Abstract
C40H36F2N2O4, monoclinic, P21/c (no. 14), a = 9.8180(16) Å, b = 19.355(3) Å, c = 17.930(3) Å, β = 102.353(3)°, V = 3328.3(9) Å3, Z = 4, Rgt(F) = 0.0564, wRref(F2) = 0.1866, T = 296(2) K.
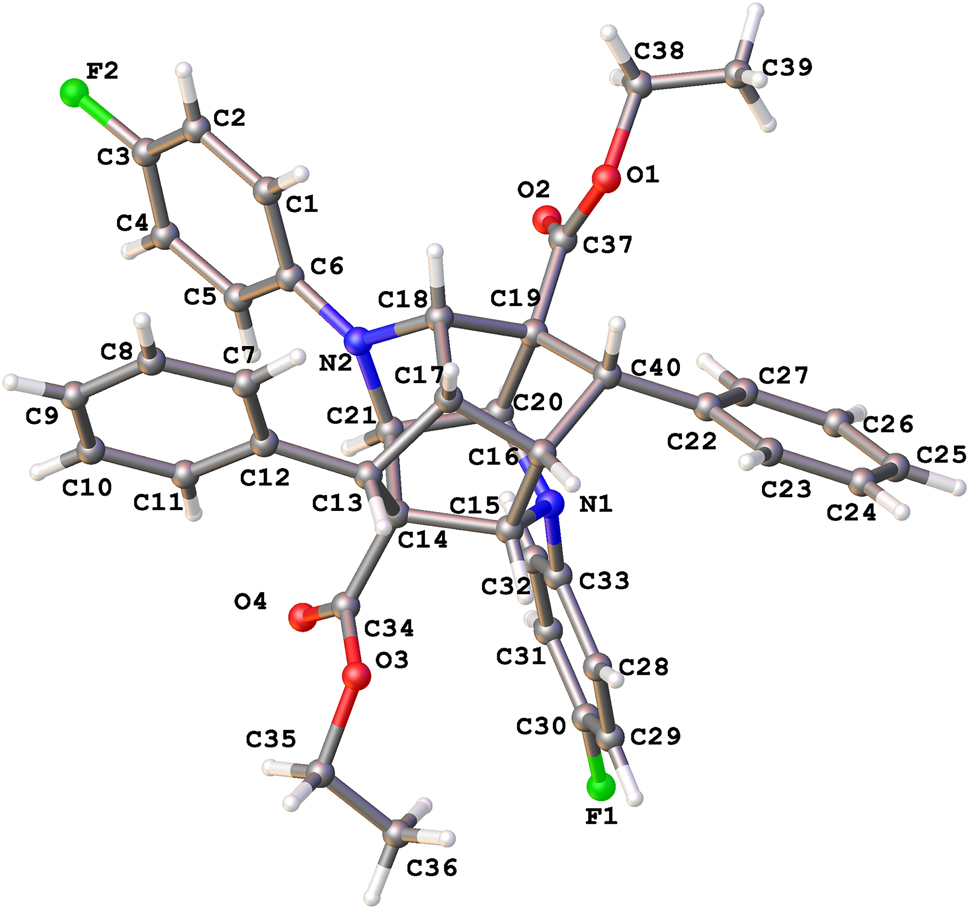
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.18 × 0.16 × 0.15 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 27.5°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 20,489, 7585, 0.034 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 4295 |
| N(param)refined: | 473 |
| Programs: | Bruker [1], SHELX [2,3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| F1 | 0.6963 (2) | 0.97589 (9) | 0.47231 (12) | 0.1322 (7) |
| C1 | 0.6467 (2) | 0.49669 (13) | 0.19420 (14) | 0.0768 (7) |
| H1 | 0.562781 | 0.474591 | 0.194619 | 0.092* |
| O1 | 0.22769 (16) | 0.61826 (10) | 0.14321 (8) | 0.0840 (5) |
| O2 | 0.41076 (18) | 0.68466 (10) | 0.13638 (9) | 0.0828 (5) |
| F2 | 0.93853 (17) | 0.47412 (9) | 0.10501 (10) | 0.1121 (6) |
| C2 | 0.7306 (3) | 0.47082 (15) | 0.14912 (16) | 0.0869 (8) |
| H2 | 0.702761 | 0.432216 | 0.118800 | 0.104* |
| O3 | 0.68921 (16) | 0.64025 (9) | 0.54550 (8) | 0.0784 (5) |
| C3 | 0.8522 (3) | 0.50090 (15) | 0.14866 (14) | 0.0770 (7) |
| N1 | 0.46538 (16) | 0.72575 (8) | 0.36222 (8) | 0.0477 (4) |
| N2 | 0.59973 (16) | 0.57907 (9) | 0.28714 (9) | 0.0540 (4) |
| O4 | 0.82344 (16) | 0.67755 (9) | 0.46835 (8) | 0.0726 (5) |
| C4 | 0.8935 (3) | 0.55757 (17) | 0.19074 (17) | 0.0977 (10) |
| H4 | 0.978888 | 0.578119 | 0.190126 | 0.117* |
| C5 | 0.8071 (3) | 0.58497 (15) | 0.23509 (15) | 0.0846 (8) |
| H5 | 0.834204 | 0.625064 | 0.262903 | 0.102* |
| C6 | 0.6825 (2) | 0.55447 (10) | 0.23906 (11) | 0.0502 (5) |
| C7 | 0.6023 (3) | 0.42589 (13) | 0.40095 (15) | 0.0741 (7) |
| H7 | 0.506494 | 0.419474 | 0.385721 | 0.089* |
| C8 | 0.6885 (3) | 0.36920 (13) | 0.40570 (16) | 0.0830 (7) |
| H8 | 0.649528 | 0.325738 | 0.393760 | 0.100* |
| C9 | 0.8263 (3) | 0.37527 (14) | 0.42702 (18) | 0.0897 (8) |
| H9 | 0.889780 | 0.345286 | 0.412287 | 0.108* |
| C17 | 0.4186 (2) | 0.55270 (11) | 0.35957 (12) | 0.0532 (5) |
| H17 | 0.362804 | 0.510689 | 0.359300 | 0.064* |
| C16 | 0.3433 (2) | 0.61699 (10) | 0.37881 (11) | 0.0510 (5) |
| H16 | 0.281579 | 0.606945 | 0.413771 | 0.061* |
| C15 | 0.46274 (19) | 0.66781 (10) | 0.41420 (11) | 0.0477 (5) |
| H15 | 0.456343 | 0.683123 | 0.465440 | 0.057* |
| C14 | 0.60060 (19) | 0.62616 (10) | 0.41450 (10) | 0.0472 (5) |
| C13 | 0.5535 (2) | 0.55083 (10) | 0.42098 (12) | 0.0538 (5) |
| H13 | 0.525529 | 0.547136 | 0.470127 | 0.065* |
| C20 | 0.5046 (2) | 0.69105 (10) | 0.29770 (11) | 0.0491 (5) |
| H20 | 0.530858 | 0.723768 | 0.261509 | 0.059* |
| C19 | 0.3840 (2) | 0.64179 (11) | 0.25869 (11) | 0.0511 (5) |
| C18 | 0.45101 (19) | 0.56865 (11) | 0.27905 (11) | 0.0523 (5) |
| H18 | 0.413455 | 0.533521 | 0.240800 | 0.063* |
| C21 | 0.6262 (2) | 0.64104 (10) | 0.33267 (11) | 0.0489 (5) |
| H21 | 0.718442 | 0.660852 | 0.333591 | 0.059* |
| C22 | 0.1754 (2) | 0.70690 (12) | 0.30082 (11) | 0.0542 (5) |
| C12 | 0.6522 (2) | 0.49109 (11) | 0.41773 (12) | 0.0573 (5) |
| C26 | 0.1030 (3) | 0.82274 (14) | 0.25998 (14) | 0.0767 (7) |
| H26 | 0.113357 | 0.861676 | 0.231320 | 0.092* |
| C25 | 0.0051 (3) | 0.82207 (14) | 0.30448 (15) | 0.0766 (7) |
| H25 | −0.051169 | 0.860493 | 0.306016 | 0.092* |
| C24 | −0.0093 (2) | 0.76401 (14) | 0.34690 (14) | 0.0714 (7) |
| H24 | −0.076096 | 0.763119 | 0.376693 | 0.086* |
| C23 | 0.0748 (2) | 0.70753 (13) | 0.34516 (12) | 0.0615 (6) |
| H23 | 0.064398 | 0.668860 | 0.374222 | 0.074* |
| C27 | 0.1860 (2) | 0.76533 (13) | 0.25795 (12) | 0.0650 (6) |
| H27 | 0.250714 | 0.766017 | 0.226917 | 0.078* |
| C32 | 0.6160 (2) | 0.82532 (11) | 0.35302 (13) | 0.0594 (6) |
| H32 | 0.636321 | 0.807797 | 0.308344 | 0.071* |
| C31 | 0.6716 (3) | 0.88846 (12) | 0.38108 (15) | 0.0729 (7) |
| H31 | 0.728432 | 0.913232 | 0.355312 | 0.087* |
| C30 | 0.6425 (3) | 0.91342 (12) | 0.44591 (16) | 0.0773 (7) |
| C29 | 0.5599 (3) | 0.87847 (13) | 0.48522 (15) | 0.0797 (7) |
| H29 | 0.542306 | 0.896424 | 0.530331 | 0.096* |
| C28 | 0.5035 (2) | 0.81682 (12) | 0.45754 (12) | 0.0640 (6) |
| H28 | 0.445575 | 0.793381 | 0.483801 | 0.077* |
| C37 | 0.3442 (2) | 0.65226 (12) | 0.17306 (12) | 0.0594 (6) |
| C36 | 0.7391 (4) | 0.7320 (2) | 0.6354 (2) | 0.171 (2) |
| H36A | 0.804493 | 0.748746 | 0.679328 | 0.256* |
| H36B | 0.649624 | 0.725914 | 0.647965 | 0.256* |
| H36C | 0.731292 | 0.764866 | 0.594538 | 0.256* |
| C38Aa | 0.1756 (9) | 0.6113 (5) | 0.0635 (4) | 0.0664 (15) |
| H38Aa | 0.128871 | 0.567260 | 0.051306 | 0.080* |
| H38Ba | 0.249765 | 0.615422 | 0.035666 | 0.080* |
| C39Aa | 0.0740 (12) | 0.6708 (5) | 0.0456 (4) | 0.129 (3) |
| H39Aa | 0.051118 | 0.678473 | −0.008512 | 0.194* |
| H39Ba | 0.115586 | 0.711697 | 0.070938 | 0.194* |
| H39Ca | −0.009363 | 0.659859 | 0.063107 | 0.194* |
| C38Bb | 0.1742 (13) | 0.6432 (7) | 0.0555 (6) | 0.0665 (16) |
| H38Cb | 0.188204 | 0.692503 | 0.051094 | 0.080* |
| H38Db | 0.224898 | 0.619266 | 0.022386 | 0.080* |
| C39Bb | 0.0296 (10) | 0.6268 (9) | 0.0350 (5) | 0.114 (4) |
| H39Db | −0.007007 | 0.640663 | −0.016849 | 0.171* |
| H39Eb | 0.017365 | 0.577878 | 0.039682 | 0.171* |
| H39Fb | −0.019139 | 0.650733 | 0.068241 | 0.171* |
| C35 | 0.7862 (3) | 0.66785 (16) | 0.61212 (14) | 0.0878 (9) |
| H35A | 0.796102 | 0.635055 | 0.653840 | 0.105* |
| H35B | 0.877080 | 0.674027 | 0.599958 | 0.105* |
| C11Bc | 0.7794 (10) | 0.4905 (5) | 0.4714 (8) | 0.075 (2) |
| H11Bc | 0.806562 | 0.527787 | 0.503928 | 0.090* |
| C10Bc | 0.8633 (10) | 0.4324 (5) | 0.4743 (8) | 0.078 (2) |
| H10Bc | 0.948072 | 0.431634 | 0.509432 | 0.094* |
| C10Ad | 0.8846 (4) | 0.4425 (2) | 0.4297 (3) | 0.0815 (14) |
| H10Ad | 0.979747 | 0.448273 | 0.433165 | 0.098* |
| C11Ad | 0.7983 (4) | 0.4992 (2) | 0.4271 (3) | 0.0709 (13) |
| H11Ad | 0.836696 | 0.543352 | 0.431570 | 0.085* |
| C34 | 0.7183 (2) | 0.65120 (11) | 0.47714 (11) | 0.0506 (5) |
| C33 | 0.53043 (19) | 0.78798 (10) | 0.39067 (10) | 0.0457 (5) |
| C40 | 0.2629 (2) | 0.64276 (11) | 0.30082 (11) | 0.0526 (5) |
| H40 | 0.198989 | 0.605594 | 0.278819 | 0.063* |
-
aOccupancy: 0.584 (11), bOccupancy: 0.416 (11), cOccupancy: 0.300 (8), dOccupancy: 0.700 (8).
1 Source of material
A 100 mL round bottom flask was sequentially charged with 50 mL of 1,2-dichloroethane, 30 mmol of 4-fluoroaniline, 30 mmol of ethyl propionate, 30 mmol of cinnamaldehyde, 12 mmol of piperazine and 0.24 mmol of p-toluenesulfonic acid. The reaction mixture was slowly heated to reflux for 12 h. At the end of the reaction the mixture was cooled, to room temperature. Then 200 mL of water were slowly added, with ethyl acetate was extracted three times (3 × 100 mL), the organic layer was separated, and saturated aqueous sodium chloride was washed twice (2 × 100 mL), and the organic layers were combined. The organic layer was dried over anhydrous sodium sulfate, filtered, distilled under reduced pressure, and separated by silica gel column chromatography (ethyl acetate:petroleum ether = 1:50) to give 1, a white powdered solid. An amount of 1 g of 1 was added to the mixed solution of methanol/tetrahydrofuran, added to a 30 mL quartz tube, and irradiated by 410 nm UV light for 72 h. The product was separated by column chromatography, and the elution solvent was petroleum ether and ethyl acetate 20:1. Finally, the obtained solution was evaporated to dryness in vacuo and recrystallized from methanol and dichloromethane to obtain the title compound.
2 Experimental details
All hydrogen atoms were placed in the calculated positions and all the non-hydrogen atoms were refined anisotropically.
3 Comment
In recent years, cage compounds, as an important class of three-dimensional polyhedral structure compounds, have been a hot topic of research due to their wide applications in antitumor, immunity enhancement and antiviral [4,5]. Photochemical reactions have been widely used in various synthetic reactions [6, 7]. In particular, six-membered rings with symmetrical double bond structure are often used as raw materials for photoreaction. A cage compound (systematic name: diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl) cyclopenta[b]pyridine-3,7(2H)-dicarboxylate) of the title structure is shown in the figure. Stereochemically the compound is the product of common enantiometric forms. It can formally be considered as resulting from the attack of one dihydropyridine molecule on the far side of the dihydropyridine ring of the second molecule (i.e., the side that does not face the aryl moiety) [8,9].
In the molecules forming the title crystal structure, several important bond angle data are involved as follows: C18–N2–C6 = 126.95(19) Å, C33–N1–C20 = 120.12(18) Å, C16–C40–C22 = 116.5(2) Å. The bond lengths and angles are in the expected ranges [10,11].
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. BRUKER. SAINT. Version 8.23B.; Bruker AXS Inc.: Madison, Wisconsin, USA, 2013.Search in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
4. Simeonova, L., Gegova, G., Galabov, A. S. Prophylactic and therapeutic combination effects of rimantadine and oseltamivir against influenza virus A (H3N2) infection in mice. Antiviral Res. 2012, 95, 172–181; https://doi.org/10.1016/j.antiviral.2012.05.004.Search in Google Scholar
5. Liu, J., Obando, D., Liao, V., Lifa, T., Codd, R. The many faces of the adamantyl group in drug design. Eur. J. Med. Chem. 2011, 46, 1949–1963; https://doi.org/10.1016/j.ejmech.2011.01.047.Search in Google Scholar
6. Han, Y., Jin, Y., Jiang, M., Yang, H., Fu, H. Photocatalyst-free visible-light photoredox dearomatization of phenol derivatives containing ketoximes: an easy access to spiropyrrolines. Org. Lett. 2019, 21, 1799–1803; https://doi.org/10.1021/acs.orglett.9b00372.Search in Google Scholar
7. Mishiro, K., Kimura, T., Furuyama, T., Kunishima, M. Phototriggered active alkyne generation from cyclopropenones with visible light-responsive photocatalysts. Org. Lett. 2019, 21, 4101–4105; https://doi.org/10.1021/acs.orglett.9b01280.Search in Google Scholar
8. Hilgeroth, A., Baumeister, U. The first functionalized 6,12-diazatetrakishomocubanes. Angew. Chem. Int. Ed. 2000, 39, 576–578; https://doi.org/10.1002/(sici)1521-3773(20000204)39:3<576::aid-anie576>3.0.co;2-g.10.1002/(SICI)1521-3773(20000204)39:3<576::AID-ANIE576>3.0.CO;2-GSearch in Google Scholar
9. Shao, H. Q., Lu, Y. M., Lin, Q. W., Wang, Y. C., Xie, Y. S. Crystal structure of diethyl 3,9-bis(4-fluorophenyl)-6,12-diphenyl-3,9-diazapentacyclo [6.4.0.02, 7.04, 11]dodecane-1,5-dicarboxylate, C40H36F2N2O4. Z. Kristallogr. N. Cryst. Struct. 2021, 2, 385–386; https://doi.org/10.1515/ncrs-2020-0552.Search in Google Scholar
10. Wang, Y.-X., Geng, S.-Q., Qin, J.-R., Zhang, W.-L., Zhong, Q.-D. Crystal structure of dimethyl 1,4,6,9-tetraphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,7(2H)-dicarboxylate, C38H34N2O4. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 463–465; https://doi.org/10.1515/ncrs-2022-0038.Search in Google Scholar
11. Fan, S., Zhang, Q., Lv, X.-D., Jin, Y.-Y., Yang, Z.-Y. Crystal structure of diethyl 4,6-diphenyl-1,9-di-p-tolylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,5(2H)-dicarboxylate, C42H42N2O4. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 455–457; https://doi.org/10.1515/ncrs-2022-0029.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3

