Abstract
C15H17FN2O4, monoclinic, P21/c (no. 14), a = 12.4266(11) Å, b = 6.6865(5) Å, c = 17.1510(15) Å, β = 99.288(2)°, V = 1406.4(2) Å3, Z = 4, Rgt(F) = 0.0422, wRref (F2) = 0.1139, T = 173 K.
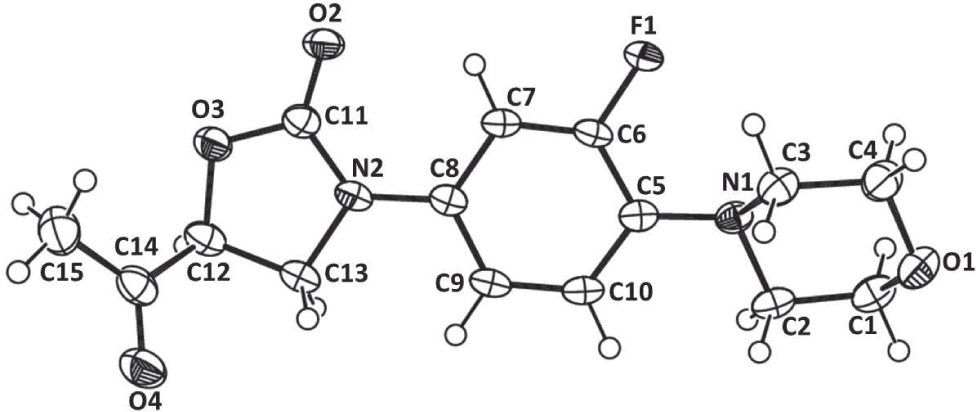
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless plate |
| Size: | 0.39 × 0.25 × 0.05 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.2 cm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, 0.5° φ and ω |
| 2θmax, completeness: | 56.6°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 16828, 3509, 0.035 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2556 |
| N(param)refined: | 200 |
| Programs: | Bruker programs [1], SHELX [2], X-SEED [3], ORTEP [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| F1 | 0.80765(8) | 0.71695(13) | 0.12697(6) | 0.0375(3) |
| O1 | 0.64862(10) | 1.22240(17) | 0.29976(7) | 0.0376(3) |
| O2 | 1.13563(9) | 0.53271(16) | 0.03838(7) | 0.0333(3) |
| O3 | 1.28472(9) | 0.69336(16) | 0.01482(7) | 0.0335(3) |
| O4 | 1.47301(10) | 1.05972(19) | 0.09716(8) | 0.0452(3) |
| N1 | 0.78112(11) | 1.08789(18) | 0.18873(7) | 0.0258(3) |
| N2 | 1.15623(10) | 0.87577(18) | 0.05818(7) | 0.0261(3) |
| C1 | 0.65539(15) | 1.3314(2) | 0.22906(10) | 0.0354(4) |
| H1A | 0.6444 | 1.4755 | 0.2384 | 0.042* |
| H1B | 0.5966 | 1.2866 | 0.1867 | 0.042* |
| C2 | 0.76414(14) | 1.3015(2) | 0.20264(9) | 0.0306(4) |
| H2A | 0.7663 | 1.3777 | 0.1534 | 0.037* |
| H2B | 0.8231 | 1.3520 | 0.2437 | 0.037* |
| C3 | 0.77146(14) | 0.9740(2) | 0.26085(9) | 0.0307(4) |
| H3A | 0.8308 | 1.0134 | 0.3038 | 0.037* |
| H3B | 0.7789 | 0.8293 | 0.2507 | 0.037* |
| C4 | 0.66271(15) | 1.0139(2) | 0.28580(10) | 0.0363(4) |
| H4A | 0.6037 | 0.9672 | 0.2440 | 0.044* |
| H4B | 0.6576 | 0.9379 | 0.3346 | 0.044* |
| C5 | 0.87668(13) | 1.0419(2) | 0.15739(8) | 0.0252(3) |
| C6 | 0.89033(12) | 0.8508(2) | 0.12708(9) | 0.0260(3) |
| C7 | 0.97882(13) | 0.7887(2) | 0.09502(9) | 0.0263(3) |
| H7 | 0.9833 | 0.6557 | 0.0763 | 0.032* |
| C8 | 1.06227(12) | 0.9263(2) | 0.09064(8) | 0.0239(3) |
| C9 | 1.05072(13) | 1.1206(2) | 0.11698(9) | 0.0284(3) |
| H9 | 1.1054 | 1.2171 | 0.1121 | 0.034* |
| C10 | 0.96062(13) | 1.1758(2) | 0.15027(9) | 0.0293(3) |
| H10 | 0.9559 | 1.3088 | 0.1688 | 0.035* |
| C11 | 1.18499(13) | 0.6879(2) | 0.03774(8) | 0.0269(3) |
| C12 | 1.32711(14) | 0.8939(2) | 0.02139(10) | 0.0316(4) |
| H12 | 1.3299 | 0.9459 | −0.0329 | 0.038* |
| C13 | 1.24476(13) | 1.0178(2) | 0.05775(9) | 0.0293(3) |
| H13A | 1.2752 | 1.0619 | 0.1119 | 0.035* |
| H13B | 1.2206 | 1.1363 | 0.0250 | 0.035* |
| C14 | 1.44106(14) | 0.9009(3) | 0.06863(10) | 0.0343(4) |
| C15 | 1.51184(16) | 0.7190(3) | 0.07280(12) | 0.0455(5) |
| H15A | 1.5824 | 0.7476 | 0.1055 | 0.068* |
| H15B | 1.5232 | 0.6819 | 0.0195 | 0.068* |
Source of material
A solution of 3-fluoro-4-(4-morpholinyl)-aniline (1.0 eq.) and carbonyldiimidazole (CDI) (1.2 eq.) in dichloromethane (DCM) (10 mL) was stirred at room temperature for 24 h. The solvent was evaporated under reduced pressure and the resulting residue was purified via column chromatography to afford pure product. Yield 65%; 1H-NMR (400 MHz, CDCl3) δ/p.p.m. = 7.43–7.41 (dd, J = 14.33, 2.20 Hz, 1H), 7.11–7.09 (d, J = 9.21 Hz, 1H), 6.94–6.91 (t, J = 9.08 Hz, 1H), 4.85–4.83 (t, J = 7.75 Hz, 1H), 4.13–4.11 (d, J = 7.16 Hz, 2H), 3.86–3.85 (t, J = 4.71 Hz, 4H), 3.06–3.04 (t, J = 4.69 Hz, 4H), 2.41 (s, 3H); 13C-NMR (CDCl3) δ = 204.6 (CO), 156.6 (CF), 155.0 (CO), 153.3 (CN), 133.0 (CN), 132.5 (CH), 119.0 (CH), 113.8 (CH), 108.3 (CH), 75.1 (CH), 68.0 (CH2), 51.3 (CH2), 47.4 (CH2), 27.4 (CH3). The title compound (3.0 mg) was dissolved in DCM in a NMR tube and the tube was covered loosely with cap to enable the solvent to slowly evaporate at ambient conditions. Crystals suitable for X-ray diffraction formed over a period of 7 days.
Experimental details
All hydrogen atoms were placed in idealised positions and refined using a riding model with Uiso assigned 1.2 or 1.5 times Ueq of their parent atoms and the C—H bond distances constrained between 0.95 and 1.00 Å. The final conventional R factor is 0.0422.
Discussion
In the past decade oxazolidonone compounds have attracted much attention because of their biologically properties [6], [7], [8], [9]. They are totally synthetic antibacterial agents with a novel mechanism of action involving the inhibition of bacterial protein synthesis at a very early stage, thus disrupting chain initiation [9, 10] . Linezolid and tedizolid are the only members of oxazolidonone compounds that are in clinical use for treatment of bacterial infection in humans [11].
Herein, we report the single crystal structure and packing analysis of a linezolid analogue, C15H17FN2O4. The title compound can be considered to be flexible as the conformation of the five membered oxazoline and six membered morpholine rings with respect to the aromatic ring (flurophenyl) can be varied. The conformational flexibility can be described in terms of two important torsion angles t1 [C13—N2—C8—C7, −176.85(13)°] and t2 [C2—N1—C5—C6, −167.18(13)°]. The value of t1 shows that the oxazolidine and fluorophenyl rings are essentially coplanar. The morpholine ring is in a chair conformation. The “downward” orientation of the morpholine ring with respect to the fluorophenyl ring can be understood in terms of minimisation of unfavourable van der Waals contacts between H (van der Waals radius 1.2 Å) and F (1.47 Å) atoms. Crystal packing analysis reveals that molecules are interconnected through C—H⋯O and C—H⋯F interactions. Pairs of molecules related by translation along the y axis are connected together through C—H⋯O [D⋯A, 3.3141(18) Å and D—H⋯A, 141°] and C—H⋯F [D⋯A, 3.1496(17) Å and D—H⋯A, 133°] interactions to form infinite columns. These columns are connected by a number of C—H⋯O interactions giving rise to an overall 3D network.
References
1 Bruker. APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, WI, USA (2009).Search in Google Scholar
2 Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar
3 Barbour, L. J.: X-Seed − A SoftwareTool for supramolecular crystallography. J. Supramol. Chem. 1 (2001) 189–191.10.1016/S1472-7862(02)00030-8Search in Google Scholar
4 Atwood, J. L.; Barbour, L. J.: Molecular graphics: from science to art. Cryst. Growth Des. 3 (2003) 3–8.10.1021/cg020063oSearch in Google Scholar
5 Farrugia, L. J.: WinGX and ORTEP for Windows: an update. J. Appl. Cryst. 45 (2012) 849–854.10.1107/S0021889812029111Search in Google Scholar
6 Thomasco, L. M.; Gadwood, R. C.; Weaver, E. A.; Ochoada, J. M.; Ford, C. W.; Zurenko, G. E.; Hamel, J. C.; Stapert, D.; Moerman, J. K.; Schaadt, R. D.; Yagi, B. H.: The synthesis and antibacterial activity of 1,3,4-thiadiazole phenyl oxazolidinone analogues. Bioorg. Med. Chem. Lett. 13 (2003) 4193–4196.10.1016/j.bmcl.2003.07.018Search in Google Scholar PubMed
7 Ebner, D. C.; Culhane, J. C.; Winkelman, T. N.; Haustein, M. D.; Ditty, J. L.; Ippoliti, J. T.: Synthesis of novel oxazolidinone antimicrobial agents. Bioorg. Med. Chem. 16 (2008) 2651–2656.10.1016/j.bmc.2007.11.040Search in Google Scholar PubMed
8 Zhu, T.; Friedrich, S. O.; Diacon, A.; Wallis, R. S.: Population pharmacokinetic/pharmacodynamic analysis of the bactericidal activities of sutezolid (PNU-100480) and its major metabolite against intracellular mycobacterium tuberculosis in ex vivo whole-blood cultures of patients with pulmonary tuberculosis. Antimicrob. Agents Chemother. 58 (2014) 3306–3311.10.1128/AAC.01920-13Search in Google Scholar PubMed PubMed Central
9 Reddy, P. K.; Mukkanti, K.; Rao, D. M.: A novel synthesis of oxazolidinone derivatives (a key intermediate of linezolid). Orient. J. Chem. 29 (2013) 1015–1019.10.13005/ojc/290322Search in Google Scholar
10 Leach, K. L.; Brickner, S. J.; Noe, M. C.; Miller, P. F.: Linezolid, the first oxazolidinone antibacterial agent. Ann. N. Y. Acad. Sci. 1222 (2011) 49–54.10.1111/j.1749-6632.2011.05962.xSearch in Google Scholar PubMed
11 Shorr, A. F.; Lodise, T. P.; Corey, G. R.; De Anda, C.; Fang, E.; Das, A. F.; Prokocimer, P.: Analysis of the phase 3 ESTABLISH trials of tedizolid versus linezolid in acute bacterial skin and skin structure infections. Antimicrob. Agents Chemother. 59 (2015) 864–871.10.1128/AAC.03688-14Search in Google Scholar PubMed PubMed Central
©2017 Marivel Samipillai et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 2,5-diiodo-4-nitro-1H-imidazole hemihydrate, C6H4I4N6O5
- Crystal structure of catena-poly[μ2-2,2′-(1,3-phenylene)diacetato-κ4O,O′:O′′,O′′′)-(μ2-1,6-bis(2-methyl-1H-benzo[d]imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C32H34CdN4O4
- Crystal structure of poly[aqua-(2,2′-bipyridine-κN,N′)-(μ4-5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalato κ8O1,O2:O3,O4:O5,O6:O7,O8)manganese(II)], C21H21MnN2O7
- Crystal structure of poly-[(μ2-((1,3-bis(benzimidazol-1-yl)propane-κ2N:N′)(μ2-4-tert-butyl-phthalato-κ2O:O′)cobalt(II)] monohydrate, C29H30CoN4O5
- Crystal structure of 2-amino-4-(3,4,5-trimethoxy-phenyl)-5-(oxo-5,6,7,8-tetrahydro-4H-chromene)-3-carbonitrile – ethanol (1/1), C21H26N2O6
- Crystal structure of ethyl 1-benzyl-5-phenyl-1H-pyrazole-3-carboxylate, C19H18N2O2
- Crystal structure of 2-(1-benzyl-3-phenyl-1H-pyrazol-5-yl)-5-(4-nitrobenzylthio)-1,3,4-oxadiazole, C25H19N5O3S
- Structure and photochromism of 1,2-bis[2-methyl-5-(2-chlorophenyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16Cl2F6S2
- The crystal structure of the Schiff base (E)-2,6-diisopropyl-N-(pyridin-4-ylmethylene)aniline, C18H22N2
- Crystal structure of (E)-2,4-dibromo-6-(((4-methyl-2-nitrophenyl)imino) methyl)phenol, C10H14Br2N2O3
- Crystal structure of (bis(2,2′-bipyridine-κ2N,N′))-(3,5-dinitrosalicylato-κ2O,O′)nickel(II), C27H18N6NiO7
- Crystal structure of 1-(diethoxy phosphonomethyl) 2-benzoyl-3-chloro-2-cyclohexen-1-ol, C18H24ClO5P
- Crystal structure of tetraaqua-bis(1,3-benzimidazol-3-ium-1,3-diacetato-κO)copper(II) hemihydrate, C22H27CuN4O12.50
- Crystal structure of 1α,11-dihydroxyeremophil-9-en-8-one, C15H24O3
- Crystal structure of 1-ferrocenylsulfonyl-1H-imidazo[4,5-b]pyridine, C16H13FeN3O2S
- Crystal structure of bis(μ2-azido-κ2N:N)-dichlorido-bis(μ2-2-(pyridin-2-yl)ethan-1-ol-κ2O,N)dicopper(II), C14H18Cl2Cu2N8O2
- Crystal structure of (5,15-cis-bis(2-hydroxy-1-naphthyl)-10-phenyl-20-(4-hydroxyphenyl)-porphyrinato)-(pyridine)-zinc(ii) pyridine solvate, C67H47N7O3Zn
- Crystal structure of (μ2-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene oxide-κ2O,P])-iodido copper(I), C44H32CuIOP2
- Crystal structure of 6,8-diphenyl-2-(4-fluorophenyl)-2,3-dihydroquinolin-4(3H)-one, C27H20FNO
- Crystal structure of 5,11,17,23-tetra(tert-butyl)-25,26,27,28-tetrahexoxycalix[4]arene, C68H104O4
- Crystal structure of N,N′–bis(pyridin-4-ylmethyl)pyrazine-2,3-dicarboxamide dihydrate, C18H20N6O4
- Crystal structure of a diaqua-bis(3,5-di(1H-imidazol-1-yl)pyridine-κN)-bis(2-(4-carboxy-phenyl)acetato-κO]manganese(II), C40H36MnN10O10
- Crystal structure of 4-(4-hydroxy-3-methoxy-phenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylic acid methyl ester, C21H25NO5
- Crystal structure of (E)-4,4′-(ethene-1,2-diyl)bis(3-nitrobenzoic acid) 1.5 hydrate, C16H13N2O9.5
- Crystal structure of (E)-2-(5-(4-fluorophenyl)-3-(furan-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-5-((4-fluorophenyl)diazenyl)-4-methylthiazole, C23H17F2N5OS
- Crystal structure of the co-crystalline adduct 4-((4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide - acetic acid (1/1), C21H24N4O4S ⋅ C2H4O2
- Synthesis and crystal structure of 2-((5-chlorobenzo[c][1,2,5]thiadiazol-4-yl)amino)-4,5-dihydro-1H-imidazol-3-ium tetraphenylborate, C33H29BClN5S
- Crystal structure of (4-chlorophenyl)(3-ferrocenyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methanone, C21H14ClF3FeN2O
- Crystal structure of (S)-benzyl 3-(benzylcarba-moyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate, C25H24N2O3
- Crystal structure of 5-acetyl-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, C15H17FN2O4
- Crystal structure of 2-(4-fluorophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16FIN2
- Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2
- Crystal structure of 2,3-diphenyl-1-[(dipropylamino)acetyl]-1,3-diazaspiro[4.5]decan-4-one, C28H37N3O2
- Crystal structure of 1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propan-1-one, C13H12N2O3
- Crystal structure of 2,9-dibromo-1,10-phenanthroline, C12H6Br2N2
- Crystal structure of 3-(adamantan-1-yl)-4-(4-fluorophenyl)-1H-1,2,4-triazole-5(4H)-thione, C18H20FN3S
- Crystal structure of trans-bis((E)-7-oxo-4-(phenyldiazenyl)cyclohepta-1,3,5-trien-1-olato)-κ2O,O′)-bis(pyridine-κN)cobalt(II), C36H28CoN6O4
- Crystal structure of 2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)malononitrile, C13H9N3S
- Crystal structure of (Z)-3-(adamantan-1-yl)-1-(3-chlorophenyl)-S-benzylisothiourea, C24H27ClN2S
- Crystal structure of chlorido{[3-(η5-cyclopenta-dienyl)-2,2,3-trimethyl-1-phenylbutylidene] azanido-κN}[η2(N,O)-N,N-dimethylhydroxylaminato]titanium(IV), C20H27ClN2OTi
- Crystal Structure of 1,1′-dimethyl-[4,4′-bipyridine]-1,1′-diium tetrachloridozincate(II), C12H14Cl4N2Zn
- Crystal structure of 5-nitro-2-(pyrrolidin-1-yl)benzaldehyde, C11H12N2O3
- Crystal structure of 2,3-diphenyl-1-(morpholin-4-ylacetyl)-1,3-diazaspiro[4.5]decan-4-one, C26H31N3O3
- Crystal structure of 3,3-dimethyl-3,4-dihydro-1H-benzo[c]chromene-1,6(2H)-dione, C15H14O3
- Crystal structure of bis(2-(2-hydroxymethyl)pyridine-κ2N,O)-bis(pivalato-κO)nickel(II), C22H32N2NiO6
- Crystal structure of (1,10-phenanthroline-κ2N,N′)-bis(1H-pyrazole-3-carboxylato-κ2N,O)manganese(II) trihydrate, C20H20N6O7Mn
- The crystal structure of 3-aminopropan-1-aminium iodide, C3H11N2I
- Crystal structure of ethyl 1-(4-chlorophenyl)-5-methyl-1H-1,2,3-triazole-4 carboxylate, C12H12ClN3O2
- Crystal structure of 4,4′-((1Z,1′Z)-2,2′-(2,5-diethoxy-1,4-phenylene)bis(ethene-2,1-diyl))dipyridine, C24H24N2O2
- Crystal structure of (16S)-12,16-epoxy-11,14-dihydroxy-17(15/16)-abeo-3a,18-cyclo-8,11,13-abietatrien-7-one, C20H24O4
- Crystal structure of aquadichlorido(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N,N′,N′′)nickel(II) monohydrate, C18H16Cl2N6NiO2
- Crystal structure of catena-poly[dichlorido-(μ-ethane-1,2-diyl-bis-(pyridyl-4-carboxylate-κN:N′)mercury(II)], C15H14Cl2HgN2O4
- Crystal structure of methyl 2-acetamido-5-chlorbenzoate, C10H10ClNO3
- Crystal structure of tetrakis(μ2-3,3-dimethylacrylato-κ2O,O′)-bis(2-aminopyrimidine-κN) dicopper(II), C28H38Cu2N6O8
- Crystal structure of 3-amino-8-methoxy-1-phenyl-1H-benzo[f]chromene-2-carbonitrile, C21H16N2O2
- Crystal structure of 4-(2-ammonioethyl)morpholin-4-ium dichloride monohydrate, C6H18Cl2N2O2
- Crystal structure of 1-(3-((5-bromo-2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime, C22H19BrN2O2
- Crystal structure of 2-(4-(dimethylamino)-2-fluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol monohydrate, C15H20FN7O2
- Crystal structure of 4-bromo-2-(1H-pyrazol-3-yl)phenol, C9H7BrN2O
- Crystal structure of 1,2,3,4,5-pentamethyl-1,3-cyclopentadiene, C10H16

