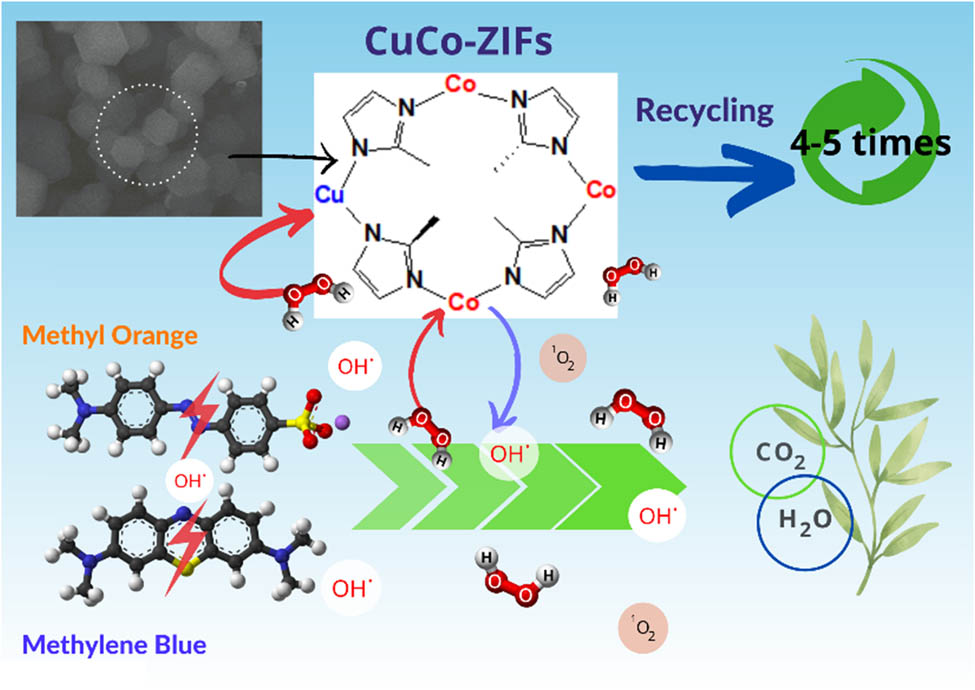
Abstract
In this study, the synthesized CuCo-zeolitic imidazolate framework (ZIF) catalyst was used to degrade methyl orange (MO) and methylene blue (MB) in water via a novel Fenton-like catalytic reaction. Effects of catalyst dosage, H2O2 concentration, initial concentration of the contaminants, and reaction time were evaluated. The results showed that MO and MB decomposition efficiencies were highly influenced by CuCo-ZIF concentration. The presence of H2O2 accelerated the degradation reaction of both MO and MB. Although it took 100 min to complete the removal of MB, it was 60 min for MO. At concentrations of MO and MB lower than 40 mg·L−1, the catalyst showed an almost complete degradation. The CuCo-ZIF catalyst presented a good recyclability with more than 90% removal of MO and MB after four times and five times reuse, respectively. These results demonstrated that MO and MB were efficiently degraded by a Fenton-like catalyst of CuCo-ZIFs and its potential in industrial wastewater treatment.
Graphical abstract
1 Introduction
Dyes are currently one of the most used substances and have been applied in many industries such as textiles, leather, paint, plastics, and paper. However, the excess number of dyes that were directly discharged into the environment is one of the main reasons for water pollution and human health problems. Dyes can cause several allergic, mutagenic, and carcinogenic effects on human body organs, namely kidneys, liver, reproductive system, or neurological system [1,2]. Furthermore, these dyes are nonbiodegradable molecules with complex aromatic structures that give optical, thermal, and physico-chemical stability [3,4,5,6].
Methyl orange (MO) and methylene blue (MB) are two dyes that are detected in large quantities in textile wastewater [7]. MO is an anionic dye, contains the stable double bond N═N in structure and has been widely used in printing, textile, food, and pharmaceutical industries, whereas MB is a cationic dye with adjacent aromatic rings and used as a dying material for wool, cotton, and silk [8]. They are both toxic and cause many harmful effects, such as cyanosis, vomiting, quadriplegia, shock, jaundice, and tissue necrosis in the human body [9] (Figure 1).
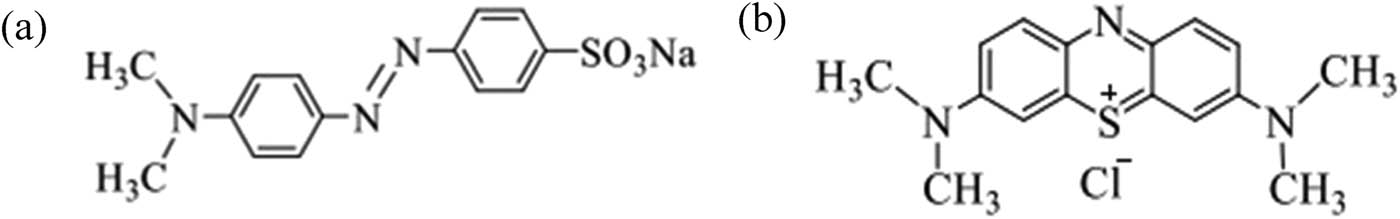
Chemical structures of (a) MO and (b) MB.
Currently, there are many investigated methods to treat the dye-polluted water such as adsorption, electrochemical oxidation, reverse osmosis, and coagulation [10]. Among these methods, adsorption is the cheapest and most straightforward of all these techniques; however, some adsorbents are expensive [11], and it does not degrade the dyes but just maintains the nondegradable pollutants on the active sites; therefore, desorption process is also required, which is costly. As a result, it is critical to creating a simpler and more efficient approach for removing dyes from aqueous solutions. Another efficient method is Fenton catalysis, which is based on the advanced oxidation processes, using metals or metal oxides as a reactive center to activate the oxidant agents and then generate the strong oxidant radicals [12]. The Fenton-like heterogeneous catalysis has much more advantages, such as environmental friendliness, energy efficiency [13], and recyclability [14], and is widely applied in degradation of many kinds of persistent organic pollutants such as dyes [15,16], fertilizers [17], benzene and its derivatives [18], and medicines [19].
Metal–organic frameworks (MOFs) is a family of crystalline porous materials, which contains both metal ions and organic linkers, and possesses unique properties such as high surface areas [20] and excellent thermal and chemical stabilities [21]. Zeolitic imidazolate frameworks (ZIFs), a typical class of MOFs, are usually constructed from the tetrahedrally coordinated divalent cations (Zn2+ or Co2+) linked by the imidazolate ligands with sodalite-type cage-like zeolites [22]. ZIF-67 is a general-applied member of ZIFs due to its good stability, excellent pore sizes and surface areas [23], and highly applicable potential in sensing, gas separation and storage, and adsorption [23,24,25,26]. The modification of ZIF-67 is also interesting to many scientists for advanced applications such as batteries or catalysts [27,28,29]. The creation of bimetallic ZIFs by adding another metal into ZIFs has been studied because of the significant increase of catalysis activity and the improvement of material stability [30,31,32]. Copper ion (Cu2+) is a divalent metal cation, which is currently used as a metal precursor, combining with cobalt cation (Co2+) to generate the CuCo-ZIFs. Because the ionic radius of Cu2+ and Co2+ in the tetrahedral arrangement are nearly equal (0.73 Å [33] and 0.72 Å [34]), it is available for random substitution of Cu2+ ions into Co2+ nodes in the structure of ZIF-67, in which the 2-methylimidazole (2-MIm) ligands form a bridge with metal ions at the position of the N atom to form a porous crystal structure (Figure 2) and remain sodalite structure (SOD). The Cu-doped phase integrated both structural features and functions of ZIF-67 [35], and it showed a high gas uptake capacity and highly efficient photocatalytic for MO degradation [23], and it is used as a selective catalyst for organic reactions [36].
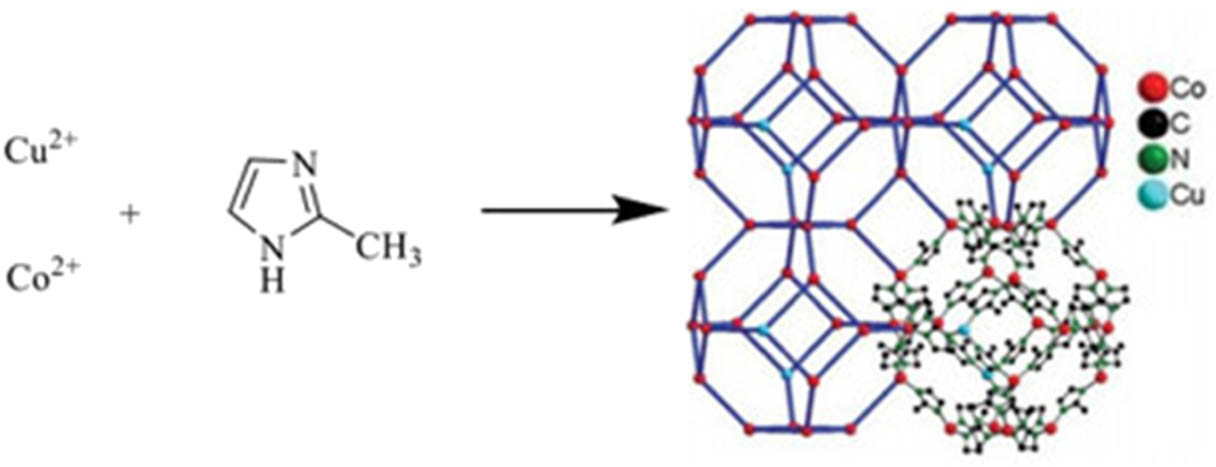
Components and SOD-type framework structure of CuCo-ZIFs.
In this study, CuCo-ZIFs was successfully synthesized in ethanol by ultrasound method and applied as a novel Fenton-like heterogeneous catalyst for the removal of MO and MB from aqueous solutions.
2 Materials and methods
2.1 Materials
2-MIm (C4H6N2, 99%) was purchased from Acros (Acros Organics, ThermoFisher Scientific). Cobalt(ii) nitrate hexahydrate (Co(NO3)2·6H2O, 99%), copper(ii) nitrate trihydrate (Cu(NO3)2·3H2O, 99%), and ethanol (C2H5OH, 99.5%) were purchased from Xilong Chemical Co., Ltd, China. All the reagents were used as received without further purification. Zeolite, activated carbon, ZIF-8, ZIF-67, and Ag/ZIF-67 were used as heterogeneous catalysts.
2.2 CuCo-ZIF catalyst preparation
Catalyst of CuCo-ZIFs was prepared by the ultrasound method at an ambient temperature in ethanol solvent as reported in the previous process [37]. To this end, Co(NO3)2·6H2O (0.582 g, 2 mmol), Cu(NO3)2·3H2O (0.483 g, 2 mmol), and 2-MIm (1.314 g, 16 mmol) were separately dissolved in ethanol (10 mL for each precursor). Subsequently, copper nitrate solution was dropped to the cobalt nitrate solution and stirred for 10 min using a magnetic stirrer to form a homogeneous mixture. Then, the homogeneous mixture was slowly dropped into the 2-MIm solution under magnetic stirring to form a purple suspension. This mixture was subjected to ultrasound for 15 min and kept at ambient temperature for 24 h. The purple precipitates were then obtained by centrifugation of 6,000 rpm within 15 min. The purple solid was immersed in 10 mL of ethanol for 4 h and then the dispersing was removed before adding the same amount of new ethanol into the sample. This step was repeated for ten times to completely remove all impurities. Finally, the sample was dried at 60°C to obtain the CuCo-ZIFs catalyst.
2.3 Degradation of MO and MB
The degradation experiments of MO and MB were conducted to illustrate the catalytic activity of CuCo-ZIFs. In the degradation experiment of MO, different dosages of CuCo-ZIF catalyst (0–250 mg·L−1) were added into 10 mL MO solution at various initial concentrations (10–70 mg·L−1) with the presence of different amount of H2O2 (0–0.25 mol·L−1). The kinetics experiment of this reaction was conducted and sampled at different time intervals (5–90 min). The residual concentration of MO was determined by the UV-visible (UV-Vis) spectrophotometer at a wavelength of 464 nm.
The degradation experiments of MB were also run in the identical procedure but at different initial concentrations of MB (10–50 mg·L−1) and sampling time intervals (5–140 min). The residual amount of MB in solution was analyzed by UV-Vis spectrophotometer (Shimadzu UV-1800 Spectrophotometer) at a wavelength of 664 nm.
The removal efficiency of MO and MB was defined as the following equation:
where C 0 is the initial concentration of MO or MB (mg·L−1), and C e is the residual concentration of MO or MB (mg·L−1).
2.4 Reusability of CuCo-ZIFs
To evaluate the reusability of CuCo-ZIF catalyst in the removal of MO and MB, the catalysis experiments were conducted at the optimal conditions of degradation reaction of MO and MB. Then the catalyst material was recovered by centrifugation, washed several times with ethanol, and then dried at 60°C in 24 h and reused. This procedure was repeated, and the recovered catalyst at the last run was analyzed by powder X-ray diffraction (PXRD) and Fourier transform-infrared (FT-IR) spectroscopy to assess its structural stability.
3 Results and discussion
The synthesized catalyst of CuCo-ZIFs was analyzed by using several characterization techniques, namely powder X-ray diffraction (PXRD), scanning electron microscope (SEM), energy dispersive X-ray (EDX), nitrogen adsorption–desorption isotherm, fourier-transform infrared spectroscopy (FT-IR), and thermogravimetric analysis (TGA) (Figures A1–A6 in Appendix). The results showed the successful preparation of CuCo-ZIF bimetallic framework. The novel Fenton-like reaction of the prepared catalytic of CuCo-ZIFs was proved by MO and MB degradation in the presence of hydrogen peroxide.
3.1 Degradation of MO and MB using a novel Fenton-like CuCo-ZIF catalyst
The catalyst dosage is critical because it plays a catalytic function in the activation of H2O2 to produce ·OH free radicals – a reducing agent for the MO and MB removal process. To determine the role of CuCo-ZIFs in the removal of both contaminants, a series of experiments were conducted with different catalyst dosages from 0 to 250 mg·L−1.
Removal yields of both MO and MB with variation of CuCo-ZIF dosage from 0 to 250 mg·L−1 are shown in Figure 3. Generally, removal yields of both dyes significantly increased with increasing CuCo-ZIF dosage. At catalyst dosage of zero, the yields of MO and MB removal were low, at 6.8% and 17.2%, respectively. The reason could be due to the instability and self-degradation of H2O2 into ·OH that could attack the MO and MB molecules, which caused the discoloring of dyes. As the catalyst dosage increased to 50 mg·L−1, the removal yield markedly increased to 91.8% for MO and 97.2% for MB. The highest yield could be achieved at 99.3% for MO and 97.2% for MB as catalyst dosage were at 100 mg·L−1. The above result showed the essential role of the catalyst in the removal process of MO and MB. The active sites Cu accelerated the decomposition of H2O2, leading to an increase of reactive oxygen species (·OH). This active species was a strong oxidant and was attributed to crack down the structure of both contaminants. However, this radical was not selective, so it attacked itself to form H2O (Eq. 2) and HO2 · (Eq. 3), leading to reduce the ·OH radicals and then decrease the contaminants removal yields. This phenomenon can be observed as catalyst dosage increased from 100 to 250 mg·L−1 and the degradation yield slightly decreased to 97.4% for MO and 96.7% for MB. Besides the above explanation, another reason that should be accounted for is the reduction of total surface area of the catalyst. When the high amount of catalyst dosage dispersed into the solution, those microparticles and nanoparticles tended to create the aggregation of such particles that limited the total active sites on the catalyst surface. Hence, to maintain a high removal efficiency in the following experiments, CuCo-ZIF dosage was chosen at 100 mg·L−1 for MO and 50 mg·L−1 for MB.
![Figure 3
Effect of catalyst dosage on the removal efficiency of MO and MB. MO: initial pH 7.0, [H2O2] = 0.2 mol·L−1, initial [MO] = 10 mg·L−1, time = 60 min, and ambient temperature. MB: initial pH 3.0, [H2O2] = 0.2 mol·L−1, initial [MB] = 10 mg·L−1, time = 60 min, and ambient temperature.](/document/doi/10.1515/gps-2022-0006/asset/graphic/j_gps-2022-0006_fig_003.jpg)
Effect of catalyst dosage on the removal efficiency of MO and MB. MO: initial pH 7.0, [H2O2] = 0.2 mol·L−1, initial [MO] = 10 mg·L−1, time = 60 min, and ambient temperature. MB: initial pH 3.0, [H2O2] = 0.2 mol·L−1, initial [MB] = 10 mg·L−1, time = 60 min, and ambient temperature.
H2O2 is the main factor that directly attacks contaminants by producing the ·OH radicals; hence, it is necessary to determine the effect of H2O2 concentration on the degradation performance. To study the effect of H2O2 concentration on the MO and MB removal efficiency, the variation of H2O2 concentration was conducted from 0 to 0.25 mol·L−1, and the results are shown in Figure 4.
![Figure 4
Effect of H2O2 concentration on the removal efficiency. MO: initial pH 7.0, catalyst dosage = 100 mg·L−1, initial [MO] = 10 mg·L−1, time = 60 min, and ambient temperature. MB: initial pH 3.0, catalyst dosage = 50 mg·L−1, initial [MB] = 10 mg·L−1, time = 60 min, and ambient temperature.](/document/doi/10.1515/gps-2022-0006/asset/graphic/j_gps-2022-0006_fig_004.jpg)
Effect of H2O2 concentration on the removal efficiency. MO: initial pH 7.0, catalyst dosage = 100 mg·L−1, initial [MO] = 10 mg·L−1, time = 60 min, and ambient temperature. MB: initial pH 3.0, catalyst dosage = 50 mg·L−1, initial [MB] = 10 mg·L−1, time = 60 min, and ambient temperature.
In the absence of H2O2, the removal efficiency of MO and MB were at 20.28% and 26.68%, respectively. The reason could be the adsorption capacity of CuCo-ZIFs due to its porous crystal structure and high surface area (1,555 m²·g−1). However, the low yields of adsorption mechanisms are not the main purpose of this research, and thus H2O2 was added before the adsorption occurred. When the H2O2 concentration increased from 0 to 0.25 mol·L−1, the MO and MB removal tended to increase, except for the MO removal at 0.25 mol·L−1 of H2O2. When the concentration of H2O2 varied from 0.05 to 0.2 mol·L−1, the removal efficiency increased from 64.57% to 98.53% for MO and from 62.90% to 96.89% for MB. At 0.25 mol·L−1 of H2O2, the removal efficiency of MO dropped down to 90.08%, whereas the MB removal efficiency maintained at 96.89%. This result can be attributed to the nonselectivity of ·OH. When a great amount of free radicals is generated but there is not enough time for MO degradation, these radicals not only attack H2O2 but also themselves, thus reducing the removal process. For MB removal, the efficiency kept constant at 96.89% when increasing the H2O2 concentration from 0.2 to 0.25 mol·L−1. These results showed that the addition of a higher concentration of H2O2 did not improve the removal efficiency. Therefore, the optimum H2O2 concentration for MO and MB removal were, respectively, found at 0.2 and 0.1 mol·L−1 for further experiments.
Determination of the initial organic dye concentration is required because in the wastewater, this concentration is also varied. The initial measured pH values of MO and MB at different initial concentrations were 7.0 and 3.0, respectively. The initial dye concentration range varied as highest as possible to measure the exceptional catalytic activity of CuCo-ZIFs. The results were depicted in Figure 5 with an initial concentration in a range of 10–70 mg·L−1 of MO (Figure 5a) and in a range of 10–50 mg·L−1 of MB (Figure 5b). In Figure 5a, while the removal of MO remained unchanged at about 97–98% in a range of 10–50 mg·L−1 and dropped down to 85% and 71.84% at 60 and 70 mg MO·L−1, respectively. Similarly, in Figure 5b, the removal of MB presented a high efficiency at 97–98% as initial concentration of MB varied from 10 to 40 mg MB·L−1 and significantly reduced to 88.1% at 50 mg MB·L−1. Basically, at the high concentration of dyes, more reaction between dye molecules and active centers was required to maintain the high removal efficiency. However, when there are more dye molecules dispersed into solution, they dominate the whole space of solution and are covered and adsorbed on the surface of catalyst particles. Therefore, the active sites were limited to interact with H2O2 leading to reduced reactive oxygen species. This could cause a decrease in degradation efficiency of both MO and MB at a very high concentration. One more reason is that 60 min is not enough for the catalyst to generate a large amount of free radicals for the degradation reaction. The different structures between MO and MB also led to the different behaviors of two dyes at high concentration, when MO structure contains only two aromatic rings whereas MB has three adjacent rings, which made MB structure more stable and then lower degrading yield compared to MO at the same concentration. Then, initial concentrations of 50 mg MO·L−1 and 40 mg MB·L−1 were chosen as optimum dye concentration for further experiments.
![Figure 5
Effect of initial concentration of (a) MO and (b) MB on the removal efficiency. MO: initial pH 7.0, catalyst dosage = 100 mg·L−1, [H2O2] = 0.2 mg·L−1, time = 60 min, and ambient temperature. MB: initial pH 3.0, catalyst dosage = 50 mg·L−1, [H2O2] = 0.1 mg·L−1, time = 60 min, and ambient temperature.](/document/doi/10.1515/gps-2022-0006/asset/graphic/j_gps-2022-0006_fig_005.jpg)
Effect of initial concentration of (a) MO and (b) MB on the removal efficiency. MO: initial pH 7.0, catalyst dosage = 100 mg·L−1, [H2O2] = 0.2 mg·L−1, time = 60 min, and ambient temperature. MB: initial pH 3.0, catalyst dosage = 50 mg·L−1, [H2O2] = 0.1 mg·L−1, time = 60 min, and ambient temperature.
The reaction time is one of the important parameters of the degradation reaction because it affects the productivity of the removal process. If the time is short and not enough, it will lead to an incomplete reaction, reducing the contaminants decomposition efficiency; in contrast, if it is too long, it will cause unnecessary waste. A series of experiments were conducted at different time periods, from 5 to 90 min for MO and from 5 to 140 min for MB. The MB degradation required longer time because of the MB complex structure. The MO removal efficiency presented an increasing tendency within the first 60 min, thereafter the yield did not markedly change. More detailed, the efficiency of MO degradation achieved 35% in the first 5 min and increased to 87.9%, 91.8%, 96.7%, and 98.3% after 15, 30, 45, and 60 min, respectively. After that, when the reaction time continuously went to 75 and 90 min, the efficiency changed at very small levels to 98.8% and 99.4%, respectively. For MB, the degradation efficiency trend seemed like the MO removal, for which the efficiency showed a positive correlation with the reaction time. Figure 6b shows that the removal of MB achieved high efficiency in the first 20 min, dramatically increased from 36% to 88%, and reached 98% at 100 min. After that, it tended to reach an equilibrium of efficiency despite the longer time.
![Figure 6
Effect of reaction time on the removal efficiency of (a) MO and (b) MB. MO: initial pH 7.0, catalyst dosage = 100 mg·L−1, [H2O2] = 0.2 mol·L−1, initial [MO] = 10 mg·L−1, and ambient temperature. MB: initial pH 3.0, catalyst dosage = 50 mg·L−1, [H2O2] = 0.1 mol·L−1, initial [MB] = 10 mg·L−1, and ambient temperature.](/document/doi/10.1515/gps-2022-0006/asset/graphic/j_gps-2022-0006_fig_006.jpg)
Effect of reaction time on the removal efficiency of (a) MO and (b) MB. MO: initial pH 7.0, catalyst dosage = 100 mg·L−1, [H2O2] = 0.2 mol·L−1, initial [MO] = 10 mg·L−1, and ambient temperature. MB: initial pH 3.0, catalyst dosage = 50 mg·L−1, [H2O2] = 0.1 mol·L−1, initial [MB] = 10 mg·L−1, and ambient temperature.
Generally, the removal of both MO and MB went fast and reached more than 90% in 30 min for MO and 40 min for MB. The optimum reaction time was chosen as 60 min for MO removal and 100 min for MB removal, with efficiency for both dyes above 98%.
The possible mechanism of MO and MB removal by CuCo-ZIFs is illustrated by the novel Fenton process and summarized in Figure 7.
The degradation of mixture of dyes was also studied at optimal parameters by using a solution of MO 50 ppm and MB 40 ppm. CuCo-ZIFs presented a good ability to accelerate H2O2 for dye mixture degradation. The results showed a high degrading performance on a two dye mixture within 60 min, demonstrating the promising potential of CuCo-ZIF material for the dye-polluted wastewater treatment (Figure 8).
![Figure 8
Mixture of MO 50 ppm and MB 40 ppm before and after degradation by Fenton-like reaction with CuCo-ZIFs. Catalyst dosage = 150 mg·L−1, [H2O2] = 0.3 mol·L−1, initial [dye mixture] = 20 mg·L−1, time = 60 min, and ambient temperature.](/document/doi/10.1515/gps-2022-0006/asset/graphic/j_gps-2022-0006_fig_008.jpg)
Mixture of MO 50 ppm and MB 40 ppm before and after degradation by Fenton-like reaction with CuCo-ZIFs. Catalyst dosage = 150 mg·L−1, [H2O2] = 0.3 mol·L−1, initial [dye mixture] = 20 mg·L−1, time = 60 min, and ambient temperature.
3.2 Comparison catalytic activity of CuCo-ZIFs with other catalysts
For both the MO and MB removal processes, the catalytic activity of CuCo-ZIFs was compared to that of various homogeneous and heterogeneous catalysts. Homogeneous catalysts included 2-MIm, cobalt nitrate salt, and copper nitrate salt, which were the reactants for CuCo-ZIF synthesis (Figure 9). Five types of heterogeneous catalysts, including zeolite, activated carbon, ZIF-8, ZIF-67, and Ag/ZIF-67, were also used to compare with CuCo-ZIFs under the identical conditions for each dye (Figure 10). The optimum conditions for MO and MB removal included catalyst dosage, H2O2 concentration, initial dye concentration, and reaction time from previous experiments were used. For MO, they were 100 mg·L−1 catalyst, 0.2 mol·L−1 H2O2, 50 mg MO·L−1, 60 min, and a pH value of 7.0 at ambient temperature, respectively. The optimal conditions of MB removal were 50 mg·L−1 catalyst, 0.1 mol·L−1 H2O2, 40 mg MB·L−1, reaction in 100 min and at a pH value of 3.0, and ambient temperature, respectively.
![Figure 9
Comparison of MO and MB removal efficiency among homogeneous catalysts and CuCo-ZIFs. MO: pH 7.0, catalyst dosage = 100 mg·L−1, [H2O2] = 0.2 mol·L−1, initial [MO] = 35 mg·L−1, time = 60 min, and ambient temperature. MB: pH 3.0, catalyst dosage = 50 mg·L−1, [H2O2] = 0.1 mol·L−1, initial [MB] = 40 mg·L−1, time = 100 min, and ambient temperature.](/document/doi/10.1515/gps-2022-0006/asset/graphic/j_gps-2022-0006_fig_009.jpg)
Comparison of MO and MB removal efficiency among homogeneous catalysts and CuCo-ZIFs. MO: pH 7.0, catalyst dosage = 100 mg·L−1, [H2O2] = 0.2 mol·L−1, initial [MO] = 35 mg·L−1, time = 60 min, and ambient temperature. MB: pH 3.0, catalyst dosage = 50 mg·L−1, [H2O2] = 0.1 mol·L−1, initial [MB] = 40 mg·L−1, time = 100 min, and ambient temperature.
![Figure 10
Comparison of MO and MB removal efficiency among other heterogeneous catalysts and CuCo-ZIFs. MO: pH 7.0, catalyst dosage = 100 mg·L−1, [H2O2] = 0.2 mol·L−1, initial [MO] = 35 mg·L−1, time = 60 min, and ambient temperature. MB: pH 3.0, catalyst dosage = 50 mg·L−1, [H2O2] = 0.1 mol·L−1, initial [MB] = 40 mg·L−1, time = 100 min, and ambient temperature.](/document/doi/10.1515/gps-2022-0006/asset/graphic/j_gps-2022-0006_fig_010.jpg)
Comparison of MO and MB removal efficiency among other heterogeneous catalysts and CuCo-ZIFs. MO: pH 7.0, catalyst dosage = 100 mg·L−1, [H2O2] = 0.2 mol·L−1, initial [MO] = 35 mg·L−1, time = 60 min, and ambient temperature. MB: pH 3.0, catalyst dosage = 50 mg·L−1, [H2O2] = 0.1 mol·L−1, initial [MB] = 40 mg·L−1, time = 100 min, and ambient temperature.
As shown in Figure 9, the MO removal efficiency of homogeneous catalysts was low. The efficiencies of using cobalt salt, 2-MIm, and copper salt achieved, respectively, 9.7%, 10.5%, and 18.3% after 60 min reaction. Obviously, they were much lower than the efficiency of using CuCo-ZIFs (98%). For MB, the catalytic activities of cobalt salt and 2-MIm were lower than 20%, whereas that of the copper salt performed a high efficiency at 80%; however, this value was still lower than that of CuCo-ZIFs (98%). Generally, the degradation of MO and MB using cobalt salt and 2-MIm was attributed to the presence of H2O2 only. In the meantime, the removal of MO and MB using copper salt in the presence of H2O2 was known as a Fenton reaction [40]. Nevertheless, the degradation of MO was lower than that of MB because the Fenton reaction was strong at low pH. That is the main reason for the higher removal performance of copper salt at pH 3.0 for MB. In terms of CuCo-ZIFs, the degradation of both MO and MB was a novel Fenton-like reaction, which is a synergistic catalyst between CuCo and ZIFs [41]. Chen et al. showed that reactive oxygen species, namely •OH and 1O2, were the dominant reactive centers accelerating the degradation of two dyes [41]. The novel Fenton-like catalysis of this catalyst was also presented by its high performance at neutral pH (pH 7.0) instead of acidic pH.
In Figure 10, the catalytic activity of CuCo-ZIFs in comparison to that of heterogeneous materials is demonstrated. The results showed that the MO and MB removal efficiency of using other heterogeneous catalysts were still lower than CuCo-ZIFs. For MO, the efficiency of using zeolite, activated carbon, ZIF-8, ZIF-67, and Ag/ZIF-67 increased in this order after 60 min. However, the catalytic efficiency of CuCo-ZIFs was still the highest one. For MB, the efficiency of using ZIF-8 was the lowest, and the efficiency orderly increased with activated carbon, zeolite, ZIF-67, Ag/ZIF-67, and CuCo-ZIFs. Zeolite showed very low and not remarkable efficiency due to the adsorption, whereas activated carbon and other ZIFs presented higher removal yields because of their interaction with H2O2 under stirring condition, which activated carbon and metal ions in ZIFs, accelerated the producing of hydroxyl radicals as presented in the following equations, and resulted in the breaking down of the structure of dyes [42,43,44].
Moreover, Zn(ii) in ZIF-8 is weaker than Co(ii) in ZIF-67, so ZIF-8 presented a lower catalytic ability compared to ZIF-67. Both Ag/ZIF-67 and ZIF-67 showed a similar catalytic ability, and this might be because Ag(i) does not involve or play any important role in this reaction when it is doped on the ZIF structure. Consequently, those compared heterogeneous catalysts possessed Fenton-like catalytic activity, but the heterogeneous catalyst (CuCo-ZIFs) in this study presented a novel Fenton-like catalytic activity via the synergistic catalysis between CuCo and ZIFs.
3.3 Reusability of CuCo-ZIF catalyst
Reusability is a crucial parameter to consider in a heterogeneous catalysis. CuCo-ZIFs used in the experiment at optimal conditions for MO and MB removal were washed with ethanol and dried after using. Figure 11 depicted the results. The high efficiency is still retained after many times of recycling processes. For MO, the removal efficiency decreased from 98% at the first use to 89% at the fourth use of the catalyst, and for MB, this value fell from 99% to 87% after five times of recycling.
![Figure 11
The removal efficiency of MO after four times (a) and MB after five times (b) reusing of CuCo-ZIFs. MO: pH 7.0, catalyst dosage = 100 mg·L−1, [H2O2] = 0.2 mol·L−1, initial [MO] = 35 mg·L−1, time = 60 min, and ambient temperature. MB: pH 3.0, catalyst dosage = 50 mg·L−1, [H2O2] = 0.1 mol·L−1, initial [MB] = 40 mg·L−1, time = 100 min, and ambient temperature.](/document/doi/10.1515/gps-2022-0006/asset/graphic/j_gps-2022-0006_fig_011.jpg)
The removal efficiency of MO after four times (a) and MB after five times (b) reusing of CuCo-ZIFs. MO: pH 7.0, catalyst dosage = 100 mg·L−1, [H2O2] = 0.2 mol·L−1, initial [MO] = 35 mg·L−1, time = 60 min, and ambient temperature. MB: pH 3.0, catalyst dosage = 50 mg·L−1, [H2O2] = 0.1 mol·L−1, initial [MB] = 40 mg·L−1, time = 100 min, and ambient temperature.
The determination of the material structure after many times of usage was also conducted by using PXRD and FT-IR analysis (Figure 12).
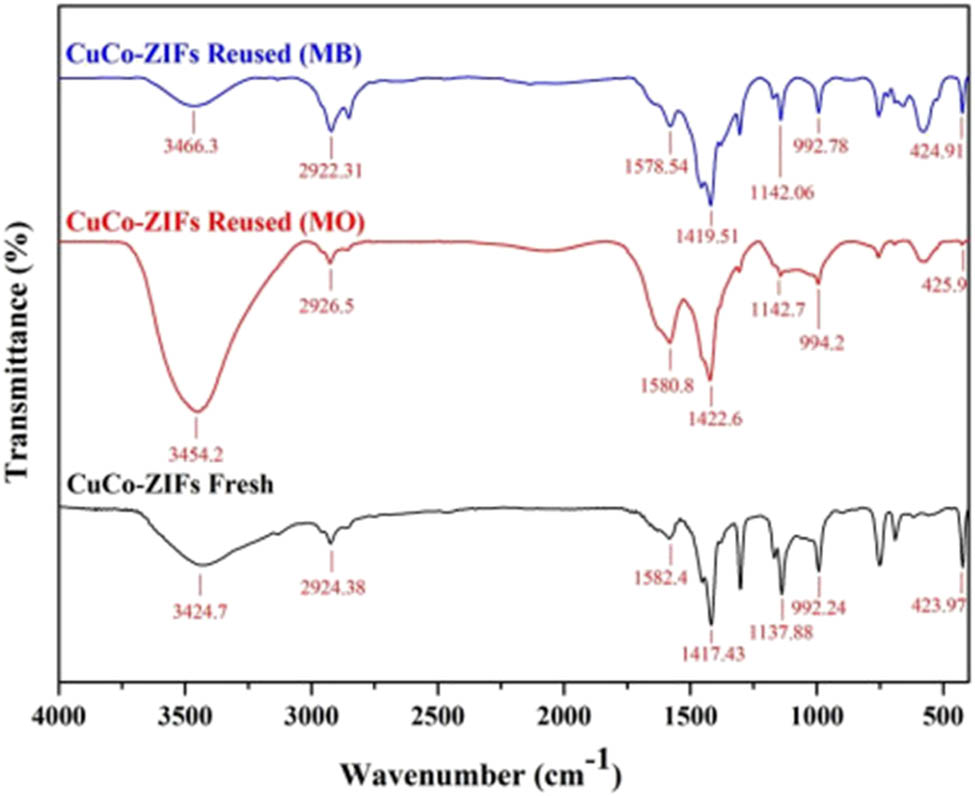
FT-IR spectrum of reused-CuCo-ZIFs, in comparison to fresh material.
Infrared spectrum of the reused CuCo-ZIFs, in comparison with the fresh one, still maintained the peaks at the featured positions, although there was a little change, this change was relatively small and can be considered as not much influence on the material structure. The stretching bonds “metal-N” at 423.97 cm−1 was considered as one of the important bonds because they proved the successful bonding of metal and organic ligands to form the material structure. In reused CuCo-ZIFs, it shifted slightly to 424.91 cm−1 (reused with MB) and 432.24 cm−1 (reused with MO). Other important bonds, the oscillation bonds of the imidazolate ring from 600 to 1,500 cm−1, were still held as beginning after recycling of the material. The spectrums at 802.4 cm−1 (the –C–H– bending of MO [45]), and 669 and 614 cm−1 (the band of C–S–C of MB [46]) in both reused materials proved the adsorption of MO and MB molecules at some positions on the surface and pores of the CuCo-ZIF material.
The PXRD spectrum showed the crystal structure of the CuCo-ZIFs materials and was presented in Figure 13. As can be seen from this result, the reused CuCo-ZIFs had more noise than the fresh material. However, at featured positions corresponding to planes (011), (002), (112), (013), (222), (114), and (134), there were still obtained the crystal structure of CuCo-ZIFs after degrading MB and MO. Therefore, it can be said that the CuCo-ZIFs catalyst showed a good recyclability as they achieved high degradation yield and stayed high crystalline after multiple usage.
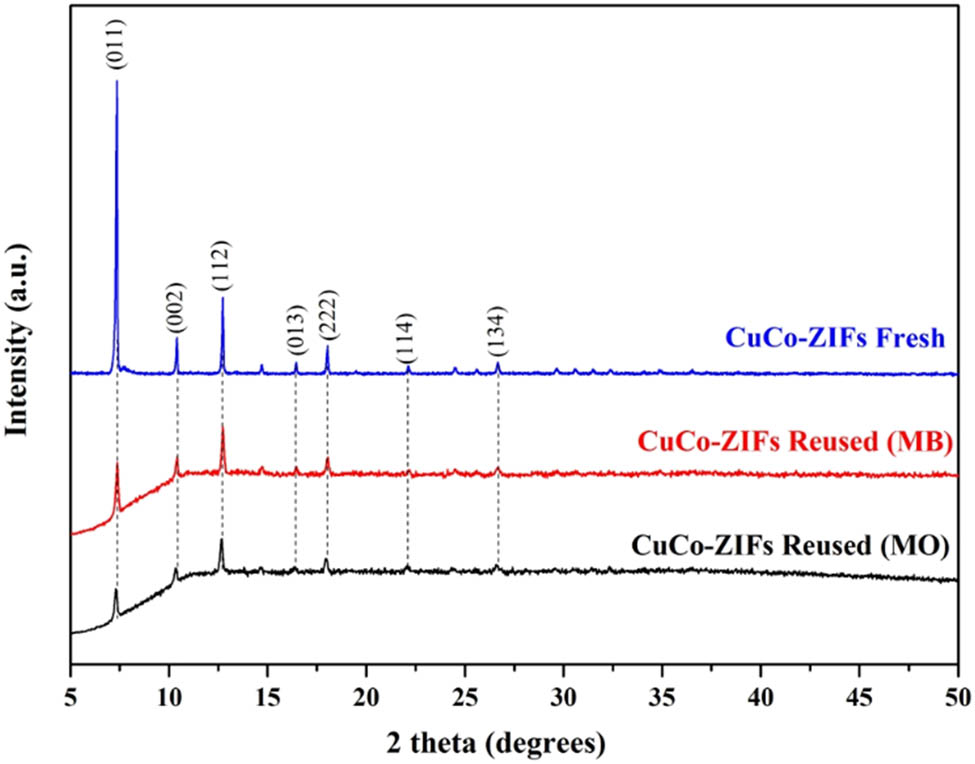
PXRD analysis of reused-CuCo-ZIFs, in comparison to fresh material.
4 Conclusion
A novel Fenton-like catalyst of CuCo-ZIFs was successfully proved as a good heterogeneous catalyst for an efficient degradation of MO and MB in the presence of H2O2. The efficient degradation of both dyes (almost 98%) was obtained at a low catalyst dosage (50–100 mg·L−1), H2O2 concentration of 0.1–0.2 mol·L−1, reaction time of 60–100 min, ambient temperature, and a pH of 3.0–7.0. The as-synthesized solid catalyst also maintained its high catalytic activity at least up to four times with MO and five times with MB degradation without significant reduction in catalytic structure. In conclusion, the CuCo-ZIFs catalyst showed a novel Fenton-like synthesis for the effective removal of both anionic and cationic dyes from aqueous solutions.
-
Funding information: This study is funded by PEER Cycle 9 project, supported by USAID and NAS (US Government).
-
Author contributions: Thanh H. V. Luong: writing – original draft, writing – review and editing, visualization; Thao H. T. Nguyen: writing – original draft, writing – review and editing, methodology, visualization, and formal analysis; Binh V. Nguyen: methodology, formal analysis; Nghia K. Nguyen: project administration; Thanh Q. C. Nguyen: resources; and Giao H. Dang: writing – original draft, writing – review and editing, methodology, resources.
-
Conflict of interest: Authors state no conflict of interest.
Appendix
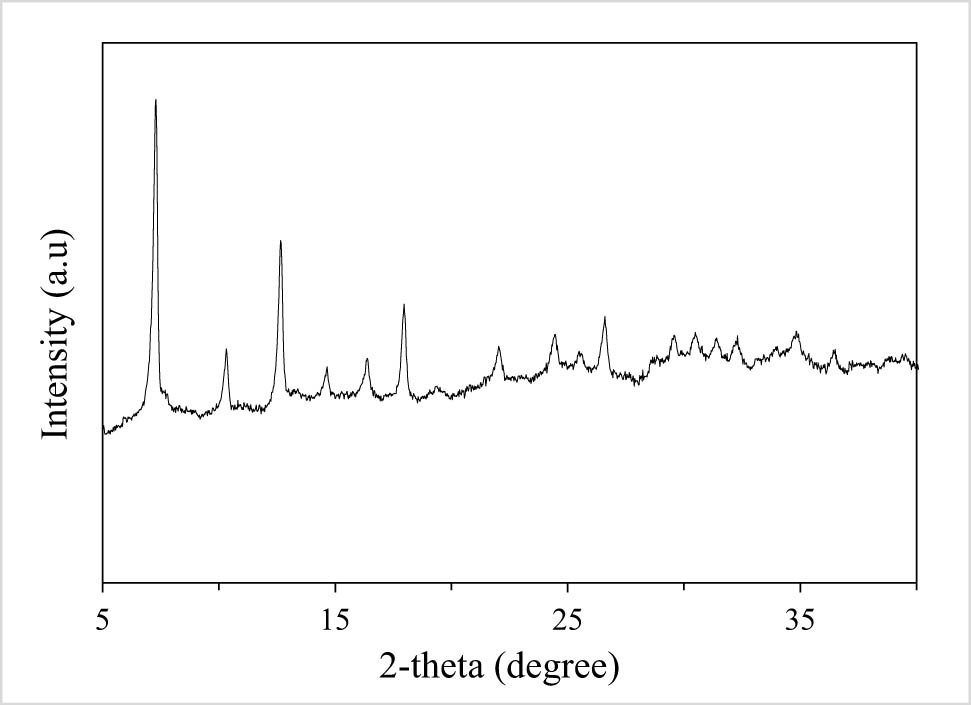
PXRD of CuCo-ZIFs.

SEM images of CuCo-ZIFs at different magnification ratios: (a) 20,000×, (b) 80,000×, and (c) 100,000×.
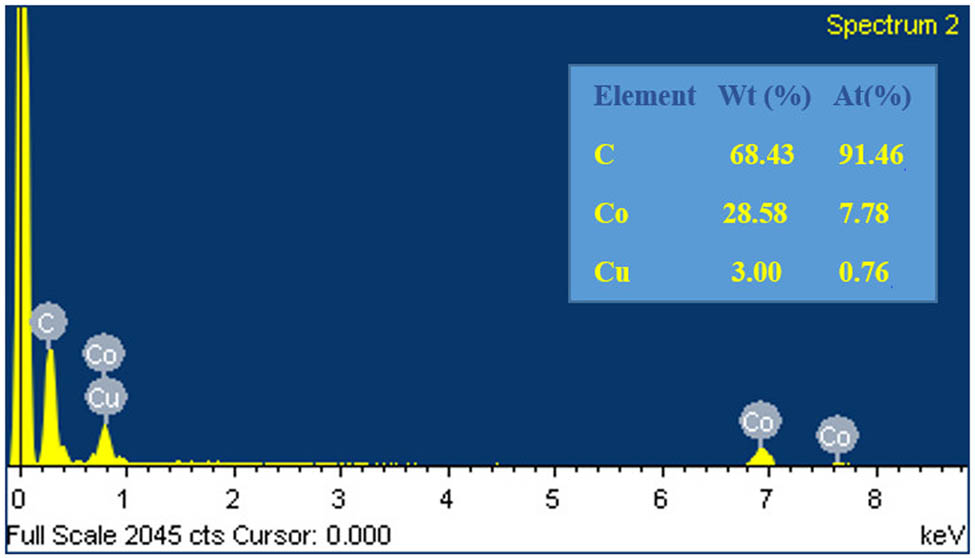
EDX spectra of CuCo-ZIFs.
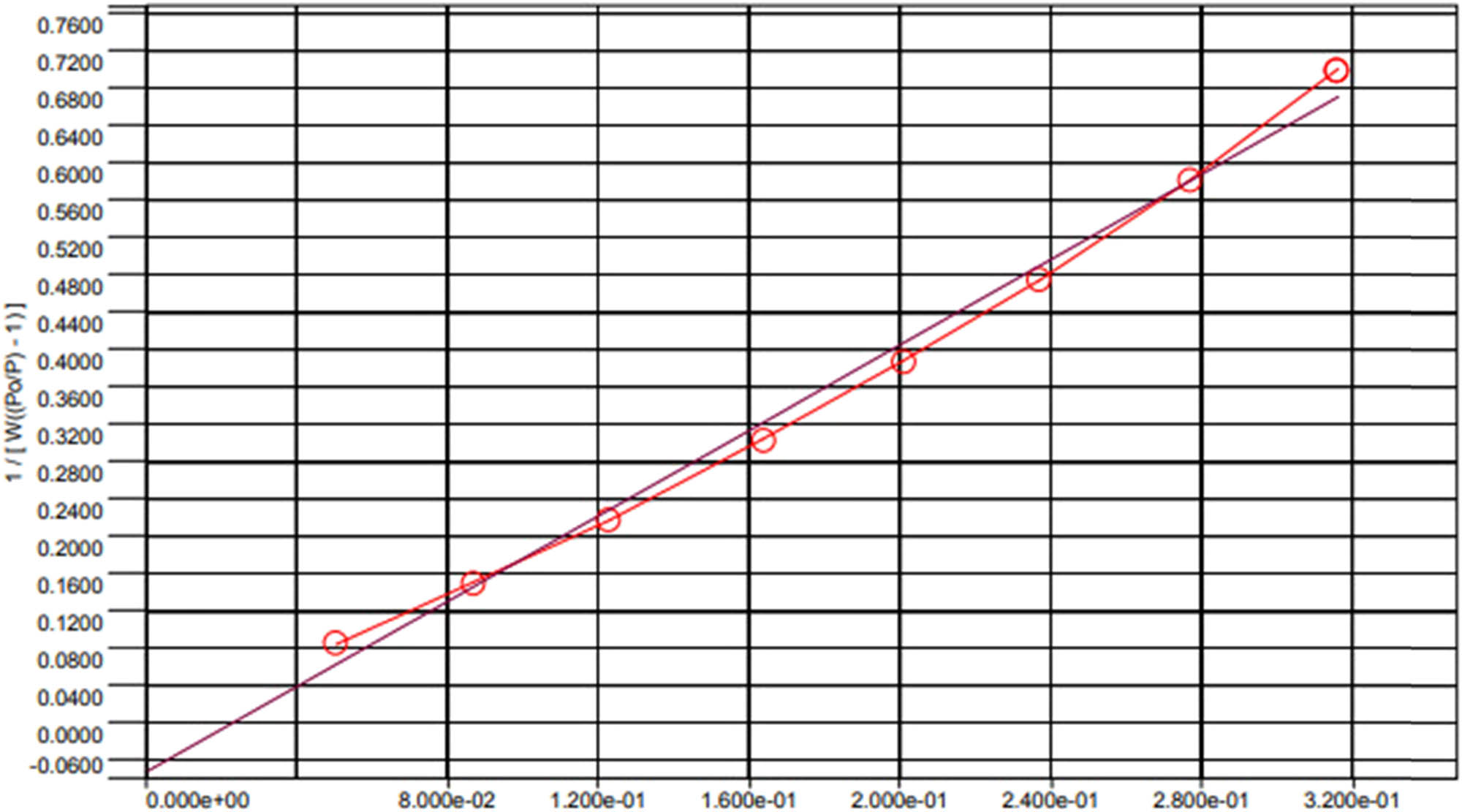
Nitrogen adsorption–desorption isotherm of CuCo-ZIFs.
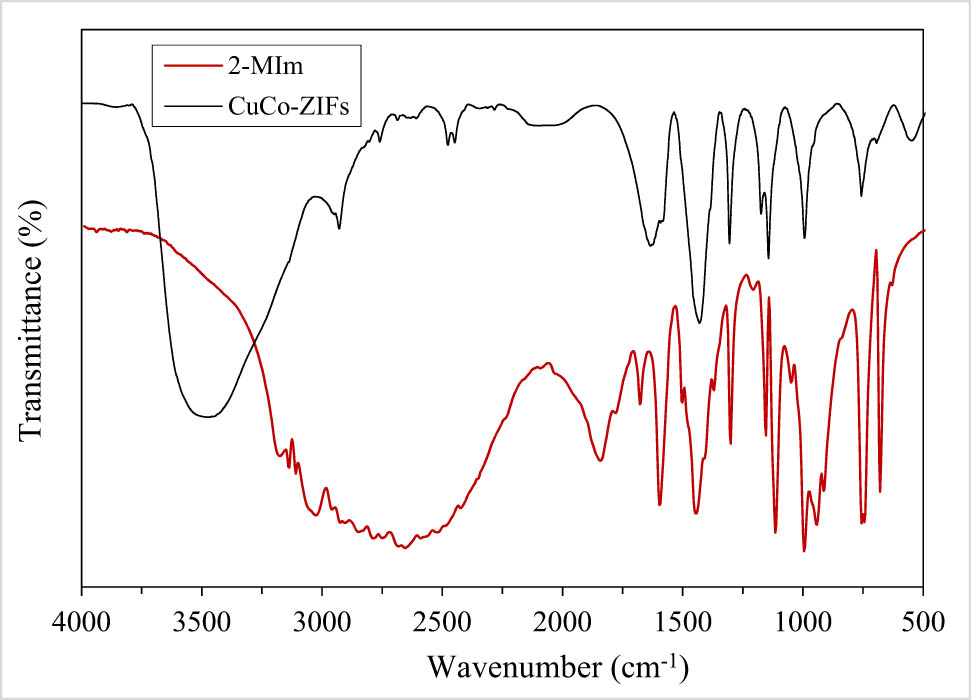
FT-IR spectra of CuCo-ZIFs and 2-MIm.
TGA analysis of CuCo-ZIFs.
References
[1] Ajji Z, Ali AM. Adsorption of methyl violet and brilliant blue onto poly (vinyl alcohol) membranes grafted with N-vinyl imidazole/acrylic acid. Nucl Instrum Meth B. 2007;265:362–5. 10.1016/j.nimb.2007.09.004.Search in Google Scholar
[2] Mahmoodi NM, Arami M. Numerical finite volume modeling of dye decolorization using immobilized titania nanophotocatalysis. Chem Eng J. 2009;146(2):189–93. 10.1016/j.cej.2008.05.036.Search in Google Scholar
[3] Ofomaja AE, Ho YS. Effect of temperatures and pH on methyl violet biosorption by Mansonia wood sawdust. Bioresour Technol. 2008;99(13):5411–7. 10.1016/j.biortech.2007.11.018.Search in Google Scholar PubMed
[4] Sriram G, Kigga M, Uthappa UT, Rego RM, Thendral V, Kumeria T, et al. Naturally available diatomite and their surface modification for the removal of hazardous dye and metal ions: a review. Adv Colloid Interfac. 2020;282:102198. 10.1016/j.cis.2020.102198.Search in Google Scholar PubMed
[5] Sriram G, Uthappa UT, Rego RM, Kigga M, Kumeria T, Jung HY, et al. Ceria decorated porous diatom-xerogel as an effective adsorbent for the efficient removal of Eriochrome Black T. Chemosphere. 2020;238:124692. 10.1016/j.chemosphere.2019.124692.Search in Google Scholar PubMed
[6] Sriram G, Bhat MP, Kigga M, Uthappa UT, Jung HY, Kumeria T, et al. Amine activated diatom xerogel hybrid material for efficient removal of hazardous dye. Mater Chem Phys. 2019;235:121738. 10.1016/j.matchemphys.2019.121738.Search in Google Scholar
[7] Zhang C, Li H, Li C, Li Z. Fe-loaded MOF-545 (Fe): peroxidase-like activity for dye degradation dyes and high adsorption for the removal of dyes from wastewater. Molecules. 2020;25(1):168. 10.3390/molecules25010168.Search in Google Scholar PubMed PubMed Central
[8] Shakya J, Mahanta T, Kumar S, Mohanty T. Photocatalytic degradation of anionic and cationic dye by using nitrogen doped MoS2. AIP Conference Proceedings. AIP Publishing LLC; Jul 11 2019.10.1063/1.5113024Search in Google Scholar
[9] Ismail M, Akhtar K, Khan MI, Kamal T, Khan MA, M Asiri A, et al. Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr Pharm Des. 2019;25(34):3645–63. 10.2174/1381612825666191021142026.Search in Google Scholar PubMed
[10] Hussain S, Kamran M, Khan SA, Shaheen K, Shah Z, Suo H, et al. Adsorption, kinetics and thermodynamics studies of methyl orange dye sequestration through chitosan composites films. Int J Biol Macromol. 2021;168:383–94. 10.1016/j.ijbiomac.2020.12.054.Search in Google Scholar PubMed
[11] Aksu Z. Application of biosorption for the removal of organic pollutants: a review. Process Biochem. 2005;40(3–4):997–1026. 10.1016/j.procbio.2004.04.008.Search in Google Scholar
[12] Nunes WB, Dantas RF, Fagnani E. Ferroin in dyes degradation by Fenton-like process: a chemical waste recycling perspective. Water Sci Technol. 2021;84(5):1217–27. 10.2166/wst.2021.311.Search in Google Scholar PubMed PubMed Central
[13] Liang D, Li N, An J, Ma J, Wu Y, Liu H. Fenton-based technologies as efficient advanced oxidation processes for microcystin-LR degradation. Sci Total Env. 2021;753:141809. 10.1016/j.scitotenv.2020.141809.Search in Google Scholar PubMed
[14] Dang GH, Le TT, Ta AK, Ho TN, Pham TV, Doan TV, et al. Removal of Congo red and malachite green from aqueous solution using heterogeneous Ag/ZnCo-ZIF catalyst in the presence of hydrogen peroxide. Green Process Synth. 2020;9(1):567–77. 10.1515/gps-2020-0060.Search in Google Scholar
[15] Hu Y, Li Y, He J, Liu T, Zhang K, Huang X, et al. EDTA-Fe (III) Fenton-like oxidation for the degradation of malachite green. J Env Manage. 2018;226:256–63. 10.1016/j.jenvman.2018.08.029.Search in Google Scholar PubMed
[16] Sun B, Li H, Li X, Liu X, Zhang C, Xu H, et al. Degradation of organic dyes over fenton-like Cu2O–Cu/C catalysts. Ind Eng Chem. 2018;57(42):14011–21. 10.1021/acs.iecr.8b02697.Search in Google Scholar
[17] Cheng M, Zeng G, Huang D, Lai C, Xu P, Zhang C, et al. Degradation of atrazine by a novel Fenton-like process and assessment of the influence on the treated soil. J Hazard Mater. 2016;312:184–91. 10.1016/j.jhazmat.2016.03.033.Search in Google Scholar PubMed
[18] Bai J, Liu Y, Yin X, Duan H, Ma J. Efficient removal of nitrobenzene by Fenton-like process with Co-Fe layered double hydroxide. Appl Surf Sci. 2017;416:45–50. 10.1016/j.apsusc.2017.04.117.Search in Google Scholar
[19] Minella M, Bertinetti S, Hanna K, Minero C, Vione D. Degradation of ibuprofen and phenol with a Fenton-like process triggered by zero-valent iron (ZVI-Fenton). Env Res. 2019;179:108750. 10.1016/j.envres.2019.108750.Search in Google Scholar PubMed
[20] Krishna R. Diffusion in porous crystalline materials. Chem Soc Rev. 2012;41(8):3099–118. 10.1039/C2CS15284C.Search in Google Scholar PubMed
[21] Howarth AJ, Liu Y, Li P, Li Z, Wang TC, Hupp JT, et al. Chemical, thermal and mechanical stabilities of metal–organic frameworks. Nat Rev Mater. 2016;1(3):1–5. 10.1038/natrevmats.2015.18.Search in Google Scholar
[22] Zhou K, Mousavi B, Luo Z, Phatanasri S, Chaemchuen S, Verpoort F. Characterization and properties of Zn/Co zeolitic imidazolate frameworks vs. ZIF-8 and ZIF-67. J Mater Chem A. 2017;5(3):952–7. 10.1039/C6TA07860E.Search in Google Scholar
[23] Zhong G, Liu D, Zhang J. The application of ZIF-67 and its derivatives: adsorption, separation, electrochemistry and catalysts. J Mater Chem A. 2018;6(5):1887–99. 10.1039/C7TA08268A.Search in Google Scholar
[24] Krokidas P, Castier M, Economou IG. Computational study of ZIF-8 and ZIF-67 performance for separation of gas mixtures. J Phys Chem C. 2017;121(33):17999–8011. 10.1021/acs.jpcc.7b05700.Search in Google Scholar
[25] Sohouli E, Karimi MS, Khosrowshahi EM, Rahimi-Nasrabadi M, Ahmadi F. Fabrication of an electrochemical mesalazine sensor based on ZIF-67. Measurement. 2020;165:108140. 10.1016/j.measurement.2020.108140.Search in Google Scholar
[26] Guo C, Zhang Y, Nan X, Feng C, Guo Y, Wang J. Efficient selective catalytic oxidation of benzylic CH bonds by ZIF-67 under eco-friendly conditions. Mol Catal. 2017;440:168–74. 10.1016/j.mcat.2017.07.013.Search in Google Scholar
[27] Liu Y, Lin D, Yang W, An X, Sun A, Fan X, et al. In situ modification of ZIF-67 with multi-sulfonated dyes for great enhanced methylene blue adsorption via synergistic effect. Micropor Mesopor Mat. 2020;303:110304. 10.1016/j.micromeso.2020.110304.Search in Google Scholar
[28] Li W, Yang F, Fang X, Rui Y, Tang B. Systematic post-synthetic modification of metal-organic framework (ZIF-67) with superior cyclability for lithium-ion batteries. Electrochim Acta. 2018;282:276–85. 10.1016/j.electacta.2018.06.066.Search in Google Scholar
[29] Chen X, Zhao JX, Wang JW, Liu Y, Wang LC, Weerasooriya R, et al. Doping ZIF-67 with transition metals results in bimetallic centers for electrochemical detection of Hg(ii). Electrochim Acta. 2021;387:138539. 10.1016/j.electacta.2021.138539.Search in Google Scholar
[30] Haider MD, Iqbal N, Rizvi SA, Noor T, Hanif S, Anwar R. ZIF-67 derived Cu-doped electrocatalyst for oxygen reduction reaction. J Electrochem Energy Convers Storage. 2021;18(2):021001. 10.1115/1.4047331.Search in Google Scholar
[31] Budi CS, Deka JR, Hsu WC, Saikia D, Chen KT, Kao HM, et al. Bimetallic Co/Zn zeolitic imidazolate framework ZIF-67 supported Cu nanoparticles: an excellent catalyst for reduction of synthetic dyes and nitroarenes. J Hazard Mater. 2021;407:124392. 10.1016/j.jhazmat.2020.124392.Search in Google Scholar
[32] Zanon A, Chaemchuen S, Verpoort F. Zn@ ZIF-67 as catalysts for the Knoevenagel condensation of aldehyde derivatives with malononitrile. Catal Lett. 2017;147(9):2410–20. 10.1007/s10562-017-2153-y.Search in Google Scholar
[33] Kayestha R, Hajela K. ESR studies on the effect of ionic radii on displacement of Mn2+ bound to a soluble β-galactoside binding hepatic lectin. FEBS Lett. 1995;368(2):285–8. 10.1016/0014-5793(95)00673-W.Search in Google Scholar
[34] Kabbur SM, Nadargi DY, Kambale RC, Ghodake UR, Suryavanshi SS. Microstructure and magnetic interactions of Co2+ substituted NiCuZn ferrites. J Magn Magn Mater. 2021;517:167376. 10.1016/j.jmmm.2020.167376.Search in Google Scholar
[35] Yang H, He XW, Wang F, Kang Y, Zhang J. Doping copper into ZIF-67 for enhancing gas uptake capacity and visible-light-driven photocatalytic degradation of organic dye. J Mater Chem. 2012;22(41):21849–51. 10.1039/C2JM35602C.Search in Google Scholar
[36] Lee JG, Yoon S, Yang E, Lee JH, Song K, Moon HR, et al. Structural evolution of ZIF-67-derived catalysts for furfural hydrogenation. J Catal. 2020;392:302–12. 10.1016/j.jcat.2020.10.014.Search in Google Scholar
[37] Ma J, Wang H, Yang X, Chai Y, Yuan R. Porous carbon-coated CuCo2O4 concave polyhedrons derived from metal–organic frameworks as anodes for lithium-ion batteries. J Mater Chem A. 2015;22:12038–43. 10.1039/c5ta00890e.Search in Google Scholar
[38] Devi LG, Kumar SG, Reddy KM, Munikrishnappa C. Photo degradation of methyl orange an azo dye by advanced fenton process using zero valent metallic iron: influence of various reaction parameters and its degradation mechanism. J Hazard Mater. 2009;164(2–3):459–67. 10.1016/j.jhazmat.2008.08.017.Search in Google Scholar PubMed
[39] Yang B, Zhou P, Cheng X, Li H, Huo X, Zhang Y. Simultaneous removal of methylene blue and total dissolved copper in zero-valent iron/H2O2 Fenton system: kinetics, mechanism and degradation pathway. J Colloid Interf Sci. 2019;555:383–93. 10.1016/j.jcis.2019.07.071.Search in Google Scholar PubMed
[40] Maezono T, Tokumura M, Sekine M, Kawase Y. Hydroxyl radical concentration profile in photo-Fenton oxidation process: generation and consumption of hydroxyl radicals during the discoloration of azo-dye Orange II. Chemosphere. 2011;82(10):1422–30. 10.1016/j.chemosphere.2010.11.052.Search in Google Scholar PubMed
[41] Chen M, Wang N, Wang X, Zhou Y, Zhu L. Enhanced degradation of tetrabromobisphenol A by magnetic Fe3O4@ ZIF-67 composites as a heterogeneous Fenton-like catalyst. Chem Eng J. 2021;413:127539–47. 10.1016/j.cej.2020.127539.Search in Google Scholar
[42] Khalil LB, Girgis BS, Tawfik TA. Decomposition of H2O2 on activated carbon obtained from olive stones. J Chem Technol Biot: International Research in Process. Environmental & Clean Technology. 2001;76(11):1132–40. 0.1002/jctb.481.Search in Google Scholar
[43] Ling SK, Wang S, Peng Y. Oxidative degradation of dyes in water using Co2 +/H2O2 and Co2 +/peroxymonosulfate. J Hazard Mater. 2010;178(1–3):385–9. 10.1016/j.jhazmat.2010.01.091.Search in Google Scholar
[44] Sato K, Akaike T, Kohno M, Ando M, Maeda H. Hydroxyl radical production by H2O2 plus Cu, Zn-superoxide dismutase reflects the activity of free copper released from the oxidatively damaged enzyme. J Biol Chem. 1992;267(35):25371–7. 10.1016/S0021-9258(19)74050-2.Search in Google Scholar
[45] Nandini R, Vishalakshi B. A study of interaction of methyl orange with some polycations. E-J Chem. 2012;9(1):1–4. 10.1155/2012/343928.Search in Google Scholar
[46] Ovchinnikov OV, Evtukhova AV, Kondratenko TS, Smirnov MS, Khokhlov VY, Erina OV. Manifestation of intermolecular interactions in FTIR spectra of methylene blue molecules. Vib Spectrosc. 2016;86:181–9. 10.1016/j.vibspec.2016.06.016.Search in Google Scholar
© 2022 Thanh H. V. Luong et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

![Figure 7
Summary of removal mechanism of (a) MO [38] and (b) MB [39].](/document/doi/10.1515/gps-2022-0006/asset/graphic/j_gps-2022-0006_fig_007.jpg)
