Abstract
C14H10N3O3·C8H5O4, triclinic,
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless needle |
| Size: | 0.47 × 0.12 × 0.11 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.12 mm−1 |
| Diffractometer, scan mode: | Bruker D8 Venture Photon, ω |
| θmax, completeness: | 28.0°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 14,217, 4561, 0.045 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 2937 |
| N(param)refined: | 299 |
| Programs: | Bruker [1], SHELX [2, 3], WinGX/ORTEP-3 [4, 5], PLATON [6] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.5449 (2) | 0.16425 (18) | 0.46116 (15) | 0.0262 (4) |
| C2 | 0.5811 (2) | 0.24257 (18) | 0.59650 (15) | 0.0242 (4) |
| C3 | 0.5145 (2) | 0.36283 (19) | 0.65133 (16) | 0.0287 (4) |
| H3 | 0.437264 | 0.399733 | 0.603863 | 0.034* |
| C4 | 0.5618 (3) | 0.4278 (2) | 0.77525 (16) | 0.0322 (5) |
| H4 | 0.514936 | 0.509225 | 0.813737 | 0.039* |
| C5 | 0.7394 (3) | 0.26249 (19) | 0.79175 (16) | 0.0306 (5) |
| H5 | 0.818914 | 0.229485 | 0.841257 | 0.037* |
| C6 | 0.6934 (2) | 0.19145 (18) | 0.66885 (15) | 0.0278 (4) |
| H6 | 0.738003 | 0.10787 | 0.633413 | 0.033* |
| C7 | 0.5057 (3) | 0.2320 (2) | 0.21158 (17) | 0.0360 (5) |
| C8 | 0.4229 (3) | 0.1658 (2) | 0.08092 (17) | 0.0389 (5) |
| C9 | 0.4670 (3) | 0.1833 (2) | −0.01673 (19) | 0.0482 (6) |
| H9 | 0.558923 | 0.2469 | −0.005792 | 0.058* |
| C10 | 0.3744 (4) | 0.1060 (3) | −0.1309 (2) | 0.0541 (7) |
| H10 | 0.402815 | 0.115695 | −0.20008 | 0.065* |
| C11 | 0.2406 (4) | 0.0145 (3) | −0.1459 (2) | 0.0607 (8) |
| H11 | 0.177295 | −0.0371 | −0.225503 | 0.073* |
| C12 | 0.1953 (3) | −0.0042 (3) | −0.0451 (2) | 0.0536 (7) |
| H12 | 0.104157 | −0.067956 | −0.054859 | 0.064* |
| C13 | 0.2909 (3) | 0.0752 (2) | 0.06797 (17) | 0.0410 (6) |
| C14 | 0.2794 (3) | 0.0800 (2) | 0.19166 (19) | 0.0374 (5) |
| N1 | 0.4383 (2) | 0.21912 (18) | 0.39431 (13) | 0.0362 (4) |
| N2 | 0.6728 (2) | 0.37790 (16) | 0.84223 (14) | 0.0301 (4) |
| N3 | 0.4174 (2) | 0.16765 (17) | 0.26974 (13) | 0.0348 (4) |
| O1 | 0.61114 (18) | 0.06184 (13) | 0.41769 (11) | 0.0355 (4) |
| O2 | 0.6216 (2) | 0.31991 (16) | 0.26162 (14) | 0.0491 (4) |
| O3 | 0.1775 (2) | 0.02278 (16) | 0.22208 (15) | 0.0500 (4) |
| H2 | 0.706 (3) | 0.425 (2) | 0.926 (2) | 0.06* |
| C15 | 0.1524 (3) | 0.4397 (2) | 0.34621 (17) | 0.0317 (5) |
| C16 | 0.0454 (2) | 0.55637 (18) | 0.35745 (15) | 0.0256 (4) |
| C17 | −0.0721 (2) | 0.57976 (18) | 0.26479 (15) | 0.0259 (4) |
| C18 | −0.1433 (3) | 0.70270 (19) | 0.29432 (17) | 0.0331 (5) |
| H18A | −0.2186 | 0.720511 | 0.23181 | 0.04* |
| C19 | −0.1093 (3) | 0.7999 (2) | 0.41054 (18) | 0.0376 (5) |
| H19A | −0.1594 | 0.883056 | 0.427275 | 0.045* |
| C20 | −0.0016 (3) | 0.7741 (2) | 0.50174 (17) | 0.0369 (5) |
| H20A | 0.0197 | 0.838038 | 0.582865 | 0.044* |
| C21 | 0.0750 (3) | 0.6555 (2) | 0.47516 (16) | 0.0313 (5) |
| H21A | 0.15074 | 0.640106 | 0.53886 | 0.038* |
| C22 | −0.1345 (3) | 0.4846 (2) | 0.13344 (16) | 0.0321 (5) |
| O4 | 0.25504 (19) | 0.44073 (15) | 0.43538 (12) | 0.0408 (4) |
| O5 | 0.1388 (2) | 0.34188 (16) | 0.24257 (14) | 0.0564 (5) |
| O6 | −0.0670 (2) | 0.37799 (15) | 0.08790 (13) | 0.0526 (5) |
| O7 | −0.2573 (2) | 0.51703 (15) | 0.07231 (12) | 0.0453 (4) |
| H5A | 0.056 (4) | 0.354 (3) | 0.174 (2) | 0.068* |
| H1 | 0.383 (3) | 0.295 (2) | 0.421 (2) | 0.049 (7)* |
1 Source of materials
All reagents were commercially available and used without further purification. An amount of 0.1123 g of isonicotinic acid hydrazide (0.819 mmol), 0.1364 g of phthalic acid (0.821 mmol), 0.1619 g of 2,2-dihydroxybenzophenone (0.818 mmol) and 3.0 mL of methanol were added into a screw-top dram vial (with rubber septum) and stirred at 300 rpm at 60 °C for 10 min. The vial was closed, and the solution was allowed to reflux at 60 °C for 24 h. The solution was then left slightly open to allow slow evaporation at room temperature. Colourless needles were observed after 3 days.
2 Experimental details
C-bound hydrogen atoms were located in the difference map then positioned geometrically and were allowed to ride on their respective parent atoms with thermal displacement parameters 1.2 times of the parent C atom. The coordinates and isotropic displacement parameters of all N-bound and O-bound H atoms were allowed to refine freely. Diagrams and publication material were generated using ORTEP-3 [4], WinGX [5] and PLATON [6].
3 Comment
For over 50 years, isoniazid has been used as a first-line pharmaceutical drug for the treatment of Mycobacterium tuberculosis. However, the mycobacterium has developed resistance to isoniazid due to certain genetic mutations (Shehzad et al. [7]). It is therefore beneficial to modify the NH2 functional group of isoniazid, as this covalent modification has often shown to be effective against multi-drug resistant tuberculosis strains (Setshedi et al. [8]). In this study, isoniazid was covalently modified with phthalic acid, and a salt was formed from an interaction with a second phthalic acid molecule, resulting in the product presented here.
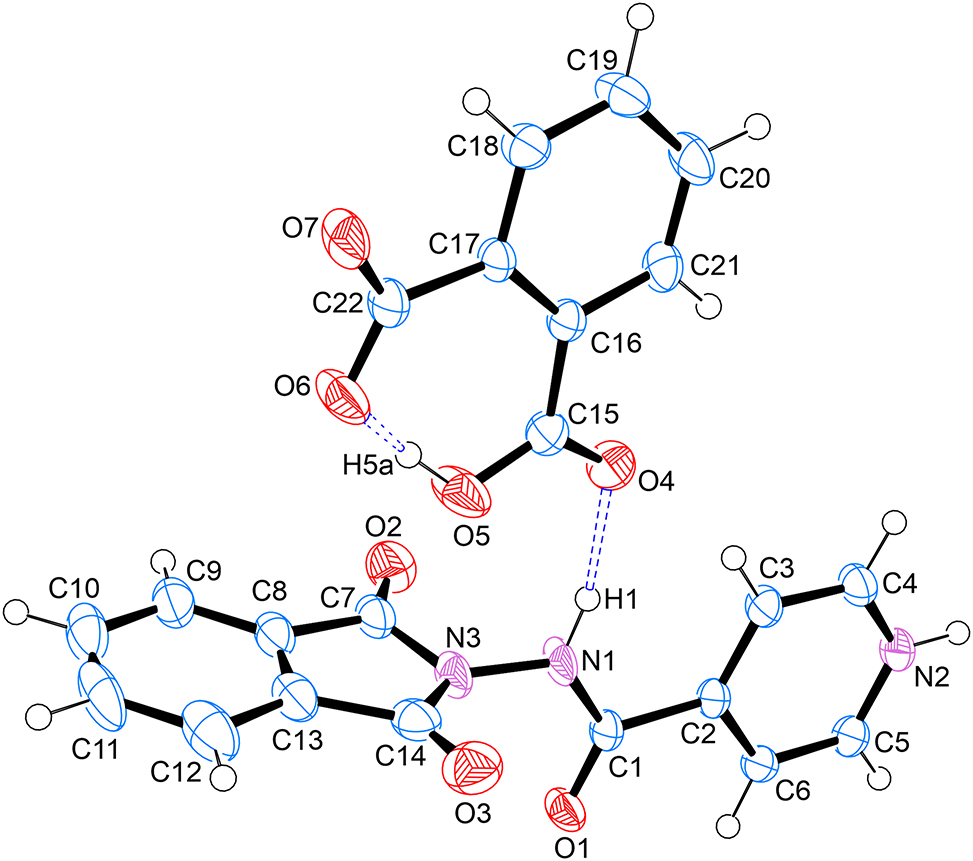
As shown in the figure, the asymmetric unit contains a salt formed via one molecule of 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium and one molecule of 2-carboxybenzoate. The two molecules are held together by a hydrogen bond between H2 on the pyridinium ring and O7 of the carboxylate moiety on the phthalic acid molecule. Furthermore, a hydrogen bond exists between the H1 donor on the carbohydrazide moiety of the modified isoniazid and the O4 acceptor atom of the carboxylic acid moiety of phthalic acid. The carboxylic acid and carboxylate moieties on phthalic acid form an expected intramolecular S(6) hydrogen bond [9], between the H5a donor and the O6 acceptor. All bond lengths and angles are as expected [10, 11].
-
Author contributions: The author has accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the University of South Africa.
-
Conflict of interest statement: The author declares no conflict of interest regarding this article.
References
1. Bruker. APEX2 (Version 2012.10-0), SAINT (Version 27B), SADABS (Version 2012/1); Bruker AXS Inc: Madison, Wisconsin, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Farrugia, L. J. WINGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
5. Farrugia, L. J. WINGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838; https://doi.org/10.1107/s0021889899006020.Search in Google Scholar
6. Spek, A. L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155; https://doi.org/10.1107/s090744490804362x.Search in Google Scholar
7. Shehzad, A., Rehman, G., Ul-Islam, M., Khattak, W. A., Lee, Y. S. Challenges in the development of drugs for the treatment of tuberculosis. Braz. J. Infect. Dis. 2013, 17, 74–81; https://doi.org/10.1016/j.bjid.2012.10.009.Search in Google Scholar PubMed PubMed Central
8. Setshedi, I. B., Lemmerer, A., Smith, M. G. Co-crystallization of N′-benzylidenepyridine-4-carbohydrazide and benzoic acid via autoxidation of benzaldehyde. Acta Crystallogr. 2023, E79, 682–685; https://doi.org/10.1107/s2056989023005698.Search in Google Scholar
9. Bernstein, J., Davis, R. E., Shimoni, L., Chang, N. L. Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew. Chem., Int. Ed. 1995, 34, 1555–1573; https://doi.org/10.1002/anie.199515551.Search in Google Scholar
10. Lemmerer, A., Bernstein, J., Kahlenberg, V. One-pot covalent and supramolecular synthesis of pharmaceutical co-crystals using the API isoniazid: a potential supramolecular reagent. CrystEngComm 2010, 12, 2856–2864; https://doi.org/10.1039/c000473a.Search in Google Scholar
11. Turdybekov, K. M., Nurkenov, O. A., Fazylov, S. D., Makhmutova, A. S., Turdybekov, D. M., Seilkhanov, T. M., Arinova, A. E. Synthesis, crystal structure, and conformation of N-isonicotinoylphthalimide. J. Struct. Chem. 2021, 62, 1279–1284; https://doi.org/10.1134/s0022476621080151.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3

