The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
-
Jingjing Sun
, Xuefeng Mao
Abstract
2,6-Methylacylnaphthalene is an important organic chemical raw material, mainly used as a precursor for synthesizing polyethylene 2,6-naphthalene dicarboxylate (PEN). The heterogeneous catalyst molecular sieve catalyzes the acylation of 2-methylnaphthalene to synthesize β,β-methylacylnaphthalene, which has good activity, is green and environmentally friendly, with simple post-treatment. Different molecular sieves and reaction solvents were selected, and Hβ molecular sieves were more suitable for the acylation reaction of 2-methylnaphthalene. The reaction results were better when sulfolane was used as a solvent in this paper. The catalytic performances of citric acid-modified Hβ molecular sieve (SiO2/Al2O3 of 25) and unmodified molecular sieve were investigated and compared. The results showed that modification with low-concentration citric acid increased the amount of mediate strong acid and Bronsted acid, the specific surface area, pore volume, and pore size of Hβ zeolite. When the concentration of citric acid was 0.3 mol·L−1, the modification time was 48 h and the calcination at 550°C for 3 h had the best catalytic activity. By further optimizing the acylation process, the conversion rate of 2-methylnaphthalene increased to 88.82%, and the yield of β,β-methyl propyl naphthalene increased to 82.12%.
1 Introduction
2,6-Naphthalene dicarboxylic acid (2,6-NDA) is an important chemical raw material. It polymerizes with ethylene glycol to form polyethylene 2,6-naphthalene dicarboxylate (PEN), which is a polyester compound with better chemical and physical properties than polyethylene glycol phthalate (PET) [1,2,3,4]. PEN has been widely used in films, sheets, hollow materials, fibers, engineering plastics, and other fields. It is one of the resin materials with the fastest developed and the broadest application prospects globally [5,6]. With the expansion of PEN’s applications, the demand for 2,6-NDA has increased sharply [7]. However, the complex preparation process of 2,6-NDA and the significant investment in production has severely restricted the development of PEN.
Currently, four preparation methods are available for the preparation of 2,6-NDA, including the Henkel method, carboxyl transfer method, dialkyl naphthalene oxidation method, and 2-methyl naphthalene (2-MN) acylation oxidation method [8]. The Henkel method and the carboxyl group transfer method use expensive and toxic metal salts as catalysts, which are costly and difficult to recycle, so there is less industrial development [9,10]. The dialkyl naphthalene oxidation method has been studied extensively. Its raw material is mainly 2,6-dimethyl naphthalene (2,6-DMN). The early preparation routes of 2,6-DMN process mainly include direct extraction of coal tar [11], toluene and pentene alkylation [12,13,14], o-xylene and butadiene alkylation [15,16], naphthalene or methylnaphthalene and methanol alkylation method [17,18], 2-MN disproportionation method [19], and so on. Some progress in dialkyl naphthalene oxidation is presented, which can produce various byproducts under acid-catalyzed conditions. Nevertheless, the physical properties of the products and byproducts are similar, and the separation process is complex, which makes it relatively difficult to produce high-purity 2,6-NDA on a large scale. However, the 2-MN acylation oxidation method for preparing 2,6-NDA technology has high yields and easy purification of the raw materials, which has become a hot research topic in the industry and academia. Since the acylates of 2-MN quickly oxidize to produce 2,6-NDA [20]. The difficulty of this method lies in the synthesis of precursor 2-methyl-6-acyl naphthalene.
The synthesis still uses liquid acid as a catalyst, such as AlCl3 and CF3SO3H [21,22]. Japan’s Mitsubishi Gas Company (MGC) first used 2-MN to produce 2-methyl-6-acyl naphthalene through HF/BF3-catalyzed acylation in 1990. Liu [23] used AlCl3 to catalyze the acylation reaction of 2-MN and propionyl chloride; the reaction effect was remarkable. However, AlCl3 needs hydrolysis to separate from the product after the reaction. The hydrolysis process produces a large amount of acidic waste gas and liquid waste, resulting in corrosion, pollution, and waste, violating the concept of green chemistry [24]. Yuan et al. [25] used an H-type zeolite to catalyze the acylation reaction of 2-MN to produce 2-methyl-6-acetyl naphthalene and 2-methyl-6-butyryl naphthalene. In the autoclave, molecular sieve coking was severe, and later, her team made some progress in intermittent atmospheric operation. The molecular sieve’s acidic properties and pore size can be adjusted by modification treatment, suitable for various aromatic ring substrates. For acylating agents (short-chain acyl chloride, carboxylic acid, and anhydride), carboxylic acid is almost inactive in acylation of 2-methylnaphthalene. The boiling point of short-chain acyl chlorides is low, and the reflux temperature is too low under atmospheric pressure to reach the required temperature of the reaction. Only anhydride has strong reactivity, and the reactivity increases with an increase in hydrocarbon chain length [26]. According to the principle of green chemistry, different molecular sieve catalysts and different solvents were discussed using acid anhydride as an acylation reagent in this paper. In addition, the selected Hβ molecular sieve was modified with acid to synthesize β,β-methyl acyl naphthalene (β,β-MPN), and the best catalyst preparation conditions and reaction conditions were determined.
2 Experimental
2.1 Preparation of the modified Hβ molecular sieve
The molecular sieve was put into a muffle furnace and calcined at 600°C for 3 h to remove impurities. A specific concentration of the acid solution was prepared, then added dropwise to a beaker containing a molecular sieve (add 10 mL solution per gram of molecular sieve), and sealed. Then, it was stirred at a constant speed at room temperature for a specific time and washed with suction until the filtrate was neutral. The solid was placed in a drying oven at 120°C for 12 h, ground into powder, roasted in a muffle furnace, and finally placed in a desiccator for later use.
2.2 Characterization of the modified Hβ molecular sieve
Acidity was determined using an automatic temperature-programmed chemisorption (NH3-TPD) unit developed by Micromeritics (AutoChem II 2920) with a thermal conductivity detector. The catalyst sample (0.2 g) was first flushed with helium (30 mL·min−1) at 700°C for 3 h, cooled to 150°C, saturated with NH3 until it equilibrated, and flushed again with helium until the baseline of the integrator was stable. The NH3-TPD treatment quickly starts from 150°C to 700°C, and the heating rate was 15°C·min−1.
Surface morphology was observed using a TESCAN MIRA4 ultrahigh resolution field-emission scanning electron microscope (SEM) manufactured in the Czech Republic. The electron gun was a Schottky field emission gun, the magnification was 20,000–100,000, and the test voltage was 200–30 eV.
The specific surface area and pore volume aperture (BET) were measured using Micromeritics ASAP 2460 3.01. About 0.12 g of the sample was weighed in a sample tube, vacuum-dried, and then devacuumed (300°C degassing for 3 h); then, N2 was adsorbed in liquid nitrogen at −196°C. After the test was completed, the specific surface area of the catalyst was calculated by the BET method, and the BJH model calculated the pore size distribution.
A PANalytical Axios X-ray fluorescence spectrometer produced by Philips, the Netherlands, was used to determine SiO2/Al2O3 of Hβ zeolites before and after modification. The instrument type was multi-channel, and the power of the X-ray ray tube was 4 kW.
Pyridine absorption infrared spectroscopy was performed using a Thermo Fisher Nicolet iS50 infrared spectrometer to measure the concentration of Bronsted and Lewis acids in the samples. The transmission method was used for testing, the number of infrared scanning was 32, the scanning wavelength was 400–4,000 cm−1, and the resolution was 4 cm−1.
2.3 Catalyst evaluation
Certain amounts of 2-MN and propionic anhydride (PA) were added to a three-necked, round-bottomed flask equipped with a condenser tube, a thermometer, a drying tube, and a metal bath. Then an appropriate amount of the solvent was added. After stirring evenly, the molecular sieve catalyst was added, and the temperature was increased and stirred continuously for a while. Then, the catalyst was separated by filtration, and the reaction solution was analyzed using a gas chromatograph GC-2014C (SHIMADZU, Japan) was equipped with an FID detector. The temperature program mode (215°C for 30 min) was used to analyze the extracted reaction solution. The chromatographic column was HP-5 50 m × 0.200 mm. N2 was used as carrier gas at a nitrogen pressure of 0.5 MPa. The detector temperature was 300°C, and the injection port temperature was 300°C. The injection quantity was 0.2 μL.
3 Results and discussion
3.1 The acylation of 2-MN catalyzed by different molecular sieves
USY (SiO2/Al2O3 = 6), HY (SiO2/Al2O3 = 6), mordenite (SiO2/Al2O3 = 10), HZSM-5 (SiO2/Al2O3 = 25), and Hβ (SiO2/Al2O3 = 25) were used under the same conditions. The acylation of 2-MN and PA was catalyzed by tetramethyl sulfone (TS) as a solvent. The results are given in Table 1.
Propionylation of 2-MN catalyzed by different molecular sieves
| Catalysts | Dimensions/channels | Conversion (%) | Selective (%) | |
|---|---|---|---|---|
| β,β-MPN | 2,6-MPN | |||
| USY (SiO2/Al2O3 = 6) | 0.72–0.80 nm three-dimensional | 0.81 | 38.01 | 30.04 |
| HY (SiO2/Al2O3 = 6) | 0.80–0.90 nm three-dimensional | 2.76 | 49.15 | 38.81 |
| Mordenite (SiO2/Al2O3 = 10) | 0.58–0.70 nm one-dimensional | 10.67 | 45.81 | 36.01 |
| HY (SiO2/Al2O3 = 25) | 0.80–0.90 nm three-dimensional | 8.30 | 30.13 | 23.73 |
| HZSM-5 (SiO2/Al2O3 = 25) | 0.52–0.58 nm three-dimensional | — | — | — |
| Hβ (SiO2/Al2O3 = 40) | 0.56–0.75 nm three-dimensional | 41.96 | 90.86 | 45.64 |
| Hβ (SiO2/Al2O3 = 25) | 58.67 | 93.42 | 46.29 | |
| Hβ (SiO2/Al2O3 = 30) | 50.87 | 91.82 | 45.51 | |
Reaction conditions: 2-MN (0.0125 mol), PA (0.025 mol), TS (10 g), catalyst (calcination at 600°C for 3 h, 2 g), 190°C, 10 h.
The pore structure and pore size of the molecular sieve significantly affect the product distribution and selectivity of the acylation reaction. Through Gaussian software simulations, the molecular diameter of 2-MN was about 0.42 nm and that of 2,6-MPN was about 0.62 nm. Since the framework of the molecular sieve was non-rigid and was always in a state of vibration during the reaction, the respiration of its pores might cause larger-sized molecules to enter the pores [26]. Furthermore, the acid center of the molecular sieve had a significant influence on its catalytic performance. Therefore, a molecular sieve with a pore size greater than 0.50 nm and more suitable for F–C acylation was selected for investigation. From Table 1, Hβ performed better in catalyzing acylation of 2-MN and PA. Even though other molecular sieves had larger pores (such as mordenite) and open three-dimensional pores (such as HY and USY), their catalytic effects are far less than those of Hβ. The reason might be that they did not have suitable acid centers.
Figure 1 shows the NH3-TPD spectra of five molecular sieves. Due to the low silicon–aluminum contents of HY and USY, there were excessive strong acidic sites on the molecular sieve, so the reactants and the corresponding complexes were strongly chemically adsorbed causing them to be rapidly deactivated [24] (Figure 1). The spectra of Hβ, HZSM-5, and mordenite at strong acidic sites were relatively similar. HZSM-5 and mordenite had more weak acidic sites than Hβ, but their catalytic effect was weak, indicating that weak acid sites did not affect the acylation reaction of 2-MN. The spectra of Hβ, HZSM-5, and mordenite were different at 350°C, and Hβ had a large amount of medium–strong acidic sites, which might be the main reason for the better performance of Hβ to catalyze 2-MN acylation. In addition, the catalytic performance of Hβ with the different silica–aluminum ratios for the acylation of 2-MN was analyzed, and it was found that the catalytic performance of Hβ with a silica–aluminum ratio of 25 (Hβ25) was better. Therefore, Hβ25 was used as a catalyst to catalyze the acylation reaction of 2-MN.
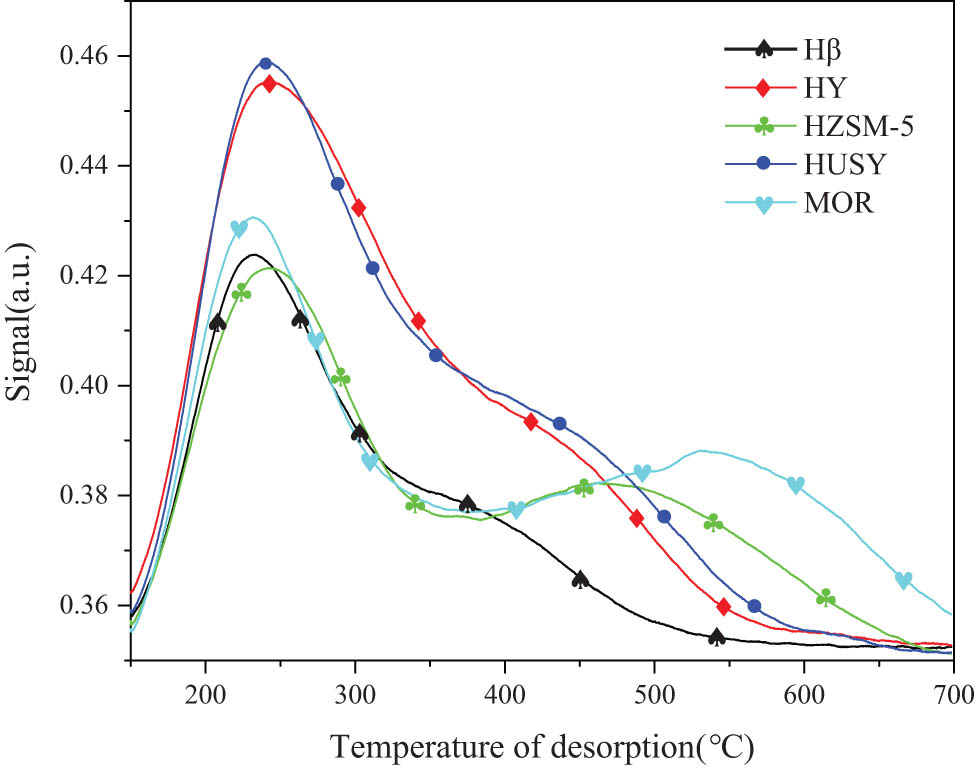
NH3-TPD spectra of five zeolite zeolites.
3.2 Choice of solvents
For the acylation reaction of 2-MN, nitrobenzene was usually used as the reaction solvent to disperse the AlCl3 plasma liquid catalyst so that the catalytic reaction proceeded under homogeneous conditions. However, the molecular sieve used in this paper was carried out under heterogeneous conditions, so the influence of various solvents on the acylation of 2-MN catalyzed by Hβ25 was investigated. Molecular sieves catalyzed 2-MN at a higher temperature, and several polar solvents that are relatively green with higher boiling points were selected. The results obtained are given in Table 2.
The influence of solvents on the 2-MN acylation catalyzed by Hβ
| Solvents | Conversion rate (%) | Selective (%) | |
|---|---|---|---|
| β,β-MPN | 2,6-MPN | ||
| Decahydro naphthalene | 26.35 | 93.88 | 47.56 |
| Nitrobenzene | 44.23 | 97.01 | 53.3 |
| Tetramethylene sulfone (TS) | 73.85 | 93.88 | 46.17 |
Reaction conditions: 2-MN (0.0125 mol), PA (0.025 mol), solvent (10 g), Hβ25 (3 g), 190°C, 10 h.
When the solvent was TS, the conversion rate of 2-MN and the yield of β,β-MPN were higher (Table 2). In a heterogeneous catalysis process, the catalytic activity was closely related to chemical adsorption. The solvent diluted the reactants during the reaction, but solvent molecules were also adsorbed on the active center of Hβ. 2-MN and PA exhibited competitive adsorption, which would reduce the reactivity of the acylation reaction to varying degrees. Compared with decahedron naphthalene and nitrobenzene, the competitive adsorption of TS with reactants was weaker. It occupied fewer catalytic active centers, and the reaction effect was better. Therefore, TS is chosen as the solvent.
3.3 Comparison of catalytic performance of modified Hβ
The catalytic performance comparison of different acid-modified Hβ catalysts is presented in Table 3. It can be seen from Table 3 that the catalytic activity of different acid-modified Hβ catalysts varied greatly. Among them, the catalytic performance of Hβ modified by inorganic acids (nitric acid [HNA], hydrochloric acid [HHA], and phosphoric acid [HPA]) was lower than that of unmodified Hβ. The catalytic performance of Hβ modified by organic acids (citric acid [HCA], tartrate acid [HTA], and acetate acid [HAA]) was significantly higher than that of unmodified Hβ. The reason may be that organic acid modification eluted part of the non-framework aluminum or the amorphous material in the pores during the pickling process. The number of micropores increased, increasing the conversion rate of 2-MN and the yield of product β,β-MPN. However, the inorganic acid had strong acidity, which directly or indirectly destroyed the framework of the molecular sieve during the preparation of the catalyst, leading to the destruction of a large number of acidic sites, thereby reducing the catalytic performance. It was worth noting that the catalytic performance of the molecular sieve modified by HHA had little change. The reason might be that the acidity of hydrochloric acid was strong, which would also cause the framework of the molecular sieve to collapse on a large scale. However, the chlorine in the solution and aluminum removed by molecular sieve pickling formed a complex with anhydride, thus promoting the reaction. Moreover, the reaction mechanism was similar to the acylation reaction of 2-MN catalyzed by AlCl3.
Comparison of catalytic performance of different acid-modified Hβ
| Catalysts | Conversion rate (%) | Selective (%) | Yield of β,β-MPN (%) | |
|---|---|---|---|---|
| β,β-MPN | 2,6-MPN | |||
| Hβ | 74.92 | 93.62 | 46.65 | 70.14 |
| HCA/Hβ | 77.12 | 92.98 | 48.17 | 71.71 |
| HTA/Hβ | 76.12 | 95.51 | 47.65 | 72.70 |
| HAA/Hβ | 76.88 | 94.81 | 47.62 | 72.89 |
| HPA/Hβ | 29.62 | 75.12 | 37.99 | 22.25 |
| HHA/Hβ | 73.69 | 92.71 | 46.41 | 68.32 |
| HNA/Hβ | 58.48 | 90.96 | 47.20 | 53.19 |
Reaction conditions: 2-MN (0.0125 mol), PA (0.025 mol), TS (10 g), Hβ (acid concentration 0.1 mol·L−1, modification 24 h, calcination at 600°C for 3 h, 3 g), 190°C, 10 h.
3.4 Effect of modification of HCA on the catalytic performance of Hβ
3.4.1 SEM characterization of HCA-modified Hβ
In order to study the effect of different concentrations of HCA modification on the micro-morphology of Hβ, SEM analysis was carried out for the modified Hβ with different concentrations. Figure 2 shows the SEM image of Hβ modified with different concentrations of HCA at a magnification of 50,000×. Hβ particles had a small cubic structure, uniform particle size, regular morphology, and high dispersion. The crystal grain shape of the modified Hβ was still regular and clear, and the particle size distribution was uniform, showing good crystal morphology. However, the crystal size of the molecular sieve became smaller after modification, and the change became more evident with an increase in HCA concentration. This might be because of the removal of non-framework aluminum from the surface of Hβ after modification, which exposed the framework of the molecular sieve and reduced the crystal size. Moreover, the modified crystal grains were more dispersed as the concentration of HCA increased. The higher concentration of HCA-modified molecular sieves may be due to excessive acid corroding the framework structure of the molecular sieve, causing a small amount of framework to collapse, resulting in more excellent dispersion.

SEM images of Hβ modified by HCA with different concentrations: (a) Hβ, (b) HCA(0.1)/Hβ, (c) HCA(0.3)/Hβ, (d) HCA(0.5)/Hβ.
3.4.2 Influence of the Si–Al composition in Hβ with HCA modification
The silicon–aluminum ratio of the molecular sieve is an important parameter that affects the activity of the catalyst. Table 4 compares the percentage content and the ratio of the silicon–aluminum components in HCA-modified and unmodified Hβ. The silicon–aluminum ratio of Hβ modified by HCA had been greatly improved (Table 4). After HCA modification, the silicon–aluminum ratio of the molecular sieve changed from 23.628 to 47.970 and 69.857, respectively. In an acidic medium, H+ could react with aluminum atoms in zeolite but could be nonreactive toward silicon. H+ located near
Silicon–aluminum content and proportion of Hβ after modification by HCA
| Catalysts | Al2O3 (wt%) | SiO2 (wt%) | SiO2/Al2O3 | Si (wt%) | Al (wt%) | Si/Al |
|---|---|---|---|---|---|---|
| Hβ | 6.665 | 92.802 | 23.628 | 43.379 | 3.528 | 11.814 |
| HCA(0.3)/Hβ | 3.401 | 91.023 | 47.970 | 44.921 | 1.800 | 23.985 |
| HCA(0.5)/Hβ | 2.163 | 89.042 | 69.857 | 46.132 | 0.998 | 34.929 |
3.4.3 Effect of modification by HCA on the particle characteristics of Hβ
In order to study the effect of different concentrations of HCA on the specific surface area and pore volume distribution of Hβ, BET characterization was carried out. Compared with unmodified Hβ, the specific surface area, pore volume, and pore diameter of HCA/Hβ showed an increasing trend (Table 5). The micropore-specific surface area and pore volume of Hβ modified with a low concentration of HCA increased, indicating that Hβ modified with a low concentration of HCA produced new micropores. It might be due to the low concentration of HCA that could elute part of the non-framework aluminum in the molecular sieve and the amorphous structure in the pores during the modification process, thereby forming new micropores. In addition, some free or suspended chemical bonds in the molecular sieve crystal recombined to form bridges. These bridges divided some macropores into several micropores, which might also increase the micropores of Hβ [27]. The increase in mesoporous pore volume indicated that HCA pickling had a specific role in pore expansion. Also, Hβ produced new mesopores during the modification process. The modification of Hβ dealumination with a low concentration of HCA solution could make the pores of the molecular sieve more abundant [28]. However, when the concentration of HCA was too high, the non-framework aluminum and transition-state aluminum could be removed, and the framework aluminum of Hβ could be removed, causing the framework to collapse and destroy the pore structure.
Specific surface area and pore volume distribution of Hβ modified by HCA
| Catalysts | Surface area (m2·g−1) | Micropore area (m2·g−1) | External surface area (m2·g−1) | Pore volume (cm3·g−1) | Micropore volume (cm3·g−1) | Mesoporous volume (cm3·g−1) | Pore size (nm) |
|---|---|---|---|---|---|---|---|
| Hβ | 530 | 358 | 173 | 0.45 | 0.165 | 0.285 | 4.63 |
| HAC(0.1)/Hβ | 559 | 384 | 175 | 0.50 | 0.169 | 0.334 | 5.13 |
| HAC(0.3)/Hβ | 573 | 377 | 197 | 0.58 | 0.196 | 0.406 | 5.34 |
| HAC(0.5)/Hβ | 603 | 365 | 239 | 0.63 | 0.174 | 0.536 | 5.56 |
3.4.4 Characterization of NH3-TPD of modified Hβ
The NH3-TPD spectra of Hβ modified by different concentrations of HCA are shown in Figure 3. Compared with unmodified Hβ, with an increase in HCA concentration, the acid strength and acid content of modified Hβ showed an apparent downward trend, and the acid strength and acid content of medium–strong acids and weak acids were opposite (Figure 3). It suggested that, during modification, HCA promoted the conversion of some strong acids in the Hβ lattice into medium–strong acids. As the concentration of HCA increased, the degree of conversion was greater. It might be because HCA could make the removed non-framework aluminum undergo isomorphous substitution to the framework aluminum, forming more bridged hydroxyl groups, thereby increasing the number of strong acids [29]. In the process of HCA modification, aluminum supplementation also occurred during dealumination. Dealumination occurred at the Si(2Al) position of the Hβ framework, and aluminum supplementation occurred at the Si(0Al) position. Due to different concentrations of HCA, the position and number of dealumination and aluminum supplementation were different, which led to the difference between the amount of acid and acid strength in the sample [30].
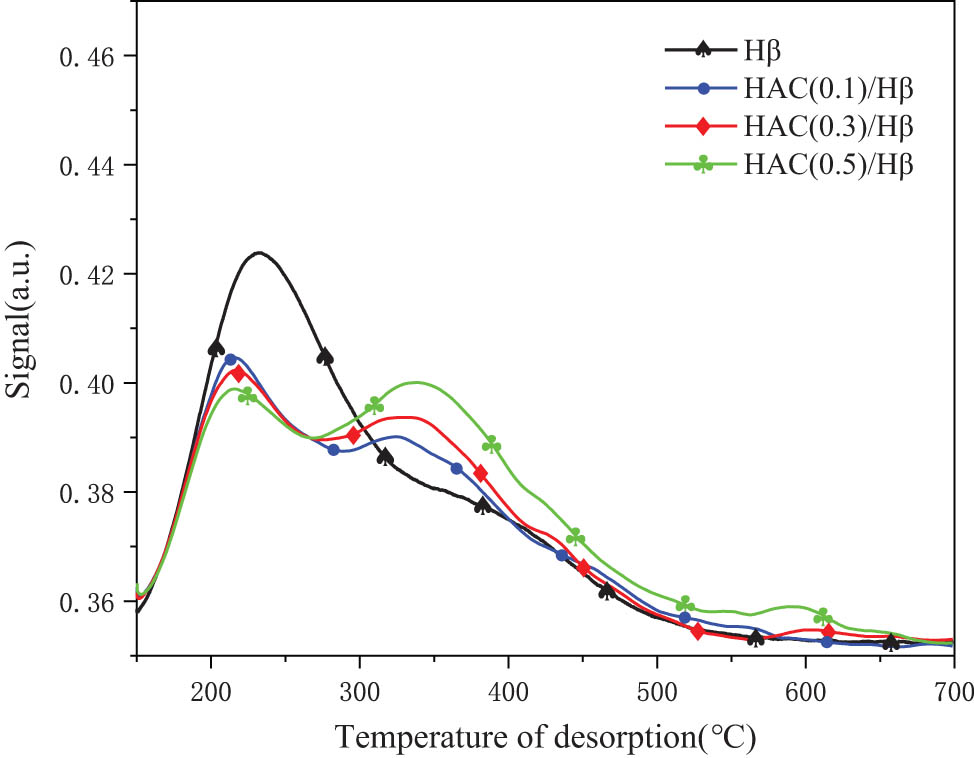
NH3-TPD spectrum of Hβ modified by HCA at different concentrations.
3.4.5 Infrared characterization of pyridine adsorption of modified Hβ
The pyridine adsorption infrared data of different concentrations of HCA-modified Hβ are given in Table 6. The amount of acid at 350°C was the amount of Lewis acids and Bronsted acids in the molecular sieve after desorption, which was the sum of the medium–strength acid amount and strong acid amount, and the amount of acid at 200°C was the total acid amount of the molecular sieve. The changing trends of the amount of Bronsted acids, Lewis acids, and total acids were the same at 200°C and 350°C (Table 6). When the concentration of HCA was low, the amount of Bronsted acids and Lewis acids showed a trend of first decreasing and then increasing. It might be because the low-concentration HCA modification could remove the non-framework aluminum in the molecular sieve, causing the silicon–aluminum ratio to increase, the Bronsted acidic site to decrease, and the acid density to decrease. At the same time, in the process of mechanical stirring, the non-framework silicon in the molecular sieve would also be eluted, resulting in a decrease in the amount of Lewis acids. However, the overall trend of the ratio of Bronsted acids to Lewis acids for pickling remained unchanged. With an increase of HCA concentration, the amount of Bronsted acids and Lewis acids increased, which might be due to the removal of non-framework aluminum and non-framework silicon from the pores of Hβ. The covered Bronsted-acid and Lewis-acid centers gradually appeared. The amount of Bronsted acids and Lewis acids increased, and the amount of Lewis acids increased slightly during aluminum replacement [31]. Therefore, the overall ratio of Bronsted acids to Lewis acids was still increasing. As the concentration of HCA continued to increase, the molecular sieve framework collapsed and part of the framework aluminum was removed and became non-framework aluminum. It significantly reduced the number of charges in the unit cell and the intensity of the electric field and destroyed the structural symmetry of the unit cell. The bond angle of the Si–O–Al bond changed, resulting in a decrease in the Bronsted acid center and the Lewis acid center.
Pyridine adsorption infrared data of Hβ before and after modification
| Catalysts | 200°C | 350°C | ||||||
|---|---|---|---|---|---|---|---|---|
| Bronsted acidity (μmol·g−1) | Lewis acidity (μmol·g−1) | Total acidity (μmol·g−1) | B/L | Bronsted acidity (μmol·g−1) | Lewis acidity (μmol·g−1) | Total acidity (μmol·g−1) | B/L | |
| Hβ | 124.88 | 30.99 | 155.87 | 4.03 | 118.33 | 17.81 | 136.14 | 6.64 |
| HAC (0.1)/Hβ | 43.77 | 6.55 | 50.32 | 6.68 | 39.47 | 4.19 | 43.66 | 9.41 |
| HAC (0.3)/Hβ | 75.52 | 10.8 | 86.32 | 6.99 | 71.69 | 5.69 | 77.38 | 12.6 |
| HAC (0.5)/Hβ | 60.5 | 5.84 | 66.34 | 10.35 | 55.56 | 3.48 | 59.04 | 15.96 |
3.5 Effect of modification conditions on Hβ-catalyzed 2-MN propionylation
3.5.1 Effect of HCA concentration on the catalytic performance of modified Hβ
The effect of HCA concentration on the ability to dealuminated with Hβ is shown in Figure 4, which results in the catalytic activity of the molecular sieve. When the concentration of HAC increased, the conversion rate of 2-MN and the yield of β,β-MPN increased (Figure 4). When it was 0.3 mol·L−1, the conversion rate of 2-MN reached the maximum of 82.45%. After that, the conversion rate of 2-MN and the yield of 2,6-MPN decreased with an increase in HCA concentration. The yield of β,β-MPN decreased more obviously with higher concentration. The reason might be that the high-concentration HAC was mainly released. The aluminum framework was removed, which caused part of the framework to collapse, the active catalytic center was destroyed, and the catalytic performance of the catalyst decreased. Therefore, the most suitable HAC concentration was 0.3 mol·L−1.
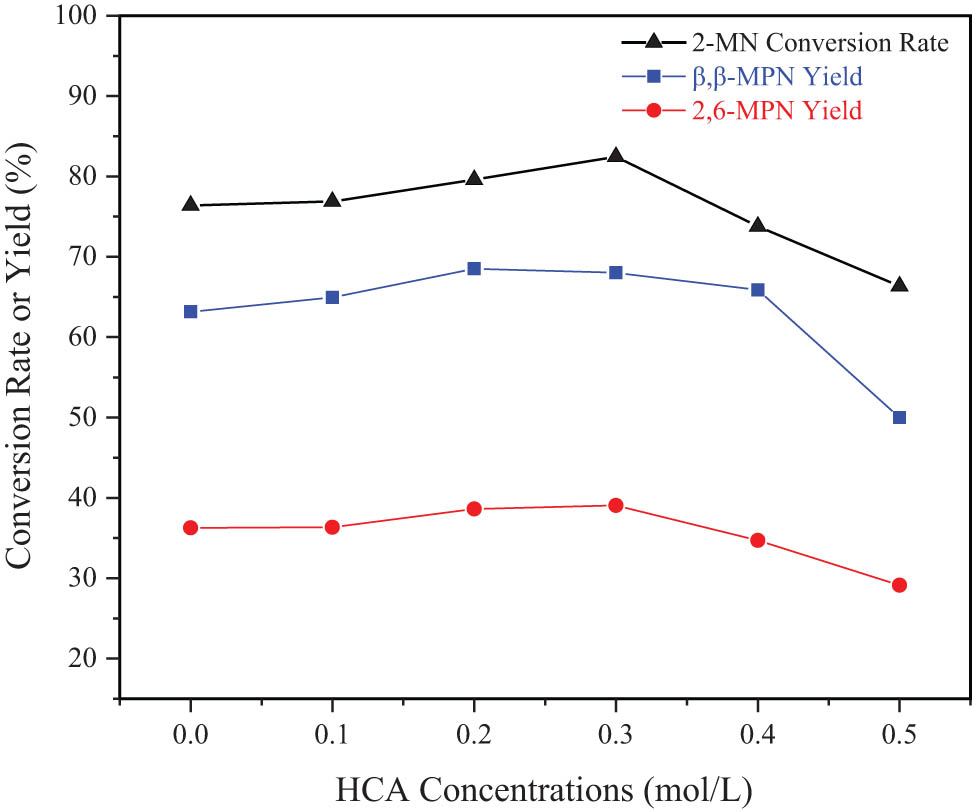
Effect of HCA concentration on the catalytic performance of modified Hβ. Reaction conditions: 2-MN (0.0125 mol), PA (0.025 mol), TS (10 g), Hβ (modification 24 h, calcination at 550°C for 3 h, 3 g), 190°C, 10 h.
3.5.2 Effect of stirring time on the catalytic performance of modified Hβ
The effect of stirring time on Hβ catalytic performance during HCA modification is also apparent. The results are shown in Figure 5. As the stirring time increased, the conversion rate of 2-MN and the yield of β,β-MPN showed increased. When the stirring time was 48 h, the conversion rate of 2-MN and the yield of β,β-MPN were the largest, and they were 87.95% and 77.16%, respectively. As the stirring time increased, the conversion rate of 2-MN no longer changed significantly, but the selectivity of β,β-MPN decreased, especially that of 2,7-MPN decreased most significantly. With the prolongation of stirring time, HCA entered the pores more deeply, and the ability to modify the pores of the molecular sieve was more vital. However, as the stirring time continued to increase, the molecular sieve pores slightly collapsed due to the increased acid immersion time. The pore size decreased slightly, resulting in a decrease in the selectivity of the molecular sieve pores to β,β-MPN. Since the molecular diameter of 2,7-MPN was larger than that of 2,6-MPN, the inhibitory effect on 2,7-MPN was more evident than that on 2,6-MPN. However, the conversion rate of 2-MN was almost unchanged, indicating that it was formed by other by-products, which aggravated the difficulty of later separation. Therefore, the optimal stirring time was 48 h.
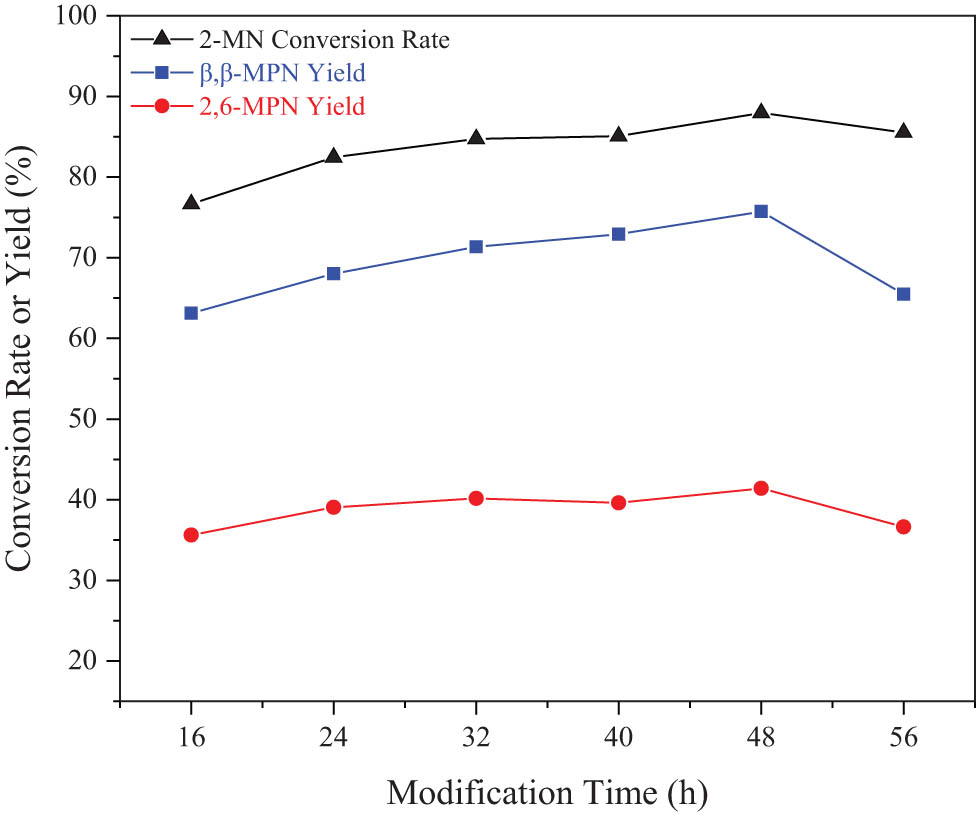
Effect of stirring time on the catalytic performance of modified Hβ. Reaction conditions: 2-MN (0.0125 mol), PA (0.025 mol), TS (10 g), HAC(0.3)/Hβ (calcination at 550°C for 3 h, 3 g), 190°C, 10 h.
3.5.3 Effect of calcination temperature on the catalytic performance of acid-modified Hβ
Figure 6 shows the effect of calcination temperature on the catalytic performance of HCA/Hβ. The conversion rate of 2-MN increased as the calcination temperature increased. When the calcination temperature was 550°C, the conversion rate of 2-MN was the largest, which was 86.95%. The calcination temperature continued to increase, but the conversion rate of 2-MN decreased. It might be due to the change in the acidity of Hβ by changing the calcination temperature. With an increase in the calcination temperature, the water inside the pores of Hβ was removed, and more acidic sites were exposed, which improved the catalytic activity. However, the molecular sieve framework was dealuminated when the calcination temperature increased.
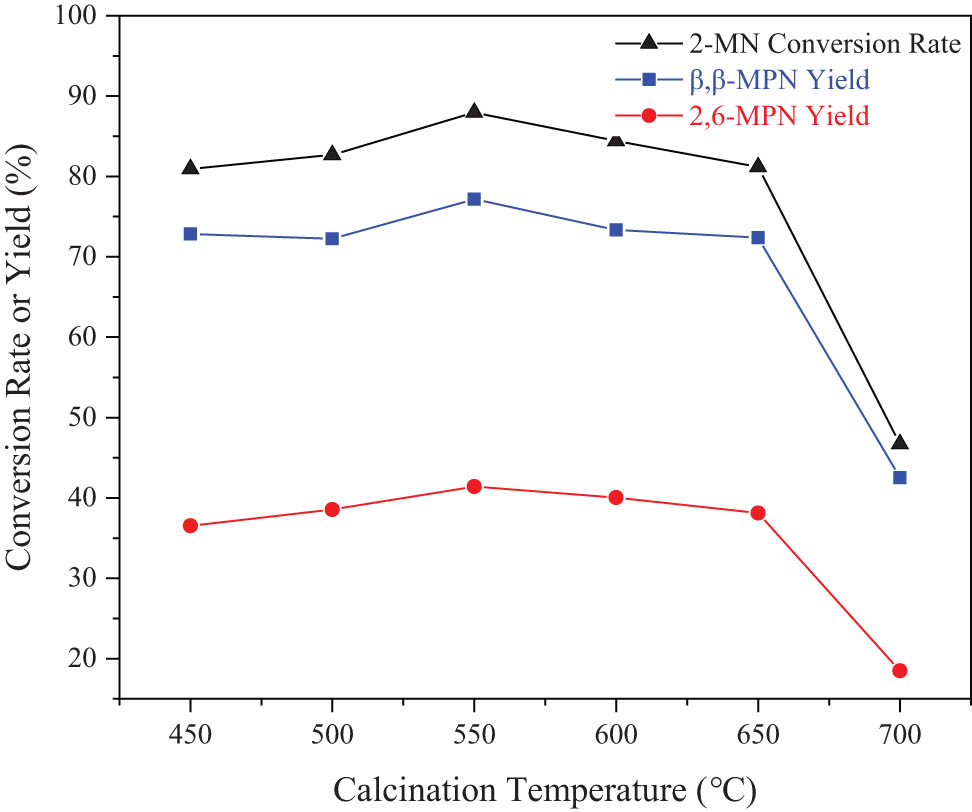
Effect of calcination temperature on the catalytic performance of acid-modified Hβ. Reaction conditions: 2-MN (0.0125 mol), PA (0.025 mol), TS (10 g), HAC(0.3)/Hβ (modification 48 h, calcination for 3 h, 3 g), 190°C, 10 h.
3.6 Optimization of acid-modified Hβ-catalyzed reaction conditions for 2-MN propionylation
3.6.1 Effect of the amount of catalyst on the 2-MN propionyl reaction
The effect of the amount of catalyst on the 2-MN propionyl reaction is shown in Figure 7. It can be seen from Figure 7 that the conversion rate of 2-MN first increased and then decreased with an increase in the amount of catalyst. When the amount of catalyst was 3 g, the conversion rate of 2-MN and the yield of β,β-MPN were the largest. With PA as the acylation agent, the reaction process mainly depended on the activity of the catalyst. When the amount of the catalyst was small, fewer acid active centers were provided, and the catalytic activity was lower; the conversion rate of 2-MN increased with an increase in the amount of the catalyst. However, when the amount of catalyst was more than 3 g, the selectivity of the target product β,β-position isomer decreased. It might be due to the excessive amount of the catalyst and the excessive number of acid centers on the outer surface, which led to the increased selectivity of product 2,7-MPN. In addition, the increase in other mono-acylation and di-acylation products would significantly increase the cost of subsequent product separation and purification. Considering the reaction conversion rate and selectivity, the most suitable amount of catalyst was 3 g.
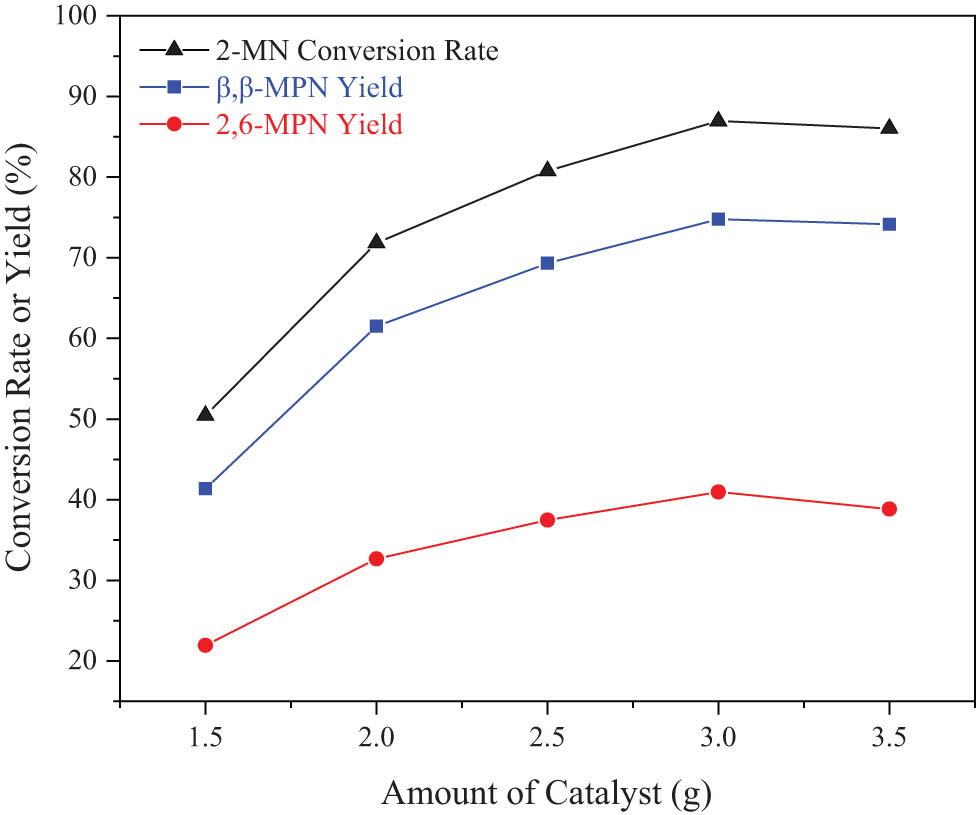
Effect of the amount of catalyst on the 2-MN propionyl reaction. Reaction conditions: 2-MN (0.0125 mol), PA (0.025 mol), TS (10 g), HAC(0.3)/Hβ (modification 48 h, calcination for 3 h), 190°C, 10 h.
3.6.2 Effect of reactant ratio on the 2-MN propionyl reaction
The amount of acylating agent PA also affected the conversion rate of 2-MN and the yield of β,β-MPN, so it was studied. As shown in Figure 8, when n (2-MN):n (PA) = 1:1.8, the conversion rate of 2-MN and the yield of β,β-MPN first increased to the optimal level and then decreased gradually with an increase of n (2-MN):n (PA). When the amount of PA was too small, the conversion rate of 2-MN was low. Too little PA made it difficult to produce enough acyl carbocations to contact the substrate 2-MN, so that 2-MN was excessive, and the conversion rate was low. With the gradual increase of the amount of PA, the acyl carbanion increased, and the conversion rate of 2-MN also increased. When n (2-MN):n (PA) = 1:2.2, the conversion rate of 2-MN (86.4%) was lower than the conversion rate of 2-MN (86.95%) when n (2-MN):n (PA) = 1:1.8; this might be caused by the dilution effect of PA; and if the amount of PA was too high, the selectivity of the target product would decrease, and the content of diacylation products and other isomers of the target product would increase. Excessive PA would be adsorbed on the active center of the molecular sieve to form excess carbonations. In addition, the amount of 2-MN was too small, and the local excess acyl carbanion continued to attack the aromatic-ring carbon atoms of the complex intermediate, causing a series of reactions to generate diacylated products [24]. Therefore, the appropriate reaction material ratio was n (2-MN):n (PA) = 1:1.8.
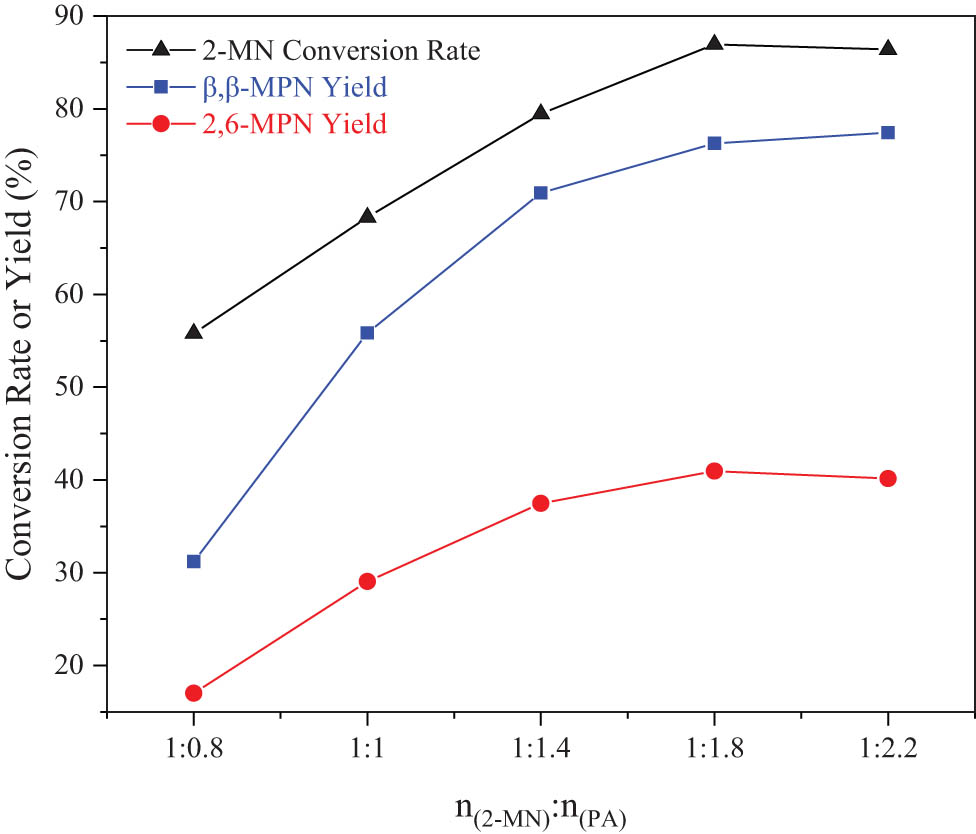
Effect of reactant ratio on the 2-MN propionylation reaction. Reaction conditions: 2-MN (0.0125 mol), TS (10 g), HAC(0.3)/Hβ (modification 48 h, calcination for 3 h, 3 g), 190°C, 10 h.
3.6.3 Effect of the solvent amount on the 2-MN propionyl reaction
The solvent had the effect of improving mass and heat transfer in the reaction. In this experiment, the amount of TS, as the solvent, in the reaction also had a specific effect, so it was investigated. As shown in Figure 9, as the amount of solvent increased, the conversion rate of 2-MN and the yield of β,β-MPN gradually decreased. The main reason was that too much solvent competes for adsorption on the active center of the molecular sieve. As the number of active centers was limited, the less the amount of solvent, the better. According to this speculation, the catalytic effect was the best when there was no solvent. However, when there was no solvent, heat and mass transfer in the kettle-type reaction process had a more significant influence, and the reaction effect was poor. Therefore, the optimal amount of TS was 5 g.
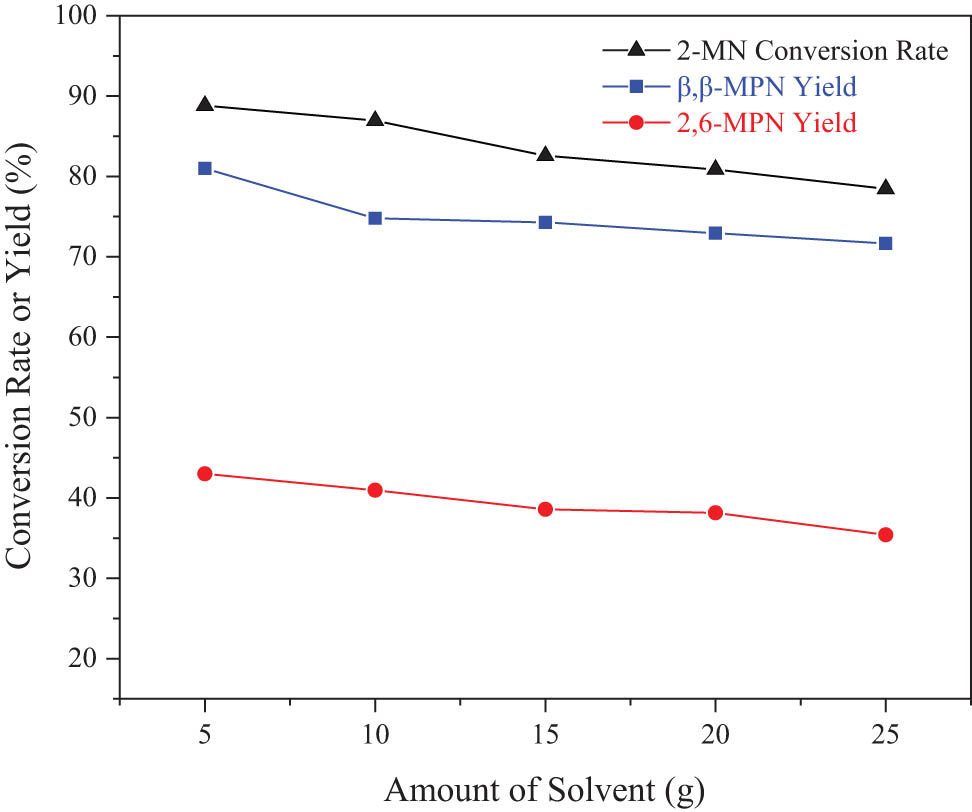
Effect of the solvent amount on the 2-MN propionyl reaction. Reaction conditions: 2-MN (0.0125 mol), PA (0.025 mol), TS, HAC(0.3)/Hβ (modification 48 h, calcination for 3 h, 3 g), 190°C, 10 h.
3.6.4 Effect of reaction temperature on the 2-MN propionyl reaction
The effect of reaction temperature on the 2-MN propionyl reaction is shown in Figure 10. With an increase in the reaction temperature, the conversion rate of 2-MN and the yield of β,β-MPN increased significantly. When the reaction temperature was increased, the conversion rate of 2-MN remained unchanged but the yield of β,β-MPN decreased. The main reason might be that as the temperature increased, a small part of the diacrylate of the macromolecule was produced. It caused the blockage of the molecular pores and reduced the reaction activity. In terms of the reaction phenomenon, as the reaction temperature increased, the reaction mixture was in the form of black viscous sludge after the reaction. It then separated and recovered Hβ that was slightly black. From this, it could be judged that when the temperature exceeded 200°C, the deactivation of carbon deposits occurred due to the excessively high temperature. The generated carbon deposits covered part of the active acid centers of the molecular sieve, and the reaction activity was also reduced. When the reaction temperature was 190°C, the conversion rate of 2-MN reached the maximum and the selectivity of β,β-MPN was also higher. Therefore, 190°C was a suitable reaction temperature.
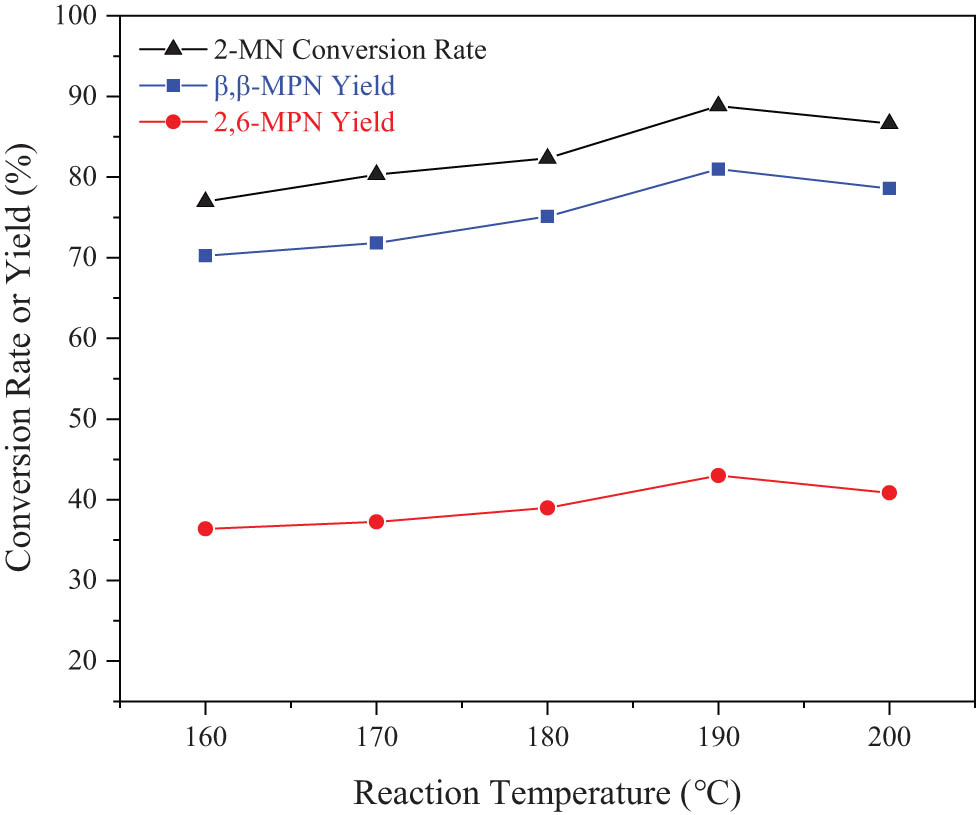
Effect of reaction temperature on the 2-MN propionyl reaction. Reaction conditions: 2-MN (0.0125 mol), PA (0.025 mol), TS (10 g), HAC(0.3)/Hβ (modification 48 h, calcination for 3 h, 3 g), 10 h.
3.6.5 Effect of reaction time on the 2-MN propionyl reaction
The effect of reaction temperature on the 2-MN propionyl reaction is shown in Figure 11. Before the reaction for 10 h, the conversion rate of 2-MN and the yield of β,β-MPN showed a slowly increasing trend. When the reaction time was 10 h, the conversion rate was maximum. After that, when the reaction time was extended, the conversion rate of 2-MN remained unchanged, the selectivity of β,β-MPN remained almost unchanged after slowly increasing with an increase of reaction time, and the isomers of other acylation products decreased. It was due to the high thermodynamic stability of 2,6-MPN and 2,7-MPN among the acylated products. Under the shape-selective action of Hβ, several other isomers had undergone isomerization. After careful consideration, the most suitable reaction time was 10 h.
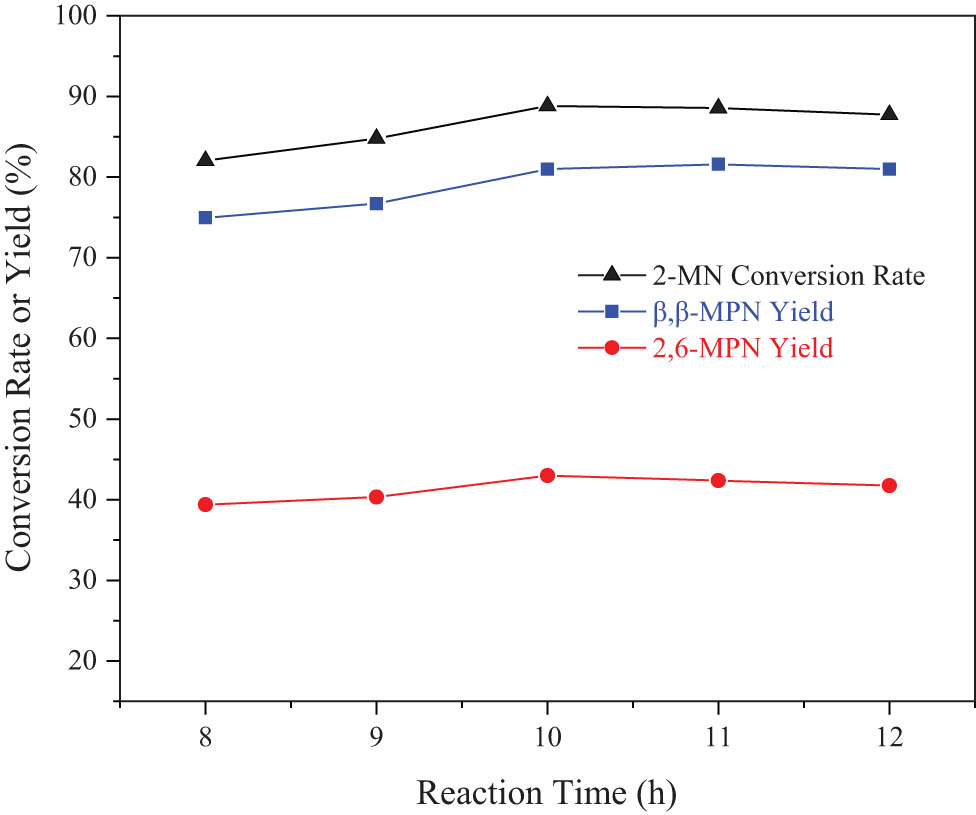
Effect of reaction time on 2-MN propionylation reaction. Reaction conditions: 2-MN (0.0125 mol), PA (0.025 mol), TS (10 g), HAC(0.3)/Hβ (modification 48 h, calcination for 3 h, 3 g), 190°C.
3.7 Regeneration experiment of the HCA/Hβ catalyst
Under the conditions of n (2-MN):n (PA) = 1:1.8, 3 g of catalyst, 5 g of TS, 190°C of reaction for 10 h, 0.3 mol·L−1 of HCA concentration, 48 h of modification time, and 550°C calcination for 3 h, the reusability of HCA/Hβ was investigated. Table 7 lists the experimental results.
Stability of HCA/Hβ catalyst after repeated use
| Catalyst recovery methods | 2-MN conversion rate (%) | Yield (%) | |
|---|---|---|---|
| β,β-MPN | 2,6-MPN | ||
| Fresh catalyst | 88.82 | 82.13 | 43.04 |
| Recovered catalyst (only dried at 120°C) | 23.53 | 21.87 | 10.73 |
| Recovered catalyst (550°C calcination for 3 h) 1 cycle | 48.15 | 45.02 | 22.29 |
| Recovered catalyst (550°C calcination for 3 h) 2 cycles | 43.86 | 40.26 | 20.01 |
Reaction conditions: 2-MN (0.0125 mol), PA (0.025 mol), TS (10 g), HAC(0.3)/Hβ (modification 48 h, calcination for 3 h, 3 g), 190°C, 10 h.
It can be seen from Table 7 that the catalytic activity of the recovered HCA/Hβ catalyst for 2-MN propyl acylation was significantly decreased after only drying. In contrast, that of the calcined HCA/Hβ catalyst was significantly increased after calcination at 550°C, but the activity was still low compared with that of the fresh catalyst. The catalytic activity decreased with an increase in recovery times. The main reason was that 1 mol PA involved in the reaction would generate 1 mol propionic acid in the reaction process, which would make the surface of the molecular sieve dealuminated; The drying temperature was lower than that of desorption, so the activity of the catalyst recovered by only drying was lower. It can be seen from Figure 12 that after calcination, some substances adsorbed on the surface of the catalyst were removed, but a coking condition appeared. Although the catalytic activity of the catalyst was improved compared with that of the catalyst recovered by only drying, it fell short of the state of the fresh catalyst.

SEM images of HCA/Hβ catalysts before and after recovery: (a) fresh catalyst, (b) recovered catalyst (550°C calcination for 3 h) 1 cycle.
3.8 Catalytic reaction mechanism
The reaction mechanism of the Friedel–Crafts acylation of 2-MN catalyzed by a molecular sieve is still ambiguous. Chiche et al. [33] believed that the Bronsted acid center of the molecular sieve was the active acid center of the reaction, while Ma et al. [35] believed that both the Lewis acid and Bronsted acid centers had catalytic activity, and the Lewis acid center was more effective. Yuan et al. [25] proposed that in the catalytic process of the molecular sieve catalyst, medium strong acids played a significant role. The ratio of Bronsted acids to Lewis acids could effectively catalyze the acylation reaction of the aromatic ring within an appropriate range. Also, the acylation reaction process of 2-MN and anhydride catalyzed by a molecular sieve was proposed, as shown in Figure 13.
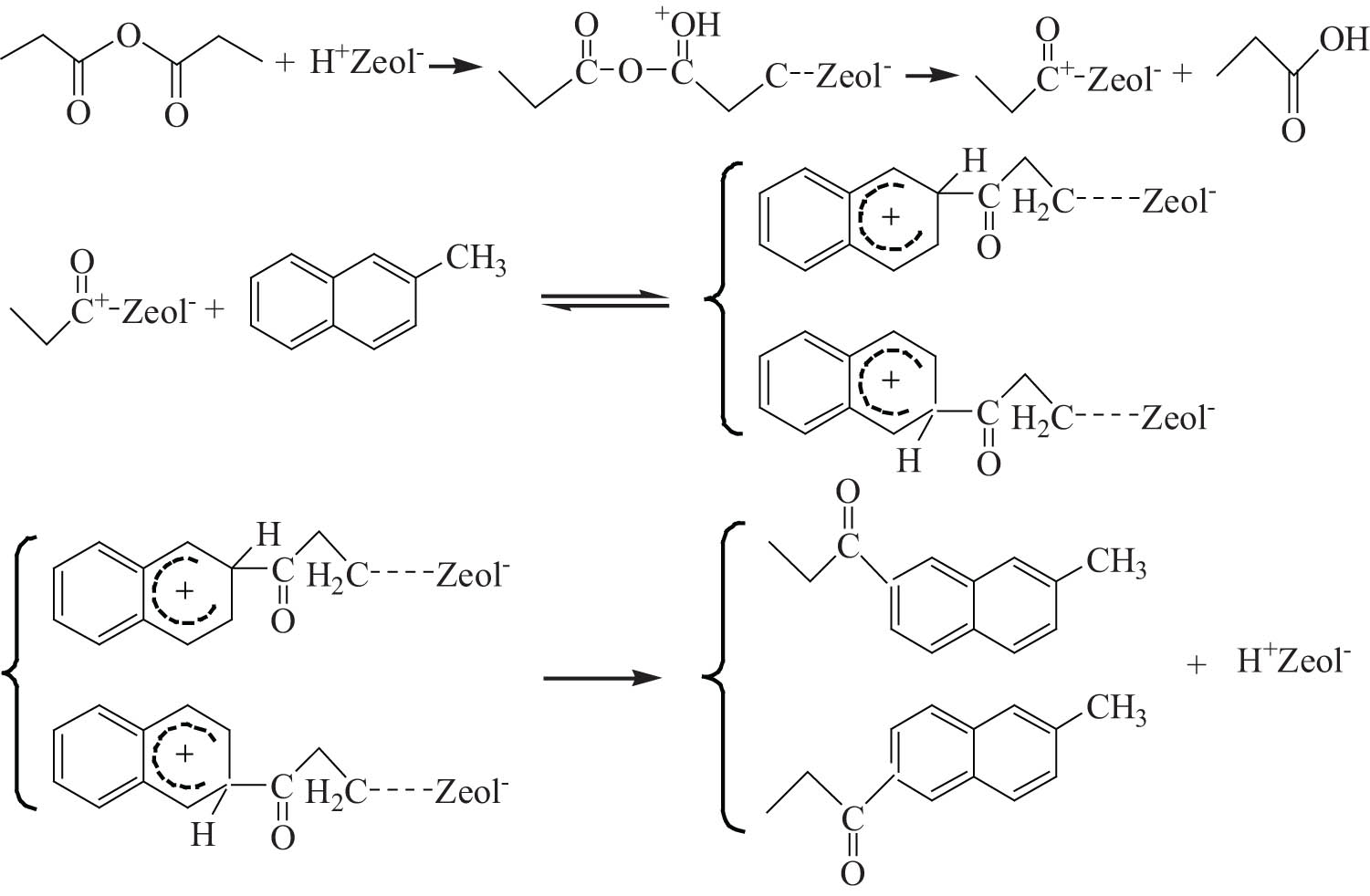
Mechanism of 2-MN propionyl catalyzed by molecular sieve.
The reaction performance and characterization results of Hβ zeolite modified by HCA with different concentrations showed that medium–strong acids played an essential role in the acylation reaction of 2-MN, and the acid centers on the surface of the HCA/Hβ catalyst were mainly Bronsted acids. However, a pure Bronsted acid was challenging to achieve an excellent catalytic effect. When the acid center of equal strength Bronsted-acid/Lewis-acid reached the appropriate value, the molecular sieve had an excellent catalytic performance. Therefore, it was concluded that medium–strong acids played a decisive role in the acylation reaction of 2-MN and PA catalyzed by the HCA/Hβ molecular sieve catalyst. Moreover, the synergistic action of the Bronsted acid center in the medium–strong acids and the appropriate Lewis acid center could achieve the best catalytic activity.
4 Conclusions
In the 2-MN propionyl reaction, compared with HY, USY, and mordenite, the Hβ molecular sieve (the molar ratio of SiO2 to Al2O3 is 25) has more vital catalytic activity and HZSM-5 has no catalytic activity. In the characterization of NH3-TPD, Hβ has a more considerable amount of medium–strong acids, which may be the main reason for the better acylation performance of 2-MN catalyzed by Hβ.
Compared with nitrobenzene and decalin, TS with a high boiling point is more suitable as a solvent for the 2-MN acylation reaction.
Acid modification can change the molecular sieve’s pore structure and active acid centers. In the acylation reaction of 2-MN, HCA modification by the organic acid has significant catalytic activity.
The modification of Hβ zeolites with a low concentration of HCA increases the specific surface area, pore volume, pore diameter, and the ratio of Si to Al. It can effectively adjust the ratio of Bronsted acids to Lewis acids, reduce the amount of strong acids, and increase the site of medium strong acid. However, a high HCA concentration will remove part of skeleton aluminum, destroy the structure of the zeolite, and reduce the catalytic activity, and the optimal HCA concentration is 0.3 mol·L−1. The catalytic activity of HCA/Hβ can be improved by increasing calcination temperature, but too high calcination temperature will lead to the sintering of the molecular sieve and the destruction of the skeleton structure, which is not conducive to the catalytic reaction; the optimum roasting temperature is 550°C.
For propionylation of 2-MN, the catalytic effect of Hβ is the best when the HCA concentration is 0.3 mol·L−1, the modification time is 48 h, and the Hβ is calcined at 550°C for 3 h. When n (2-MN):n (PA) is 1:1.8, the amount of solvent TS is 5 g, the amount of catalyst is 3 g, the reaction temperature is 190°C, and the reaction time is 10 h, the conversion rate of 2-MN is 88.82%, the yield of 2,6-MPN is 82.12%, and the yield of 2,6-MPN is 43%.
HCA modification is targeted to increase the favorable medium–strong acids in the acylation reaction of 2-MN and effectively adjust the ratio of Bronsted acids to Lewis acids of the Hβ molecular sieve, increasing the entrance aperture, and the preparation process is simple. No other ion in the reaction liquid simplifies the operation and is environmentally friendly, the renewable performance of modified Hβ compared to that of unmodified Hβ has improved. Hence, it is feasible to use a zeolite molecular sieve instead of the traditional AlCl3 catalyst, which is easier to operate and more environmentally friendly.
-
Funding information: Financial supported by the National Natural Science Foundation of China (91634101) and the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges under Beijing Municipality (IDHT20180508).
-
Author contributions: Jingjing Sun: writing – original draft, writing – review and editing, methodology, formal analysis; Nan Zhang: formal analysis; Haibo Jin: writing – review and editing, resources, funding acquisition, conceptualization; Xuefeng Mao: formal analysis; Guangxiang He: writing – review and editing, methodology; Junfang Li: resources, validation; Zihao Yan: validation; Fating Hu: investigation; Lei Ma: formal analysis, investigation; Xiaoyan Guo: supervision; Suohe Yang: software.
-
Conflict of interest: Authors state no conflict of interest.
References
[1] Hoppe M, de Voogt P, Franz R. Oligomers in polyethylene naphthalene and polybutylene terephthalate identification and exploring migration. Food Packag Shelf. 2018;17:171–8. 10.1016/j.fpsl.2018.07.001.Search in Google Scholar
[2] Zhang SZ, Zhang XJ, Zhou XY, Tian ZW. Study on the extraction of 2,6-dimethyl naphthalene from coal tar wash oil. Contemp Chem Ind. 2018;47(10):2037–40. 10.13840/j.cnki.cn21-1457/tq.2018.10.010.Search in Google Scholar
[3] Li JF, Mao XF, Hu FT, Li WB. Synthesis and characterization of 2-methyl-6-propyl naphthalene. J China Coal Soc. 2020;45(12):4184–90. 10.13225/j.cnki.jccs.2019.1647. Search in Google Scholar
[4] Mebarki F, David E. Dielectric characterization of thermally aged recycled polyethylene terephthalate and polyethylene naphthalate reinforced with inorganic fillers. Polym Eng Sci. 2018;58(5):701–12. 10.1002/pen.24602.Search in Google Scholar
[5] Seokgyu R, Kiho K, Jooheon K. Silane surface treatment of boron nitride to improve the thermal conductivity of polyethylene naphthalate requiring high-temperature molding. Polym Composite. 2018;39(S3):1692–700. 10.1002/pc.24680.Search in Google Scholar
[6] Ren XH, Yang HH, Zhe DM, Gao YJ. Application status and the prospect of polyethylene naphthalenediate. Petrochem Technol. 2021;50(6):616–21. 10.3969/j.issn.1000-8144.2021.06.018.Search in Google Scholar
[7] Zhang CR, Zhou B. Progress in the synthesis of 2,6-naphthale nediformic acid. Chem Adhes. 2012;34(6):54–7.Search in Google Scholar
[8] Cui FX. Progress in synthesis technology of 2,6-naphthale nediformic acid. Polyest Ind. 2013;26(6):4–7. 10.3969/j.issn.1008-8261.2013.06.002.Search in Google Scholar
[9] Meng MY, Ma Y, Wang YC, Liu D, Gao JX, Yang WD. Method for synthesis of 2,6-naphthale nediformic acid. Dyest Color. 2013;50(4):48–50 + 3.Search in Google Scholar
[10] Amoco C. Process for preparing purified 2,6-naphthalene-carboxylic acid. US: 5629446(A); 1997.Search in Google Scholar
[11] Ban H, Cheng Y, Wang L, Li X, Zhou X, Zhang X. Preparation of high-purity 2,6-naphthalene dicarboxylic acid from coal tar distillate. Chem Eng Technol. 2019;42(6):1188–98. 10.1002/ceat.201800129.Search in Google Scholar
[12] Chen CY, Schinski WL, O’Rear DJ, Harris TV. Method of making dimethi naphthelenes. US: 5955641; 1999.Search in Google Scholar
[13] Vahteristo K, Halme E, Koskimies S, Csicsery SM, Laatikainen M, Niemi V. Manufacturing of 2,6-dimethyl naphthalene. US: 5952534; 1999.Search in Google Scholar
[14] Matsumoto T, Kumagai Y, Kumata F. Method of alkylating the side chain of alkyl-substituted aromatic hydrocarbon. US: 5625102; 1997.Search in Google Scholar
[15] Bai XF, Liu WB, Xia YL. Preparation of 2,6-dimethyl naphthalene. CN: 1151107C; 2004.Search in Google Scholar
[16] Iwakura T, Nishiyama T. Production of 2,6-dimethyl naphthalene. JPH: 0717879A; 1995.Search in Google Scholar
[17] Wang XX, Guo F, Wei XX, Liu ZM, Zhang W, Guo SQ, et al. The catalytic performance of methylation of naphthalene with methanol over SAPO-11 zeolites synthesized with different Si content. Korean J Chem Eng. 2016;33(7):2034–41. 10.1002/ceat.201800129.Search in Google Scholar
[18] Zhang C, Guo XW, Song CS, Zhao SQ, Wang XS. Effects of steam and TEOS modification on HZSM-5 zeolite for 2,6-dimethyl naphthalene synthesis by methylation of 2-methylnaphthalene with methanol. Catal Today. 2009;149(1):196–201. 10.1016/j.cattod.2009.04.015.Search in Google Scholar
[19] Li YW. Preparation of 2,6-dimethyl naphthalene by disproportionation of β-methylnaphthalene catalyzed by the ionic liquid. MA thesis. Harbin Engineering University; 2005 (in China).Search in Google Scholar
[20] Wang P, Jian XG. Synthesis of 2-methyl-6-acyl naphthalene. J Dalian Marit Univ. 2008;1:99–102. 10.16411/j.cnki.issn1006-7736.2008.01.007. Search in Google Scholar
[21] Tachibana Y, Tate K, Ono M. Process for the preparation of naphthalene carboxylic acids. US: 5055612; 1991.Search in Google Scholar
[22] Mitamura S, Fujishiro K, Inoue H. Process for preparation of naphthalene 2,6-dicarboxylic acid dialkalic metal salts. US: 4820868; 1989.Search in Google Scholar
[23] Liu L. Synthesis of 2,6-naphthalenedioic acid by oxidation reaction of 2-methylnaphthalene acylation. MA thesis. East China University of Technology; 2012.Search in Google Scholar
[24] Wei H, Xie S, Liu K, Xin W, Li X, Liu S, et al. CTAB modification of MCM-49 zeolite containing HMI and its acylation of anisole. Chin J Catal. 2015;36:1766–76. 10.1016/S1872-2067(15)60887-7.Search in Google Scholar
[25] Yuan B, Li ZS, Liu YJ, Zhang SS. Liquid phase acylation of 2-methylnaphthalene catalyzed by H-beta zeolite. J Mol Catal A-Chem. 2007;280(1):210–8. 10.1016/j.molcata.2007.11.004.Search in Google Scholar
[26] Bejblov M, Proch DZ, Cejka J. Acylation reactions over zeolites and mesoporous catalysts. Chem Sus Chem. 2009;2(6):486–99. 10.1002/cssc.200900007.Search in Google Scholar PubMed
[27] Yan XM, Ke M, Song ZZ, Jiang QZ, Yu P. Study on the improvement of low-temperature etherification activity of Hβ zeolite by citric acid. J China Univ Pet. 2016;40(4):154–60. 10.3969/j.issn.1673-5005.2016.04.021.Search in Google Scholar
[28] Wang YY, Song H, Song HL, Sun XL, Wang XQ. Kinetic studies on the tert-butylated toluene over H-beta zeolite. Prog React Kinet Mec. 2016;41(2):126–34. 10.3184/146867816X14623650404891.Search in Google Scholar
[29] Gunduz G, Dimitrova R, Yilmaz S. Isomerisation of α-pinene over Beta zeolites synthesized by different methods. J Mol Catal A-Chem. 2005;225(2):253–8. 10.1016/j.molcata.2004.09.018.Search in Google Scholar
[30] Kumar GS, Vishnuvarthan M, Palanichamy M, Murugesan V. SBA-15 supported HPW: Effective catalytic performance in the alkylation of phenol. J Mol Catal A-Chem. 2006;260(1):49–55. 10.1016/j.molcata.2006.07.050.Search in Google Scholar
[31] Li SK, Zhao ZC, Zhao RR, Zhou DH, Zhang WP. Aluminum location and acid strength in an aluminum‐rich Beta zeolite catalyst: a combined density functional theory and solid‐state NMR study. Chem Cat Chem. 2017;9(8):1494–502. 10.1002/cctc.201601623.Search in Google Scholar
[32] Wang YY, Song H, Yuan DD, Sun XL, Liu YX. Alkylation of toluene and tert-butanol catalyzed by H-beta modified by citric acid. Chem Ind Eng Prog. 2022;41(1):237–43. 10.16085/j.issn.1000-6613.2021-0284.Search in Google Scholar
[33] Chiche B, Finiels A, Gauthier C, Geneste P. Friedel-Crafts acylation of toluene and p-xylene with carboxylic acids catalyzed by zeolites. J Org Chem. 1986;51(11):2128–30. 10.1021/jo00361a039.Search in Google Scholar
[34] Ma YD, Wang QL, Jiang W, Zuo BJ, Qiu Q. Zeolite-catalyzed Friedel-Crafts acylation of aromatics. Nature of the active sites of zeolites. Chin Chem Lett. 1996;7(9):841–4.Search in Google Scholar
© 2022 Jingjing Sun et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

