Abstract
C12H12ClN3O2, monoclinic, P21/c (no. 14), a = 13.5673(5) Å, b = 7.7549(3) Å, c = 12.4911(5) Å, β = 102.468(2)°, V = 1283.23(9) Å3, Z = 4, Rgt(F) = 0.047, wRref(F2) = 0.123, T = 293(2) K.
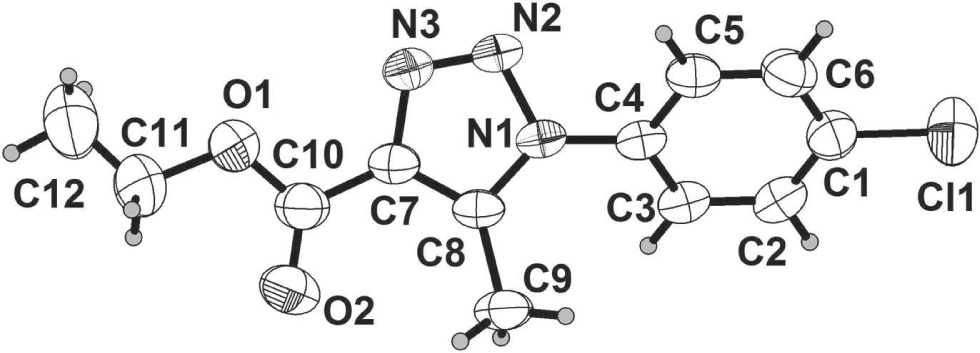
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless parallelepiped |
| Size: | 0.18 × 0.10 × 0.08 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 26.4 cm−1 |
| Diffractometer, scan mode: | Bruker AXS, φ and ω |
| 2θmax, completeness: | 118°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 12946, 1835, 0.056 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1498 |
| N(param)refined: | 166 |
| Programs: | Bruker [1], SHELX [2], PLATON [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Cl1 | 0.15384(6) | 0.38969(14) | −0.01408(8) | 0.1067(4) |
| N1 | 0.53730(14) | 0.3725(2) | 0.31511(14) | 0.0476(5) |
| N2 | 0.53072(16) | 0.3264(3) | 0.41950(15) | 0.0584(6) |
| N3 | 0.62191(16) | 0.3290(3) | 0.47923(16) | 0.0586(6) |
| O1 | 0.82490(14) | 0.3722(3) | 0.56269(16) | 0.0861(7) |
| O2 | 0.85300(15) | 0.4195(4) | 0.39692(18) | 0.1050(9) |
| C1 | 0.2672(2) | 0.3879(4) | 0.0817(2) | 0.0641(7) |
| C2 | 0.3509(2) | 0.3161(3) | 0.0543(2) | 0.0597(7) |
| H2 | 0.3469 | 0.2703 | −0.0153 | 0.072* |
| C3 | 0.44105(19) | 0.3122(3) | 0.13057(19) | 0.0523(6) |
| H3 | 0.4981 | 0.2632 | 0.1130 | 0.063* |
| C4 | 0.44588(18) | 0.3818(3) | 0.23331(18) | 0.0474(6) |
| C5 | 0.36158(19) | 0.4554(3) | 0.2603(2) | 0.0577(7) |
| H5 | 0.3656 | 0.5028 | 0.3295 | 0.069* |
| C6 | 0.2721(2) | 0.4577(4) | 0.1843(2) | 0.0678(8) |
| H6 | 0.2149 | 0.5061 | 0.2018 | 0.081* |
| C7 | 0.68700(18) | 0.3756(3) | 0.41528(19) | 0.0506(6) |
| C8 | 0.63409(18) | 0.4042(3) | 0.31011(19) | 0.0470(6) |
| C9 | 0.6665(2) | 0.4649(4) | 0.2102(2) | 0.0649(8) |
| H9B | 0.6841 | 0.3675 | 0.1708 | 0.097* |
| H9A | 0.7240 | 0.5392 | 0.2311 | 0.097* |
| H9C | 0.6122 | 0.5272 | 0.1643 | 0.097* |
| C10 | 0.7955(2) | 0.3913(4) | 0.4558(2) | 0.0658(8) |
| C11 | 0.9336(3) | 0.3817(7) | 0.6062(3) | 0.1351(19) |
| H11A | 0.9683 | 0.3255 | 0.5555 | 0.162* |
| H11B | 0.9542 | 0.5017 | 0.6118 | 0.162* |
| C12 | 0.9620(3) | 0.3057(8) | 0.7070(4) | 0.1393(18) |
| H12C | 0.9325 | 0.1929 | 0.7048 | 0.209* |
| H12B | 0.9392 | 0.3748 | 0.7607 | 0.209* |
| H12A | 1.0342 | 0.2962 | 0.7262 | 0.209* |
Source of material
The title compound was obtained using the synthetic method described by Kalmaraj et al. [4]. A mixture of para-chloroaniline (7.24 mmol) in 4.50 mL of HCl /water (1:1) was cooled at 0–5 °C and added dropwise a solution of sodium nitrite 525 mg (7.60 mmol) in 30 mL of water. The resulting mixture was stirred for 10 minutes, then it was slowly added a solution of sodium azide (612 mg, 9.41 mmol) in 60 mL of cold water. The mixture was stirred for 30 minutes and extracted with 10 ml of diethyl ether and washed successively with water (3 × 6 mL). The organic phase was dried over anhydrous sodium sulfate and concentrated in vacuum at 30 °C affording the phenylazide. The phenylazide obtained was dissolved in DMSO and 7.96 mmol (1.1 eq) of ethyl acetoacetate and 1.0 g of K2CO3 (1 eq) were added. The mixture was stirred for 3 h and the reaction stopped by adding 30 mL of cold water. The precipitate obtained was filtered under vacuum, washed with cold water and vacuum drying, obtaining 1.98 g (yield 78%) of the title compound. Finally, crystallization from ethanol gave colourless crystals, mp. 168–170 °C. IR (KBr) cm−1: 3096 and 3066 (CAr–H), 1716 (C = O), 1560 (N = N), 1223 (CO–O), 1026 (Ar–Cl), 981 (N–N = N). 1H-NMR (500 MHz, CDCl3) δ: 7.54 (d, J = 6.9 Hz, 2H), 7.40 (d, J = 7.3 Hz, 2H), 4.44 (q, J = 6.5 Hz, 2H), 2.58 (s, 3H), 1.43 (t, J = 6.7 Hz, 3H). 13C-NMR (126 MHz, CDCl3) δ: 161.7 (C = O), 139.0 (C), 137.0 (C), 136.4 (C), 134.0 (C), 130.1 (CH), 126.8 (CH), 61.3 (CH2), 14.5 (CH3), 10.2 (CH3). HRESIMS: m/z 266.0680 for C12H13ClN3O2 [M + H]+, calculated: m/z 266.0696.
Experimental details
H atoms were located in the difference Fourier map, but refined with fixed individual displacement parameters, using a riding model with C—H distances of 0.93 Å (for aromatic rings), 0.92 Å; 0.96 Å (for CH2 and CH3 respectively), with U(H) values of 1.2Ueq(C) (for CH in aromatic moiety and CH2), and 1.5Ueq(C) (for CH3).
Discussion
1,2,3-Triazoles are an important class of heterocyclic compounds due to their wide range of applications as pharmaceutical agents [5, 6] . The diversity of chemical structures of the 1,2,3-triazole family and their useful biological activities made these compounds attractive targets in synthetic organic chemistry and many studies have been reported on the synthesis of these compounds [7], [8], [9].
The title compound is isomorphous with the ethyl 1-(4-bromophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate analogue, reported by Singh, et al. [10]. The triazole ring make angles of 7.1(3)° with the lateral chain and 42.41(18)° with the phenyl ring. The crystal packing is stabilized only by van der Waals interactions. All distances and angles are in the normal ranges.
Acknowledgement
IB Thanks to fondequip grant N° (EQM13–0021). VK thanks to European Union Project ChemBioFight (Grant 269301).
References
1 Bruker. APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA (2007).Search in Google Scholar
2 Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Cryst. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar
3 Spek, A. L.: Structure validation in chemical crystallography. Acta Cryst. D65 (2009) 148–155.10.1107/S090744490804362XSearch in Google Scholar
4 Kalmaraj, V. R.; Senthil, S.; Kannan, P.: One pot synthesis and the fluorescent bahavior of 4-acetyl-5-methyl-1,2,3-triazole regioisomers. J. Mol. Struct. 892 (2008) 210–215.10.1016/j.molstruc.2008.05.028Search in Google Scholar
5 Holla, B. S.; Mahalinga, M.; Karthikeyan, M. S.; Poojary, B.; Akberali, P. M.; Kumari, N. S.: Synthesis, characterization and antimicrobial activity of some substituted 1,2,3-triazoles. Eur. J. Med. Chem. 40 (2005) 1173–1178.10.1016/j.ejmech.2005.02.013Search in Google Scholar
6 Dabak, K.; Sezer, O.; Akar, A.; Anac, O.: Synthesis and investigation of tuberculosis inhibition activities of some 1,2,3-triazole derivatives. Eur. J. Med. Chem. 38 (2003) 215–218.10.1016/S0223-5234(02)01445-9Search in Google Scholar
7 Lewis, W. G.; Green, L. G.; Grynszpan, F.; Radic, Z.; Carlier, P. R.; Taylor, P.; Finn, M. G.; Sharpless. K. B.: Click chemistry in situ: acetylcholinesterase as a reaction vessel for the selective assembly of a femtomolar inhibitor from an array of building blocks. Angew. Chem. Int. Ed. 41 (2002) 1053–1057.10.1002/1521-3773(20020315)41:6<1053::AID-ANIE1053>3.0.CO;2-4Search in Google Scholar
8 Link, A. J.; Tirrelli. D. A.: Cell surface labeling of escherichia coli via copper(I)-catalyzed [3 + 2] cycloaddition. J. Am. Chem. Soc. 125 (2003) 11164–11165.10.1021/ja036765zSearch in Google Scholar
9 Nelson, R.; Kesternich, V.; Pérez-Fehrmann, M.; Jaldin, S.; Marcourt, L.; Christen, P.: Regiospecific synthesis of 1,4,5-trisubstituted-1,2,3-triazoles by enolate-azide cicloadition between aryl azides and 1,3-dicarbonyl compounds. J. Chem. Res. 40 (2016) 453–457.10.3184/174751916X14656662266973Search in Google Scholar
10 Singh, H.; Sindhu, J.; Khurana, J. M.: Efficient, green and regioselective synthesis of 1,4,5-trisubstituted- 1,2,3-triazoles in ionic liquid [bmim]BF4 and in task-specific basic ionic liquid [bmim]OH. J. Iran. Chem. Soc. 10 (2013) 883–888.10.1007/s13738-013-0224-6Search in Google Scholar
©2017 Iván Brito et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16


