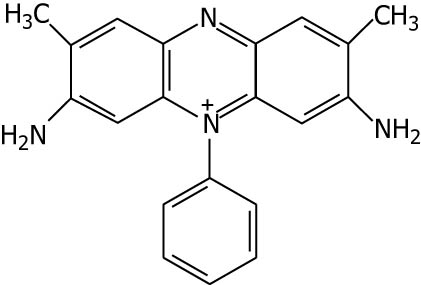
|
| Crystal violet |
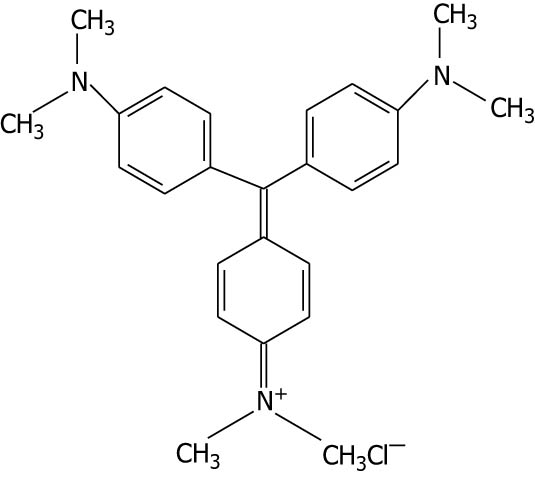
|
| Metanil yellow |
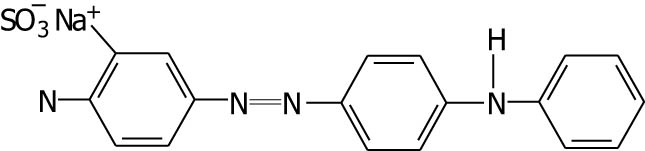
|
| Methylene blue |
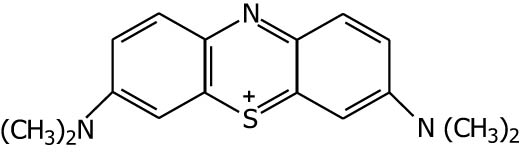
|
| Light green |
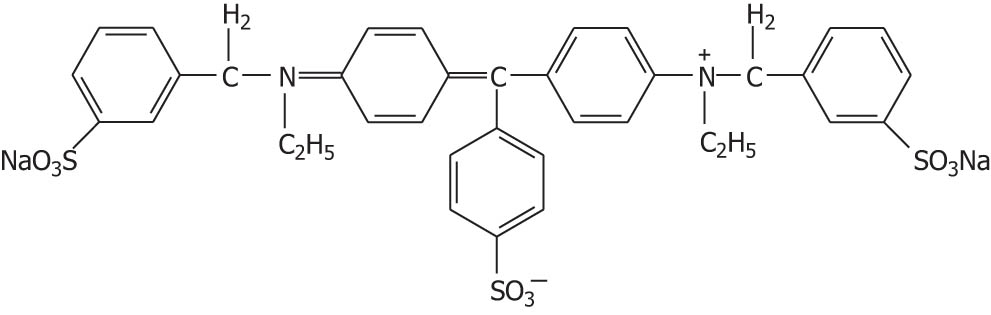
|
| Methyl-thymol blue sodium salt | C37H40N2Na4O13S |
2.2 Major classification of dyes
Dyes are used as color for fabric, foodstuff, textiles, paper, pharmaceuticals, polymers, cosmetics, lacquers, printing inks, oils, paints, and detergents. Apart from their apparent function as coloring agents, dyes are also employed to evaluate the surface area and cation exchange capacity of oxides and clays [35,36].
The global level of high dye manufacture and widespread use generate effluent, which pollutes the environment. It diminishes the penetration of sunlight, which reduces the life span of aquatic biota [37] and affects the photosynthetic activity of aquatic plants and maybe terrestrial plants [38], decreasing the cation exchange capacity of the soil.
2.2.1 Categories of synthetic organic dyes
Acid dyes: These are azo dyes and anionic (negative charge) water-soluble dyes used to color wool, silk, nylon, and certain acrylic fibers and have no affinity for cotton. A positively charged oxygen or sulfur atom may occasionally substitute nitrogen. They are acids containing one or more acid functions. The red congo could be considered a representative member of this dye family. Acid dyes cannot be used on cellulosic fiber due to structural differences, compared to direct dyes. Acid dyes are more responsive to protein and polyamide fibers. They are machine washable and light resistant. Acid dyes are available in a wide range of colors. They are cost effective and characterized by the salts of sulphonic or carboxylic acids, e.g., methyl orange, orange I, orange II, naphthol yellow, and Martius yellow.
Basic dyes: These dyes are water soluble in their natural state (with the help of acetic acid), contain amino groups in unsubstituted (–NH2) or substituted form (–NR2), and are cationic (with a positive charge). These dyes are widely used on acrylic, paper, and nylon substrates, but they can also be utilized on modified polyester substrates. These are used to dye modified orlon materials nylons, paper, polyesters, leather, cotton, wool, e.g., aniline yellow and malachite green.
Direct dyes: They belong to the class of azo dyes and dye the fabrics directly by placing them in an aqueous solution of the dye in a neutral environment. They are used to dye cellulose fibers without the use of a mordant in a variety of brilliant colors. Cotton, wool, paper, leather, silk, and nylon are all dyed using direct dyes. These form H-bond with fabrics. These are used to dye wool, silk, nylon, rayon, and cotton, e.g., Martius yellow and Congo red.
Disperse dyes: These dyes are not water insoluble, which are dispersed in the chemicals like phenol, cresol, etc. They are developed to dye cellulose acetate, nylon, polyesters and polyacrylonitrile, cellulose triacetate, and acrylic fibers. They create high-temperature disperse dyes, and most outdoor furniture fabric used disperse dyes, e.g., celliton fast pink B and celliton fast blue B.
Reactive dyes: These are reactive groups that react directly with the hydroxyl or amino group of the material’s fiber. The color is rapid and has a longer duration because of the irreversible chemical process. These are used to dye wool, nylon, silk, and cotton socks.
Azoic dyes: These dyes are unique dyes directly synthesized on the surface of the fiber. By controlling the diazoic and coupling reaction, the alkaline solution of phenol or naphthol is treated with a solution of diazotised amine to produce different shades. Because the chemicals used in the procedure are hazardous, this method of dyeing is not recommended. These are used to dye silk, nylon, cotton, polyester (e.g., nitroaniline red).
Vat dyes: These are water insoluble and cannot color any fabric directly. They are generally soluble in hot water although some of them can also be dissolved in modest volumes of Na2CO3. This class of dyes is used because of their affinity for cellulosic fibers and their application to nanofibers, which are first reduced to colorless leuco compounds by alkaline reducing agents like sodium hyposulphite and then by an oxidation reaction before being applied to the fabric. Indigo, also known as the blue in blue jeans, is the most used vat dye cotton fibers.
Mordant dyes: Mordant dyes, which are almost identical to azoic dyes, operate as a binding agent between the dye and the cloth, improving wash and light fastness. The mordant is commonly a heavy metal hydroxide compound. These dyes do not directly color a cloth; instead, they require a mordant. Dyes produce a variety of colors depending on the mordant employed, e.g., alizarin (a mordant dye) gives a red color with Al and Sn salts, black-violet with Fe mordant, and brownish red with Cr mordant.
Sulfur dyes: These dyes were applied to cotton textiles in darker colors. They may also be used to color leather. Sulfur dyes are water insoluble, highly colored chemicals that must be converted to water-soluble forms (lucoforms) before being utilized on textiles. Organic molecules are sulfurized to produce sulfur colors, which have a high molecular weight. To complete the conversion, a reducing agent, such as dilute aqueous Na2S, is used. Because the sulfur lucoform dye is vital to cellulosic materials (air exposure), oxidation is carried out using an oxidizing agent such as sodium dichromate.
3 Technologies for treating waste water
Wastewater treatment methods were initially created in reaction to the negative consequences of releasing untreated wastewater directly into the environment, as well as concerns about human health. This has resulted in the extinction of numerous species [39]. Our contemporary lifestyle makes use of a variety of luxury things to make our lives more pleasant and convenient, but it comes at a cost. Wastewater is a common result of our present lifestyle, and this wastewater is unfit for human consumption. Physical water treatment, chemical treatment, biological water treatment, and sludge treatment are four typical methods for treating wastewater. Solids are removed using methods such as screening, sedimentation, and skimming. There are no chemicals used in this method. Sedimentation, a process of suspending insoluble/heavy particles from wastewater, is one of the most used physical wastewater treatment processes. Once the insoluble matter has settled to the bottom, you can separate the clean water. Aeration is another excellent physical water treatment method. To deliver oxygen to the water, this procedure involves moving air through it. The third approach, filtration, is used to filter out pollutants by bypassing wastewater through specific filters that separate the contaminants and insoluble particles present in it. The most popular filter is the sand filter.
3.1 Preliminary treatment methods
This technology uses the removal of grits, rags, clarity, papers, coarse screening, and comminution of big materials, as well as the removal of gas and oil, all of which obstruct efficient wastewater treatment and reduce the biological oxygen requirement of the wastewater. Secondary treatment is the further handling of the discharge from primary treatment to eradicate the residual organics and suspended solids [40].
3.2 Secondary treatment method
It entails the removal of colloidal, biodegradable organic matter using aerobic treatment methods (such as activated sludge, aerated lagoons, membrane bioreactors, aerobic bioreactors, microalgae bioreactors, anaerobic bioreactors, vermifilters, trickling filters, rotating biological contactors, and biooxidation). Physical therapy is used in advanced wastewater treatment systems such as adsorption, sedimentation, reverse osmosis, coagulation-flocculation, ultrafiltration, microfiltration, and nanofiltration, and chemical reactions such as ion exchange, chemical precipitation, ozonation, photolysis, photocatalysis, solar-driven, photo-Fenton/electro-Fenton oxidation, and microbial degradation echnologies for the treatment of wastewater containing dyes and other contaminants such as heavy metals, radioactive materials, additional suspended solids, refractory organics, and dissolved materials [41].
3.3 Silica gel
Silica gel is most commonly used as adsorbent as well as in stationary phase in chromatography. Due to the presence of hydroxyl groups, the oxygen atoms visible on the surface of silica gel particles are coupled with protons, making the surface of silica gel extremely polar (Figure 2).
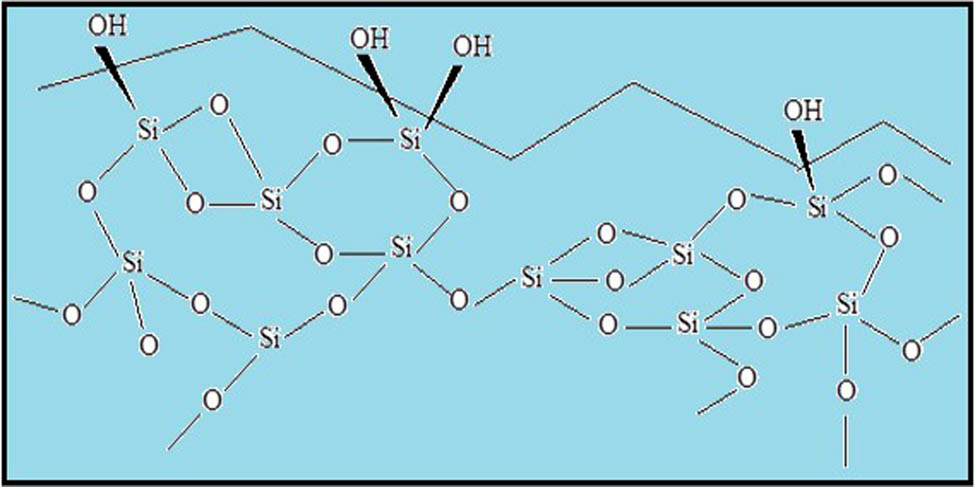
Silica gel chemical structure.
Thus, polar functionality in the analyte molecule can bind to silica gel strongly with the surface of the silica gel particle and weak interaction with nonpolar functionality. Polar functionality can bind the silica gel by two means: via dipole–dipole interactions and hydrogen bonding. Hence, a polar group interacting with the surface of the adsorbent will interact more strongly than an analyte that plays the same polar functionality.
3.4 Polymer-based adsorbents
In recent years, because of the wide range of porous structures of a particular chemical system that can develop within the framework, the development of polymer-based adsorbents served as an alternative source for natural and waste material-based adsorbents with better adsorption capacity with low-cost polymeric adsorbents. However, several other types of adsorbent materials have been exploited as low-cost alternatives; the key drawbacks of these adsorbents are their poor thermal and mechanical stabilities and weak interactions between separating molecules.
3.5 Polyaniline-modified silica gel nanocomposite preparation
Polyaniline-modified silica gel was made by simple in situ oxidative polymerization of aniline in the presence of varying quantities of silica gel using potassium persulfate as an oxidizing agent. In an aqueous HCl solution, the aniline monomer was dissolved (1 M). In the aniline solution, different quantities of silica gel were added. The aniline solution was then polymerized with potassium persulfate (5–6 g) and diluted in HCl (1 M) for 22 h, and the reaction mixture was continuously stirred [42,43]. The emeraldine salt-containing mixture gradually transformed into a greenish-black slurry, which was filtered, extensively washed with DDW, and undoped with 500 mL of 1 M ammonia solution to produce Pani@SG emeraldine base in a neutral form. To eliminate contaminants, the filtrate was washed with DDW and methanol until it was neutral and colorless. The nanocomposites were dried in an air oven at 70–80°C for 12 h before being transformed into fine powders for application [44,45].
3.6 Adsorbent-based polyaniline-silica gel
Polyaniline is one of the most promising alternating adsorbing materials now having a conducting nature due to the presence of –NH-groups on its conjugate structure. Polyaniline is one of the most important studied polymers as they are very easy to synthesize, have superior physicochemical characteristics, have the feasibility of doping, and have mechanical flexibility. Its monomer is readily available, and it is environmentally stable. Polyaniline are most extensively utilized for the chemical modification of silica to create adsorbents. Due to the presence of active groups, they interact with molecules of various pollutants prevalent in water bodies.
Pani has a number of distinct features, including ease of synthesis, mechanical and chemical flexibility, pH and temperature tolerance, polar groups, hydrophobicity, conjugated structure, and ion exchange capacity. The mechanism allows for the retention of multiple groups of analytes, both charged and uncharged cationic and anionic forms, and polyaniline was chosen to cover silica. The physicochemical characteristics of the sorbent, such as polyaniline, are derived from the fact that silica is a porous material with interior pores that are larger than outer pores. Pani@SG is used as an adsorbent in wastewater treatment. Adsorption of colors and heavy metals was shown to be successful using polyaniline silica gel-modified forms [46]. Pure and modified polymeric materials are now being explored as an adsorbent because of their excellent adsorption effectiveness from decontaminated water [47]. Adsorption is a frequently used method for removing heavy metals from wastewater because it is simple, inexpensive, and effective. The adsorbent is the most important component impacting performance in this process; as a result, major efforts have been undertaken to develop highly efficient and selective adsorbents with exceptional characteristics.
Polymeric hybrid materials are one of the most effective adsorbents for the adsorption of the dye molecule. As a result, scientists have increasingly concentrated on polymer-based materials as adsorbents for pollutant removal from wastewater. Different forms of polymeric materials [48] in their pure state or as demonstrated in the pernigraniline form of polyaniline are shown in Figure 3. Pernigraniline and emeraldine are two types of Pani that may be found as salts or bases.
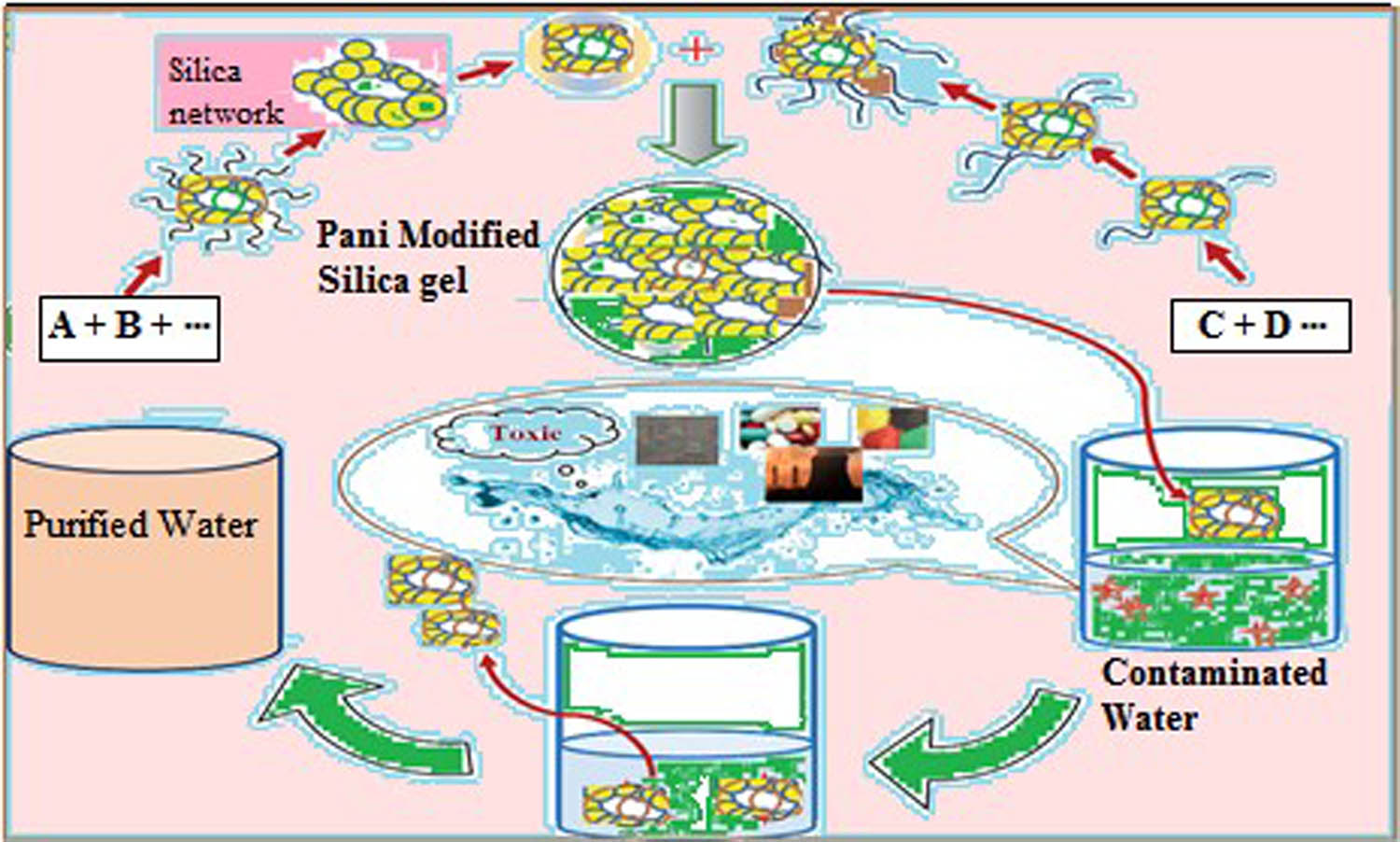
Overview of Pani@SG materials focuses on their adsorption strategies for water decontamination.
3.7 Methods of adsorption and choice of adsorbent
One of the most often used methods is adsorption, which is the effective and broadly accepted technique for the treatment of wastewater to drop off hazardous organic and inorganic materials in the form of waste matter due to its convenient, cheap, most effective, proficient, by-producing simple operational design and nontoxic method [49,50]. Dye elimination should possess a variety of attractive characteristics such as large surface area, porosity, and high adsorption capability to be a suitable adsorbent. The properties such as easy availability, mechanical stability, economically feasible, compatibility, ease of regeneration, eco-friendly, and high selectivity are needed to remove a wide range of dyes [51]. Organic dye adsorption mechanisms on polyaniline impregnated silica gel-based adsorbents. Various kind of functional groups present in adsorbent and adsorbate, electrostatic force of contact, textural and surface qualities of the adsorbent, dispersion or diffusion behavior of adsorbate towards adsorbent, and manner of their interaction all influence the adsorption mechanism [52].
In comparison to SG, Pani@SG was more effective in separating a mixture of organic dyes. It is easy to see how a modified silica gel surface causes a partial conversion of surface silanol groups into new organo-functional groups, giving the SG surface significantly different qualities than the original [53]. Silica gel lone pairs of oxygen interact with Pani polarons, and lone pairs of nitrogen interact with Pani polarons interact with silica hydrogen (Figure 4). The positive and negative centers of Pani with SG are produced that are capable of interacting with the dyes in the wastewater, and adsorption occurs [54]. Electrostatic interactions between the dye and the polymer, hydrogen bonding (between O, H, and N atoms in the dye molecule and N and H atoms in the polymer), and van der Waals forces (between the aromatic nature of the polymeric matrix and the aromatic rings of the dyes) are all responsible for differential dye migration, which eventually leads to adsorption [46]. There are three types of forces that contribute to this long-range interaction between polar molecules, which are collectively known as van der Waals interactions:
dipole–dipole (orientation) interactions,
dipole–induced dipole (induction) interactions,
induced dipole–induced dipole (dispersion) interactions.
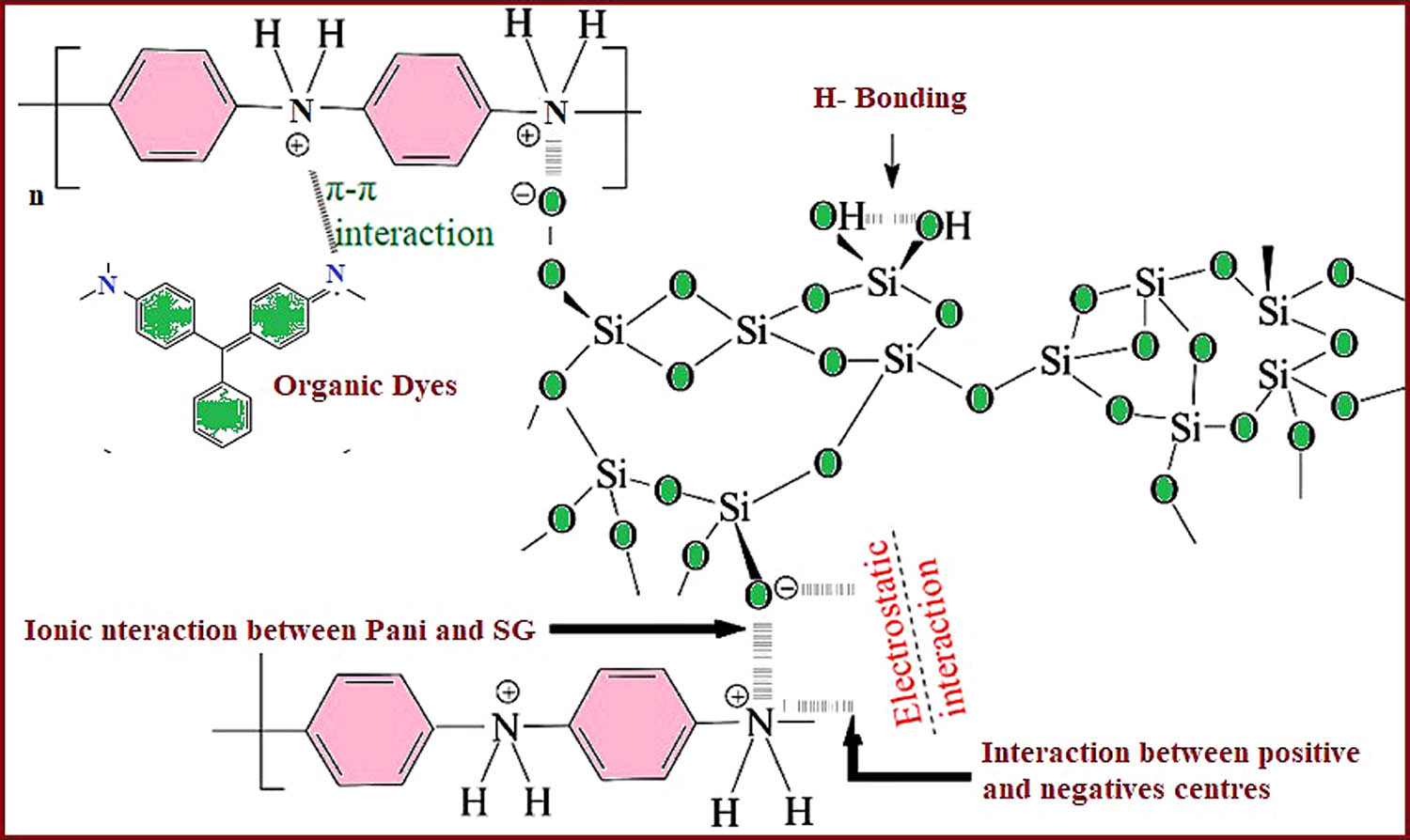
Mechanism of adsorption of organic dyes onto polyaniline-modified silica gel showing different interactions.
The principal adsorption mechanism of dyes on Pani-based silica adsorbents relies on strong electrostatic interactions between the dye-dissociated sulfonate groups and the Pani-based silica adsorbents protonated amino groups. Many studies show that the aforementioned technique works best in acidic environments where the overall “charge” of the Pani-based silica adsorbents is higher (due to the stronger protonation of amino groups at acidic pH values).
Adsorption of dye occurs by physisorption, chemisorption, or both depending on the type of mutual interaction between the adsorbate and adsorbent molecules. Dye adsorption on polyaniline-based materials is common in many instances. As a result, pH has a considerable impact on the total adsorption process (Figure 5).
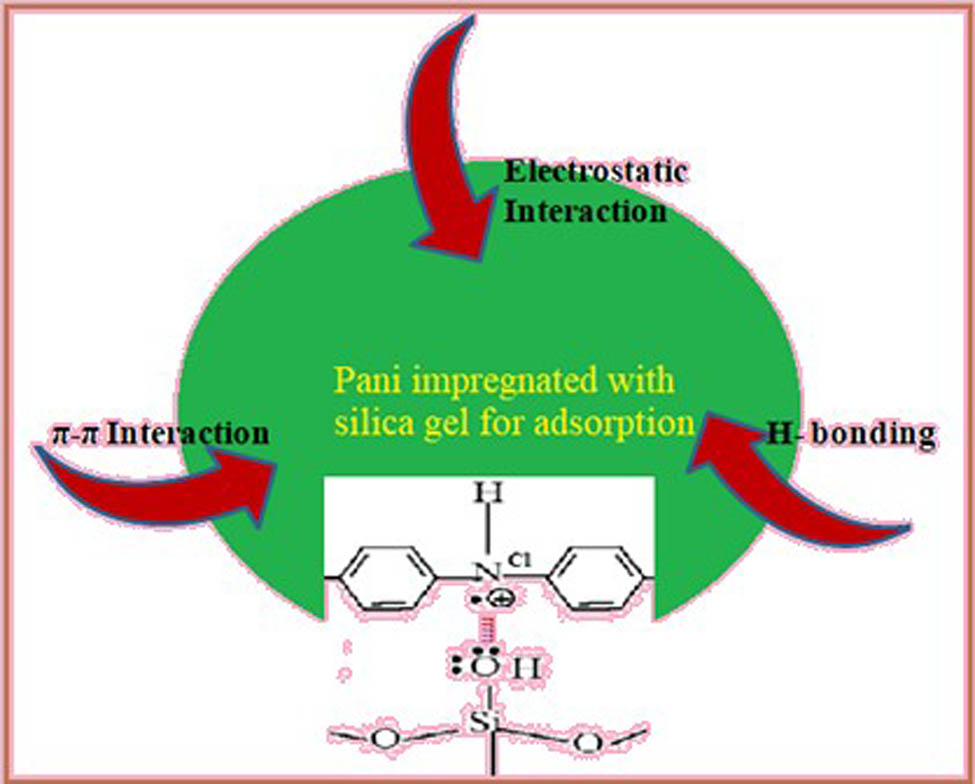
Scheme showing electrostatic interaction between Pani@SG possible adsorption mechanisms of dyes.
3.7.1 Solution pH
The adsorption capacity is affected by the pH of the solution. The experimental equilibrium adsorption capacity falls when the pH of the solution drops from 4 to 1.
3.7.2 Ionic strength
Ionic strength affects adsorption if increasing ionic strength decreases adsorption, and the potential in the plane of adsorption must be positive. Also, if increasing ionic strength increases adsorption, the potential must be negative.
3.7.3 Temperature
Temperature is a critical parameter. Adsorption decreases with the increasing temperature according to the adsorption principle, and molecules adsorbed earlier on a surface tend to desorb from the surface at higher temperature.
3.8 Adsorption kinetics and isotherms of adsorbents
The rate of adsorption and desorption kinetics are mathematical models that describe this behavior. Adsorption kinetic models are usually categorized into adsorption reaction models and adsorption diffusion models.
Adsorption isotherms mean different mathematical forms of kinetics rate equations have been developed. Compressing microporous or mesoporous materials into pellets of different shapes generally obtains adsorbent pellets. The different phases are involved in the diffusion of gas in an adsorbent. Hence, there are not only micro/mesopores in the pellets but also macropores.
4 Some other important adsorbents
4.1 Activated carbon
The surface adsorption of heavy metal ions is the principle of activated carbon adsorption. It has a surface area of 500–2,000 m2‧g−1. Activated carbon is often made from coal, lignite, coconut shells, and wood, and it is produced using both physical and chemical methods. Carbonized materials will be heated to high temperatures, resulting in long-short activated carbon. Adding chemical reagents to carbonized materials is a method of chemical preparation.
4.2 Chitosan
Chitin is the world’s second-largest biological polymer. Chitosan, which has a molecular structure comparable to cellulose, is more important than chitin. The adsorption ability of chitosan has gained a lot of interest recently. Chitin changed by alkali loses its deacetylation and becomes chitosan, which is found mostly in crab exoskeletons. Many scholars have been studying chitosan extensively in recent years. Someone used chitosan to remove cadmium for the first time in 1988, with an adsorption capability of 5.93 mg‧g−1 and a pH range of 4.0–8.3. As a result, the maximum absorbance of chitosan adsorption in the heavy metal study [8] is 815, 222, 164, and 75 mg‧g−1, and the maximum absorbance of chitosan adsorption Hg2+, Cu2+, Ni2+, Zn2+ is 815, 222, 164, and 75 mg‧g−1, respectively [55]. The greatest number of adsorption of chitosan is 430 mg‧g−1.
4.3 Zeolite
Zeolite is a common mineral adsorbent whose adsorption properties are determined by the ion exchange power. The zeolite structure has a lot of space and can do ion exchange with other metal ions. The original zeolite crystals are aluminate crystals, which are made up of oxygen atoms in a tetrahedron form. The zeolite cation exchange capacity has been examined by many scientists. The ability of zeolite to boost cation exchange capacity. NaOH treatment of zeolite improves adsorption efficiency, with its ability to adsorb Pb2+ and Cd2+ exceeding 100 mg‧g−1, which is higher than untreated zeolite.
4.4 Clay minerals
They can be used to make adsorbents. Humic acid can be prepared as an adsorbent with corresponding resins and can adsorb a wide range of heavy metal ions. The Freundlich adsorption process model was used to adsorb Cd2+ ions from coal [56] with an adsorption amount of 0.91 mg‧g−1. Because the soluble characteristic is low when pH > 10, the adsorption quantity of hydrogen and oxygen compounds decreases. The results show that using nitric acid for coal pretreatment can improve the adsorption ability, Hg2+ ions, and adsorption sites of adsorption reaction speed, adsorption occurred on the surface of coal, with pH between 7.0 and 8.5.
5 Regeneration performance of adsorbents
The adsorption effectiveness of the exhausted/spent adsorbent for water treatment can be improved through regeneration. To renew spent or dye-adsorbed a variety of procedures have been devised, including chemical treatment, thermal, photocatalytic, supercritical extraction, and biological degradation. Pani-based silica adsorbents have strong regeneration capacity in addition to good adsorption potential. Pani-based silica-modified adsorbent materials have superior regeneration and adsorption capabilities and selectivity when compared to other adsorbents, as well as being low cost and porous with a large surface area. These adsorbents have strong regeneration capacity in addition to good adsorption potential.
6 Conclusion and future outlook
Organic dye pollution has become a major environmental issue throughout the world as a result of the massive volume of manufacturing and use of organic pollutants during the last few decades. An extremely effective method for removing highly hazardous organic substances such as colors from water and wastewater has piqued curiosity. Adsorption is a cost-effective and efficient method for removing organic pollutants from water and wastewater. This chapter focused on the adsorption of organic pollutants using a polyaniline silica gel-based nanocomposite of natural and synthetic adsorbents. Because of its cationic character, the adsorption phenomenon is one of the most successful processes of adsorbate onto the polyaniline impregnated silica gel. Pani undergoes protonation, which can adsorb dye molecules through a variety of interacting mechanisms and demonstrates increased adsorption of an anionic dye. Polyaniline may be modified to vary its surface charge, making it useful for the adsorption of both cationic and anionic dyes. The ability of modified Pani to adsorb dyes from wastewater was improved, as was the adsorption efficiency due to increased porosity or improved interaction due to the formation of a new functional group, the number of active binding sites, and the ion exchange properties, all of which improved dye uptake. To evaluate the adsorption rate, adsorption mechanism, and efficiency of polyaniline composite in removing dyes from wastewater, various parameters such as pH of the solution, contact time between adsorbate and adsorbent, concentration and adsorption isotherm, temperature, kinetics, and thermodynamic study have been demonstrated. After conducting large-scale studies and assessing the operational costs of real-world wastewater treatment. this article takes a careful look into green adsorption. Many different topics are discussed, including (i) adsorption capacity, (ii) kinetic modeling (with the ultimate goal of scaling up) data to fixed-bed column calculations for designing/optimizing commercial processes), and (iii) critical techno-economical data of green adsorption processes to scale up experiments with economic analysis and perspectives of the use of green adsorbents. Polyaniline composites are effective adsorbent materials with numerous promising future profits due to their large effective surface area, high adsorption efficiency, low cost, high selectivity, easy accessibility, appropriate pore size and volume, compatibility, reusability, high mechanical, chemical, environmentally friendly, and thermal stability, which can be demonstrated in future research.
-
Funding information: The authors state no funding involved.
-
Author contributions: Mahfoozurrahman Khan: writing – original draft, writing – review and editing, methodology, formal analysis; Syed Wazed Ali: writing – original draft, formal analysis, visualization; Mohammad Shahadat: formal analysis, data curation, visualization, and validation; Suresh Sagadevan: formal analysis, data curation, validation and reviewing, and editing.
-
Conflict of interest: One of the corresponding authors (Suresh Sagadevan) is a member of the Editorial Board of Green Processing and Synthesis.
References
[1] Ali I, Aboul-Enein HY. Chiral pollutants: Distribution, toxicity and analysis by chromatography and capillary electrophoresis. John Wiley & Sons; 2004.10.1002/0470867825Search in Google Scholar
[2] Santhosh C, Velmurugan V, Jacob G, Jeong SK, Grace AN, Bhatnagar A. Role of nanomaterials in water treatment applications: a review. Chem Eng J. 2016;306:1116–37.10.1016/j.cej.2016.08.053Search in Google Scholar
[3] Rezakazemi M, Khajeh A, Mesbah M. Membrane filtration of wastewater from gas and oil production. Environ Chem Lett. 2018;16(2):367–88.10.1007/s10311-017-0693-4Search in Google Scholar
[4] Ali I, Khan TA, Asim M. Removal of arsenic from water by electrocoagulation and electrodialysis techniques. Sep & Purif Rev. 2011;40(1):25–42.10.1080/15422119.2011.542738Search in Google Scholar
[5] Saleh TA, Gupta VK. Column with CNT/magnesium oxide composite for lead (II) removal from water. Environ Sci Pollut Res. 2012;19(4):1224–8.10.1007/s11356-011-0670-6Search in Google Scholar PubMed
[6] Gupta VK, Nayak A. Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem Eng J. 2012;180:81–90.10.1016/j.cej.2011.11.006Search in Google Scholar
[7] Chong MN, Jin B, Chow CW, Saint C. Recent developments in photocatalytic water treatment technology: a review. Water Res. 2010;44(10):2997–3027.10.1016/j.watres.2010.02.039Search in Google Scholar PubMed
[8] Pendergast MM, Hoek EM. A review of water treatment membrane nanotechnologies. Energy & Environ Sci. 2011;4(6):1946–71.10.1039/c0ee00541jSearch in Google Scholar
[9] Gayathiri E, Prakash P, Selvam K, Awasthi MK, Gobinath R, Karri RR, et al. Plant microbe based remediation approaches in dye removal: A review. Bioengineered. 2022;13(3):7798–828.10.1080/21655979.2022.2049100Search in Google Scholar PubMed PubMed Central
[10] Khan FSA, Mubarak NM, Tan YH, Khalid M, Karri RR, Walvekar R, et al. A comprehensive review on magnetic carbon nanotubes and carbon nanotube-based buckypaper-heavy metal and dyes removal. J Hazard Mater. 2021;413:125375.10.1016/j.jhazmat.2021.125375Search in Google Scholar PubMed
[11] Qureshi SS, Shah V, Nizamuddin S, Mubarak NM, Karri RR, Dehghani MH, et al. Microwave-assisted synthesis of carbon nanotubes for the removal of toxic cationic dyes from textile wastewater. J Mol Liq. 2022;356:119045.10.1016/j.molliq.2022.119045Search in Google Scholar
[12] Khan FSA, Mubarak NM, Tan YH, Karri RR, Khalid M, Walvekar R, et al. Magnetic nanoparticles incorporation into different substrates for dyes and heavy metals removal—A review. Environ Sci Pollut Res. 2020;27(35):43526–41.10.1007/s11356-020-10482-zSearch in Google Scholar
[13] Christie RM. Colour chemistry. Cambridge: Royal Society of Chemistry; 2001. 10.1039/9781847550590.Search in Google Scholar
[14] Dehghani MH, Omrani GA, Karri RR. Solid Waste – Sources, toxicity, and their consequences to human health. Book Chapter 11 – Soft computing techniques in solid waste and wastewater management. Elsevier; 2021. p. 205–13.10.1016/B978-0-12-824463-0.00013-6Search in Google Scholar
[15] Karri RR, Ravindran G, Dehghani MH. Wastewater—sources, toxicity, and their consequences to human health. Book Chapter 1 – Soft computing techniques in solid waste and wastewater management. Elsevier; 2021. p. 3–33.10.1016/B978-0-12-824463-0.00001-XSearch in Google Scholar
[16] Fricke J, Tillotson T. Aerogels: production, characterization, and applications. Thin Solid Films. 1997;297(1–2):212–23.10.1016/S0040-6090(96)09441-2Search in Google Scholar
[17] Fang WZ, Zhang H, Chen L, Tao WQ. Numerical predictions of thermal conductivities for the silica aerogel and its composites. Appl Therm Eng. 2017;115:1277–86.10.1016/j.applthermaleng.2016.10.184Search in Google Scholar
[18] Gurav JL, Rao AV, Nadargi DY, Park HH. Ambient pressure dried TEOS-based silica aerogels: good absorbents of organic liquids. J Mater Sci. 2010;45(2):503–10.10.1007/s10853-009-3968-8Search in Google Scholar
[19] Deb A, Debnath A, Saha B. Sono-assisted enhanced adsorption of eriochrome Black-T dye onto a novel polymeric nanocomposite: kinetic, isotherm, and response surface methodology optimization. J Dispers Sci Technol. 2021;42(11):1579–92.10.1080/01932691.2020.1775093Search in Google Scholar
[20] Deb A, Debnath A, Bhowmik K, Rudra Paul S, Saha B. Application of polyaniline impregnated mixed phase Fe2O3, MnFe2O4 and ZrO2 nanocomposite for rapid abatement of binary dyes from aqua matrix: response surface optimisation. Int J Environ Anal Chem. 2021;101:1–19.10.1080/03067319.2021.1946683Search in Google Scholar
[21] Das P, Nisa S, Debnath A, Saha B. Enhanced adsorptive removal of toxic anionic dye by novel magnetic polymeric nanocomposite: optimization of process parameters. J Dispers Sci Technol. 2020;43(6):880–95.10.1080/01932691.2020.1845958Search in Google Scholar
[22] Deb A, Debnath A, Bhattacharjee N, Saha B. Ultrasonically enhanced dye removal using conducting polymer functionalised ZnO nanocomposite at near neutral pH: kinetic study, isotherm modelling and adsorbent cost analysis. Int J Environ Anal Chem. 2020;100:1–20.10.1080/03067319.2020.1843649Search in Google Scholar
[23] Das P, Debnath A, Saha B. Ultrasound‐assisted enhanced and rapid uptake of anionic dyes from the binary system onto MnFe2O4/polyaniline nanocomposite at neutral pH. Appl Organomet Chem. 2020;34(8):e5711.10.1002/aoc.5711Search in Google Scholar
[24] Anirudhan TS, Suchithra PS. Heavy metals uptake from aqueous solutions and industrial wastewaters by humic acid-immobilized polymer/bentonite composite: kinetics and equilibrium modeling. Chem Eng J. 2010;156(1):146–56.10.1016/j.cej.2009.10.011Search in Google Scholar
[25] Carvallho MN, da Silva KS, Sales DC, Freire EM, Sobrinho MA, Ghislandi MG. Dye removal from textile industrial effluents by adsorption on exfoliated graphite nanoplatelets: kinetic and equilibrium studies. Water Sci Technol. 2016;73(9):2189–98.10.2166/wst.2016.073Search in Google Scholar PubMed
[26] Ansari R, Mosayebzadeh Z. Application of polyaniline as an efficient and novel adsorbent for azo dyes removal from textile wastewaters. Chem Pap. 2011;65(1):1–8.10.2478/s11696-010-0083-xSearch in Google Scholar
[27] Ado A, Yahaya H, Kwalli AA, Abdulkadir RS. Dyeing of textiles with eco-friendly natural dyes: a review. Int J Environ Monit Prot. 2014;1(5):76–81.Search in Google Scholar
[28] Prabhu KH, Bhute AS. Plant based natural dyes and mordants: A Review. J Nat Prod Plant Resour. 2012;2(6):649–64.Search in Google Scholar
[29] Hamdy DM, Hassabo AG. Various natural dyes from different sources. J Textiles Coloration Polym Sci. 2021;18(2):171–90.10.21608/jtcps.2021.79786.1066Search in Google Scholar
[30] Mahbubul Bashar M, Khan MA. An overview on surface modification of cotton fiber for apparel use. J Polym Environ. 2013;21(1):181–90.10.1007/s10924-012-0476-8Search in Google Scholar
[31] Molinari R, Lavorato C, Argurio P. Recent progress of photocatalytic membrane reactors in water treatment and in synthesis of organic compounds. A review. Catal Today. 2017;281:144–64.10.1016/j.cattod.2016.06.047Search in Google Scholar
[32] Venil CK, Zakaria ZA, Ahmad WA. Bacterial pigments and their applications. Process Biochem. 2013;48(7):1065–79.10.1016/j.procbio.2013.06.006Search in Google Scholar
[33] Nagendrappa G. Sir William Henry Perkin: the man and his ‘mauve’. Resonance. 2010;15(9):779–93.10.1007/s12045-010-0088-3Search in Google Scholar
[34] Khan M. Preparation, characterization and applications of polyaniline impregnated silica gel for the identification and separation of some important organics. PhD thesis, Aligarh Muslim University; 2018.Search in Google Scholar
[35] Hunger K, editor. Industrial dyes: chemistry, properties, applications. John Wiley & Sons; 2007.Search in Google Scholar
[36] Leslie E. Synthetic worlds: nature, art and the chemical industry. Reaktion Books; 2006.Search in Google Scholar
[37] Ramesh M, Manavalaramanujam R. Alterations in lactate dehydrogenase activity induced by dye effluent in a freshwater fish Labeo Rohita. Pollution Res. 1993;12(2):105–8.Search in Google Scholar
[38] Inbaraj BS, Selvarani K, Sulochana N. Evaluation of a carbonaceous sorbent prepared from Pearl Millet Husk for its removal of basic dyes. J Sci Ind Res. 2002;61:971–8.Search in Google Scholar
[39] Sonune A, Ghate R. Developments in wastewater treatment methods. Desalination. 2004;167:55–63.10.1016/j.desal.2004.06.113Search in Google Scholar
[40] Stasinakis AS, Thomaidis NS, Arvaniti OS, Asimakopoulos AG, Samaras VG, Ajibola A, et al. Contribution of primary and secondary treatment on the removal of benzothiazoles, benzotriazoles, endocrine disruptors, pharmaceuticals and perfluorinated compounds in a sewage treatment plant. Sci Total Environ. 2013;463:1067–75.10.1016/j.scitotenv.2013.06.087Search in Google Scholar PubMed
[41] Ahmed MB, Zhou JL, Ngo HH, Guo W, Thomaidis NS, Xu J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: a critical review. J Hazard Mater. 2017;323:274–98.10.1016/j.jhazmat.2016.04.045Search in Google Scholar PubMed
[42] Khan M, Mohammad A, Ullah Q, Mohammad F. Polyaniline modified silica gel coupled with green solvent as eco favourable mobile phase in thin layer chromatographic analysis of organic dyes. Appl Chem Eng. 2021;4(1):41–55.10.24294/ace.v4i1.747Search in Google Scholar
[43] Mohammad A, Khan M, Mobin R, Mohammad F. A new thin-layer chromatographic system for the identification and selective separation of brilliant blue food dye: Application of a green solvent. JPC-J Planar Chromatograp-Modern TLC. 2016;29(6):446–52.10.1556/1006.2016.29.6.7Search in Google Scholar
[44] Khan M, Anwer T, Mohammad F. Sensing properties of sulfonated multi-walled carbon nanotube and graphene nanocomposites with polyaniline. J Sci Adv Mater Devices. 2019;4(1):132–42.10.1016/j.jsamd.2019.02.002Search in Google Scholar
[45] Khan M, Anwer T, Mohammad F. Sulphonated polyaniline/MWCNTs nanocomposite: preparation and promising thermoelectric performance. Int Nano Lett. 2018;8(3):213–20.10.1007/s40089-018-0246-2Search in Google Scholar
[46] Mohammad A, Khan M, Ullah Q, Mohammad F. Effective separation of organic dyes using ionic liquids as green mobile phase and polyaniline-modified silica gel nanocomposite-based thin-layer chromatography. J Anal Sci Technol. 2017;8(1):1–14.10.1186/s40543-017-0127-8Search in Google Scholar
[47] Pan B, Pan B, Zhang W, Lv L, Zhang Q, Zheng S. Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem Eng J. 2009;151(1–3):19–29.10.1016/j.cej.2009.02.036Search in Google Scholar
[48] Nasar A. Polyaniline (PANI) based composites for the adsorptive treatment of polluted water. Smart Polym Compos. 2018;21:41–64.10.21741/9781945291470-2Search in Google Scholar
[49] Lee JW, Choi SP, Thiruvenkatachari R, Shim WG, Moon H. Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dye Pigment. 2006;69(3):196–203.10.1016/j.dyepig.2005.03.008Search in Google Scholar
[50] Raval NP, Shah PU, Shah NK. Adsorptive amputation of hazardous azo dye Congo red from wastewater: a critical review. Environ Sci Pollut Res. 2016;23(15):14810–53.10.1007/s11356-016-6970-0Search in Google Scholar
[51] Yagub MT, Sen TK, Afroze S, Ang HM. Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interfac. 2014;209:172–84.10.1016/j.cis.2014.04.002Search in Google Scholar
[52] Zare EN, Motahari A, Sillanpää M. Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: a review. Environ Res. 2018;162:173–95.10.1016/j.envres.2017.12.025Search in Google Scholar
[53] Shimada T, Aoki K, Shinoda Y, Nakamura T, Tokunaga N, Inagaki S, et al. Functionalization on silica gel with allylsilanes. A new method of covalent attachment of organic functional groups on silica gel. J Am Chem Soc. 2003;125(16):4688–9.10.1021/ja034691lSearch in Google Scholar
[54] Ahmad S, Sultan A, Raza W, Muneer M, Mohammad F. Boron nitride based polyaniline nanocomposite: Preparation, property, and application. J Appl Polym Sci. 2016;133(39):89–97.10.1002/app.43989Search in Google Scholar
[55] Maleki A, Pajootan E, Hayati B. Ethyl acrylate grafted chitosan for heavy metal removal from wastewater: Equilibrium, kinetic and thermodynamic studies. J Taiwan Inst Chem Eng. 2015;51:127–34.10.1016/j.jtice.2015.01.004Search in Google Scholar
[56] Lakatos J, Brown SD, Snape CE. Coals as sorbents for the removal and reduction of hexavalent chromium from aqueous waste streams. Fuel. 2002;81(5):691–8.10.1016/S0016-2361(01)00159-4Search in Google Scholar
© 2022 Mahfoozurrahman Khan et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

