Abstract
C29H34N2O13, triclinic, P
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
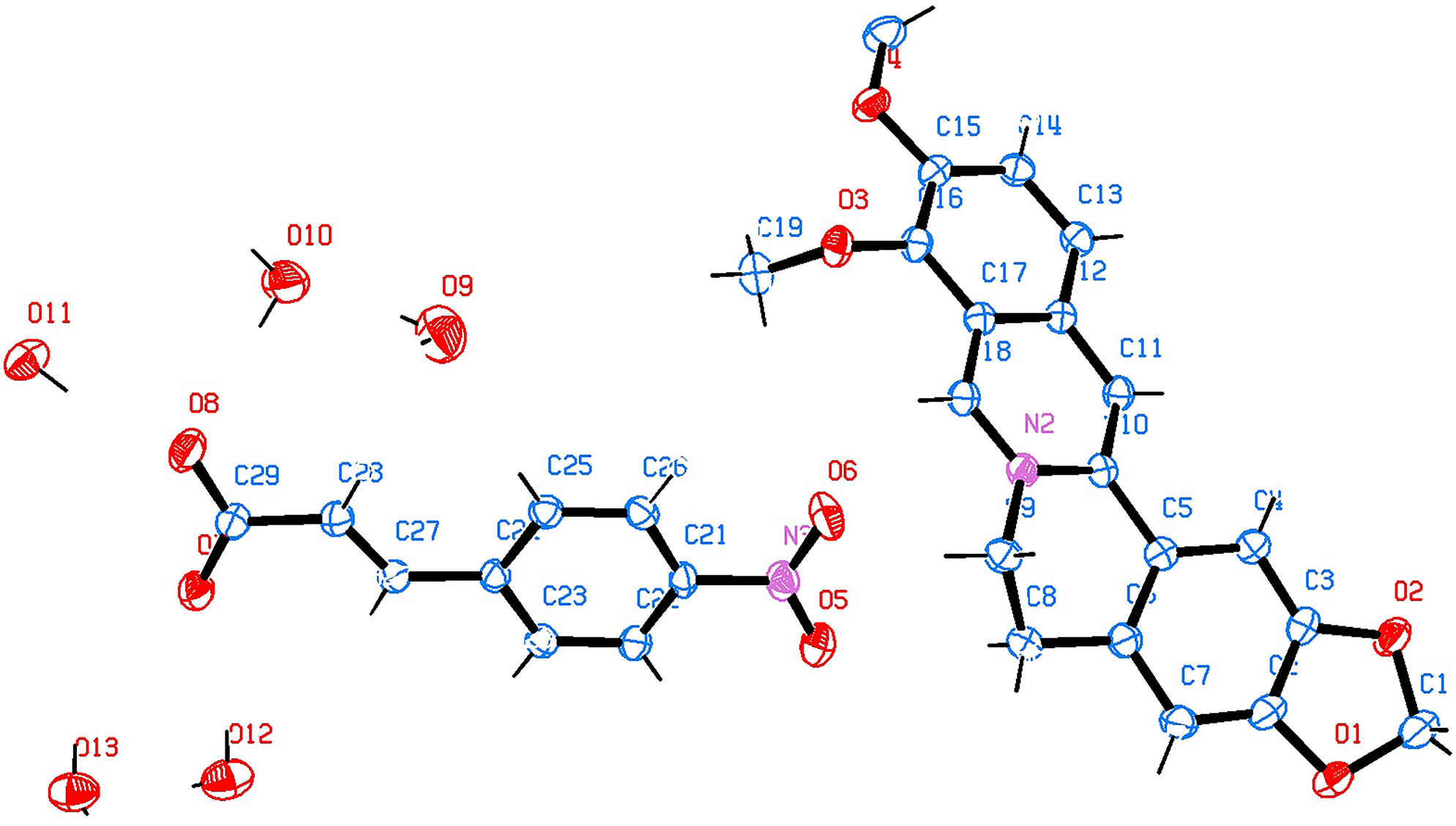
Data collection and handling.
| Crystal: | Brown block |
| Size: | 0.13 × 0.12 × 0.10 mm |
| Wavelength: | Ga Kα radiation (1.34139 Å) |
| μ: | 0.61 mm−1 |
| Diffractometer, scan mode: | Bruker D8 VENTURE PHOTON II, φ and ω |
| θ max, completeness: | 60.3°, % |
| N(hkl)measured , N(hkl)unique, R int: | 13,791, 6231, 0.043 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 4466 |
| N(param)refined: | 217 |
| Programs: | Bruker [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.6625 (2) | 0.40911 (15) | 1.46588 (13) | 0.0412 (4) |
| H1A | 0.747871 | 0.444740 | 1.517461 | 0.049* |
| H1B | 0.586977 | 0.371344 | 1.500452 | 0.049* |
| C2 | 0.6403 (2) | 0.34556 (13) | 1.30742 (12) | 0.0306 (4) |
| C3 | 0.5421 (2) | 0.43061 (12) | 1.30916 (12) | 0.0284 (4) |
| C4 | 0.43528 (19) | 0.45607 (12) | 1.22622 (12) | 0.0270 (4) |
| H4 | 0.369421 | 0.515013 | 1.227851 | 0.032* |
| C5 | 0.42706 (19) | 0.39111 (11) | 1.13817 (12) | 0.0251 (3) |
| C6 | 0.52366 (19) | 0.30398 (12) | 1.13722 (12) | 0.0274 (4) |
| C7 | 0.6338 (2) | 0.28074 (13) | 1.22376 (13) | 0.0315 (4) |
| H7 | 0.700907 | 0.222242 | 1.223845 | 0.038* |
| C8 | 0.5055 (2) | 0.23647 (12) | 1.04141 (13) | 0.0330 (4) |
| H8A | 0.540452 | 0.166848 | 1.056801 | 0.040* |
| H8B | 0.577267 | 0.262328 | 0.996564 | 0.040* |
| C9 | 0.3276 (2) | 0.23416 (11) | 0.98930 (13) | 0.0304 (4) |
| H9A | 0.316687 | 0.192185 | 0.924650 | 0.037* |
| H9B | 0.257110 | 0.203051 | 1.031915 | 0.037* |
| C10 | 0.31815 (19) | 0.41631 (11) | 1.04520 (11) | 0.0238 (3) |
| C11 | 0.26007 (19) | 0.51182 (11) | 1.02772 (12) | 0.0253 (3) |
| H11 | 0.291059 | 0.565790 | 1.078011 | 0.030* |
| C12 | 0.15580 (18) | 0.53236 (11) | 0.93742 (11) | 0.0234 (3) |
| C13 | 0.09069 (19) | 0.62873 (12) | 0.91769 (12) | 0.0270 (4) |
| H13 | 0.112214 | 0.683439 | 0.968104 | 0.032* |
| C14 | −0.00325 (19) | 0.64415 (12) | 0.82653 (12) | 0.0279 (4) |
| H14 | −0.045617 | 0.709768 | 0.814243 | 0.033* |
| C15 | −0.03842 (19) | 0.56429 (12) | 0.75035 (12) | 0.0265 (3) |
| C16 | 0.01737 (19) | 0.46816 (11) | 0.76812 (12) | 0.0254 (3) |
| C17 | 0.11489 (18) | 0.45095 (11) | 0.86228 (11) | 0.0237 (3) |
| C18 | 0.17555 (19) | 0.3546 (11) | 0.88341 (12) | 0.0253 (3) |
| H18 | 0.147483 | 0.299175 | 0.834481 | 0.030* |
| C19 | 0.0734 (2) | 0.38137 (14) | 0.61956 (13) | 0.0399 (4) |
| H19A | 0.039269 | 0.321176 | 0.573711 | 0.060* |
| H19B | 0.190719 | 0.376514 | 0.648847 | 0.060* |
| H19C | 0.056803 | 0.442436 | 0.582190 | 0.060* |
| C20 | −0.1790 (2) | 0.67549 (13) | 0.63255 (14) | 0.0396 (4) |
| H20A | −0.244978 | 0.672231 | 0.564698 | 0.059* |
| H20B | −0.080289 | 0.718245 | 0.634678 | 0.059* |
| H20C | −0.245318 | 0.704600 | 0.681145 | 0.059* |
| C21 | 0.5776 (2) | 0.21634 (11) | 0.66085 (11) | 0.0267 (4) |
| C22 | 0.7251 (2) | 0.19164 (12) | 0.62999 (12) | 0.0293 (4) |
| H22 | 0.827770 | 0.206847 | 0.673009 | 0.035* |
| C23 | 0.7197 (2) | 0.14409 (12) | 0.53465 (12) | 0.0287 (4) |
| H23 | 0.820046 | 0.127074 | 0.511580 | 0.034* |
| C24 | 0.56855 (19) | 0.12092 (11) | 0.47211 (11) | 0.0250 (3) |
| C25 | 0.4213 (2) | 0.14556 (11) | 0.50737 (12) | 0.0272 (4) |
| H25 | 0.317842 | 0.128657 | 0.465831 | 0.033* |
| C26 | 0.4251 (2) | 0.19401 (11) | 0.60161 (12) | 0.0273 (4) |
| H26 | 0.325478 | 0.211620 | 0.625287 | 0.033* |
| C27 | 0.5693 (2) | 0.06989 (12) | 0.37173 (12) | 0.0269 (3) |
| H27 | 0.674421 | 0.056009 | 0.353619 | 0.032* |
| C28 | 0.4377 (2) | 0.04150 (12) | 0.30429 (12) | 0.0292 (4) |
| H28 | 0.331429 | 0.053183 | 0.321511 | 0.035* |
| C29 | 0.4472 (2) | −0.00769 (12) | 0.20301 (12) | 0.0288 (4) |
| N2 | 0.27136 (16) | 0.33904 (9) | 0.97017 (9) | 0.0242 (3) |
| N3 | 0.58185 (19) | 0.26806 (10) | 0.76089 (11) | 0.0332 (3) |
| O1 | 0.73853 (15) | 0.34079 (10) | 1.40050 (9) | 0.0391 (3) |
| O2 | 0.57185 (15) | 0.48065 (9) | 1.40372 (8) | 0.0373 (3) |
| O3 | −0.02383 (14) | 0.38722 (8) | 0.69845 (8) | 0.0309 (3) |
| O4 | −0.12997 (14) | 0.57516 (8) | 0.65761 (8) | 0.0331 (3) |
| O5 | 0.71451 (18) | 0.27646 (11) | 0.81835 (10) | 0.0506 (4) |
| O6 | 0.45331 (17) | 0.30309 (10) | 0.78304 (10) | 0.0456 (3) |
| O7 | 0.58137 (15) | −0.04250 (10) | 0.18472 (9) | 0.0405 (3) |
| O8 | 0.31460 (15) | −0.01130 (10) | 0.13977 (9) | 0.0433 (3) |
| O9 | 0.0204 (2) | 0.07935 (13) | 0.37249 (12) | 0.0627 (4) |
| H9C | 0.025552 | 0.094081 | 0.311390 | 0.094* |
| H9D | −0.015205 | 0.016615 | 0.365972 | 0.094* |
| O10 | 0.06230 (18) | 0.12005 (10) | 0.17255 (12) | 0.0526 (4) |
| H10A | 0.141171 | 0.078422 | 0.163261 | 0.079* |
| H10B | −0.023277 | 0.096687 | 0.129675 | 0.079* |
| O11 | 0.21619 (15) | −0.01936 (10) | −0.05997 (9) | 0.0407 (3) |
| H11A | 0.263852 | −0.020782 | 0.002549 | 0.061* |
| H11B | 0.295529 | −0.003191 | −0.091552 | 0.061* |
| O12 | 0.87790 (16) | −0.12151 (11) | 0.28528 (11) | 0.0495 (4) |
| H12A | 0.786091 | −0.091699 | 0.262688 | 0.074* |
| H12B | 0.935291 | −0.120417 | 0.236799 | 0.074* |
| O13 | 1.01618 (16) | −0.13927 (10) | 0.10689 (11) | 0.0481 (4) |
| H13A | 1.116802 | −0.114675 | 0.118380 | 0.072* |
| H13B | 0.956934 | −0.091730 | 0.077932 | 0.072* |
1 Source of materials
Berberine sulfate (hydrate) (0.86 g, 2 mmol) was dissolved in water (50 mL) at 50 °C to obtain a clear solution. A solution of NaOH (0.08 g, 2 mmol) and 4-nitro-cinnamic acid (PNCA) (0.38 g, 2 mmol) in water (20 mL) were added. The mixture was heated to 50 °C to get a brown coloured solution. Crystals of the title compound were obtained by slow evaporation within 4 days.
2 Experimental details
Data collection was performed on a Bruker D8 VENTURE Metaljet PHOTON II diffractometer at 193 K, operating at 50 kV and 30 mA. Data were processed by the BRUKER AXS Crystal Structure Analysis Package [1]. Data collection, BRUKER APEX3; cell refinement and data reduction by BRUKER SAINT V8.40B; Structure solution: SHELXT 2014/5 [2]; Structure refinement: SHELXL-2018/3 [3]; Molecular graphics and publication materials by BRUKER SHELXTL [2]. All H-atoms were placed geometrically and refined using a riding model with common isotropic displacement factors, Uiso values were set to 1.2U eq of the parent atoms.
3 Comment
Berberine hydrochloride (BBR–HCl) is contained in the Chinese Pharmacopoeia (2020) for gastrointestinal infections such as gastroenteritis and bacillary dysentery caused by sensitive pathogens. But, it presents poor intestine absorption via oral administration. Additionally, BBR–HCl is highly hygroscopic and presents an extremely bitter taste. Cocrystals have been prepared in attempts to solve these problems. Recently, A wide range of coformers, such as flavonoids (myricetin, dihydromyricetin [4], chrysin [5]); anthraquinones (emodin [6], rhein [7]); API (Ibuprofen [8], rosiglitazone [9, 10]), aromatic carboxylic [11] (3,4,5-methoxycinnamic acid [12], carboxylic acids [13]) and small molecular organic acid (aliphatic dicarboxylic acids [14], L(+)-lactic acid [15]) can be if hydrogen bonding formation is possible, providing many options to develop pharmaceutical products with improved pharmaceutical properties, including solubility.
It is worth noting that, by reacting Berberine sulfate and ((4-nitrophenyl) acrylic acid) via a liquid phase reaction, the title salt has been obtained. The asymmetric unit of the title structure contains one 9,10-dimethoxy-5,6-dihydro-[1, 3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium cation, one (E)−3-(4-nitrophenyl) acrylate anion and five water molecules. All moieties are connected by hydrogen bonds. All bond lengths are in the expected ranges. The proportions of lattice water in each crystal lattice account for 14.5%. The cavity can be clearly observed when the lattice waters were removed from the crystal lattice, which plays a critical role in supporting the three dimensional structures. This means lattice water is an important hydrogen donor acceptor in the title salt.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: We gratefully acknowledge support by The Key project of Anhui Provincial Department of Education, China (NO. KJ2020A672 and 2022AH040077) and The Open Fund Project of Anhui Key Laboratory of Pharmaceutical Preparation Technology and Application (NO. 2021KFKT08). Co-authors would like to thank Zhang CX from Shiyanjia Lab (www.shiyanjia.com) for the SXRD analysis.
References
1. BRUKER. APEX3, SAINT; Bruker AXS Inc: Madison, Wisconsin, USA, 2016.Search in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Li, P. Z., Ramaiah, T., Zhang, M., Zhang, Y., Huang, Y., Lou, B. Two cocrystals of berberine chloride with myricetin and dihydromyricetin: crystal structures, characterization, and antitumor activities. Cryst. Growth Des. 2020, 20, 157–166; https://doi.org/10.1021/acs.cgd.9b00939.Search in Google Scholar
5. Sa, R. J., Zhang, Y. J., Deng, Y. P., Huang, Y., Zhang, M., Lou, B. Novel salt cocrystal of chrysin with berberine: preparation, characterization, and oral bioavailability. Cryst. Growth Des. 2018, 18, 4724–4730; https://doi.org/10.1021/acs.cgd.8b00696.Search in Google Scholar
6. Deng, Y. P., Zhang, Y. J., Huang, Y. L., Zhang, M., Lou, B. Preparation, crystal structures, and oral bioavailability of two cocrystals of emodin with berberine chloride. Cryst. Growth Des. 2018, 18, 7481–7488; https://doi.org/10.1021/acs.cgd.8b01257.Search in Google Scholar
7. Yang, D. Z., Wang, H. J., Liu, Q. W., Yuan, P., Chen, T., Zhang, L., Yang, S., Zhou, Z., Lu, Y., Du, G. Structural landscape on a series of rhein: berberine cocrystal salt solvates: the formation, dissolution elucidation from experimental and theoretical investigation. Chin. Chem. Lett. 2022, 33, 3207–3211; https://doi.org/10.1016/j.cclet.2021.10.012.Search in Google Scholar
8. Wang, M., Xu, R., Liu, X. L., Zhang, L., Qiu, S., Lu, Y., Zhang, P., Yan, M., Zhu, J. A co-crystal berberine-ibuprofen improves obesity by inhibiting the protein kinases TBK1 and IKKε. Commun. Biol. 2022, 5, 807; https://doi.org/10.1038/s42003-022-03776-0.Search in Google Scholar PubMed PubMed Central
9. He, Q. C., Chen, B., Wang, G., Zhou, D., Zeng, H., Li, X., Song, Y., Yu, X., Liang, W., Chen, H., Liu, X., Wu, Q., Wu, L., Zhang, L., Li, H., Hu, X., Zhou, W. Co-crystal of rosiglitazone with berberine ameliorates hyperglycemia and insulin resistance through the PI3K/AKT/TXNIP pathway in vivo and in vitro. Front. Pharm. 2022, 13, 842879; https://doi.org/10.3389/fphar.2022.842879.Search in Google Scholar PubMed PubMed Central
10. Guan, X. S., Jiang, L., Cai, L. H., Zhang, L., Hu, X. A new co-crystal of synthetic drug rosiglitazone with natural medicine berberine: Preparation, crystal structures, and dissolution. Molecules 2020, 25, 4288; https://doi.org/10.3390/molecules25184288.Search in Google Scholar PubMed PubMed Central
11. Gao, Z. Y., Liu, S. Y., Sun, C. C. Complexation with aromatic carboxylic acids expands the solid-state landscape of berberine. Int. J. Pharm. 2022, 617, 121587; https://doi.org/10.1016/j.ijpharm.2022.121587.Search in Google Scholar PubMed
12. Han, N., Huang, X., Tian, X., Li, T., Liu, X., Li, W., Huo, S., Wu, Q., Gu, Y., Dai, Z., Xu, B., Wang, P., Lei, H. M. Self-assembled nanoparticles of natural phytochemicals (berberine and 3,4,5-methoxycinnamic acid) originated from traditional Chinese medicine for inhibiting multidrug-resistant Staphylococcus aureus. Curr. Drug Deliv. 2021, 18, 914–921; https://doi.org/10.2174/1567201817666201124121918.Search in Google Scholar PubMed
13. Huang, X. M., Wang, P. L., Li, T., Tian, X., Guo, W., Xu, B., Huang, G., Cai, D., Zhou, F., Zhang, H., Lei, H. Self-assemblies based on traditional medicine berberine and cinnamic acid for adhesion-induced inhibition multidrug-resistant Staphylococcus aureus. ACS Appl. Mater. Interfaces 2020, 12, 227–237; https://doi.org/10.1021/acsami.9b17722.Search in Google Scholar PubMed
14. Wang, L. L., Liu, S. Y., Chen, J. M., Wang, Y.-x., Sun, C. C. Novel salt-cocrystals of berberine hydrochloride with aliphatic dicarboxylic acids: odd-even alternation in physicochemical properties. Mol. Pharm. 2021, 18, 1758–1767; https://doi.org/10.1021/acs.molpharmaceut.0c01250.Search in Google Scholar PubMed
15. Wang, L. L., Liu, S. Y., Gao, Z. Y. Crystal structure, dissolution and hygroscopicity of a novel cocrystal hydrate of berberine hydrochloride with L(+)-lactic acid. Pharmazie 2020, 75, 483–487; https://doi.org/10.1691/ph.2020.0079.Search in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3

