Abstract
Bacterial cellulose (BC) produced from coconut water, commonly known as nata de coco, is a biopolymer with enormous properties. Compared to plant cellulose, BC has better mechanical strength and a greater degree of polymerization. BC’s high purity and high porosity make it a suitable candidate for the embedding and dispersion template for silver nanoparticles (AgNPs). This study investigated a facile and scalable method of making BC from coconut water and impregnated them with AgNO3 solution to produce AgNPs templated BC. The resulting materials were characterized by Fourier transform infra-Red (FTIR), scanning electron microscope (SEM), energy dispersive X-ray (EDX), and X-ray diffraction (XRD). The thermal stability was also investigated by thermogravimetric analysis (TGA). The antibacterial activity of AgNPs templated BC was challenged in cultures of gram-positive bacteria Staphylococcus aureus and gram-negative bacteria Escherichia coli and showed an inhibition zone of growth in agar media. This study proves that the resulting AgNPs templated BC sheets are potential materials for antibacterial and industrial application that are low cost and easy to produce.
1 Introduction
As the most common biopolymer material with a production of 1.5 × 1012 tons per year, cellulose is considered an unlimited source of raw material, which has the potential to increase the need for biocompatible and environmentally friendly products [1]. Compared to plant cellulose, bacterial cellulose (BC) has several superior characteristics such as high purity, high porosity, high water retention capacity, high crystallinity, better mechanical strength, and fibers of BC have a high aspect ratio [2]. Cellulose produced from bacteria (such as Acetobacter xylinum, Gluconacetobacter hansenii, and Gluconacetobacter medellinensis [3,4]) has abundant hydroxyl groups making it a material that has high biocompatibility [3,4,5], which is easy to combine with other materials to form a composite. In addition, the structure of BC has extraordinary mechanical strength and a high specific surface area [5]. Recent studies have shown improvements in the mechanical [6], hydrophilicity [1], durability [7], and antibacterial [8,9,10] properties of BC composite via chemical and surface modification. Some of the most exciting applications of BC as a biomaterial is the wound dressing, temporary skin substitute, antibacterial textile, and sterile package. In these applications, the antibacterial properties of BC become the most crucial properties to be obtained. Because of the high biocompatibility, BC is considered as a suitable matrix for the assimilation of metals. Among the several metals that could be inserted in the BC matrix, silver has drawn interest due to the wellknown antibacterial properties.
Nanoparticles with a size range between 1 and 100 nm show various improved properties compared to bulk form due to changes the in shape, size, size distribution, and surface area [11]. Various specific applications of nanoparticles refer to their large surface area for chemical reactions [12]. The antibacterial characteristics of silver nanoparticles (AgNPs) are much improved compared to the bulk form due to the increased physicochemical and mechanical properties [10,12]. The synthesis of AgNPs could be done with the help of microorganisms [5,8] and plant extracts [9,10,11,13,14]. The microorganisms and plant extracts play a role of reducing and capping agent in forming of AgNPs [11,13,15]. The advantages of this method are that they are simple, environmentally friendly, inexpensive, and the application of non-toxic reactants [13] compared to the conventional processes. On the other hand, AgNPs’ usage is limited by their stability depending on the surface charge of the suspending media and its rapid oxidation [16]. As a result, AgNPs have been integrated into composites to improve their stability and overcome rapid oxidation [17].
Recent studies proved that AgNPs could be impregnated into BC matrix via in-situ [18,19] or ex-situ synthesis [20,21]. In general, reductant chemicals are needed to reduce Ag+ from bulk solution into Ag0 of AgNPs and cap them in cellulose matrix for better stability, for example, adding sodium borohydride (NaBH4) [8,15,22,23], triethanolamine (TEA) [24], and urea [25] to produce cellulose-AgNPs composites. To avoid the utilization of chemicals that may be hazardous to the environment, Han et al. [25] explained that cellulose was capable of reducing Ag+ to Ag0 at room temperature in strong alkali conditions. Also, the existence of alkali residue promotes the generation of Ag2O, which was a deposit in the cellulose matrix and gave another easier reaction route to form AgNPs. Besides, the abundant hydroxyl group of BC itself was sufficient to supply the energy required for metal reduction [15].
BC has vast potential in many sectors because of its superior characteristics. However, the applications of BC on a large scale are constrained by its relatively low productivity and high cost of culture medium. The focus of this present study was to synthesize BC in a relatively low-cost medium and utilize it for the embedding and dispersion of AgNPs. Acetobacter xylinum strain grown in a simple coconut water medium was sterilized beforehand and used as a template for reducing and capping AgNPs. This method was simple, low cost, and used reduced chemicals, which has not been investigated. The most notable benefit of this study was providing sustainable nanoparticles composite biomaterials to meet the need for environmentally friendly products and utilize cheap and native resources. The resulting AgNPs templated BC products were characterized by Fourier transform infra-red (FTIR) to confirm the functional groups that play a role in forming and binding AgNPs in the BC matrix, X-ray diffraction (XRD), scanning electron microscope (SEM), and energy dispersive X-ray (EDX) to confirm the presence of AgNPs in BC matrix. The thermal stability of AgNPs templated BC was also assessed by thermogravimetric analysis (TGA). The antibacterial activities were measured by the inhibition zone of gram-positive bacteria (S. aureus) and gram-negative bacteria (E. coli).
2 Materials and methods
2.1 Materials
Silver nitrate (AgNO3), sodium hydroxide (NaOH), and acetic acid glacial (CH3COOH) were analytical grades and purchased from Merck, Germany. The coconut water was supplied by the local market in Yogyakarta, Indonesia. The white sugarcane crystals were purchased from PT. Sweet Indo Lampung, Indonesia, and (NH4)2SO4 were purchased from PT. Petrokimia Gresik, Indonesia.
2.2 Preparation and purification of BC
BC from coconut water, commonly known as nata de coco, was produced by boiling 1 L of coconut water, 10 g sugarcane, and 5 g (NH4)2SO4. Acetic acid was added dropwise to adjust the pH of the solution to 5.0. After the coconut water medium cooled down, about 10 mL of Acetobacter xylinum solution starter was added, then the solution was left for fermentation at room temperature for 4 days. BC produced was harvested and rinsed with running water until the pH of the rinsing water became neutral. BC was then put in boiling water for 5 min to kill the remaining bacteria. The resulting BC sheet with a thickness of 4 mm, as seen in Figure 1, was immersed in distilled water and stored at 4°C until subsequent use.

Bacterial cellulose nata de coco fermented for 4 days.
2.3 Impregnation of Ag into BC matrix
The BC sheet obtained initially was treated by soaking in various concentrations of AgNO3 solution (2, 5, 10, and 15 mM) with constant mixing for 1 h, then leaving it to rest for a night to make sure the Ag+ ions fully adsorb into the BC matrix. BC sheet treated with AgNO3 was then rinsed with distilled water for 5 min to eliminate the excess AgNO3 solution, followed by reduction process by immersing in 0.01 N NaOH solution for 10 min. Browny BC sheet indicated the formation of AgNPs in the BC matrix [8,23,25]. AgNPs templated BC was then dried in Memmert oven at 60°C until dry sheet was obtained (±2 h). The composite sheet of AgNPs templated BC was denoted as AgNP2@BC, AgNP5@BC, AgNP10@BC, and AgNP15@BC according to the concentration of AgNO3 used for the treatment at the initial stage.
2.4 Characterization of AgNPs templated BC
FTIR data were collected using the Nicolet Avatar 360 IR instrument, measured at a wavelength of 4,000–500 cm−1. XRD pattern of pristine BC and AgNP@BC was identified using the Bruker D2 Phaser X-ray Diffraction instrument with a value of 2θ from 10° to 80°. The crystallinity index (CI) was measured as:
where I 002 is the maximum intensity, and I am is the intensity of the amorphous region [26]. Surface morphology and elemental composition of AgNPs templated BC were seen using SEM-EDX Phenom Desktop ProXL. The presence of AgNPs in the BC matrix was measured by TGA Linseis PT1000 with an analytical temperature from 0°C to 600°C, heating rate value of 10°C·min−1, and nitrogen gas flow rate of 50 mL·min−1 to avoid degradation of the sample by thermal oxidation.
2.5 Assay of antibacterial activity
The potential antibacterial activity of AgNP@BC was measured by gram-positive bacteria (S. aureus) and gram-negative bacteria (E. coli). For this investigation, bacteria were grown in agar nutrients placed in a 10 cm diameter Petri dish. First, a nutrient agar medium was prepared with a concentration of 20 g·L−1. Agar medium was sterilized at 121°C for 15 min. After the agar medium was cooled at 40°C, bacteria were inoculated up to 107. Agar medium was then stirred until homogeneous, poured into a Petri dish (±20 mL), and left until hardened. A small piece of AgNPs templated BC was placed on the surface of the agar medium. This Petri dish was cooled at 4–10°C for 1 h and then incubated at 37°C for 24 h (or until the inhibition zone was clearly visible). The formation of the inhibition zone was then measured for each sample.
3 Results and discussion
3.1 Impregnation of Ag into BC matrix
BC is produced from the fermentation process of sugars, especially fructose and glucose, by exopolysaccharide-producing bacteria [27]. Coconut water was selected as the primary ingredient in the fermentation media in this study because it contains nutrients and minerals that Acetobacter xylinum needs for metabolism [28]. During fermentation, Acetobacter xylinum converts sucrose into glucose and fructose with the help of the sucrase enzyme and produces a white sheet of cellulose due to metabolism activities. The thickness of the resulting BC membrane increased with the increase in the fermentation time [29]. After 4 days of fermentation, a milky white BC sheet with a thickness of 4 mm was formed, as shown in Figure 1.
Wet BC sheets were immersed in distilled water at 4°C for several days to obtain a consistent grey or brown color when impregnated in AgNO3 solution. This step was done to ensure that the BC pores were completely filled with water so that when the BC sheet was immersed in AgNO3 solution, it was expected that Ag particles would be easier to fill in the BC matrix. The immersion of the BC sheet in the AgNO3 solution was carried out for a day to ensure that Ag ions were completely adsorbed in the BC matrix. Abundant hydroxyl groups in BC can reduce Ag ions and provide anchoring sites for Ag+ [5]. After the immersion step, the BC sheet looks slightly blackish, as seen in Figure 2a. The following process was immersion in 0.01N NaOH solution to facilitate strong alkaline conditions, so the BC could reduce Ag+ to Ag0 and simultaneously produce AgNPs, which is capped in the BC matrix [25]. After immersion in NaOH solution, the BC sheet turned brownish in color (Figure 2b), indicating the formation of AgNPs [8,23,25].

Bacterial cellulose nata de coco: (a) immersed in AgNO3 solution and (b) reduced by NaOH solution.
The formation of AgNPs can be explained as an interaction with the hydroxyl groups of cellulose. The diffusion of hydrated silver ions (Ag(H2O)2)+ to the BC matrix leads to coordination with the hydroxyl groups of cellulose [24]. This can be indicated by FTIR spectra. Once the AgNPs are made, the BC matrix forms a thin layer or film that acts as a capping agent, stabilizer, and agglomeration preventer [30]. In the end, AgNPstemplated BC with a small and tight size distribution was obtained. The final step was to dry the sheet in an oven at 60°C to obtain a dry sheet.
3.2 FTIR analysis
FTIR analysis was carried out to ascertain the spectrum shift indicating the functional groups that play a role in forming and binding AgNPs in the BC matrix structure. FTIR spectra of pristine BC and AgNPs templated BC is presented in Figure 3.
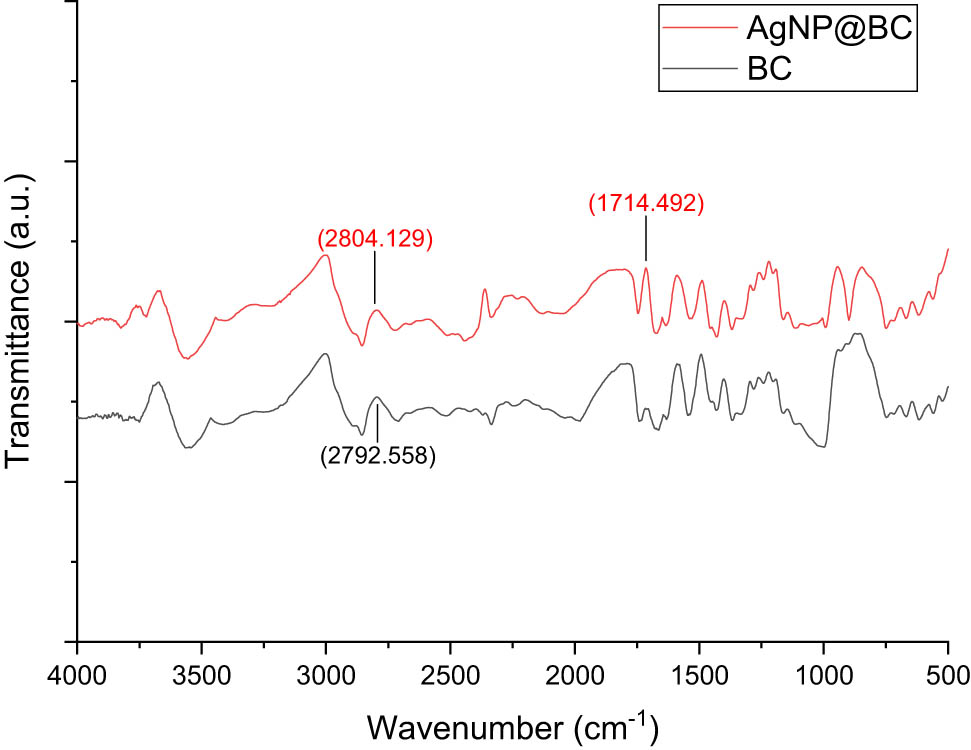
FTIR spectra of pristine BC and sample AgNP10@BC.
FTIR spectra of both the pristine BC and AgNP@BC in Figure 3 showed the typical spectrum of cellulose. The spectrum detected around 3,400–3,600 cm−1 in both the graphs indicated an abundance of OH group, which may play a significant role in reducing Ag+ from AgNO3 to Ag0 of AgNPs and stabilizing AgNPs in the BC matrix by acting as a capping agent [23,30,31], and also hydroxyl groups have been shown to have the ability to coordinate with metal ions. As a result of reducing Ag+ ions in the BC matrix, AgNP@BC FTIR spectra exhibited spectrum shift from 2,792 to 2,804 cm−1 corresponding to the shift of carbonyl group of aldehyde group. Additionally, a spectrum appeared at 1,714 cm−1 assigned to C═O of the carbonyl group. These significant spectrum changes in the FTIR spectrum reflected the considerable factors in the synthesis of metal nanoparticles: the oxidation of hydroxyl and aldehyde groups to form carbonyl groups [30,31]. Previously published studies also stated that the band appearing in the range of 1,700–1,600 cm−1 indicated the formation of AgNPs capped in biostructure [11,32,33].
3.3 X-ray diffraction
XRD analysis indicates the crystal structure, crystallinity index, and silver content of the BC matrix. Diffraction was measured at 2θ value from 10° to 80°. Diffraction peaks of pristine BC and AgNPs templated BC are shown in Figure 4.

XRD graph of pristine BC and AgNP10@BC.
Those diffraction peaks showed the pattern of typical cellulose I. Diffraction of BC showed prominent peaks at 2θ = 14.46° which correspond to triclinic structure (Iα) = 110 and monoclinic structure (Iβ) = 100, 17.11° which correspond to Iα = 010 and Iβ = 110, and 22.69° which correspond to Iα = 110 and Iβ = 200 [34,35]. The diffraction peaks of AgNP@BC at 32.04°, 45.9°, 54.4°, and 78.36° corresponded to the lattice plane values (111), (200), (220), and (311) of a face centered cubic (FCC) metallic silver crystal fitted with a JCPDS card of Ag (No. 4-783) [10,11,36], demonstrating the presence of AgNPs in the BC matrix. These samples’ crystallinity index (CI) was 73.47% for pristine BC and 72.57% for AgNPs templated BC. These results were conformable with crystallinity index data for BC products from previously published articles [3,35,37] and indicated that deposition of AgNPs did not affect the crystal structure of BC.
3.4 Morphology of AgNP templated BC
SEM analysis was performed to obtain the morphological surface information. As seen in Figure 5, it was shown that pristine BC was composed of nanofiber networks that form evenly distributed micro spaces. The typical network structure of BC is all dependent on porosity, compaction, and water tightness [3]. All studies reveal that the morphology BC was composed of a homogeneous network of cellulose microfibrils as presented in Figure 5a, similar to those reported in previous studies [37,38]. Meanwhile, the AgNPs templated BC sample showed spherical (white dot) AgNPs embedded between the nanofiber networks of BC as presented in Figure 5b. The spherical AgNPs formed in the BC matrix depend on the porosity and surface crystallography [8,9,15,24].
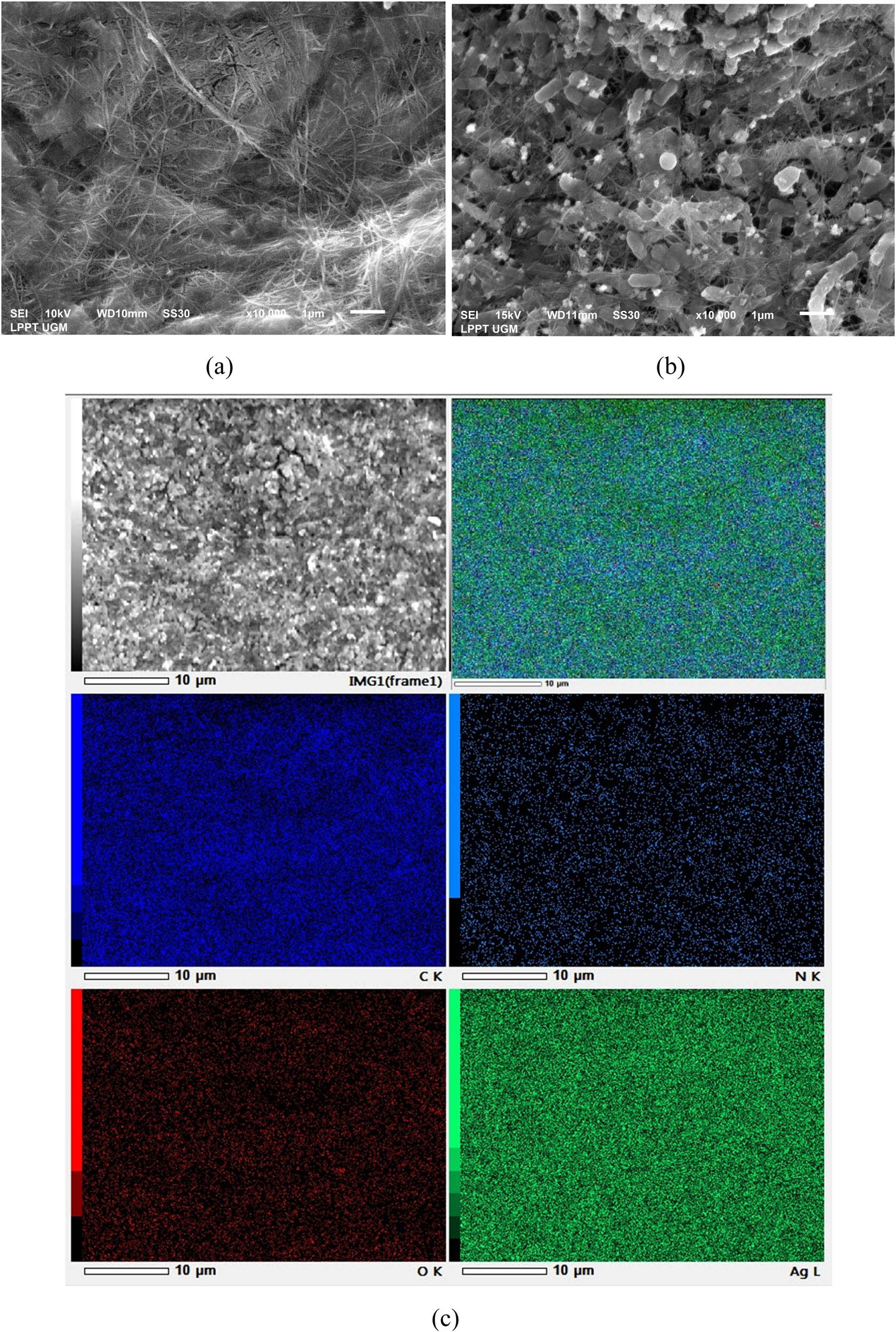
SEM image of: (a) pristine BC, (b) AgNP15@BC, and (c) its elemental mapping.
EDX scanning can be used to determine the elements and chemical composition of a material. The presence of AgNPs embedded in the BC matrix was clearly visualized in EDX mapping, as presented in Figure 5c. Additionally, EDX quantitative analysis was done to determine the concentration of AgNPs in the BC matrix. As illustrated in Figure 6, a strong peak at 3 keV of EDX quantitative analysis confirmed the presence of AgNPs. Table 1 summarizes the elements found in AgNPs templated BC based on the EDX analysis. The maximum Ag content was possessed by the AgNP15@BC sample with 33.21% mass, the remaining components, including C, O, and N, were derived from the BC sheet. This result is in accordance with the thermal stability data, which confirmed that there was 33% mass residue of AgNPs templated BC sample after heating up to 600°C indicating the presence of AgNPs.
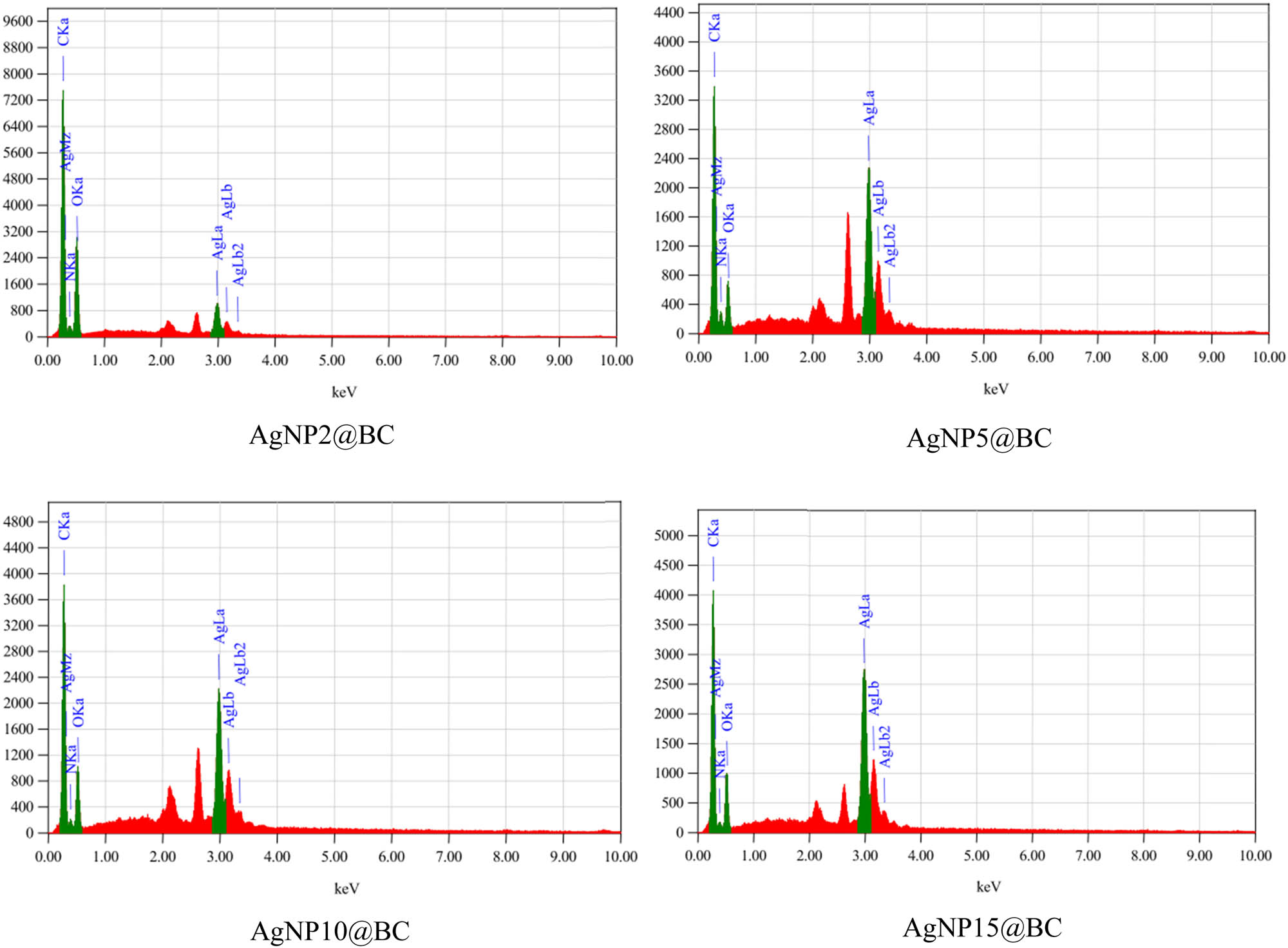
EDX quantitative analysis of AgNPs templated BC.
Elements detected in AgNPs templated BC samples by EDX analysis
| Sample | Element (%) | |||
|---|---|---|---|---|
| Ag | C | O | N | |
| AgNP2@BC | 8.13 | 37.55 | 33.48 | 20.83 |
| AgNP5@BC | 31.36 | 28.09 | 17.39 | 23.16 |
| AgNP10@BC | 28.74 | 30.47 | 22.42 | 18.37 |
| AgNP15@BC | 33.21 | 29.15 | 20.78 | 16.86 |
3.5 Thermal stability
TGA was performed to investigate the thermal stability of BC and AgNPs templated BC sheets. The results are displayed in Figure 7.
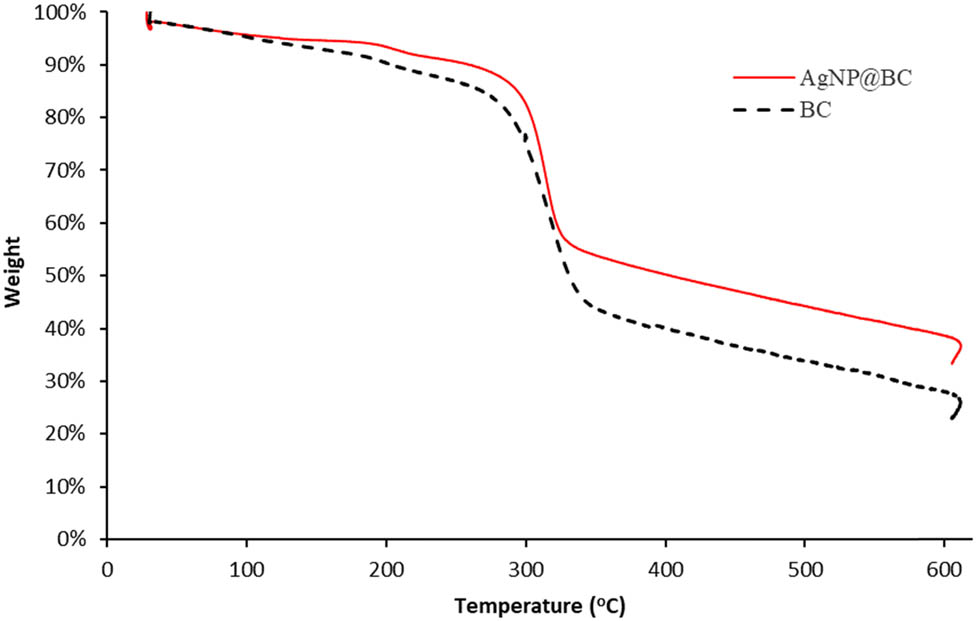
Thermal stability of pristine BC and AgNP10@BC by TGA analysis.
The first significant weight loss occurred when heated up to 100°C. The remaining weight of both the BC and AgNPs templated BC sheets was 95%. This weight loss happened due to the evaporation of the remaining water in the BC matrix. The subsequent significant weight loss occurred at 270°C, which may be associated with the degradation of cellulose, including depolymerization, dehydration, and decomposition of glucose units [3]. When heated over 600°C, around 20% of the remaining weight of the BC sample corresponded to burnt ashes, similar to the other reported studies [3,34,38]. Based on the TGA curve in Figure 7, AgNPs’ presence can be detected as the residue around 33% on heating above 600°C. The residue may be attributed to the remaining ashes from combustion and silver particles which do not degrade due to heating. Based on this investigation, both the pristine BC and AgNPstemplated BC were relatively stable at thermal heating up to 250°C. Considering the requirement for industrial application materials that remain intact at high temperatures of 250°C [3], this BC exhibits good thermal stability. Thermal degradation is one of the critical criteria of BC as a biopolymer for industrial material. Characteristic of the BC such as crystallinity, molecular weight, hydrophilicity, and functionalized with an inorganic nanoparticle are primary factors that could affect the thermal degradation of BC [39].
3.6 Antibacterial activities
Recently, AgNPs have been applied as antibacterial agents for several commercial products such as food storage, body care, pharmaceuticals, water treatment, and paint [10]. The antibacterial activity of pristine BC and AgNPs templated BC was measured by the inhibition zone of gram-positive bacteria (S. aureus) and gram-negative bacteria (E. coli), presented in Figures 8 and 9.
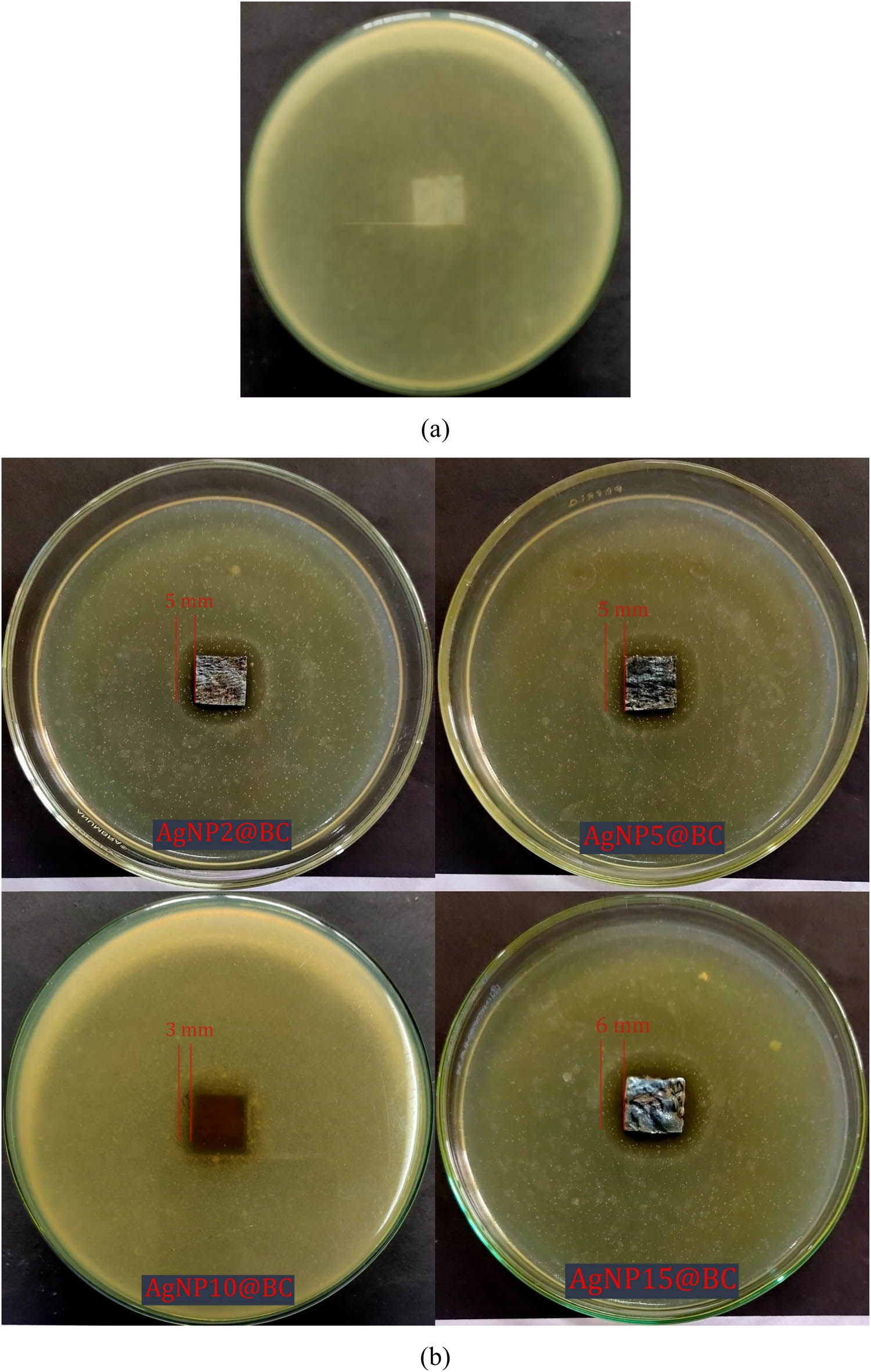
Antibacterial activity against S. aureus of: (a) pristine BC and (b) AgNPs templated BC.

Antibacterial activity against E. coli of: (a) pristine BC and (b) AgNPs templated BC.
The visual difference of agar media for each sample was caused only by incubation and observation time. The incubation time does not directly affect the inhibition zone, so this visual difference could be neglected. During this incubating time, bacteria grew normally in the area around the pristine BC. On the other hand, the inhibition zone was clearly formed around AgNPs templated BC. The antibacterial strength of AgNPs templated BC against S. aureus (Figure 8b) and E. coli (Figure 9b) were tested with different concentrations of AgNO3 solution, which were used in the initial treatment of synthesis of AgNPs templated BC. The concentration of AgNO3 solution affects the antibacterial strength of the sheet. It was observed that AgNP15@BC has the maximum antibacterial activity against both S. aureus and E. coli. Figure 10 exhibited a bar diagram of the inhibition zone formed by each sample, which proved that AgNPs templated BC generated a larger inhibition zone against gram-positive bacteria compared to gram-negative bacteria.

Inhibition zone of BC and AgNPs templated BC against S. aureus and E. coli.
Previous studies demonstrated that AgNP/BC composites were effective in controlling pathogenic bacteria such as E. coli [22,40], S. aureus [19,21,22,40], P. aeruginosa [21], V. harveyi, and V. parahaemolyticus [18]. A similar result was obtained in this study where the AgNPs templated BC showed a good antibacterial activity compared to previously published studies, as shown in Table 2. It was noticeable that AgNPs templated BC is a potential material for the antibacterial application and could be effectively utilized in pharmaceutical, biotechnological, and biomedical applications.
Comparison of antibacterial activity of several AgNP/BC composite materials
| BC | Synthesis method | Antibacterial activity | References |
|---|---|---|---|
| Gluconacetobacter xylinus grown in medium containing mannitol, tryptone, and yeast extract | UV irradiation | Inhibition zone of 6.5 mm against S. aureus | [19] |
| Acetobacter xylinum grown in medium containing d-glucose anhydrous, yeast extract powder, and distilled water | NaBH4 reduction | Inhibition zone of 2 mm against E. coli and 3.5 mm against S. aureus | [22] |
| Gluconacetobacter intermedius grown in HS medium | TEMPO oxidation, followed by sodium citrate reduction | Inhibition zone of 3.7 mm against E. coli and 3.2 mm against S. aureus | [40] |
| Gluconacetobacter xylinum grown in HS medium | TEMPO oxidation | Diameter of inhibition area was 19 mm in V. harveyi and 21 mm in V. parahaemolyticus | [18] |
| Komagataeibacter xylinus grown in medium containing glucose, yeast extract, KH2PO4, MgSO4·7H2O, and ethanol | Montmorillonite incorporation | Inhibition zone of 2.0 mm for S. aureus and 2.5 mm for P. aeruginosa | [21] |
| Acetobacter xylinum grown in coconut water medium | Alkali reduction | Inhibition zone of 6 mm against both E. coli and S. aureus | This study |
4 Conclusion
Investigation of synthesis of AgNPs templated BC sheet as a potential material for the antibacterial and industrial application has been done. The AgNPs templated BC was prepared via a simple and greener route. This work demonstrates that Ag ions in 2–15 mM AgNO3 solution can successfully be incorporated into BC matrix as AgNPs with the help of reducing in 0.01N NaOH solution. The presence of AgNPs in the BC matrix was verified by FTIR, XRD, SEM, EDX, and TGA analyses. In addition, the presence of AgNPs in the BC matrix showed the antibacterial activity on both S. aureus and E. coli, which is relevant for controlling pathogenic bacteria related to several human diseases. Finally, synthesis of BC from Acetobacter xylinum grown in coconut water medium and utilized as a template for the formation of AgNPs provides a low-cost and straightforward method for developing biomaterial for antibacterial and industrial applications.
Acknowledgment
All authors gratefully acknowledge the Directorate of Research and Community Service, Gadjah Mada University, for funding this research project.
-
Funding information: This work was funded by the Directorate of Research and Community Service, Gadjah Mada University, under contract number 3143/UN1.P.III/DIT-LIT/PT/2021 (final assignment recognition 2021).
-
Author contributions: Tintin Mutiara: writing – original draft, conceptualization, methodology, investigation, and visualization; Hary Sulistyo: writing – review, investigation, and supervision; Moh. Fahrurrozi: writing – review, investigation, and supervision; Muslikhin Hidayat: writing – review, conceptualization, investigation, supervision, project administration, and funding acquisition.
-
Conflict of interest: Authors state no conflict of interest.
References
[1] Sai H, Jin Z, Wang Y, Fu R, Wang Y, Ma L. Facile and green route to fabricate bacterial cellulose membrane with superwettability for oil–water separation. Adv Sustain Syst. 2020;4(7):1–9.10.1002/adsu.202000042Search in Google Scholar
[2] Wang J, Tavakoli J, Tang Y. Bacterial cellulose production, properties and applications with different culture methods – A review. Carbohydr Polym. 2019;219:63–76.10.1016/j.carbpol.2019.05.008Search in Google Scholar PubMed
[3] Galdino CJS, Maia AD, Meira HM, Souza TC, Amorim JDP, Almeida FCG, et al. Use of a bacterial cellulose filter for the removal of oil from wastewater. Process Biochem. 2019;1:288–96.10.1016/j.procbio.2019.12.020Search in Google Scholar
[4] Lehtonen J, Chen X, Beaumont M, Hassinen J, Orelma H, Dumée LF, et al. Impact of incubation conditions and post-treatment on the properties of bacterial cellulose membranes for pressure-driven filtration. Carbohydr Polym. 2020;251:51.10.1016/j.carbpol.2020.117073Search in Google Scholar PubMed
[5] Yang Y, Chen Z, Wu X, Zhang X, Yuan G. Nanoporous cellulose membrane doped with silver for continuous catalytic decolorization of organic dyes. Cellulose. 2018;25(4):2547–58.10.1007/s10570-018-1710-xSearch in Google Scholar
[6] Romling U, Galperin MY. Bacterial cellulose biosynthesis: diversity of operons, subunits, products and functions. Trends Microbiol. 2015;23(9):545–57.10.1016/j.tim.2015.05.005Search in Google Scholar PubMed PubMed Central
[7] Tian D, Guo Y, Huang M, Zhao L, Deng S, Deng O, et al. Bacterial cellulose/lignin nanoparticles composite films with retarded biodegradability. Carbohydr Polym. 2021;274:118656.10.1016/j.carbpol.2021.118656Search in Google Scholar PubMed
[8] Yang G, Xie J, Hong F, Cao Z, Yang X. Antimicrobial activity of silver nanoparticle impregnated bacterial cellulose membrane: Effect of fermentation carbon sources of bacterial cellulose. Carbohydr Polym. 2021;87(1):839–45.10.1016/j.carbpol.2011.08.079Search in Google Scholar PubMed
[9] Hamedi S, Shojaosadati AS, Mohammad A. Evaluation of the catalytic, antibacterial and anti-biofilm activities of the Convolvulus arvensis extract functionalized silver nanoparticles. J Photochem Photobiol B Biol. 2017;167:36–44.10.1016/j.jphotobiol.2016.12.025Search in Google Scholar PubMed
[10] Renuka R, Devi KR, Sivakami M, Thilagavathi T, Uthrakumar R, Kaviyarasu K. Biosynthesis of silver nanoparticles using Phyllanthus emblica fruit extract for antimicrobial application. Biocatal Agric Biotechnol. 2020;24:101567.10.1016/j.bcab.2020.101567Search in Google Scholar
[11] Chand K, Cao D, Eldin Fouad D, Hussain Shah A, Qadeer Dayo A, Zhu K, et al. Green synthesis, characterization and photocatalytic application of silver nanoparticles synthesized by various plant extracts. Arab J Chem. 2020;13(11):8248–61.10.1016/j.arabjc.2020.01.009Search in Google Scholar
[12] Marimuthu S, Antonisamy AJ, Malayandi S, Rajendran K, Tsai PC, Pugazhendhi A, et al. Silver nanoparticles in dye effluent treatment: A review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J Photochem Photobiol B Biol. 2020;205:111823.10.1016/j.jphotobiol.2020.111823Search in Google Scholar PubMed
[13] Khodadadi B, Bordbar M, Yeganeh-Faal A, Nasrollahzadeh M. Green synthesis of Ag nanoparticles/clinoptilolite using Vaccinium macrocarpon fruit extract and its excellent catalytic activity for reduction of organic dyes. J Alloy Compd. 2017;719:82–8.10.1016/j.jallcom.2017.05.135Search in Google Scholar
[14] Ravichandran V, Vasanthi S, Shalini S, Shah SAA, Tripathy M, Paliwal N. Green synthesis, characterization, antibacterial, antioxidant and photocatalytic activity of Parkia speciosa leaves extract mediated silver nanoparticles. Results Phys. 2019;15:102565.10.1016/j.rinp.2019.102565Search in Google Scholar
[15] Yang G, Xie J, Deng Y, Bian Y, Hong F. Hydrothermal synthesis of bacterial cellulose/AgNPs composite: A ‘green’ route for antibacterial application. Carbohydr Polym. 2012;87(4):2482–7.10.1016/j.carbpol.2011.11.017Search in Google Scholar
[16] Hillenkamp M, Domenicantonio GD, Eugster O, Félix C. Instability of Ag nanoparticles in SiO2 at ambient conditions. Nanotechnology. 2006;18:015702.10.1088/0957-4484/18/1/015702Search in Google Scholar
[17] Jeevanandam J, Krishnan S, Hii YS, Pan S, Chan YS, Acquah C. Synthesis approach-dependent antiviral properties of silver nanoparticles and nanocomposites. J Nanostruc Chem. 2022;1–23.10.1007/s40097-021-00465-ySearch in Google Scholar PubMed PubMed Central
[18] Elayaraja S, Zagorsek K, Li F, Xiang J. In situ synthesis of silver nanoparticles into TEMPO-mediated oxidized bacterial cellulose and their antivibriocidal activity against shrimp pathogens. Carbohydr Polym. 2017;166:329–37.10.1016/j.carbpol.2017.02.093Search in Google Scholar PubMed
[19] Yang G, Wang C, Hong F, Yang X, Cao Z. Preparation and characterization of BC/PAM-AgNPs nanocomposites for antibacterial applications. Carbohydr Polym. 2015;115:636–42.10.1016/j.carbpol.2014.09.042Search in Google Scholar PubMed
[20] Nicoara AI, Stoica AE, Ene DE, Vasile BS, Holban AM, Neacsu I In situ and ex situ designed hydroxyapatite: bacterial cellulose materials with biomedical applications. Mater (Basel). 2020;13(21):4793.10.3390/ma13214793Search in Google Scholar PubMed PubMed Central
[21] Horue M, Cacicedo ML, Fernandez MA, Rodenak-Kladniew B, Torres Sánchez RM, Castro GR. Antimicrobial activities of bacterial cellulose – Silver montmorillonite nanocomposites for wound healing. Mater Sci Eng C. 2020;116:111152.10.1016/j.msec.2020.111152Search in Google Scholar PubMed
[22] Maneerung T, Tokura S, Rujiravanit R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr Polym. 2008;72(1):43–51.10.1016/j.carbpol.2007.07.025Search in Google Scholar
[23] Isik Z, Unyayar A, Dizge N. Filtration and antibacterial properties of bacterial cellulose membranes for textile wastewater treatment. Avicenna J Env Heal Eng. 2018;5(2):106–14.10.15171/ajehe.2018.14Search in Google Scholar
[24] Barud HS, Barrios C, Regiani T, Marques RFC, Verelst M, Dexpert-Ghys J, et al. Self-supported silver nanoparticles containing bacterial cellulose membranes. Mater Sci Eng C. 2008;28(4):515–8.10.1016/j.msec.2007.05.001Search in Google Scholar
[25] Han Y, Wu X, Zhang X, Zhou Z, Lu C. Reductant-Free Synthesis of Silver Nanoparticles-Doped Cellulose Microgels for Catalyzing and Product Separation. ACS Sustain Chem Eng. 2016;4(12):6322–31.10.1021/acssuschemeng.6b00889Search in Google Scholar
[26] Segal L, Creely JJ, Martin AE, Conrad CM. An empirical method for estimating the degree of crystallinity of native cellulose using the X-Ray diffractometer. Text Res J. 1959;29(10):786–94.10.1177/004051755902901003Search in Google Scholar
[27] Thakur K, Kumar V, Kumar V, Kumar S. Genomic characterization provides genetic evidence for bacterial cellulose synthesis by Acetobacter pasteurianus RSV-4 strain. Int J Biol Macromol. 2020;156:598–607.10.1016/j.ijbiomac.2020.04.078Search in Google Scholar PubMed
[28] Barlina R, Palma B. Bioselulosa Dari Nata De Coco Sebagai Bahan Baku Edible Film. Kementrian Pertanian, Badan Litbang Pertanian. 2011 [cited 2021 April 23]. https://www.litbang.pertanian.go.id/info-teknologi/1915/file/Bioselulosa-dari-Nata-De-C.pdf.Search in Google Scholar
[29] Luo H, Zhang J, Xiong G, Wan Y. Evolution of morphology of bacterial cellulose scaffolds during early culture. Carbohydr Polym. 2014;111:722–8.10.1016/j.carbpol.2014.04.097Search in Google Scholar PubMed
[30] Escárcega-González CE, Garza-Cervantes JA, Vázquez-Rodríguez A, Morones-Ramírez JR. Bacterial exopolysaccharides as reducing and/or stabilizing agents during synthesis of metal nanoparticles with biomedical applications. Int J Polym Sci. 2018;2018:1–15.10.1155/2018/7045852Search in Google Scholar
[31] Dong H, Snyder JF, Tran D, Leadore J. Hydrogel, aerogel and film of cellulose nanofibrils functionalized with silver nanoparticles. Carbohydr Polym. 2013;95:760–7.10.1016/j.carbpol.2013.03.041Search in Google Scholar PubMed
[32] Sharma P, Pant S, Rai S, Yadav RB, Dave V. Green synthesis of silver nanoparticle capped with allium cepa and their catalytic reduction of textile dyes: an ecofriendly approach. J Polym Env. 2017;26:1795–803.10.1007/s10924-017-1081-7Search in Google Scholar
[33] Sathishkumar P, Preethi J, Vijayan R, Yusoff ARM, Ameen F, Suresh S, et al. Anti-acne, anti-dandruff and anti-breast cancer efficacy of green synthesized silver nanoparticles using Coriandrum sativum leaf extract. J Photochem Photobiol. 2016;B163:69–76.10.1016/j.jphotobiol.2016.08.005Search in Google Scholar PubMed
[34] Leonarski E, Cesca K, Zanella E, Stambuk BU, de Oliveira D, Poletto P. Production of kombucha-like beverage and bacterial cellulose by acerola byproduct as raw material. Lwt. 2020;135:1–8.10.1016/j.lwt.2020.110075Search in Google Scholar
[35] He F, Yang H, Zeng L, Hu H, Hu C. Production and characterization of bacterial cellulose obtained by Gluconacetobacter xylinus utilizing the by‑products from Baijiu production. Bioprocess Biosyst Eng. 2020;43:0123456789-936.10.1007/s00449-020-02289-6Search in Google Scholar PubMed
[36] Jiang X, Xie Y, Lu J, Zhu L, He W, Qian Y. Preparation, characterization, and catalytic effect of CS2-stabilized silver nanoparticles in aqueous solution. Science. 2001;D21:3795–9.10.1021/la001361vSearch in Google Scholar
[37] Gomes FP, Silva NH, Trovatti E, Serafim LS, Duarte MF, Silvestre AJ, et al. Production of bacterial cellulose by Gluconacetobacter sacchari using dry olive mill residue. Biomass Bioenergy. 2013;55:205–11.10.1016/j.biombioe.2013.02.004Search in Google Scholar
[38] Corzo Salinas DR, Sordelli A, Martínez LA, Villoldo G, Bernal C, Pérez MS, et al. Production of bacterial cellulose tubes for biomedical applications: Analysis of the effect of fermentation time on selected properties. Int J Biol Macromol. 2021;189:1–10.10.1016/j.ijbiomac.2021.08.011Search in Google Scholar PubMed
[39] Torgbo S, Sukyai P. Biodegradation and thermal stability of bacterial cellulose as biomaterial: The relevance in biomedical applications. Polym Degrad Stab. 2020;179:109232.10.1016/j.polymdegradstab.2020.109232Search in Google Scholar
[40] Feng J, Shi Q, Li W, Shu X, Chen A, Xie X, et al. Antimicrobial activity of silver nanoparticles in situ growth on TEMPO-mediated oxidized bacterial cellulose. Cellulose. 2014;21:4557–67.10.1007/s10570-014-0449-2Search in Google Scholar
© 2022 Tintin Mutiara et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

