Abstract
Traditional fertilization not only enhances the acidification of soil but also leads toward eutrophication. Here stimulatory and inhibitory effects of foliar fertilization of Cordia based silver nanoparticles (AgNPs) were studied on lettuce (Lactuca sativa L.) biomass accumulation, antioxidant activity, and morphological and anatomical modifications. The AgNPs were foliar supplied with a concentration of 25, 50, and 100 ppm along with control (deionized water) and negative control AgNO3 for consecutive 25 days. The L. sativa fresh and dry biomass accumulation were significantly higher by 53%, leaf area by 25%, and leaf water holding capacity by 207% for foliar sprayed at 25 ppm concentration of AgNPs. The application of AgNPs and AgNO3 had significantly shortened the shoot length (52%), while foliar spray of AgNPs promoted the root length (40%). Among different concentrations of AgNPs, the 50 ppm improved the thickness of stem epidermis (44%), hypodermis (130%), and cortex cell area (372%). For antioxidant studies, the 25 ppm of AgNPs depicted the highest anti-oxidative activity for 2,2-diphenyl-1-picryl hydrazyl radical scavenging activity (75%), total antioxidant capacity (167%), total phenolic content (292%), and total reducing power (60%), while 50 ppm showed the maximum activity for total flavonoid content (243%) as compared to control. Thus, we may conclude that the AgNPs have both stimulatory and inhibitory effects on L. sativa. These effects were dependent on the concentration of the nanoparticles and have varied for different growth, antioxidants, and anatomical traits of the plant.
Graphical abstract
Schematic illustration of AgNPs synthesis, foliar application on lettuce with morpho-anatomical characterizations.
1 Introduction
Excessive utilization of fertilizers for requirement of enhancing population for food has become the challenging task for researcher in the field of agriculture. The health index of soil alters by rapid use of artificial fertilizers which leads toward the loss of fertility of soil thereby decrease in the production rate [1,2]. The soil conditions in the agro-ecosystem have been changed by the anthropogenic activities, extensive cropping system, and intensive land use management. Furthermore, these human activities have also influenced the diversity of soil bacteria which ultimately leads toward the loss of soil fertility [3,4]. To combat this, nanotechnology, an emerging science, has been recently spreading all over the world with a fast rate of research and scientific development. The role of nanoparticles (NPs) has been studied extensively in different fields including agriculture [5]. Researchers have reported its beneficial effects, phytotoxicity, growth promoters, and their role as nano fertilizers contribution in plants [6]. Although the silver nanoparticles (AgNPs) application trend is increasing in the field of biotechnology, very few documented record is present on their method of preparation and concentration of dose [7,8]. Approximately, 25% of the total NPs used in the nanotechnology-based products are comprised of silver owing to its antifungal, antibacterial, and antimicrobial utilizations [9,10,11]. In agriculture, the silver-based NPs are chiefly used in nano-pesticides to encounter plant diseases [12]. The main problem with AgNPs is their concentration and exposure time which determined the fate of crops.
Certain applications regarding the environmental issues involve the implementation of AgNPs. It has been observed that AgNPs are found to be very effective in the sunscreens, fiber industry, food industry, and certain agricultural and biomedical industries due to their powerful bioactivity [13,14]. Nano-agriculture is the implementation of nanotechnology in the field of crop and agriculture in which nano-sized particles are used to suppress the pests population and to enhance the production by focusing on the targeted farming [15]. Nanoparticles in the form of nanoemulsion and as nano-fertilizer increases the availability of micronutrients to the plants which enhances the direct implementation of required nutrients to the system of plants, improve their growth at seedling stage, and decreases the fertilizer waste [16,17]. But the rapid use of nano-fertilizers through foliar spray increases the nutrient bioavailability with fast response which enhanced the growth and production of plants [18,19]. As the reactivity of nano-fertilizers is very high, these fertilizers can penetrate in the cuticle and manage the controlled release and delivery of nutrients to the targeted part [20]. This foliar technique of nano-fertilizers greatly affects the quality, production, growth, abiotic stress alleviation, toxicity of heavy metals, and nutrient use efficiency of the crops [21]. Stampoulis et al. observed that Ag content in Cucurbita pepo shoots was approximately 5 times higher in plants exposed to 10–1,000 m·L−1 AgNPs than those treated with bulk Ag powder at similar concentrations, due to higher levels of ion release from AgNPs [22]. Easy penetration of NPs into plants and specific properties of NPs can cause a strong reaction at various levels, including alterations in metabolic processes [23].
Due to the diversified properties of NPs, i.e., composition, structure, size, and stability, nanoparticles affect the plants in both negative and positive way [24]. Positive effects are greatly studied in the crops production in which efficient NPs are synthesized for the bioactivity against pests, growth regulation, nutrition, and soil conditioners [25]. Silver nanoparticles greatly influenced the production and efficiency of certain hormones of plants such as auxins and cytokinins [26]. Negative effects of NPs are also widely observed and resulted in a decrease in growth, development, pigments quantity, and yield [27,28]. Exposure to AgNPs can lead to an oxidative stress in plant, for instance, higher concentrations of AgNPs produced excessive quantity of reactive oxygen species (ROS) including superoxide radicals and H2O2 that created oxidative stress in Brassica rapa seedlings [29,30]. Different concentrations of NPs, morphology, size, and time duration have influenced the cell and plant morphological and physiological response in various plants. There are many benefits of green synthesized AgNPs in different disciplines such as medical, food, health care centers [31,32], environmental science, cosmetics, and many other industries [33]. However, agriculture is very special and need unique characteristics of AgNPs for controlling pesticides, fungicides, and insecticides [34,35]. Exposure to higher concentration of NPs generally results in over production of ROS and consequently cytotoxicity, while exposure to lower concentration of NPs usually stimulates nontoxic modulation of redox signaling which favors plants against different environmental stresses. For these reasons, the current study was designed to investigate the impact of green synthesized AgNPs on morphological and physiological parameters of lettuce’ (Lactuca sativa L.). Lettuce is a well-known annual herb belonging to Asteraceae family, which is used commonly in most parts of the world as leaf vegetable. Therefore, the study was used to observe the changes in concentration of NPs with respect to metabolite marker in response to AgNPs. The growth parameters, biomass accumulation, and leaf contents were also measured. The antioxidant activity, phenolic content, and anatomical traits of leaf, stem, and root of L. sativa against silver nitrate and AgNPs were determined. Furthermore, a comparison of effect of different concentrations as well as on possible alterations in the plant response from stimulatory to inhibitory have been observed as well.
2 Materials and methods
2.1 Chemicals and equipment
Silver nitrate, Cordia myxa plant, coconut peat medium, plastic pots, 90% absolute alcohol, safranine, dimethyl sulfoxide DMSO, hydroponic apparatus, magnetic stirrer, UV-Vis spectroscopy, centrifuge machine, seedling tray, and Eppendorf tubes.
2.2 Cordia myxa based synthesis of AgNPs
The AgNPs were synthesized using the Cordia myxa branches extract as previously reported [9,31]. First, 1 M aqueous stock solution of AgNO3 was prepared and mixed with C. myxa branches extract in a 1:1 ratio. The whole mixture was heated and stirred with a magnetic stirrer (100 rpm) at 70°C for 30–50 min. Continuous visual observation of mixtures was recorded by changing the color change. The bio reduction of Ag ions in the aqueous solution was periodically monitored by UV-Vis spectroscopy. When the reaction was completed, end product was collected via centrifuge (30 min at 7,000 rpm). The supernatant was stored in Falcon tubes and kept at 4°C.
2.3 Foliar application of AGNPs on L. sativa
The seeds of L. sativa were purchased from market and hand sown in a seedling tray having coconut peat medium, porous peat mainly consisting of holocellulose and lignin content. As the root system developed, the seedlings started absorbing water. The seeds starting germination after 5–7 days. As the seeds attained maximum germination, sprinkling of water spray continued everyday as per requirement. All the seedlings were tagged with specific concentration experimental group and kept in net pot which were placed in hydroponic environment. Plastic containers were filled with 100 mL of Hoagland’s solution. Lettuce seedlings were labelled with five groups, i.e., control group without AgNPs, 25, 50, and 100 ppm NPs, and silver nitrate as negative group. The experiment was carried out in triplets with each group having 8–10 plants. 12 mL of AgNPs solution were supplied in different concentrations every day for 25 days along with control having distilled water and negative control with silver nitrate solution (0.01 M). All the groups were placed in an open atmosphere and maintained on 14 h light/10 h dark cycle at ∼22°C. The foliar application of all concentrations in the experimental group were continuous till the harvesting for analysis of morpho-anatomical parameter.
2.4 Plant morphological studies
Lettuce seedling morphological characteristics were recorded as in our previous reports by slight modification for height of plant (cm), dry weight accumulation (g), fresh weight accumulation (g), shoot length (cm), and root length (cm), leaf area, leaf frequency per plant by following a standard agronomic procedure.
2.5 Anatomical characterization of L. sativa
Anatomical studies were performed for leaf, stem, and root separately. The sectioning of the L. sativa parts was carried after complete dehydration process containing double stains to prepare permanent slides. Then, microscope was used to observe these permanent slides. Different fast green solutions and certain staining solutions were prepared which had the concentrations of about 90% absolute alcohol, 70% alcohol, 50% alcohol, 30% alcohol, and 70% alcohol along with safranine. The photographs were captured by a digital camera, which was 24 mega pixels, mounted on the microscope. Furthermore, the anatomical characterization of root, stem, and leaves included: thickness of lamina (µm), thickness of palisade cells (µm), thickness of cuticle (µm), thickness of spongy cells (µm), thickness of mid rib (µm), thickness of abaxial epidermis (µm), thickness of adaxial epidermis (µm), thickness of bundle sheath (µm), and number of vascular bundles. Cell area of leaf tissues were measured for the area of palisade cells (µm2), bundle sheath cells (µm2), abaxial cells (µm2), adaxial cells (µm2), and spongy cells (µm2). Root anatomical characterization included: thickness of pericycle (µm), thickness of cortex (µm), thickness of epidermis at 10× (µm), thickness of endodermis (µm), thickness of hypodermis (µm), and number of vascular bundles. Cell area of root tissues were measured for the hypodermis cells (µm2), cortical cells (µm2), epidermis cells (µm2), and endodermis cells (µm2). Stem anatomical characterization included: thickness of hypodermis (µm), thickness of epidermis (µm), thickness of endodermis (µm), thickness of cortex (µm), thickness of pith (µm), diameter of stem, and number of vascular bundles. Cell area of stem tissues were measured for the hypodermal cells (µm2), endodermal cells (µm2), epidermal cells (µm2), and cortex cells (µm2).
2.6 Determination of L. sativa antioxidant activity
The anti-oxidant potential of lettuce seedling in response to different concentrations of cordia-stabilized AgNPs were determined. Dimethyl sulfoxide (DMSO) (10 mg·mL−1) was used to prepare suspension in Eppendorf tubes. Every sample was kept at room temperature for 48 h and then centrifuged at 12,000 rpm for 15 min. The supernatant obtained from each group was used to determine the activities of different antioxidants. 1,1-di-phenyl-2-picryl-hydrazyl (DPPH) assay was used to analyze the antioxidants. The capability of sample to absorb DPPH ion was analyzed by the following formula:
where Abs represents the absorbance of DPPH solution with sample and Abc represents the absorbance of negative control (having solvents and reagents only). The ability to reduce other elements was determined by the methods carried out by previously reported method [36,37]. To determine the total antioxidant capacity, phosphomolybdenum method, already reported in ref. [38], was adopted. Methanol (0.3 mL) was used as a blank in place of plant extract. The total antioxidant capacity was expressed as number of grams equivalent of ascorbic acid. Folin-Denis reagent method was used to calculate the total phenolic content (TPC) [39]. Gallic acid or trihydroxy benzoic acid was taken as standard and DMSO was used as blank and they were run in parallel. The obtained TPC was analyzed as microgram trihydroxy benzoic acid equivalent per mg FW (µg GAE per mg FW). AlCl3 colorimetric method was used to estimate the total flavonoid content (TFC) of crude extract as used by previous researcher [40]. Absorbance was calculated by using microtiter plate at 415 nm after 15 min of incubation.
2.7 Statistical analysis
The effect of different concentrations of cordia-based stabilized AgNPs significance testing was analyzed by using the least significant difference (LSD) test; differences were determined to be statistically significant when p-value <0.05. The results were represented as a mean value ± standard deviation.
3 Results
3.1 Lettuce growth studies
The foliar application of AgNPs with 25, 50, and 100 ppm NPs concentration and their impact on morphology, physiological, and anatomical changes and their antioxidant response in the lettuce plant are shown in Figure 1. In our previous reports, the AgNPs were applied hydroponically and their influence was observed. In the current study, the foliar application of AgNPs was applied for 25 days to investigate the morpho-anatomical and antioxidant responses. Initially, the plant height and number of leaves were higher in the control group.
Illustration of foliar spray of silver nanoparticles on L. sativa and their morpho-anatomical changes.
There was a decrease of 30% in the number of leaves per plant and 36% in plant height in the negative control. The application of AgNPs progressively declined the above studied growth traits. The response of lettuce was concentration dependent as mean values of plant height and number of leaves per plant were gradually decreased with an increase in AgNPs concentration (25, 50, and 100 ppm). On contrary to plant height, lettuce fresh and dry biomass accumulation were significantly higher for foliar sprayed AgNPs over control (Figure 2a). The low concentration of AgNPs had accumulated almost 53% higher fresh and dry weight as compared to control.

Foliar application of silver nanoparticles with 25, 50, and 100 ppm on L. sativa morpho-anatomical changes (a), thickness of root tissue (b), thickness of stem tissue (c), and thickness of leaf tissue (d).
The AgNO3 proved fatal and lowered plant dry biomass accumulation by 83%. With an increase in AgNPs concentration, the lettuce biomass accumulation capacity gradually decreased but still remained higher than both positive and negative controls. Shoot length of the lettuce displayed a similar trend as that of plant height (Figure 2a). The effect of AgNPs was antagonistic for shoot length to root length. The application of AgNPs and AgNO3 has significantly shortened shoot length (52%), while foliar spray of AgNPs promoted root length (40%). It is concluded that the application of AgNPs negatively affected leaf emergence, shoot length, and plant height, while it promoted plant capacity for biomass accumulation and roots elongation. The foliar application of AgNPs has significantly improved leaf area and water content as compared to control (Figure 2a). The application of AgNO3 exhibited a damaging effect on both these traits. The lowest concentration (25 ppm) of AgNPs displayed an astonishing improvement in leaf water holding capacity (207%) with a nominal increase in leaf area (25%). The response of leaf area and water content were significantly altered with the change in concentrations of AgNPs.
3.2 Morpho-anatomical characterization of lettuce
The data of thickness of root diameter and number of vascular bundles are presented in Figure 2b. The application of AgNO3 and AgNPs has significantly declined the number of vascular bundles and root diameter, while 25 ppm of AgNPs marginally improved root diameter. The application of all concentrations of AgNPs severely dropped the root epidermis and hypodermis thickness (Figure 2b). On the contrary, the application of AgNO3 had improved thickness of epidermis by 101% but it did not affect the thickness of endodermis. The application of 100 ppm of AgNPs improved the thickness of pericycle by 64%. The application of AgNPs displayed a severe negative effect on all root anatomical characteristics except for the thickness of pericycle and diameter of root.
3.3 Anatomy of stem tissue
Stem anatomical studies reported thickness of endodermis and hypodermis of about 0.1 µm; however, the thickness of epidermis was around 0.16 µm (Figure 2c). The foliar application of AgNO3 improved thickness of epidermis and hypodermis by about 25–130%, while the thickness of endodermis remained unchanged. The application of AgNPs displayed a variable response to the thickness of stem dermis layers. Their response varied significantly depending upon the concentration of the AgNPs. For example, the 25–50 ppm concentration of AgNPs improved thickness of endodermis by about 30% and 100 ppm concentration of AgNPs reduced it by about 40%. The medium dose of AgNPs incremented the thickness of hypodermis by 130% and its highest dose brought no change in thickness of hypodermis as compared to the control. The observed thickness of epidermis of the lettuce plants, which were treated with AgNPs concentration of 25 and 100 ppm, showed 19% decrease, while the plants treated with 50 ppm concentration showed 44% increase (Figure 2c).
Figure 2c displays the effect of AgNO3 and AgNPs on stem diameter, cortex thickness, pith thickness, and number of vascular bundles. The application of AgNO3 positively improved all these traits. The application of AgNPs also promoted these traits except for stem diameter that significantly declined (18%) at all concentrations. The application of 50 ppm of AgNPs promoted the production of the number of vascular bundles by 58%. The observed thickness of pith of the stem, which were treated with 25 and 50 ppm concentrations, were 5.93 and 6.13 µm, as compared to the control group (3 µm), these showed 97% and 104% upsurge in pith thickness, respectively. Similarly, 25 and 50 ppm concentrations of AgNPs showed 126% and 187% increase in cortex thickness, respectively. The 100 ppm dose of AgNPs proved toxic and significantly declined in all anatomical characteristics of the stem (Figure 2c).
3.4 Anatomy of leaf tissue
Figure 2d presents the data for leaf thickness parameters. All studied leaf tissues displayed a variable response to foliar application of AgNPs in different concentrations. The lower concentration of AgNPs showed a significant improvement in cuticle thickness, while the plants which were implemented by the 50 and 100 ppm concentrations completely lost their cuticle. The application of AgNO3 improved the thickness of midrib and lamina, while the AgNPs gradually decreased the thickness of these traits. Silver nitrate improved the adaxial epidermis thickness by 284% and decreased the abaxial epidermis thickness by 60% as compared to the control. The AgNPs concentration of 25, 50, and 100 ppm showed 100%, 338%, and 207% decrease in adaxial epidermis thickness and 51%, 0%, and 60% decrease in abaxial epidermis thickness, respectively. The application of AgNO3 and 100 ppm AgNPs improved the palisade thickness by 69% and 117%, respectively as compared to the control (Figure 2d). The low and medium levels of AgNPs slightly declined the thickness of palisade cells. On the contrary, the 25–50 ppm AgNPs positively improved the thickness of spongy cells, while 100 ppm concentration sharply declined it. The foliar spray of AgNO3 had the thickness of spongy cells of about 1 µm that was 65% higher than control. Except for 50 ppm concentration of AgNPs that improved thickness of bundle sheath cells by 230%, the remaining treatments of silver had a non-significant consequence on bundle sheath cells.
3.5 Cell area plant vegetative part
Cell area of root tissues (epidermis, hypodermis, endodermis, and cortex) is presented in Figure 3a. Control group had the area of cortex cells of about 0.31 µm2. It was studied that cortex cell area of negative control group, i.e., AgNO3 was 0.48 µm2 which showed about 55% increase as compared to the control. Similarly, AgNO3 positively affected root epidermis and hypodermis cell area. The application of AgNPs at a concentration of 50 ppm favored the endodermis cell area.

Effect of different concentrations of Ag NPs and AgNO3 (1 M) on area of endodermis, epidermis, cortex, and hypodermis cell: (a) root tissue, (b) stem tissue, and (c) area of spongy cell, abaxial, bundle sheath, and palisade cell in leaf.
The effect of AgNO3 and all concentrations of AgNPs were non-significant for the area of epidermis, endodermis, and hypodermis of stem except for 100 ppm that displayed a severe decline in hypodermis area (Figure 3b). The application of AgNO3 produced the maximum cortex cell area that was 460% higher as compared to the control. Among the AgNPs, the 50 ppm concentration improved the cortex cell area by 372% as compared to control. Thus, it is concluded that stem cortex area is an important trait to study the effect of silver on lettuce plant. Area of adaxial and abaxial epidermis depicted almost similar trend against the foliar application of both AgNO3 and AgNPs (Figure 3c). The application of 25–50 ppm AgNPs decreased the area of adaxial and abaxial epidermis by 29–56%, while the foliar spray of 100 ppm improved these traits by 129–433%, respectively. The application of AgNO3 stretched the area of adaxial and abaxial epidermis by 400–1,500% approximately. The application of AgNO3 significantly enhanced the area of spongy cell by 384% and palisade by 370%, while the application of AgNPs significantly declined the area of spongy cell and palisade cell. The concentration of AgNPs had an inverse relationship with the area of spongy cell and palisade cell. All levels of AgNPs displayed negative effect on both the areas of spongy cell and palisade cell. The negative effect of AgNPs was more severe on the area of spongy cell as compared to the area of palisade cell. The effect of AgNO3 and AgNPs were non-significant to the area of bundle sheath cells; however, it decreased slightly with the application of AgNPs.
3.6 Foliar application of AgNPs and silver nitrate on lettuce
Foliar application and the uptake of AgNPs in root, stem, and leaves were performed using anatomical microscopic studies. These microscopic studies exposed the uptake and retention of the AgNPs via leaf route and their anatomical changes in cells of root, stem, and leaves. The presence of AgNPs in the root, stem, and leaves are marked by red arrows in Figure 4. The foliar application of 25 ppm AgNPs showed maximum absorption in the leaf tissue as compared to stem and root (Figure 4a). Similarly, the influence of 50 ppm was also observed in the vegetative part of lettuce via foliar application but the leaf shows maximum uptake of NPs because of direct exposure and having stomata. Significant changes in cell morphology, structure, and physiology have been observed in root, stem, and leaves section (Figure 4b). In case of Figure 4c, NPs-induced cell death via apoptosis was observed based on the anatomy results. Significant changes in the anatomy of all parts of plant were observed.
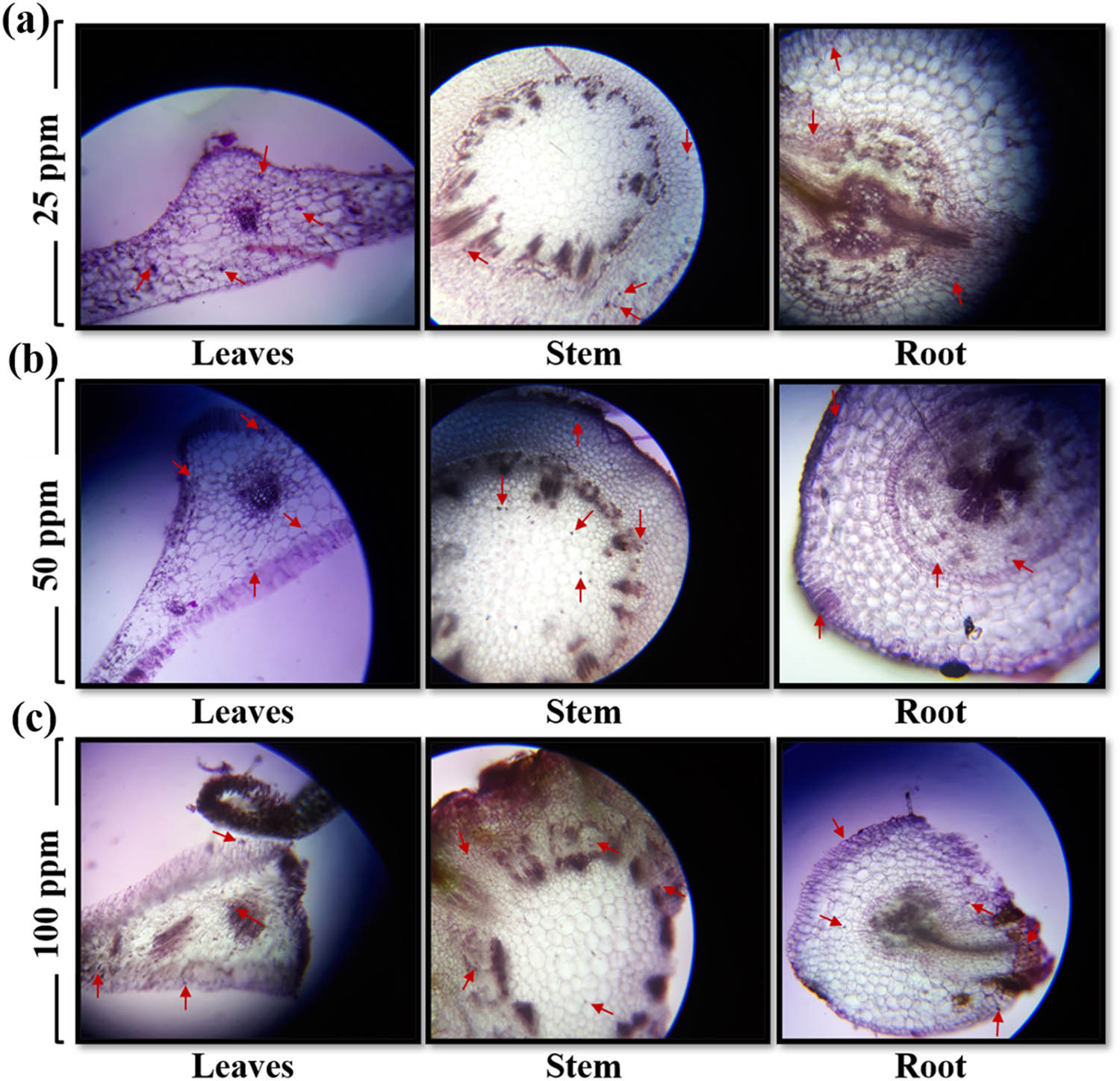
Morpho-anatomical analysis of L. sativa leaves, stem, and root: (a) 25 ppm, (b) 50 ppm, and (c) 100 ppm. The images were recorded under a light microscope (Nikon 104, Japan) at a resolution of 1,500× with the help of a 20 mega pixel digital camera.
3.7 Anti-oxidative response of lettuce in foliar application of AgNPs and AgNO3
The anti-oxidative response of lettuce was measured for DPPH activity, total antioxidant capacity (TAC), TPCTPC, TFC, and total reducing power (TRP). Control group had DPPH of 28.2 µg·mg−1 and TAC of 18.3 µg·mg−1 and observations showed an increase of 34% in DPPH inhibition (Figure 5a) and 59% in TAC for foliar application of AgNO3 as compared to the control (Figure 5b). The observed DPPH and TAC inhibition of the plants, which were treated with 25, 50, and 100 ppm concentrations of AgNPs, were increased by approximately 75–167%, 61–120%, and 44–28%, respectively, as compared to the control.

Effect of different concentrations of AgNPs and AgNO3 (1 M) on: (a) free radical scavenging activity, (b) total antioxidant activity, (c) total phenolic content, (d) total flavonoid content, and (e) total reducing power levels in roots and leaves of lettuce plant.
The application of AgNPs and AgNO3 significantly improved the activity of TPC, TFC, and TRP (Figure 5c–e). Among different concentrations of AgNPs, the 25 ppm depicted the highest anti-oxidative activity for TPC (292%) and TRP (60%) (Figure 5e), while 50 ppm showed the maximum activity for TFC (243%) as compared to control (Figure 5d). The anti-oxidative activity of L. sativa decreased considerably at 100 ppm concentration of AgNPs.
4 Discussion
Recently, the trend of foliar spray of NPs prepared by chemical and biological methods as nanofertilizers has increased in the agricultural industry [41,42]. These NPs have enhanced the effectiveness of nanofertilizers and nanopesticides emulsion as compared to the traditional soil root application [43]. The effectiveness of foliar application helps plants to avoid nutrient from leaching, soil contamination, and economically feasible. Lettuce is a green fleshy vegetable having good source of essential nutrient and antioxidant molecules which has been used as soup, salads, and sandwiches throughout the world [44,45]. To increase the growth and production of lettuce, nanotechnological tools, and techniques have been effective for uptake of nutrients and ions by direct interaction with soil, air, and water [46]. The current study evaluated the response of L. sativa against the foliar exposure of AgNO3 and AgNPs with various concentrations to investigate the optimal conditions as nanofertilizers or toxicants. Explicitly the effects induced by AgNPs were studied on L. sativa growth, biomass accumulation, leaf, stem, and root thickness, area, and anatomical traits. The effects were also studied on anti-oxidative activity and phenolic contents of L. sativa in response to biologically synthesized AgNPs and AgNO3. The L. sativa response significantly varied depending upon the concentration of the NPs. The knowledge provided us relevant information for the evaluation of the positive effects on plants and potential risks of toxicity. The findings of this study displayed that AgNPs have negatively affected leaf emergence, shoot length, and plant height, while it promoted plant capacity for biomass accumulation and root length. Foliar application of AgNPs at 60 ppm concentration improved the fresh and dry weight accumulation of Phaseolus vulgaris by 27–30% [47] and Zea mays by 33–35%, while at 100 ppm it significantly declined the fresh and dry weight of both plants [48]. On the contrary, negative effects of AgNPs are also frequently reported in different plants. Application of AgNPs declined the growth and biomass accumulation of Oryza sativa and Triticum aestivum [49,50]. In the authors’ opinion, the growth promotory potential of NPs depends on the concentration of AgNPs and it varies for each growth parameter. For example, plant height significantly decreased at 25 ppm concentration, while leaf water holding capacity considerably improved at this concentration. Although the results presented exhibited some variation in the anti-oxidative activity of L. sativa leaves treated with AgNPs at different concentrations. In general, the source of silver ions was responsible for the increment of enzymatic activities in the treated plants, but the degree of this increase was dependent on the concentration of the applied NPs. The low and medium concentration of AgNPs reported the highest anti-oxidative activity (DPPH, TAC, TPC, TFC, and TRP). Similar kind of reports were reported earlier and showed that TiO2 NPs enhanced the activity of superoxide dismutase, ascorbate peroxidase (APX), and glutathione peroxidase in Spinacia oleracea plant [51]. In another report, the APX activity improved in Solanum tuberosum plants by the application of AgNPs [52]. Upregulation of genes was documented in A. thaliana involved in the synthesis of glutathione when treated with the AgNPs [53]. To the best of our knowledge, the effect of AgNPs on DPPH, TAC, TPC, TFC, and TRP have not yet been presented in detail and the mechanisms of increase in the activity of these antioxidants observed in our experiment requires further in-depth research to explore the key physiochemical mechanisms regulating these antioxidants under foliar application of AgNPs. Application of higher concentrations of AgNPs enhanced the scavenging activity of DPPH by 37–44% as compared to control, while lower concentration of AgNPs did not produce any effect on DPPH [54]. Antioxidative activity of Corchorus olitorius increased in a concentration dependent manner for the AgNPs when mixed in the soil [55]. In another report, DPPH free radical scavenging activity slightly improved at 20 ppm of AgNPs and tremendously enhanced at 50 ppm in Echium amoenum, which agrees with our findings [56]. Furthermore, one more researcher revealed that AgNPs and PtNPs at 40 ppm concentration have improved the total phenolic contents by 17–15% as compared to the control. Similarly, the AgNPs and PtNPs increased carotenoids content in L. sativa by 13–17% when applied with 20–40 ppm solutions [57]. Total phenolic contents were found maximum in Triticum aestivum seedlings that were treated with the 50 ppm concentration of AgNPs. Review of previous literature suggests that there is generally a positive correlation between the plant exposure to AgNPs and total phenolic contents. In our study, the same relationship was confirmed at the medium AgNP concentrations. Current study’s results concluded that there is a need to investigate the nature of functional biomolecules which are responsible for the reduction process and play key role in the formation of NPs [58]. Furthermore, there is also a need to investigate the mechanism of stimulatory and inhibitory effect of AgNPs in model plants. This positive or negative effect will help to understand the all-molecular processes, signaling and action of AgNPs may open up new perspectives in the practical approach of scientist and new possibilities of creating beneficial combinations with other biologically active agents. Recently, green synthesized AgNps were used as potential biomass feedstock for the sustainable production of biodiesel [59]. In another report, Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment [60]. There is also a need to investigate the combinational use of AgNPs with other antimicrobial and antifungal agents for solving the problem of toxicity and avoid the possible risk of microbial resistance development.
5 Conclusion
The study evaluated the response of green synthesized AgNPs (25, 50, and 100 ppm) on lettuce growth, antioxidant activity, and morpho-anatomical changes in leaf, stem, and root cells. The response of lettuce plant depended on the applied concentration of the AgNPs. The lower concentrations of the NPs 25–50 ppm promoted different studied traits, while at 100 ppm it inhibited the functioning of the same traits. The higher dose of AgNPs proved fatal and resulted in phytotoxicity such as failure to develop cuticle cells, but in limited cases, the full dose of AgNPs (100 ppm) promoted the studied traits such as the thickness of pericycle in root tissues. Finally, it an be concluded from this research that in-depth research is needed to explore the key physiochemical mechanisms regulating these morpho-anatomical changes in lettuce under foliar application of green synthesized AgNPs.
Acknowledgments
The authors acknowledge the financial support provided by The Islamia University of Bahawalpur, Pakistan, Higher Education Commission Pakistan and NRPU Project (9458), Guangdong Science and Technology Planning Project (2016A020210066), and Guangzhou Science and Technology Planning Project (201707010461).
-
Funding information: The Islamia University of Bahawalpur, Pakistan, Higher Education Commission Pakistan and NRPU Project (9458), Guangdong Science and Technology Planning Project (2016A020210066), and Guangzhou Science and Technology Planning Project (201707010461).
-
Author contributions: Murtaza Hasan: writing – original draft; Muhammad Sajjad: methodology and experimental design; Ayesha Zafar: writing – draft and review; Riaz Hussain: visualization, and software application; Syed Ishtiaq Anjum: designing of project; Muhammad Zia: methodology and draft of data; Zahid Ihsan: methodology and idea creating; Xugang Shu: resources of experimental work.
-
Conflict of interest: Authors state no conflict of interest.
References
[1] Li J, Han G, Wang G, Liu X, Zhang Q, Chen Y, et al. Imbalanced nitrogen–phosphorus input alters soil organic carbon storage and mineralisation in a salt marsh. Catena. 2022;208:105720. 10.1016/j.catena.2021.105720.Search in Google Scholar
[2] Chen X, Yan X, Wang M, Cai Y, Weng X, Su D, et al. Long-term excessive phosphorus fertilization alters soil phosphorus fractions in the acidic soil of pomelo orchards. Soil Tillage Res. 2022;215:105214. 10.1016/j.still.2021.105214.Search in Google Scholar
[3] Sui X, Zhang R, Frey B, Yang L, Li MH, Ni H. Land use change effects on diversity of soil bacterial, acidobacterial and fungal communities in wetlands of the Sanjiang Plain, northeastern China. Sci Rep. 2019;9(1):55063–4. 10.1038/s41598-019-55063-4.Search in Google Scholar PubMed PubMed Central
[4] Yang T, Siddique KHM, Liu K. Cropping systems in agriculture and their impact on soil health – a review. Glob Ecol Conserv. 2020;23:e01118. 10.1016/j.gecco.2020.e01118.Search in Google Scholar
[5] Hasan M, Rafique S, Zafar A, Loomba S, Khan R, Hassan SG, et al. Physiological and anti-oxidative response of biologically and chemically synthesized iron oxide: Zea mays a case study. Heliyon. 2020;5:e04595. 10.1016/j.heliyon.2020.e04595.Search in Google Scholar PubMed PubMed Central
[6] Yan A, Chen Z. Impacts of silver nanoparticles on plants: a focus on the phytotoxicity and underlying mechanism. Int J Mol Sci. 2019;20:1003. 10.3390/ijms20051003.Search in Google Scholar PubMed PubMed Central
[7] Fatima F, Hashim A, Anees S. Efficacy of nanoparticles as nanofertilizer production: a review. Environ Sci Pollut Res. 2021;28:1292–303. 10.1007/s11356-020-11218-9.Search in Google Scholar PubMed
[8] Hasan M, Mahmood K, Mustafa G, Zafar A, Tariq T, Hassan SG, et al. Phytotoxic evaluation of phytosynthesized silver nanoparticles on lettuce. Coatings. 2021;11:225. 10.3390/coatings11020225.Search in Google Scholar
[9] Zulfiqar H, Zafar A, Rasheed MN, Ali Z, Mehmood K, Mazher A, et al. Synthesis of silver nanoparticles using: Fagonia cretica and their antimicrobial activities. Nanoscale Adv. 2019;1:1707–13. 10.1039/c8na00343b.Search in Google Scholar
[10] Hasan M, Zafar A, Shahzadi I, Luo F, Hassan SG, Tariq T, et al. Fractionation of biomolecules in Withania coagulans extract for bioreductive nanoparticle synthesis, antifungal and biofilm activity. Molecules. 2020;25:3478. 10.3390/molecules25153478.Search in Google Scholar PubMed PubMed Central
[11] Hasan M, Altaf M, Zafar A, Hassan SG, Ali Z, Mustafa G, et al. Bioinspired synthesis of zinc oxide nano-flowers: a surface enhanced antibacterial and harvesting efficiency. Mater Sci Eng C. 2020;119:111280. 10.1016/j.msec.2020.111280.Search in Google Scholar PubMed
[12] Mishra S, Singh HB. Biosynthesized silver nanoparticles as a nanoweapon against phytopathogens: exploring their scope and potential in agriculture. Appl Microbiol Biotechnol. 2015;99:1097–107. 10.1007/s00253-014-6296-0.Search in Google Scholar PubMed
[13] Das CGA, Kumar VG, Dhas TH, Karthick V, Govindaraju K, Joselin M, et al. Antibacterial activity of silver nanoparticles (biosynthesis): a short review on recent advances. Biocatal Agric Biotechnol. 2020;27:101593. 10.1016/j.bcab.2020.101593.Search in Google Scholar
[14] Vigneswari S, Amelia TSM, Hazwan MH, Mouriya GK, Bhubalan K, Amirul AAA, et al. Transformation of biowaste for medical applications: Incorporation of biologically derived silver nanoparticles as antimicrobial coating. Antibiotics. 2021;10:229. 10.3390/antibiotics10030229.Search in Google Scholar PubMed PubMed Central
[15] Shahzad K, Manzoor F. Nanoformulations and their mode of action in insects: a review of biological interactions. Drug Chem Toxicol. 2021;44:1–11. 10.1080/01480545.2018.1525393.Search in Google Scholar PubMed
[16] Upinder, Kumar, R. Nanotechnology: advancement for agricultural sustainability. In: Singh P, Singh R, Verma P, Bhadouria R, Kumar A, Kaushik M, editors. Plant-microbes-engineered nano-particles (PM-ENPs) nexus in agro-ecosystems. Advances in Science, Technology & Innovation. Cham: Springer; 2021.10.1007/978-3-030-66956-0_2Search in Google Scholar
[17] Mustafa G, Hasan M, Yamaguchi H, Hitachi K, Tsuchida K, Komatsu S. A comparative proteomic analysis of engineered and bio synthesized silver nanoparticles on soybean seedlings. J Proteom. 2020;224:103833. 10.1016/j.jprot.2020.103833.Search in Google Scholar PubMed
[18] He J, Zhang L, He SY, Ryser ET, Li H, Zhang W. Stomata facilitates foliar sorption of silver nanoparticles by Arabidopsis thaliana. Environ Pollut. 2022;292:118448. 10.1016/j.envpol.2021.118448.Search in Google Scholar PubMed
[19] Janmohammadi M, Amanzadeh T, Sabaghnia N, Dashti S. Impact of foliar application of nano micronutrient fertilizers and titanium dioxide nanoparticles on the growth and yield components of barley under supplemental irrigation. Acta Agric Slovenica. 2016;107:265–76. 10.14720/aas.2016.107.2.01.Search in Google Scholar
[20] Hong J, Wang C, Wagner DC, Gardea-Torresdey JL, He F, Rico CM. Foliar application of nanoparticles: mechanisms of absorption, transfer, and multiple impacts. Environ Sci Nano. 2021;8(5):1196–210. 10.1039/d0en01129k.Search in Google Scholar
[21] Salehi H, Chehregani A, Lucini L, Majd A, Gholami M. Morphological, proteomic and metabolomic insight into the effect of cerium dioxide nanoparticles to Phaseolus vulgaris L. under soil or foliar application. Sci Total Environ. 2018;617:1540–51. 10.1016/j.scitotenv.2017.10.159.Search in Google Scholar PubMed
[22] Stampoulis D, Sinha SK, White JC. Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol. 2009;24:9473–9. 10.1021/es901695c.Search in Google Scholar PubMed
[23] Kumari R, Singh DP. Ameliorating effect of surfactants against silver nanoparticle toxicity in crop Fagopyrum esculentum L. Environ Nanotechnol Monit Manag. 2019;12:100254. 10.1016/j.enmm.2019.100254.Search in Google Scholar
[24] Czyżowska A, Barbasz A. A review: zinc oxide nanoparticles–friends or enemies? Int J Environ Health Res. 2020;32:885–901. 10.1080/09603123.2020.1805415.Search in Google Scholar PubMed
[25] Mahyoub JA. Bioactivity of two marine algae extracts and their synthesized silver nanoparticles as safe controls against Musca domestica housefly. Entomol Res. 2021;51:323–30. 10.1111/1748-5967.12512.Search in Google Scholar
[26] Ismail G, Abou-Zeid H. The role of priming with biosynthesized silver nanoparticles in the response of Triticum aestivum L. to salt stress. Egypt J Bot. 2018:58:73–85. 10.21608/ejbo.2017.1873.1128.Search in Google Scholar
[27] Jiráková K, Moskvin M, Urdzikova LM, Rossner P, Elzeinova F, Chudichova M, et al. The negative effect of magnetic nanoparticles with ascorbic acid on peritoneal macrophages. Neurochem Res. 2020;45(1):159–70. 10.1007/s11064-019-02790-9.Search in Google Scholar PubMed
[28] Goswami P, Yadav S, Mathur J. Positive and negative effects of nanoparticles on plants and their applications in agriculture. Plant Sci Today. 2019;6(2):232–42. 10.14719/pst.2019.6.2.502.Search in Google Scholar
[29] Khan SA, Shahid S, Lee CS. Green synthesis of gold and silver nanoparticles using leaf extract of clerodendrum inerme; characterization, antimicrobial, and antioxidant activities. Biomolecules. 2020;10:835. 10.3390/biom10060835.Search in Google Scholar PubMed PubMed Central
[30] Thiruvengadam M, Gurunathan S, Chung IM. Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanoparticles in turnip (Brassica rapa ssp. rapa L.). Protoplasma. 2015;252:1031–46. 10.1007/s00709-014-0738-5.Search in Google Scholar PubMed
[31] Na-Phatthalung W, Keaonaborn D, Jaichuedee J, Keawchouy S, Sinyoung S, Musikavong C. Effect of silver nanoparticles and chlorine reaction time on the regulated and emerging disinfection by-products formation. Environ Pollut. 2022;292:118400. 10.1016/j.envpol.2021.118400.Search in Google Scholar PubMed
[32] Naseer QA, Xue X, Wang X, Dang S, Din SU, Jamil J. Synthesis of silver nanoparticles using lactobacillus bulgaricus and assessment of their antibacterial potential. Braz J Biol. 2022;82:232434. 10.1590/1519-6984.232434.Search in Google Scholar PubMed
[33] Yonathan K, Mann R, Mahbub KR, Gunawan C. The impact of silver nanoparticles on microbial communities and antibiotic resistance determinants in the environment. Environ Pollut. 2022;293:118506. 10.1016/j.envpol.2021.118506.Search in Google Scholar PubMed
[34] Kaziem AE, Yang L, Lin Y, Song Z, Xu H, Zhang Z. Efficiency of mesoporous silica/carboxymethyl β-glucan as a fungicide nano-delivery system for improving chlorothalonil bioactivity and reduce biotoxicity. Chemosphere. 2022;287:131902. 10.1016/j.chemosphere.2021.131902.Search in Google Scholar PubMed
[35] Bapat MS, Singh H, Shukla SK, Singh PP, Vo DN, Yadav A, et al. Evaluating green silver nanoparticles as prospective biopesticides: An environmental standpoint. Chemosphere. 2022;286:131761. 10.1016/j.chemosphere.2021.131761.Search in Google Scholar PubMed
[36] Zhao P, Guo Y, Zhang W, Chai H, Xing H, Xing M. Neurotoxicity induced by arsenic in Gallus Gallus: regulation of oxidative stress and heat shock protein response. Chemosphere. 2017;166:238–45. 10.1016/j.chemosphere.2016.09.060.Search in Google Scholar PubMed
[37] Zafar A, Tariq T, Hasan M, Nazar M, Rasheed MN, Mehmood N, et al. Green-maturation of cobalt-oxide nano-sponges for reinforced bacterial apoptosis. Colloids Interface Sci Commun. 2021;45:100531. 10.1016/j.colcom.2021.100531.Search in Google Scholar
[38] Luo F, Wang W, Chen M, Zheng Z, Zeng D, Hasan M, et al. Synthesis and efficacy of the n-carbamoyl-methionine copper on the growth performance, tissue mineralization, immunity, and enzymatic antioxidant capacity of Nile tilapia (oreochromis niloticus). ACS Omega. 2020;35:22578–86. 10.1021/acsomega.0c03220.Search in Google Scholar PubMed PubMed Central
[39] Kaur C, Kapoor HC. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol. 2002;37:153–61. 10.1046/j.1365-2621.2002.00552.x.Search in Google Scholar
[40] Zhang M, Wang S, Sun L, Gun L, Lin Y, Shao J, et al. Ammonia induces changes in carbamoyl phosphate synthetase I and its regulation of glutamine synthesis and urea cycle in yellow catfish Pelteobagrus fulvidraco. Fish Shellfish Immunol. 2022;120:1–6. 10.1016/j.fsi.2021.11.023.Search in Google Scholar PubMed
[41] Luo F, Fu Z, Wang M, Ke Z, Wang M, Wang W, et al. Growth performance, tissue mineralization, antioxidant activity and immune response of oreochromis niloticus fed with conventional and gluconic acid zinc dietary supplements. Aquacult Nutr. 2021;27:897–907. 10.1111/anu.13234.Search in Google Scholar
[42] Wasaya A. Improving growth and yield of Mungbean (Vigna radiata L.) through foliar application of silver and zinc nanoparticles. Pure Appl Biol. 2020;9:790–7. 10.19045/bspab.2020.90085.Search in Google Scholar
[43] Shahid M, Dumat C, Khalid S, Rabbani F, Farooq AB, Amjad M, et al. Foliar uptake of arsenic nanoparticles by spinach: an assessment of physiological and human health risk implications. Environ Sci Pollut Res. 2019;26:20121–31. 10.1007/s11356-018-3867-0.Search in Google Scholar
[44] Kim MJ, Moon Y, Tou JC, Mou B, Waterland NL. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J Food Compos Anal. 2016;49:19–34. 10.1016/j.jfca.2016.03.004.Search in Google Scholar
[45] Lei C, Engeseth NJ. Comparison of growth characteristics, functional qualities, and texture of hydroponically grown and soil-grown lettuce. LWT. 2021;150:790–7. 10.1016/j.lwt.2021.111931.Search in Google Scholar
[46] Najafi S, Razavi SM, Khoshkam M, Asadi A. Effects of green synthesis of sulfur nanoparticles from Cinnamomum zeylanicum barks on physiological and biochemical factors of Lettuce (Lactuca sativa). Physiol Mol Biol Plants. 2020;26(5):1055–66. 10.1007/s12298-020-00793-3.Search in Google Scholar
[47] Zea L, Salama HMH. Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn. Int Res J Biotechnol. 2012;3:190–7.Search in Google Scholar
[48] Rajkumar T, Sapi A, Das G, Debnath T, Ansari AZ, Patra JK. Biosynthesis of silver nanoparticle using extract of Zea mays (corn flour) and investigation of its cytotoxicity effect and radical scavenging potential. J Photochem Photobiol B Biol. 2019;193:1–7. 10.1016/j.jphotobiol.2019.01.008.Search in Google Scholar
[49] Yang Q, Shan W, Hu L, Zhao Y, Hou Y, Yin Y, et al. Uptake and transformation of silver nanoparticles and ions by rice plants revealed by dual stable isotope tracing. Environ Sci Technol. 2019;53:625–33. 10.1021/acs.est.8b02471.Search in Google Scholar
[50] Iqbal M, Raja NI, Mishwani ZR, Wattoo FH, Hussain M, Ejaz M, et al. Assessment of AgNPs exposure on physiological and biochemical changes and antioxidative defence system in wheat (Triticum aestivum L) under heat stress. IET Nanobiotechnol. 2019;13:230–6. 10.1049/iet-nbt.2018.5041.Search in Google Scholar
[51] Azmat R, Altaf I, Moin S. The reflection of the photocatalytic properties of TiO2 nanoparticles on photosynthetic activity of spinacia oleracea plants. Pak J Bot. 2020;52:1229–34. 10.30848/PJB2020-4(2).Search in Google Scholar
[52] Bagherzadeh Homaee M, Ehsanpour AA. Physiological and biochemical responses of potato (Solanum tuberosum) to silver nanoparticles and silver nitrate treatments under in vitro conditions. Ind J Plant Physiol. 2015;20:353–9. 10.1007/s40502-015-0188-x.Search in Google Scholar
[53] Ke M, Li Y, Qu Q, Ye Y, Peijnenburg WJGM, Zhang Z, et al. Offspring toxicity of silver nanoparticles to Arabidopsis thaliana flowering and floral development. J Hazard Mater. 2020;15:121975. 10.1016/j.jhazmat.2019.121975.Search in Google Scholar PubMed
[54] Bawazeer S, Rauf A, Shah SUA, Shawky AM, Awthan YS, Bahattab OS, et al. Green synthesis of silver nanoparticles using Tropaeolum majus: phytochemical screening and antibacterial studies. Green Process Synth. 2021;10:85–94. 10.1515/gps-2021-0003.Search in Google Scholar
[55] Azeez L, Lateef A, Wahab AA, Rufai MA, Salau AK, Ajai EIO, et al. Phytomodulatory effects of silver nanoparticles on Corchorus olitorius: its antiphytopathogenic and hepatoprotective potentials. Plant Physiol Biochem. 2019;136:109–117. 10.1016/j.plaphy.2018.12.006.Search in Google Scholar PubMed
[56] Abbasi F, Jamei R. Effects of silver nanoparticles and silver nitrate on antioxidant responses in echium amoenum. Russ J Plant Physiol. 2019;66:488–94. 10.1134/S1021443719030026.Search in Google Scholar
[57] Mares-Briones F, Barragán-Mares O, López-Miranda JL, Esparza R, Rosas G. Bimetallic Ag@Pt core-shell nanoparticles and their catalytic activity by a green approach. Mater Res Exp. 2019;6:ab299c. 10.1088/2053-1591/ab299c.Search in Google Scholar
[58] Hasan M, Zhongqiu T, Iqbal J, Umer A, Shiying M, Rongji D, et al. Assessment of bioreducing and stabilizing potential of dragon’s blood (dracaena cochinchinensis, Lour. S. C. Chen) resin extract in synthesis of silver nanoparticles. Nanosci Nanotechnol Lett. 2013;5:780–4. 10.1166/nnl.2013.1600.Search in Google Scholar
[59] Molnár Z, Bodai V, Szakacs G, Erdelyi B, Fograssy Z, Safran G, et al. Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci Rep. 2018;8(1);8:3943. 10.1038/s41598-018-22112-3.Search in Google Scholar PubMed PubMed Central
[60] Rambabu K, Bharath G, Banat F, Show PL. Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J Hazard Mater. 2021;402:123560. 10.1016/j.jhazmat.2020.123560.Search in Google Scholar PubMed
© 2022 Murtaza Hasan et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

