Abstract
C31H28N2O2, triclinic,
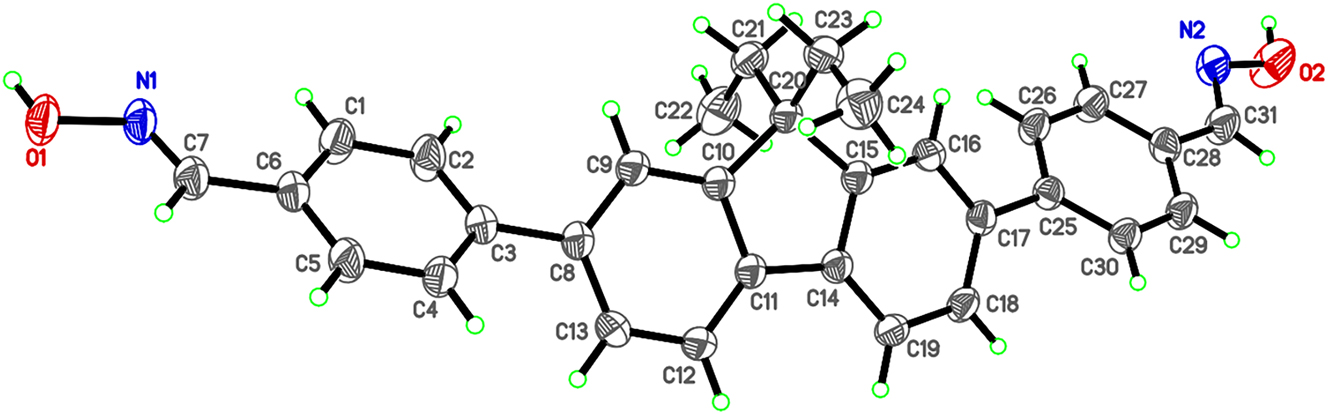
The molecular structure is shown in the figure. Displacement ellipsoids are drawn at the 40 % probability level. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.50 × 0.32 × 0.30 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 29.1°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 9746, 5521, 0.025 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 3677 |
| N(param)refined: | 320 |
| Programs: | Olex2 [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.95431 (17) | 0.34140 (16) | 0.41397 (9) | 0.0538 (4) |
| H1 | 1.016939 | 0.329062 | 0.457292 | 0.081* |
| O2 | 0.8604 (3) | −1.4046 (2) | −0.64363 (11) | 0.0854 (6) |
| H2 | 0.901996 | −1.467785 | −0.619567 | 0.128* |
| N1 | 0.94996 (19) | 0.23455 (18) | 0.34110 (10) | 0.0455 (4) |
| N2 | 0.8489 (2) | −1.29613 (19) | −0.57669 (11) | 0.0505 (4) |
| C1 | 0.8971 (2) | 0.0298 (2) | 0.18022 (13) | 0.0497 (5) |
| H1A | 0.968374 | 0.011979 | 0.228069 | 0.060* |
| C2 | 0.8699 (2) | −0.0590 (2) | 0.09905 (13) | 0.0495 (5) |
| H2A | 0.923299 | −0.135602 | 0.093451 | 0.059* |
| C3 | 0.7643 (2) | −0.0363 (2) | 0.02539 (12) | 0.0392 (4) |
| C4 | 0.6877 (2) | 0.0798 (2) | 0.03721 (13) | 0.0476 (5) |
| H4 | 0.617453 | 0.098946 | −0.010806 | 0.057* |
| C5 | 0.7135 (2) | 0.1674 (2) | 0.11862 (13) | 0.0505 (5) |
| H5 | 0.658762 | 0.242766 | 0.124802 | 0.061* |
| C6 | 0.8196 (2) | 0.1450 (2) | 0.19135 (12) | 0.0400 (4) |
| C7 | 0.8469 (2) | 0.2444 (2) | 0.27519 (13) | 0.0446 (5) |
| H7 | 0.787393 | 0.316246 | 0.280171 | 0.053* |
| C8 | 0.7342 (2) | −0.1327 (2) | −0.06144 (12) | 0.0390 (4) |
| C9 | 0.7321 (2) | −0.2835 (2) | −0.06069 (12) | 0.0398 (4) |
| H9 | 0.747484 | −0.324380 | −0.005177 | 0.048* |
| C10 | 0.7074 (2) | −0.3730 (2) | −0.14132 (11) | 0.0366 (4) |
| C11 | 0.6874 (2) | −0.3118 (2) | −0.22579 (11) | 0.0354 (4) |
| C12 | 0.6862 (2) | −0.1626 (2) | −0.22743 (12) | 0.0426 (5) |
| H12 | 0.670435 | −0.121764 | −0.282926 | 0.051* |
| C13 | 0.7086 (2) | −0.0745 (2) | −0.14595 (13) | 0.0444 (5) |
| H13 | 0.706498 | 0.025345 | −0.147455 | 0.053* |
| C14 | 0.67471 (19) | −0.42972 (19) | −0.29884 (11) | 0.0344 (4) |
| C15 | 0.68186 (19) | −0.5615 (2) | −0.25867 (11) | 0.0347 (4) |
| C16 | 0.6827 (2) | −0.6894 (2) | −0.31057 (11) | 0.0374 (4) |
| H16 | 0.685650 | −0.776398 | −0.282822 | 0.045* |
| C17 | 0.67916 (19) | −0.6891 (2) | −0.40501 (11) | 0.0350 (4) |
| C18 | 0.6680 (2) | −0.5578 (2) | −0.44492 (12) | 0.0395 (4) |
| H18 | 0.662138 | −0.557004 | −0.507926 | 0.047* |
| C19 | 0.6655 (2) | −0.4286 (2) | −0.39318 (12) | 0.0392 (4) |
| H19 | 0.657710 | −0.342482 | −0.421102 | 0.047* |
| C20 | 0.6987 (2) | −0.5385 (2) | −0.15493 (11) | 0.0373 (4) |
| C21 | 0.8424 (2) | −0.5888 (2) | −0.10902 (13) | 0.0498 (5) |
| H21A | 0.853585 | −0.565556 | −0.043512 | 0.060* |
| H21B | 0.829584 | −0.694816 | −0.120295 | 0.060* |
| C22 | 0.9865 (3) | −0.5198 (3) | −0.14161 (16) | 0.0681 (7) |
| H22A | 0.977213 | −0.542955 | −0.206335 | 0.102* |
| H22B | 1.070648 | −0.557678 | −0.110776 | 0.102* |
| H22C | 1.002842 | −0.415054 | −0.128321 | 0.102* |
| C23 | 0.5595 (2) | −0.6226 (2) | −0.12067 (13) | 0.0488 (5) |
| H23A | 0.550497 | −0.726523 | −0.138796 | 0.059* |
| H23B | 0.576579 | −0.610095 | −0.054397 | 0.059* |
| C24 | 0.4117 (3) | −0.5753 (3) | −0.15540 (16) | 0.0612 (6) |
| H24A | 0.403517 | −0.566857 | −0.219968 | 0.092* |
| H24B | 0.408392 | −0.481869 | −0.124638 | 0.092* |
| H24C | 0.329117 | −0.647002 | −0.143683 | 0.092* |
| C25 | 0.69764 (19) | −0.8232 (2) | −0.45854 (11) | 0.0353 (4) |
| C26 | 0.7939 (2) | −0.9130 (2) | −0.42002 (12) | 0.0420 (5) |
| H26 | 0.843610 | −0.888367 | −0.360266 | 0.050* |
| C27 | 0.8180 (2) | −1.0369 (2) | −0.46732 (13) | 0.0462 (5) |
| H27 | 0.883256 | −1.094308 | −0.439487 | 0.055* |
| C28 | 0.7449 (2) | −1.0768 (2) | −0.55685 (12) | 0.0407 (4) |
| C29 | 0.6485 (2) | −0.9883 (2) | −0.59586 (12) | 0.0425 (5) |
| H29 | 0.598771 | −1.013409 | −0.655560 | 0.051* |
| C30 | 0.6246 (2) | −0.8635 (2) | −0.54820 (12) | 0.0396 (4) |
| H30 | 0.559291 | −0.806111 | −0.576092 | 0.047* |
| C31 | 0.7709 (3) | −1.2050 (2) | −0.61017 (14) | 0.0538 (6) |
| H31 | 0.728291 | −1.220746 | −0.671662 | 0.065* |
1 Source of material
Raw materials 2,7-dibromo-9,9-diethyl-9H-fluorene (3.80 g, 10.0 mmol), 4-formylphenylboronic acid (3.31 g, 22 mmol), K2CO3 (8.28 g, 60 mmol) and Pd(PPh3)4 (0.76 g, 0.3 mmol) were introduced into 100 mL three-necked flask and degassed for three times. The mixture solution of toluene, ethanol and H2O (3:2:1, v/v/v 80 mL) was slowly added under N2 atmosphere. The mixture was stirred and refluxed under N2. The progress of reaction was monitored by thin-layer chromatography. After 40 h, the organic phase was evaporated under vacuum. The residue was purified by column chromatography with dichloromethane/petroleum ether (1:1 v/v) as eluent. The intermediate 4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde was obtained as a light yellow powder.
The intermediate (0.431 g, 1 mmol) and hydroxylamine hydrochloride (0.139 g, 2 mmol) were dissolved in anhydrous methanol (20 mL). Two drops of triethylamine was added to the above solution as a catalyst. The mixture solution was refluxed for 4 h (monitored by TLC). When the reaction is complete, light gray precipitates were filtered and washed with cold methanol. The crystals of the title compound were grown by slow evaporation of its methanol/dichloromethane solution at room temperature.
2 Experimental details
Using Olex2 [1], the structure was solved with the SHELXT [2] structure solution program and refined with the SHELXL [3] refinement package. The C-bound H atoms were geometrically placed (C–H = 0.93–0.98 Å) and refined as riding with Uiso(H) = 1.2–1.5Ueq(C). The O-bound H atom was located in a difference map and refined with O–H = 0.82 Å, and with Uiso(H) = 1.5Ueq(O).
3 Comment
Fluorene and its derivatives have drawn much attention because of their wide variety of application in photophysics and photochemistry [4], [5], [6]. This unique structure leads to the result that fluorene segment are rigidly coplanar with the delocalized π-conjugated system. Simultaneously, the five-membered ring (C-9 position) and two benzene rings are easily modified by some functional groups and/or solubilizing groups. The functional structures make fluorene compounds showing high photoluminescence quantum yields, thermal stabilities and good solubility in water [7, 8]. However, the short excitation and emission wavelengths of fluorene dyes limit their wide applications in biological systems [9]. In order to solve this problem, it is important that the π-bridge conjugated systems of fluorene compounds will be extended by the structural modification [10]. Suitable substitutions can not only enhance the intensities of their absorption and emission peaks but also produce a red shift. Based on the above considerations, the title compound containing fluorene moiety and aromatic rings was synthesized by SUZUKI couple and Schiff base reaction [11].
X-ray single crystal diffraction analysis reveals that the title compound crystallizes in the triclinic space group
In the title compound, the oxime functional group could be chosen as a highly selective recognition group for ClO−. Previous results have shown that the oxime can be oxidized into aldehyde group [15, 16]. The corresponding fluorescence intensity and position will undergo significant changes.
-
Research ethics: This work don’t involve research ethics.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Competing interests: The authors declare no conflicts of interest regarding this article.
-
Research funding: This work was supported by the Project of the Shandong Province Higher Educational Science and Technology Program (No. J18KA092).
-
Data availability: All relevant data are within the paper and its Supporting Information files.
References
1. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Chen, H., Wang, X., Ren, H., Lu, M., Cui, X., Wang, L., Wang, H., Tang, Q. Fluorene-based polymers of intrinsic microporosity as fluorescent probes for metal ions. React. Funct. Polym. 2022, 181, 105431; https://doi.org/10.1016/j.reactfunctpolym.2022.105431.Search in Google Scholar
5. Han, S.-Y., Niu, X., Wang, J., Jin, G.-X., Liu, A., Ma, J.-P. Characterization and gas adsorption of a novel three-dimensional metal-organic framework (MOF) generated from a new polydentate fluorene-bridged ligand. Acta Crystallogr. 2018, C74, 212–217; https://doi.org/10.1107/s2053229618000815.Search in Google Scholar
6. Wei, Y., Yan, Y., Li, X., Xie, L., Huang, W. Covalent nanosynthesis of fluorene-based macrocycles and organic nanogrids. Org. Biomol. Chem. 2022, 20, 73–97; https://doi.org/10.1039/d1ob01558c.Search in Google Scholar PubMed
7. Pecnikaj, I., Orlandi, S., Pozzi, G., Cappellari, M. V., Marzari, G., Fernández, L., Zensich, M. A., Hernandez, L., Fungo, F. Improving the electropolymerization properties of fluorene-bridged dicarbazole monomers through polyfluoroalkyl side chains. Langmuir 2019, 35, 8732–8740; https://doi.org/10.1021/acs.langmuir.9b01141.Search in Google Scholar PubMed
8. Baheti, A., Justin Thomas, K. R., Li, C.-T., Lee, C.-P., Ho, K.-C. Fluorene-based sensitizers with a phenothiazine donor: effect of mode of donor tethering on the performance of dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2015, 7, 2249–2262; https://doi.org/10.1021/am506149q.Search in Google Scholar PubMed
9. Xia, S., Gao, Y., Wang, P., Ma, Y., Zhu, D., Niu, H., Zhou, T., Wang, W., Zhao, X. Three dimensional fluorene-based polyamides facile to transfer ion designed for near-infrared electrochromic application and detection for explosive. Chem. Eng. J. 2022, 437, 135108; https://doi.org/10.1016/j.cej.2022.135108.Search in Google Scholar
10. Shaya, J., Corridon, P. R., Al-Omari, B., Aoudi, A., Shunnar, A., Mohideen, M. I. H., Qurashi, A., Michel, B. Y., Burger, A. Design, photophysical properties, and applications of fluorene-based fluorophores in two-photon fluorescence bioimaging: a review. J. Photochem. Photobiol., C 2022, 52, 100529; https://doi.org/10.1016/j.jphotochemrev.2022.100529.Search in Google Scholar
11. Tian, X., Shoyama, K., Würthner, F. Nitrogen-doped polycyclic aromatic hydrocarbons by a one-pot Suzuki coupling/intramolecular SNAr reaction. Chem. Sci. 2023, 14, 284–290; https://doi.org/10.1039/d2sc05409d.Search in Google Scholar PubMed PubMed Central
12. Seidel, P., Schwarzer, A., Mazik, M. Crystal structure of 9,9-diethyl-9H-fluorene-2,4,7-tricarbaldehyde. Acta Cryst. 2021, E77, 1029–1032; https://doi.org/10.1107/S2056989021009464.Search in Google Scholar PubMed PubMed Central
13. Robin, J., Audebrand, N., Poriel, C., Canivet, J., Calvez, G., Roisnel, T., Dorcet, V., Roussel, P. A series of chiral metal-organic frameworks based on fluorene di- and tetra-carboxylates: syntheses, crystal structures and luminescence properties. CrystEngComm 2017, 19, 2042–2056; https://doi.org/10.1039/c7ce00108h.Search in Google Scholar
14. Ibragimov, B. T., Weber, E., Beketovn, K. M., Makhkamov, K. K. Crystal structure of 2′H,7′H-dispiro[fluorene-9,2′-dibenzo[c,e]- oxepine-7′ ,9′′-fluorene]. Z. Kristallogr. - N. Cryst. Struct. 2003, 218, 215–216; https://doi.org/10.1524/ncrs.2003.218.jg.215.Search in Google Scholar
15. Ding, Y., Xu, C., Li, Z., Qin, W., Han, X., Han, X., Zhang, C., Yu, C., Wang, X., Li, L., Huang, W. Fast-response fluorogenic probe for visualizing hypochlorite in living cells and in zebrafish. ChemBioChem 2019, 20, 831–837; https://doi.org/10.1002/cbic.201800659.Search in Google Scholar PubMed
16. Enbanathan, S., Manickam, S., Munusamy, S., Jothi, D., Manoj Kumar, S., Kulathu Iyer, S. A phenanthridine-based probe for selective detection of hypochlorite ions. New J. Chem. 2022, 46, 6570–6576; https://doi.org/10.1039/d1nj06023f.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3

